Abstract
Visual signal transduction takes place on the surface of flat membrane vesicles called photoreceptor discs, which reside inside the light-sensitive outer segment organelle of vertebrate photoreceptor cells. While biochemical studies have indicated that discs are built with a handful of highly specialized proteins, proteomic studies have yielded databases consisting of hundreds of entries. We addressed this controversy by employing protein correlation profiling which allows identification of unique components of organelles that can be fractionated but not purified to absolute homogeneity. We subjected discs to sequential steps of fractionation and identified the relative amounts of proteins in each fraction by label-free quantitative mass spectrometry. This analysis demonstrated that the photoreceptor disc proteome contains only eleven components, which satisfy the hallmark criterion for being unique disc-resident components: the retention of a constant molar ratio among themselves across fractionation steps. Remarkably, one of them is PRCD, a protein whose mutations have been shown to cause blindness, yet cellular localization remained completely unknown. Identification of PRCD as a novel disc-specific protein facilitates understanding its functional role and the pathobiological significance of its mutations. Our study provides a striking example how protein correlation profiling allows a distinction between constitutive components of cellular organelles and their inevitable contaminants.

Keywords: Photoreceptor disc proteome, quantitative proteomics, PRCD, protein correlation profiling
INTRODUCTION
Photoreceptors are specialized neurons which generate electrical signals in response to light stimuli. In vertebrates, photoreceptors are represented by two cell types, rods and cones, with the former dominating in most mammalian retinas.1 Rods and cones are characterized by a high degree of compartmentalization, with all biochemical reactions involved in generating light responses being confined to their ciliary outer segment organelle. The outer segment is a cylinder filled with flat membrane vesicles called photoreceptor discs (see2, 3 for recent reviews of outer segment structure). This morphological organization is thought to play two functional roles. First, discs form hundreds of membrane layers tightly packed with visual pigment, an arrangement ensuring highly efficient photon capture by photoreceptor cells. Second, discs provide vast membrane surfaces for housing phototransduction proteins responsible for propagating and amplifying visual signals.
Rod outer segments can be easily detached from the rest of the cell and obtained in preparative amounts sufficient for biochemical experiments;4, 5 discs can be further purified from these preparations, following simple membrane fractionation procedures. This ease of membrane purification facilitated progress in understanding visual signal transduction and placed photoreceptors among the most productive model systems for studying general principles of cell signaling.6 Several decades of intensive studies have identified a number of outer segment-specific proteins, those involved in visual signaling and those responsible for maintaining the outer segment structure. However, systematic analysis of outer segment and disc proteomes has become possible only in recent years, following advances in mass spectrometry.
Proteomes of rod outer segments have been identified in three independent studies,7–9 with the latter two addressing the proteomes of photoreceptor discs as well. These studies reported very large databases, including many hundreds of individual proteins. Notably, even the smaller proteomes of photoreceptor discs contained over 200 entries. These results do not appear intuitive: the outer segment is a highly-specialized organelle fulfilling a single physiological function. Furthermore, biochemical studies consistently stressed that photoreceptor discs have a relatively simple protein composition. One plausible explanation for such a discrepancy is that membrane preparations analyzed in these proteomic studies were contaminated by other membranes present in retinal homogenates. This explanation is consistent with each published proteome containing multiple entries for proteins normally confined to mitochondria, nuclei, endoplasmic reticulum, or synaptic terminals, rather than outer segments. We should stress that these contaminants were identified not because outer segments or discs analyzed in these studies lacked traditionally acceptable biochemical purity, but due to the very high sensitivity of modern mass spectrometry techniques. Therefore, the actual number of proteins residing specifically in photoreceptor outer segments and discs is likely to be significantly smaller than reported, and non-traditional methodologies should be employed for their reliable identification.
In this study, we revisited the photoreceptor disc proteome by utilizing a mass spectrometry approach known as protein correlation profiling.10 We subjected photoreceptor discs to three sequential purification steps, identified protein compositions of each preparation, and determined the relative amounts of identified proteins across disc preparations using label-free quantitative proteomics. We reasoned that the abundance of unique disc-resident proteins must remain constant across all disc preparations, whereas the abundance of contaminating or non-unique proteins should decrease at each purification step. The total number of proteins identified in disc fractions ranged from 1000 to over 300 hundred. However, only eleven satisfied the hallmark criterion of being unique disc-resident components. Ten of these proteins have been well-characterized in previous studies and can be divided into three groups: phototransduction proteins, lipid flippases, and proteins supporting the structure of disc rims. The eleventh member of this group is PRCD (Progressive Rod and Cone Degeneration) – a protein linked to retinal degenerations in humans and dogs,11, 12 whose localization in the retina was previously unknown.
MATERIALS AND METHODS
Antibodies
Polyclonal anti-PRCD antibody was generated in rabbit against the peptide: CDGTVVGSGSDTDLQSTGREKGPVK representing the mouse PRCD sequence. Polyclonal anti-peripherin antibody was generated in sheep against the peptide: CKGNQVEAEGEDAGQAPAAG representing the bovine peripherin sequence. Each antibody was affinity-purified using the corresponding peptides attached to SulfoLink beads (Thermo Scientific) via the N-terminal cysteine residues.
Isolation of Osmotically Intact Rod Outer Segments from Bovine Retinas
All procedures were performed under dim red light illumination at 4°C, following the strategy described in13 with modifications. 100 frozen retinas (T.A. & W.L. Lawson Co., Lincoln, NE) were thawed, re-suspended in 180 ml of the Ringer’s solution (10 mM HEPES, 130 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, and 0.02 mM EDTA pH 7.4) containing 20% OptiPrep (Axis-Shield PoC AC, Oslo). Outer segments were detached from the retinas by swirling in 1 liter Erlenmeyer flask and separated from retinal debris by centrifugation at 3,700g for 6 min. The supernatant was diluted with an equal volume of Ringer’s, and rod outer segments were pelleted by centrifugation at 6,300g for 8 min. The pellet was re-suspended in Ringer’s, applied on the 12/20% step gradient of OptiPrep and centrifuged for 1 h in a SW-28 rotor at 27,000 rpm. Rod outer segments were collected from the 12/20% OptiPrep interphase and used to prepare disc membranes.
Photoreceptor Disc Purification
The overall disc purification scheme is illustrated in Figure 1. The initial disc preparation (D1) was obtained from bovine rod outer segments as described in4 by subjecting rod outer segments to osmotic shock in water and floating discs on the 6% Ficoll solution in water. Discs were next enriched on a 1–7% continuous gradient of Ficoll in water, using a SW-28 rotor at 27,000 rpm for 2 h. Discs migrated as a pink band located ~⅓ from the top of the gradient just above another diffused whitish band representing microsomal contaminations. A small grayish pellet of additional contaminating material was sedimented at the bottom of the tube. This pink band was collected in the dark, diluted with 40 ml buffer containing 10 mM HEPES, 120 mM NaCl, 8 mM MgCl2 (pH 7.5) (this buffer was used at all subsequent disc purification steps) and spun down at 20,000 rpm for 20 min. To remove the residual Ficoll, the membrane pellet was washed in 45 ml buffer. The pellet was re-suspended in 1–2 ml buffer and was defined as D2. The final disc preparation (D3) was obtained by immunoprecipitation of D2 with antibodies against the disc-specific protein, peripherin. D2 membranes containing 450 μg of total protein were incubated overnight with 150 μg anti-peripherin antibodies, followed by pull-down with Protein A/G magnetic beads (200 μl the 50/50 slurry; Thermo Scientific). The slurry was gently shaken for 2 h at 20°C and the beads were collected using a magnet, washed 3 times with the disc purification buffer and once with the same buffer containing 0.5 M NaCl. To maximize protein recovery from the beads, we solubilized discs attached to the beads with SDS-PAGE sample buffer (100 μl of 0.125 M Tris-HCl (pH 6.8), 2% SDS and 10% glycerol). Total protein concentration in all disc fractions was determined using a BCA assay (Thermo Scientific).
Figure 1.

A schematic illustration of the photoreceptor disc purification procedure employed in this study. D1 preparation was obtained from osmotically shocked bovine rod outer segments; D2 was obtained by subjecting D1 to a centrifugation step on a linear Ficoll gradient; D3 was obtained by immunoprecipitating D2 with anti-peripherin antibodies. Discs and are marked in red. The number of proteins confidently identified by mass spectrometry in each disc preparation is indicated at the bottom.
Measurements of Protein Concentration
Rhodopsin concentration in D1 and D2 disc preparations was determined by difference spectroscopy at 500 nm before and after sample bleaching using the molar extinction coefficient of 40,500 M−1·cm−1.14 Because rhodopsin in D3 was denatured by SDS, its concentration could not be measured spectrophotometrically and instead was quantified by Coomassie-staining rhodopsin bands alongside with rhodopsin standards. The amount of PRCD in D2 preparation was determined by quantitative Western blotting. The synthetic full-length PRCD standard peptide was purchased from Alpha Diagnostic. The peptide concentration was determined spectrophotometrically using the calculated molar extinction coefficient of 5,580 M−1·cm−1 at 280 nm Various amounts of standards were run on the same gel alongside D2 disc samples containing known amounts of rhodopsin.
Sample Preparation and LC-MS/MS Analysis
Aliquots from disc preparations containing 15 μg rhodopsin were solubilized in 2% SDS, 100 mM Tris-HCl (pH 6.8) and 100 mM DTT and subjected to tryptic hydrolysis using the filter-aided sample preparation (FASP) protocol as described in.15 Digests were vacuum-dried and dissolved in 60 μl of 0.1% trifluoroacetic acid containing 3% acetonitrile. 2 μl aliquots of tryptic digests were analyzed by LC-MS/MS using a nanoAcquity UPLC system coupled to a Synapt G2 HDMS mass spectrometer (Waters Corp, Milford, MA). Peptides were initially trapped on a 180 μm × 20 mm Symmetry C18 column (at the 5 μl/min flow rate for 5 min in 99.9% water, 0.1% formic acid). Peptide separation was then performed on a 100 μm × 100 mm column filled with the 1.7 μm C18 BEH resin (Waters) using the 5 to 32% acetonitrile gradient with 0.1% formic acid for 2 h at the flow rate of 0.3 μl/min at 35°C. Eluting peptides were sprayed into the ion source of Synapt G2 using the 10 μm PicoTip emitter (Waters) at the voltage of 3.0 kV.
For each disc preparation, we conducted three data-independent (MSE) analyses used for simultaneous peptide identification and quantification. MSE runs were performed at the cycle time of 1.5 sec, alternating between the low collision energy (6 V) and high collision energy ramps (20 to 35 V). In order to increase the peptide identification rate, MSE analyses were complemented with additional LC-MS/MS runs conducted in the data-dependent analysis (DDA) mode. For the latter, proteins from each disc preparation were separated by SDS-PAGE, the gel was cut into 12 slices, and each slice was subjected to trypsin in-gel digestion. Extracted peptides were analyzed by LC-MS/MS, using 0.8 sec MS scans of eluting peptides followed by the MS/MS acquisition of the top four ions with charges greater than one. An isolation window for each ion was 3 Da, maximum acquisition time per precursor was 1.5 sec and the dynamic exclusion for each precursor was 90 sec within the mass window of 1 Da. Complete mass spectrometry analysis was performed with two independently obtained biochemical preparations of D1, D2 and D3. The number of technical repeats was three.
DDA Protein Identification
Initial protein identification in disc fractions was performed for the DDA data. Raw data were exported to the ProteinLynx Global Server (PLGS 2.5.1) and searched against the International Protein Index (IPI) bovine database (v. 3.67) using the Mascot search engine (v. 2.2; Matrix Science) with a concatenated reversed decoy database. The final database used for this search contained 34,283 protein entrees. Precursor ion mass tolerance was 10 ppm and product ion tolerance – 0.2 Da. Enzyme specificity was set to trypsin with one missed cleavage allowed. Carbaminomethyl (Cys) was selected as a fixed modification, and oxidation (Met), acetylation (protein N-terminus) and deamidation (Asp, Gln) were selected as variable modifications. Peptide filtering and protein assembly were conducted by the Scaffold 3.3.6 (Proteome Software Inc.). Protein false discovery rate (FDR) was set to 0.1% and peptide FDR to 1.8%. Parsimony rules were applied to generate a minimal list of proteins satisfying these criteria. Proteins containing identical sets of peptides were annotated as a protein group named after a protein with the highest probability and the largest associated number of spectra. A list of annotated peptides with their Mascot scores is reported in Supplemental Table 2.
Label-Free Protein Quantification by LC/MS
Protein quantification followed the strategies described in our previous publication,16 except that we used the Progenesis-LC-MS software (Nonlinear Dynamics) rather than Rosetta Elucidator. For robust peak detection and alignment of individual peptides across all MSE runs representing disc fractions from each purification set, we performed automatic alignment and manual correction of ion chromatography peaks representing the same mass/retention time features. To perform peptide assignment to the features, we utilized both DDA and MSE data. For DDA acquisition files, mgf searchable files were produced in Progenesis LC-MS and searches were submitted to and then exported from the Mascot search engine. For MSE data, ProteinLynx Global Server (PLGS) 2.5.1 (Waters) was used to generate searchable files that were submitted to the IdentityE search engine (Waters) and then imported back into Progenesis LC-MS. Both DDA and MSE data were searched against the sub-database of 1017 proteins identified in the D1 disc fraction by the DDA analysis.
Relative abundance of each feature was next calculated across individual MSE runs. To account for small variations in experimental conditions and absolute amounts of protein material in individual MS runs, integrated peak area for each identified peptide was corrected using the automatic Progenesis LC-MS data normalization algorithm. This algorithm corrected peptide peak areas by the factor calculated from the mean of the abundance ratio among the top thirty peptides of ABCA4 (a known disc-resident protein) measured in the same run. Under-cleaved and modified ABCA4 peptides were excluded from this calculation. We chose ABCA4 over rhodopsin because rhodopsin produced only two well-quantifiable peptides, whereas ABCA4 produced over thirty. Relative amount of each protein in each disc preparation was next calculated as a sum of normalized peak areas representing all peptides assigned to a given protein. Conflicting peptides were excluded from calculations. Protein quantitation data are summarized in the Supplementary Table 3.
Statistical Analysis
Protein ranking analysis
To determine statistical significance between the top eleven and the rest of the proteins ranked in Figure 3, we compared proteins #12-335 with the mean of the proteins #1-11 using a linear mixed effects model, which accounted for correlation between the 6 repeated measurements and for variation of proteins #1-11 around the mean of this group. The overall test was significant with p<0.0001. Each of the individual comparisons was also significant with p<0.0001. We used the Bonferroni adjustment to account for 324 individual tests and found the adjusted p-values still being significant at the 0.05 level (adjusted p=0.0324).
Figure 3.
335 proteins identified in D3 ranked according to their relative abundances between the D3 and D1 disc preparations (mean±s.d.; n=6). The top 11 entries are listed in the figure in their ranking order. The ranking order within the “group of five” is: TMEM30A, PDE6α, PDE6β, Gαt and prominin-1. The specific values of D3/D1 ratios for all proteins are listed in Supplementary Table 4.
Self-organizing maps (SOM) calculation
The amounts of proteins from each analytical and biological replicate were utilized as inputs for statistical analysis, using Spotfire DecisionSite with Functional Genomics Package v.9.1.2. The data were log2 scaled in order to obtain a near-normal distribution of protein quantities. The analysis was performed separately for each independently purified set of disc preparations and the amount of each protein was z-score normalized. The aggregate z-score normalized data were then directly utilized for SOM calculation. A 2×2 matrix was calculated using a linear learning function with 12,500 training steps. The matrix was restricted to four clusters due to the limited number of hypothetical protein profiles in the data; essentially, either a protein should decrease in abundance with purification or it should not.
Immunofluorescence Microscopy
Immunofluorescence detection of PRCD was performed as in.17 Briefly, posterior eyecups from the CD-1 mice (Charles River) were fixed in 4% paraformaldehyde in PBS for 1 h at 22°C and embedded in 4% agarose (Invitrogen). 100 μm agarose sections were collected, blocked in 5% goat serum in PBS with 0.5% Triton X-100 for 1 h, incubated overnight in the primary antibody diluted in blocking solution, washed three times in PBS with 0.5% Triton X-100, and stained for 2 h with secondary rabbit antibodies conjugated to Alexa Fluor 488 or 594 (Invitrogen). WGA Alexa Fluor 594 (Invitrogen) was used to stain photoreceptor outer segments, PNA Alexa Fluor 488 (Invitrogen) was used to stain cones, and 10 mg/ml Hoechst 33342 (Invitrogen) to label nuclei. All samples were mounted with Fluoromount (EMS) under glass coverslips and visualized using a Nikon Eclipse 90i microscope and a C1 confocal scanner controlled by EZ-C1, version 3.10, software (Nikon). Manipulation of images was limited to adjusting the brightness level, image size, and cropping using either EZ-C1 v 3.10 Viewer or Adobe Photoshop.
Quantitative Western Blotting
PRCD quantification in the D2 disc preparation was determined essentially as described in.18 Disc aliquots containing known amounts of rhodopsin were analyzed alongside with synthetic full-length bovine PRCD peptide (purchased from Alpha Diagnostic Int.). The peptide concentration was determined spectrophotometrically using the molar extinction coefficient ε280=5,580. PRCD bands were visualized with our anti-PRCD antibody and secondary goat anti-rabbit antibodies conjugated with Alexa Fluor 680 (Invitrogen). Protein bands quantification was performed using the Odyssey Infrared Imaging System (LI-COR Biosciences).
RESULTS AND DISCUSSION
Protein Composition of Disc Membranes Subjected to Multiple Purification Steps
The goal of this study was to identify the proteome of transmembrane or otherwise tightly membrane-associated proteins uniquely residing in photoreceptor discs. The initial disc preparation (D1; Figure 1) was obtained by osmotically shocking rod outer segments, followed by flotation of sealed disc membranes in 6% Ficoll – the methodology used for decades to purify discs in biochemical studies.4 These discs were subjected to two additional fractionation steps designed to remove or reduce their contamination by other organelles and membrane fragments. We then subjected D1 to centrifugation on a linear 1–7% Ficoll density gradient, which produced a distinct band of pink-colored discs just above another white-colored band containing membrane material of slightly higher density. Discs collected from this gradient (preparation D2) were then precipitated by antibodies against peripherin, a protein uniquely located at disc rims, to yield the final disc preparation (D3).
Proteins from each disc preparation were subjected to trypsin digestion, followed by peptide identification by LC/MS-MS using the data-dependent analysis (DDA) workflow described in Materials and Methods. Proteins and their corresponding peptides, confidently identified in each disc fraction, are summarized in Supplementary Tables 1 and 2, respectively. Consistent with increasing disc purity, the number of confidently identified proteins (protein FDR<0.1%, minimum 2 peptides) was 1017, 526 and 335 in D1, D2 and D3, respectively. However, even D3, likely the purest photoreceptor disc membranes analyzed to date, contained a large number of proteins residing in other membrane organelles, such as mitochondria, synapses, or ER. This convinced us that conventional membrane purification techniques are unlikely to ever allow removing disc contaminants below the detection level of modern mass spectrometers.
Protein Correlation Profiling Across Disc Preparations of Various Purity
A powerful alternative approach to analyzing multi-protein complexes or organelles that can be fractionated but not purified to homogeneity is protein correlation profiling. This approach was introduced to identify resident components of centrosomes by analyzing the relative protein compositions of sucrose gradient fractions containing these organelles.10 The method was subsequently expanded for protein assignment to other organelles,19–21 and additional applications such as conducting subcellular proteomic profiling of a layered tissue.16
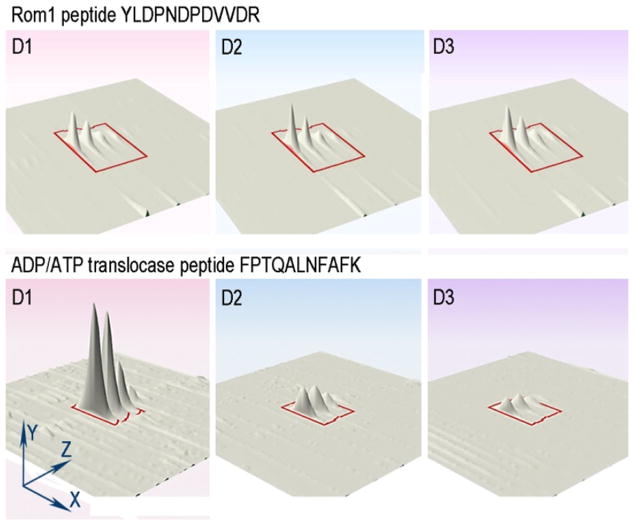
In the context of the disc proteome, we reasoned that unique disc-resident proteins should remain at a constant molar ratio among themselves across the D1–D3 preparations, whereas the relative content of contaminating proteins should decrease as disc purity increases. We illustrate this principle in Figure 2, which shows how the quantities of representative peptides from either resident or contaminating protein vary across the disc preparations. The upper panel of Figure 2 shows that the relative amount of a peptide from the disc-specific protein Rom1 remains nearly unchanged across disc preparations. In contrast, the abundance of another peptide representing the ADP/ATP translocase, a contaminating protein residing in the inner mitochondrial membrane, decreases at each disc purification step (Figure 2; lower panel).
Figure 2.
A 3D graphic representation of ion chromatograms obtained for peptides from a putative disc-resident protein (Rom1; top) and a putative impurity (mitochondrial ADP/ATP transporter; bottom) across D1–D3 disc preparations. The images are generated by the Progenesis LC-MS software. The axes are: X, time; Y, ion intensity; Z, mass. The red contour lines represent the isotope cluster area used for calculating the integrated ion intensity for each peptide.
Assuming that proteins absent from the purest D3 preparation cannot represent unique constitutive disc components, we devoted the subsequent quantitative analysis to 335 proteins identified in D3. The relative amount of each protein in each disc preparation was calculated as a sum of ion intensities from the LC/MS runs for all peptides confidently assigned to this protein (see Materials and Methods and ref.16 for details) and normalized by the amount of disc-resident protein ABCA4 (which produced at least 36 quantifiable peptides). To maximize our chances to identify minor disc components, we did not restrict this analysis to any minimal number of quantifiable peptides since all proteins in this group have been already identified by DDA with high stringency.
Once the relative quantities of all 335 proteins were calculated (Supplementary Table 3), we ranked them based on their molar ratios between the most pure (D3) and the least pure (D1) disc preparations (Figure 3). The coefficient of variation for the entire dataset (an average of the standard deviation-to-mean ratios for all proteins) was 23.7%. Remarkably, only eleven proteins (listed in Figure 3) yielded the normalized D3/D1 ratio within this variation range from the value of 1 and, therefore, are disc-resident protein candidates. The difference between the D3/D1 ratio of these eleven proteins and any other of the remaining 324 proteins was highly statistically significant (p<0.0001; see Material and Methods for details of statistical analysis). Notably, five proteins outside these top eleven formed a distinct ranking group with a D3/D1 ratio of 0.61–0.71. The identities of proteins forming the “top eleven” and the “group of five” will be discussed in the next section.
An alternative statistical analysis was to correlate the relative behavior of the 335 proteins across all disc preparations, using a Self-Organizing-Maps (SOM) algorithm originally designed to find proteins which have a similar expression pattern within a dataset. Each protein was assigned to a group with other proteins which exhibit similarity in relative abundance across all individual mass spectrometry experiments (Figure 4). As expected for the majority of proteins, three of the four SOM clusters displayed patterns of gradual decrease from D1 to D3. The small cluster which did not follow this rule (cluster #3 in Figure 4) contained nine proteins annotated in the margin to the left. All of them belong to the top eleven group established by the protein ranking algorithm in Figure 3.
Figure 4.
Self-Organizing Maps (SOM) algorithm used to group all 335 proteins found in the purest disc fraction (D3) according to the patterns of their co-purification in D1, D2 and D3 preparations. Details of the statistical analysis and data normalization are described in Materials and Methods. Each point on the X-axis represents data from a single technical repeat of the LC/MS-MS experiment; prep #1 and prep #2 refer to two independently fractionated disc preparations. Each protein was allowed to belong to a single cluster of the 2×2 SOM matrixes. The identities of proteins which did not trend to decrease with purification stages (D1–D3) are indicated next to their unique cluster.
Protein Components of the Photoreceptor Disc
Ten out of eleven proteins with the highest D3/D1 ratio represent previously characterized disc proteins (Figure 5). Six of them are critical components of the phototransduction pathway (see22–24 for reviews on structure and function of phototransduction proteins). They include: the visual pigment rhodopsin; retinal guanylate cyclase isoforms 1 and 2 (RetGC1&2) responsible for the synthesis of cGMP which is the second messenger in phototransduction; three proteins comprising the RGS9·Gβ5·R9AP GTPase activating complex for transducin. Interestingly, RGS9 and Gβ5 displayed the lowest values of the D3/D1 ratio in this group and did not appear in the disc protein SOM cluster. Unlike all other proteins in this group, RGS9 and Gβ5 do not contain transmembrane domains and instead are tethered on the disc surface by the membrane anchor R9AP. Thus, it is conceivable that a small RGS9·Gβ5 fraction is lost from the membranes upon disc purification. This example suggests that protein ranking analysis is a more informative method for analyzing results of protein correlation profiling than SOM by providing a continuous trend of protein behavior vs. dividing them into large groups with sharp thresholds defining each cluster.
Figure 5.

Cartoon representation of the 11 unique disc-resident proteins.
It is important to stress that several other phototransduction proteins contain neither transmembrane domains nor transmembrane anchor proteins and are instead attached to discs via lipid modifications. These proteins could be readily washed from the discs upon multiple steps of purification (e.g.25), which explains why they display low values of the D3/D1 ratio. For example, farnesylated phototransduction proteins, transducin βγ-subunit and rhodopsin kinase, have ratios between 0.33 and 0.38. Somewhat unique in this regards is cGMP phosphodiesterase (PDE6), whose α- and β-subunits belong to the “group of five” in Figure 3. This is because each of these tightly associated subunits has its own isoprenoid modification,26 making the membrane association of the PDE6 holoenzyme stronger than that of any singly-lipidated proteins.
Another lipidated phototransduction protein found in the “group of five” is the acylated α-subunit of transducin Gαt. This result is not intuitive because the membrane affinity of Gαt is significantly lower than that of PDE6 or even its cognate βγ-subunit.27–30 We believe that such an unexpected Gαt behavior is explained by a fraction of this protein being tightly associated with RGS9·Gβ5·R9AP.31 While the free Gαt present in D1 could be readily washed away, the Gαt associated with RGS9·Gβ5·R9AP is expected to remain on the discs upon their fractionation, thereby positioning Gαt in the intermediate protein group on the ranking plot.
The structural proteins found in the top eleven are peripherin (also known as peripherin-2 or rds protein) and rom-1. These proteins form large hetero-oligomeric complexes responsible for maintaining the curvature of disc edges (reviewed in32–34). In addition, peripherin was shown to possess membrane fusogenic activity in model systems,35 which suggest its potential involvement in disc morphogenesis.
The last two known proteins found in top eleven entries are the lipid flippases ABCA4 and ATP8A2. ABCA4 is responsible for transporting all-trans-retinol and its phosphatidylethanolamine conjugates from the intradiscal to the cytoplasmic leaflet of the disc membrane.36, 37 This process is thought to play a critical role in detoxification of disc membranes from compounds that may poison retinal pigment epithelium, the cell type performing phagocytosis of photoreceptor outer segment tips.38, 39 ATP8A2 is a relatively new member of the P-type ATPase family localized in disc membranes.40 It is thought to be involved in the ATP-dependent translocation of amino-phospholipids across disc membranes and in maintaining the phosphatidylserine asymmetry across the membrane, which could be important for normal phototransduction. Interestingly, ATP8A2 in photoreceptor discs exist as a stoichiometric complex with TMEM30A, a protein responsible for correct folding, trafficking and regulation of ATP8A2 activity.41 Yet, TMEM30A was found in the “group of five” (D3/D1=0.71), rather than in the top eleven as ATP8A2. This result is, in fact, predictable from the difference in cellular expression patterns of these proteins41: while ATP8A2 is found exclusively in photoreceptor outer segments, TMEM30A is present in both outer segments and other photoreceptor compartments and therefore is not a disc-unique protein.
The last protein to mention is prominin-1, which belongs to the “group of five”. Prominin-1, a transmembrane protein thought to be involved in the biogenesis of newly formed basal discs.42 It is confined exclusively to discs located at the outer segment base,43 which suggests that our procedure for disc fractionating is biased for enrichment of mature vs. nascent discs.
PRCD is a Novel Disc-Specific Protein
The eleventh protein satisfying the most stringent criteria for being an integral part of photoreceptor discs is PRCD (progressive rod-cone degeneration) – a protein linked to retinal degenerations in humans and dogs11, 12 whose intracellular localization and cellular function have not previously been addressed. The Cys2Tyr mutation of the PRCD gene is associated with blindness in 18 different dog breeds and a human patient.11 Recently, another mutation in PRCD (Arg22Ter) was shown to cause retinitis pigmentosa in 18 patients.12 PRCD contains only 54 amino acids, with a putative N-terminal α-helix that was proposed to span the disc membrane.11
To confirm that PRCD indeed resides in photoreceptor discs, we generated polyclonal antibodies against the PRCD C-terminus and conducted immunohistochemical detection of this protein in the mouse retina (Figure 6). PRCD immunostaining nearly completely overlapped with that of the lectin, WGA, decorating primarily rod outer segments. We next interrogated whether PRCD is also expressed in the outer segments of cones, as may be predicted from the disease phenotype affecting both photoreceptor types. Retina cross-sections were stained for PRCD and another lectin, PNA, which decorates primarily cone inner segments. The images in Figure 6 demonstrate strong PRCD staining in cone outer segments, which appears as an extension of the PNA-positive cone inner segment structures.
Figure 6.
Localization of PRCD to the outer segments of rods and cones. Retinal cross-sections were co-stained with the anti-PRCD antibody and WGA (upper panels) or the anti-PRCD antibody and PNA (lower panels). Cone outer segments are marked by yellow arrowheads. Nuclei were counter-stained with Hoechst (blue). To the left are schematics of rod and cone cells, illustrating the relative positions of their outer segments (OS) in mouse retinal cross-sections.
We next determined the amount of PRCD in bovine photoreceptor discs (D2) by quantitative Western blotting, using a synthetic PRCD peptide standard to obtain a calibration curve (Figure 7). These measurements revealed that the molar ratio between PRCD and rhodopsin is 1:290±40 (SD), which is on the same order as the corresponding ratios for PDE644 and the members of the GTPase activating complex.45
Figure 7.
Quantification of PRCD in bovine photoreceptor discs. D2 aliquots containing indicated amounts of rhodopsin were separated by SDS-PAGE alongside indicated amounts of PRCD peptide standards and immunoblotted using anti-PRCD antibody (top). The calibration curve is shown below the blot.
The Detection Limit of Protein Correlation Profiling
A critical question regarding the completeness of the disc-specific proteome is the detection limit of protein correlation profiling methodology. Our study identified each previously known unique disc-resident protein, including the least abundant ATP8A2, guanylate cyclase 1 and guanylate cyclase 2 expressed at ~1:3,000, 1:1,400 and 1:5,800 molar ratios with rhodopsin, respectively.40, 46 Provided that a mammalian rod photoreceptor disc contains fewer than 100,000 rhodopsin molecules,47, 48 these molar ratios suggest that we were able to confidently identify proteins expressed in the amount of at least ~15–50 copies/disc. While we cannot exclude that less abundant disc-specific proteins escaped our detection limits, there is no evidence in the literature that they may exist.
Do Photoreceptor Discs Contain SNARE and Rab Proteins?
A central finding of the previous proteome study by Molday and colleagues9 was that photoreceptor discs contain multiple members of the SNARE and Rab protein families engaged in the regulation of membrane fusion and vesicular trafficking. While multiple representatives of both protein types were reliably identified in the D3 preparation, none of them came close to satisfying the criterion of being a unique disc component. This could be explained, at least in part, by all identifiable SNAREs being shared between discs and other membrane compartments of photoreceptors, an idea consistent with immunohistochemical data in that study.9 It is also possible that SNARE proteins residing in the outer segment are confined to the plasma membrane and do not sequester into discs upon their morphogenesis, which concurs with outer segments containing significantly larger quantities of SNARE proteins than purified discs.9 But irrespective of any specific explanation, our data clearly demonstrate that discs do not contain a unique SNARE protein constituent.
The total number of Rab proteins identified in the D3 disc preparation was nine, with their D3/D1 ratios ranging from 0.46 to 0.25. This does not exactly match the result from ref.9 demonstrating that, despite none of the Rab proteins being uniquely expressed in rod outer segments, at least four of them were found in equal amounts in outer segments and discs. This discrepancy may be explained by the gradual loss of lipidated Rabs from the disc membranes subjected to multiple purification steps in our study.
What could be the functional role of Rab proteins in the outer segment? While it is tempting to speculate that they are involved in disc morphogenesis, the number of Rab family members found in disc membranes is surprisingly large for performing this single cellular function. Therefore, their presence in D3 may be explained by alternative reasons. For example, it is possible that lipidated Rab proteins, involved in guiding membrane transport vesicles from the trans-Golgi network to the base of the outer segment, are not completely released upon vesicle fusion and become stuck in the discs. This is consistent with D3 containing both Rab11 and Rab8, small GTPases critical for rhodopsin transport.49 If this is true, the identity of seven other Rab proteins found in D3 (Rab1a, Rab5c, Rab7a, Rab3a, Rab18, Rab2a, and Rab1b) may provide valuable leads about the intracellular trafficking pathways responsible for outer segment delivery of other resident proteins. Another explanation is that an extremely high disc membrane density serves as a “trap” for lipidated Rab proteins occasionally diffusing into the outer segment. Interestingly, Molday and colleagues9 observed a high outer segment content of both isoforms of RabGDI responsible for solubilizing Rabs in their inactive GDP-bound form.50 Perhaps RabGDIs serve to aid the recycling of Rabs trapped in the membranes of photoreceptor discs. Regardless, the role of Rab proteins in the outer segment remains an interesting area for future studies.
Concluding Remarks
This study provides a striking example how protein correlation profiling can distinguish between unique and shared/contaminating components of cellular organelles, and discover their novel constituencies. We demonstrated that the number of unique disc-resident proteins is indeed very small and is represented by ten well-studied proteins and PRCD. Despite the PRCD gene being recognized as a locus affected in degenerative diseases of the retina, nothing was known about the protein that this gene encodes. Our discovery that PRCD belongs to a handful of unique protein components of the photoreceptor discs will undoubtedly facilitate progress toward understanding its functional role and the pathobiological significance of its mutations.
Supplementary Material
Acknowledgments
We thank Dr. G.D. Aguirre for stimulating discussions and Drs. S. Farsiu and Y. Lokhnygina for their help with statistical analysis. The work was supported by NIH grants EY12859 (VYA) and Core Grant for Vision Research EY5722 (to Duke University), and an unrestricted grant from Research to Prevent Blindness.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Four supplementary tables describe: a list of proteins identified in photoreceptor disc preparations D1, D2 and D3; a complete list of peptides used as the basis of this protein identification; data used for protein quantification across disc preparations; protein ranking sequence based on the D3/D1 ratio.
References
- 1.Rodieck RW. The first steps in seeing. Sinauer Associates; Sunderland: 1998. p. 562. [Google Scholar]
- 2.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237 (8):1982–92. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010;190 (6):953–63. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith HG, Jr, Stubbs GW, Litman BJ. The isolation purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res. 1975;20 (3):211–7. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- 5.Papermaster DS. Preparation of retinal rod outer segments. Methods Enzymol. 1982;81:48–52. doi: 10.1016/s0076-6879(82)81010-0. [DOI] [PubMed] [Google Scholar]
- 6.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–87. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr, Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007;6 (8):1299–317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panfoli I, Musante L, Bachi A, Ravera S, Calzia D, Cattaneo A, Bruschi M, Bianchini P, Diaspro A, Morelli A, Pepe IM, Tacchetti C, Candiano G. Proteomic analysis of the retinal rod outer segment disks. J Proteome Res. 2008;7 (7):2654–69. doi: 10.1021/pr7006939. [DOI] [PubMed] [Google Scholar]
- 9.Kwok MC, Holopainen JM, Molday LL, Foster LJ, Molday RS. Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol Cell Proteomics. 2008;7 (6):1053–66. doi: 10.1074/mcp.M700571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426 (6966):570–4. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 11.Zangerl B, Goldstein O, Philp AR, Lindauer SJ, Pearce-Kelling SE, Mullins RF, Graphodatsky AS, Ripoll D, Felix JS, Stone EM, Acland GM, Aguirre GD. Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics. 2006;88 (5):551–63. doi: 10.1016/j.ygeno.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nevet MJ, Shalev SA, Zlotogora J, Mazzawi N, Ben-Yosef T. Identification of a prevalent founder mutation in an Israeli Muslim Arab village confirms the role of PRCD in the aetiology of retinitis pigmentosa in humans. J Med Genet. 2010;47 (8):533–7. doi: 10.1136/jmg.2009.073619. [DOI] [PubMed] [Google Scholar]
- 13.McDowell JH. Preparing rod outer segment membranes, regenerating rhodopsin, and determining rhodopsin concentration. Methods Neurosci. 1993;15:123–130. [Google Scholar]
- 14.Bownds D, Gordon-Walker A, Gaide-Huguenin AC, Robinson W. Characterization and analysis of frog photoreceptor membranes. J Gen Physiol. 1971;58 (3):225–37. doi: 10.1085/jgp.58.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6 (5):359–62. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 16.Reidel B, Thompson JW, Farsiu S, Moseley MA, Skiba NP, Arshavsky VY. Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol Cell Proteomics. 2011;10(3) doi: 10.1074/mcp.M110.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobanova ES, Herrmann R, Finkelstein S, Reidel B, Skiba NP, Deng WT, Jo R, Weiss ER, Hauswirth WW, Arshavsky VY. Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light. J Neurosci. 2010;30 (20):6815–24. doi: 10.1523/JNEUROSCI.0613-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobanova ES, Finkelstein S, Herrmann R, Chen YM, Kessler C, Michaud NA, Trieu LH, Strissel KJ, Burns ME, Arshavsky VY. Transducin γ-subunit sets expression levels of α- and β-subunits and is crucial for rod viability. J Neurosci. 2008;28 (13):3510–3520. doi: 10.1523/JNEUROSCI.0338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster LJ, de Hoog CL, Zhang Y, Xie X, Mootha VK, Mann M. A mammalian organelle map by protein correlation profiling. Cell. 2006;125 (1):187–99. doi: 10.1016/j.cell.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Wiese S, Gronemeyer T, Ofman R, Kunze M, Grou CP, Almeida JA, Eisenacher M, Stephan C, Hayen H, Schollenberger L, Korosec T, Waterham HR, Schliebs W, Erdmann R, Berger J, Meyer HE, Just W, Azevedo JE, Wanders RJ, Warscheid B. Proteomics characterization of mouse kidney peroxisomes by tandem mass spectrometry and protein correlation profiling. Mol Cell Proteomics. 2007;6 (12):2045–57. doi: 10.1074/mcp.M700169-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Borner GH, Antrobus R, Hirst J, Bhumbra GS, Kozik P, Jackson LP, Sahlender DA, Robinson MS. Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J Cell Biol. 2012;197 (1):141–60. doi: 10.1083/jcb.201111049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 2008;48 (20):2052–61. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palczewski K. Chemistry and biology of vision. J Biol Chem. 2012;287 (3):1612–9. doi: 10.1074/jbc.R111.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arshavsky VY, Burns ME. Photoreceptor signaling: Supporting vision across a wide range of light intensities. J Biol Chem. 2012;287 (3):1620–6. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baehr W, Morita EA, Swanson RJ, Applebury ML. Characterization of bovine rod outer segment G-protein. J Biol Chem. 1982;257 (11):6452–60. [PubMed] [Google Scholar]
- 26.Anant JS, Ong OC, Xie HY, Clarke S, O’Brien PJ, Fung BK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267 (2):687–90. [PubMed] [Google Scholar]
- 27.Bigay J, Faurobert E, Franco M, Chabre M. Roles of lipid modifications of transducin subunits in their GDP-dependent association and membrane binding. Biochemistry. 1994;33 (47):14081–90. doi: 10.1021/bi00251a017. [DOI] [PubMed] [Google Scholar]
- 28.Seitz HR, Heck M, Hofmann KP, Alt T, Pellaud J, Seelig A. Molecular determinants of the reversible membrane anchorage of the G-protein transducin. Biochemistry. 1999;38 (25):7950–60. doi: 10.1021/bi990298+. [DOI] [PubMed] [Google Scholar]
- 29.Fukada Y, Matsuda T, Kokame K, Takao T, Shimonishi Y, Akino T, Yoshizawa T. Effects of carboxyl methylation of photoreceptor G protein gamma-subunit in visual transduction. J Biol Chem. 1994;269 (7):5163–70. [PubMed] [Google Scholar]
- 30.Kosloff M, Alexov E, Arshavsky VY, Honig B. Electrostatic and lipid anchor contributions to the interaction of transducin with membranes: Mechanistic implications for activation and translocation. J Biol Chem. 2008;283 (45):31197–207. doi: 10.1074/jbc.M803799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu G, Wensel TG. R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9-1. Proc Natl Acad Sci U S A. 2002;99 (15):9755–60. doi: 10.1073/pnas.152094799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farjo R, Naash MI. The role of Rds in outer segment morphogenesis and human retinal disease. Ophthalmic Genet. 2006;27 (4):117–22. doi: 10.1080/13816810600976806. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg AF. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol. 2006;253:131–75. doi: 10.1016/S0074-7696(06)53004-9. [DOI] [PubMed] [Google Scholar]
- 34.Molday RS, Warren R, Loewen C, Molday L. Cyclic GMP-gated channel and peripherin/rds-rom-1 complex of rod cells. Novartis Found Symp. 1999;224:249–61. doi: 10.1002/9780470515693.ch14. [DOI] [PubMed] [Google Scholar]
- 35.Boesze-Battaglia K, Goldberg AF, Dispoto J, Katragadda M, Cesarone G, Albert AD. A soluble peripherin/Rds C-terminal polypeptide promotes membrane fusion and changes conformation upon membrane association. Exp Eye Res. 2003;77 (4):505–14. doi: 10.1016/s0014-4835(03)00151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98 (1):13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 37.Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274 (12):8269–81. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 38.Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10 (9):802–23. doi: 10.2174/156652410793937813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology. 2010;25 (1):8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman JA, Kwok MC, Molday RS. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284 (47):32670–9. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman JA, Molday RS. Critical role of the beta-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2. J Biol Chem. 2011;286 (19):17205–16. doi: 10.1074/jbc.M111.229419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, Chen H, Zhao C, Al-Sheikh YT, Karan G, Corbeil D, Escher P, Kamaya S, Li C, Johnson S, Frederick JM, Zhao Y, Wang C, Cameron DJ, Huttner WB, Schorderet DF, Munier FL, Moore AT, Birch DG, Baehr W, Hunt DM, Williams DS, Zhang K. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest. 2008;118 (8):2908–16. doi: 10.1172/JCI35891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han Z, Anderson DW, Papermaster DS. Prominin-1 localizes to the open rims of outer segment lamellae in Xenopus laevis rod and cone photoreceptors. Invest Ophthalmol Vis Sci. 2012;53 (1):361–73. doi: 10.1167/iovs.11-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pentia DC, Hosier S, Cote RH. The glutamic acid-rich protein-2 (GARP2) is a high affinity rod photoreceptor phosphodiesterase (PDE6)-binding protein that modulates its catalytic properties. J Biol Chem. 2006;281 (9):5500–5. doi: 10.1074/jbc.M507488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martemyanov KA, Krispel CM, Lishko PV, Burns ME, Arshavsky VY. Functional comparison of RGS9 splice isoforms in a living cell. Proc Natl Acad Sci U S A. 2008;105 (52):20988–93. doi: 10.1073/pnas.0808941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peshenko IV, Olshevskaya EV, Savchenko AB, Karan S, Palczewski K, Baehr W, Dizhoor AM. Enzymatic properties and regulation of the native isozymes of retinal membrane guanylyl cyclase (RetGC) from mouse photoreceptors. Biochemistry. 2011;50 (25):5590–600. doi: 10.1021/bi200491b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Res. 2004;44 (28):3235–51. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177 (5):917–25. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deretic D, Wang J. Molecular assemblies that control rhodopsin transport to the cilia. Vision Res. 2012;75:5–10. doi: 10.1016/j.visres.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oesterlin LK, Goody RS, Itzen A. Posttranslational modifications of Rab proteins cause effective displacement of GDP dissociation inhibitor. Proc Natl Acad Sci U S A. 2012;109 (15):5621–6. doi: 10.1073/pnas.1121161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.