Abstract
Methionine metabolism is disrupted in patients with alcoholic liver disease, resulting in altered hepatic concentrations of S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), and other metabolites. The present study tested the hypothesis that reductive stress mediates the effects of ethanol on liver methionine metabolism. Isolated rat livers were perfused with ethanol or propanol to induce a reductive stress by increasing the NADH/NAD+ ratio, and the concentrations of SAM and SAH in the liver tissue were determined by high-performance liquid chromatography. The increase in the NADH/NAD+ ratio induced by ethanol or propanol was associated with a marked decrease in SAM and an increase in SAH liver content. 4-Methylpyrazole, an inhibitor the NAD+-dependent enzyme alcohol dehydrogenase, blocked the increase in the NADH/NAD+ ratio and prevented the alterations in SAM and SAH. Similarly, co-infusion of pyruvate, which is metabolized by the NADH-dependent enzyme lactate dehydrogenase, restored the NADH/NAD+ ratio and normalized SAM and SAH levels. The data establish an initial link between the effects of ethanol on the NADH/NAD+ redox couple and the effects of ethanol on methionine metabolism in the liver.
Keywords: Perfused rat liver, Alcohol, Redox state, Methionine metabolism, Reductive stress
1. Introduction
Ethanol exposure affects a wide spectrum of biochemical and molecular processes in the liver leading to alcoholic liver disease. One prominent feature of the ethanol-exposed liver is altered metabolism of the essential amino acid methionine, manifested as changes in the intracellular concentrations of S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH) and other downstream metabolites [1,2]. SAM is synthesized from methionine and ATP by the enzyme methionine adenosyltransferase (MAT; EC 2.5.1.6), and SAH is formed when the methyl group of SAM is transferred to one of a variety of substrates, including proteins, DNA, RNA and lipids. SAH is a powerful feedback inhibitor of these methyltransferase reactions, and hepatocytes typically keep SAH levels low relative to SAM levels through the action of SAH hydrolase (SAHH; EC 3.3.1.1.) which catalyzes the reversible hydrolysis of SAH to homocysteine and adenosine. Ethanol can alter the expression and activities of these enzymes [3,4], but the precise mechanisms by which this occurs are still unclear.
Another characteristic response to ethanol exposure is the conversion of NAD+ to NADH as a consequence of the metabolism of ethanol to acetaldehyde by the NAD+-dependent enzyme alcohol dehydrogenase (EC 1.1.1.1). The resulting increase in the ratio of NADH/NAD+ constitutes a reductive stress [5] and has been observed following chronic administration of ethanol to laboratory animals and acute administration to isolated perfused liver [6–12]. Ethanol-induced reductive stress has received less attention than ethanol-induced oxidative stress, but interest has been growing in recent years due to accumulating evidence that the NADH/NAD+ system has functions beyond serving as a cofactor for oxidation–reduction reactions catalyzed by NAD-dependent dehydrogenases. The discovery of NAD+-consuming enzymes that contribute to epigenetic regulation [13] and the identification of proteins that respond to changes in the ratio of NADH/NAD+ [14] have broadened our understanding of the ways in which NADH/NAD+ contribute to the regulation of cellular processes.
In this study, we tested the hypothesis that reductive stress plays an important role in the modulation of the intracellular concentrations of SAM and SAH. The experiments we report here were performed on isolated perfused rat livers in which we manipulated the redox state of the NADH/NAD+ system by means of administration of ethanol and other compounds that change the NADH/NAD+ ratio, and measured the levels of SAM and SAH in liver tissue. Our results indicate for the first time that a short-term shift of the redox state of the NADH/NAD+ system rapidly modulates the intracellular concentration of methionine metabolites in the perfused rat liver.
2. Materials and methods
2.1. Animals and their treatment
Male, Sprague–Dawley rats, weighing 300–350 g, were maintained under standard conditions (12-hour dark/light cycle, 20–22 °C, constant humidity, and Purina rat chow and water ad libitum). The animals were taken for experimentation between 8.00 and 11.00 a.m. without overnight fasting. The animals were treated in accordance with the Guide for Care and Use of Laboratory Animals The experimental protocol of the study was approved by the Institutional Animal Care and Use Committee of the University of Louisville.
2.2. Liver perfusion
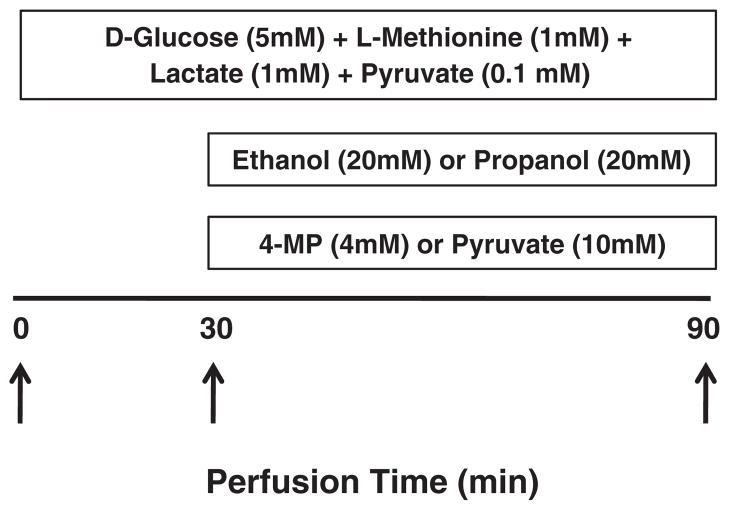
The perfusion medium was Krebs–Henseleit bicarbonate buffer continuously gassed with O2:CO2 (95:5%), at 36 °C, in a flow-through system. Gassing of the perfusate was ensured at two sites of the perfusion system: in the pre-heating, pre-gassing, water jacketed vessel and in an in-line rotary disc oxygenator as described by Scholz [15]. The animals were anesthetized with sodium pentobarbital (NembutalR; 80 mg•kg−1 body weight, intraperitoneally), the abdominal cavity opened and the portal vein cannulated at a flow rate of 6–8 mL•min−1. Immediately after cannulation and ligation in place of the portal cannula, the infrarenal segment of the inferior vena cava was severed and the flow rate increased to 32 mL•min−1. This rate was maintained for the entire perfusion time using a peristaltic pump (Cole Parmer Instrument Co., Model 7554-80; Vernon Hills, IL). The portal vein pressure was measured with the aid of an in-line pressure gauge (Model 900A Micropressure System (World Precision Instruments, Sarasota, FL) according to manufacturer’s instructions, and fluctuated between 12 and 14 mm Hg. A second, outflow cannula was inserted through the right atrium in the supradiaphragmatic segment of the inferior vena cava and ligated in place. At this point, the infrarenal segment of the inferior vena cava was ligated and the perfusate diverted entirely through the outflow cannula. The perfusion continued in a flow-through mode for 90 min. At the beginning of the perfusion, which occurred approximately 6 min after opening the abdominal cavity, the caudate lobe of the liver was tied off and immersed in liquid nitrogen. Then, a continuous infusion of a mixture of D-glucose (5 mM) and L-methionine (1 mM; both final concentrations in the perfusate) was started and maintained until the end of perfusion. Glucose and methionine were included in all perfusates to ensure an adequate supply of ATP and methionine, the substrates for SAM synthesis by MAT. Livers were perfused for 30 min to establish equilibrium, then concentrated solutions of test compounds (i.e., ethanol, propanol, 4-methylpyrazole and/or pyruvate) were added with the aid of microprocessor-assisted infusion pump (KD Scientific, Model 200, New Hope, PA) into a 2 mL infusion chamber placed in line with the perfusion line at a constant rate of 0.5 mL•min−1. The stock solutions were prepared such that they would be diluted to the appropriate concentrations in the infusion chamber. Influent and effluent perfusate samples were collected every 10 min throughout the entire perfusion time and immediately processed for lactate and pyruvate measurements by deproteinization in perchloric acid. Thirty minutes after the start of D-glucose+L-methionine infusion, the papilliform lobe of the liver was tied off and immersed in liquid nitrogen. Sampling of the lobes was made such that no leakage of the liver occurred during the perfusion. Immediately after the sampling of the papilliform lobe, infusion of other compounds was started and continued for the remaining 60 min of perfusion. The 90-min time point marked the end of the perfusion when a third part of the liver, the bipartite lobe, was tied off and immersed in liquid nitrogen. A scheme of the liver perfusion is given in Fig. 1.
Fig. 1.
Scheme of the rat liver perfusion experiment. The 90-min time line represents the duration of the perfusion in flow-through mode. The position of each box indicates the time period over which each component was infused (final concentrations in the influent perfusate are indicated in parentheses). Liver samples were taken by tying off lobules at the beginning of the experiment (0 time point) and after 30 and 90 min of perfusion, as indicated by arrows. n=6 animals per group.
2.3. Liver biochemical assays
Liver tissue was analyzed for SAM and SAH using a high-performance liquid chromatography procedure [16]. Fifty- to one hundred-milligram pieces of frozen liver stored at −85 °C were treated with 10 volumes of 5% (w/v) metaphosphoric acid on ice, homogenized with a glass-to-glass Potter-Elvehjem homogenizer, centrifuged for 30 min at 15,000×g, and the supernatant used for metabolite assays. All operations were performed at ice temperature.
2.4. Perfusate biochemical assays
The effluent perfusate was deproteinized in perchloric acid, and lactate and pyruvate concentrations were determined using enzymatic methods described in Ref. [17]. The deproteinization step was included to prevent interconversion between lactate and pyruvate catalyzed by lactate dehydrogenase that may have been released during perfusion.
2.5. Statistical analysis
The statistical analysis was performed using Graph-Pad Prism version 5.01 for Windows. Data are expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) and Student’s t test were performed to evaluate significant differences between the different experimental conditions. p-Values≤0.05 were considered significant.
3. Results
3.1. Ethanol and propanol induced a marked shift of the NADH/NAD+ redox couple to a more reduced state in the rat liver
In the isolated perfused rat liver model used in the present studies, the redox state of the cytoplasmic NADH/NAD+ redox couple was determined by measuring the concentrations of lactate and pyruvate in the effluent perfusate [6,13]. Because lactate and pyruvate are in equilibrium with free NADH and NAD+through the reaction catalyzed by lactate dehydrogenase (EC 1.1.1.27; reaction 1), the ratio of lactate/pyruvate is proportional to the ratio of NADH/NAD+. Therefore, we used the lactate/pyruvate ratio in the effluent perfusate as a measure of NADH/NAD+, and by altering the concentrations of lactate and pyruvate in the influent perfusate we could control the intracellular NADH/NAD+ ratio.
| (Reaction 1) |
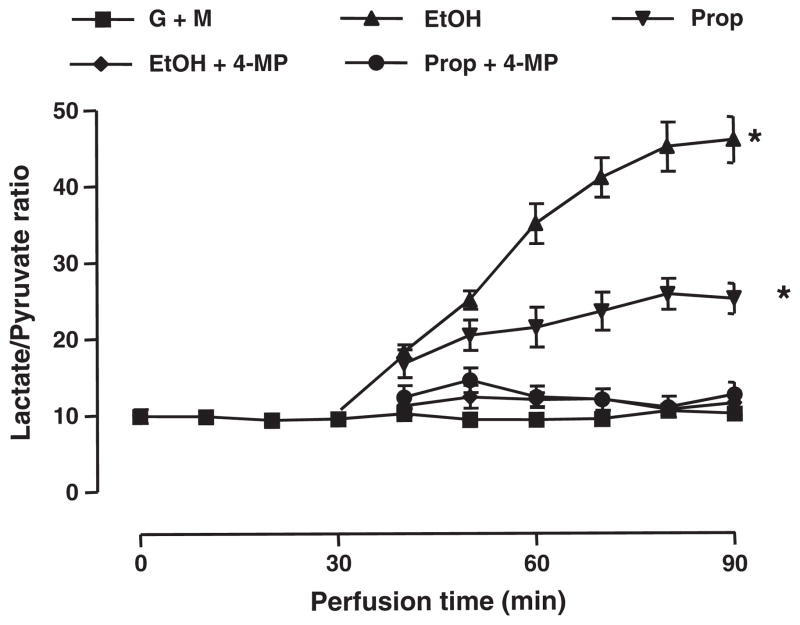
When a mixture of lactate and pyruvate at physiological concentrations (1 mM and 0.1 mM, respectively) and at a ratio of 10:1 was infused, the ratio of lactate/pyruvate in the effluent was maintained close to 10:1 during the entire perfusion (Fig. 2). Infusion of either ethanol or propanol induced a marked increase of the lactate to pyruvate ratio in the effluent, reaching 45.9±3.0 and 25.1±2.0, respectively, at the end of perfusion. The effect of both alcohols was markedly blocked by co-infusion of 4-methylpyrazole, an inhibitor of alcohol dehydrogenase [18].
Fig. 2.
Infusion of ethanol or propanol shifts the NADH/NAD+ ratio to a more reduced state. Isolated rat liver was perfused with ethanol (EtOH, 20 mM) or propanol (Prop, 20 mM), and the cytoplasmic NADH/NAD+ ratio was determined by measuring the concentrations of lactate and pyruvate in the effluent perfusate (i.e., the metabolite indicator method). Effluent perfusate samples were collected every 10 min throughout the entire perfusion time. Ethanol and propanol induced an increase in the lactate to pyruvate ratio. The effect of both alcohols was blocked by 4-methylpyrazole (4-MP, 4 mM), an inhibitor of alcohol dehydrogenase. *Indicates a significant difference (p<0.05) versus the basal value, established by infusion of glucose, methionine and a mixture of lactate and pyruvate at a ratio of 10 (G+M).
3.2. Ethanol and propanol decreased SAM content of the liver
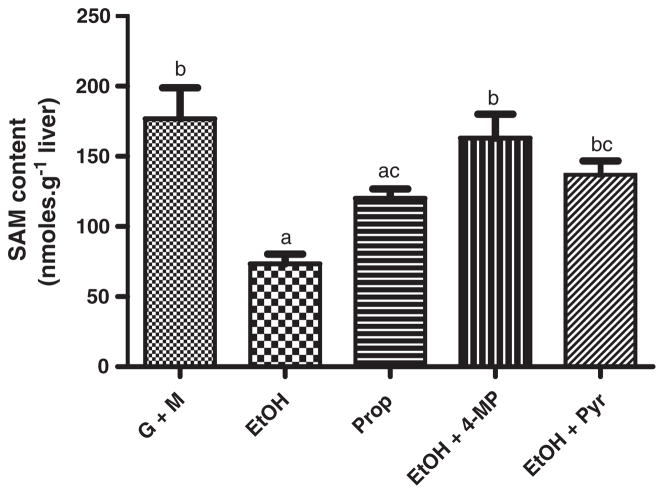
In the absence of alcohols, a relatively stable concentration of hepatic SAM was reached within 30 min after the start of perfusion and was maintained until the end of the perfusion. As described in Materials and methods, all perfusion media contained glucose and methionine to ensure that neither ATP nor methionine, the two substrates for SAM synthesis, became limiting over the course of the experiments. Upon infusion of ethanol or propanol, SAM content in the liver at the end of perfusion was decreased (Fig. 3). Thus, ethanol produced a decrease from 176.7±22.1 nmol•g−1 liver to 73.4±7.0 nmol•g−1 liver (p<0.05), while propanol produced a smaller decrease, i.e., to 120.0±6.7 nmol•g−1 liver (p<0.05). The effect of ethanol was blocked by 4-methylpyrazole. The inhibitor alone did not affect the intracellular content of SAM or SAH (not shown).
Fig. 3.
Infusion of ethanol or propanol decreased hepatic SAM concentration. Isolated rat liver was infused with ethanol (EtOH, 20 mM) or propanol (Prop, 20 mM) for 60 min, and S-adenosylmethionine (SAM) content was measured in liver lobules. Ethanol or propanol infusion resulted in a significant decrease of the liver SAM. The effect of ethanol was markedly attenuated by co-infusion of 4-methylpyrazole (4-MP, 4 mM), an inhibitor of alcohol dehydrogenase, and pyruvate (Pyr, 10 mM). Groups that do not share a common letter are significantly different (p<0.05).
Inhibition of alcohol dehydrogenase by 4-methylpyrazole provides evidence that the effects of ethanol and propanol are due to the oxidation of both alcohols by alcohol dehydrogenase. We also used a physiological approach to counteract the effect of ethanol by infusing pyruvate, a metabolite which would rapidly consume NADH through the reverse of Reaction 1, thus shifting the redox state of the NADH/NAD+ system to a more oxidized state. The pyruvate concentration in the influent perfusate was increased from 0.1 mM to 10 mM, resulting in a lactate/pyruvate ratio of 0.1. Under these conditions, the decrease in SAM concentrations induced by ethanol was blunted; hepatic SAM levels were 1.8-fold higher in the presence of 10 mM pyruvate than with ethanol alone (Fig. 3a). A similar pattern was obtained with propanol and pyruvate (not shown).
3.3. Ethanol and propanol increased SAH content of the perfused liver
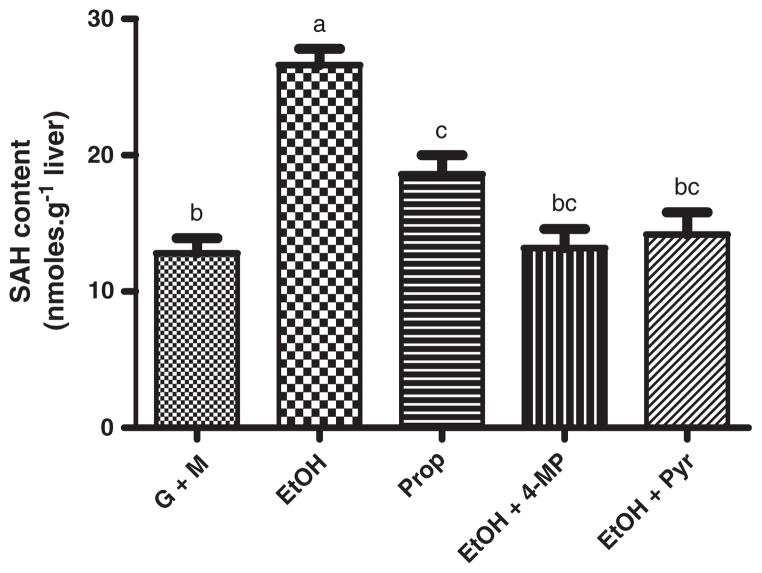
Like SAM, hepatic SAH reached a stable concentration within 30 min of the start of glucose+methionine infusion. Both ethanol and propanol increased the content of SAH in the liver, and the effect was more pronounced with ethanol (Fig. 4). Ethanol increased the SAH level from 12.8±1.1 nmol•g−1 liver to 26.6±1.2 nmol•g−1 liver (p<0.05), while propanol increased the level to 18.6±1.4 nmol•g−1 liver (p<0.05). Infusion of either 4-methylpyrazole or 10 mM pyruvate completely blocked the effect of ethanol and propanol on the SAH content.
Fig. 4.
Ethanol and propanol increased hepatic SAH concentrations. The effect of ethanol (EtOH, 20 mM) and propanol (Prop, 20 mM) on the S-adenosylhomocysteine (SAH) content was assessed in the perfused rat liver. Ethanol and propanol resulted in a significant increase of liver SAH. The effect of ethanol was markedly attenuated by co-infusion of 4-methylpyrazole (4-MP, 4 mM) and pyruvate (Pyr, 10 mM). Groups that do not share a common letter are significantly different (p<0.05).
3.4. NADH/NAD+ redox shift induced changes in SAM to SAH ratio
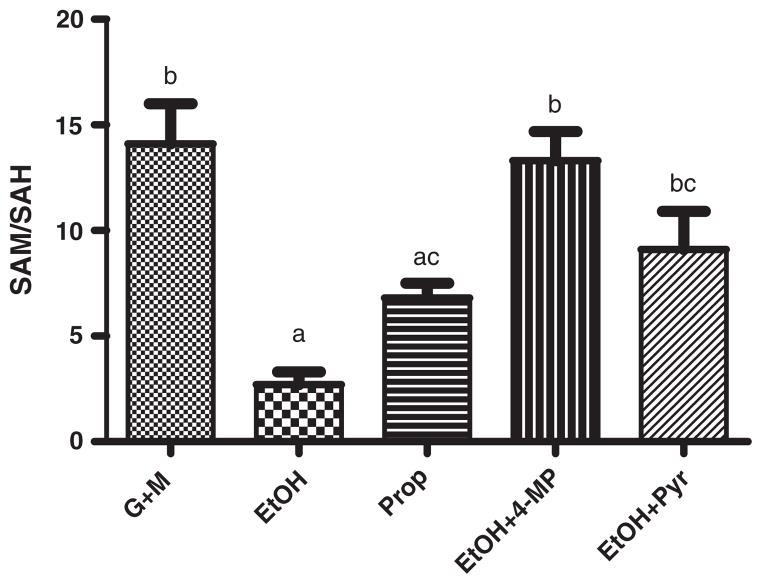
Changes in the SAM and SAH content in the liver have an impact on the SAM/SAH ratio. This ratio is taken as a marker of cellular methylation potential because SAM serves as a donor of methyl group while SAH inhibits methyl transfer reactions. Ethanol and propanol significantly (p<0.05) decreased the SAM/SAH ratio compared to the basal experimental conditions: 2.7±0.6, 6.8±0.7 and 14.1±1.9, respectively. Pyruvate and 4-methylpyrazole counteracted the effects of alcohols (Fig. 5).
Fig. 5.
The SAM/SAH ratio decreased in rat liver perfused with ethanol (EtOH, 20 mM) or propanol (Prop, 20 mM). The SAM/SAH ratio was calculated from the hepatic concentrations of SAM and SAH. The ratio was restored by infusion of 4-methylpyrazole (4-MP, 4 mM) or pyruvate (10 mM). Groups that do not share a common letter are significantly different (p<0.05).
4. Discussion
The goal of this study was to test the hypothesis that ethanol-induced dysregulation of liver methionine metabolism may be ascribed, at least in part, to ethanol-induced changes in the redox state of the NADH/NAD+ couple. To determine this, we used isolated perfused rat livers in which we manipulated the free NADH/NAD+ redox couple by infusing substrates of NADH- and NAD+-dependent dehydrogenases and measuring the tissue levels of SAM and SAH. These experiments confirmed earlier findings that ethanol infusion increased the ratio of NADH/NAD+ [8,11] and, through the use of 4-methylpyrazole, demonstrated that the increase in NADH was largely due to the activity of alcohol dehydrogenase. Consistent with this finding, a second alcohol dehydrogenase substrate, propanol, had a similar effect on the NADH/NAD+ ratio. In support of our hypothesis, conditions that increased the NADH/NAD+ ratio (ethanol and propanol infusion) led to a decrease in SAM, an increase in SAH, and a decrease in the SAM/SAH ratio, whereas conditions that normalized the NADH/NAD+ ratio (4-methylpyrazole and pyruvate) blocked the perturbations in methionine metabolism. The observation that 4-methylpyrazole blocked the effects of ethanol and propanol demonstrated that formation of NADH by the NAD+-dependent alcohol dehydrogenase is necessary for the changes in SAM and SAH content rather than other factors related to the molecular properties of the two alcohols such as their effects on membrane fluidity.
It is not clear whether a change in the NADH/NAD+ ratio is sufficient to induce changes in SAM and SAH. In addition to NADH, aldehydes are also products of the alcohol dehydrogenase-catalyzed oxidation of ethanol and propanol, and acetaldehyde (ethanal) and propionaladehyde (propanal) may have contributed to the changes in methionine metabolism. We consistently observed that the effects of propanol on NADH/NAD+ and SAM/SAH were weaker than the effects of ethanol even though rat liver alcohol dehydrogenase has the same Km for both ethanol and propanol, and propanol is oxidized by the perfused rat liver at the same velocity as ethanol [19]. One potential explanation for the observed differences between ethanol and propanol may be that subsequent metabolic steps occur at different rates. The second enzyme in the metabolism of both ethanol and propanol is aldehyde dehydrogenase (EC 1.2.1.3), which is also NAD+-dependent. If propionaldehyde is oxidized at a lower rate than acetaldehyde, then less NADH would be produced. When excess pyruvate was infused, the rates of metabolism of both the parent alcohol and the product aldehyde by alcohol dehydrogenase and aldehyde dehydrogenase, respectively, would be expected to increase due to increased re-oxidation of NADH to NAD+. Others have shown that when glycerate was used to increase the rate of NADH re-oxidation in ethanol-fed rats, the clearance of ethanol was increased and there was a trend toward increased acetaldehyde levels [20], suggesting that oxidation of ethanol to acetaldehyde was faster than oxidation of acetaldehyde to acetate. We did not measure aldehyde concentrations as part of this study, but it is unlikely that aldehyde concentrations decreased significantly upon infusion of pyruvate. Therefore, the effects of pyruvate on ethanol- and propanol-induced changes in SAM and SAH levels are probably not the result of decreased aldehyde levels.
Earlier studies showed that chronic exposure to ethanol decreased SAM and increased SAH [3,4,16,21–24]. We reproduced these effects with an acute exposure to ethanol as well as propanol. It is not clear whether similar mechanisms are responsible for both the acute and chronic effects. Little is known about the effects of ethanol on SAM and SAH at very early time points; it is possible that the SAM/SAH ratio decreases transiently as an early response to ethanol exposure, coincident with an early shift in the NADH/NAD+ ratio [7]. The shift in the NADH/NAD+ ratio tends to be less pronounced in chronic ethanol exposure models than in acute models [25,26] as a result of increased re-oxidation of NADH and/or increased metabolism of ethanol through enzymes other than alcohol dehydrogenase [27]. While acute ethanol exposure can produce more dramatic changes in the NADH/NAD+ ratio, changes similar to those described here have been observed in chronic ethanol models. Direct measurement of the NADH/NAD+ ratio in rats fed ethanol intragastrically for 1 month showed a 3- to 10-fold increase in the ratio, depending on whether the measurements were made when blood alcohol levels were at their highest or lowest points [11]. Similarly, Baraona et al. reported that although the lactate/pyruvate ratio was only 30% higher in chronic alcohol-fed baboons than in pair-fed controls, the ratio increased 15-fold in response to acute exposure to ethanol in both groups [28]. Therefore, changes in the NADH/NAD+ ratio similar to or greater than the 2.5- to 5-fold increase we observed in our liver perfusion model are achievable in chronic ethanol exposure models and may be sufficient to sustain prolonged changes in methionine metabolism.
The enzymes that directly contribute to SAM concentrations are MAT (SAM synthesis), methyltransferases (substrate methylation), and SAM decarboxylase (polyamine synthesis). It is not clear which of these enzymes are responsible for the decrease in SAM levels that we observed following infusion of ethanol or propanol. Decreased SAM synthesis by MAT is certainly a possibility. The MAT substrates, methionine and ATP, are probably present in sufficient concentrations (methionine was infused throughout the experiments, and ATP depletion was not observed in an earlier study using similar conditions [29]), but MAT activity is subject to transcriptional and allosteric regulation [3,30]. In light of this, it will be interesting to test the hypothesis that the NADH/NAD+ ratio modulates MAT activity. Oxidation or alkylation of nucleophilic cysteine residues within the MAT enzyme results in loss of activity [30–32]. Therefore, it will be important to determine whether NADH/NAD+-dependent alterations in activity are mediated by intracellular reactive oxygen species or electrophilic aldehydes such as acetaldehyde or lipid peroxidation byproducts. An increase in the use of SAM as a methyl donor in methyltransferase reactions would also account for the decrease of SAM and the increase of SAH. Another possibility is that SAM is used in other reactions such as polyamine formation which would divert a part of its pool from SAH formation, but this pathway has not been tested in ethanol exposed liver.
SAH is a product of SAM-dependent methyltransferases (EC 2.1.1.X) and is a feedback inhibitor of many of these enzymes [33]. Using the average total water content of 0.74 mL•g−1 liver measured at the beginning and end of the perfusion (not shown), we calculated the intracellular concentration of SAH after 90-min infusion of ethanol to be approximately 43 μM. This concentration is within the range at which SAH inhibits several methyltransferase reactions, including RNA methyltransferases (EC 2.1.1.X) and glycine methyltransferase (EC 2.1.1.20) [34,35]. An increase in SAH relative to SAM could therefore lead to decreased methylation of various cellular components, including proteins, nucleic acids and lipids. In addition, SAH is the main precursor of circulating homocysteine whose increased levels are implicated as a risk factor in a number of diseases, particularly of the cardiovascular system [36]. Therefore, an increase in SAH may have far reaching effects on cell function and disease progression.
The precise mechanisms underlying the increase in intracellular concentrations of SAH in the liver of alcohol exposed animals are not fully understood. The intracellular concentration of SAH reflects the balance between the rate of formation and the rate of elimination by SAHH-mediated hydrolysis to homocysteine and adenosine. SAH can be formed in two ways: by SAM-dependent methyltransferases, as discussed above, and by SAHH-mediated synthesis from homocysteine and adenosine (the reverse of the hydrolysis reaction). A potential mechanism for the increase in SAH that we have observed may be a decrease in SAH hydrolysis catalyzed by SAHH. Decreased activity of SAHH has been reported in the livers of micropigs fed alcohol, but this was most likely due to a decrease in expression levels of the enzyme [4]. An intriguing possibility with respect to the current studies is that SAHH activity may be sensitive to changes in the redox state of the NADH/NAD+ system. In SAHH, NAD+ is tightly bound to the enzyme and acts catalytically in both the forward and reverse reactions [37,38]. Replacing NAD+ with NADH in the active site inhibits SAHH hydrolysis activity by increasing its affinity for adenosine [39]. The active site of SAHH is not in rapid equilibrium with the pool of free NADH/NAD+, but under conditions of chronic reductive stress this regulatory mechanism may become more important. Newly synthesized SAHH must bind NAD+ in order to become catalytically active. During this time, therefore, the redox state of the free NADH/NAD+ pool may have an impact on the redox state of the enzyme bound NADH/NAD+. Such a scenario may be encountered in long-term alcohol exposure of the liver as happens in human ethanol abusers and in animals chronically exposed to alcohol.
5. Conclusions
The data presented in this study suggest that the shift in the redox state of the NADH/NAD+ system to a more reduced state causes rapid changes in liver methionine metabolism, reflected by decreased SAM and increased SAH content. These observations provide a totally new mechanism for the metabolically important changes in SAM and SAH observed in alcoholic liver disease. Furthermore, the data add a novel metabolic pathway to an already large list of processes that are regulated by the redox state of the NADH/NAD+ system [13,40].
Acknowledgments
The work presented in this study was supported by NIH grants R21 AA014611 and R21 AA015611 (IVD), K01 AA 015344 (ZS), R01 AA010486, R37 AA010762, R01 AA0015970, P01 AA017103, and the Department of Veterans Affairs (CJM).
Abbreviations
- SAM
S-adenosylmethionine
- MAT
methionine adenosyltransferase
- SAHH
S-adenosylhomocysteine hydrolase
- G+M
glucose+methionine
- 4-MP
4-methylpyrazole
- Pyr
pyruvate
- EtOH
ethanol
- Prop
propanol
References
- 1.McClain CJ, Hill DB, Song Z, Chawla R, Watson WH, Chen T, Barve S. S-Adenosylmethionine, cytokines, and alcoholic liver disease. Alcohol. 2002;27:185–192. doi: 10.1016/s0741-8329(02)00224-0. [DOI] [PubMed] [Google Scholar]
- 2.Kharbanda KK. Alcoholic liver disease and methionine metabolism. Semin Liver Dis. 2009;29:155–165. doi: 10.1055/s-0029-1214371. [DOI] [PubMed] [Google Scholar]
- 3.Lu SC, Huang ZZ, Yang H, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G178–G185. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva JA, Halsted CH. Hepatic transmethylation reactions in micropigs with alcoholic liver disease. Hepatology. 2004;39:1303–1310. doi: 10.1002/hep.20168. [DOI] [PubMed] [Google Scholar]
- 5.Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B. Ethanol-induced oxidative stress: basic knowledge. Genes Nutr. 2010;5:101–109. doi: 10.1007/s12263-009-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guynn RW, Pieklik JR. Dependence on dose of the acute effects of ethanol on liver metabolism in vivo. J Clin Invest. 1975;56:1411–1419. doi: 10.1172/JCI108222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frosander OA, Raeihae N, Salaspuro M, Maeenpaeae P. Influence of ethanol on the liver metabolism of fed and starved rats. Biochem J. 1965;94:259–265. doi: 10.1042/bj0940259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreisberg RA. Effect of alcohol on glucose production and lactate, pyruvate and ketone body metabolism by the isolated perfused rat liver. Diabetes. 1967;16:784–790. doi: 10.2337/diab.16.11.784. [DOI] [PubMed] [Google Scholar]
- 10.Hassinen IE, Ylikahri RH, Kahonen MT. Effect of ethanol, thyrxine and fructose on the intracellular redox state of a perfused liver as studied by surface fluorometry. Ann Med Exp Biol Fenn. 1970;48:176–183. [PubMed] [Google Scholar]
- 11.Bardag-Gorce F, French BA, Li J, Riley NE, Yuan QX, Valinluck V, Fu P, Ingelman-Sundberg M, Yoon S, French SW. The importance of cycling of blood alcohol levels in the pathogenesis of experimental alcoholic liver disease in rats. Gastroenterology. 2002;123:325–335. doi: 10.1053/gast.2002.34177. [DOI] [PubMed] [Google Scholar]
- 12.Jauhonen VP, Baraona E, Lieber CS, Hassinen IE. Dependence of ethanol-induced redox shift on hepatic oxygen tensions prevailing in vivo. Alcohol. 1985;2:163–167. doi: 10.1016/0741-8329(85)90036-9. [DOI] [PubMed] [Google Scholar]
- 13.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 15.Scholz R. Untersuchungen zur Redox compartmentierung der hemeoglobinfrei perfundierten Leber. In: Staib W, editor. Stoffwechesel der isolierten perfundierten Leber. Springer Verlag; Berlin: 1968. pp. 24–38. [Google Scholar]
- 16.Song Z, Zhou Z, Song M, Uriarte S, Chen T, Deaciuc I, McClain CJ. Alcohol-induced S-adenosylhomocysteine accumulation in the liver sensitizes to TNF hepatotoxicity: possible involvement of mitochondrial S-adenosylmethionine transport. Biochem Pharmacol. 2007;74:521–531. doi: 10.1016/j.bcp.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutman I. Lactat und pyruvat, Methoden der Enzymatischen Analyse. Verlag Chemie, Weinheim/Bergsrasse; 1974. pp. 1510–1513. [Google Scholar]
- 18.Blomstrand R, Ostling-Wintzell H, Lof A, McMartin K, Tolf BR, Hedstrom KG. Pyrazoles as inhibitors of alcohol oxidation and as important tools in alcohol research: an approach to therapy against methanol poisoning. Proc Natl Acad Sci U S A. 1979;76:3499–3503. doi: 10.1073/pnas.76.7.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson GP, Olson RM. Comparison of the metabolism of alcohols by rat hepatic and pulmonary alcohol dehydrogenase. Biochem Mol Biol Int. 1995;37:65–71. [PubMed] [Google Scholar]
- 20.Eriksson CJ, Saarenmaa TP, Bykov IL, Heino PU. Acceleration of ethanol and acetaldehyde oxidation by D-glycerate in rats. Metabolism. 2007;56:895–898. doi: 10.1016/j.metabol.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Barak AJ, Beckenhauer HC, Mailliard ME, Kharbanda KK, Tuma DJ. Betaine lowers elevated S-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutr. 2003;133:2845–2848. doi: 10.1093/jn/133.9.2845. [DOI] [PubMed] [Google Scholar]
- 22.Halsted CH, Villanueva J, Chandler CJ, Stabler SP, Allen RH, Muskhelishvili L, James SJ, Poirier L. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology. 1996;23:497–505. doi: 10.1002/hep.510230314. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol. 2007;46:314–321. doi: 10.1016/j.jhep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Seo JM, Jung YS, Kwak HE, Kim YC. Alterations in hepatic metabolism of sulfur-containing amino acids induced by ethanol in rats. Amino Acids. 2003;24:103–110. doi: 10.1007/s00726-002-0324-6. [DOI] [PubMed] [Google Scholar]
- 25.Salaspuro MP, Lindros KO, Pikkarainen P. Ethanol and galactose metabolism as influenced by 4-methylpyrazole in alcoholics with and without nutritional deficiencies. Preliminary report of a new approach to pathogenesis and treatment in alcoholic liver disease. Ann Clin Res. 1975;7:269–272. [PubMed] [Google Scholar]
- 26.Salaspuro MP, Shaw S, Jayatilleke E, Ross WA, Lieber CS. Attenuation of the ethanol-induced hepatic redox change after chronic alcohol consumption in baboons: metabolic consequences in vivo and in vitro. Hepatology. 1981;1:33–38. doi: 10.1002/hep.1840010106. [DOI] [PubMed] [Google Scholar]
- 27.Salaspuro MP, Lindros KO, Pikkarainen PH. Effect of 4-methylpyrazole on ethanol elimination rate and hepatic redox changes in alcoholics with adequate or inadequate nutrition and in nonalcoholic controls. Metabolism. 1978;27:631–639. doi: 10.1016/0026-0495(78)90001-x. [DOI] [PubMed] [Google Scholar]
- 28.Baraona E, Jauhonen P, Miyakawa H, Lieber CS. Zonal redox changes as a cause of selective perivenular hepatotoxicity of alcohol. Pharmacol Biochem Behav. 1983;18(Suppl 1):449–454. doi: 10.1016/0091-3057(83)90216-2. [DOI] [PubMed] [Google Scholar]
- 29.Strubelt O, Deters M, Pentz R, Siegers CP, Younes M. The toxic and metabolic effects of 23 aliphatic alcohols in the isolated perfused rat liver. Toxicol Sci. 1999;49:133–142. doi: 10.1093/toxsci/49.1.133. [DOI] [PubMed] [Google Scholar]
- 30.Pajares MA, Duran C, Corrales F, Pliego MM, Mato JM. Modulation of rat liver S-adenosylmethionine synthetase activity by glutathione. J Biol Chem. 1992;267:17598–17605. [PubMed] [Google Scholar]
- 31.Corrales F, Cabrero C, Pajares MA, Ortiz P, Martin-Duce A, Mato JM. Inactivation and dissociation of S-adenosylmethionine synthetase by modification of sulfhydryl groups and its possible occurrence in cirrhosis. Hepatology. 1990;11:216–222. doi: 10.1002/hep.1840110210. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Chantar ML, Pajares MA. Role of thioltransferases on the modulation of rat liver S-adenosylmethionine synthetase activity by glutathione. FEBS Lett. 1996;397:293–297. doi: 10.1016/s0014-5793(96)01201-x. [DOI] [PubMed] [Google Scholar]
- 33.Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med. 2007;45:1694–1699. doi: 10.1515/CCLM.2007.341. [DOI] [PubMed] [Google Scholar]
- 34.Kerr SJ. Competing methyltransferase systems. J Biol Chem. 1972;247:4248–4252. [PubMed] [Google Scholar]
- 35.James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. J Nutr. 2002;132:2361S–2366S. doi: 10.1093/jn/132.8.2361S. [DOI] [PubMed] [Google Scholar]
- 36.Asfar S, Safar HA. Homocysteine levels and peripheral arterial occlusive disease: a prospective cohort study and review of the literature. J Cardiovasc Surg (Torino) 2007;48:601–605. [PubMed] [Google Scholar]
- 37.Palmer JL, Abeles RH. The mechanism of action of S-adenosylhomocysteinase. J Biol Chem. 1979;254:1217–1226. [PubMed] [Google Scholar]
- 38.Yang X, Hu Y, Yin DH, Turner MA, Wang M, Borchardt RT, Howell PL, Kuczera K, Schowen RL. Catalytic strategy of S-adenosyl-L-homocysteine hydrolase: transition-state stabilization and the avoidance of abortive reactions. Biochemistry. 2003;42:1900–1909. doi: 10.1021/bi0262350. [DOI] [PubMed] [Google Scholar]
- 39.Kloor D, Ludtke A, Stoeva S, Osswald H. Adenosine binding sites at S-adenosylhomocysteine hydrolase are controlled by the NAD+/NADH ratio of the enzyme. Biochem Pharmacol. 2003;66:2117–2123. doi: 10.1016/s0006-2952(03)00581-1. [DOI] [PubMed] [Google Scholar]
- 40.Ying W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]