Synopsis
The clinical severity, impact on development, and poor prognosis of Childhood Onset Schizophrenia (COS) may represent a more homogeneous group. Positive symptoms in children are necessary for the diagnosis and hallucinations are more often multi modal. Both in healthy children, as well as in children with a variety of other psychiatric illnesses, hallucinations are not uncommon [1] and diagnosis should not be based on these alone. COS is an extraordinarily rare illness which is poorly understood but appears continuous with the adult onset disorder. Additionally, as seen in other areas of medicine, early onset populations have more prominent progressive brain changes, and genetic risk factors [2].
Diagnosing a child with schizophrenia has profound effects on the treatment course, including the potential for neglecting another disorder, as psychosis often becomes the primary focus. Since onset is almost always insidious, the “episodes” so common in later onset disorder are rarely seen. The gold standard for diagnosis remains the use of unmodified DSM criteria, based on extensive collateral information. Once a diagnosis is affirmed, aggressive medication treatment, in majority of cases with Clozapine, combined with family education and individual counseling may defer further deterioration.
Keywords: Schizophrenia, Childhood Onset Schizophrenia, Childhood Psychosis
Introduction
The clinical severity, impact on development, and poor prognosis of Childhood Onset Schizophrenia (COS) may represent more homogeneous forms of the disorder. Additionally, the deleterious effects of incorrectly diagnosing COS are equally important to recognize. Despite the relatively high (up to 5%) prevalence of psychotic symptoms in otherwise healthy children [3, 4], COS is very rare and so epidemiologic incidence data with diagnoses based on standardized clinical assessments are lacking. It is generally accepted that the incidence of COS is less than 0.04% based on the observations from the National Institutes of Mental Health (NIMH) cohort. Approximately 30 to 50% of patients with affective or other atypical psychotic symptoms are misdiagnosed as COS [5-9], and over 90% of the initial referrals to the NIMH study of COS to date received alternate diagnoses. Because our attempt is to study schizophrenia in its most homogeneous form, we exclude children with a diagnosis of schizoaffective disorder. In general, we have had very few schizoaffective children over the years, precluding any meaningful data analyses.
Although neurobiologically and phenomenologically continuous with its adult counterpart, COS represents a more severe form of the disorder [10, 11], with more prominent pre-psychotic developmental disorders, brain abnormalities and genetic risk factors [2, 9]. The use of various screening and diagnostic tools has not proven to be as valuable as the longitudinal assessment by a judicious clinician. A unique benefit of the NIMH COS study is the washout period, where patients are observed inpatient, medication free for up to 3 weeks. If a provisional diagnosis of COS is appropriate based on the screening process (clinical interview, records review, structured interview), the patient is admitted to the unit and begins the rigorous process of tapering all medications (up to 4 weeks). During this period, and the subsequent medication free phase (up to 3 weeks), patients are observed by staff, receive weekly ratings and have the support of up to 2 individually assigned staff members (i.e. 2:1 staffing). This process has ruled out COS in almost 40% of the children provisionally diagnosed as COS.
Realizing the framework and limitations of the environment in which psychiatric providers operate, our model is not feasible outside of the NIMH. However, it has taught us that the keys to attaining accurate diagnoses and optimizing treatment planning lie in evaluating children suspected of having COS for speech/language/educational deficits, obtaining extensive collateral information, and observing patients and their families over several visits. Furthermore, COS carries with it a commitment to use a class of medications with a significant side effect profile and significant long term health risks [12]. Given the implications of the diagnosis, it is important for clinicians to exercise a considerable amount of caution and care when evaluating children with COS, being careful not to focus solely on addressing the psychotic symptoms and subsequently overlooking common comorbidities such as receptive and expressive language disorders.
Research on the effects of a delayed diagnosis in COS is sparse, and our study design excludes children whose diagnosis may have been delayed, occurring after the age of 13. Additionally, even the adult literature is limited by the lack of a standardized measurement [13, 14]. However, in adults, it has been shown that a delay in diagnosis results in a longer duration of untreated psychosis; having a robust but moderate effect on clinical outcome [14, 15]. Although we advocate a measured, thoughtful approach to diagnosis; making a timely diagnosis is also important.
Premorbid Phenotype
67% of children with COS show premorbid disturbances in social, motor, and language domains as well as demonstrate learning disabilities and have what seem to be comorbid mood or anxiety disorders. Additionally, although not reported in studies of the premorbid history of adult-onset schizophrenia[16, 17], 27% have met criteria for Autism/Autism Spectrum Disorders prior to the onset of their psychotic symptoms [18]. Outcome and prognosis have been positively correlated with the presence and severity of these developmental abnormalities [19-21] with some studies suggesting the severity of these deficits may actually represent a premorbid phenotype for COS [22-27].
The data on the premorbid functioning and symptomatology of the NIMH patients confirms and extends these findings. A review of our cohort (n=47) in 2000 showed that 55% had language abnormalities, 57% had motor abnormalities, and 55% had social abnormalities several years before the onset of psychotic symptoms. There was also a high rate of failed grades and special education placement [24, 28]. Gender, familial psychopathology, and familial eye-tracking dysfunction have shown significant relationships with at least some aspect of the probands’ premorbid development; Table 1 [24].
Table 1.
Relation of Premorbid Impairments to Schizophrenia Risk Factors for 49 Patients with COS.
| Premorbid Impairment and Risk Factor | Present (N) | Absent (N) | p Value |
|---|---|---|---|
|
| |||
| Speech and Language Impairment | |||
| Sex | 27 | 22 | 0.57 |
| Score for family loading for schizophrenia spectrum disorders | 27 | 21 | 0.04 |
| Mean family score for eye tracking | 22 | 17 | 0.04 |
|
| |||
| Motor Impairment | |||
| Sex | 28 | 21 | 0.009 |
| Score for family loading for schizophrenia spectrum disorders | 28 | 20 | 0.50 |
| Mean family score for eye tracking | 22 | 17 | 0.25 |
|
| |||
| Social Impairment | |||
| Sex | 27 | 22 | 0.56 |
| Score for family loading for schizophrenia spectrum disorders | 27 | 21 | 0.15 |
| Mean family score for eye tracking | 19 | 20 | 0.37 |
These results have been strengthened by a 2012 review of our cohort (n=118). Of the 118 children in the cohort, 65 (55.08%) had premorbid academic impairments, 85 (72.03%) had premorbid social/behavioral impairments, 60 (50.85%) had premorbid language impairments, 52 (44.07%) had premorbid motor impairments, and 24 (20.34%) screened positive for pervasive developmental disorder. (Table 2) The average number of abnormalities (15 domains) in each child was 3.89 and 103 (87.29%) of the children had premorbid impairment in at least one domain. Additionally, 47% of children who did not have a pervasive developmental disorder (e.g., Autism, Asperger, PDD-NOS), received pre-psychotic mental health treatment and/or a psychiatric or psychological evaluation.
Table 2.
Realms of premorbid developmental problems based on 2012 chart review
| N (%) | |
|---|---|
| Social/Behavioral | 85 (72.03%) |
| Academic | 65 (55.08) |
| Language | 60 (50.85%) |
| Motor | 52 (44.07%) |
| Pervasive Developmental Disorder | 24 (20.34%) |
**Definition/Symptom Criteria
Since Kolvin’s classic studies, it is generally agreed upon that Childhood-onset schizophrenia can be diagnosed with the unmodified DSM IV-TR Criteria for Schizophrenia (Table 3) [29]. In addition, the NIMH study has defined COS where the onset of psychotic symptoms is before the 13th birthday, combined with a premorbid IQ of 70 or above and absence of any significant neurological problem. The DSM V proposes a reorganization to reflect a gradient of psychopathology, from least to most severe, and updated severity dimensions. [30].
Table 3.
DSM IV-TR Criteria for Schizophrenia
| A. Characteristic symptoms: Two (or more) of the following, each present for a significant portion of time during a 1-month period (or less if successfully treated): |
|
| B. Social/occupational dysfunction: | For a significant portion of the time since the onset of the disturbance, one or more major areas of functioning such as work, interpersonal relations, or self-care are markedly below the level achieved prior to the onset (or when the onset is in childhood or adolescence, failure to achieve expected level of interpersonal, academic, or occupational achievement). |
| C. Duration: | Continuous signs of the disturbance persist for at least 6 months. This 6-month period must include at least 1 month of symptoms (or less if successfully treated) that meet Criterion A (i.e., active-phase symptoms) and may include periods of prodromal or residual symptoms. During these prodromal or residual periods, the signs of the disturbance may be manifested by only negative symptoms or two or more symptoms listed in Criterion A present in an attenuated form (e.g., odd beliefs, unusual perceptual experiences). |
| D. Schizoaffective and Mood Disorder exclusion: | Schizoaffective Disorder and Mood Disorder With Psychotic Features have been ruled out because either (1) no Major Depressive, Manic, or Mixed Episodes have occurred concurrently with the active-phase symptoms; or (2) if mood episodes have occurred during active-phase symptoms, their total duration has been brief relative to the duration of the active and residual periods. |
| E. Substance/general medical condition exclusion: | The disturbance is not due to the direct physiological effects of a substance (e.g., a drug of abuse, a medication) or a general medical condition. |
| F. Relationship to a Pervasive Developmental Disorder: | If there is a history of Autistic Disorder or another Pervasive Developmental Disorder, the additional diagnosis of Schizophrenia is made only if prominent delusions or hallucinations are also present for at least a month (or less if successfully treated). |
Note: Only one Criterion A symptom is required if delusions are bizarre or hallucinations consist of a voice keeping up a running commentary on the person’s behavior or thoughts, or two or more voices conversing with each other.
Clinical Findings
Physical examination
The diagnosis of Childhood-onset schizophrenia requires exclusion of an underlying medical or psychiatric illness. It is only after all other identifiable causes of ‘organic psychosis’ have been excluded a diagnosis of COS can appropriately be considered. Details regarding the components of the physical examination of individuals suspected of having a primary psychiatric illness are discussed in this volume by Kumra and Goerke: Substance abuse and psychosis: etiological contribution and clinical considerations. A physical and thorough neurologic exam is essential to the diagnostic process and clinicians should be vigilant to any abnormal physical and/or neurologic findings as COS is a diagnoses of exclusion. It is also important to have in mind the rare medical etiologies and frequently missed diagnoses during the evaluation. Although discussed elsewhere in this volume, a select summary list is provided in Table 4.
Table 4.
Differential Diagnoses of Childhood-onset schizophrenia
| Medical Etiologies |
|
| Misdiagnosed Psychiatric Illnesses |
|
**Rating scales and Diagnostic modalities
Frequently used rating scales will be discussed in subsequent chapters. For the NIMH COS study we use the Social Communication Questionnaire (SCQ), previously known as the Autism Screening Questionnaire (ASQ), and Kiddie-Sads-Present and Lifetime Version (K-SADS-PL), using the supplemental ratings as indicated by the results of the K-SADS-PL, for all probands. The Schedule for Affective Disorders and Schizophrenia (SADS) and the Structured Interview for DSM-III Personality (SIDP) are used to evaluate all family members for Axis I and Axis II disorder respectively. During follow-up visits the probands are evaluated using the Scale for the Assessment of Positive Symptoms (SAPS), Scale for the Assessment of Negative Symptoms (SANS), Brief Psychiatric Rating Scale for Children (BPRS – C), Clinical Global Impressions Scale (CGIS), Children’s’ Global Impressions Scale (CGAS), Bunny-Hamburg Global Ratings, Simpson-Angus Scale (SAS), and Abnormal Involuntary Movement Scale (AIMS) [34].
As previously mentioned, it is simply not feasible to apply what is done at NIMH in the community. In clinical practice, we routinely recommend to outpatient providers they use the SAPS and SANS to monitor clinical progress and the AIMS to monitor for potential side effects of the medication regimen. (Table 5)
Table 5.
Tools used by the NIMH Child Branch in the Evaluation of COS
| Tool | Description |
|---|---|
| Initial Evaluation: | |
| • Social Communication Questionnaire (SCQ) previously known as the Autism Screening Questionnaire (ASQ) | Brief instrument helps evaluate communication skills and social functioning in children who may have autism or autism spectrum disorders. Completed by a parent or other primary caregiver in less than 10 minutes |
| • Kiddie-Sads-Present and Lifetime Version (K-SADS-PL) | A semi-structured diagnostic interview designed to assess current and past episodes of psychopathology in children and adolescents according to DSM-III-R and DSM-IV criteria. |
| Supplements to the K-SADS-PL used for diagnostic exploration and clarification; administered in the order in which symptoms appeared. | |
| Follow-up: | |
| • Scale for the Assessment of Positive Symptoms (SAPS)* | Assessment of positive symptoms of psychosis devised primarily to focus on schizophrenia |
| • Scale for the Assessment of Negative Symptoms (SANS)* | Assessment of negative symptoms of psychosis devised primarily to focus on schizophrenia |
| • Brief Psychiatric Rating Scale for Children (BPRS - C) | A 21-item, clinician-based rating scale designed for use in evaluating psychiatric problems of children and adolescents. |
| • Clinical Global Impressions Scale (CGIS)* | A primary outcome frequently used in medical care and clinical research to measure in studies evaluating the efficacy of treatments. |
| • Children’s Global Impressions Scale (CGAS)* | An adaptation of the CGIS for children. |
| • Bunny-Hamburg Global Ratings | Two subscales that, when used together, best exclude COS as a viable diagnosis (62% accuracy at screening, 85% accuracy at the medication-free period)ˆ |
| • Simpson-Angus Scale (SAS) | An established instrument for neuroleptic-induced parkinsonism |
| • Abnormal Involuntary Movement Scale (AIMS) | 12 item clinician administered and scored anchored scale used to detect and follow the occurrence of tardive dyskinesia (TD) in patients receiving neuroleptic medications |
available in the Handbook of Psychiatric Measures (Book with CD-ROM for Windows) by the American Psychiatric Association
Used as indicated by the results of the K-SADS-PL
[34]
**Imaging
Structural brain abnormalities are an established feature of schizophrenia, characterized by decreased total gray matter (GM) volume reduction in cortex, hippocampus, and amygdala [35-43]. The number of imaging studies of Childhood and Early Onset Schizophrenia is growing with most them coming from the NIMH cohort. Advances in computational image analysis permit regional GM density, or cortical thickness measurements, which, when automated, can be applied to large samples, increasing statistical power [44-47]. This provides unprecedented anatomic detail of cortical GM change across both the entire cortex and time (Figure 1) [43, 47]. Prospective longitudinal brain MRI rescan measures for the NIMH COS sample show, progressive changes in COS, particularly during adolescence, highlighting this period as critical, and particularly vulnerable to treatment influences. These changes occur only during a limited period as the rate and degree of cortical loss if continued would resemble the extreme loss seen in some dementias [43, 47]. As it is, the GM volume of COS is 8-10% less than that of age matched controls.
Figure 1.
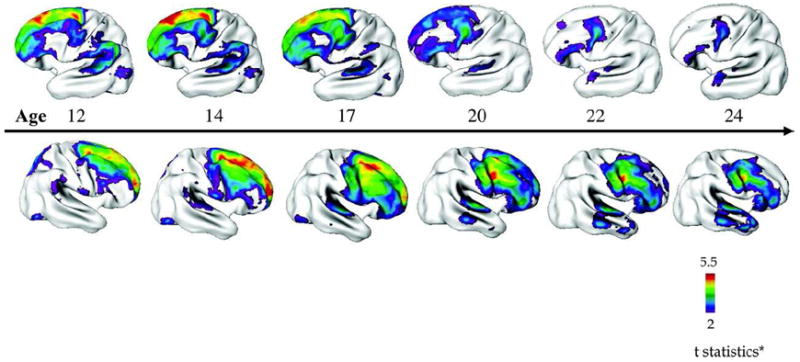
Progression of Cortical Gray Matter (GM) Loss in Childhood-Onset Schizophrenia (COS) (n = 70, 162 scans) Relative to Age-, Sex-, and Scan Interval–Matched Healthy Controls (n = 72, 168 Scans) From Adolescence to Young Adulthood (age 12–24 years).
[48, 49] Progression of Cortical Gray Matter (GM) Loss in Childhood-Onset Schizophrenia (COS) (n = 70, 162 scans) Relative to Age-, Sex-, and Scan Interval–Matched Healthy Controls (n = 72, 168 Scans) From Adolescence to Young Adulthood (age 12–24 years). Analyses were done using mixed model regression statistics and covaried from mean cortical thickness. Side bar shows t statistic with threshold to control for multiple comparisons using the false discovery rate procedure with q = 0.05. Differences are from mixed model regression with age centered at approximate 3-year intervals for middle 80% of the age range, and colors represent areas of statistically significant thinning in COS.81 [43].
Longitudinal analysis of quantitative brain imaging data suggests the rate of gray matter loss slows as these COS patients reach age 20 as shown in [50-52]. These studies also support the previous findings that, although representing a more severe form, COS is continuous with its adult onset counterpart. The total, frontal, temporal, and parietal GM loss, not seen in healthy children and adolescents nor in those with atypical psychosis, appears to be diagnostically specific for COS. [50]
Pathology
Identifying the neurobiological basis and pathophysiology of schizophrenia is an essential future goal for establishing its diagnostic validity, delineating meaningful subtypes or alternate diagnoses, and finding causative mechanisms and novel targets for drug development [53, 54]. To date, the etiology of schizophrenia is unknown. There is general agreement that this is a brain disease, with alterations of white and gray matter, disconnectivity, and in vivo brain function. Research measures such as neural synchrony, sleep architecture, smooth pursuit eye movements (SPEM), and pre-pulse inhibition (PPI), all reflect widespread disorder. The few narrower models are discussed in this publication by Frazier, Dvir, and Cochran in Autism and Schizophrenia and by Dvir, Frazier, and Deneitolis in Trauma and Psychosis.
The general model of schizophrenia as a neurodevelopmental disorder is widely held. One version focused on schizophrenia as a static lesion, occurring during fetal brain development [55], while others argued that schizophrenia occurs as a result of a second “hit” in the form of abnormal brain development during adolescence such as excessive synaptic and/or dendritic elimination resulting in aberrant neuronal connectivity [9, 56, 57]. These theories have merged and it is now generally understood that COS is a multifactorial illness, characterized by multiple genetic elements, each contributing a modest degree of risk [58] an interacting with the environment. There are also various other hypotheses focused on the cortical amino acid neurotransmitter systems (i.e., dopamine, glutamate, GABA, serotonin) [59, 60].
Alterations in genetics, neurodevelopment, and neurotransmitter systems [61] remain among the most promising directions for further research. Schizophrenia risk genes are associated with transcripts that are enriched in, or unique to, the human brain. Some also show preferential expression in the fetal brain [62]. Studies have revealed aberrant neuronal development, specifically localized to prefrontal and temporal cortices [56]. Alterations in timing of developmental disruption of GABAergic interneurons as the basis for several different neurodevelopmental disorders are gaining increasing support [63]. It is almost certain that both dopamine and glutamate transmission are abnormal in this disorder [64-66] and striatal dopamine over-activity may be critical to conversion to psychosis or psychotic symptoms generally [56, 67, 68].
Not only does the etiology of COS/EOS elude us, several roadblocks to progress toward finding one remain. First, the phenotypic, biological and etiological heterogeneity of schizophrenia may account for the fact that the effect size of these individual risks do not support any single neurobiological finding as a core deficit in the illness [54, 69, 70]. Second, we remain handicapped by the difficulty in studying the human brain and the lack of good animal models. Recent post-mortem studies, indicate time specific developmental genetic effects It remains clear however that schizophrenia, including COS/EOS, has no clearly definable neuropathologic markers (e.g. demyelinated neurons in Multiple Sclerosis) [54]. While the study if COS suggests it may have more salient genetic effects [71], there is no finding of even a rare form of genetic dominant transmission for COS.
Diagnostic Dilemmas
The diagnosis of childhood onset schizophrenia (COS) is a difficult, time consuming process. Although early developmental abnormalities in social, motor, and language domains in COS are more striking compared to the later onset cases[22-24, 28, 72], they are not diagnostic and do not cumulatively represent a reliable premorbid phenotype. Additionally, not only do healthy children experience hallucinations, but children with various other psychiatric and behavioral disturbances present with positive symptoms [73, 74]. Pressure from families, the severity of the clinical picture, and time limitations placed on providers coalesce to make the diagnosis of COS a tedious process fraught with pitfalls. The most common disorder misdiagnosed as COS are affective disorders, organic psychosis, pervasive developmental disorders, and a group referred to as “Atypical Psychosis” or “Multi Dimensionally Impaired (MDI).” Details regarding these disorders, the latter of which is an important differential and is described in detail below, and achieving diagnostic clarity will be described elsewhere in this volume.
The NIMH cohort has been going on since 1990, using nationwide recruitment. Over the past 22 years, over 3000 charts have been reviewed. Of these, 90% are rejected from further consideration as they fail to meet the criteria for childhood onset schizophrenia. Over 300 children have been screened in person, of whom approximately 60% receive other psychiatric diagnoses such as affective disorders, anxiety, or behavioral disorders. Over 200 children who appeared likely to meet criteria for COS were admitted to the research unit and underwent an initial observation period followed by complete medication washout. After being observed off medications for up to three weeks, an additional 20% of children did not meet criteria for childhood onset schizophrenia and received alternative diagnosis. A 4- to 6-year follow up study of the ‘ruled out’ cases indicated good stability of the alternative diagnoses and non schizophrenic status[75]. The most frequent alternative diagnosis have been affective disorders, and anxiety disorders. A subgroup of children has also shown a form of atypical psychosis; provisionally labeled as “Multi Dimensionally Impaired (MDI) [76-78] based on a unique set of features which warrants further description.
The “Multi Dimensionally Impaired (MDI)” group
To-date 33 children have been given the provisional diagnosis of “MDI” after the medication washout period and have been followed prospectively along with the COS children. This heterogeneous group of children, in general, has severe functional impairment associated with transient psychotic symptoms, multiple developmental abnormalities, abnormal neuropsychological test profiles, eye movement abnormalities, and familial risk factors that are not adequately characterized by existing DSM-IV categories [76, 79, 80]. Despite the presence of overlapping symptoms with childhood and early onset schizophrenia, there are distinct features which have been used as the ‘operational diagnostic criteria’ by the NIMH group to distinguish these individuals. [76, 79]:
Brief, transient episodes of psychosis and perceptual disturbance, typically in response to stress.
Nearly daily periods of emotional lability disproportionate to precipitants.
Impaired interpersonal skills despite the desire to initiate peer friendships (distinction from childhood onset schizophrenia).
Cognitive deficits as indicated by multiple deficits in information processing.
-
No clear thought disorder (clinically can be difficult to define, especially in presence of communication disorder).
**ADHD is highly comorbid in the MDI group.
At first glance, the symptom cluster these patients present suggests these children will likely progress to develop schizophrenia spectrum disorders; in the current DSM these patients could be considered as psychosis NOS. These children are similar in some way to some of the other syndromes described such as the Multiple complex developmental disorder (MCDD), Borderline Syndrome of Childhood or other Borderline Disorders of Childhood [80-83]. However, contrary to MDI, these other syndromes have more predominant symptoms of pervasive developmental disorder; greater evidence of formal thought disorder, and onset before age five [80, 84, 85]. The MDI group appears to have a distinct course, with none progressing to schizophrenia at long term follow-up [86], but strikingly, 38% developing Bipolar Disorder, Type I [87]. This long term data emphasizes to us that when diagnosing a child with schizophrenia, there are significant short and long term implications, including the potential for neglecting other disorders, as psychosis often becomes the primary focus.
Process of elimination
It has long been known that hallucinations, delusions, and ‘disordered thoughts’ can occur in healthy non psychotic children [88] but usually diminish after age 6 [89]. Transient anxiety and stress related visual hallucinations are also occasionally reported in preschool children [90], and the prognosis of these phenomena is benign. However, when psychotic phenomena occur in school age children, they generally tend to be more persistent and associated with drug toxicity or more significant mental illness [1, 91-93].
Comorbidities
Childhood-onset schizophrenia is highly correlated with other illness and disorders (Table 6) [24]. During the evaluation of a child with suspected COS, it is imperative they are screened, with a high index of suspicion, for other comorbid illnesses and disorders, both psychiatric [18] and medical [94] (Table 5) [95], the latter of which account for almost 60% of premature deaths not related to suicide in adult schizophrenia patients [94, 96].
Table 6.
Select Comorbidities for Childhood-onset schizophrenia
Psychiatric Comorbidities
|
| Medical Comorbidities associated with treatment |
highly correlated with the treatment of Schizophrenia [95].
Conclusions / Summary: Implications for Clinical Practice
Schizophrenia is a devastating illness, particularly when presenting in childhood or adolescence. Despite the presence of premorbid characteristics, a reliable pre-morbid phenotype has not been defined and research into the pathophysiology of the syndrome remains ongoing without a substantial target demonstrated in a systematic way. The frequency and duration of psychotic episodes has deleterious neuropsychological, neurophysiological, and neurostructural effects [97-101], making prompt, aggressive treatment an important component of care. Once the diagnosis is established and other comorbid conditions are adequately assessed, clinicians should treat this illness aggressively. Treatment planning should encompass psychopharmacological, psychotherapeutic and early psychosocial intervention such as support and education of the family about the disorder, particularly during the first years of the evolution of the disease, as these can actually improve the course of illness [102]. Additionally, clinicians should not shy away from the use of Clozapine, as evidenced by the epidemiological studies demonstrating that its use occurs even much later than that recommended by the clinical guidelines [102].
Key Points.
Childhood-Onset Schizophrenia (COS) is an extraordinarily rare illness with an incidence less than 0.04%. In both healthy children and children with a variety of other psychiatric illnesses, hallucinations are not uncommon; diagnosis should not be based on these alone.
The evaluation of a child with suspected COS, includes collecting extensive collateral information, observing patients/families over several visits, excluding underlying medical illnesses and evaluating, with a high index of suspicion, for speech/language/educational deficits and comorbid mood or anxiety disorders.
Once the diagnosis is established and other comorbidities are addressed, treatment planning should encompass aggressive psychopharmacological, psychotherapeutic and psychosocial interventions.
Clozapine is an excellent third line medication for use in COS. Epidemiological studies demonstrate that its use often occurs much later than recommended by the clinical guidelines.
Abbreviations
- AIMS
Abnormal Involuntary Movement Scale
- ASQ*
Autism Screening Questionnaire* See SCQ for new name
- BPRS-C
Brief Psychiatric Rating Scale for Children
- CGAS
Childrens’ Global Impressions Scale
- CGIS
Clinical Global Impressions Scale
- COS
Childhood Onset Schizophrenia
- K-SADS-PL
Kiddie-Sads-Present and Lifetime Version
- MDI
Multi Dimensionally Impaired
- NIMH
National Institutes of Mental Health
- PPI
Pre-pulse inhibition
- SADS
Schedule for Affective Disorders and Schizophrenia
- SANS
Scale for the Assessment of Negative Symptoms
- SAPS
Scale for the Assessment of Positive Symptoms
- SAS
Simpson-Angus Scale
- SCQ*
Social Communication Questionnaire* Formerly ASQ
- SPEM
Smooth pursuit eye movements
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David I. Driver, Email: david.driver@nih.gov.
Nitin Gogtay, Email: gogtayn@mail.nih.gov.
Judith L. Rapoport, Email: rapoporj@mail.nih.gov.
References
- 1.Schreier HA. Hallucinations in nonpsychotic children: more common than we think? J Am Acad Child Adolesc Psychiatry. 1999;38(5):623–5. doi: 10.1097/00004583-199905000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Childs B, Scriver CR. Age at onset and causes of disease. Perspect Biol Med. 1986;29(3 Pt 1):437–60. doi: 10.1353/pbm.1986.0056. [DOI] [PubMed] [Google Scholar]
- 3.Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 41(1):1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- 4.Kelleher I, et al. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 42(9):1857–63. doi: 10.1017/S0033291711002960. [DOI] [PubMed] [Google Scholar]
- 5.Werry JS. Child and adolescent (early onset) schizophrenia: a review in light of DSM-III-R. J Autism Dev Disord. 1992;22(4):601–24. doi: 10.1007/BF01046330. [DOI] [PubMed] [Google Scholar]
- 6.McKenna K, Gordon CT, Rapoport JL. Childhood-onset schizophrenia: timely neurobiological research. J Am Acad Child Adolesc Psychiatry. 1994;33(6):771–81. doi: 10.1097/00004583-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Gordon CT, et al. Childhood-onset schizophrenia: an NIMH study in progress. Schizophr Bull. 1994;20(4):697–712. doi: 10.1093/schbul/20.4.697. [DOI] [PubMed] [Google Scholar]
- 8.Gogtay N, et al. Psychotic symptoms and gray matter deficits in clinical pediatric populations. Schizophr Res. 140(1-3):149–54. doi: 10.1016/j.schres.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapoport JL, Gogtay N. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int J Dev Neurosci. 2010 doi: 10.1016/j.ijdevneu.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolson R, et al. Obstetrical complications and childhood-onset schizophrenia. Am J Psychiatry. 1999;156(10):1650–2. doi: 10.1176/ajp.156.10.1650. [DOI] [PubMed] [Google Scholar]
- 11.Nicolson R, et al. Clinical and neurobiological correlates of cytogenetic abnormalities in childhood-onset schizophrenia. Am J Psychiatry. 1999;156(10):1575–9. doi: 10.1176/ajp.156.10.1575. [DOI] [PubMed] [Google Scholar]
- 12.De Hert M, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 26(3):144–58. doi: 10.1016/j.eurpsy.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Large M, et al. Measurement and reporting of the duration of untreated psychosis. Early Interv Psychiatry. 2008;2(4):201–11. doi: 10.1111/j.1751-7893.2008.00080.x. [DOI] [PubMed] [Google Scholar]
- 14.Singh SP. Outcome measures in early psychosis; relevance of duration of untreated psychosis. Br J Psychiatry Suppl. 2007;50:s58–63. doi: 10.1192/bjp.191.50.s58. [DOI] [PubMed] [Google Scholar]
- 15.Black K, et al. Duration of untreated psychosis predicts treatment outcome in an early psychosis program. Schizophr Res. 2001;47(2-3):215–22. doi: 10.1016/s0920-9964(00)00144-4. [DOI] [PubMed] [Google Scholar]
- 16.Done DJ, et al. Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ. 1994;309(6956):699–703. doi: 10.1136/bmj.309.6956.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones P, et al. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344(8934):1398–402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport J, et al. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48(1):10–8. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, et al. Premorbid adjustment as a predictor of phenomenological and neurobiological indices in schizophrenia. Schizophr Res. 1995;16(3):189–97. doi: 10.1016/0920-9964(94)00073-h. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, et al. Neurological soft signs in neuroleptic-naive and neuroleptic-treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry. 1995;152(2):191–6. doi: 10.1176/ajp.152.2.191. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK, et al. Effect of alosetron (a new 5-HT3 receptor antagonist) on the pharmacokinetics of haloperidol in schizophrenic patients. J Clin Pharmacol. 1995;35(2):202–7. doi: 10.1002/j.1552-4604.1995.tb05012.x. [DOI] [PubMed] [Google Scholar]
- 22.Hollis C. Child and adolescent (juvenile onset) schizophrenia A case control study of premorbid developmental impairments. Br J Psychiatry. 1995;166(4):489–95. doi: 10.1192/bjp.166.4.489. [DOI] [PubMed] [Google Scholar]
- 23.Alaghband-Rad J, et al. Childhood-onset schizophrenia: the severity of premorbid course. J Am Acad Child Adolesc Psychiatry. 1995;34(10):1273–83. doi: 10.1097/00004583-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Nicolson R, et al. Premorbid speech and language impairments in childhood-onset schizophrenia: association with risk factors. Am J Psychiatry. 2000;157(5):794–800. doi: 10.1176/appi.ajp.157.5.794. [DOI] [PubMed] [Google Scholar]
- 25.Asarnow JR, Ben-Meir S. Children with schizophrenia spectrum and depressive disorders: a comparative study of premorbid adjustment, onset pattern and severity of impairment. J Child Psychol Psychiatry. 1988;29(4):477–88. doi: 10.1111/j.1469-7610.1988.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 26.Russell A, Bott L, Sammons C. The phenomena of schizophrenia occurring in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:399–407. doi: 10.1097/00004583-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Watkins JM, Asarnow RF, Tanguay PE. Symptom development in childhood onset schizophrenia. J Child Psychol Psychiatry. 1988;29(6):865–78. doi: 10.1111/j.1469-7610.1988.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 28.Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46(10):1418–28. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- 29.Kolvin I. Studies in the childhood psychoses. I. Diagnostic criteria and classification. Br J Psychiatry. 1971;118(545):381–4. doi: 10.1192/bjp.118.545.381. [DOI] [PubMed] [Google Scholar]
- 30.Association AP. Recent Updates to Proposed Revisions for DSM-5. 2012 [cited 2012 11/28/2012]; Recent Updates to Proposed Revisions for DSM-5]. Available from: http://www.dsm5.org/Pages/RecentUpdates.aspx.
- 31.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56(10):940–5. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 32.Arinami T, et al. Screening for 22q11 deletions in a schizophrenia population. Schizophr Res. 2001;52(3):167–70. doi: 10.1016/s0920-9964(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 33.Green T, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1060–8. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 34.Gochman P, Miller R, Rapoport JL. Childhood-onset schizophrenia: the challenge of diagnosis. Curr Psychiatry Rep. 13(5):321–2. doi: 10.1007/s11920-011-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–20. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 36.Wright IC, et al. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 37.Shenton ME, et al. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1-2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantelis C, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31(3):672–96. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 39.Mathalon DH, et al. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(2):148–57. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 40.Gur RE, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55(2):145–52. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 41.Lieberman J, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49(6):487–99. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 42.DeLisi LE. Regional brain volume change over the life-time course of schizophrenia. J Psychiatr Res. 1999;33(6):535–41. doi: 10.1016/s0022-3956(99)00028-x. [DOI] [PubMed] [Google Scholar]
- 43.Gogtay N. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull. 2008;34(1):30–6. doi: 10.1093/schbul/sbm103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luders E, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26(2):493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Thompson PM, et al. Detecting Disease Specific Patterns of Brain Structure using Cortical Pattern Matching and a Population-Based Probabilistic Brain Atlas. In: Insana M, Leahy RM, editors. Lecture Notes in Computer Science (LNCS); IEEE Confernce on Information Processing in Medical Imaging (IPMI); UC Davis. 2001; Springer-Verlag; 2001. pp. 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson PM, et al. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404(6774):190–3. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 47.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenstein D, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47(10):1003–12. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 49.Gogtay N, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64(7):772–80. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 50.Gogtay N, et al. Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry. 2004;61(1):17–22. doi: 10.1001/archpsyc.61.1.17. [DOI] [PubMed] [Google Scholar]
- 51.Sporn AL, et al. Progressive Brain Volume Loss During Adolescence in Childhood-Onset Schizophrenia. Am J Psychiatry. 2003;160(12):2181–2189. doi: 10.1176/appi.ajp.160.12.2181. [DOI] [PubMed] [Google Scholar]
- 52.Gogtay N, et al. Structural brain MRI abnormalities in healthy siblings of patients with childhood-onset schizophrenia. Am J Psychiatry. 2003;160(3):569–71. doi: 10.1176/appi.ajp.160.3.569. [DOI] [PubMed] [Google Scholar]
- 53.Keshavan MS, et al. Neurobiology of early psychosis. Br J Psychiatry Suppl. 2005;48:s8–18. doi: 10.1192/bjp.187.48.s8. [DOI] [PubMed] [Google Scholar]
- 54.Keshavan MS, et al. Schizophrenia, “just the facts”: what we know in 2008 Part 3: neurobiology. Schizophr Res. 2008;106(2-3):89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 55.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 56.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 58.Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27(4):226–33. doi: 10.1016/j.tips.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto S, et al. Recent advances in the neurobiology of schizophrenia. Mol Interv. 2003;3(1):27–39. doi: 10.1124/mi.3.1.27. [DOI] [PubMed] [Google Scholar]
- 60.Weinberger DR. The biological basis of schizophrenia: new directions. J Clin Psychiatry. 1997;58(Suppl 10):22–7. [PubMed] [Google Scholar]
- 61.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296(5568):692–5. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 62.Kleinman JE, et al. Genetic neuropathology of schizophrenia: new approaches to an old question and new uses for postmortem human brains. Biol Psychiatry. 69(2):140–5. doi: 10.1016/j.biopsych.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marin O. Interneuron dysfunction in psychiatric disorders. Nature reviews. Neuroscience. 2012;13(2):107–20. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 64.Henn FA. Dopamine: a marker of psychosis and final common driver of schizophrenia psychosis. Am J Psychiatry. 168(12):1239–40. doi: 10.1176/appi.ajp.2011.11091346. [DOI] [PubMed] [Google Scholar]
- 65.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33(1):141–65. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 66.Beneyto M, Lewis DA. Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 29(3):295–304. doi: 10.1016/j.ijdevneu.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howes OD, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 168(12):1311–7. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102(1-3):1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 70.Tsuang MT, Faraone SV. The case for heterogeneity in the etiology of schizophrenia. Schizophr Res. 1995;17(2):161–75. doi: 10.1016/0920-9964(95)00057-s. [DOI] [PubMed] [Google Scholar]
- 71.Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–43. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 72.Green WH, et al. Schizophrenia with childhood onset: a phenomenological study of 38 cases. J Am Acad Child Adolesc Psychiatry. 1992;31(5):968–76. doi: 10.1097/00004583-199209000-00027. [DOI] [PubMed] [Google Scholar]
- 73.Garralda ME. Hallucinations in children with conduct and emotional disorders: II. The follow-up study. Psychol Med. 1984;14(3):597–604. doi: 10.1017/s0033291700015208. [DOI] [PubMed] [Google Scholar]
- 74.Garralda ME. Hallucinations in children with conduct and emotional disorders: I. The clinical phenomena. Psychol Med. 1984;14(3):589–96. doi: 10.1017/s0033291700015191. [DOI] [PubMed] [Google Scholar]
- 75.Calderoni D, et al. Differentiating childhood-onset schizophrenia from psychotic mood disorders. J Am Acad Child Adolesc Psychiatry. 2001;40(10):1190–6. doi: 10.1097/00004583-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 76.McKenna K, et al. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry. 1994;33(5):636–44. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Kumra S, et al. Including children and adolescents with schizophrenia in medication-free research. Am J Psychiatry. 1999;156(7):1065–8. doi: 10.1176/ajp.156.7.1065. [DOI] [PubMed] [Google Scholar]
- 78.Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46(10):1418–28. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- 79.Kumra S, et al. “Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia? J Am Acad Child Adolesc Psychiatry. 1998;37(1):91–9. doi: 10.1097/00004583-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 80.Towbin KE, et al. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry. 1993;32(4):775–82. doi: 10.1097/00004583-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Dahl EK, Cohen DJ, Provence S. Clinical and multivariate approaches to the nosology of pervasive developmental disorders. J Am Acad Child Psychiatry. 1986;25(2):170–80. doi: 10.1016/s0002-7138(09)60223-5. [DOI] [PubMed] [Google Scholar]
- 82.Petti TA, Vela RM. Borderline disorders of childhood: an overview. J Am Acad Child Adolesc Psychiatry. 1990;29(3):327–37. doi: 10.1097/00004583-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 83.Van der Gaag RJ, et al. A controlled multivariate chart review of multiple complex developmental disorder. J Am Acad Child Adolesc Psychiatry. 1995;34(8):1096–106. doi: 10.1097/00004583-199508000-00021. [DOI] [PubMed] [Google Scholar]
- 84.Cohen DJ, Paul R, Volkmar FR. Issues in the classification of pervasive and other developmental disorders: toward DSM-IV. J Am Acad Child Psychiatry. 1986;25(2):213–20. doi: 10.1016/s0002-7138(09)60228-4. [DOI] [PubMed] [Google Scholar]
- 85.Ad-Dab’bagh Y, Greenfield B. Multiple complex developmental disorder: the “multiple and complex” evolution of the “childhood borderline syndrome” construct. J Am Acad Child Adolesc Psychiatry. 2001;40(8):954–64. doi: 10.1097/00004583-200108000-00018. [DOI] [PubMed] [Google Scholar]
- 86.Nicolson R, et al. Children and adolescents with psychotic disorder not otherwise specified: a 2- to 8-year follow-up study. Compr Psychiatry. 2001;42(4):319–25. doi: 10.1053/comp.2001.24573. [DOI] [PubMed] [Google Scholar]
- 87.Gogtay N, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48(9):852–62. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 88.Lukianowicz N. Hallucinations in non-psychotic children. Psychiatr Clin (Basel) 1969;2(6):321–37. doi: 10.1159/000278582. [DOI] [PubMed] [Google Scholar]
- 89.Caplan R. Thought disorder in childhood. J Am Acad Child Adolesc Psychiatry. 1994;33(5):605–15. doi: 10.1097/00004583-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 90.Rothstein A. Hallucinatory phenomena in childhood. A critique of the literature. J Am Acad Child Psychiatry. 1981;20(3):623–35. doi: 10.1016/s0002-7138(09)61649-6. [DOI] [PubMed] [Google Scholar]
- 91.Abramowicz M. Drugs that cause psychiatric symptoms. Med Lett Drugs Ther. 1993;35:65–70. [PubMed] [Google Scholar]
- 92.Davison K. Schizophrenia-like psychoses associated with organic cerebral disorders: a review. Psychiatr Dev. 1983;1(1):1–33. [PubMed] [Google Scholar]
- 93.McGee R, Williams S, Poulton R. Hallucinations in nonpsychotic children. J Am Acad Child Adolesc Psychiatry. 2000;39(1):12–3. doi: 10.1097/00004583-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Lambert TJ, Velakoulis D, Pantelis C. Medical comorbidity in schizophrenia. Med J Aust. 2003;178(Suppl):S67–70. doi: 10.5694/j.1326-5377.2003.tb05311.x. [DOI] [PubMed] [Google Scholar]
- 95.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 96.Goff DC, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183–94. doi: 10.4088/jcp.v66n0205. quiz 147, 273-4. [DOI] [PubMed] [Google Scholar]
- 97.Ienciu M, et al. First episode psychosis and treatment delay--causes and consequences. Psychiatr Danub. 22(4):540–3. [PubMed] [Google Scholar]
- 98.Franz L, et al. Stigma and treatment delay in first-episode psychosis: a grounded theory study. Early Interv Psychiatry. 4(1):47–56. doi: 10.1111/j.1751-7893.2009.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Norman RM, et al. Does treatment delay predict occupational functioning in first-episode psychosis? Schizophr Res. 2007;91(1-3):259–62. doi: 10.1016/j.schres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 100.Compton MT, Esterberg ML. Treatment delay in first-episode nonaffective psychosis: a pilot study with African American family members and the theory of planned behavior. Compr Psychiatry. 2005;46(4):291–5. doi: 10.1016/j.comppsych.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 101.Harrigan SM, McGorry PD, Krstev H. Does treatment delay in first-episode psychosis really matter? Psychol Med. 2003;33(1):97–110. doi: 10.1017/s003329170200675x. [DOI] [PubMed] [Google Scholar]
- 102.Vera I, et al. Clozapine as treatment of first choice in first psychotic episodes. What do we know? Actas Esp Psiquiatr. 40(5):281–9. [PubMed] [Google Scholar]


