Abstract
Alterations in arousal states are associated with multiple neuropsychiatric disorders including generalized anxiety disorders, addiction, schizophrenia, and depression. Therefore, elucidating the neurobiological mechanisms controlling the boundaries between arousal, hyperarousal, and hypoarousal is a crucial endeavor in biological psychiatry. Substantial research over several decades has identified distinct arousal-promoting neural populations in the brain; however, how these nuclei act individually and collectively to promote and maintain wakefulness and various arousal states is unknown. We have recently applied optogenetic technology to the repertoire of techniques used to study arousal. Here, we discuss the recent results of these experiments and propose future use of this approach as a way to understand the complex dynamics of neural circuits controlling arousal and arousal-related behaviors.
Keywords: sleep, wakefulness, arousal, hypothalamus, locus coeruleus, optogenetics
Introduction
Although states of sleep and wakefulness are qualitatively and quantitatively easy to characterize, it is relatively difficult to define arousal. Sleep is characterized as a period of relative inactivity with stereotyped posture, higher sensory threshold, and unconsciousness. Wakefulness is a conscious state in which an animal can perceive and interact with its environment. States of both sleep and wakefulness can be quantitatively identified and characterized by patterns of cortical activity on an electroencephalogram (EEG) and intensity of motor activity on an electromyogram (EMG). The term “arousal” refers to the degree of vigilance and alertness during wakefulness. In mammals it can be described as a state of wakefulness with increased motor activation, responsiveness to sensory inputs, emotional reactivity, and enhanced cognitive processing (1). Depending on the environmental circumstances, arousal systems increase the vigilance of an animal so that an animal can properly react to a stressor, initiate the flight-or-fight response, courtship and reproductive behaviors, or engage in goal-directed behaviors that are mandatory for behavioral adaptation and survival.
Mild perturbations of arousal in humans are considered symptoms of many psychiatric disorders (2). For example, hyperarousal is a symptom of schizophrenia, addiction, and generalized anxiety disorder (GAD) (3). In contrast, hypoarousal correlates with aggressiveness and attention deficit hyperactivity disorder (ADHD) (4, 5). Although hypocretins, as well as other arousal circuits, participate to the setting of arousal threshold in hyper- or hypo-arousal (6), it remains unknown whether those changes are causally linked to such symptoms. Therefore, elucidating the neurobiological substrates of wakefulness and arousal is important for understanding the intensity of vigilance, sensory responsiveness, and emotional reactivity that are often impaired in psychiatric conditions. Despite the enormous progress in identifying neural populations that regulate wakefulness and arousal, it is still unclear how these circuits are anatomically and functionally interposed to affect behavior in different environmental contexts.
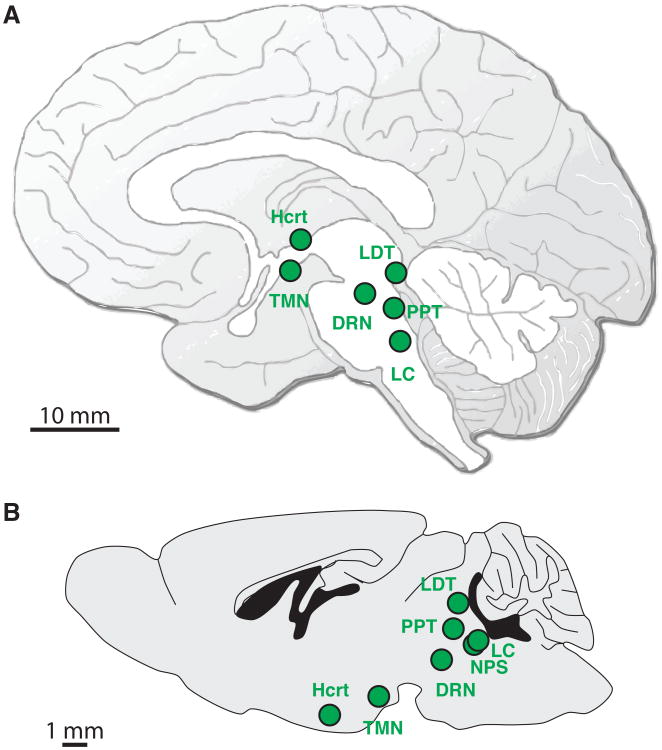
Arousal is regulated by distinct neural populations in the brain (Figure 1A, B). Activity in these nuclei is correlated with arousal: not only does their activity increase when an animal is awake compared to asleep, but this activity also increases during states of enhanced arousal, such as moments of high alertness or stress (7). These arousal systems include:
The hypocretin (Hcrt)-expressing neurons in the lateral hypothalamus
The noradrenergic locus coeruleus (LC)-expressing neurons in the brainstem
The neuropeptide S neurons (NPS) in the brainstem
The serotoninergic dorsal raphe nuclei (DRN) in the brainstem
The histaminergic tuberomammilary nucleus (TMN) in the posterior hypothalamus
The cholinergic pedunculopontine (PPT) and laterodorsal tegmental (LDT) nuclei in the midbrain, as well as cholinergic neurons in the basal forebrain
Glutamatergic and GABAergic neurons located in the above mentioned nuclei and brain areas.
Figure 1.
Schematic representation of the evolutionary conserved arousal systems in the human (A) and mouse (B) brain. These systems include the hypocretin (Hcrt) neurons (lateral hypothalamus), the noradrenergic neurons (locus coeruleus), the neuropeptide S neurons (brainstem), the serotoninergic neurons (dorsal raphe nuclei), the histaminergic neurons (tuberomammilary nucleus in the posterior hypothalamus), the cholinergic neurons (pedunculopontine and laterodorsal tegmental nuclei in the midbrain, as well as basal forebrain). Local glutamatergic and GABAergic neurons located in the vicinity of these arousal systems are not represented.
Activation of these systems not only promotes wakefulness, but also engages other arousal-related behavioral outputs such as reward-seeking, sexual activity, flight-or-fight responses, etc. Interestingly, specific behavioral outputs are not necessarily consistent from one arousal system to another. For example, activation of the LC-norepinephrine system increases arousal and can cause anxiety-like behaviors (8). In contrast, the NPS system also increases arousal but decreases anxiety (9). Thus, to support such diverse behaviors, these arousal systems must be fine-tuned based on their specific afferent and efferent connections into extremely specific circuits to regulate specific arousal-related behaviors.
How do each of these arousal systems specifically affect wakefulness and arousal? How do they functionally interact to promote and maintain particular arousal states in specific contexts? We and others have recently begun to address these questions, especially focusing on the Hcrt and LC systems. Here, we summarize recent optogenetic experiments that test the hypothesis that Hcrt and LC neurons cause arousal state transitions and maintenance. First, we briefly highlight and summarize previous reports about these systems using traditional genetic and pharmacological techniques. Next we integrate our own findings using optogenetic probes to selectively stimulate or inhibit these systems in freely moving mice. Finally, we discuss unresolved questions and speculate on future anatomical and functional dissections of arousal circuits.
The Hypocretin System
Hypocretins (10) are a pair of secreted neuropeptides, hypocretin-1 and hypocretin-2 (Hcrt1 and Hcrt2) that are cleaved from the same genetic precursor. These peptides are exclusively expressed by a population of glutamatergic neurons located in the lateral hypothalamus (Figure 2A) and bind with different affinities to two Hcrt receptors, Hcrt-r1 and Hcrt-r2 (11). These receptors are located on postsynaptic terminals in a pattern consistent with the efferent projections of Hcrt neurons (Figure 2A).
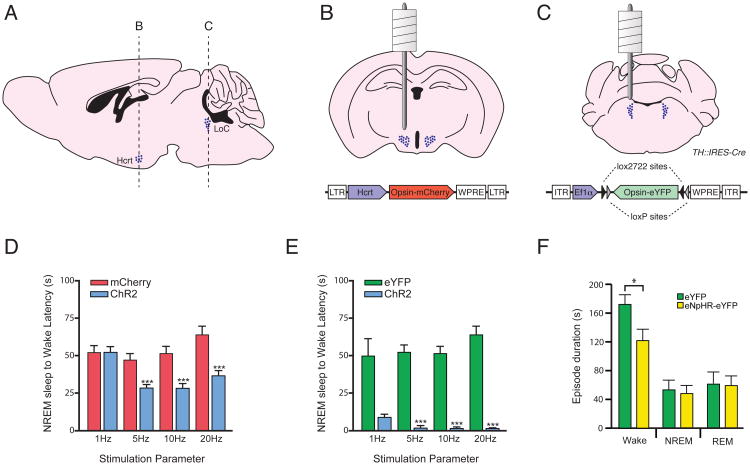
Figure 2.
Optogenetic modulation of Hcrt and NA neurons in vivo. Schematic drawings representing the anatomical location of Hcrt- and noradrenaline (NE)-producing neurons in sagittal (A) and coronal (B, C) sections through the mouse brain. B and C (top panels) show the stereotactic placement of cannula guide for deep brain light delivery through optical fibers in freely-moving animals. Note that the tip of the optical fiber has a short projection from the cannula guide to target the entire hcrt or NA fields. B and C (bottom panels) represent the backbone of the virus vector used to genetically target Hcrt and NE neurons. (D) Optogenetic stimulation at high frequencies (>5 Hz) of Hcrt neurons increase the probability of NREM sleep-to-wake transitions (modified from Adamantidis et al. 2007 (36)). (E) Optogenetic stimulation of LC-NE neurons at all frequencies increases the probability of NREM sleep-to-wake transitions (modified from Carter et al. 2010 (38)). (F) The duration of individual wake, NREM, and REM episodes during 1 h photoinhibition during the active period. *p<0.05, ***p<0.0001, Student's t-test between transduced animals.
Hypocretin neurons show high discharge activity during arousal elicited by environmental stimuli (e.g. an auditory stimulus) and behavior accompanied with a strong locomotor activity (e.g. goal-oriented behaviors) (12-14). These recordings suggest that in an awake state, Hcrt neurons may participate in the neurobiological mechanisms underlying alertness and in the increase in arousal observed during various goal-oriented behaviors. Hcrt neurons are generally silent during quiet wakefulness, non-rapid eye movement (NREM) sleep, and REM sleep and are reactivated during REM sleep-to-wake transitions.
Studies that block or suppress Hcrt signaling demonstrate that Hcrts are necessary for maintaining wakefulness or emerging from anesthesia in mice, rats, dogs, and humans (15). The most compelling loss-of-function evidence comes from the link between Hcrt deficiency and the symptoms of narcolepsy (16, 17). Narcoleptic patients with cataplexy have non- or barely-detectable levels of Hcrt in the cerebrospinal fluid and an absence of Hcrt gene transcripts in the hypothalamus. Doberman narcoleptic dogs bear a mutation in Hcrt-r2, and all genetically engineered rodents with either a deletion of Hcrt, Hcrt-r2, or Hcrt cells present behavioral arrests that resemble cataplexy, the hallmark of narcolepsy (17).
Intracerebroventricular (i.c.v.) infusion of Hcrt peptides or Hcrt agonists causes an increase in the time spent awake and a decrease in NREM and REM sleep (15). Stereotactic injections of the peptide in the LC, LTD, basal forebrain, and lateral hypothalamus each also cause increased wakefulness and locomotor activity accompanied by a marked reduction in NREM and REM sleep. Interestingly, Hcrt administration can reverse behavioral attacks in narcoleptic dogs. More recently, genetic disinhibition of Hcrt neurons using a selective GABA-B receptor gene deletion only in Hcrt neurons induced severe fragmentation of sleep/wake states during both the light and dark periods without showing an abnormality in total sleep/wake durations or signs of cataplexy (18). Although one should not expect the occurrence of cataplexy upon disinhibition of Hcrt neurons, it is puzzling that the total duration of sleep/wake duration remains unchanged in those conditions. A possible explanation for this sleep/wake fragmentation comes from the persistent genetic disinhibition of Hcrt neurons, and thus the increased transitions from sleep to wakefulness. This is somewhat consistent with our optogenetic studies (see below).
The Locus Coeruleus / Norepinephrine System
The LC is located adjacent to the 4th ventricle in the brainstem and synthesizes the monoamine norepinephrine (NE) (19-22). At least four other cell populations produce norepinephrine (the A1, A2, A5, and A7 cell groups), but the LC produces approximately 50% of the brain's total NE and is the only source to the cortex (19-22). There are many functional NE receptors located throughout the brain, with α1 and β receptors usually causing excitatory postsynaptic potentials and α2 receptors usually causing inhibitory postsynaptic potentials (19-22). α2 receptors are densely found on LC neurons themselves and serve as inhibitory autoreceptors to suppress intrinsic activity.
Recordings in awake, behaving animals show that LC neurons fire tonically at 1-3 Hz during awake states, fire less during NREM sleep, and are virtually silent during REM sleep (23, 24). The LC also fires phasically in short bursts of 8-10 Hz during the presentation of salient stimuli that prolong wake states (23, 25). Like Hcrt neurons, alterations in discharge rate precede changes in sleep-to-wake transitions (23, 24).
Interestingly, physical lesions of the LC do not elicit consistent changes in cortical EEG or behavioral indices of arousal (26-28). Genetic ablation of dopamine beta-hydroxylase, an enzyme required for NE synthesis, also does not disrupt sleep-wake states (29). However, central injections of pharmacological antagonists of α1 and β noradrenergic receptors or agonists of inhibitory α2 autoreceptors have substantial sedative effects (30). In contrast, central administration of NE directly into the ventricles or forebrain promotes wakefulness (31, 32). Stimulation of neurons in the LC using local microinjections of the cholinergic agonist bethanechol produces rapid activation of the forebrain EEG in halothane-anesthetized rats (33). Recently, the LC-norepinephrine system was shown to be critical for maintaining the increased membrane potential of cortical neurons in awake compared to sleep states (34). Taken together, these studies imply that the LC-NE system desynchronizes cortical activity and increases cortical membrane potential to increase arousal.
Acute Control of Arousal Using Optogenetics
Experimental evidence reviewed in the previous sections consistently suggests that both the Hcrt and NE systems represent important arousal circuits of the brain. However, it remains unknown whether (1) these systems are permissive or sufficient to promote or maintain arousal; (2) if Hcrt and NE neurons have defined patterns of discharge (e.g., spike frequency) for arousal control, and (3) if selective activation of these systems is causally associated with increased arousal. In this section, we summarize the main advantage of the optogenetic technology to dissect neural circuits controlling arousal, as illustrated by recent studies.
Selectively stimulating or inhibiting specific cell populations without affecting surrounding cells or fibers-of-passage has been difficult using traditional pharmacological, electrical, and physical techniques (35). Therefore, we recently applied optogenetic techniques to reversibly and selectively manipulate the activity of Hcrt (36, 37) and LC (38) neurons in freely moving animals to probe their function in sleep and wakefulness. Optogenetics is a recent technology in which a genetically-encoded neuromodulatory probe is expressed in a specific cell type of interest and then activated by a specific wavelength of light (39-48). For example, Channelrhodopsin-2 (ChR2) is a nonspecific cation channel that depolarizes neurons upon to blue light illumination; Halorhodopsin (NpHR) is a chloride pump that hyperpolarizes neurons in response to yellow light. To deliver these probes to Hcrt or LC neurons, we used lentiviral or adeno-associated viral (AAV) gene delivery tools, respectively, under the control of cell-type specific promoters (Figure 2B,C) (36, 37). To deliver light to the brain regions containing the Hcrt- or NE-expressing cells, we place fiber optic cables inside surgically implanted cannulae in the brain (Figure 2 A-C) (36). Further information about optogenetic technology can be found in many other excellent reviews (49-58).
We first genetically delivered ChR2 to Hcrt neurons and demonstrated millisecond-precise stimulation in vitro(36). The high temporal and spatial precision of stimulation allowed us to mimic the physiological range of hypocretin neuronal spike (1-30 Hz) and thus overcome the limitations of previous techniques. Indeed, parameters of the optogenetic stimulation we used were based on actual frequency analysis of Hcrt (12, 59) and LC (23, 25) neurons in vivo. We found that direct optical stimulation of Hcrt neurons increased the probability of transitions to wakefulness from either NREM or REM sleep (Figure 2D). Interestingly, photostimulation using 5-30 light pulse trains reduced the latency to wakefulness whereas 1 Hz trains did not. We also showed that the effects of stimulating Hcrt neurons could be blocked by injection of a Hcrt-R1 antagonist or by genetic deletion of the Hcrt gene (see (36)). These results demonstrated a causal link between Hcrt neuron activation and sleep-to-wake transitions, as suggested by previous single unit recording studies. They also show that Hcrt release from Hcrt-expressing neurons is necessary for the wake-promoting properties of these neurons.
A recent pharmacogenetic study confirmed these results. Sasaki and collaborators used a Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) approach to activate and suppress Hcrt neural activity (60). DREADD technology allows bimodal modulation of neural activity with temporal resolution of several hours (61). Sasaki et al. found that activation of Hcrt neural activity increased wakefulness while suppression of Hcrt activity promoted NREM. The latter result was corroborated by inhibition of Hcrt neurons using NpHR (62).
We further showed that Hcrt-mediated sleep-to-wake transitions are blocked by sleep pressure caused by sleep deprivation (63). Furthermore, downstream arousal centers such as the LC and TMN increase activity as measured by c-Fos expression in response to Hcrt stimulation, as previously suggested (64). Because the behavioral effects of Hcrt stimulation are not mediated by the histamine system in those experimental conditions (37), we focused our experimental investigations on the noradrenergic LC as an alternative target of the Hcrt system (64) for optogenetic manipulation.
Optogenetic stimulation of LC neurons caused immediate sleep-to-wake transitions from both NREM and REM sleep (38). Interestingly, the effects of LC stimulation occurred within seconds of the onset of stimulation while the effects of Hcrt stimulation took an average of 20-30 seconds to cause awakenings (Figure 2 D vs E). Stimulation of LC neurons during wakefulness increased locomotor activity and the total time spent awake. Inhibition of LC neurons with NpHR decreased the duration of wake episodes but did not block sleep-to-wake transitions when animals were asleep (Figure 2F). Taken together, these results demonstrate that LC activity is sufficient to promote wakefulness from sleep and general locomotor arousal, but is not necessary for animals to wake from sleep, probably due to lack of activity (or disinhibition) in other arousal systems.
It is interesting that acute stimulation of Hcrt neurons causes sleep-to-wake transitions over an average time period of 10-30 s while acute stimulation of LC neurons causes sleep-to-wake transitions in less than 5 s. There are at least three possible reasons for this difference: (1) The Hcrt study was the first to use in vivo optogenetics through unilateral stimulation, while the LC study uses bilateral stimulation. Bilateral stimulation of Hcrt neurons reduces latency of sleep-to-wake transitions even more, although to a less dramatic effect than NE (A. Adamantidis, personal communication) (2) The effect of Hcrt neurotransmission on downstream targets (mediated by the dynamics of Hcrt receptors and interactions with other ion channels) may not be as potent as the effect of NE neurotransmission. (3) Hcrt neurons is farther upstream in the neural circuitry of arousal, with the LC, TMN, and other populations (dorsal raphe, pedunculo-pontine tegmentum-PPT) acting as the main effectors in a putative “hierarchy” (65), suggesting the importance of Hcrt activation of not only the LC neurons but also the dorsal raphe and the PPT. (4) Perhaps the effects of photostimulation of ChR2-transduced neurons in Hcrt and LC neurons are different due to the different gene delivery methods used to target each structure. We used a lentivirus carrying an endogenous 3.1 kb Hcrt promoter to target Hcrt neurons and an adeno-associated virus using a strong EF1 α promoter, in combination with a Tyrosine Hydroxylase (TH)∷Cre knockin mouse to target LC neurons. Expression of reporter genes is substantially higher in the LC than in Hcrt neurons, indicating a much lower expression of ChR2 in Hcrt cells. It is unclear whether this reduced expression results in reduced effects of photostimulation.
It is also interesting that relatively long periods of photostimulation over 1-4 hours caused differential effects on arousal between Hcrt and LC neurons. Long-term stimulation of Hcrt neurons increased sleep-to-wake transitions but did not increase the total duration of wakefulness (36, 37). In contrast, long-term stimulation of LC neurons significantly reduced the total duration of wakefulness with an increase in locomotor behavior (38). One explanation is that Hcrt neurons play a more specialized role in regulating the boundaries between sleep-wake transitions based on hypothalamic-related functional inputs (circadian rhythms, metabolic status and associated stress response, homeostatic sleep pressure), while LC neurons have a greater impact on increasing cortical membrane potential and desynchronizing the cortical EEG to promote wakefulness, attention or hyperarousal.
An important question for future investigation is whether Hcrt and LC neurons promote wakefulness in parallel or participate in the same arousal circuit. For example, Hcrt-neurons project strongly to the LC. It is likely that Hcrt neurotransmission is relayed by the LC to the cortical microcircuitry where NE induces desynchronization and raises the membrane potential of individual neurons.
Aroused, not just awake
The ability to target and selectively manipulate Hcrt and LC neurons allows the opportunity to study these nuclei in different contexts of hyperarousal. For example, in addition to promoting wakefulness, Hcrts have also been implicated in rodent models of food intake, addiction, stress, attention, and male sexual arousal (15). LC neurons have been implicated in many similar behaviors including stress, addiction, attention, learning and memory, and depression(20). These studies beg the question, which stand for most modulatory systems of the brain: How can these arousal systems play a role in such a wide spectrum of behaviors? How are these circuits anatomically and functionally organized to modulate the diverse forms of arousal?
One possibility is that Hcrt and LC neurons are organized into subpopulations such that each “module” projects to different downstream structures and therefore mediates different functions. For example, Harris and Aston-Jones (2006) proposed that Hcrt neurons are functionally organized such that reward and cue-processing is mediated by the lateral Hcrt field by projecting to the ventral tegmental area, while drug-related stress or aversive behaviors are mediated by dorso-median and perifornical Hcrt neurons (66, 67). However, such a dichotomy has not been verified anatomically (65, 68). In addition, although subpopulations of Hcrt neurons exhibit distinct electrophysiological properties and can be differentiated on their integration of metabolic changes (e.g., glucose) (69-71), this functional dichotomy has not been matched with anatomical territories of the lateral hypothalamus. Recent studies have also found that Hcrt neuron response to to NE in vitro varies with previously experienced sleep pressure (72, 73). Furthermore, the functional characterization of Hcrt neurons using immuno-histochemical detection of the immediate early gene c-Fos after behavioral challenges has not presented evidence for functional subpopulations of neurons (74-76). As already documented in zebrafish and mice (19), exciting new circuit-labeling tools such as Brainbow technology (77) may help to anatomically or functionally reconstruct the connectivity of individual Hcrt neurons in intact mammalian systems which may ultimately reveal precise anatomical features of these neurons.
An alternative hypothesis to explain how arousal systems may differentially affect arousal-related behaviors is through different release of peptides and neuromodulators depending on specific patterns of activity. For example, it is possible that Hcrt neurons may release different contents from synaptic vesicles during synaptic neurotransmission depending on phasic or tonic activity. In addition to Hcrt, these neurons secrete glutamate, dynorphin, and likely other transmitters/modulators (78). Thus, the vesicular content from Hcrt-containing terminals includes small vesicles with glutamate and large, dense core vesicles with Hcrt and other modulators. Small vesicles are thought to be release upon slow tonic firing of the cell, whereas the release of large vesicles happens upon firing of the cell in burst mode. Although this hypothesis has never been tested in regards to the Hcrt system, it is reasonable to propose that low frequency firing of Hcrt neurons (as it occurs during quiet wakefulness) may induce the release of glutamate. In contrast, burst firing of the neurons during sleep-to-wake transitions or goal-oriented behaviors may preferentially provoke the release of neuropeptide-containing vesicles (e.g. hypocretins, dynorphin, and others). Interestingly, similar mechanisms have been observed for other peptidergic neurons (79-82), however, it remains unknown whether such mechanisms have significant behavioral consequences.
In addition, the composition of vesicular glutamate transporters in modulatory systems of the brain, such as the serotoninergic system, and the vesicular synergy—a process for enhanced packaging and the release of the “primary” transmitter—has been recently highlighted as a possible mechanism of synapse specialization (83). Thus, it is possible that the local composition of vesicular glutamate transporters in the Hcrt terminals may condition the vesicular composition of the Hcrt terminals, and thus, its synaptic transmission. This may define new anatomical “terminals” territories in which Hcrt neurons would differentially affect postsynaptic cells depending on the firing mode of the cells and the targeted brain area. Although this is purely speculative, it may diversify the functional repertoire of Hcrt and NE neuron modulation by releasing a combination of different transmitters that would result in a regionalized, yet highly specific, post-synaptic response, and possibly behavioral output as well. Optogenetics makes it possible to stimulate arousal systems with different frequency patterns and examine the output at different synapses, and this will be an important opportunity for future study.
Arousal in neuropsychiatric disease
Changes in arousal states and thresholds are at the core of most neuropsychiatric disorders, including depression, anxiety, schizophrenia and post-traumatic stress disorder The control of arousal in the mammalian brain results from a balance between inhibitory and excitatory networks highly organized on both temporal and spatial scales. Subtle imbalance in the temporal dynamics of arousal circuits impede the parameters of the sleep-wake cycle, which frequently results in altered cognitive functions (84-86). Hyperarousal is associated with schizophrenia, addiction, generalized anxiety disorder (GAD) (3) and post-traumatic stress disorder, while hypoarousal correlates with depression, aggressiveness and attention deficit hyperactivity disorder (ADHD) (4, 5).
The boundary between hyper- and hypoarousal in a given psychiatric disorder might be less clear as it is the case in narcolepsy. Narcolepsy is characterized by a cluster of distinct symptoms including hypnagogic hallucinations, sleepiness, sleep paralysis and cataplexy. Cataplexy is complete loss of skeletal muscle tone during awake or alert states upon athletic activity, laughter, anger or other rapid emotions trigger (87). Thus, human narcoleptic patients exhibit both hyperarousal during the night (fragmented sleep) and hypoarousal during the day (inability to maintain long wake periods) (87). Although the underlying mechanism of cataplexy remains unknown, increasing experimental evidence using a wide variety of tools has identified the Hcrt system as necessary for proper sleep-wake cycle and muscle tone (15, 88). In addition to the lack of Hcrt function, NE neurons in the LC cease firing during cataplexy (89), which support a role for NE in maintaining appropriate muscle tone, as well as arousal, during goal-oriented behaviors in mammals.
Although the hcrt and NE systems modulate arousal with different kinetics (as discussed above), our optogenetic approach suggest they are systems linked with hyperarousal. Our results are in agreement with the hypothesis that Hcrt and NE neurons are necessary to maintain appropriate arousal levels during strong positive or negative emotions (e.g., stress, joy, laughter) (90) that typically trigger cataplexy when the hcrt system is not functional (87). The role of the NE neurons in narcolepsy remains unclear, however, the lack of a functional “Hcrt-NE” link to transfer emotion or motivational state into hyperarousal might explain some of the cataplexy symptoms, as suggested by our recent studies (37, 38).
In addition to sleep, Hcrt neurons have recently been shown to tune motivational states related to food intake (91-93), sexual behavior (94), drug intake (67, 95-97), stress (98) and depressive-like behaviors (99, 100) . These studies induced hypoarousal by decreasing hcrt tone. In contrast, NE transmission has been shown to be crucial for attention and cognitive function (21). Activation of the NE system increases arousal and causes anxiety-like behaviors (8). Although a causal role for imbalance of Hcrt or NE activity and hypo- or hyperarousal is strongly suggested by the literature, it remains unknown whether dysfunction of these systems is responsible for the hypo- or hyperarousal states associated with neuropsychiatric conditions. Which comes first is debatable and requires extended pre-clinical investigation.
Conclusion
Optogenetics has allowed us to make major advances in our understanding of the Hcrt and LC –NE systems, and this technology should be used to dissect other arousal systems as well. Due to the multi-tasking properties of the hcrt and NE systems in a wide variety of brain functions, it is conceptually and experimentally difficult to separate the arousal from the motivational consequence upon activation of those systems. Optogenetic tools now provide a better alternative for controlling neural circuits in vivo to examining sleep/wake boundaries. In future research, it will be important to determine the mechanisms by which each arousal system affects hypo- and hyperarousal based on anatomical projections, synaptic neurotransmission, and the frequency patterns of stimulation. Such experiments will undoubtedly shine light on how neural circuits share such functions in the normal and the pathological brain.
Acknowledgments
L.d.L. is supported by grants from the Defense Advanced Research Projects Agency, the National Alliance for Research on Schizophrenia and Depression, and the Klarman Family Foundation. M.E.C. is supported by a fellowship from the Hilda and Preston Davis Foundation. A.A. is supported by the Douglas Foundation, the Canadian Institute for Health Research, the Canadian Fund for Innovation, the Canadian Research Chair and the NARSAD.
Footnotes
Financial Disclosure Statement: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfaff DW, Kieffer BL. Molecular and biophysical mechanisms of arousal, alertness, and attention. Preface. Ann N Y Acad Sci. 2008;1129:xi. doi: 10.1196/annals.1417.034. [DOI] [PubMed] [Google Scholar]
- 2.Bryant RA, Harvey AG, Guthrie RM, Moulds ML. A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. J Abnorm Psychol. 2000;109:341–344. [PubMed] [Google Scholar]
- 3.Carlsson A. Neurocircuitries and neurotransmitter interactions in schizophrenia. Int Clin Psychopharmacol. 1995;10(3):21–28. [PubMed] [Google Scholar]
- 4.Haller J, Toth M, Halasz J. The activation of raphe serotonergic neurons in normal and hypoarousal-driven aggression: a double labeling study in rats. Behav Brain Res. 2005;161:88–94. doi: 10.1016/j.bbr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Miano S, Donfrancesco R, Bruni O, Ferri R, Galiffa S, Pagani J, et al. NREM sleep instability is reduced in children with attention-deficit/hyperactivity disorder. Sleep. 2006;29:797–803. [PubMed] [Google Scholar]
- 6.Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol (Oxf) 2010;198:223–235. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 8.Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–361. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- 9.Pape HC, Jungling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology. 2010;58:29–34. doi: 10.1016/j.neuropharm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 12.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 14.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 16.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 17.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, et al. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci U S A. 2009;106:4459–4464. doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissman TA, Sanes JR, Lichtman JW, Livet J. Generating and imaging multicolor Brainbow mice. Cold Spring Harb Protoc. 2011;2011:763–769. doi: 10.1101/pdb.top114. [DOI] [PubMed] [Google Scholar]
- 20.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 21.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 22.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 23.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobson RW, 2nd, Wright CB, Lamoy RE. Adrenergic mechanisms in the cephalic and cerebral circulations of the subhuman primate. Surgery. 1975;77:304–310. [PubMed] [Google Scholar]
- 25.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco-Centurion C, Gerashchenko D, Shiromani PJ. Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci. 2007;27:14041–14048. doi: 10.1523/JNEUROSCI.3217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones BE, Harper ST, Halaris AE. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 1977;124:473–496. doi: 10.1016/0006-8993(77)90948-9. [DOI] [PubMed] [Google Scholar]
- 28.Lidbrink P. The effect of lesions of ascending noradrenaline pathways on sleep and waking in the rat. Brain Res. 1974;74:19–40. doi: 10.1016/0006-8993(74)90109-7. [DOI] [PubMed] [Google Scholar]
- 29.Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–526. [PubMed] [Google Scholar]
- 30.Berridge CW, Espana RA. Synergistic sedative effects of noradrenergic alpha(1)- and beta-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience. 2000;99:495–505. doi: 10.1016/s0306-4522(00)00215-3. [DOI] [PubMed] [Google Scholar]
- 31.Flicker C, Geyer MA. Behavior during hippocampal microinfusions. I. Norepinephrine and diversive exploration. Brain Res. 1982;257:79–103. doi: 10.1016/0165-0173(82)90006-6. [DOI] [PubMed] [Google Scholar]
- 32.Segal DS, Mandell AJ. Behavioral activation of rats during intraventricular infusion of norepinephrine. Proc Natl Acad Sci U S A. 1970;66:289–293. doi: 10.1073/pnas.66.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 36.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 41.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Peca J, Matsuzaki M, Matsuzaki K, Noguchi J, Qiu L, et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci U S A. 2007;104:8143–8148. doi: 10.1073/pnas.0700384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 44.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 45.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Boyden ES. A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 Biol Rep. 2011;3:11. doi: 10.3410/B3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueiredo M, Lane S, Tang F, Liu BH, Hewinson J, Marina N, et al. Optogenetic experimentation on astrocytes. Exp Physiol. 2011;96:40–50. doi: 10.1113/expphysiol.2010.052597. [DOI] [PubMed] [Google Scholar]
- 53.Knopfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30:14998–15004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miesenbock G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- 55.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sjulson L, Miesenbock G. Photocontrol of neural activity: biophysical mechanisms and performance in vivo. Chem Rev. 2008;108:1588–1602. doi: 10.1021/cr078221b. [DOI] [PubMed] [Google Scholar]
- 57.Stehfest K, Hegemann P. Evolution of the channelrhodopsin photocycle model. Chemphyschem. 2010;11:1120–1126. doi: 10.1002/cphc.200900980. [DOI] [PubMed] [Google Scholar]
- 58.Wyart C, Del Bene F. Let there be light: zebrafish neurobiology and the optogenetic revolution. Rev Neurosci. 2011;22:121–130. doi: 10.1515/RNS.2011.013. [DOI] [PubMed] [Google Scholar]
- 59.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One. 2011;6:e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong S, Rogan SC, Roth BL. Directed molecular evolution of DREADDs: a generic approach to creating next-generation RASSLs. Nat Protoc. 2010;5:561–573. doi: 10.1038/nprot.2009.239. [DOI] [PubMed] [Google Scholar]
- 62.Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31:10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter M, McCaughey E, Annaz D, Hill CM. Sleep problems in a Down syndrome population. Arch Dis Child. 2009;94:308–310. doi: 10.1136/adc.2008.146845. [DOI] [PubMed] [Google Scholar]
- 64.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 68.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Schone C, Venner A, Knowles D, Karnani MM, Burdakov D. Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol. 2011;589:2767–2779. doi: 10.1113/jphysiol.2011.208637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uschakov A, Grivel J, Cvetkovic-Lopes V, Bayer L, Bernheim L, Jones BE, et al. Sleep-deprivation regulates alpha-2 adrenergic responses of rat hypocretin/orexin neurons. PLoS One. 2011;6:e16672. doi: 10.1371/journal.pone.0016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grivel J, Cvetkovic V, Bayer L, Machard D, Tobler I, Muhlethaler M, et al. The wake-promoting hypocretin/orexin neurons change their response to noradrenaline after sleep deprivation. J Neurosci. 2005;25:4127–4130. doi: 10.1523/JNEUROSCI.0666-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 77.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 78.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trudel E, Bourque CW. Central clock excites vasopressin neurons by waking osmosensory afferents during late sleep. Nat Neurosci. 2010;13:467–474. doi: 10.1038/nn.2503. [DOI] [PubMed] [Google Scholar]
- 80.Brown CH, Bourque CW. Mechanisms of rhythmogenesis: insights from hypothalamic vasopressin neurons. Trends Neurosci. 2006;29:108–115. doi: 10.1016/j.tins.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Ellis JL, Burnstock G. Neuropeptide Y neuromodulation of sympathetic co-transmission in the guinea-pig vas deferens. Br J Pharmacol. 1990;100:457–462. doi: 10.1111/j.1476-5381.1990.tb15828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hardebo JE. Influence of impulse pattern on noradrenaline release from sympathetic nerves in cerebral and some peripheral vessels. Acta Physiol Scand. 1992;144:333–339. doi: 10.1111/j.1748-1716.1992.tb09302.x. [DOI] [PubMed] [Google Scholar]
- 83.El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 84.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, et al. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci U S A. 2011;108:13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 86.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 87.Guilleminault C. Narcolepsy syndrome. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. W. B. Saunders; Philadelphia: 1994. pp. 145–162. [Google Scholar]
- 88.Kroeger D, de Lecea L. The hypocretins and their role in narcolepsy. CNS Neurol Disord Drug Targets. 2009;8:271–280. doi: 10.2174/187152709788921645. [DOI] [PubMed] [Google Scholar]
- 89.Wu MF, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 91.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 92.McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31:15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winsky-Sommerer R, Boutrel B, de Lecea L. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- 99.Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, et al. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 2011;222:289–294. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]