Abstract
Contrary to expectations derived from preclinical studies of the effects of stress, and imaging studies of adults with PTSD, there is no evidence of hippocampus atrophy in children with PTSD. Multiple pediatric studies have reported reductions in the corpus callosum – the primary white matter tract in the brain. Consequently, in the present study, Diffusion Tensor Imaging was used to assess corpus callosum white matter integrity in 17 maltreated children with PTSD and 15 demographically matched normal controls. Children with PTSD had reduced fractional anisotropy in the medial and posterior corpus, a region which contains interhemispheric projections from brain structures involved in circuits that mediate the processing of emotional stimuli and various memory functions --- core disturbances associated with a history of trauma. Further exploration of the effects of stress on corpus callosum and white matter development appears a promising strategy to better understanding the pathophysiology of PTSD in children.
Keywords: Posttraumatic Stress Disorder, Imaging, DTI, children
1. INTRODUCTION
Child abuse is frequently associated with long-term significant psychiatric sequelae, and currently little is known about the mechanisms that initiate and maintain the various forms of psychopathology associated with early trauma. While not all abused children develop difficulties, many experience a chronic course of psychopathology, with PTSD one of the most common psychiatric sequelae of child maltreatment (Molnar et al 2001).
Pre-clinical studies suggest that stress early in life can promote long-term changes in stress reactivity and brain development (Kaufman et al 2000). These studies provide a valuable heuristic in understanding the pathophysiology of PTSD in adults, with many of the biological alterations associated with early stress in preclinical studies reported in adults with PTSD and other stress related disorders. The application of research findings from these preclinical studies in understanding the neurobiology of PTSD in children, however, is somewhat limited.
One of the best replicated findings in adults with PTSD is reduction in hippocampal volume (Bremner 2006). Pediatric studies have failed to detect this (Carrion et al 2001; De Bellis et al 1999; De Bellis et al 2002; Tupler and De Bellis 2006). Instead, children with PTSD have been found in two independent samples to have reduction in the medial and posterior region of the corpus callosum (CC) (De Bellis et al 1999). Reduction in CC area has likewise been reported in psychiatric inpatients with a history of maltreatment when compared to psychiatric and healthy controls without a history of early childhood trauma, with half the children in the maltreatment group meeting criteria for PTSD at discharge (Teicher et al 2004), and recently documented in adults with PTSD as well (Villarreal et al 2004).
To the best of our knowledge, there is only one published structural MRI study in prepubescent non-human primates subjected to early stress (Sanchez et al 1998). Consistent with the child investigations described above, this study also failed to find evidence of hippocampal atrophy, and instead reported reductions in the medial and posterior CC.
Given prior results suggesting children, adolescents, and adults with PTSD show atrophy of the medial and posterior CC – the primary white matter tract in the brain -- we utilized Diffusion Tensor Imaging (DTI) in the current investigation to assess possible changes in myelination or white matter coherence in these regions. DTI is a relatively new technique that provides data on white matter microstructure based on properties of diffusion. Fractional anisotropy (FA), a scale- and orientation-independent measure of diffusion derived with DTI (Pierpaoli and Basser, 1996), is the primary outcome measure examined in the current report. FA is not a direct measure of myelination, but a measure of anisotropy in water diffusion, which increases with myelination (McGraw et al., 2002; Snook et al., 2005). To the best of our knowledge, this is the first investigation to utilize DTI in maltreated children. Given exposure to excessive levels of stress hormones has been found to suppress glial cell division critical for myelination (Lauder 1983), it was hypothesized that compared to normal controls, maltreated children with PTSD would have reduced FA in the medial and posterior regions of the CC.
2. METHOD
2.1. Subjects
The sample included 32 children: 17 maltreated children (7 males and 10 females) who met criteria for PTSD secondary to intrafamilial abuse; and 15 demographically matched normal controls with no current or lifetime history of psychiatric illness (7 males and 8 females). All the children within the PTSD group had a history of protective services intervention for indicated allegations of child maltreatment, and the absence of maltreatment in the controls was verified by parent- and child-report and review of State records. The children were a mean age of 10.6 (SD 2.3, Range: 6.3–14.4), mixed ethnically (25% Caucasian, 41% African American, 18% Hispanic, and 16% Biracial), and from low-income families (Hollingshead SES Score 30.8 ± 11.7, Range: 14–50). The children had a mean IQ of 93.2 (SD=14.4, Range: 71–123), and there were no differences between the two groups in terms of age, sex, race, SES, or IQ. In addition, all children were right handed, and no children were taking psychotropic or other medication with CNS effects.
As previously described (Kaufman et al 2006), multiple informants and multiple methods were used to assess children’s maltreatment experiences. Only one child experienced a single form of maltreatment; the majority experienced three or more types: 71% had a history of physical abuse, 29% sexual abuse, 47% neglect, 59% emotional maltreatment, and 94% witnessed domestic violence. Major depression was the most common co-occurring diagnosis (41%) in this sample, followed by other depressive diagnoses (30%), oppositional defiant disorder (12%), and attention deficit hyperactivity disorder (6%).
2.2. Procedures
Both Yale University Human Investigations Committee and Department of Children Families Institutional Review Board approved this study. The majority of children from the present study were recruited from our ongoing studies of genetic and environmental predictors of risk and resiliency in maltreated children (Kaufman et al 2006), and a subset of the children with PTSD was recruited from a local child guidance agency. The children’s legal guardian provided written consent, and all children provided written assent for study participation. When the children’s legal guardian was not their birth parent, written assent was also obtained from the birth parent if they were available.
Clinical Evaluation
The Schedule for Affective Disorders and Schizophrenia for School Aged Children – Present and Lifetime Version (K-SADS-PL) (Kaufman et al 1997), a semi-structured psychiatric interview, was administered to each child and one guardian. A number of standardized and well validated clinical symptomatology rating scales were also administered and psychiatric diagnoses were derived using best estimate procedures as described previously (Pine et al 2005).
MRI Acquisition
Imaging data was acquired on a GE Signa 1.5T scanner. DTI was performed using echo-planar imaging. Thirty-five coronal slices were obtained using the following parameters: matrix 128 × 128, TE= minimum, TR= 11000 ms, FOV=24 cm, NEX=1, no gap, resolution = 1.875 mm × 1.875 mm × 5 mm, across 6 runs. The diffusion sensitizing gradients were applied along 6 non-collinear directions, with a b-value of 1000 s/mm2. T2-weighted images were also obtained for each run. It has been suggested that if DTI voxel dimensions larger than 3 × 3 × 3 mm are acquired, analysis should only be performed for the thickest white matter tracts (Smith et al 2007). Since the corpus callosum is a thick fiber tract characterized by tightly packed bundles that run in a similar direction, the acquisition parameters utilized in the current investigation provide adequate voxel resolution and minimal artifact effects (Oouchi et al 2007). Structural MR images were acquired using a 3D SPGR coronal acquisition series (TR = 18 ms, TE = 5 ms, flip angle = 30°, NEX = 2, matrix size = 256 × 160, FOV = 24 cm, 124 slices, thickness= 1.5 mm).
Image Analysis
The corpus was outlined at the midsaggital slice and segmented into seven sections with a semi-automated program generated using the guidelines delineated by Witelson (Witelson 1989). The seven corpus regions were defined on the structural data, and brought into the DTI space to obtain the region of interest FA assessments. ICC inter-rater reliability was > .90 for all corpus sections.
The DTI data were analyzed using a custom software package, part of the Yale BioImage Suite (Papademetris et al 2006) using an encoding gradient set containing 6 directions based on an energy minimization procedure (Papadakis et al 1999). Each diffusion-weighted series was registered to a single T2 image volume. A 12 parameters -affine registration was performed for each individual SPGR to the same T2 image. Computation of FA was performed for each voxel within each region of the CC.
Statistical Analyses
Prior to conducting analyses, measures were examined for normality using the Shapiro Wilk’s test. As standardized transformations failed to normalize skewed measures, rank transformed values were utilized in analyses as indicated. The association of DTI measures with age, sex, IQ, SES, and ratings of motion were also examined, but were not included in the model examining group differences as they did not relate to the FA values. As groups differ on total white matter volume, with the PTSD group being significantly smaller (F=5.3, p < .03), we corrected for white matter volume using it as a covariate within the multiple analysis of covariance (MANCOVA). MANCOVA was used to test for the effects of diagnostic and gender on the FA of the seven corpus regions. If the MANCOVA was significant (Wilks’ Lamda, F=3.10, p < .04), follow up univariate analyses of covariance (ANCOVA) were conducted.
3. RESULTS
3.1 Group Differences on DTI Measures
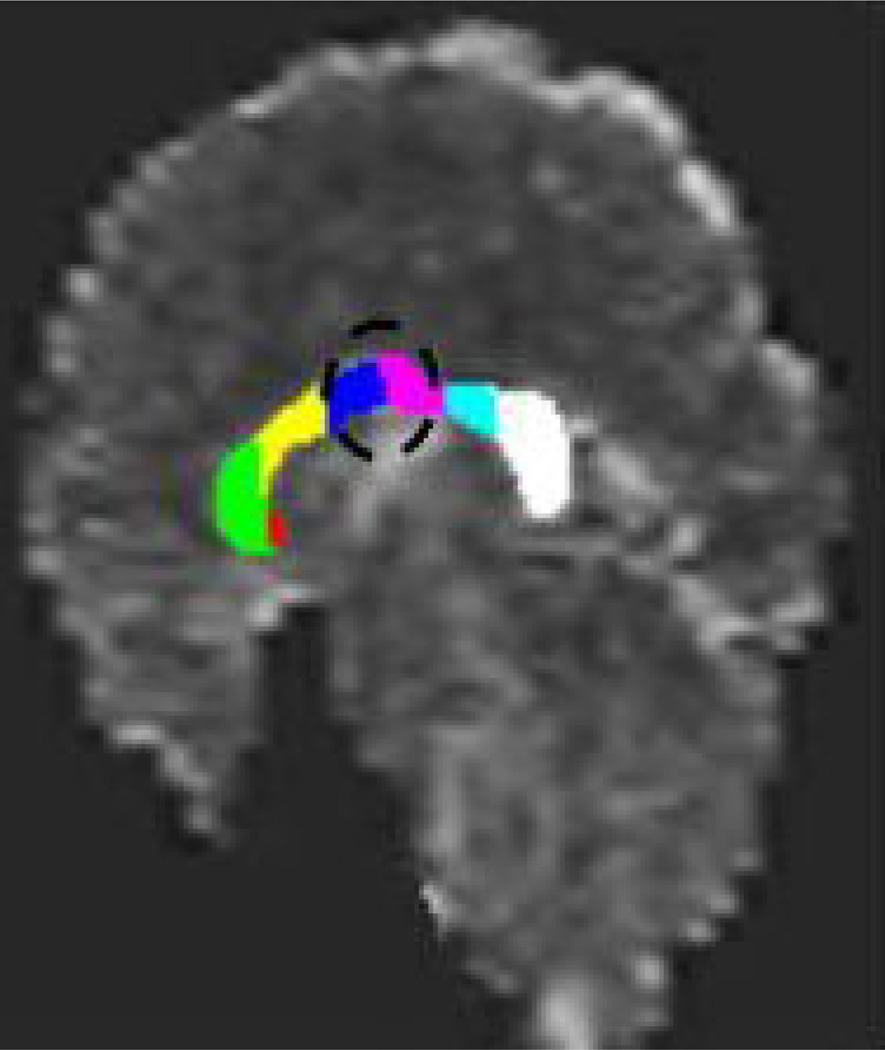
Table 1 presents group means, effect sizes (Cohen’s d) and univariate comparisons for corpus DTI data. Compared to controls, maltreated children with PTSD had significantly reduced FA in two of the four predicted corpus regions (anterior and posterior midbody), and a trend toward significantly reduced FA in a third region (splenium) (Figure 1).
Table 1.
Fractional Anisotropy Assessments of the Corpus Callosum1
| PTSD N=17 |
Controls N=15 |
F-Value Statistic |
p-value | Cohen’s d Effect size |
|
|---|---|---|---|---|---|
| Region 1 – Rostrum | .23 ± .02 | .24 ± .02 | 0.01 | ns | −0.13 |
| Region 2 – Genu | .43 ± .02 | .46 ± .02 | 1.00 | ns | −0.37 |
| Region 3 – Rostral Body | .36 ± .022 | .36 ± .032 | 0.01 | ns | 0 |
| Region 4 – Ant. Midbody | .37 ± .02 | .43 ± .02 | 4.55 | .04 | −0.75 |
| Region 5 – Post. Midbody | .36 ± .02 | .43 ± .02 | 4.35 | .05 | −0.87 |
| Region 6 – Isthmus | .38 ± .022 | .42 ± .032 | 0.90 | ns | −0.50 |
| Region 7 – Splenium | .41 ± .022 | .48 ± .032 | 2.78 | .10 | −0.70 |
Notes:
= DTI measures are adjusted for total white matter volume (X ± SE).
= Raw scores are presented in the table, rank transformed scores were used in the analyses.
Figure 1. Subregions of Corpus Callosum.
Overlay of the seven corpus callosum regions onto a fractional anisotropy (FA) map. Circle: callosal areas (anterior and posterior midbody) of reduced FA in maltreated children with PTSD compared to controls.
3.2 Correlation Between DTI Measures and Clinical Ratings
FA measures in regions 2 (Rho=−0.40, p < .02), 4 (Rho= −0.43, p < .02), and 7 (Rho= −0.38, p < .03) of the corpus callosum correlated significantly with total anxiety scores obtained using the Screen for Child Anxiety and Related Disorders (Birmaher et al 1997). FA values in these same regions also correlated with the Panic and Separation Anxiety subscale scores of this measure (Rho Range: −0.48 to −0.52, p < .01, all comparisons), but were not related to depression or dissociation indices.
4. DISCUSSION
As predicted, the maltreated children had reduced FA in the medial and posterior regions of the CC. The FA changes in the CC observed in this study may be due to reduced myelination or other subtle alterations in axonal structure (e.g., neurofilaments, microtubules). CC myelination occurs in a rostral-caudal sequence and continues throughout childhood into early adulthood (Giedd et al., 1996). Consequently, it has been suggested that different regions of the CC might have different windows of vulnerability to the effects early trauma (Teicher et al., 2002; 2004).
The medial and posterior portions of the CC contain interhemispheric projections from the auditory cortices, posterior cingulate, insula, and somatosensory and visual cortices to a lesser extent. It also includes connections from the inferior parietal lobe to the contralateral inferior parietal lobe, superior temporal sulcus, cingulate, retrosplenial cortex, and parahippocampal gyrus (Pandya and Seltzer 1986). Several of the regions with interhemispheric projections through the medial and posterior CC have connections with prefrontal cortical areas, and are involved in circuits that mediate the processing of emotional stimuli and various memory functions --- core disturbances associated with PTSD.
In the current investigation, however, it is impossible to determine if the CC alterations are a function of PTSD per se, or maltreatment, as all subjects had PTSD secondary to physical abuse, sexual abuse, or exposure to domestic violence. Teicher and colleagues (1997) in their study of maltreated inpatients did not find the presence of a PTSD diagnosis to contribute in predicting corpus callosum area above and beyond the effects of neglect, although the study was not fully powered to investigate this (Teicher et al 2002). There is preliminary evidence to suggest that sociopathy is associated with increased CC area, and that this effect is independent of childhood history of abuse (Raine et al., 2003), but further work in this area is warranted. The use of two (e.g., maltreatment vs. no maltreatment) by two (e.g., PTSD vs. no PTSD) research designs in carefully characterized samples will help to determine the specificity of CC changes observed in association with trauma, PTSD, and other psychiatric diagnoses.
It is possible that CC changes observed in PTSD represent a pre-existing vulnerability factor, present prior to exposure to stress predisposing individuals to develop PTSD. This question has been raised in association with hippocampal findings reported in adults with PTSD given results of a neuroimaging study conducted with 35 identical twin pairs that were discordant for combat exposure (Gilbertson et al, 2002). Twelve of the thirty-five combat exposed twins developed PTSD. Combat exposed twins with PTSD and their unexposed co-twins were found to have smaller hippocampal volumes than combat exposed twins without PTSD and their unexposed co-twins. Given that the identical twins that were not exposed to combat had hippocampal volumes that were comparable to that of their combat-exposed co-twins who developed PTSD, and significantly smaller hippocampi than combat-exposed men who did not develop PTSD, the authors concluded that the reduced hippocampal volume represented a preexisting, inherent vulnerability factor, rather than a consequence of trauma exposure. This interpretation has to be accepted with caution, however, as the combat-exposed veterans who developed PTSD and their combat unexposed co-twins were significantly more likely to have a history of alcohol dependence than the combat-exposed veterans who did not develop PTSD and their combat un-exposed co-twins. Moreover, childhood histories of sexual and physical abuse were also higher in the combat-exposed veterans who developed PTSD and their combat unexposed co-twins than the other twin pairs. More than likely the structural and functional alterations reported in individuals with PTSD are a result of genetic and environmental factors. Additional use of twin designs (de Geus et al., 2007) and other molecular genetic approaches (Canli et al., 2006; Hairi & Weinberger, 2003), will help to untangle these effects.
There is emerging preclinical and clinical evidence to suggest that the neurobiological effects of stress vary at different developmental periods, with white matter changes more prominent than subcortical gray (e.g., hippocampus) matter changes in juvenile cohorts. Developmental differences in the neurobiological correlates of PTSD across the lifecycle have raised questions as to whether PTSD is one and the same disorder in children and adults. With minimum changes, however, the criteria required to diagnose PTSD can be applied to children as young a six years of age (Stover & Berkowitz, 2005). Emerging data on neurobiological correlates and treatment response of depressed children, adolescents, and adults likewise demonstrate similarities and differences across the lifecycle, with pharmacological treatments (e.g. tricyclic antidepressants) that are highly effective in adults, found to be no more effective than placebo in children with depression (Kaufman, Blumberg, & Young, 2004). Developmental differences in the neural underpinning of PTSD will likewise hamper the ability to extrapolate from adult treatment studies in guiding intervention strategies for children with PTSD, highlighting the need for further work in this area.
Although promising, the significance of our results is limited by the small sample studied, and the poor resolution of the 1.5T DTI data collected which prohibited track tracing and examination of the circuits affected by the FA changes in the corpus. Smaller and isotropic voxel size will certainly be beneficial for fiber tracking as well as for voxel-wise analyses techniques that have become available and can enhance the quality of future work in this area.
Further exploration of the effects of stress on the development of the CC, corticolimbic circuitry, and white matter tracts appears a promising strategy to better understanding the etiology and pathophysiology of PTSD in children. Developmental differences in the neurobiological correlates of PTSD across the lifecycle highlight the need for more work in this area.
Acknowledgements
The authors wish to thank the children and families, the staff at the State Department of Children and Families, and the clinicians at Clifford Beers Clinic that facilitated the completion of this work. The authors also wish to acknowledge Deborah Lipschitz, M.D. and Damion Grasso, M.A. for their help in the collection of the clinical data and derivation of best estimate psychiatric diagnoses. This research was funded by the NIMH: 1R01MH65519-01 (JK), P50 AA-12870-04 (JHK, JK); support from the National Center for Posttraumatic Stress Disorder, Veterans Administration, West Haven, Connecticut, and Yale University General Clinical Research Center Grant (M01RR06022).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birmaher B, Khetarpol S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Properties. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bremner JD. The relationship between cognitive and brain changes in posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:80–86. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, et al. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, et al. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- de Geus EJ, van't Ent D, Wolfensberger SP, Heutink P, Hoogendijk WJ, Boomsma DI, et al. Intrapair differences in hippocampal volume in monozygotic twins discordant for the risk for anxiety and depression. Biol Psychiatry. 2007;61(9):1062–1071. doi: 10.1016/j.biopsych.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. British Medical Bulletin. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky P, Nemeroff C, Charney D. Effects of early adverse experience on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Brain-Derived Neurotrophic Factor-5-HTTLPR Gene Interactions and Environmental Modifiers of Depression in Children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Blumberg HP, Young CM. Neurobiology of Early Onset Mood Disorders. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. Second ed. New York, New York: Oxford University Press; 2004. [Google Scholar]
- Lauder JM. Hormonal and humoral influences on brain development. Psychoneuroendocrinology. 1983;8:121–155. doi: 10.1016/0306-4530(83)90053-7. [DOI] [PubMed] [Google Scholar]
- Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health. 2001;91:753–760. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oouchi H, Yamada K, Sakai K, et al. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. Am J Neuroradiol. 2007;28:1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commisural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two Hemispheres - One Brain: Functions of the Corpus Callosum. Vol 17. New York: Alan R. Liss, Inc; 1986. pp. 47–74. [Google Scholar]
- Papadakis NG, Xing D, Huang CLH, et al. A comparative study of acquisition schemes for diffusion tensor imaging using MRI. J Magn Reson. 1999;137:67–82. doi: 10.1006/jmre.1998.1673. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski M, Rajeevan N, Constable RT, Staib L. Bioimage Suite, Yale Department of Diagnostic Imaging. 2006 http://www.bioimagesuite.org (ed) [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, et al. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Taylor K, et al. Corpus callosum abnormalities in psychopathic antisocial individuals. Arch Gen Psychiatry. 2003;60(11):1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with Tract-Based Spatial Statistics. Nature Protocols. 2007;2:499–504. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpol S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Properties. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bremner JD. The relationship between cognitive and brain changes in posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:80–86. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, et al. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky P, Nemeroff C, Charney D. Effects of early adverse experience on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Brain-Derived Neurotrophic Factor-5-HTTLPR Gene Interactions and Environmental Modifiers of Depression in Children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health. 2001;91:753–760. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. The topography of commisural fibers. In: Lepore F, Ptito M, Jasper HH, editors. Two Hemispheres - One Brain: Functions of the Corpus Callosum. Vol 17. New York: Alan R. Liss, Inc.; 1986. pp. 47–74. [Google Scholar]
- Papademetris X, Jackowski M, Rajeevan N, Constable RT, Staib L. Bioimage Suite, Yale Department of Diagnostic Imaging. 2006 http://www.bioimagesuite.org (ed) [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, et al. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD. Segmented Hippocampal Volume in Children and Adolescents with Posttraumatic Stress Disorder. Biological Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Graham DP, et al. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131:227–235. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, et al. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann NY Acad Sci. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, et al. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Graham DP, et al. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131:227–235. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]