Supplemental Digital Content is available in the article.
Key Words: Astrocytes, Endothelium, Ischemic stroke, Lacunar infarct, Macrophages, Microglia, Neurons, Sulfonylurea receptor 1, Sur1
Abstract
In animal models of stroke, sulfonylurea receptor 1 (Sur1), a member of the adenosine triphosphate binding cassette transporter gene family, is transcriptionally upregulated in neural and vascular cells in which it plays a leading role in edema formation and necrotic cell death. To date, expression of Sur1 in the brains of humans with cerebral infarcts has not been systematically evaluated. We examined Sur1 expression in postmortem specimens obtained from 13 patients within the first 31 days after focal infarcts, 5 patients with lacunar infarcts, and 6 normal control brains using immunohistochemistry. Elevated immunoreactivity for Sur1 was detected in all cases of focal infarcts, with 3 distinct temporal patterns of expression: 1) neurons and endothelium showed the greatest elevation during the first week, after which levels declined; 2) astrocytes and microglia/macrophages showed progressive increases during the first 31 days; and 3) neutrophils near the infarct showed prominent immunoreactivity that did not change over time. Upregulation of Sur1 was corroborated using in situ hybridization for Abcc8 mRNA. Sulfonylurea receptor 1 immunoreactivity in lacunar infarcts was less prominent and more sporadic than in nonlacunar infarcts. In conjunction with previous studies, these data suggest that Sur1 may be a promising treatment target in patients with acute cerebral infarction.
INTRODUCTION
Cerebrovascular disease is a leading cause of death (1, 2). Stroke accounts for 5.5 million deaths or approximately 10% of all deaths worldwide each year. Two thirds of these deaths occur in people living in developing countries, and 40% of stroke patients are younger than 70 years. Cerebrovascular disease also is a leading cause of disability in adults. Many surviving stroke patients never regain functional independence, and each year, millions of stroke survivors are forced to adapt to a life burdened by severe restrictions in activities of daily living. Long-standing efforts to identify pharmacotherapeutic targets to reduce the burden of ischemic stroke, including death and disability, have yet to bear fruit. Recombinant tissue plasminogen activator is the only medication approved by national regulatory agencies specifically for use in acute ischemic stroke and, for a variety of reasons, it is used in less than 20% of stroke victims in developed countries (3–6).
The adenosine triphosphate (ATP) binding cassette (ABC) transporters comprise a large superfamily of membrane proteins, with more than 48 genes encoding human ABC transporters (7–10). Most ABC proteins couple ATP hydrolysis to the translocation of solutes, transporting endogenous substances, xenobiotics, or drugs across biologic membranes (11). Among ABC proteins, the 2 sulfonylurea receptors, Sur1/Abcc8 and Sur2/Abcc9, are exceptional; they are not transporters but instead they coassociate with heterologous pore-forming subunits to form ion channels. For sulfonylurea receptor 1 (Sur1), the most widely recognized association is with the ATP-sensitive K+ channel, Kir6.2/Kcnj11, with which it forms KATP channels in pancreatic β cells and neurons (8, 12, 13). Sulfonylurea receptor 1 also associates with an ATP- and calcium-sensitive nonselective cation channel, recently identified as a transient receptor potential melastatin (Trpm) 4, to form Sur1-Trpm4 channels (14). Although both KATP (Sur1-Kir6.2) and Sur1-Trpm4 channels are regulated by Sur1, these 2 channels have opposite functional effects in CNS injury, that is, opening of KATP channels hyperpolarizes the cell and may be neuroprotective (15), whereas opening of Sur1-Trpm4 channels depolarizes the cell and, if unchecked, is associated with cytotoxic edema and necrotic cell death (16, 17).
Sulfonylurea receptor 1 has been found to be transcriptionally upregulated in neurons, astrocytes, oligodendrocytes, and microvascular endothelial cells in several rodent models of stroke (18, 19). Recently, Sur1 also was identified in microglia in regions of cerebral ischemia (20, 21). To date, however, the expression of Sur1 has not been investigated systematically in human brains after cerebral ischemia. Here, we sought to determine whether cerebral ischemic insults in humans would be associated with increased expression of Sur1 protein and Abcc8 mRNA.
MATERIALS AND METHODS
Human Brain Tissue
The tissue collection protocol was approved by the institutional review board of the University of Maryland, Baltimore, MD. Patients dying within 31 days of documented cerebral ischemia between January 2010 and December 2012 and who underwent autopsy were identified retrospectively by reviewing the records of the Department of Pathology, University of Maryland School of Medicine. A total of 24 ischemic lesions were obtained from 15 men and 5 women, with age at death ranging from 32 to 95 years (mean, 61 years). The lesions consisted of 15 focal infarcts originating from 13 patients and, for comparison, 9 subacute subcortical lacunar infarcts originating from 5 patients. Patients with global ischemia were excluded. In cases of focal infarcts, contralateral cortex was evaluated as control tissue. For lacunar infarcts, adjacent uninvolved tissue more than 1 cm from the lesion and/or a contralateral subcortical nucleus was used as control. Normal control brain specimens were obtained from the brains of 4 men and 2 women who had died rapidly from non-neurological diseases (i.e. acute cardiovascular or respiratory disorders) (Table). Postmortem intervals ranged between 12 and 80 hours. Additional patient demographics are shown in the Table. Histologic validation of the presence of an ischemic lesion was made in all cases by a neuropathologist. For additional validation of antibodies, sections from 2 normal pancreata and 2 normal hippocampi also were evaluated. Standard postmortem fixation (7–10 days in formalin) was applied.
TABLE.
Patient Demographics
Representative paraffin-embedded tissue blocks were selected from each ischemic lesion for further evaluation. For normal brains, 3 blocks encompassing representative frontal and/or parietal cortex, basal ganglia with thalamus, and pons were studied. Blocks were sectioned at 6 μm, and the sections were stained with hematoxylin and eosin or were prepared for immunohistochemistry.
Antibodies
The 2 custom anti-Sur1 antibodies used for this study have been described (14). The antigenic peptide was the intracellular nucleotide-binding domain 1 of Sur1 (rat Sur1 cDNA amino acids 598 to 965 of NP_037171). This region shares 92% identity with the human sequence. Anti-Sur1 antibodies were raised in rabbit and in goat and were used at 1:200 dilution. Other primary antibodies included guinea pig anti-insulin (prediluted, 760-2655; Ventana, Tucson, AZ) for pancreatic β cells; mouse anti–glial fibrillary acidic protein (GFAP) (1:500, CY3 conjugated, C-9205; Sigma, St. Louis, MO) for astrocytes; mouse anti-NeuN (1:100, MAB377; Chemicon, Temecula, CA) for neurons; goat anti-CD31 (PECAM-1, 1:200, sc-1506; Santa Cruz Biotechnology, Santa Cruz, CA) for endothelial cells; rabbit anti-myeloperoxidase (MPO) (1:200, A0398; Dako, Carpinteria, CA) for neutrophils; and mouse anti-CD68 (790-2937; Ventana) for microglia/macrophages.
Immunohistochemistry
Deparaffinized sections were rinsed in ethanol and washed with PBS (10 mmol/L, pH 7.4). For antigen retrieval, slides were placed in citrate buffer (10 mmol/L, pH 8.0) and heated in a microwave oven at 900 W for 10 minutes, then washed in PBS. Slides were incubated with a mixture of 5% goat serum (Sigma) and 0.2% Triton X-100 for 1 hour at room temperature before incubation overnight at 4°C with anti-Sur1 antibody and, in some cases, with a cell-specific primary antibody. The slides were rinsed again in PBS.
Single-label immunohistochemistry for Sur1 was developed using biotin-conjugated secondary antibody. Sections were incubated for 30 minutes in PBS with 20% Tween containing 0.3 % H2O2 to block endogenous peroxidase activity. After overnight incubation with primary antibody, sections were incubated with biotinylated secondary antibody (BA-1000, 1:500, goat anti-rabbit; Vector Laboratories, Burlingame, CA) for 2 hours. After washing in PBS, sections were incubated in avidin-biotin solution (Vector Laboratories), and the color was developed in diaminobenzidine chromogen solution (0.02% diaminobenzidine in 0.175 mol/L sodium acetate) activated with 0.01% H2O2. Cresyl violet was used as a counterstain to visualize cell nuclei. The sections were rinsed, mounted, dehydrated, and coverslipped with DPX mounting medium (Electron Microscopy Services, Fort Washington, PA). Omission of primary antibody was used as a negative control.
To show localization of Sur1 within identified cell types, immunofluorescence was performed using the standard protocol with anti-Sur1 antibody with one of the cell-specific primary antibodies listed above. Slides were incubated for 1 hour with fluorescent-labeled species-appropriate secondary antibodies (1:500, Alexa Fluor 488 and Alexa Fluor 555; Invitrogen/Molecular Probes, Eugene, OR) at room temperature. Omission of primary antibody was used as a negative control. The sections were coverslipped with polar mounting medium containing antifade reagent and 4′,6-diamidino-2-phenylindole ([DAPI] Invitrogen, Eugene, OR). Immunolabeled sections were visualized using epifluorescence microscopy (Nikon Eclipse 90i; Nikon Instruments Inc., Melville, NY).
In Situ Hybridization
A digoxigenin labeled probe (antisense, 5′-TGCAGGGGTCAGGGTCAGGGCGCTGTCGGTCCACTTGGCCAGCCAGTA-3′), designed to hybridize to nucleotides 3217-3264 located within coding sequence of human Abcc8 gene (NM_00352), was supplied by GeneDetect (Brandenton, FL). In situ hybridization (ISH) was performed on 10-μm-thick sections using an IsHyb ISH Kit (Biochain Institute, Inc., Newark, CA) according to the manufacturer’s protocol. Deparaffinization, rehydration, and antigen retrieval were performed as described above. Sections were then incubated in diethyl pyrocarbonate (DEPC)–treated PBS and fixed in 4% paraformaldehyde in PBS for 20 minutes. After rinsing twice with DEPC-PBS, the slides were treated with 10 μg/mL proteinase K at 37°C for 10 minutes. Slides were then washed in DEPC-PBS, rinsed with DEPC-H2O, and prehybridized with ready-to-use prehybridization solution (BioChain Institute) for 3 hours at 50°C. The DIG-labeled probe was diluted in hybridization buffer (BioChain Institute) and applied at 4 ng/μL. Sections then were incubated at 45°C for 16 hours. Posthybridization washing and immunological detection, using anti-DIG-alkaline phosphatase with NBT/BCIP as substrates, were performed as recommended by the manufacturer. Alkaline phosphatase–conjugated anti-DIG antibodies (1:100 PBS diluted; BioChain Institute) were incubated with slides for 2 hours. Finally, slides were rinsed in distilled H2O and then were immunolabeled for Sur1 using a fluorescent secondary antibody, as above. The dark purple reaction product represents Abcc8 mRNA; red fluorescence indicates immunohistochemical staining for Sur1.
Semiquantitative Analysis of Sur1 Protein Expression
Semiquantitative analysis of Sur1 immunoreactivity was performed for each cell type–specific marker for each case. For semiquantitative immunohistochemistry, all sections were immunolabeled as a single batch, and all images were collected using uniform parameters of magnification and exposure, as previously described (22, 23). Two areas encompassing ischemic lesions or controls were randomly selected for construction of a montage, with each montage composed of 16 images, each 300 × 400 μm, that were acquired at 20× magnification.
Images were independently evaluated by 3 observers blinded to demographic and clinical data, including the postischemic interval—the time that had elapsed between the clinical diagnosis of stroke and death. Specific Sur1 immunoreactivity associated with each cell type–specific marker for each case was evaluated. Areas of maximum labeling were scored for each case using a semiquantitative scale ranging from 0 to 4 (0, none; 1, +, present in rare cells; 2, ++, punctate staining in scattered cells or aggregates; 3, +++, punctate staining in most cells; and 4, ++++, present in all cells, with intense labeling). The overall concordance between observers was more than 90%. In cases of disagreement, independent reevaluation was performed by all observers to arrive at the final score.
Statistical Analysis
Scores for Sur1 expression in different cell types were analyzed as a function of time by calculating Spearman correlation coefficient. Calculations were performed using OriginPro version 8 (Origin Lab Corp., Northampton, MA).
RESULTS
Positive Controls
The custom anti-Sur1 antibodies used here were validated previously in CNS tissues from wild-type and Abcc8-/- mice using immunoblots as well as mass spectrometry of immunoisolated protein (14); they also have been used to immunolabel human tissues after spinal cord injury (24). These antibodies were further validated here using human pancreas and human hippocampal tissues, both of which are known to express Sur1 (25–27). Strong cytoplasmic and membranous immunoreactivity was seen in human pancreatic islet cells. Labeling was most intense in and colocalized with insulin-positive β cells (Fig. 1A–D). In human hippocampal tissue, there was intense labeling within granule cells of the dentate gyrus (Fig. 1E) and adjacent pyramidal neurons (Fig. 1F). No labeling was found in pancreatic serous glands adjacent to the islets (Fig. 1A–D).
FIGURE 1.
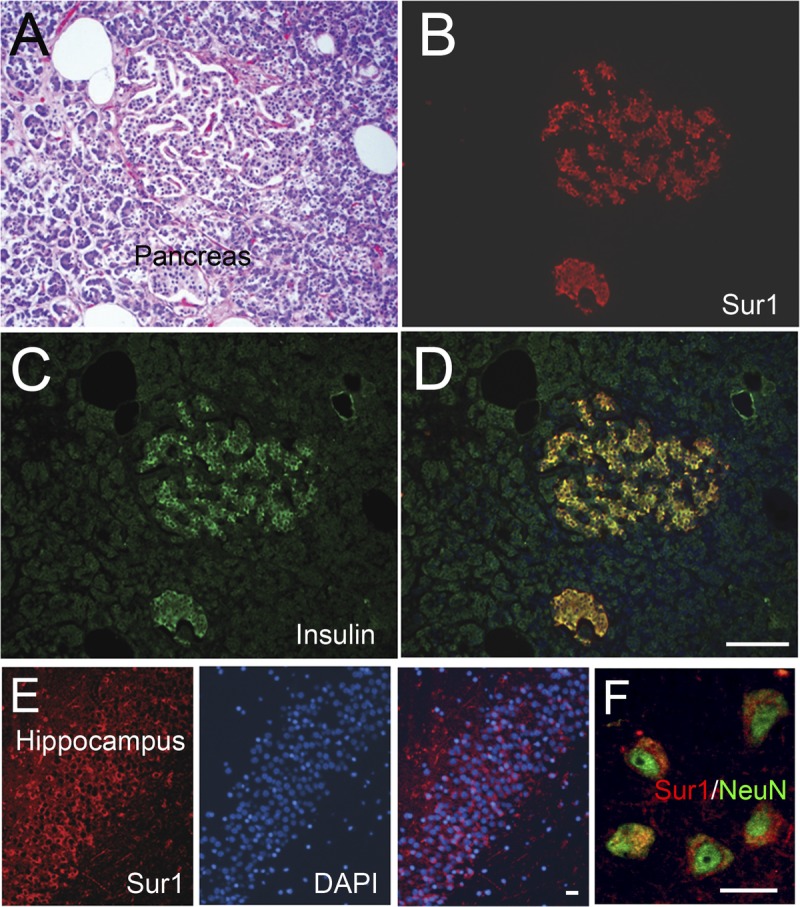
Sulfonylurea receptor 1 (Sur1)–positive controls. (A) A hematoxylin and eosin–stained section of pancreas showing pancreatic islet cells with adjacent fat and serous glands. (B–D) Immunohistochemistry using primary antibodies against Sur1 protein shows labeling of pancreatic islet cells (B), which colocalizes with insulin in β cells (C); merged fluorescent image is shown in (D). (E, F) Immunohistochemistry using primary antibodies against Sur1 shows labeling of dentate granule cells (E) or pyramidal cells (F) of the hippocampus; nuclei are stained with DAPI. In (F), the tissues were double immunolabeled using anti-NeuN antibody, with the merged image shown. Scale bars = (D) 100 μm (for A–D); (E, F) 10 μm. Red/CY3, Sur1; green/FITC, insulin and NeuN; blue, DAPI.
Focal Infarcts
We studied 15 cortices from frontal (n = 4), parietal (n = 5), or occipital (n = 6) infarcts (Table). Patients had died within 1 to 31 days from onset of ischemia (Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A483). Hematoxylin and eosin–stained sections of early lesions showed evolving ischemic changes with variable degrees of neutrophil response and scattered perivascular petechial microhemorrhages (Fig. 2A). Subacute lesions showed cavitation with neovascularization, macrophages, and evolving astrogliosis around the cavities. Many degenerating neuronal cells showed eosinophilia or were shrunken, with a condensed chromatin pattern. Scattered ischemic neurons showed mild nuclear and cytoplasmic enlargement with pale chromatin staining (ghost neurons) or vesicular changes, including swelling and clearing of the cytoplasm and scalloping of the nuclear borders. Extracellular edema and accompanying microvacuolization of the neuropil were variable in each case.
FIGURE 2.
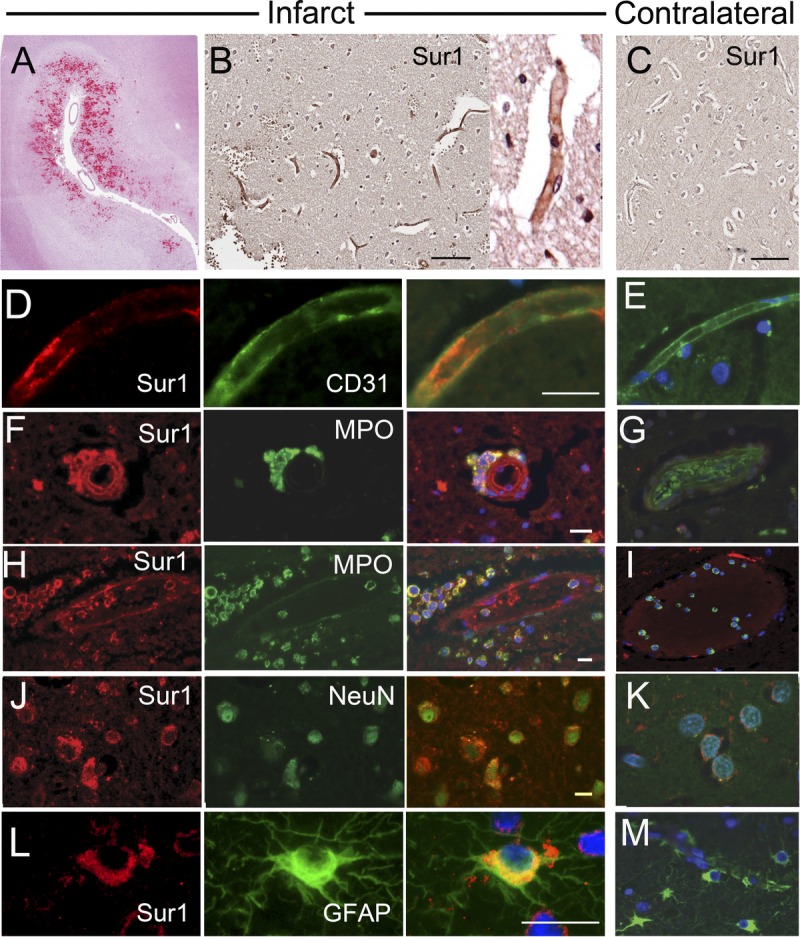
Sulfonylurea receptor 1 (Sur1) immunoreactivity is upregulated in neural and vascular cells in acute ischemic infarcts. (A) Hematoxylin and eosin–stained section of an acute ischemic infarct showing scattered reperfusion microhemorrhages. (B, C) By immunohistochemistry with diaminobenzidine chromogen, upregulated Sur1 immunoreactivity is observed in all neural and vascular cells (B), relative to contralateral cortex (C). (D–L) With fluorescent double labeling, greater Sur1 immunolabeling is observed within CD31-positive capillaries (D), myeloperoxidase (MPO)-positive neutrophils (F, H), NeuN-positive neurons (J), and glial fibrillary acidic protein (GFAP)–positive astrocytes (L) in the ischemic tissues (D, F, H, J, L) versus contralateral control tissues (E, G, I, K, M). Merged images of double labeling are shown in the third and fourth columns. Original magnifications = (A–C) 10×; (D–L) and inset and (B) 40×. Scale bars = (B, C) 100 μm; (D, F, H, J, L) 10 μm. Red/CY3, Sur1; green/FITC, PECAM-1 (CD31), MPO, NeuN, and GFAP; blue, DAPI. The figures are from cases 3 and 6.
Sur1 in Acute Infarcts
Sulfonylurea receptor 1 protein expression was analyzed in lesional tissues and contralateral cortices using immunohistochemistry. Representative sections of acute infarcts (i.e. infarcts 1–7 days in age) are shown in Figures 2 and 3. In immunoperoxidase preparations, Sur1 immunoreactivity was evident in neural and endothelial cells in acute infarcts (Fig. 2B), whereas adjacent tissues and control specimens consistently demonstrated only faint or no Sur1 immunoreactivity in background neuropil (Fig. 2C; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A483).
FIGURE 3.
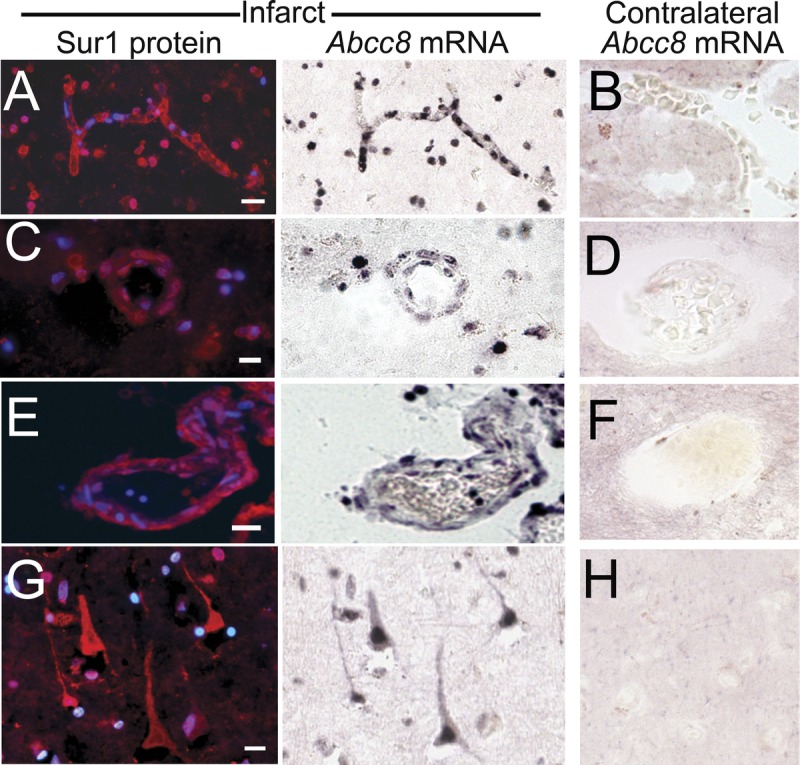
In situ hybridization shows upregulated Abcc8 mRNA in acute ischemic infarcts. (AYH) Tissues from recently infarcted cortex were hybridized with antisense probe directed against Abcc8 and immunolabeled for sulfonylurea receptor 1 (Sur1); greater dual labeling for Abcc8 mRNA and Sur1 protein was identified within endothelial cells in capillaries (A), arterioles (C), and venules (E), and in scattered neutrophils (A, C) and neurons (G) in the ischemic tissues (A, C, E, G) versus contralateral control tissues (B, D, F, H); no counterstain was used. Original magnification = (AYH) 20. Scale bar = 10 Km. These figures are from cases 3 and 6.
In immunofluorescence preparations, there was marked Sur1 immunolabeling in all neural and vascular cells in acute ischemic infarcts (Fig. 2D, F, H, J, L). Endothelial cells of capillaries, arterioles, and venules within ischemic territories showed diffuse membranous and cytoplasmic immunoreactivity for Sur1 and colabeled for the endothelial cell marker PECAM (CD31) (Fig. 2D). In ischemic tissues, immunolabeling also was prominent in intravascular and perivascular neutrophils, which colabeled for MPO (Fig. 2F, H). Intravascular neutrophils in the contralateral hemisphere generally were Sur1 negative (Fig. 2I). Granular or diffuse intense labeling for Sur1 was observed on somata and apical processes of NeuN-positive neurons (Fig. 2J). Perinuclear dotlike staining was seen in scattered perivascular and subpial astrocytes that colabeled for GFAP (Fig. 2L). Sulfonylurea receptor 1 was essentially negative in all neural and vascular cells of noninfarcted contralateral cortices (Fig. 2; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A483).
Sulfonylurea receptor 1 is transcriptionally upregulated after an ischemic insult (17). In situ hybridization was performed to localize Abcc8 mRNA, which encodes Sur1. These experiments showed strong labeling in neurons; endothelial cells of capillaries, venules, and arterioles; and neutrophils (Fig. 3, middle column). In situ hybridization signal for Abcc8 was observed in cells that were immunopositive for Sur1 protein (Fig. 3, left column). Remote and contralateral control tissues were essentially negative for Abcc8 mRNA (Fig. 3, right column).
Sur1 in Subacute Infarcts
Sulfonylurea receptor 1 protein expression was also analyzed in lesional tissues and contralateral cortices of patients with subacute infarcts (i.e. infarcts 15–31 days in age). Despite strong membranous and cytoplasmic staining in acute infarcts, endothelial and neuronal staining was entirely absent in necrotic tissues in the cores of subacute lesions after approximately 16 days postinfarction (arrow, Fig. 4B). However, Sur1 expression was prominent in neutrophils, macrophages, and astrocytes in the vicinity of the lesions (Fig. 4D–F, respectively).
FIGURE 4.
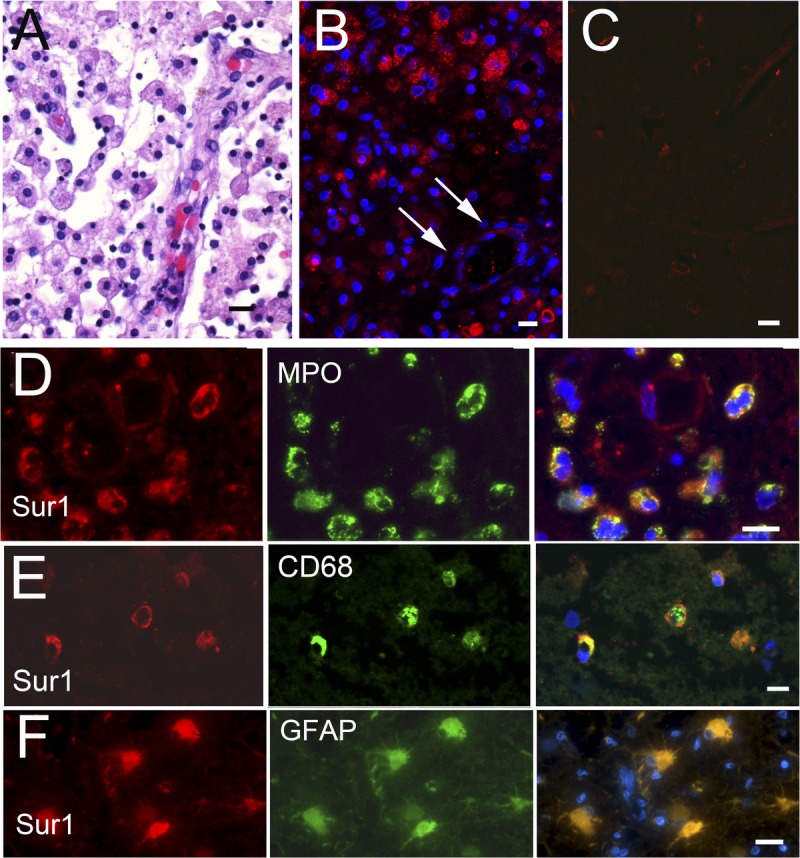
Sulfonylurea receptor 1 (Sur1) immunoreactivity is upregulated in subacute ischemic infarcts only in inflammatory cells. (A) Hematoxylin and eosinYstained section of subacute ischemic infarct, showing abundant microglia/macrophages with neovascularization. Sur1 protein is upregulated in scattered cells within a subacute infarct (B) but not in the contralateral cortex (C). Fluorescent double labeling of subacute infarcts shows colocalization of Sur1 with myeloperoxidase (MPO)-positive neutrophils (D), CD68-positive microglia/macrophages (E), and glial fibrillary acidic protein (GFAP)Ypositive astrocytes (F). There was essentially no staining in CD31-positive endothelial cells or NeuN-positive neurons (data not shown). Merged images are shown in the right column; nuclei were counterstained with DAPI. Original magnifications = (AYC) 10; (DYF) 40. Scale bar = 10 Km. Red/CY3, Sur1; green/FITC, PECAM-1 (CD31), NeuN, MPO, CD68, and GFAP; blue, DAPI. Figures are from cases 11 and 13a.
Time Course of Sur1 Immunoreactivity
Sulfonylurea receptor 1 expression was evaluated semiquantitatively in different cell types at various times after the ischemic event (Fig. 5). In neurons, Sur1 immunopositivity did not correlate with the postmortem interval but did correlate with the postischemic interval (Fig. 5A). Sulfonylurea receptor 1 was evident in neurons and endothelium as early as 1 day after clinical onset of acute infarction and remained strongly positive for the first 7 to 10 days (Fig. 5A, B). Sulfonylurea receptor 1 expression began to diminish at approximately 7 days and reached control levels by 20 to 30 days after infarction. Concomitantly, Sur1 immunolabeling was minimal early on and intensified in hyperplastic and hypertrophied reactive astrocytes and microglia/macrophages in reorganizing infarcted tissues (Fig. 5C). Extravasated neutrophils in ischemic territories remained intensely and uniformly positive at all times, thereby serving as internal controls in these specimens (Fig. 5D; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A483).
FIGURE 5.
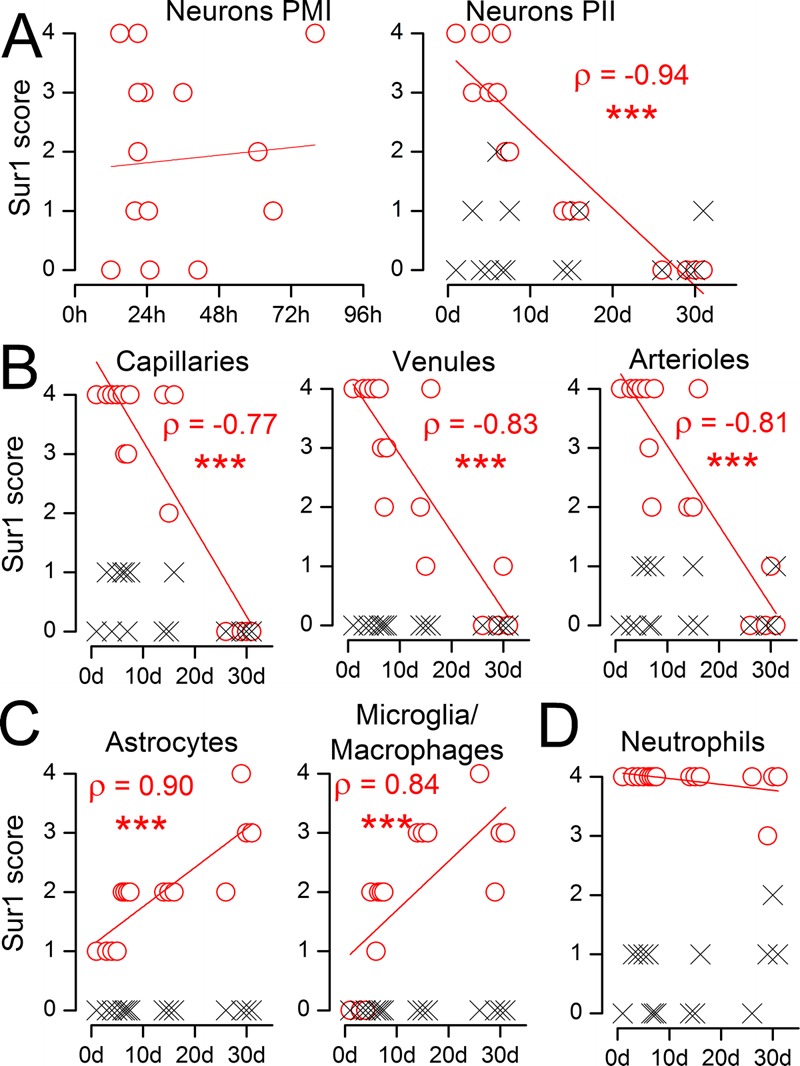
Semiquantitative analysis of the time course of sulfonylurea receptor 1 (Sur1) immunoreactivity in acute and subacute infarcts. (A) The abundance of Sur1 in NeuN-positive neurons from the ischemic region (red circles) and from contralateral cortex (black x) is plotted against the postmortem interval (PMI) (left panel) and against the postischemic interval (PII), the time between the clinical presentation of stroke and death (right panel); Spearman correlation coefficient (ρ) is given; *** p < 0.001. (B–D) The abundance of Sur1 immunoreactivity in CD31-positive endothelium of capillaries, venules, and arterioles (B); in glial fibrillary acidic protein (GFAP)–positive astrocytes and CD68-positive microglia/macrophages (C); and in myeloperoxidase (MPO)-positive neutrophils (D) from the ischemic region (red circles) and from contralateral cortex (black x) is plotted against the postischemic interval (PII).
Pathologic Correlates of Sur1 Immunoreactivity
In acute lesions, scattered Sur1-positive endothelial cells showed thickened vesicular cytoplasm with prominent membrane irregularities, cell blebbing, and frank necrosis (Fig. 6A). These features are consistent with karyolysis associated with evolving cytotoxic edema. Areas of endothelial blebbing (zeiosis) were associated with acute inflammatory infiltrates and early petechial microhemorrhages within central regions of ischemic infarcts. In addition, early blebbing was seen in neuronal cell bodies, extending into proximal dendrites and axons (Fig. 6C). Cellular membrane blebbing was not identified within Sur1-negative neural or vascular cells in adjacent tissue, controls, or infarcted brain (Fig. 6B, D).
FIGURE 6.
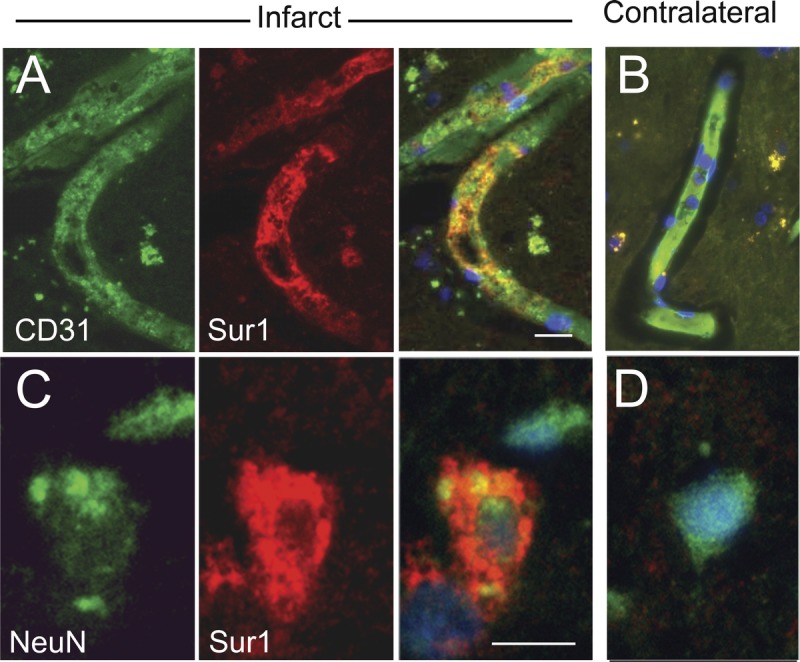
Oncosis and cell blebbing in sulfonylurea receptor 1 (Sur1)–immunopositive endothelial cells and neurons in acute cerebral infarcts. (A, B) Recently infarcted cortex shows degenerating capillaries with CD31-positive endothelial cells (A) that immunolabel intensely for Sur1 (merged image on the right); vessels in the contralateral control tissues are negative for Sur1 (B). (C, D) Recently infarcted cortex shows degenerating neurons with variable labeling intensity for NeuN (C) and marked immunoreactivity for Sur1; neurons in the contralateral control tissues are negative for Sur1 (D); merged images are shown in the right columns of (A, C) and in (B, D). Note the cytoplasmic swelling with membrane irregularities, that is, features of osmotic necrosis (blebbing), within Sur1-positive endothelial cells and neurons (A, C); nuclei are counterstained with DAPI. Original magnification = (A–D) 100×. Scale bar = 10 μm. Red/CY3, Sur1; green/FITC, PECAM-1 (CD31) and NeuN; blue, DAPI. The figures are from cases 3 and 6.
Subcortical Lacunar Infarcts
Lacunar infarcts were present within the caudate (n = 6), putamen (n = 1), thalamus (n = 1), and basis pontis (n = 1) and ranged from 2 to 12 mm in diameter (Table). Hematoxylin and eosin–stained sections showed focal cavitation composed predominantly of foamy macrophages. Recent hemorrhage and scant neutrophils were evident to variable extents in each case. Evolving astrogliosis was seen at the periphery of some lesions. There were few or moderate numbers of axonal spheroids. Intralesional microvessels were present. The postinfarction intervals in these cases was unknown, although the histologic appearances favored subacute to chronic infarcts.
Distribution of Sur1 in Lacunar Infarcts
Sulfonylurea receptor 1 protein expression was analyzed in lesional tissues and contralateral subcortical nuclei, as above. A representative section of a lacunar infarct is shown in Figure 7A. Expression of Sur1 was evident to varying degrees in lacunar infarcts (Fig. 7B), whereas control specimens consistently demonstrated only faint or no Sur1 immunoreactivity in background neuropil (Fig. 7C; Table, Supplemental Digital Content 1, http://links.lww.com/NEN/A483). At high power, Sur1 immunolabeling was detected in endothelial cells of scattered vessels, neurons, inflammatory cells, and glia (Fig. 7D–H). Partial membranous staining was noted in endothelial cells. Punctate granular membranous staining was observed within neuronal cell bodies and apical processes. Neutrophils, microglia/macrophages, and astrocytes were variably positive. Cell blebbing was not found in Sur1-positive or Sur1-negative cells within lacunar infarcts.
FIGURE 7.
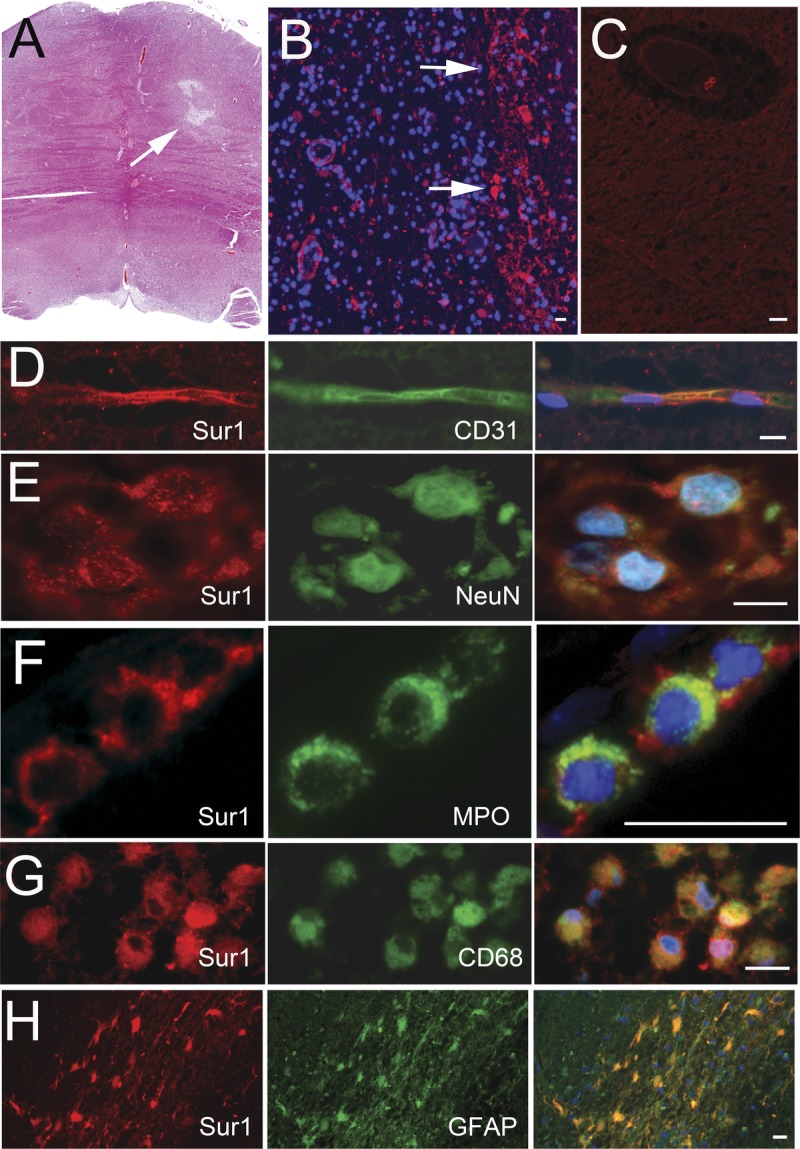
Variable upregulation of sulfonylurea receptor 1 (Sur1) immunoreactivity within lacunar infarcts. (A) Hematoxylin and eosin–stained section of a lacunar infarct showing abundant microglia/macrophages, with neovascularization and evolving gliosis. (B, C) Sur1 protein immunoreactivity is upregulated in several cells in the region of the infarct (B) but is absent in a contralateral control subcortical nucleus (C). (D–H) Fluorescent double labeling of lacunar infarcts shows punctate staining within CD31-positive capillaries (D) and NeuN-positive neurons (E) and marked labeling of inflammatory cells, including myeloperoxidase (MPO)-positive neutrophils, CD68-positive microglia/macrophages, and glial fibrillary acidic protein (GFAP)–positive astrocytes (F–H, respectively); merged images are shown in the right column; nuclei are counterstained with DAPI. Original magnifications = (A–C, H) 10×; (D–G) 40×. Scale bar = 10 μm. Red/CY3, Sur1; green/FITC, PECAM-1/CD31, MPO, NeuN, CD68, and GFAP; blue, DAPI. The figures are from cases 16a and 16b.
DISCUSSION
This is the first report of systematic evaluation of Sur1 expression in adult human brains after an acute ischemic event. The findings indicate that focal infarcts are associated with upregulation of Abcc8 mRNA and Sur1 protein and that expression of Sur1 changes with time in different ways depending on the cell type. A single previous study examining tissues from 12 infants with germinal matrix hemorrhage also reported Sur1 upregulation (28). In that study, regionally specific upregulation of Abcc8 mRNA and Sur1 protein was found in the germinal matrix and was correlated with expression of the transcription factor, hypoxia inducible factor 1 (Hif-1), which was used as a marker of hypoxia. Other reports of Sur1 in human cerebral ischemia have been limited to single cases (17, 29). The findings reported here showing early upregulation in focal infarcts are in accord with observations in animal models of stroke in which Sur1 has been found to be upregulated within a few hours of the onset of focal ischemia (17).
This is the first report in any species to show that the temporal pattern of Sur1 expression after cerebral ischemia differs in different cell types. We speculate that the different patterns of expression observed may reflect the fact that different transcriptional programs are responsible for Sur1 upregulation in different cell types. We found that Sur1 was maximally upregulated in neurons and endothelium of arterioles, capillaries, and veins during the first several days after the event and showed progressive decline thereafter, reaching levels found in controls by 20 to 30 days (or earlier) after the ischemic event.
In animal brains subjected to ischemic insults, endothelial cells are the first cell type to demonstrate Sur1 upregulation. This occurs within 3 hours of the onset of ischemia and is followed somewhat later by Sur1 upregulation in neurons (30). In neurons and endothelial cells, Hif-1 and specificity protein (Sp) 1 play prominent roles in transcriptional upregulation of Abcc8/Sur1. Ischemia induces hypoxia, thereby activating Hif-1. Hypoxia inducible factor 1 activation causes transcriptional upregulation of Sp1, and Sp1 in turn causes transcriptional upregulation of Abcc8/Sur1 (18, 30). In neurons and endothelium, Sur1 upregulation in the context of focal ischemia has been shown to be associated with de novo expression of Sur1-Trpm4 channels (18, 30).
By contrast, we found that Sur1 expression in astrocytes and microglia/macrophages was minimal initially but increased progressively over the subsequent 30 days. Astrocytes and microglia/macrophages participate in a reactive or inflammatory response after a sterile insult such as the extravasation of blood, which induces Toll-like receptor 4–mediated nuclear factor-κB (NF-κB) signaling (31–33). Experimental studies have shown that, when the brain is subjected to a proinflammatory stimulus such as hemorrhage, NF-κB signaling causes transcriptional upregulation of Abcc8/Sur1, which progresses during the course of several days (22, 34, 35). In reactive astrocytes, Sur1 upregulation is known to be associated with de novo expression of Sur1-Trpm4 channels (34, 35). In microglia/macrophages, Sur1 upregulation is associated with KATP (Sur1-Kir6.2) channels (20, 21).
Finally, we found that intravascular as well as extravasated neutrophils in ischemic territories express abundant Sur1, whereas intravascular neutrophils in the contralateral hemisphere showed little or no Sur1. Activated neutrophils exhibited a consistently high extent of Sur1 immunoreactivity that did not diminish with time. These observations are consistent with the hypothesis that Sur1 upregulation in neutrophils may be associated with neutrophil activation. Based largely on their response to the Sur1 inhibitor, glibenclamide, it is believed that neutrophils involved in an inflammatory response (36) or ischemia/reperfusion (37, 38) express Sur1-Kir6.2 (KATP) channels. To our knowledge, molecular validation of the presence of Sur1-Kir6.2 channels in neutrophils has not been presented, and transcriptional regulation of Abcc8/Sur1 after neutrophil activation has not been studied.
The importance of Sur1 upregulation in humans with focal infarcts is underscored by 2 retrospective studies of patients with type 2 diabetes mellitus presenting with acute ischemic stroke (29, 39). In both studies, outcomes in patients whose diabetes was managed with and who remained on a sulfonylurea drug during hospitalization were compared with outcomes in patients whose diabetes was managed without sulfonylurea drugs. In the first study, the primary outcome measure was a “major neurological improvement,” defined as an improvement on the National Institutes of Health stroke scale of 4 points or more, or reaching 0 by the time of discharge (40). Among patients with focal strokes, it was found that 42% of those in the sulfonylurea group achieved the criterion of “major neurological improvement,” whereas none did in the control group. In the second study, the primary outcome measures were symptomatic hemorrhagic transformation and death. It was found that no patient in the sulfonylurea group experienced either event compared with 11% incidence of hemorrhagic transformation and 10% death in the control group. These studies complement the findings of the present report and support the hypothesis that the target of sulfonylurea drugs, that is, Sur1, may be involved in the pathophysiologic response to cerebral ischemia in humans.
We found that Sur1 was upregulated in some cases with lacunar infarcts, albeit less prominently and less consistently than in focal infarcts. It is possible that the variability in Sur1 expression that we observed was caused by differences in the ages of the infarcts, given that the time of onset of ischemia was not well documented with the lacunar infarcts. However, it is also possible that the molecular pathophysiology of lacunar infarcts differs from that of a more severe focal infarct (46, 47).
In animal models of stroke, accidental necrosis in the setting of ATP depletion results in oncosis caused by dysregulated influx of Na+ ions (18–21, 41–45). This process is distinct from regulated necrosis and results not only in cytoplasmic volume increase but also membrane blebbing and eventual membrane rupture. Trpm4, a monovalent cation channel, is activated in pathologic states and has been shown to play a critical role in this process of oncosis, likely functioning as an end-executioner in accidental necrotic cell death (16). This channel has recently been shown to coassociate with Sur1, which regulates its activity (14).
Important progress in the prevention and treatment of stroke has been made in recent years, but optimizing the outcomes of patients with cerebral infarction remains a major clinical challenge. Affected patients are at high risk for progressive neurologic deterioration and death caused by irreversible cerebral edema. Identifying promising treatment targets for patients with stroke remains a critical goal. Recent work identifying the important role of Sur1 in necrotic cell death and edema formation after cerebral ischemia suggests that inhibiting Sur1 may be beneficial in patients with acute stroke.
Supplementary Material
Footnotes
Send correspondence and reprint requests to: J. Marc Simard, MD, PhD, Department of Neurosurgery, 22 S. Greene St., Suite S12D, Baltimore, MD 21201-1595; E-mail: msimard@smail.umaryland.edu
This work was supported by grants to J. Marc Simard from the National Heart, Lung and Blood Institute (HL082517) and the National Institute of Neurological Disorders and Stroke (NS061808).
J. Marc Simard holds a US patent (7,285,574), “A novel nonselective cation channel in neural cells and methods for treating brain swelling.” J. Marc Simard is a member of the scientific advisory board and holds shares in Remedy Pharmaceuticals. No support, direct or indirect, was provided to J. Marc Simard or for this project by Remedy Pharmaceuticals. All other authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jneuropath.com).
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License, where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially.
REFERENCES
- 1. Truelsen T, Begg S, Mathers C. The global burden of cerebrovascular disease. Available at: http://www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf. Accessed January 10, 2013
- 2. Go AS, Mozaffarian D, Roger VL, et al. Executive summary: Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013; 127: 143– 52 [DOI] [PubMed] [Google Scholar]
- 3. Adeoye O, Hornung R, Khatri P, et al. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: A doubling of treatment rates over the course of 5 years. Stroke 2011; 42: 1952– 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kleindorfer D, Lindsell CJ, Brass L, et al. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008; 39: 924– 28 [DOI] [PubMed] [Google Scholar]
- 5. Singer OC, Hamann GF, Misselwitz B, et al. Time trends in systemic thrombolysis in a large hospital-based stroke registry. Cerebrovasc Dis 2012; 33: 316– 21 [DOI] [PubMed] [Google Scholar]
- 6. Jauss M, Schutz HJ, Tanislav C, et al. Effect of daytime, weekday and year of admission on outcome in acute ischaemic stroke patients treated with thrombolytic therapy. Eur J Neurol 2010; 17: 555– 61 [DOI] [PubMed] [Google Scholar]
- 7. Aguilar-Bryan L, Clement JP, Gonzalez G, et al. Toward understanding the assembly and structure of KATP channels. Physiol Rev 1998; 78: 227– 45 [DOI] [PubMed] [Google Scholar]
- 8. Aittoniemi J, Fotinou C, Craig TJ, et al. Review. SUR1: A unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci 2008; 364: 257– 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lefer DJ, Nichols CG, Coetzee WA. Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med 2009; 19: 61– 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol 2005; 39: 51– 60 [DOI] [PubMed] [Google Scholar]
- 11. Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol 2007; 17: 412– 18 [DOI] [PubMed] [Google Scholar]
- 12. Bryan J, Munoz A, Zhang X, et al. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch 2007; 453: 703– 18 [DOI] [PubMed] [Google Scholar]
- 13. Burke MA, Mutharasan RK, Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ Res 2008; 102: 164– 76 [DOI] [PubMed] [Google Scholar]
- 14. Woo SK, Kwon MS, Ivanov A, et al. The sulfonylurea receptor 1 (sur1)-transient receptor potential melastatin 4 (trpm4) channel. J Biol Chem 2013; 288: 3655– 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamada K, Inagaki N. Neuroprotection by KATP channels. J Mol Cell Cardiol 2005; 38: 945– 49 [DOI] [PubMed] [Google Scholar]
- 16. Simard JM, Woo SK, Gerzanich V. Transient receptor potential melastatin 4 and cell death. Pflugers Arch 2012; 464: 573– 82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simard JM, Woo SK, Schwartzbauer GT, et al. Sulfonylurea receptor 1 in central nervous system injury: A focused review. J Cereb Blood Flow Metab 2012; 32: 1699– 717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med 2006; 12: 433– 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simard JM, Tsymbalyuk N, Tsymbalyuk O, et al. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke 2010; 41: 531– 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, et al. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp Neurol 2012; 235: 282– 96 [DOI] [PubMed] [Google Scholar]
- 21. Ortega FJ, Jolkkonen J, Mahy N, et al. Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia. J Cereb Blood Flow Metab 2013; 33: 356– 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simard JM, Geng Z, Woo SK, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2009; 29: 317– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerzanich V, Ivanov A, Ivanova S, et al. Alternative splicing of cGMP-dependent protein kinase I in angiotensin-hypertension: Novel mechanism for nitrate tolerance in vascular smooth muscle. Circ Res 2003; 93: 805– 12 [DOI] [PubMed] [Google Scholar]
- 24. Simard JM, Woo SK, Norenberg MD, et al. Brief suppression of Abcc8 prevents autodestruction of spinal cord after trauma. Sci Transl Med 2010; 2: 28ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guiot Y, Stevens M, Marhfour I, et al. Morphological localisation of sulfonylurea receptor 1 in endocrine cells of human, mouse and rat pancreas. Diabetologia 2007; 50: 1889– 99 [DOI] [PubMed] [Google Scholar]
- 26. Holemans S, Javoy-Agid F, Agid Y, et al. Sulfonylurea binding sites in normal human brain and in Parkinson’s disease, progressive supranuclear palsy and Huntington’s disease. Brain Res 1994; 642: 327– 33 [DOI] [PubMed] [Google Scholar]
- 27. Ikeda M, Dewar D, McCulloch J. Differential alterations of ion channel binding sites in temporal and occipital regions of the cerebral cortex in Alzheimer’s disease. Brain Res 1993; 630: 50– 56 [DOI] [PubMed] [Google Scholar]
- 28. Simard JM, Castellani RJ, Ivanova S, et al. Sulfonylurea receptor 1 in the germinal matrix of premature infants. Pediatr Res 2008; 64: 648– 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kunte H, Busch MA, Trostdorf K, et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol 2012; 72: 799– 806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woo SK, Kwon MS, Geng Z, et al. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab 2012; 32: 525– 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teng W, Wang L, Xue W, et al. Activation of TLR4-mediated NFkappaB signaling in hemorrhagic brain in rats. Mediators Inflamm 2009; 2009: 473276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma CX, Yin WN, Cai BW, et al. Toll-like receptor 4/nuclear factor-kappa B signaling detected in brain after early subarachnoid hemorrhage. Chin Med J (Engl) 2009; 122: 1575– 81 [PubMed] [Google Scholar]
- 33. Lin S, Yin Q, Zhong Q, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation 2012; 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen M, Simard JM. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J Neurosci 2001; 21: 6512– 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen M, Dong Y, Simard JM. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J Neurosci 2003; 23: 8568– 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Da Silva-Santos JE, Santos-Silva MC, Cunha FQ, et al. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J Pharmacol Exp Ther 2002; 300: 946– 51 [DOI] [PubMed] [Google Scholar]
- 37. Pompermayer K, Amaral FA, Fagundes CT, et al. Effects of the treatment with glibenclamide, an ATP-sensitive potassium channel blocker, on intestinal ischemia and reperfusion injury. Eur J Pharmacol 2007; 556: 215– 22 [DOI] [PubMed] [Google Scholar]
- 38. Pompermayer K, Souza DG, Lara GG, et al. The ATP-sensitive potassium channel blocker glibenclamide prevents renal ischemia/reperfusion injury in rats. Kidney Int 2005; 67: 1785– 96 [DOI] [PubMed] [Google Scholar]
- 39. Kunte H, Schmidt S, Eliasziw M, et al. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke 2007; 38: 2526– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581– 7 [DOI] [PubMed] [Google Scholar]
- 41. Simard JM, Yurovsky V, Tsymbalyuk N, et al. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke 2009; 40: 604– 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wali B, Ishrat T, Atif F, et al. Glibenclamide administration attenuates infarct volume, hemispheric swelling, and functional impairments following permanent focal cerebral ischemia in rats. Stroke Res Treat 2012; 2012: 460909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simard JM, Woo SK, Tsymbalyuk N, et al. Glibenclamide-10-h treatment window in a clinically relevant model of stroke. Transl Stroke Res 2012; 3: 286– 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simard JM, Geng Z, Silver FL, et al. Does inhibiting Sur1 complement rt-PA in cerebral ischemia? Ann N Y Acad Sci 2012; 1268: 95– 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nistico R, Piccirilli S, Sebastianelli L, et al. The blockade of K(+)-ATP channels has neuroprotective effects in an in vitro model of brain ischemia. Int Rev Neurobiol 2007; 82: 383– 95 [DOI] [PubMed] [Google Scholar]
- 46. Del BA, Palumbo V, Lamassa M, et al. Progressive lacunar stroke: Review of mechanisms, prognostic features, and putative treatments. Int J Stroke 2012; 7: 321– 29 [DOI] [PubMed] [Google Scholar]
- 47. Knottnerus IL, Ten CH, Lodder J, et al. Endothelial dysfunction in lacunar stroke: A systematic review. Cerebrovasc Dis 2009; 27: 519– 26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


