Abstract
Using real time qPCR, we examined the expression of mRNAs for the five somatostatin receptors (SSTRs) in the caudate putamen of male C57BL/6J and 129P3/J mice. Animals were exposed to multiple injections of heroin, or saline, in the setting of a conditioned place preference study. The relative expression levels of the five SSTR mRNAs differed between the two strains. In both strains, SSTR-1 mRNA was expressed at the highest levels and SSTR-5 at the lowest. Interestingly, in 129P3/J mice SSTR-3 mRNA was not detected in the caudate putamen. We confirmed this finding in the frontal cortex, hypothalamus, nucleus accumbens and a region containing the substantia nigra and ventral tegmental area. We also found strain differences in the mRNA levels of SSTR-2 and -4. Intermittent heroin administration had a dose-dependent effect on the levels of SSTR-1 and -3mRNAs. These results demonstrate strain differences in the expression of specific mRNAs and a heroin-induced dose-dependent elevation of SSTR-1 and -3 mRNAs in the mouse caudate putamen.
Keywords: C57BL/6J, 129P3/J, Somatostatin receptor, mRNA
1. Introduction
The ventromedial (nucleus accumbens; NAc) and dorsolateral (caudate putamen; CPu) striata, are major sites of action for drugs of abuse (e.g. Di Chiara and Imperato, 1988; Volkow et al., 2002). Drugs of abuse activate the mesolimbic and nigrostriatal dopaminergic systems, elevating extracellular dopamine in the NAc and CPu. Opioids interact with the mu opioid receptor on GABAergic interneurons in the midbrain, inhibiting GABAergic activity, thus disinhibiting dopaminergic projection neurons (e.g. Johnson and North, 1992; Xi et al., 2002). The NAc is a major locus mediating the rewarding effects of drugs of abuse (e.g. Wise, 1989). The CPu receives a topologically ordered projection from the cortex (e.g. McGeorge and Faull, 1989; Willuhn et al., 2003), is involved in habitual learning and motivated behavior (e.g. Packard and Knowlton, 2002; White and McDonald, 2002), and thus may play a role in the development of addictions.
Somatostatin (a.k.a. somatotropin release inhibiting factor; SRIF) is a neuropeptide originally isolated from the hypothalamus and shown to inhibit the secretion of growth hormone from the pituitary (Brazeau et al., 1973). Two forms of SRIF are known, the original tetradecapeptide and an N terminal extended form, SRIF-28 (Noe and Spiess, 1983). SRIF has a wide distribution, both in the central nervous system and in peripheral tissues (e.g. Johansson et al., 1984; Patel et al., 1995; Reichlin, 1983a,b). Most SRIF is found in extrahypothalamic regions (for review see e.g. Vale et al., 1977), including the basal ganglia and limbic regions (e.g. Johansson et al., 1984; Vale et al., 1977; Weiss and Chesselet, 1989).
SRIF has exocrine, endocrine, neuromodulatory and neurotransmitter functions. There is a strong interaction between the dopaminergic system and SRIF. Exogenous SRIF, administered i.c.v. to rats, resulted in increased synthesis and utilization of dopamine (and serotonin) (Garcia-Sevilla et al., 1978). SRIF dose dependently increased dopamine levels in the mouse striatum when administered locally (Hathway et al., 2004). SRIF increased dopamine release in superfused rat or cat striatum (Chesselet and Reisine, 1983) and elevated extracellular dopamine in the mouse striatum (Hathway et al., 2004). Antagonism of either dopamine D1 or D2 receptors decreased levels of SRIF mRNA in the rat striatum (Augood et al., 1991). In addition to effects on dopamine release, SRIF also stimulates the release of GABA and glutamate from striatal neurons (e.g. Hathway et al., 1998).
SRIF has a dose-dependent behavioral effect (e.g. Rezek et al., 1977; Vecsei and Widerlov, 1990). Low doses of SRIF infused directly into the rat CPu result in a behavioral activation characterized by increased locomotor activity and the expression of behavioral stereotypy (Rezek et al., 1977). In rats, low doses of SRIF administered i.c.v. also resulted in increased locomotor activity and behavioral stereotypy, while higher doses produced motor deficits including catatonia, paraplegia-in-extension and tonic–clonic seizures (Havlicek et al., 1976). In mice, SRIF administration unilaterally into the CPu, resulted in contralateral turning behavior (Hathway et al., 2004). Depletion of SRIF with cysteamine administered systemically blunted apomorphine-induced behavioral stereotypy and amphetamine-induced locomotor activation (Martin-Iverson et al., 1986). Local infusion of cysteamine into the nucleus accumbens also attenuated the locomotor stimulating effects of amphetamine, but had no effect on amphetamine-induced conditioned place preference (Martin-Iverson et al., 1986).
SRIF acts through binding to membrane bound G protein coupled receptors. There are five distinct somatostatin receptors (SSTR-1–5), each encoded by its own gene (for review see e.g. Patel et al., 1995). In the rat, mRNAs for all SSTRs are located throughout the brain and expression levels are region specific (e.g. Breder et al., 1992; Bruno et al., 1993; Patel et al., 1995). mRNA for all five SSTRs are found in the rat striatum, with modest levels of SSTR-3 and -5, somewhat lower levels of SSTR-2 and -4 and low levels of SSTR-1 (for review see Patel et al., 1995). Morphine (a mu opioid receptor (MOP-r) agonist) binds to SSTR-2 (Hatzoglou et al., 1995) and the formation of heterodimers of SSTRs and dopaminergic and opioidergic receptors have been reported in vitro (for review see Duran-Prado et al., 2008). However, it is important to note that there is no evidence, to date, to indicate the formation of such heterodimers in vivo (Duran-Prado et al., 2008). Additionally, a previous study from our laboratory demonstrated a significant increase in SSTR-2 mRNA in the CPu of rats exposed to cocaine (Yuferov et al., 2003).
Opioid administration to rodents also causes behavioral activation (e.g. Babbini and Davis, 1972; Castellano et al., 1976; Schlussman et al., 2008). Strain differences in the behavioral effects of opioids have been reported. In C57BL/6J mice, opioids have a locomotor stimulatory effect (e.g. Crawley et al., 1997; Oliverio and Castellano, 1981; Schlussman et al., 2008). C57BL/6J mice show high levels of oral morphine self-administration (Belknap et al., 1993). 129P3/J mice did not show naloxone-induced jumping following chronic infusion of morphine, suggesting a lack of development of physical dependence (Kest et al., 2002b; Metten et al., 2009), nor did they develop tolerance to analgesic effects of morphine (Kest et al., 2002a). However, another study suggested that 129P3/J mice did develop physical dependence to morphine (Metten et al., 2009) and we have shown that 129P3/J mice develop conditioned place preference to heroin, although at higher doses than in C57BL/6J animals (Schlussman et al., 2008), indicating that heroin is rewarding in this strain of mice. The C57BL/6J and 129P3/J strains also differ in the locomotor and pDynmRNA stimulatory effects of cocaine (Schlussman et al., 2003a,b). These studies demonstrate that the C57BL/6J and 129P3/J strains of mice show significant differences in their behavioral response to drugs of abuse.
To more fully characterize the C57BL/6J and 129P3/J mouse strains, we examined mRNA levels of the five SSTRs within the CPu in mice following four administrations of various doses of heroin alternated with four injections of saline, in a conditioned place preference paradigm.
2. Results
2.1. Strain effects
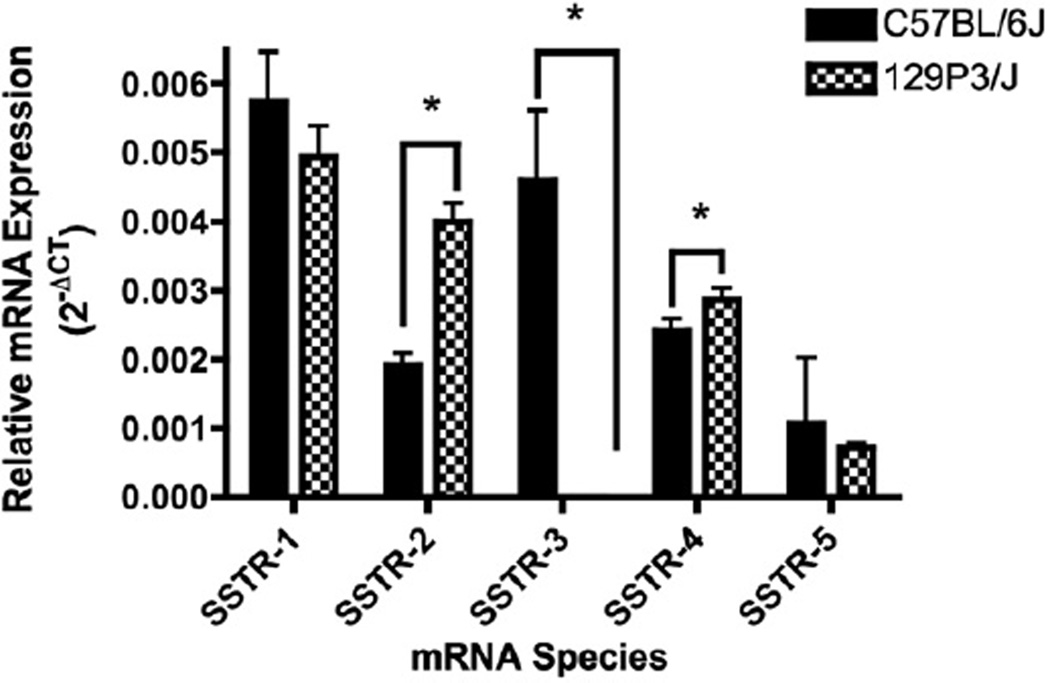
mRNA for all five somatostatin receptors was detected in the CPu of C57BL/6J mice (Fig. 1). Significant differences in the levels of mRNA expression of these receptors were observed (F(4,164)=24.08, p<0.00001). SSTR-1 had the highest level of expression, followed by SSTR-3. SSTR-5 showed the lowest level of expression. Significant differences in the levels of individual SSTR mRNAs were also observed in the CPu of 129P3/J mice (F(4,224)=104.1, p<0.001). mRNA for SSTR-1, -2, -4 and -5 was observed (Fig. 1) with SSTR-1 showing the highest level of expression, followed by SSTR-2 and -4, with SSTR-5 showing the lowest detectable levels of mRNA expression. SSTR-3 mRNA was not detected in the CPu of 129P3/J mice. In addition to this lack of expression of SSTR-3 mRNA in 129P3/J mice (Fig. 1), we also found significant strain differences in the expression levels of SSTR-2 (F(1,92)=49.81, p<0.0001; Fig. 1) and SSTR-4 (F(1,111)=0.01; Fig. 1). In the CPu, levels of mRNA for SSTR-2 and SSTR-4 were greater in 129P3/J mice than in C57BL/6J animals.
Fig. 1.
Relative expression levels of all five SSTR mRNAs in the CPu of C57BL/6J and 129P3/J mice. Levels of SSTR-2 and -4 mRNA were higher in the CPu of 129P3/Jmice than in C57BL/6J animals (p<0.0001 and 0.01 respectively). SSTR-3 was not detectable in the CPu of 129P3/Jmice.
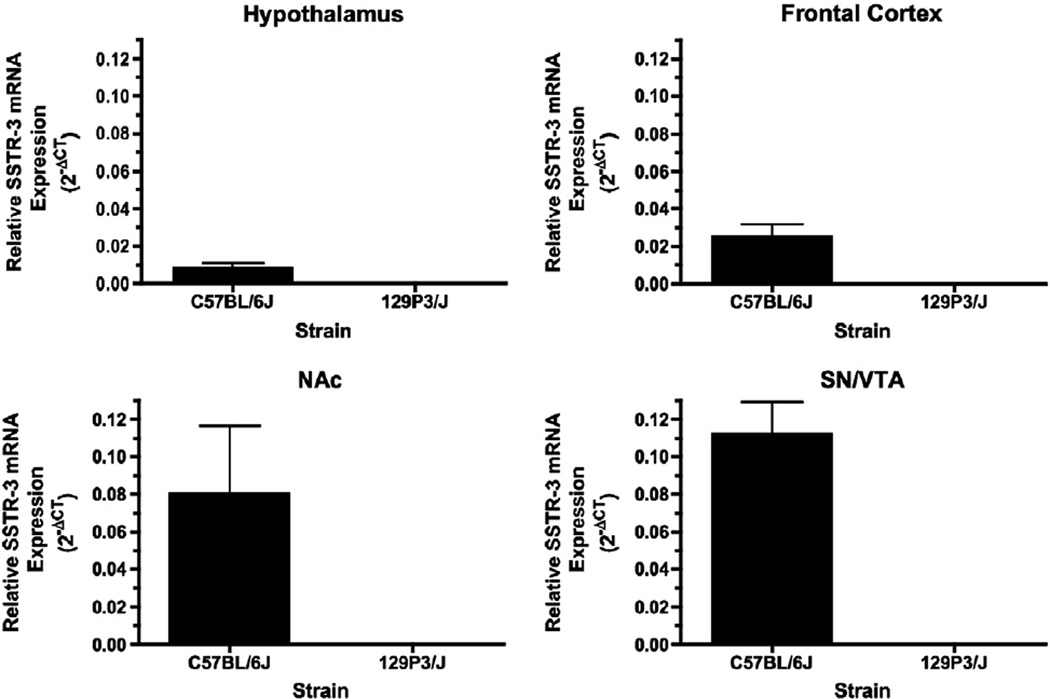
To further investigate the apparent lack of SSTR-3 mRNA in 129P3/J mice, we measured SSTR-3 mRNA levels in the hypothalamus, frontal cortex, nucleus accumbens and a region containing the substantia nigra and ventral tegmental area. SSTR-3 mRNA was observed in all these brain regions of C57BL/6J mice but no measurable expression of SSTR-3 mRNA was found in 129P3/J mice (Fig. 2).
Fig. 2.
SSTR-3 mRNA was abundant in the hypothalamus, frontal cortex, nucleus accumbens and substantia nigra/ventral tegmental area of C57BL/6J mice but was below the limits of detection in these brain regions from 129P3/J animals.
2.2. Drug effects
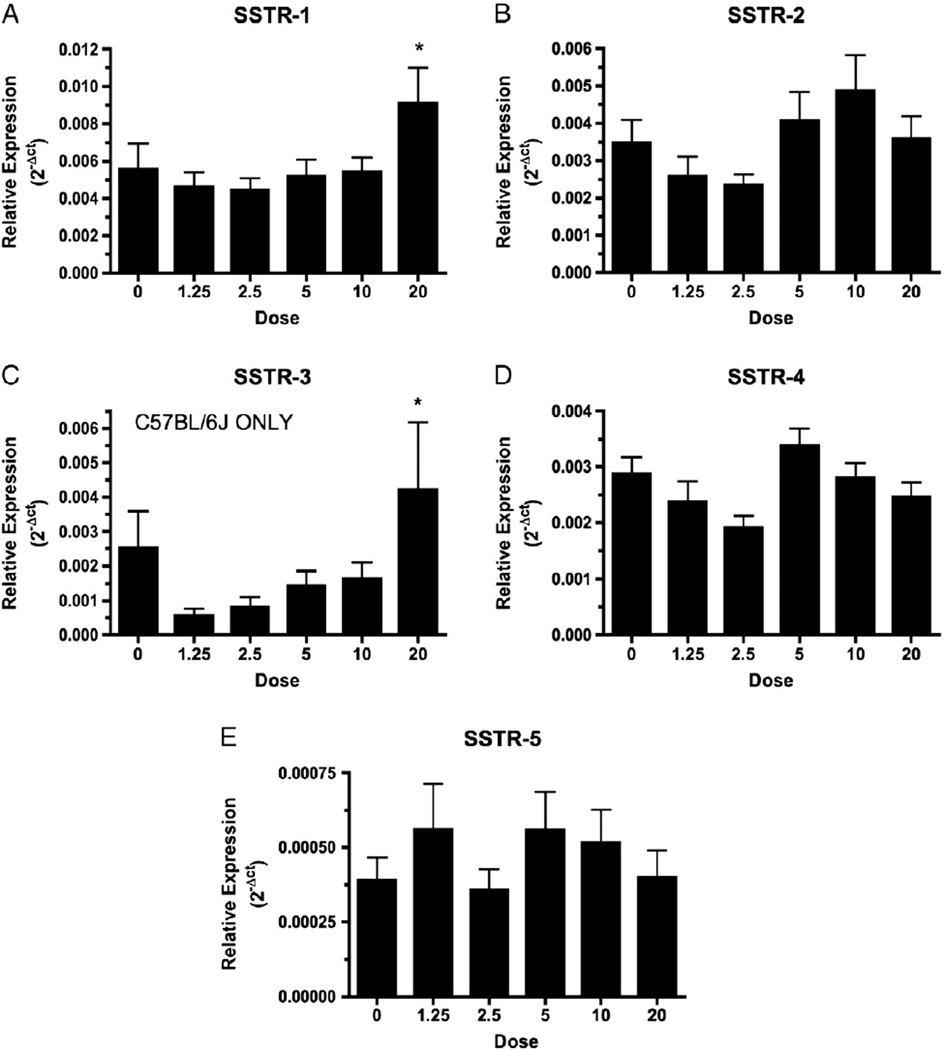
A significant main effect of dose was observed in the expression of SSTR-1 (F(5,108)=3.76, p<0.005), SSTR-2 (F(5,92)=4.76, p<0.001), SSTR-3 (F(5,106)=3.12, p<0.05) and SSTR-4 mRNAs (F(5,111)=3.03, p<0.05); however the only significant change relative to saline controls was found in SSTR-1 and SSTR-3 (Fig. 3). mRNA levels of SSTR-1 and -3 were significantly higher in mice given the 20 mg/kg dose of heroin relative to saline controls (Newman–Keuls post hoc tests p<0.05 and p<0.005, respectively).
Fig. 3.
Heroin effects on mRNA levels of specific SSTRs. Heroin dose dependently elevated SSTR-1 (A) and SSTR-3 (C) mRNAs. The highest dose of heroin (20 mg/kg) significantly elevated the levels of SSTR-1 (p<0.005) and SSTR-3 (p<0.005) relative to saline controls. Heroin did not significantly affect the expression of SSTR-2 (B), SSTR-4 (D) or SSTR-5 (E) relative to saline controls. Note: Y-axis scale differs in all graphs.
3. Discussion
The pattern of SSTR mRNA expression in the CPu reported here differs significantly from reports on relative expression levels in the rat. In the CPu of both C57BL/6J and 129P3/J mice we found that SSTR-1 mRNA has the highest levels of expression and SSTR-5 has the lowest. This is in sharp contrast to the relative expression levels reported in the rat striatum. In adult Sprague–Dawley rat striatum there were relatively low levels of SSTR-1 mRNA expression (Bruno et al., 1993). In the CPu of adult Wistar rats, SSTR-1 mRNA was at background levels (Thoss et al., 1995). Similar to our findings in the mouse, SSTR-5 mRNA levels in the CPu of Wistar rats were also at low levels of expression (Thoss et al., 1995). In contrast, in the striatum of Sprague–Dawley rats SSTR-5 had the highest level of expression along with SSTR-3 (Bruno et al., 1993). These differences are likely related to strain and species differences, as well as to technical considerations concerning tissue isolation and mRNA quantification.
Interestingly, SSTR-3 mRNA was not detected in the CPu of 129P3/J mice whereas we found relatively abundant expression of this mRNA in the CPu of C57BL/6J mice, and others have reported relatively high levels of SSTR-3 mRNA in the rat CPu or striatum (e.g. Bruno et al., 1993; Thoss et al., 1995). Furthermore, SSTR-3 mRNA was not detected in the frontal cortex, hypothalamus, nucleus accumbens or a region containing both the substantia nigra and ventral tegmental area of 129P3/J mice, but was found to be expressed in of all those regions in C57BL/6J animals. Additional studies are required to determine if 129P3/J mice are indeed completely lacking in SSTR-3 mRNA expression. Further studies will also be required to determine the etiology of this deficit. In the absence of appropriate behavioral studies utilizing SSTR-3 knockout mice or specific SSTR-3 agonists or antagonists, it is difficult to determine the functional significance of the lack of SSTR-3 mRNA in 129P3/J mice.
SSTR-3 is localized in primary cilia of neurons throughout the CNS (Berbari et al., 2008; Handel et al., 1999). Studies using SSTR-3 knockout mice and specific SSTR-3 agonists and antagonists have shown that SSTR-3 in the hippocampus is critical for recall of object information and synaptic plasticity (Einstein et al., 2010). We did not examine SSTR-3 mRNA in the hippocampus; however compared to C57BL/6J mice, 129/J (now 129P3/J) mice showed increased escape latencies in distally and proximally cued Morris Water Maze tasks, (e.g. Montkowski et al., 1997) which are, at least partially, dependent on hippocampal function (e.g. Morris et al., 1982). These deficits in Morris Water Maze performance may be partially due to loss of SSTR-3 mediated signaling in these mice. However, it may also be related to poor visual acuity associated with the pink-eyed dilution allele carried by 129P3/Jmice (see e.g. Montkowski et al., 1997).
We also found significant strain differences in the expression of SSTR-2 and SSTR-4 mRNA. Levels of both were higher in the CPu of 129P3/J mice than in age-matched C57BL/6J counterparts. SSTR-2 has been suggested to mediate SRIF/dopamine/glutamate interactions. Mice with lifelong deletion (knockout) of the SSTR-2 gene show higher basal levels of glutamate and attenuated SRIF-induced release of dopamine and glutamate in the striatum (Allen et al., 2003). We have reported that, compared to C57BL/6J mice, 129P3/J mice show augmented levels of striatal dopamine following a single “binge” administration of cocaine (Zhang et al., 2001). It is possible that this strain difference is related to differential levels of striatal SSTR-2 receptors.
SSTR-2 knockout mice also show increased anxiety-like behavior and decreased levels of exploratory behaviors when stressed (Viollet et al., 2000). The report of lower levels of anxiety-like behavior in an elevated plus maze test in 129P3/J mice compared to C57BL/6J mice (Montkowski et al., 1997) might be related to differential expression of striatal SSTR-2.
It is difficult to know the functional consequence of differential expression of striatal SSTR-4 mRNA. It has been shown that hippocampal SSTR-4 are critical for the selection of memory strategies, mediating the switch between declarative, hippocampal-based memory and procedural, striatal-based memory strategies (Gastambide et al., 2009) which may be relevant to drug seeking or self-administration behavior. The SSTR-2 and -4 receptors interact in the mouse hippocampus (Cammalleri et al., 2006; Moneta et al., 2002). It is not clear whether this interaction is the result of direct coupling of the receptors (Cammalleri et al., 2006; Moneta et al., 2002) or a functional interaction (Gastambide et al., 2010). It also is not known whether such interactions occur in the striatum.
We identified a significant increase in the mRNA levels of SSTR-1 and SSTR-3 in the CPu as a result of exposure to heroin at the highest (20 mg/kg) dose, but not of SSTR-2, 4 or 5. It is important to emphasize that animals were sacrificed 24.5 h after the last conditioning session; therefore there was no heroin on board at the time of tissue collection. This suggests that heroin, at this dose, has a relatively long-term effect on SSTR-1 and 3 mRNA levels or that elevations in these mRNA levels are the result from acute withdrawal from heroin. The latter is unlikely however, since animals received a single daily dose of heroin, every other day for a total of eight days (four heroin injections (Schlussman et al., 2008)). Additionally, while somatic signs of heroin withdrawal were not monitored in this study, nor were they grossly apparent.
In the basal ganglia, SSTR-1 is thought to function as an inhibitory autoreceptor on somatostatinergic neurons (Thermos et al., 2006) and increased mRNA levels may represent a countermodulatory mechanism related to elevated levels of dopamine.
Unlike the other SSTRs, SSTR-3 is coupled to adenylate cyclase (Yasuda et al., 1992). In vitro, SRIF acting through SSTR-3 inhibits dopamine D1 receptor cAMP formation (Yasuda et al., 1992). The increase in SSTR-3 mRNA observed in this study may be related to a heroin-induced increase in dopaminergic tone and activity at the dopamine D1 receptor.
The lack of a heroin-induced effect on mRNA levels of SSTR-2 is, perhaps, unexpected. SSTR-2 has been shown to bind MOP-r agonists in vitro (e.g. Hatzoglou et al., 1995) and, also in vitro, SSTR-2 and MOP-r were shown to form functional heterodimers (e.g. Pfeiffer et al., 2002).
The C57BL/6J and 129P3/J strains of mice differ in their behavioral and neurochemical response to “binge” cocaine (Schlussman et al., 2003a,b; Zhang et al., 2001) and to opioids (Kest et al., 2002a,b; Schlussman et al., 2008) in a manner that suggests that the 129P3/J mice are relatively less sensitive to the effects of drugs of abuse. This examination of all the SSTR mRNA species demonstrates a significant strain-specific expression of SSTR mRNAs in the CPu. These strain-specific differences in mRNA expression may underlie some of the behavioral differences observed in these strains.
4. Experimental procedures
A total of 125 age-matched male mice (6 weeks old on arrival, Jackson Laboratory, Bar Harbor, ME), 55 C57BL/6J and 70 129P3/J were studied. All animals were individually housed in an environmentally controlled room dedicated to this study. Food and water were available ad lib and animals were allowed two weeks to acclimate prior to the start of the experiments. Mice of each strain were randomly assigned to one of six groups, each administered a specific dose (0, 1.25, 2.5, 5, 10 or 20 mg/kg) of heroin (3, 6 diacetyl-morphine HCl, obtained from NIH-NIDA). This study was approved by the Rockefeller University Institutional Animal Care and Use Committee and included provisions to minimize pain and discomfort.
Mice used in this study were froma study of heroin-induced conditioned place preference, which has been reported else-where (Schlussman et al., 2008). Animals in the 0mg/kg group received i.p. injections of isotonic saline on all days of the study. Animals in the other groups received i.p. injections of heroin or saline on alternate days for a total of 8 days (for details see Schlussman et al., 2008). Animals were sacrificed immediately following the testing session, 24.5 h following the last conditioning session, by decapitation following brief CO2 exposure (<20 s). Brains were rapidly removed and placed on ice. Brains were placed into a chilled rodent brain matrix (ASI Instruments, Warren, MI) and 1.0mm coronal sections were cut. The CPu, frontal cortex, hypothalamus, nucleus accumbens and a region containing the substantia nigra and ventral tegmental area were rapidly dissected, on ice, from the 1.0mm coronal sections under a stereoscope and homogenized in guanidine thiocyanate as previously described (Branch et al., 1992). RNA was isolated from homogenates of the CPu, frontal cortex and hypothalamus, with the RNAqueous system (Ambion [ABI], Austin TX) according tomanufacturer's instructions. RNA from the nucleus accumbens and the region containing both the substantia nigra and ventral tegmental area were isolated using acid phenolic extractions as previously described (Chomczynski and Sacchi, 1987) Following RNA isolation, all samples were treated with DNase (Turbo DNA-free™, Ambion [ABI], Austin, TX). The quantity and quality of RNA in each extract were determined using the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA).
cDNA was synthesized from each sample using the Super Script™ III first strand synthesis kit (Invitrogen, Carlsbad, CA). One µg of RNA from the CPu, frontal cortex and hypothalamus was used for reverse transcription. The entire RNA from the nucleus accumbens and the region containing both the substantia nigra and ventral tegmental area was used to synthesize cDNAs. All cDNAs were diluted 1:10 for real time PCR analysis.
Real time PCR analysis of the relative mRNA expression levels of SSTR-1–5 was conducted using commercially available primers and master mix (RT2 qPCR™ primer assays and RT2 Real Time™ SYBR® Green PCR Master Mix; SA Bioscience, Frederick, MD) according to manufacturer's directions in an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA).
All samples were assayed in duplicate.Water controls were included in each assay. Any sample with a cycle threshold (CT) greater than that of the water control or a CT of 35 or higher was not included in the analysis. All data were normalized to the expression level of GAPDH and reported as 2−ΔCT where ΔCT is the cycle threshold of the mRNA of interest minus the cycle threshold of GAPDH.
Data for each mRNA of interest were analyzed by two-way ANOVA, Strain×Dose, followed by Newman–Keuls post hoc analysis where appropriate. Any sample that was ≥2.5 standard deviations from the mean was considered an outlier and dropped from the analysis.
Acknowledgment
3, 6 diacetyl-morphine HCl was generously provided by NIH-NIDA Division of Drug Supply and Analytical Services. This work was supported by grants from NIH-NIDA (DA05130) and the Arcadia Charitable Trust to MJK.
Abbreviations
- CPu
caudate putamen
- SRIF
somatostatin (a.k.a. somatotropin release inhibiting factor)
- SSTR
somatostatin receptors
- NAc
nucleus accumbens
REFERENCES
- Allen JP, Hathway GJ, Clarke NJ, Jowett MI, Topps S, Kendrick KM, Humphrey PP, Wilkinson LS, Emson PC. Somatostatin receptor 2 knockout/lacZ knockin mice show impaired motor coordination and reveal sites of somatostatin action within the striatum. Eur. J. Neurosci. 2003;17:1881–1895. doi: 10.1046/j.1460-9568.2003.02629.x. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Kiyama H, Faull RL, Emson PC. Dopaminergic D1 and D2 receptor antagonists decrease prosomatostatin mRNA expression in rat striatum. Neuroscience. 1991;44:35–44. doi: 10.1016/0306-4522(91)90249-n. [DOI] [PubMed] [Google Scholar]
- Babbini M, Davis WM. Time–dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br. J. Pharmacol. 1972;46:213–224. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Riggan J, O'Toole LA. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:352–358. doi: 10.1007/BF02244932. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantitation of preproenkephalin mRNA levels in brain regions from male Fischer rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res. Mol. Brain Res. 1992;14:231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Breder CD, Yamada Y, Yasuda K, Seino S, Saper CB, Bell GI. Differential expression of somatostatin receptor subtypes in brain. J. Neurosci. 1992;12:3920–3934. doi: 10.1523/JNEUROSCI.12-10-03920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JF, Xu Y, Song J, Berelowitz M. Tissue distribution of somatostatin receptor subtype messenger ribonucleic acid in the rat. Endocrinology. 1993;133:2561–2567. doi: 10.1210/endo.133.6.8243278. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Cervia D, Dal Monte M, Martini D, Langenegger D, Fehlmann D, Feuerbach D, Pavan B, Hoyer D, Bagnoli P. Compensatory changes in the hippocampus of somatostatin knockout mice: upregulation of somatostatin receptor 2 and its function in the control of bursting activity and synaptic transmission. Eur. J. Neurosci. 2006;23:2404–2422. doi: 10.1111/j.1460-9568.2006.04770.x. [DOI] [PubMed] [Google Scholar]
- Castellano C, Filibeck L, Oliverio A. Effects of heroin, alone or in combination with other drugs, on the locomotor activity in two inbred strains of mice. Psychopharmacology (Berl) 1976;49:29–31. doi: 10.1007/BF00427467. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Reisine TD. Somatostatin regulates dopamine release in rat striatal slices and cat caudate nuclei. J. Neurosci. 1983;3:232–236. doi: 10.1523/JNEUROSCI.03-01-00232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Prado M, Malagon MM, Gracia-Navarro F, Castano JP. Dimerization of G protein-coupled receptors: new avenues for somatostatin receptor signalling, control and functioning. Mol. Cell. Endocrinol. 2008;286:63–68. doi: 10.1016/j.mce.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Einstein EB, Patterson CA, Hon BJ, Regan KA, Reddi J, Melnikoff DE, Mateer MJ, Schulz S, Johnson BN, Tallent MK. Somatostatin signaling in neuronal cilia is critical for object recognition memory. J. Neurosci. 2010;30:4306–4314. doi: 10.1523/JNEUROSCI.5295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Magnusson T, Carlsson A. Effect of intracerebroventricularly administered somatostatin on brain monoamine turnover. Brain Res. 1978;155:159–164. doi: 10.1016/0006-8993(78)90318-9. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Lepousez G, Viollet C, Loudes C, Epelbaum J, Guillou JL. Cooperation between hippocampal somatostatin receptor subtypes 4 and 2: functional relevance in interactive memory systems. Hippocampus. 2010;20:745–757. doi: 10.1002/hipo.20680. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Viollet C, Lepousez G, Epelbaum J, Guillou JL. Hippocampal SSTR4 somatostatin receptors control the selection of memory strategies. Psychopharmacology (Berl) 2009;202:153–163. doi: 10.1007/s00213-008-1204-x. [DOI] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Emson PC, Humphrey PP, Kendrick KM. Somatostatin potently stimulates in vivo striatal dopamine and gamma-aminobutyric acid release by a glutamate-dependent action. J. Neurochem. 1998;70:1740–1749. doi: 10.1046/j.1471-4159.1998.70041740.x. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Humphrey PP, Kendrick KM. Somatostatin induces striatal dopamine release and contralateral turning behaviour in the mouse. Neurosci. Lett. 2004;358:127–131. doi: 10.1016/j.neulet.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Hatzoglou A, Ouafik L, Bakogeorgou E, Thermos K, Castanas E. Morphine cross-reacts with somatostatin receptor SSTR2 in the T47D human breast cancer cell line and decreases cell growth. Cancer Res. 1995;55:5632–5636. [PubMed] [Google Scholar]
- Havlicek V, Rezek M, Friesen H. Somatostatin and thyrotropin releasing hormone: central effect on sleep and motor system. Pharmacol. Biochem. Behav. 1976;4:455–459. doi: 10.1016/0091-3057(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Johansson O, Hokfelt T, Elde RP. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience. 1984;13:265–339. doi: 10.1016/0306-4522(84)90233-1. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: a survey of 11 inbred mouse strains. Pharmacol. Biochem. Behav. 2002a;73:821–828. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic-morphine physical-dependence. Neuroscience. 2002b;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Radke JM, Vincent SR. The effects of cysteamine on dopamine-mediated behaviors: evidence for dopamine–somatostatin interactions in the striatum. Pharmacol. Biochem. Behav. 1986;24:1707–1714. doi: 10.1016/0091-3057(86)90509-5. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection fromthe cerebral cortex to the striatumin the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC, Belknap JK. Genetic correlates of morphine withdrawal in 14 inbred mouse strains. Drug Alcohol Depend. 2009;99:123–131. doi: 10.1016/j.drugalcdep.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneta D, Richichi C, Aliprandi M, Dournaud P, Dutar P, Billard JM, Carlo AS, Viollet C, Hannon JP, Fehlmann D, Nunn C, Hoyer D, Epelbaum J, Vezzani A. Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice. Eur. J. Neurosci. 2002;16:843–849. doi: 10.1046/j.1460-9568.2002.02146.x. [DOI] [PubMed] [Google Scholar]
- Montkowski A, Poettig M, Mederer A, Holsboer F. Behavioural performance in three substrains of mouse strain 129. Brain Res. 1997;762:12–18. doi: 10.1016/s0006-8993(97)00370-3. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Noe BD, Spiess J. Evidence for biosynthesis and differential post-translational proteolytic processing of different (pre)prosomatostatins in pancreatic islets. J. Biol. Chem. 1983;258:1121–1128. [PubMed] [Google Scholar]
- Oliverio A, Castellano C. Behavioral effects of opiates: a pharmacogenetic analysis. Curr. Dev. Psychopharmacol. 1981;6:45–64. doi: 10.1007/978-94-011-8123-5_2. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Patel YC, Greenwood MT, Panetta R, Demchyshyn L, Niznik H, Srikant CB. The somatostatin receptor family. Life Sci. 1995;57:1249–1265. doi: 10.1016/0024-3205(95)02082-t. [DOI] [PubMed] [Google Scholar]
- Pfeiffer M, Koch T, Schroder H, Laugsch M, Hollt V, Schulz S. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem. 2002;277:19762–19772. doi: 10.1074/jbc.M110373200. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N. Engl. J. Med. 1983a;309:1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts) N. Engl. J. Med. 1983b;309:1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Rezek M, Havlicek V, Leybin L, Pinsky C, Kroeger EA, Hughes KR, Friesen H. Neostriatal administration of somatostatin:differential effect of small and large doses on behavior and motor control. Can. J. Physiol. Pharmacol. 1977;55:234–242. doi: 10.1139/y77-034. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic“binge” cocaine in C57BL/6J and 129/J mice. Pharmacol. Biochem. Behav. 2003a;75:123–131. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Yuferov V, LaForge KS, Ho A, Kreek MJ. Acute ‘binge’ cocaine administration elevates dynorphin mRNA in the caudate putamen of C57BL/6J but not 129/J mice. Brain Res. 2003b;974:249–253. doi: 10.1016/s0006-8993(03)02561-7. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Hsu NM, Allen JM, Ho A, Kreek MJ. Heroin-induced locomotor activity and conditioned place preference in C57BL/6J and 129P3/J mice. Neurosci. Lett. 2008;440:284–288. doi: 10.1016/j.neulet.2008.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermos K, Bagnoli P, Epelbaum J, Hoyer D. The somatostatin sst1 receptor: an autoreceptor for somatostatin in brain and retina? Pharmacol. Ther. 2006;110:455–464. doi: 10.1016/j.pharmthera.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Thoss VS, Perez J, Duc D, Hoyer D. Embryonic and postnatal mRNA distribution of five somatostatin receptor subtypes in the rat brain. Neuropharmacology. 1995;34:1673–1688. doi: 10.1016/0028-3908(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Brown M. Regulatory peptides of the hypothalamus. Annu. Rev. Physiol. 1977;39:473–527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]
- Vecsei L, Widerlov E. Preclinical and clinical studies with somatostatin related to the central nervous system. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1990;14:473–502. doi: 10.1016/0278-5846(90)90003-y. [DOI] [PubMed] [Google Scholar]
- Viollet C, Vaillend C, Videau C, Bluet-Pajot MT, Ungerer A, L'Heritier A, Kopp C, Potier B, Billard J, Schaeffer J, Smith RG, Rohrer SP, Wilkinson H, Zheng H, Epelbaum J. Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur. J. Neurosci. 2000;12:3761–3770. doi: 10.1046/j.1460-9568.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol. Learn. Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Weiss LT, Chesselet MF. Regional distribution and regulation of preprosomatostatin messenger RNA in the striatum, as revealed by in situ hybridization histochemistry. Brain Res. Mol. Brain Res. 1989;5:121–130. doi: 10.1016/0169-328x(89)90003-x. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol. Learn. Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: relationship to cortical inputs and role of behavioural context. Eur. J. Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci. Biobehav. Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Wu G, Stein EA, Li SJ. GABAergic mechanisms of heroin-induced brain activation assessed with functional MRI. Magn. Reson. Med. 2002;48:838–843. doi: 10.1002/mrm.10282. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Rens-Domiano S, Breder CD, Law SF, Saper CB, Reisine T, Bell GI. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J. Biol. Chem. 1992;267:20422–20428. [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, LaForge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schlussman SD, Ho A, Kreek MJ. Effect of acute binge cocaine on levels of extracellular dopamine in the caudate putamen and nucleus accumbens in male C57BL/6J and 129/J mice. Brain Res. 2001;923:172–177. doi: 10.1016/s0006-8993(01)03032-3. [DOI] [PubMed] [Google Scholar]