Abstract
Inhalation exposure to fine Concentrated Ambient Particles (CAPs) increases cardiac oxidants by mechanisms involving modulation of the sympathovagal tone on the heart. Angiotensin-II is a potent vasoconstrictor and a sympatho-excitatory peptide involved in the regulation of blood pressure. We hypothesized that increases in angiotensin-II after fine PM exposure could be involved in the development of cardiac oxidative stress. Adult rats were treated with an angiotensin converting enzyme (ACE) inhibitor (Benazepril ®), or an angiotensin receptor blocker (ARB, Valsartan ®) before exposure to fine PM aerosols or filtered air. Exposures were carried out for 5 hours in the chamber of the Harvard Fine Particle Concentrator (fine PM mass concentration: 440 ± 80 μg/m3). At the end of the exposure the animals were tested for in situ chemiluminescence (CL) of the heart, TBARS and for plasma levels of angiotensin-II. Also, continuous ECG measurements were collected on a subgroup of exposed animals. PM exposure was associated with statistically significant increases in plasma angiotensin concentrations. Pretreatment with the ACE inhibitor effectively lowered angiotensin concentration, whereas ARB treatment led to increases in angiotensin above the PM-only level. PM exposure also led to significant increases in heart oxidative stress (CL, TBARs), and a shortening of the T-end to T-peak interval on the ECG that were prevented by treatment with both the ACE inhibitor and ARB. These results show that ambient fine particles can increase plasma levels of angiotensin-II and suggest a role of the renin-angiotensin system in the development of particle-related acute cardiac events.
INTRODUCTION
Ambient air pollution is a recognized risk factor for cardiovascular morbidity and mortality (Brook et al. 2004). Short-term elevations in ambient particulate matter (PM) have been specifically implicated in the triggering of acute cardiovascular events including myocardial infarction (D’Ippoliti et al. 2003; Peters et al. 2001; Zanobetti and Schwartz 2005), ventricular arrhythmias (Dockery et al. 2005; Peters et al. 2000) (Rich et al. 2005), heart failure exacerbations (Dominici et al. 2006; Schwartz and Morris 1995), and ischemic stroke (Hong et al. 2002; Tsai et al. 2003; Wellenius et al. 2005).
The mechanisms underlying these observations are only partially understood. One important mechanistic pathway for cardiac health effects appears to be autonomic nervous system dysfunction. Short-term exposure to PM is associated with changes in heart rate variability (Creason et al. 2001; Devlin et al. 2003; Godleski et al. 2000; Gold et al. 2000; Holguin et al. 2003; Liao et al. 1999; Pope et al. 1999), a quantitative, non-invasive marker of cardiac autonomic nervous system control. The changes reported in these studies are consistent with perturbations of both sympathetic and parasympathetic nervous system activity. We have previously shown that instillation exposure of rats to PM results in oxidant-dependent increases in both sympathetic and parasympathetic activity (Rhoden et al. 2005), at least in part, by activation of pulmonary unmyelinated C-fibers (Ghelfi et al. 2008).
Cohort and panel studies have found that increases in the PM levels are associated not only with decreased heart rate variability and other cardiac outcomes, but also with changes in vascular parameters i.e. blood viscosity, increased blood pressure, and increase levels of thrombosis markers in circulation (reviewed in (Godleski 2006)). The mechanistic link between activation of pulmonary reflexes and these outcomes remains to be characterized. Angiotensin-II, the final active messenger of the renin–angiotensin system, has multiple biological actions including vasoconstriction, stimulation of myocytes, and facilitation of norepinephrine release from sympathetic neurons (Martin et al. 2004). These actions are mediated through the binding of Angiotensin-II to Angiotensin-II type 1 receptors (AT1), which belong to the G protein coupled receptor (GPCR) superfamily (Martin et al. 2004; Zisman et al. 1998). Angiotensin-II interacts with the sympathetic nervous system both peripherally and centrally to increase vascular tone (Brown and Vaughan 1998). Animal studies show that Angiotensin-II has effects on both limbs of the autonomic nervous system, simultaneously facilitating sympathetic activity and inhibiting vagal activity on the heart (Joy and Lowe 1970; Rechtman and Majewski 1993; Zimmerman 1993).
Angiotensin-II increases the production of superoxide anion via stimulation of NAD(P)H oxidase, and the resulting oxidative stress has been postulated as an important mediator of Angiotensin-II signaling (Hanna et al. 2002; Zhang et al. 1999). Angiotensin-II also upregulates mRNA and protein expression of most NAD(P)H oxidase subunits in vitro (Rueckschloss et al. 2002) and in vivo (Mollnau et al. 2202).
Thus angiotensin-II is a possible important link between the pulmonary and cardiovascular effects of PM. In this paper we investigated angiotensin-II involvement in the cardiotoxicity of PM by using inhibitors of its synthesis or binding.
MATERIALS AND METHODS
Adult Sprague Dawley rats were maintained and studied in accordance with the National Institutes of Health guidelines for the care and use of animals in research and all protocols were approved by the Harvard Medical Area Standing Committee on Animals. In a first set of experiments, a total of 80 unrestrained, conscious animals were exposed once for 5 hours to either fine PM or filtered air. At the end of the exposure the animals were tested for oxidative stress measure by in situ chemiluminescence (CL) and lipid peroxidation measured by thiobarbituric acid reactive substances (TBARS), as described below. Blood samples were also taken to measure angiotensin-II and creatinine levels in plasma. A total of 14 exposures, each on a different day, were run over a period of 6 months. In a separate series of experiments an additional 8 rats were exposed for 5 hours to either fine PM (CAPs) or filtered air (sham). A total of 11 exposures were performed repeatedly over a 4-month period. Rats were housed at the Harvard School of Public Health animal facility during the 7-14 days between one exposure and the other. During each exposure we used radio telemetry to record the electrocardiogram (ECG) and assessed cardiac function. In both experiments, the indicated number of rats were randomly assigned to pre-treatment with Valsartan or Benazepril in order to test the hypothesis that observed responses to fine PM are mediated at least in part by angiotensin-II.
Fine concentrated ambient particles (CAPs)
The Harvard Fine Particle Concentrator (HFPC) used in these studies concentrates ambient fine particles (0.15-2.5 μm aerodynamic diameter) about 30-fold for subsequent aerosol exposure of animals without altering particle composition or size distribution (Sioutas et al. 1997; Sioutas et al. 1995). During each exposure, we measured integrated fine CAPs mass concentration gravimetrically, trace metal concentrations using X-ray fluorescence (Chester LabNet, Tigrad, Oregon), black carbon, a surrogate for elemental carbon, continuously (Aethalometer Model AE-9, Magee Scientific, Berkeley, CA), and particle number concentration continuously (CPC Model 3022A, TSI Incorporated, Shoreview, MN). The averages for mass concentration and composition of the fine PM aerosols used in this study is presented in Table 1. The average fine PM composition in the present study was similar to the average composition in previous exposures carried out between 2001-2006 (Ghelfi et al. 2008; Gurgueira et al. 2002; Rhoden et al. 2004; Rhoden et al. 2005) except with 2-fold higher concentration of chlorine and half the concentration for vanadium. The fine PM mass concentrations in this study ranged from 100-1200 μg/m3.
Table 1.
Average composition of fine PM aerosols
| All exposures | Exp 1 | Exp 2 | |
|---|---|---|---|
| CAPs mass concentration | 440 ± 80 | 390±110 | 510±110 |
| Black carbon concentration | 8 ± 1 | 7.6 ± 1.7 | 8.5 ± 2.0 |
| Particle number concentration | 16,000 ± 1,000 | 11,000 ± 1,000 | 22,000 ± 2,000 |
| Na | 10 ± 2 | 4 ± 1 | 17 ± 4 |
| Mg | 1.3 ± 0.2 | 0.8 ± 0.1 | 1.9 ± 0.4 |
| Al | 5 ± 1 | 4 ± 1 | 5 ± 1 |
| Si | 11 ± 1 | 10 ± 1 | 12 ± 2 |
| S | 45 ± 13 | 48 ± 19 | 42 ± 13 |
| Cl | 6 ± 3 | 3 ± 2 | 11 ±15 |
| K | 2.7 ± 0.3 | 2.4 ± 0.2 | 3.2 ± 0.6 |
| Ca | 5 ± 1 | 4.6 ± 0.4 | 6 ± 1 |
| Ti | 0.41 ± 0.05 | 0.37 ± 0.04 | 0.45 ± 0.08 |
| V | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.03 ± 0.01 |
| Cr | 0.035 ± 0.005 | 0.03 ± 0.01 | 0.04 ± 0.01 |
| Mn | 0.24 ± 0.03 | 0.22 ± 0.03 | 0.27 ± 0.04 |
| Fe | 9 ± 1 | 9 ± 1 | 9 ± 1 |
| Ni | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Cu | 0.22 ± 0.03 | 0.23 ± 0.04 | 0.21 ± 0.03 |
| Zn | 0.9 ± 0.1 | 0.8 ±Ê0.1 | 1.0 ± 0.2 |
| As | 0.014 ± 0.004 | 0.016 ± 0.006 | 0.013 ± 0.006 |
| Se | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 |
| Br | 0.13 ± 0.03 | 0.11 ± 0.03 | 0.17 ± 0.04 |
| Sr | 0.042 ± 0.005 | 0.036 ± 0.06 | 0.050 ± 0.006 |
| Zr | 0.05 ± 0.01 | 0.05 ±Ê001 | 0.05ʱ 0.01 |
| Cd | 0.09 ± 0.03 | 0.007 ± 0.004 | 0.012 ± 0.004 |
| Sn | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.06 ± 0.04 |
| Ba | 0.41 ± 0.05 | 0.46 ± 0.07 | 0.34 ±Ê004 |
| Pb | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.13 ±Ê002 |
All measurements are given in μg/m3 except for particle number that is given in particles/cm3.
Exp 1: experiment 1 for oxidative stress and blood measurements. Values represent the averages of 14 exposures carried out between April and Nov 2006.
Exp 2: experiment 2 for ECG measurements. Values represent the averages of 11 exposures carried out between February and May 2007.
Rats were exposed to fine PM aerosols (CAPs) or filtered air (sham) in the chamber of the HFPC at 25 °C as previously described (Gurgueira et al. 2002). Each animal was placed inside an individual polycarbonate chamber (10cm diameter × 18 cm long) with the nose pointing to the PM/filtered air outlet. On each exposure day, eight animals were exposed simultaneously. Flow through each chamber was maintained at 1.5 liters per minute. The animals were awake and unrestrained during the exposures.
Angiotensin Converting Enzyme inhibitor (ACE) and Angiotensin Receptor blocker (ARB) treatments
Angiotensin-II converting enzyme (ACE) catalyzes the conversion of the decapeptide angiotensin I to the octapeptide angiotensin II by removing a carboxy-terminal dipeptide (Riordan 2003). Functional inhibitors of ACE have been extensively used to inhibit the renin-angiotensin system (Brown and Vaughan 1998). We choose benazepril hydrochloride (Lotensin ®), a carboxyl-containing ACE inhibitor, among other ACE inhibitors for its absence of sulfhydryl groups that may confer antioxidant properties to the drug.
The biological actions of Angiotensin-II are mediated by AT1 receptors, present in rodents as two highly homologous subtypes: AT1A and AT1B, both of them recognized by functional inhibitors. Using angiotensin receptor blockers (ARB), such as valsartan (Diovan® ), has the advantage of blocking both ACE and non-ACE pathways (such as chymases and endopeptidases (Berl 2004)) thus providing a more complete attenuation of Angiotensin-II effects.
Benazepril, a carboxyl-containing ACE inhibitor, and valsartan, a competitive inhibitor of AT1 receptors, were purchased from Novartis. Both drugs were dissolved in sterile PBS. Rats were lightly anesthetized with Isoflurane 4% (Isoflurane usp AErrane® Baxter, USA) before they received 0.5 mL gavage of either 10mg/mL Benazepril or 40mg/mL Valsartan . Benazepril treatment was repeated on the 3 consecutive days before the exposure to fine PM. Valsartan treatment consisted of a single dose 2 hours prior to PM exposure.
The treatments protocols used for Benazepril or Valsartan have been reported to effectively block blood pressure responses to Angiotensin II, and to decrease blood pressure in hypertensive rats. However, in normotensive rats, neither treatment showed an effect on resting blood pressure or heart rate at the doses employed in this study (Barker TA et al. 2006; Criscione L et al. 1993; Ledingham JM and R. 2002; Tanaka M et al. 1991).
Organ chemiluminescence
In this study we used measurements of in situ chemiluminescence (CL) to evaluate the ability of fine PM to increase ROS concentration in intact animal in real time in a non-invasive manner. CL is a low intensity emission in the visible range mainly due to the decay of excited state of molecular oxygen (singlet oxygen and excited carbonyls; Boveris et al. 1980, Cadenas et Sies 1984), which are formed during the termination steps of the chain reaction of lipid peroxidation (Halliwell et Gutteridge 1990). The spontaneous CL of organs in situ increases with intracellular H2O2 and precedes oxidative damage. Adult Sprague-Dawley rats (weight 300 ± 20g) were anesthetized with sodium pentobarbital (50 mg/kg i.p.). The trachea was cannulated and connected to an animal ventilator (2.5 ml/breath, 80 breaths/min (Harvard Apparatus Model ‘687’ Mouse Ventilator, Cambridge, MA)). Rats were kept under general anesthesia and artificially ventilated throughout the surgery. The chest was open via a sternotomy, the surrounding tissues were covered by means of an aluminum foil and the surface of the heart was exposed for the measurement of CL. The animals were placed in the measurement compartment and spontaneous CL of the surface of heart was measured as previously described (Gurgueira et al. 2002). A Thorn EMI CT1 single-photon counting apparatus with an EMI 9816B photomultiplier cooled at −20 °C was used. Body temperature was kept at 37 °C using isothermal pads (Braintree Scientific, Braintree, MA). Emission data were expressed as counts per second per unit of tissue surface (cps/cm2). An optical filter (red, Wratten number 25; Eastman Kodak, Rochester, NY) with a cut-off of 600 nm was placed in the optical path to avoid hemoglobin interference.
Determination of thiobarbituric acid reactive substances (TBARS)
Immediately after measuring CL, the animals were euthanized and the heart were excised, washed in saline, and flash frozen in a liquid nitrogen bath. For the determination of TBARS, heart tissue samples were homogenized in 7 volumes of 120 mM KCl, 30 mM phosphate buffer (pH = 7.4) added with proteinase inhibitors (1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 μg/ml soybean trypsin inhibitor, 1 μg/ml pepstatin and 0.5 mM PMSF) at 0−4 °C. The suspensions were centrifuged at 700 x g for 10 min at 0−4 °C to remove nuclei and cell debris. The pellets were discarded and the supernatants were used as homogenates.
TBARS were measured in heart homogenates. Homogenates were precipitated with 10% TCA, centrifuged, and incubated with thiobarbituric acid (Sigma, Chem. Co.) for 1 h at 100 °C. TBARS were extracted using butanol (1:1) to eliminate most interferents. After centrifugation, the fluorescence of the butanol layer was measured at 515 nm (excitation) and 555 nm (emission) using a PTI spectrofluorometer (Photon Technology International, Lawrenceville, NJ, USA). The amount of TBARS formed was expressed in picomoles per milligram of protein. Malondialdehyde standards were prepared from 1,1,3,3, -tetramethoxypropane (Esterbauer and Cheeseman 1990). Protein concentration in homogenates was measured by the Lowry method (Lowry et al. 1951) using bovine serum albumin as standard. Measurements were carried out in a Perkin-Elmer Lambda 40 spectrophotometer.
Plasma levels of angiotensin-II and creatinine
Plasma samples (4 mL) were collected from the vena cava in pre-chilled venous blood collection tubes (BD vacutainer® K2 EDTA 7.2 mg) containing EDTA. Bestatin (ALPCO Diagnostics, Windham, NH, USA; final concentration: 10μM) was added to the tubes in order to inhibit angiotensin-II degrading enzymes. The tubes were kept at 0-4°C and centrifuged at 1500 x g for 15 min in a refrigerated centrifuge. The procedure was carried out in less than 15 min. Plasma samples were immediately transferred to pre-chilled polypropylene tubes and stored at −80 C until analysis. Samples were then shipped to Anilytics Incorporated Laboratories (North Grafton, MA) for the measurement of angiotensin-II by radioimmunoassay and creatinine.
Cardiac function
Rats used for this experiment were implanted with a radio telemetry transmitter (DSI PhysioTel® Transmitter ETA-F20) for the measurement of the ECG. Electrodes were implanted subcutaneously in a Lead II configuration. Prior to each exposure, rats received by gavage either saline (Control and CAPs groups), 10mg/mL Benazepril or 40mg/mL Valsartan . As mentioned before Benazepril treatment was repeated on the 3 consecutive days before the exposure to fine PM, whereas Valsartan was given in a single dose 2 hours prior to fine PM exposure. The 6 groups (Saline/CAPs, Saline/Sham, Valsartan /Sham, Valsartan /CAPs, Benazepril /Sham, Benazepril /CAPs) were exposed and tested simultaneously by using 2 animals per day of exposure for each of the sham and CAPs groups and one for each treatment group. Baseline measurements were recorded for each animal before the beginning of the exposures. Real-time ECG waveforms were continuously recorded using a PC-based system (Dataquest ART, Data Sciences, Inc.). Standard ECG intervals and waveform amplitudes were measured from the recorded ECGs using a commercial software package (Physiostat ECG Analysis version 4.0, Data Sciences, Inc.) as previously described (Ghelfi et al. 2008). For each parameter of interest, 2 data points were contributed per animal per exposure day, one 15 min after the start of the exposure and one segment 4 h 45 min after the start of the exposure. The intervals considered in this study were: PR (time interval between the beginning of the P-wave to the peak of the R-wave), QT (time interval between the beginning of the Q-wave and the end of the T-wave), QRS (time interval between the beginning of the Q wave and the peak of the S-wave), RTp (time interval between the peak of the R-wave and the peak of the T-wave), Tpe (time interval between the peak of the T-wave and the end of the T-wave) and P duration (Pdur, time interval between the beginning and the end of the P-wave). We choose to focus on these parameters based on our previous observation that rats exposed to Boston fine CAPs for 5 hours show decreases in the length of the RT, QT, QRS and Tpe intervals of the ECG and increases in Pdur (Ghelfi et al. 2008).
Statistical Analyses
Values are expressed as means ± SEM. CL and TBARS were analyzed statistically by factorial analysis of variance (ANOVA) followed by Student-Newman-Keuls’ test for comparison of the means. Heart rate and ECG interval data were analyzed using linear mixed models with treatment group (6 categories: Sham or CAPs exposure in animals pre-treated with saline, Valsartan , or Benazepril ), time (2 categories: 15 min, 4 hr 45 min), and time-by-treatment interactions as fixed effects and random rat-specific intercepts. This modeling approach provides a framework for evaluating effects of treatments and time while accounting for the correlation between repeated measures on the same animal. Analyses were carried out using SAS v9 (Cary, NC) and Statview for Macintosh. Statistical significance was accepted at p < 0.05.
RESULTS
Inhalation exposure to fine PM increases angiotensin levels in plasma
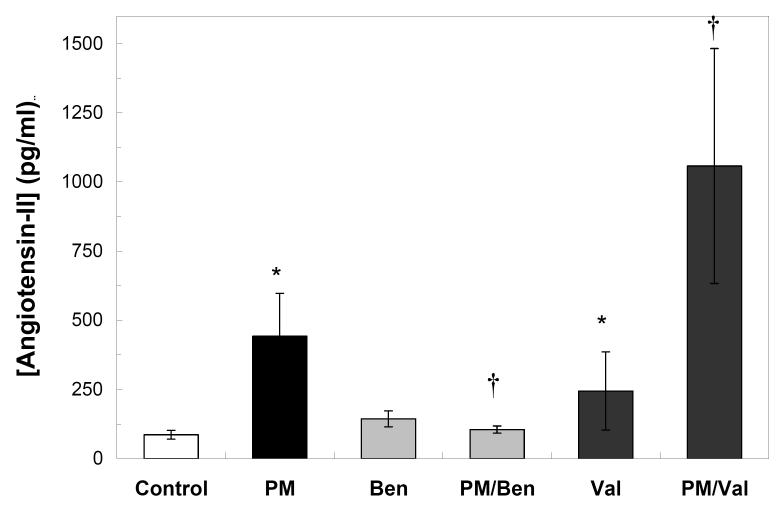
As a first step to test the hypothesis that increases in angiotensin-II after fine PM exposure are involved in the development of cardiac oxidative stress we measured angiotensin-II levels in rats exposed to fine PM or filtered air. We found that the levels of Angiotensin-II in plasma samples taken at the end of the exposure were significantly higher in PM-exposed rats than in the filtered air controls (Fig. 1). We also measured Angiotensin-II levels in rats treated with the inhibitors to be used in the following experiments to confirm their expected effects. Pretreatment of rats with the ACE inhibitor Benazepril prevented the increases in Angiotensin-II seen in the PM only group (Fig. 1). Conversely, the Angiotensin receptor blocker Valsartan led to further increases of Angiotensin-II blood levels as compared with the PM only group (Fig. 1). Controls for the effectiveness of the protocols of treatment with Benazepril or Valsartan are also presented as a reference. Benazepril -only treatment did not significantly change the circulating levels of Angiotensin-II as compared to untreated control. Valsartan -only treatment, on the other hand, prevented binding and degradation and therefore increased the blood levels of Angiotensin-II (Fig. 1).
Fig. 1. Angiotensin-II plasma levels in rats exposed to fine PM.
Adult Sprague Dawley rats treated with saline, Benazepril or Valsartan were exposed to fine PM or filtered air for 5 hours in the HFPC. Blood samples were collected at the end of the exposure and processed for the determination of angiotensin-II in plasma, as described in the Methods section. Bars represent the mean value of 4-5 independent experiments. * p <0.002 vs. control. † p< 0.05 vs. PM.
Some important functions of Angiotensin-II center on the kidney. To test for possible changes in renal function in these treatment groups we measured creatinine levels in the same samples assayed for Angiogenesis-II. Plasma creatinine was not significantly different in rats treated with saline (0.37 ± 0.058 mg/dl), Benazepril (0.35 ± 0.058 mg/dl) or Valsartan (0.35 ± 0.058 mg/dl), or in animals exposed to fine PM (0.25 ± 0.10 mg/dl) (p > 0.05). In all groups, creatinine concentration was within physiological range for rats (0.2-0.8 mg/dl).
Prevention of PM-induced cardiac oxidative stress by Angiotensin-II inhibitors
Inhibition of Angiotensin synthesis
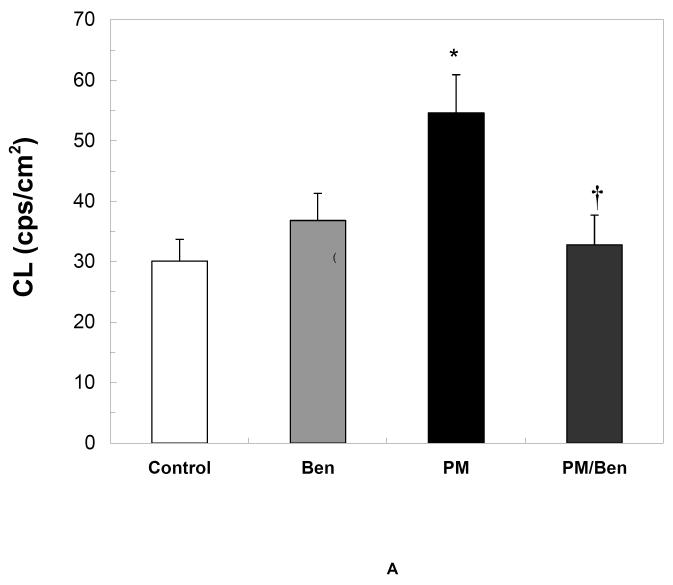
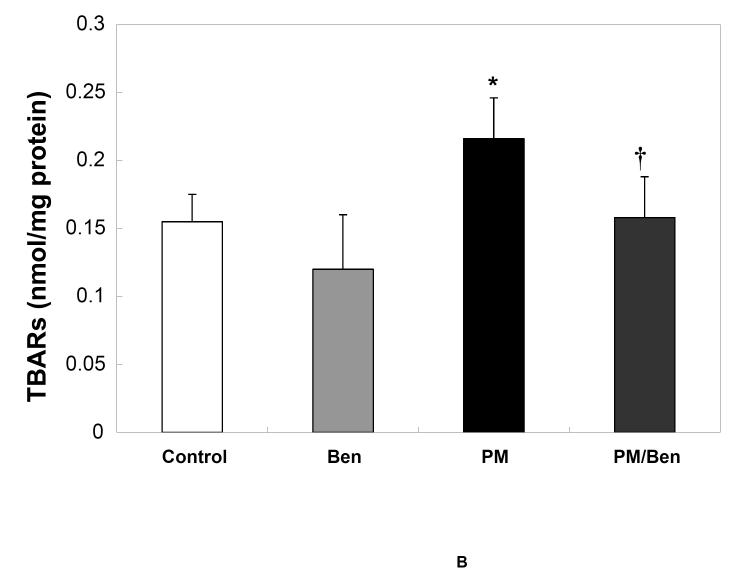
PM-induced increases in cardiac oxidants (measured by heart CL, Fig 2A) and accumulation of oxidized lipids (measured by TBARS, Fig 2B) were prevented by inhibition of ACE with Benazepril . Benazepril -only treatment did not alter oxidative stress (Fig 2A) or damage (Fig 2B) in the heart of sham rats exposed to filtered air.
Fig. 2. Inhibition of Angiotensin-II production prevents cardiac oxidative stress by fine PM.
Adult Sprague Dawley rats were given 0.5 mL of either saline of 10mg/mL Benazepril via gavage 3 consecutive days before the exposure to fine PMor filtered air. At the end of the 5-hours exposure rats were immediately assessed for heart CL (A) * p <0.006 vs. control or Benazepril . † p< 0.006 vs. PM. After CL determination, the hearts were excised and processed for TBARS (B) as described in the Methods section. * p <0.03 vs control or Benazepril . Values represent the mean of 6-8 independent experiments ± SEM.
Functional inhibition of Angiotensin
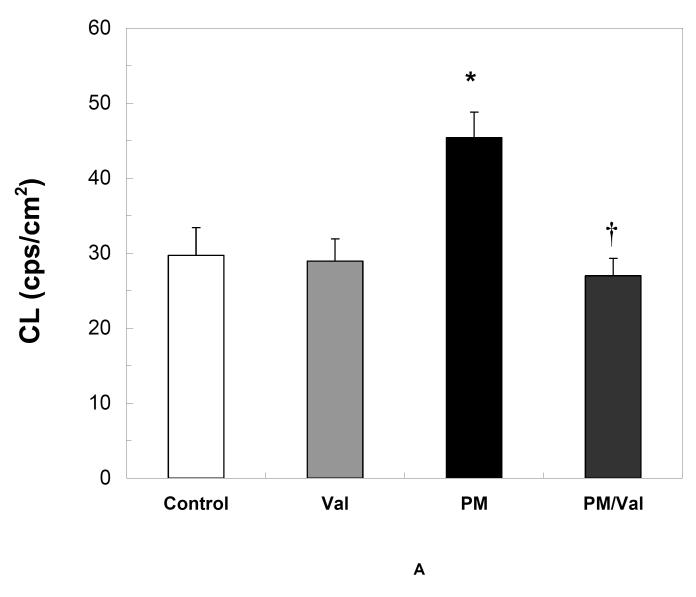
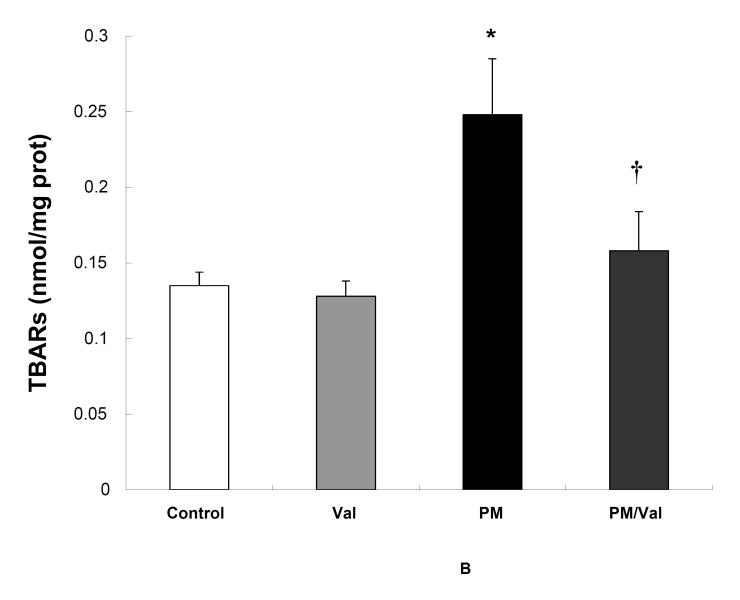
Pre-treatment of rats with Valsartan , a blocker of Angiotensin-II binding to the AT1 receptors, effectively prevented oxidative stress (Fig 3A) and accumulation of oxidized lipids (Fig. 3A) by fine PM. Valsartan -only treatment did not alter oxidative stress (Fig 3A) or lipid peroxidation (Fig 3B) in the heart of sham controls.
Fig. 3. Inhibition of Angiotensin-II binding prevents cardiac oxidative stress by fine PM.
Adult Sprague Dawley rats were given 0.5 mL of either saline or 40mg/mL Valsartan 2 hours prior to exposure to fine PM or filtered air. At the end of the 5-hours exposure rats were immediately assessed for heart CL (A) * p <0.003 vs. control or Valsartan . † p< 0.003 vs. PM. After CL determination, the hearts were excised and processed for TBARS (B) as described in the Methods section. *p <0.002 vs. control or Valsartan . † p< 0.008 vs. CAPs Values represent the mean of 8-10 independent experiments ± SEM.
Effect of Angiotensin-II inhibitors on the electrophysiological changes induced by fine PM inhalation
We evaluated rats for changes in heart rate, and in the length of the QRS, QT, Pdur, Tpe and RTp intervals of the ECG, the intervals that showed significant changes in previous studies (Ghelfi et al. 2008).
Most of the treatments tested here lead to no significant differences in HR (Table 2). In rats pre-treated with Benazepril and exposed to filtered air HR was reduced at t=15min by an average of 64.4 bpm (p=0.04).
Table 2.
Effects of exposure to fine CAPs on electrocardiographic parameters in rats pre-treated with saline or angiotensin inhibitors.
|
Pre- Treatment |
Exposure | HR | QRS | QT | Pdur | Tpe | RTp |
|---|---|---|---|---|---|---|---|
| 15 min | |||||||
| Saline | Sham | 343.8 ± 14.5 | 18.5 ± 0.4 | 47.9 ± 1.8 | 18.9 ± 1.1 | 17.8 ± 0.9 | 18.8 ± 2.0 |
| Saline | PM | 344.4 ± 18.5 | 19.0 ± 0.5 | 50.8 ± 2.2 | 20.5 ± 1.3 | 14.6 ± 1.12 | 23.8 ± 2.5 |
| Valsartan | Sham | 307.0 ± 26.3 | 19.3 ± 0.6 | 50.0 ± 3.8 | 16.9 ± 2.3 | 18.3 ± 2.1 | 20.0 ± 4.3 |
| Valsartan | PM | 292.7 ± 20.4 | 19.3 ± 0.5 | 50.1 ± 2.6 | 18.7 ± 1.6 | 19.7 ± 1.3 | 18.7 ± 3.0 |
| Benazepril | Sham | 279.3 ± 29.3 | 20.6 ± 0.7 | 52.5 ± 4.3 | 15.0 ± 2.6 | 19.1 ± 2.4 | 21.6 ± 4.9 |
| Benazepril | PM | 345.1 ± 18.42 | 18.3 ± 0.52 | 49.1 ± 2.2 | 16.0 ± 1.3 | 22.1 ± 1.1 | 16.8 ± 2.5 |
| 4 h 45 min | |||||||
| Saline | Sham | 350.8 ± 14.5 | 18.9 ± 0.4 | 48.7 ± 1.8 | 18.3 ± 1.1 | 18.6 ± 0.9 | 18.5 ± 2.1 |
| Saline | PM | 331.7 ± 18.5 | 19.1 ± 0.5 | 49.0 ± 2.2 | 21.4 ± 1.31 | 12.1 ± 1.12 | 24.2 ± 2.51 |
| Valsartan | Sham | 295.2 ± 26.3 | 19.0 ± 0.6 | 42.0 ± 3.8 | 15.1 ± 2.3 | 12.8 ± 2.1 | 17.7 ± 4.3 |
| Valsartan | PM | 318.8 ± 21.1 | 19.9 ± 0.5 | 47.9 ± 2.7 | 19.9 ± 1.6 | 16.8 ± 1.4 | 19.0 ± 3.1 |
| Benazepril | Sham | 332.2 ± 29.3 | 18.9 ± 0.7 | 45.8 ± 4.3 | 18.0 ± 2.6 | 13.8 ± 2.4 | 20.5 ± 4.9 |
| Benazepril | PM | 327.9 ± 18.1 | 18.3 ± 0.5 | 49.3 ± 2.1 | 16.0 ± 1.3 | 21.8 ± 1.02 | 17.1 ± 2.4 |
Values represent mean ± SEM. Values in bold denote that the mean response in the PM group is marginally significantly different or statistically significantly different (1p<0.1; 2p<0.05) versus the Sham-exposed group with the same pretreatment.
HR: Heart rate.
QRS: Time interval between the beginning of the Q wave and the peak of the S-wave.
QT: Time interval between the beginning of the Q-wave and the end of the T-wave.
Pdur: P duration, time interval between the beginning and the end of the P-wave.
Tpe: Time interval between the peak of the T-wave and the end of the T-wave.
RTp: Time interval between the peak of the QRS complex and the peak to the T-wave
In rats pre-treated with saline, PM exposure was associated with a 3.2 ms (p=0.02) decrease in Tpe after 15 min of exposure and a 6.5 ms (p<0.001) decrease after 4 hr 45 min of exposure (Table 2). Fine PM inhalation also increased Pdur by 3.1 ms (p=0.06) and RTp by 5.7 ms (p=0.07) at the 4 hr 45 min time point.
Blockade of angiotensin synthesis with Benazepril pre-treatment reversed the effects of PM exposure on Tpe (4.3 ms increase in Tpe after 15 min of exposure and 3.2 ms increase at 4 hr 45 min (p=0.002) compared to PM-only) and prevented PM-induced changes in Pdur or RTp. Consistently, following blockade of AT1 receptors with Valsartan , fine PM exposure was not associated with statistically significant changes in Pdur, Tpe or RTp at either time point.
In rats pre-treated with Benazepril and exposed to filtered air QRS duration was increased by 2.1 ms (p=0.003).
DISCUSSION
The cardiotoxicity of ambient air particles is mediated, at least in part, by increased production of ROS due to changes in the sympathovagal tone on the heart (Ghelfi et al. 2008; Rhoden et al. 2004). In this study we report that exposure to fine PM increases the blood levels of angiotensin-II, a potent vasoconstrictor and sympatho-excitatory peptide with strong ROS-dependent cardiotoxicity (Mehta and Griendling 2007). Our data show that increases in circulating Angiotensin II are accompanied by increased production of ROS in the heart, and changes in ion currents leading to altered ventricular repolarization. Changes in conduction velocity and ventricular depolarization are also suggested. Furthermore, inhibition of angiotensin-II synthesis or binding prevents PM effects on heart oxidants and electrophysiology.
CL is an early and sensitive marker of oxidative stress, and a predictor of cellular, subcellular, or tissue damage caused by ROS. Increases in heart CL in the order reported here (1.6- to 2.0-fold) are associated with significant mitochondrial damage and post-reperfusion arrhythmias in patients undergoing revascularization surgery (González-Flecha et al. 1991). Open-heart surgery requires that the heart is subjected to ischemia/reperfusion, and the damage caused by this procedure can be accurately estimated by measuring the differences in CL in heart biopsies taken before and after the surgery. In this model, increases in heart CL correlated with increases in the levels of mitochondrial damage measured by electron microscopy (r= 0.88, p< 0.001 (González-Flecha et al. 1991)). Consistently, treatment with antioxidants prevented the increases in CL and was associated with a decrease number of reperfusion arrhythmias (Ferreira et al. 1988; Llesuy et al. 1995).
Provided that possible interferents (such as sugars) are removed from the samples and antioxidants are used during the processing, TBARS measurements provide an estimate of the amount of oxidized lipids in a tissue, i.e. of the extent to which ROS (estimated by CL) have reacted with intracellular components. The results presented here show a significant amount of lipid peroxidation in the heart after exposure to fine PM confirming the heart CL data and the occurrence of significant oxidative damage in the heart of rats exposed to fine PM.
Consistent with our previous work (Ghelfi et al. 2008), we found that inhalation exposure to Boston fine PM leads to significant and specific alterations in heart electrophysiology. In the present study, we found that fine PM exposure was associated with statistically significant shortening of the Tpe interval, and increase in the length of the Pdur and RTp intervals that were not statistically significant.
Although changes in ECG intervals of the magnitude observed here are not likely of clinical significance, these findings may provide some insight into novel potential pathophysiological mechanisms for the cardiac effects of ambient particles. The Tpe interval, an electrocardiographic marker of the transmural dispersion of repolarization (Antzelevitch 2001), has been linked to the genesis of torsade de pointes, a life-threatening form of ventricular tachycardia associated with increased risk of sudden death in experimental models of the long-QT syndrome (Viitasalo et al. 2002). The PM-related decrease in Tpe found in the current study suggests changes in myocardial Na channel activation leading to decreases in the length of the cardiac action potential and the time required for ventricular repolarization. The clinical significance of this finding, if any, remains uncertain.
P-wave duration (Pdur) is a marker of intra-atrial conduction times and is influenced by changes in autonomic tone (Cheema et al. 1995). The marginally statistically significant increase in P-wave duration reported here and in our previous work (Ghelfi et al. 2008) in PM-exposed rats suggests a shift in sympathovagal balance towards parasympathetic dominance resulting in decreased velocity of propagation.
The RTp interval, as the QT interval, is a measure of ventricular depolarization and repolarization (Pladys et al. 2000). Although RTp is not as widely used as QT, the results for RTp may be more consistent since the peak of the T-wave is technically easier to locate than the end of the T-wave. Frampton et al. (Frampton et al. 2004) found a decrease in QT interval following exposure to laboratory-generated ultrafine carbon particles in healthy exercising subjects, but not in those with mild asthma. Two observational epidemiologic studies in patients with coronary artery disease found that exposure to traffic-related particles and organic carbon was associated with a lengthening of QT (Henneberger et al. 2005; Yue et al. 2007). In our previous study in rats, we found consistent shortening of the RTp, RT and QT intervals (Ghelfi et al. 2008) following 5-hr exposure to fine PM. The differences in the responses between these studies could be attributable to differences in aerosol composition and/or species. On the other hand, the fact that we observed changes in RTp but not QT interval in the current study may suggest that the RTp changes observed here represent a chance finding.
The observation that only a few ECG intervals are altered by exposure to fine PM suggests that the response observed is not the result of a massive effect of ROS on the myocardium (as it would be expected from massive lipid peroxidation), but rather a specific inactivation of a few ion channels resulting in current abnormalities and modest changes in conduction velocity and ventricular depolarization/repolarization.
The renin-angiotensin system plays an important role regulating arterial pressure and blood volume. In the classical system the enzyme renin is released into the circulation from kidney juxtaglomerular cells in response to sympathetic stimulation, renal artery hypotension or decreased level of sodium in distal tubules. Renin converts angiotensinogen from liver to the decapeptide angiotensin-I, which in turn undergoes proteolytic cleavage to the biologically active octapeptide angiotensin-II. The latter step is carried out by angiotensin converting enzyme (ACE) which is highly expressed on vascular endothelium, particularly in the lungs (Kurdi et al. 2005).
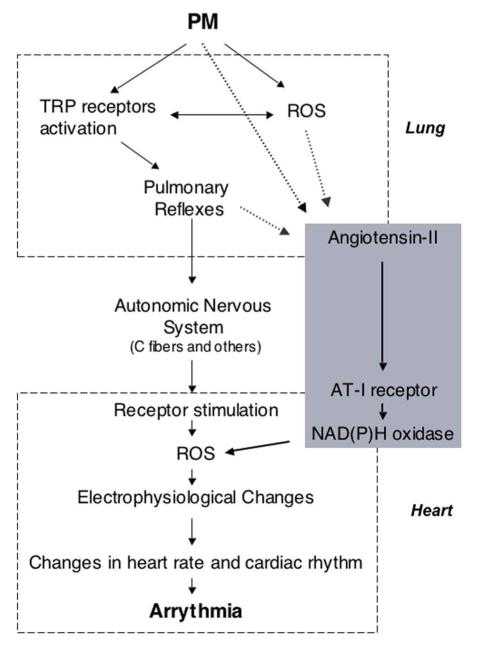
A possible mechanism linking the observations reported here is presented in Figure 4. Pulmonary reflexes triggered by PM and acting on the pulmonary or systemic vasculature could be envisioned as possible causes for increases in angiotensin levels. As mentioned above, the vascular endothelium in the lung is rich in ACE and is an easy suspect for the initiation of this response. In vitro evidence shows that PM induces phosphorylation of ERK and p38 kinases and vasoconstriction in pulmonary artery rings and arterial endothelial cells. These responses are inhibited by AT I receptor inhibitors, suggesting that angiotensin-II may play an important role, and are observable within minutes (Li et al. 2005). Although this response requires relatively high doses of PM to reach endothelial cells, it could be speculated that such doses could be reach at the hot spots of particles deposition. Another possible link between PM exposure and increased Angiotensin-II is the potent vasoconstrictor endothelin-1. Increased expression of endothelin-1 in lung cells has been reported in response to PM exposure in rodents (Bouthillier et al. 1998; Thomson et al. 2004) and humans (Calderón-Garcidueñas et al. 2007),(Brook et al. 2002). Endothelin-1 is a stimulator of the sympathetic nervous system, and of the renin-angiotensin system (Giannessi et al. 2001) and could in that way mediate the increase in angiotensin-II reported here. However, as pointed out by Li et al (Li et al. 2005), endothelin increases occur 24 hours after PM exposure and therefore can not explain acute PM effects of the ones reported here.
Fig. 4.
Schematic representation of the possible mechanism(s) connecting PM exposure, increases in Angiotensin-II blood levels, and cardiac alterations. PM: particulate matter; ROS: reactive oxygen species; TRP receptors: Transient Receptor Potential receptors.
In addition to its physiological role in the regulation of blood pressure, angiotensin-II is also known to have pathological effects in the cardiovascular system, mostly due to its ability to increase production of ROS (Mehta and Griendling 2007). ROS are implicated in the etiology of cardiovascular diseases, such as atherosclerosis, hypertension, fibrosis, myocardial infarction, and congestive heart failure. Angiotensin-II stimulates ROS production through the G protein-coupled AT1 receptor expressed in its target organs, such as vascular tissues, heart, and kidney (reviewed in (Mehta and Griendling 2007)). In our experimental model we were particularly looking at cardiac responses. In this tissue, the increase in ROS mediated by ATI/NAD(P)H oxidase activation could lead to electrophysiological changes, changes in heart rate and cardiac rhythm and possibly arrhythmia (Fig 4) by mechanism similar to those initiated by activation of pulmonary reflexes (Ghelfi et al. 2008).
Lowering angiotensin levels with ACE inhibitors or blocking the angiotensin responses with ARBs is an important therapeutic strategy to provide cardioprotection in individuals with hypertension, myocardial infarction, or congestive heart failure. Consistently, we found that exposure to fine PM aerosols increased the levels of angiotensin-II in plasma, and inhibition of both synthesis and binding of angiotensin prevents cardiac oxidative stress by PM. Furthermore, fine PM exposure led to alterations in cardiac electrophysiology that were prevented by inhibition of angiotensin synthesis or binding. In other words, blockade of the angiotensin pathway either at the initiation point (probably the lung) or at the target receptors (in the heart) prevents some of the previously described cardiac responses to fine PM.
The aerosols used in this and other studies are a mix of fine PM and gaseous components and therefore it would be plausible to think that the observed effects were caused by components other that fine PM. However, since the changes we report here are differences between filtered air (containing the gaseous components present in the original aerosol) and concentrated aerosols (enriched in particles but otherwise unaltered) the possible contribution of the gaseous fraction can be ruled out. The possibility of a direct effect of soluble components or particles on the cardiovascular system seems also unlikely based on previous calculations of the amount needed to have detectable outcomes (estimated amount of Fe delivered by fine PM: 14 nmol vs. millimolar levels needed for detectable responses)(Ghelfi et al. 2008). Similarly, the amount of ultrafine particles which could access the blood stream and reach the heart or vessels estimated in Boston fine PM samples (~ 0.4% of the PM mass, i.e. ~ 5 μg/m3)(Ghelfi et al. 2008) is about 30-fold lower than the one used experimentally to model episodes of increases of ultrafine particles in urban air (Elder et al. 2004; Elder et al. 2000).
CAPs aerosols are complex mixtures that vary in composition and concentrations from day to day. These changes are a consequence of variations in composition in the urban atmosphere that is modeled making this system very appealing as a realistic exposure. However, this same characteristic dictates the need of appropriate statistical analyses to identify outcomes that produce responses that will show consistent changes even when altered in magnitude from one set of exposures to another. CL has proven to be a reliable outcome, showing consistent increases after fine PM exposure in a variety of exposures over a number of years (Ghelfi et al. 2008; Gurgueira et al. 2002; Rhoden et al. 2004; Rhoden et al. 2005). ECG interval length has only been measured in a few studies thus far and will require more experimentation before its relation to PM exposure can be completely understood.
To compute the day-to-day variability in PM composition we worked with a total of 25 exposures and 88 rats. Of necessity we divided the study into two experiments, one to evaluate oxidative stress and blood parameters (80 rats, 14 exposures), and the other to study cardiac function (8 rats, 11 exposures). On average, the aerosols used for these two sets of exposures have 2-fold differences in fine PM mass concentrations, black carbon, and particle number concentrations (Table 1), three parameters that are strongly associated with changes in ECG interval length (Ghelfi et al. 2008). A more detailed analysis of these associations and those with elemental components is beyond the scope of this article and will be presented in a separate manuscript. Nonetheless, the effects on Tpe, Pdur and RTp found in this study were prevented by inhibition of angiotensin synthesis or binding suggesting a role for angiotensin-II in the mechanism of cardiotoxicity by PM.
Since the postulated mechanism for the cardiovascular effects of PM involves multiple compartments (lung, heart and the vasculature) it seems unlikely that a response at the target site would affect the dose-response at the deposition/initiation site. However, this cannot be either proven or ruled out with the experimental data available.
In summary, activation of the renin-angiotensin system may play a critical role in the genesis of atrial and ventricular arrhythmias, and treatment with angiotensin inhibitors and blockers may be beneficial (reviewed in (Garg et al. 2006)). PM exposure leads to some of the same alterations in cardiac function and structure (reviewed in (Godleski 2006)) by mechanisms that seem to involve increases in ROS (Ghelfi et al. 2008; Rhoden et al. 2005). The PM-induced increases in angiotensin-II reported here, and the association between those increases and cardiac oxidative stress and dysfunction provide another plausible mechanistic component to the complex responses of the heart to particles.
ACKNOWLEDGMENTS
This work was supported in part by grant R-832416 from the US Environmental Protection Agency (US EPA) and grant R00-ES015774 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the US EPA, NIEHS or the National Institutes of Health
Abbreviations
- PM
particulate matter
- PM2.5
fine particles, diameter < 2.5 μm CAPs: concentrated ambient particles
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive substances CL: chemiluminescence
- HFPC
Harvard fine particle concentrator TCA: trichloroacetic acid
- NO
nitric oxide
- ACE
angiotensin converting enzyme
- ARB
angiotensin receptor blocker
REFERENCES
- Antzelevitch C. Transmural dispersion of repolarization and the T wave. Cardiovasc Res. 2001;50:426–431. doi: 10.1016/s0008-6363(01)00285-1. [DOI] [PubMed] [Google Scholar]
- Barker TA, Massett MP, Korshunov VA, Mohan AM, Kennedy AJ, BC. B. Angiotensin II type 2 receptor expression after vascular injury: differing effects of angiotensin-converting enzyme inhibition and angiotensin receptor blockade. Hypertension. 2006;5:942–9. doi: 10.1161/01.HYP.0000241061.51003.b7. [DOI] [PubMed] [Google Scholar]
- Berl T. Angiotensin-converting enzyme inhibitors versus AT1 receptor antagonist in cardiovascular and renal protection: the case for AT1 receptor antagonist. J Am Soc Nephrol. 2004;15(Suppl 1):S71–6. doi: 10.1097/01.asn.0000093235.09769.9c. [DOI] [PubMed] [Google Scholar]
- Bouthillier L, Vincent R, Goegan P, Adamson IY, Bjarnason S, Stewart M, Guénette J, Potvin M, Kumarathasan P. Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1. Am J Pathol. 1998;153:1873–84. doi: 10.1016/S0002-9440(10)65701-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–6. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr., Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brown N, Vaughan D. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Henríquez-Roldán C, Barragán-Mejía G, Garrido-García L, Camacho-Reyes L, Valencia-Salazar G, Paredes R, R. L, Osnaya H, Villarreal-Calderón R, Torres-Jardón R, Hazucha MJ, Reed W. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Env Health Perspect. 2007;115:1248–53. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema A, Ahmed MW, Kadish AH, Goldberger J. Effects of autonomic stimulation and blockade on signal-averaged P wave duration. J Am Coll Cardiol. 1995;26:497–502. doi: 10.1016/0735-1097(95)80028-f. [DOI] [PubMed] [Google Scholar]
- Creason J, Neas L, Walsh D, Williams R, Sheldon L, Liao D, Shy C. Particulate matter and heart rate variability among elderly retirees: the Baltimore 1998 PM study. J Expo Anal Environ Epidemiol. 2001;11:116–22. doi: 10.1038/sj.jea.7500154. [DOI] [PubMed] [Google Scholar]
- Criscione L, de Gasparo M, Bühlmayer P, Whitebread S, Ramjoué HP, J. W. Pharmacological profile of valsartan: a potent, orally active, nonpeptide antagonist of the angiotensin II AT1-receptor subtype. Br J Pharmacol. 1993;110:761–71. doi: 10.1111/j.1476-5381.1993.tb13877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippoliti D, Forastiere F, Ancona C, Agabiti N, Fusco D, Michelozzi P, Perucci CA. Air pollution and myocardial infarction in Rome: a case-crossover analysis. Epidemiology. 2003;14:528–35. doi: 10.1097/01.ede.0000082046.22919.72. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concent rate d air pollution particles have decreased heart rate variability. Eur Resp. J. 2003;40:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Luttmann-Gibson H, Rich DQ, Link MS, Mittleman MA, Gold DR, Koutrakis P, Schwartz JD, Verrier RL. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113:670–4. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–34. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder A, Gelein R, Azadniv M, Frampton M, Finkelstein J, Oberdörster G. Systemic effects of inhaled ultrafine particles in two compromised, aged rat strains. Inhal Toxicol. 2004;16:461–71. doi: 10.1080/08958370490439669. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Finkelstein JN, Cox C, Oberdörster G. Pulmonary inflammatory response to inhaled ultrafine particles is modified by age, ozone exposure, and bacterial toxin. Inhal Toxicol. 2000;12:227–46. doi: 10.1080/089583700750019585. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods in Enzymology. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Llesuy S, Milei J, Scordo D, Hourquebie H, Molteni L, de Palma C, Boveris A. Assessment of myocardial oxidative stress in patients after myocardial revascularization. American Heart Journal. 1988;115:307–12. doi: 10.1016/0002-8703(88)90475-9. [DOI] [PubMed] [Google Scholar]
- Frampton M, Utell MJ, Zareba W, Oberdörster G, Cox C, Huang LS, Morrow PE, Lee FE, Chalupa D, Frasier LM, Speers DM, Stewart J. Effects of exposure to ultrafine carbon particles in healthy subjects and subjects with asthma. Res Rep Health Eff Inst. 2004;126:1–47. [PubMed] [Google Scholar]
- Garg S, Narula J, Marelli C, Cesario D. Role of angiotensin receptor blockers in the prevention and treatment of arrhythmias. Am J Cardiol. 2006;97:921–5. doi: 10.1016/j.amjcard.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, González-Flecha B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated air particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci. 2008;102:328–36. doi: 10.1093/toxsci/kfn005. [DOI] [PubMed] [Google Scholar]
- Giannessi D, Del Ry S, Vitale R. The role of endothelins and their receptors in heart failure. Pharmacol Res. 2001;43:111–26. doi: 10.1006/phrs.2000.0758. [DOI] [PubMed] [Google Scholar]
- Godleski J. Responses of the heart to ambient particle inhalation. Clinics in Occupational and Environmental Medicine. 2006;3:849–864. doi: 10.1016/j.coem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, Lovett EG, Lawrence J, Murthy GG, Wolfson JM, Clarke RW, Nearing BD, Killingsworth C. Mechanisms of morbidity and mortality from exposure to ambient air particles. Research Report - Health Effects Institute. 2000;91:5–88. [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–73. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- González-Flecha B, Llesuy S, A. B. Hydroperoxide-initiated chemiluminescence: an assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radical Biology & Medicine. 1991;10:93–100. doi: 10.1016/0891-5849(91)90002-k. [DOI] [PubMed] [Google Scholar]
- Gurgueira SA, Lawrence J, Coull B, Krishna Murthy GG, González-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environm. Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna I, Taniyama S, Szocs K, Rocic P, Griendling K. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- Henneberger A, Zareba W, Ibald-Mulli A, Ruckerl R, Cyrys J, Couderc JP, Mykins B, Woelke G, Wichmann HE, Peters A. Repolarization Changes Induced by Air Pollution in Ischemic Heart Disease Patients. Environmental Health Perspectives. 2005;113:440–446. doi: 10.1289/ehp.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin F, Tellez-Rojo MM, Hernandez M, Cortez M, Chow JC, Watson JG, Mannino D, Romieu I. Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology. 2003;14:521–7. doi: 10.1097/01.ede.0000081999.15060.ae. [DOI] [PubMed] [Google Scholar]
- Hong YC, Lee JT, Kim H, Kwon HJ. Air pollution: a new risk factor in ischemic stroke mortality. Stroke. 2002;33:2165–9. doi: 10.1161/01.str.0000026865.52610.5b. [DOI] [PubMed] [Google Scholar]
- Joy M, Lowe R. Evidence that the area postrema mediates the central cardiovascular response to angiotensin II. Clin. Sci. 1970;41:89–100. doi: 10.1038/2281303a0. [DOI] [PubMed] [Google Scholar]
- Kurdi M, DeMello W, Booz G. Working outside the system: an update on the unconventional behavior of the renin–angiotensin system components. Int J Biochem Cell Biol. 2005;37:1357–1367. doi: 10.1016/j.biocel.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Ledingham JM, R. L. Fluvastatin remodels resistance arteries in genetically hypertensive rats, even in the absence of any effect on blood pressure. Clin Exp Pharmacol Physiol. 2002;10:931–4. doi: 10.1046/j.1440-1681.2002.03752.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Carter JD, Dailey LA, YC. H. Pollutant particles produce vasoconstriction and enhance MAPK signaling via angiotensin type I receptor. Environ Health Perspect. 2005;113:1009–14. doi: 10.1289/ehp.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Creason J, Shy C, Williams R, Watts R, Zweidinger R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect. 1999;107:521–5. doi: 10.1289/ehp.99107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llesuy S, Milei J, Picone V, Gonzalez-Flecha B, Beigelman R, Boveris A. Effect of vitamins A and E on ischemia-reperfusion damage in rabbit heart. Molecular & Cellular Biochemistry. 1995;145:45–51. doi: 10.1007/BF00925712. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosembrough AL, Farr AL, Randall R. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Martin S, Boucard AA, Clement M, Escher E, Leduc R, Guillemette G. Analysis of the third transmembrane domain of the human type 1 angiotensin II receptor by cysteine scanning mutagenesis. J Biol Chem. 2004;279:51415–23. doi: 10.1074/jbc.M407965200. [DOI] [PubMed] [Google Scholar]
- Mehta P, Griendling K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of Angiotensin II Infusion on the Expression and Function of NAD(P)H Oxidase and Components of Nitric Oxide/cGMP Signaling. Circ Res. 2202;90:e58–e64. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery D, Muller J, Mittleman M. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–5. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–7. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Pladys P, Maison-Blanche P, Gout B, Badilini F, Bril A, Carré F. Influence of sympathetic heart rate modulation on RT interval rate adaptation in conscious dogs. Pacing Clin Electrophysiol. 2000;23:1604–10. doi: 10.1046/j.1460-9592.2000.01604.x. [DOI] [PubMed] [Google Scholar]
- Pope CA, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. Am. Heart J. 1999;138:890–9. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Rechtman M, Majewski H. A facilitatory effect of anti-angiotensin drugs on vagal bradycardia in the pithed rat and guinea-pig. Br. J. Pharmacol. 1993;110:289–296. doi: 10.1111/j.1476-5381.1993.tb13807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoden CR, Lawrence J, Godleski JJ, Gonzalez-Flecha B. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol. Sci. 2004;79:209–303. doi: 10.1093/toxsci/kfh122. [DOI] [PubMed] [Google Scholar]
- Rhoden CR, Wellenius G, Ghelfi E, Lawrence J, Gonzalez-Flecha B. PM-Induced Cardiac Oxidative Stress Is Mediated by Autonomic Stimulation. Biochem Biophys Acta. 2005;1725:305–313. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, Speizer FE, Dockery DW. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Riordan J. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4:225. doi: 10.1186/gb-2003-4-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–51. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 1995;142:23–25. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Koutrakis P, Godleski JJ, Ferguson ST, Kim CS, Burton R. Fine particle concentrators for inhalation exposures-- Effect of particle size and composition. J. Aerosol Sci. 1997;28:1057–1071. [Google Scholar]
- Sioutas C, Koutrakis P, Burton RM. A technique to expose animals to concentrated fine ambient aerosols. Environm. Health Perpect. 1995;103:172–177. doi: 10.1289/ehp.95103172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Yamamoto S, Nakao K, Inukai T, Ogawa N, Katsumura H, Ohara N, Shukunobe K, H. O. Antihypertensive action of the novel angiotensin converting enzyme inhibitor benazepril hydrochloride in hypertensive rat models. Arzneimittelforschung. 1991;6:608–12. [PubMed] [Google Scholar]
- Thomson E, Goegan P, Kumarathasan P, Vincent R. Air pollutants increase gene expression of the vasoconstrictor endothelin-1 in the lungs. Biochim Biophys Acta. 2004;1689:75–82. doi: 10.1016/j.bbadis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Goggins WB, Chiu HF, Yang CY. Association Between Air Pollution and Daily Stroke Admissions in Kaohsiung, Taiwan. Stroke. 2003;34:2612–16. doi: 10.1161/01.STR.0000095564.33543.64. [DOI] [PubMed] [Google Scholar]
- Viitasalo M, Oikarinen L, Swan H, Väänänen H, Glatter K, Laitinen PJ, Kontula K, Barron HV, Toivonen L, Scheinman M. Ambulatory electrocardiographic evidence of transmural dispersion of repolarization in patients with long-QT syndrome type 1 and 2. Circulation. 2002;106:2473–8. doi: 10.1161/01.cir.0000036369.16112.7d. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36:2549–53. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Yue W, Schneider A, Stölzel M, Rückerl R, Cyrys J, Pan X, Zareba W, Koenig W, Wichmann HE, Peters A. Ambient source-specific particles are associated with prolonged repolarization and increased levels of inflammation in male coronary artery disease patients. Mutat Res. 2007;621:50–60. doi: 10.1016/j.mrfmmm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113:978–82. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schmeisser A, Garlichs CD, Plötze K, Damme U, Mügge A, Daniel W. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999;44:215–22. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman BG. Adrenergic facilitation by angiotensin: does it serve a physiological function? Clin. Sci. 1993;60:343–348. doi: 10.1042/cs0600343. [DOI] [PubMed] [Google Scholar]
- Zisman L, Asano K, Dutcher DL, Ferdensi A, Robertson AD, Jenkin M, Bush EW, Bohlmeyer T, Perryman MB, Bristow M. Differential regulation of cardiac angiotensin converting enzyme binding sites and AT1 receptor density in the failing human heart. Circulation. 1998;98:1735–41. doi: 10.1161/01.cir.98.17.1735. [DOI] [PubMed] [Google Scholar]