Abstract
Osteoarthritis (OA) is a complex and painful disease of the whole joint. At present there are no satisfying agents for treating OA. The current standard of care mainly involves managing and alleviating its symptoms. Mechanisms of OA pain have been studied in rodent knee OA models produced by intra-knee injection of the chondrocyte glycolytic inhibitor mono-iodoacetate, surgery, or spontaneous development in some species. These models are clinically relevant in terms of histological damage and functional changes, and are used to study mechanisms underlying mechanical, thermal, ambulatory, body weight supporting-evoked, and ongoing OA pain. Recent peripheral, spinal, and supraspinal biochemical and electrophysiological studies in these models suggest that peripheral pro-inflammatory mediators and neuropeptides sensitize knee nociceptors. Spinal cytokines and neuropeptides promote OA-associated pain, and peripheral and spinal cannabinoids inhibit OA pain respectively through cannabinoid-1 (CB1) and CB1/CB2 receptors. TRPV1 and metalloproteinases contribute and supraspinal descending facilitation of 5-HT/5-HT 3 receptors may also contribute to OA pain. Conditioned place preference tests demonstrate that OA pain induces aversive behaviors suggesting brain involvement in OA pain. During OA, brain functional connectivity is enhanced, but at present it is unclear how this change is related to OA pain.
Keywords: Osteoarthritis, pain, hyperalgesia, allodynia, spinal cord, monoiodoacetate
1. Introduction
Osteoarthritis (OA), a complex disease of the whole joint, is characterized by structural degradation of the articular cartilage, peri-articular bone, synovial joint lining, and adjacent supporting connective tissue elements. It manifests as joint pain and loss of joint function. There are currently no satisfying treatments to this disease. The current standard of care is to manage and alleviate symptoms 1, but despite treatment with conventional analgesic drugs most individuals with OA continue to experience pain 2. A recent study demonstrated that subjects with chronic back pain and complex regional pain syndrome had significantly less bilateral hippocampal volume compared to controls, while those with OA did not 3. This suggests that OA-induced pain might be related to unique mechanisms and this has attracted researchers’ attention in recent years. Since the most common joints affected by OA are large weight-bearing joints such as hip and knee 4, intra-knee injection of the chondrocyte glycolytic inhibitor mono-iodoacetate (MIA)-, surgically induced, and spontaneous knee OA models have been used to investigate mechanisms of OA-induced pain 5–8.
In the MIA model, histological examination shows chondrocyte degeneration/ necrosis at days 1–7 post-MIA, increased osteoclasts and osteoblasts in subchondral bone by day 7, focal fragmentation and collapse of bony trabeculae with fibrosis by day 28, and large areas of bone remodeling by day 56 9, 10. In vivo microCT-arthrography clearly detects cartilage degeneration in the injected knee 11.
Tramadol, celecoxib, and diclofenac improve movement-induced pain behavior evaluated with compressive hind limb grip force in the MIA model 12, and subcutaneous morphine and gabapentin significantly decrease the mechanical and thermal sensitivity and ambulation-evoked pain 13. MIA-induced articular cartilage loss, progressive subchondral bone lesions, and the efficacy of clinical analgesics in inhibiting MIA-induced pain indicate that this model is clinically relevant and will continue to be useful for the development of better therapeutic strategies and better understanding of the mechanisms of chronic OA pain.
Surgery-induced medial meniscal tear (MMT), partial medial meniscectomy (PMM), destabilization of the medial meniscus (DMM), and anterior cruciate ligament transection (ACLT) have been used to induce knee OA 14, 15. The MMT results in a progressive cartilage lesion 16. The MMT plus ACLT-induced OA model presents bone and cartilage remodeling, infiltration of immune cells into joint tissues, and pain 17. The ACLT+ PMM model showed no hind-limb difference in gait analysis or mechanical allodynia over a period of a month 18, making it analogous to patients who show radiological changes but no pain. PMM in female C57BL/6 mice produces progressive degenerative joint damage and OA-related pain 19. The DMM-induced OA model displays a time-dependent cartilage lesion between 2 and 12 weeks, including cartilage surface fibrillations, loss of superficial cartilage and ulceration of subchondral bone, and produces pain assessed 12 weeks after surgery 7. In that model, opioid receptor antagonists led to pain onset 4 weeks earlier than in vehicle-treated animals, and opioid receptors increased in the peripheral nerves that innervate the joint in naloxone-responsive mice 7, suggesting that endogenous opioids might inhibit early-stage OA pain.
Guinea pigs, particularly the Dunkin-Hartley strain, STR/1N, STR/ort, and C57 black mice, and several transgenic and genetically altered strains of mice develop characteristics of arthritic joints, but rats rarely develop them spontaneously (for a review, see D’Souza et al. 2011). In Duncan-Hartley guinea pigs, age-dependent cartilage degeneration can be assessed by T(1ρ) MRI from 3 to 9 months 20.
All three model types have been used to investigate the mechanisms of OA pain, which has been assessed with various methods, including mechanical, thermal, ambulatory, and body weight supporting-evoked methods (for a review, see D’Souza et al. 2011). MIA has been reported to be differentially potent: weight-bearing ≥ von Frey filaments>running wheel 21. Von Frey filaments are a set of calibrated filaments used to perpendicularly stimulate skin. Recently, the MIA model has also been used to study ongoing pain assessed with conditioned place preference 22, 23. Additionally, it has been reported that biglycan (BGN) and fibromodulin (FMOD) play roles in regulating chondrogenesis and extracellular matrix turnover 24 and that doubly deficient BGN/FMOD mice develop premature temporomandibular joint OA 25. Peripheral, spinal, and supraspinal l mechanisms (Fig. 1) have recently been discovered using these rodent models.
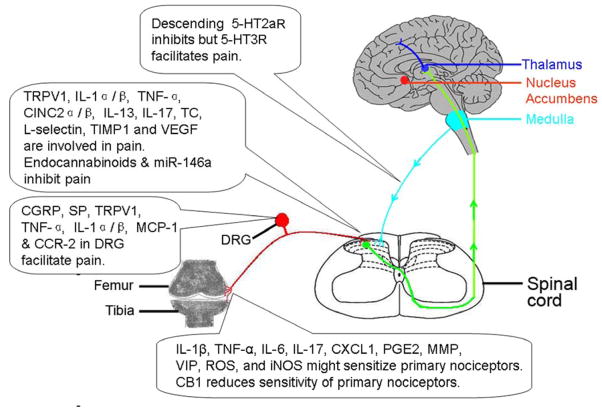
Fig. 1.
Diagram illustrating mechanisms of osteoarthritis-induced pain. Bioactive chemicals are involved in OA-induced pain at peripheral, spinal and supraspinal levels. 5-HT; 5-hydroxytryptamine; 5-HT2aR, 5-HT 2a receptors; 5-HT3R, 5-HT 3 receptors; CB1, cannabinoid-1; CINC2α/β, chemoattractant 2 α/β ; CCR-2, chemokine (C-C motif) receptor-2; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; iNOS, inducible NO synthase; IL-6, interleukin-6; IL-13, interleukin-13; IL-17, interleukin-17; IL-1α/β, interleukin alpha/beta; KC/CXCL1, keratinocyte-derived chemokine; MCP-1, monocyte chemoattractant protein-1; miR-146a, microRNAs-146a; MMP, matrix metalloproteinases; PGE2, prostaglandin E2; ROS, reactive oxygen species; SP, substance P; TC, thymus chemokines; TIMP1, tissue inhibitor of metalloproteinases 1; TRPV-1, transient receptor potential vanilloid 1; TNF-α, tumor necrosis factor-αlpha; VEGF, vascular endothelial growth factor; VIP, vasoactive intestinal peptide.
2. Peripheral mechanisms
2.1. Cytokines
Synovitis is highly correlated to OA patients’ pain 26, 27 and plays an important role in such pain 28, 29. An intra-articular MIA injection significantly increased tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the knee synovium and capsule between days 1 and 28 post-MIA; the levels of TNF-α and IL-6 peaked at day 4. The injection also induced mechanical allodynia of the ipsilateral hind paw, which was significantly mitigated by local application of nonsteroidal anti-inflammatory drugs (NSAIDs) 30, 31. A TNF-α injection into the normal knee joint caused significant and persistent sensitization of nociceptive sensory fibers to mechanical stimuli that was abolished by co-administration of etanercept, a TNF-α inhibitor; and TNF-α induced excitation of isolated dorsal root ganglion (DRG) neurons with C-fiber axons 32 and increased mechanosensitivity and peripheral receptive fields of DRG neurons 33.
An electrophysiological study demonstrated increased spontaneous activity in C-mechanosensitive fibers and increased mechanical sensitivity in A-mechanosensitive fibers of the knee in MIA-treated rats 34. Furthermore, knee primary afferents, thinly myelinated type III and unmyelinated type IV fibers, showed significant mechanosensitivity in response to normal and noxious joint rotation as compared to saline controls in an MIA model 35, 36. This was reduced by local NSAID application 36. Collectively, these studies indicate that increased TNF-α sensitizes primary afferents to facilitate joint pain and that NSAIDs can alleviate OA-induced pain by inhibiting TNF-α expression.
IL-6 increased intracellular calcium in cultured DRG neurons in calcium-imaging studies. Additionally, glycoprotein 130, to which the IL-6/IL-6R complex binds, was found in almost all DRG neurons cultured from adult rats. This suggests functional IL-6 receptors in DRG neurons 37. Moreover, an intra-articular IL-6 (20 ng/joint) injection into the normal knee joint increased C-fiber response to noxious outward rotation, and co-administration of IL-6 and its soluble receptor significantly increased response to innocuous outward rotation 38. These studies indicate that IL-6 sensitizes primary nociceptors in the knee.
IL-1β expression was higher between days 120 and 180 in cartilage, menisci, synovia, and subchondral bone in OA-prone Hartley than in OA-resistant Strain 13 guinea pigs. This parallels the fact that OA develops faster in Hartley than in Strain 13 animals 39. It has been shown that an IL-1β application increases DRG neuron mechanosensitivity 33. IL-1β treatment for 5–6 days increased the excitability of medium- and small-diameter isolectin B(4) (IB(4))-positive DRG neurons through its receptor, IL-1RI 40. Further, in an electrophysiological study, the mechanical threshold required to initiate afferent firing was significantly lower in aged (9–12 months) guinea pigs than in young (12–14 weeks) ones 41. Thus, IL-1β might sensitize primary nociceptors in the knee.
Other pro-inflammatory cytokines such as IL-7, IL-17, and IL-18 have also been implicated in synovitis, subchondral bone damage, and cartilage homeostasis alteration in spontaneous or surgically induced OA animal models and in transgenic mice primed to develop OA 42. Of these, IL-17 plays a critical role in nociception during antigen-induced arthritis 43. Local IL-17 is significantly higher in a mouse model of arthritis, and treatment with the antibody against IL-17 inhibits hypernociception. Intra-articular IL-17 also induced nociception and upregulation of TNF-α, IL-1β, keratinocyte-derived chemokine, prostaglandin 2 (PGE2), matrix metalloproteinases-9 (MMP-9) activity, and cyclooxygenase-2 (COX-2) in synovial membranes 43. Consistent with these data, treatment with the non-specific MMP inhibitor doxycycline, the COX inhibitor indomethacin, the anti-TNF antibody infliximab, or an IL-1 receptor antagonist inhibited IL-17-induced hypernociception 43. Intra-articular IL-17 also elicited slow-developing, long-lasting sensitization of nociceptive C fibers of the joint to mechanical stimuli 44. IL-17A receptors were found in most rat DRG neurons, and IL-17 enhanced excitability of cultured DRG neurons 44. Therefore, although the role of IL-17 in OA pathophysiology is not entirely established, IL-17 appears to play a role in OA-induced pain.
In accordance with studies in animals, synovial tissue from patients with all grades of OA show inflammatory cell infiltration and cytokine production 45. Human OA synovial cell cultures demonstrated that macrophages produce TNF-α and IL-1 46. When DRG from adult rats were co-cultured with normal or knee OA synovial the mRNA levels of substance P (SP), neurokinin/tachykinin receptors (NK1, NK2), neuropeptide Y receptors (NPYR1, NPYR2), the calcium channel α2δ1, and inflammation mediators such as COX2, IL-6, and interferon β2) were clearly elevated in these DRG compared to control synovia 47. This suggests that human OA synovium-produced cytokines sensitize primary sensory neurons and that blocking peripheral cytokine activity might alleviate OA pain. Indeed, clinical researchers have been investigating a novel IL-1 inhibitor for OA treatment 48. It was showed that human monoclonal antibody to IL-1RI, AMG 108, showed greater improvements in pain than placebo. Although some studies showed no statistically significant difference of WOMAC score between IL-1 receptor antagonist, orthokine, and placebo groups, some data demonstrated that orthokine considerably improved clinical signs and symptoms of knee OA patients.48
2.2. Neuropeptides
Inflamed synovia also produce vasoactive intestinal peptide (VIP) to cause pain 49. Local application of VIP to normal knees significantly increased afferent firing in response to normal rotation and hyper-rotation that was blocked by pre-administration of the VIP receptor antagonist VIP (6–28), and VIP (6–28) significantly reduced afferent firing in MIA-injected rats 50. Behaviorally, VIP induced hind limb incapacity and mechanical alodynia in the hind paw, while VIP(6–28) diminished these symptoms 51. This suggests that VIP sensitizes primary knee joint nociceptors during knee OA.
2.3. Cannabinoids
Studies show that activation of peripheral cannabinoid-1 (CB1) receptors with local application of the CB1 receptor agonist arachidonyl-2-chloroethylamide (ACEA) reduces mechanosensitivity of afferent nerve fibers in control and OA knee joints. The CB1 receptor antagonist AM251 significantly increased mechanosensitivity in the OA joint but not in controls. This indicates that MIA-induced OA activates endogenous CB1 receptors in the knee that in turn decrease the excitability of afferent fibers. Further, a transient receptor potential vanilloid 1 (TRPV1) ion channel antagonist significantly reduced the efficacy of ACEA, which suggests that TRPV1 is involved in CB1 receptor-mediated anti-nociception 35.
A local injection of the endocannabinoid hydrolysis inhibitor URB597, which blocks anandamide catabolism, significantly inhibited afferent nerve activity in MIA-treated joints but had no effect on saline-treated joints. Inhibition was prevented by CB1 but not CB2 antagonist pretreatment. Behaviorally, URB597 produced CB1- but not CB2-dependent analgesia. Similarly, URB597 inhibited afferent nerve activity in the aged guinea pig knee OA animal model but not in the young guinea pig 41. Those data confirm that peripheral CB1 activation can relieve OA-induced pain.
CB2 and TRPV1 receptor co-localization in synoviocytes of sham- and MIA-treated rats suggest that CB2 receptors play a role in pain 52. Local application of the CB2 receptor agonist GW405833 significantly reduced joint afferent firing rate in control knees but potentiated firing in OA knee joint mechanoreceptors. This illustrates the paradoxical effects of CB2 in healthy and pathological joints. GW405833 also increases calcitonin gene-related peptide (CGRP) release via a TRPV1 channel-dependent mechanism to facilitate pain 52. These studies show that CB1 and CB2 have different effects on OA-associated pain.
2.4. Matrix metalloproteinases (MMPs)
MMPs are zinc-dependent endopeptidases that play an important role in OA pathogenesis 53. Recent studies demonstrate the analgesic efficacy of MMP inhibitors in OA pain. A non-selective and equipotent MMP- 2, 8, 9, 12, and 13 inhibitor reduced osteochondral vascularity, chondropathy, and weight-bearing asymmetry in a rat knee OA model induced by transecting the medial collateral ligament and cutting the full thickness of the meniscus of the knee 54. A specific MMP-13 inhibitor not only significantly reduced MIA-induced cartilage damage but also improved weight bearing in an MIA-injected hind limb 55. Additionally, the protease inhibitor MG132 reduces pain and reverses cartilage MMP-3 upregulation 56. These studies indicate that protease inhibitors such as selective MMP inhibitors produce both chondroprotection and analgesia during OA.
2.5. Other bioactive agents
In the MIA-induced knee OA model, ABC294640 57, a selective inhibitor of sphingosine kinase-2; AS1892802 58, 59, which rarely penetrates the central nervous tissue and is a selective Rho kinase inhibitor; and MEN16132 60, a kinin B(2) receptor antagonist, significantly improved weight bearing of the injected limb and alleviated cartilage degradation. Systemic co-administration of the selective positive allosteric modulator NS-9283 enhanced the analgesic potency of the nicotinic acetylcholine receptor (nAChR) α4β2 agonist ABT-594 5-fold 61. These studies indicate that sphingosine kinase-2, Rho kinase, kinin B(2) receptors, and nAChR are involved in OA pain. Rebamipide, a free radical scavenger 62, significantly inhibited MIA-induced pain and cartilage degeneration by decreasing MMP-13, IL-1β, hypoxia-inducible factor-2alpha (HIF-2α), inducible NO synthase (iNOS), and nitrotyrosine expression in OA cartilage and by increasing tissue inhibitor expression of MMP-1 and MMP-3 63. This suggests that reactive oxygen species (ROS) are involved in OA pain.
In the ACLT-induced knee OA model, nitrite levels in joint exudates and iNOS in synovia were significantly increased. Systemic pretreatment with the non-selective iNOS inhibitor L-N(G)-nitroarginine methyl ester or the selective iNOS inhibitor 1400W reduced joint pain 64, showing that NO release is associated with such pain. Zoledronate inhibition of osteoclasts prevented cartilage loss and pain in MIA and MMT models 65, which suggests osteoclast involvement in OA pain.
In the Dunkin-Hartley guinea pig model of spontaneous OA, long-term (one month) administration of AZ12606133, a selective cathepsin K inhibitor, significantly reduced mechanosensitivity in response to both noxious and non-noxious joint movement 66.
Additionally, PGEs are involved in OA pain (for a review, see D’Souza et al. 2011). Nerve growth factor, sodium channels, angiogenesis inhibitors, and hyaluronic acid are also involved in OA pain 67. One study of Affymetrix Gene Chip expression arrays and articular chondrocytes from an anterior cruciate ligament transection rat model showed 1,619 differentially expressed genes 68, which suggests that more bioactive agents are involved in OA pain. The aforementioned bioactive chemicals might work in concert to produce OA pain.
3. Spinal mechanisms
The report of variable links between injury, pain, and spreading pain in OA patients indicates that spinal and supraspinal processing of painful inputs are altered during OA.
3.1. Glia cells/cytokines
In the MIA-induced OA model, microglia show significant hyperactivity between days 7–28, while reactive astrocytosis were seen at day 28, the late stage of OA. Nimesulide, a COX inhibitor, and minocycline attenuated pain behavior assessed with weight bearing, mechanical hind paw allodynia, and microglia and astrocyte activation in the ipsilateral spinal cord 69. MIA-injected rats displayed reduced hind limb grip force 1, 2, and 3 weeks post-MIA, gradual increase of phospho-extracellular signal-regulated kinase 1/2 (ERK1/2) in neurons with a significant increase 3 weeks post-MIA, and rapid increase of phosphorylation of p38 mitogen-activated protein kinases in microglia and neurons that peaked a week post-MIA. Intrathecal injection of the mitogen-activated protein kinase 1 inhibitor PD98059 blocked hind-limb grip force reduction and pERK1/2 induction in MIA-OA rats 70. These studies demonstrate that spinal glia are involved in OA-induced pain.
MIA injection at 5 weeks resulted in significant protein increases in the lumbar spinal dorsal horn of multiple pro-inflammatory cytokines and chemokines such as IL-1α/β, chemokine (C-C motif) ligand 5, cytokine-induced neutrophil chemoattractant 2 α/β, IL-13, IL-17, thymus chemokines, TNF-α, L-selectin, tissue inhibitor of metalloproteinases-1, and vascular endothelial growth factor. It decreased the protein levels of IL-4 and IL-10, fractalkine, an acute spinal injury pain-associated chemokine, and granulocyte–macrophage colony-stimulating factor in the spinal dorsal horn. Consistent with the protein data, TNF-α mRNA was increased in the spinal dorsal horn at week 5 but not at week 2. In DRG, TNF-α and IL-1α/β mRNA increased in MIA, ACLT, and DMM models71.
MicroRNAs-146a (miR-146a) mRNA from the lumbar DRG and the spinal dorsal horn of MIA-injected rats significantly decreased 2 and 4 weeks post-MIA compared to sham controls, and human astrocytes transfected with miR-146a significantly decreased mRNA expression of TNF-α, COX-2, iNOS, IL-6, IL8, and TRPV1 72. Most of these bioactive chemicals are involved in pain, so a miR-146a decrease during OA might facilitate pain.
A recent study in mice demonstrated that monocyte chemoattractant protein (MCP)-1 (CCL2) and its high-affinity receptor, chemokine (C-C motif) receptor 2 (CCR2), significantly increased in L3-5 DRG neurons at 8 weeks and returned to base levels at 16 weeks post-DMM surgery. Movement-provoked pain behaviors appeared at 8 weeks and were maintained for at least 16 weeks. A systemic CCR2 receptor antagonist administered to wild type DMM mice 9 weeks after surgery reversed movement-evoked pain. After DMM, CCR2-null mice showed an absence of movement-evoked pain behaviors, and rapid recovery from mechanical allodynia occurred at 4 weeks in both wild and CCR2-null mice. CCR2-null mice also had less DRG infiltration by macrophages that express numerous algogenic molecules that contribute to pain. These data indicate that DRG neuronal MCP-1 and its receptor, CCR2, participate in the development of movement-evoked pain and the maintenance of mechanical allodynia 73.
3.2. Cannabinoids
Endocannabinoids show adaptive changes in the spinal cord during the development of MIA-induced OA. MIA injection into the knee not only decreased weight-bearing force and induced mechanical allodunia in the ipsilateral hind paw but also significantly facilitated spinal wide dynamic range (WDR) neuron response to innocuous and noxious mechanical stimulation. The relationship between weight-bearing force and WDR neuron response to hind paw stimulation were significantly correlated on day 28 after the MIA injection. Further, anandamide and 2-arachidonoyl glycerol (2-AG) as well as NAPE-PLD and DAGLα, which synthesize anandamide and 2-AG, respectively, increased in spinal cords of MIA-treated rats. Spinal administration of CB1 (<10 μg/50 μl) and CB2 (0.001–0.1μg/50 μl) receptor antagonists significantly facilitated innocuous and noxious mechanically evoked responses of WDR neurons in MIA-treated but not saline-injected rats. Administration of the endocannabinoid hydrolysis inhibitor URB597 significantly inhibited mechanically evoked WDR neuron response in MIA-treated rats compared to saline-treated rats 74. These data demonstrate that spinal endocannabinoids adaptively dampen nociceptive transmission through CB1 and CB2 receptors.
3.3. TRPV1
An early investigation showed that the protein levels of CGRP and TRPV1 was higher in primary neurons innervating the knee in MIA-induced OA rat model than in those of control animals 75. In more recent studies, spinal TRPV1 activities were enhanced 76, and a TRPV1 antagonist alleviated hind limb grip force impairment in an MIA OA model 76, 77. A TRPV1 antagonist inhibited OA-enhanced glutamate and CGRP release in the spinal cord 76 and spinal WDR and nociceptive specific (NS) neuron response to 300-g von Frey stimulation of the MIA-OA knee joint 77. Moreover, mechanical allodynia was relieved in MIA-induced OA rat pretreated with the TRPV1 agonist capsaicin 78, likely due to dysfunction of nociceptive fibers, and a systemic TRPV1 antagonist effectively blocked thermal hypersensitivity in MIA-induced OA model23. These studies confirm that spinal TRPV1 is involved in OA-related ambulatory, mechanical, and thermal pain.
Although systemic administration of the TRPV1 receptor antagonist A-889425 reduced enhanced spontaneous firing of WDR neurons in OA rats 77, the systemic TRPV1 antagonist AMG9810 did not block ongoing pain assessed with conditioned place preference 23. Inconsistency between the electrophysiological and behavioral studies might be the result of the difference in MIA dosage (3 vs. 4.8 mg). The assessed ongoing pain behavior involves supraspinal mechanisms. It is possible that theTRPV1 antagonist does not modulate activity of supraspinal neurons such as those of nucleus accumbens which is associated with OA-induced spontaneous pain 79. Furthermore, a TRPA1 antagonist did not reduce the spontaneous activity of spinal WDR neurons in OA rats 80, nor did a systemic or intra-articular TRPA1 antagonist block weight asymmetry and ongoing pain. These data suggest that MIA-induced ongoing pain is independent of TRPV1 and TRPA1 activation 23.
Additionally, since surgery-induced OA and spontaneously-developed OA models also mimic some characteristics of human OA, the involvement of TRPV1 in those models-induced pain warrant further investigation.
3.4. Neuropeptides
Intra-articular MIA induced significant mRNA expression of SP and CGRP in DRG neurons, and significant increase of SP and CGRP-immunoreactivity in synovia, periosteum, and subchondral bone on day 21 post-MIA compared to control 56. Notably, the hip joint capsule and soft tissue in patients with painful osteoarthritis also show SP and CGRP upregulation 81. Another study showed CGRP and SP upregulation starting on days 7 and 28, respectively, and dynorphin (1–32) downregulation on day 14 in the spinal cord 19, 82. At day 35 post-MIA, DRG CGRP and SP declined while galanin, neuropeptide Y and downstream regulatory element antagonist modulator increased 71. Double labeling showed that intra-articular MIA induced CGRP upregulation in DRG neurons that innervate the knee on day 31 83. These studies indicate that OA pain can be modulated by spinal neuropeptides. Celecoxib improved gait parameters such as swing speed and swing phase duration, relieved mechanical allodynia, and decreased spinal CGRP but not SP in MIA-induced OA rat model 19. Eugenol, the main constituent of clove oil, improved dynamic gait parameters (swing speed, swing phase duration and duty cycle) and mechanical allodynia of the affected limb. Concomitantly, it decreased spinal SP and CGRP and increased spinal dynorphin in MIA-induced OA rat model 84. Furthermore, intrathecal injection of the peptide antagonist CGRP(8–37) mitigated MIA-induced mechanical allodynia 85. These studies suggest that chemicals that reverse spinal neuropeptide regulation alleviate OA pain.
3.5. Neuropathic components of OA pain
Because of alterations in the central nervous system and the peripheral nerves that innervate the joints, OA-associated pain gradually develops the characteristics of neuropathic pain86. Previous studies show that activation of a marker of nerve injury, transcript factor (ATF-3) in the DRG, significantly increased between days 8 and 14 post-MIA and that NSAID analgesia peaked on day 14 and decreased in efficacy thereafter86. This indicates a transition at days 8–14 from early inflammatory pain to late-stage inflammatory-neuropathic pain. Another study also showed that ATF3-immunoreactive growth-associated protein-43-immunoreactive DRG neurons significantly increased in the ipsilateral DRG 14 days after the injection 30. Additionally, an MIA injection resulted in a significant reduction in intra-epidermal nerve fiber density of the plantar hind paw post-MIA between days 7 and 14 87. Microgliosis, a characteristic of neuropathic pain 88, also occurs in the spinal cord 7–21 days post-MIA 30, 87. Taken together, these data suggest that neuronal damage occurs during the development of OA, which might explain why OA pain treatment and management is unsuccessful. It is not known whether surgery-induced and spontaneously-developed OA models show any characteristics of nerve injure, which warrant further investigation.
4. Supraspinal mechanisms
A study showed that electroacupuncture (EA) significantly improved MIA-induced hind limb incapacity within 7 days of the MIA injection; the effect was prevented by 5-HT 2A/C antagonism. This suggests that EA activates descending inhibitory 5-HT to inhibit early-stage OA-pain 89.
Studies in rats demonstrate that, compared to a saline injection, spinal WDR neurons show stimulus intensity-dependent and significantly enhanced response to mechanical stimulation 15–19 days after an intra-articular MIA injection. Response to thermal stimulation was also higher in MIA- than in saline-injected rats, although this response was not statistically significant. Further, spinal 5-HT3 antagonism significantly decreased the neuron response induced by tactile mechanical stimulus and produced greater inhibition of response to 45°C thermal stim ulation in MIA rats than in control. These data indicate tactile mechanical and noxious thermal stimulation-evoked responses are facilitated by spinal 5-HT3 receptors. Since spinal 5-HT is supraspinally produced, descending 5-HT/5-HT3 receptor facilitation might contribute to OA-induced pain. Moreover, the α2δ-1 subunit of voltage-gated calcium channels (VGCCs) increased in ipsilateral DRG in MIA rats compared to control. Consistent with these results, pregabalin inhibited spinal neuronal responses evoked by noxious electrical stimulation and innocuous and noxious natural stimulation in MIA but not in saline control rats. This inhibition was prevented by 5-HT3 receptor antagonism. Therefore, descending 5-HT/5-HT3 facilitation coupled with upregulation of α2δ-1 VGCC subunit expression might contribute to OA pain 90.
Recently, a study in the MIA model demonstrated that intraarticular lidocaine induces conditioned place preference for the lidocaine-paired compartment, indicating OA pain has an aversive component23. This suggests that OA pain also involves supraspinal mechanisms. Although clinical imaging studies demonstrate brain changes during chronic pain, image studies have not been performed in OA pain animal models until recently. In a MMT animal model, increased functional connectivity was observed 3–5 weeks post MMT in nucleus accumbens- and ventral posterior lateral thalamus-based functional connectivity analyses. Those changes were attenuated by sustained treatment with a broad-spectrum MMP inhibitor or acute treatment with celecoxib 91. In humans, gray matter decreases in the ACC, dorsolateral prefrontal cortex, amygdala, and brainstem were reversed when patients feel no hip OA-induced pain after hip replacement surgery. This suggests that brain changes are related to the pain 92. Additionally, it has been reported that nucleus accumbens activity is associated with spontaneous OA-induced pain 79 and facilitates nociception 93. Whether this increased functional connectivity during OA contributes to chronic pain requires further investigation.
5. Conclusion
OA is the most common form of joint disease. Recent studies on OA-induced pain demonstrate that peripheral pro-inflammatory mediators and neuropeptides can sensitize knee nociceptors (Fig. 1). The pro-nociceptive plasticity of spinal cytokines and neuropeptides promotes OA-associated pain. Peripheral and spinal cannabinoids might respectively inhibit OA pain through CB1 and CB1/CB2 receptors. TRPV1 contributes to OA pain (Fig. 1). MMP-13 inhibitor reduces MIA-induced cartilage damage and indirectly alleviates pain 55. Other bioactive chemicals such as sphingosine kinase-2, Rho kinase, kinin B(2) receptors, ROS, iNOS, cathepsin K, PGEs, nerve growth factor, and sodium channels are all involved in OA pain, and supraspinal descending facilitation of 5-HT/5-HT 3 receptors might contribute to OA pain. During OA, brain functional connectivity is enhanced, but how connectivity changes are related to OA pain remains elusive.
Acknowledgments
The authors’ work is supported by NIH Grants R21AT005474, P01AT002605, NS060735, and DE021804.
Role of funding source
The funding bodies had no role in the writing of this article.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Ruixin Zhang contributed to drafting, revisions and final approval of the article. Ke Ren and Ronald Dubner contributed to revisions and final approval of the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rui-Xin Zhang, Email: rzhan001@umaryland.edu.
Ke Ren, Email: kren@umaryland.edu.
References
- 1.Rainbow R, Ren W, Zeng L. Inflammation and Joint Tissue Interactions in OA: Implications for Potential Therapeutic Approaches. Arthritis. 2012;2012:741582. doi: 10.1155/2012/741582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology (Oxford) 2011;50:2157–2165. doi: 10.1093/rheumatology/ker283. [DOI] [PubMed] [Google Scholar]
- 3.Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg MC, Hunter DJ, Felson DT. Osteoarthritis. Br Med J. 2006;332:640–642. [Google Scholar]
- 5.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Bove SE, Laemont KD, Brooker RM, Osborn MN, Sanchez BM, Guzman RE, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthr Cartil. 2006;14:1041–1048. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Inglis JJ, McNamee KE, Chia S-L, Essex D, Feldmann M, Williams RO, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis Rheum. 2008;58:3110–3119. doi: 10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- 8.Marker CL, Pomonis JD. The monosodium iodoacetate model of osteoarthritis pain in the rat. Methods Mol Biol. 2012:851. doi: 10.1007/978-1-61779-561-9_18. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, et al. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003;65:1195–1199. doi: 10.1292/jvms.65.1195. [DOI] [PubMed] [Google Scholar]
- 10.Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003;31:619–624. doi: 10.1080/01926230390241800. [DOI] [PubMed] [Google Scholar]
- 11.Piscaer TM, Waarsing JH, Kops N, Pavljasevic P, Verhaar JA, van Osch GJ, et al. In vivo imaging of cartilage degeneration using microCT-arthrography. Osteoarthr Cartil. 2008;16:1011–1017. doi: 10.1016/j.joca.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Chandran P, Pai M, Blomme EA, Hsieh GC, Decker MW, Honore P. Pharmacological modulation of movement-evoked pain in a rat model of osteoarthritis. Eur J Pharmacol. 2009;613:39–45. doi: 10.1016/j.ejphar.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Vonsy JL, Ghandehari J, Dickenson AH. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain. 2009;13:786–793. doi: 10.1016/j.ejpain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza WN, Ng GY, Youngblood BD, Tsuji W, Lehto SG. A review of current animal models of osteoarthritis pain. Curr Pharm Biotechnol. 2011;12:1596–1612. doi: 10.2174/138920111798357320. [DOI] [PubMed] [Google Scholar]
- 15.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis”--the need for consensus? Osteoarthr Cartil. 2012;20:261–267. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthr Cartil. 2002;10:785–791. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- 17.Henry JL. Molecular events of chronic pain: from neuron to whole animal in an animal model of osteoarthritis. Novartis Found Symp. 2004;260:139–145. discussion 145–153, 277–139. [PubMed] [Google Scholar]
- 18.Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97:603–610. doi: 10.1016/j.pbb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Knights CB, Gentry C, Bevan S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain. 2012;153:281–292. doi: 10.1016/j.pain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Fenty MC, Dodge GR, Kassey VB, Witschey WR, Borthakur A, RR Quantitative cartilage degeneration associated with spontaneous osteoarthritis in a guinea pig model. J Magn Reson Imaging. 2012;35:891–898. doi: 10.1002/jmri.22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, et al. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011;98:35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, et al. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett. 2011;493:72–75. doi: 10.1016/j.neulet.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Embree MC, Kilts TM, Ono M, Inkson CA, Syed-Picard F, Karsdal MA, et al. Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am J Pathol. 2010;176:812–826. doi: 10.2353/ajpath.2010.090450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadhwa S, Embree M, Ameye L, Young M. Mice deficient in biglycan and fibromodulin as a model for temporomandibular joint osteoarthritis. Cells Tissues Organs. 2005;181:136–143. doi: 10.1159/000091375. [DOI] [PubMed] [Google Scholar]
- 26.Roemer FW, Kassim JM, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthr Cartil. 2010;18:1269–1274. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology (Oxford) 2011;50:2157–2165. doi: 10.1093/rheumatology/ker283. [DOI] [PubMed] [Google Scholar]
- 30.Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12:134. doi: 10.1186/1471-2474-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, et al. Percutaneously absorbed NSAIDs attenuate local production of proinflammatory cytokines and suppress the expression of c-Fos in the spinal cord of a rodent model of knee osteoarthritis. J Orthop Sci. 2012;17:77–86. doi: 10.1007/s00776-011-0175-7. [DOI] [PubMed] [Google Scholar]
- 32.Richter F, Natura G, Löser S, Schmidt K, Viisanen H, Schaible H-G. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum. 2010;62:3806–3814. doi: 10.1002/art.27715. [DOI] [PubMed] [Google Scholar]
- 33.Özaktay AC, Kallakuri S, Takebayashi T, Cavanaugh J, Asik I, DeLeo J, et al. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15:1529–1537. doi: 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 34.Kelly S, Dunham JP, Murray F, Read S, Donaldson LF, Lawson SN. Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthr Cartil. 2010;20:305–313. doi: 10.1016/j.joca.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Schuelert N, McDougall JJ. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum. 2008;58:145–153. doi: 10.1002/art.23156. [DOI] [PubMed] [Google Scholar]
- 36.Schuelert N, McDougall JJ. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett. 2009;465:184–188. doi: 10.1016/j.neulet.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 37.von Banchet GS, Kiehl M, Schaible HG. Acute and long-term effects of IL-6 on cultured dorsal root ganglion neurones from adult rat. J Neurochem. 2005;94:238–248. doi: 10.1111/j.1471-4159.2005.03185.x. [DOI] [PubMed] [Google Scholar]
- 38.Schaible HG, von Banchet GS, Boettger MK, Bräuer R, Gajda M, Richter F, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 39.Santangelo KS, Pieczarka EM, Nuovo GJ, Weisbrode SE, Bertone AL. Temporal expression and tissue distribution of interleukin-1β in two strains of guinea pigs with varying propensity for spontaneous knee osteoarthritis. Osteoarthr Cartil. 2011;19:439–448. doi: 10.1016/j.joca.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stemkowski PL, Smith PA. Long-term IL-1β exposure causes subpopulation-dependent alterations in rat dorsal root ganglion neuron excitability. J Neurophysiol. 2012;107:1586–1597. doi: 10.1152/jn.00587.2011. [DOI] [PubMed] [Google Scholar]
- 41.Schuelert N, Johnson MP, Oskins JL, Jassal K, Chambers MG, McDougall JJ. Local application of the endocannabinoid hydrolysis inhibitor URB597 reduces nociception in spontaneous and chemically induced models of osteoarthritis. Pain. 2011;152:975–981. doi: 10.1016/j.pain.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Malemud CJ. Anticytokine therapy for osteoarthritis: evidence to date. Drugs Aging. 2010;27:95–115. doi: 10.2165/11319950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Pinto LG, Cunha TM, Vieira SM, Lemos HP, Verri WA, Jr, Cunha FQ, et al. IL-17 mediates articular hypernociception in antigen-induced arthritis in mice. Pain. 2010;148:247–256. doi: 10.1016/j.pain.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Richter F, Natura G, Ebbinghaus M, von Banchet GS, Hensellek S, König C, et al. Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis Rheum. 2012;64:4125–4134. doi: 10.1002/art.37695. [DOI] [PubMed] [Google Scholar]
- 45.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 46.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Kim J-S, Wijnen A, Im H-J. Osteoarthritic tissues modulate functional properties of sensory neurons associated with symptomatic OA pain. Mol Biol Rep. 2011;38:5335–5339. doi: 10.1007/s11033-011-0684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Role of Interleukin-1 Inhibitors in Osteoarthritis: An Evidence-Based Review. Drugs Aging. 2012;29:343–358. doi: 10.2165/11599350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Sutton S, Clutterbuck A, Harris P, Gent T, Freeman S, Foster N, et al. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet J. 2009;179:10–24. doi: 10.1016/j.tvjl.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Schuelert N, McDougall JJ. Electrophysiological evidence that the vasoactive intestinal peptide receptor antagonist VIP6-28 reduces nociception in an animal model of osteoarthritis. Osteoarthr Cartil. 2006;14:1155–1162. doi: 10.1016/j.joca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 51.McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123:98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Schuelert N, Zhang C, Mogg AJ, Broad LM, Hepburn DL, Nisenbaum ES, et al. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthr Cartil. 2010;18:1536–1543. doi: 10.1016/j.joca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 54.Mapp PI, Walsh DA, Bowyer J, Maciewicz RA. Effects of a metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis. Osteoarthr Cartil. 2010;18:593–600. doi: 10.1016/j.joca.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baragi VM, Becher G, Bendele AM, Biesinger R, Bluhm H, Boer J, et al. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: Evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 2009;60:2008–2018. doi: 10.1002/art.24629. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed AS, Li J, Erlandsson-Harris H, Stark A, Bakalkin G, Ahmed M. Suppression of pain and joint destruction by inhibition of the proteasome system in experimental osteoarthritis. Pain. 2012;153:18–26. doi: 10.1016/j.pain.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Fitzpatrick LR, Green C, Maines LW, Smith CD. Experimental osteoarthritis in rats is attenuated by ABC294640, a selective inhibitor of sphingosine kinase-2. Pharmacology. 2011;87:135–143. doi: 10.1159/000323911. [DOI] [PubMed] [Google Scholar]
- 58.Takeshita N, Yoshimi E, Hatori C, Kumakura F, Seki N, Shimizu Y. Alleviating effects of AS1892802, a Rho kinase inhibitor, on osteoarthritic disorders in rodents. J Pharmacol Sci. 2011;115:481–489. doi: 10.1254/jphs.10319fp. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimi E, Kumakura F, Hatori C, Hamachi E, Iwashita A, Ishii N, et al. Antinociceptive effects of AS1892802, a novel Rho kinase inhibitor, in rat models of inflammatory and noninflammatory arthritis. J Pharmacol Exp Ther. 2010;334:955–963. doi: 10.1124/jpet.110.167924. [DOI] [PubMed] [Google Scholar]
- 60.Cialdai C, Giuliani S, Valenti C, Tramontana M, Maggi CA. Effect of Intra-articular 4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-8-quinolyloxymethyl)phenylsulfonamido]-tetrahydro-2H-4-pyranylcarbonyl} piperazino)-5-oxopentyl](trimethyl)ammonium chloride hydrochloride (MEN16132), a kinin B2 receptor antagonist, on nociceptive response in monosodium iodoacetate-induced experimental osteoarthritis in rats. J Pharmacol Exp Ther. 2009;331:1025–1032. doi: 10.1124/jpet.109.159657. [DOI] [PubMed] [Google Scholar]
- 61.Zhu CZ, Chin C-l, Rustay NR, Zhong C, Mikusa J, Chandran P, et al. Potentiation of analgesic efficacy but not side effects: Co-administration of an α4β2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochem Pharmacol. 2011;82:967–976. doi: 10.1016/j.bcp.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Naito Y, Yoshikawa T, Tanigawa T, Sakurai K, Yamasaki K, Uchida M, et al. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 63.Moon SJ, Woo YJ, Jeong JH, Park MK, Oh HJ, Park JS, et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthr Cartil. 2012;20:1426–1438. doi: 10.1016/j.joca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Castro RR, Cunha FQ, Silva FSJ, Rocha FA. A quantitative approach to measure joint pain in experimental osteoarthritis--evidence of a role for nitric oxide. Osteoarthr Cartil. 2006;14:769–776. doi: 10.1016/j.joca.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 65.Strassle BW, Mark L, Leventhal L, Piesla MJ, Jian LX, Kennedy JD, et al. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthr Cartil. 2010;18:1319–1328. doi: 10.1016/j.joca.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 66.McDougall JJ, Schuelert N, Bowyer J. Cathepsin K inhibition reduces CTXII levels and joint pain in the guinea pig model of spontaneous osteoarthritis. Osteoarthr Carti. 2010;18:1355–1357. doi: 10.1016/j.joca.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 67.Schaible HG. Mechanisms of Chronic Pain in Osteoarthritis. Curr Rheumatol Rep. 2012;14:549–556. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]
- 68.Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56:1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 69.Sagar DR, Burston JJ, Hathway GJ, Woodhams SG, Pearson RG, Bennett AJ, et al. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol Pain. 2011;7:88. doi: 10.1186/1744-8069-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee Y, Pai M, Brederson JD, Wilcox D, Hsieh G, Jarvis MF, et al. Monosodium iodoacetate-induced joint pain is associated with increased phosphorylation of mitogen activated protein kinases in the rat spinal cord. Mol Pain. 2011;7:39. doi: 10.1186/1744-8069-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Im H-J, Kim J-S, Li X, Kotwal N, Sumner DR, van Wijnen AJ, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010;62:2995–3005. doi: 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, Gibson G, Kim J-S, Kroin J, Xu S, van Wijnen AJ, et al. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene. 2011;480:34–41. doi: 10.1016/j.gene.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 2012;109:20602–20627. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sagar DR, Staniaszek LE, Okine BN, Woodhams S, Norris LM, Pearson RG, et al. Tonic modulation of spinal hyperexcitability by the endocannabinoid receptor system in a rat model of osteoarthritis pain. Arthritis Rheum. 2010;62:3666–3676. doi: 10.1002/art.27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388:75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 76.Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, et al. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydronaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010;150:319–326. doi: 10.1016/j.pain.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Chu KL, Chandran P, Joshi SK, Jarvis MF, Kym PR, McGaraughty S. TRPV1-related modulation of spinal neuronal activity and behavior in a rat model of osteoarthritic pain. Brain Res. 2011;1369:158–166. doi: 10.1016/j.brainres.2010.10.101. [DOI] [PubMed] [Google Scholar]
- 78.Kalff K-M, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, et al. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010;641:108–113. doi: 10.1016/j.ejphar.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 79.Parks EL, Geha PY, Balikil MN, Katzl J, Schnitzerl TJ, Apkarianl AV. Brain activity for chronic knee osteoarthritis: Dissociating evoked pain from spontaneous pain. Eur J Pain. 2011;15:843, e1–14. doi: 10.1016/j.ejpain.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saxler G, Loer F, Skumavc M, Pfortner J, Hanesch U. Localization of SP- and CGRP-immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur J Pain. 2007;11:67–74. doi: 10.1016/j.ejpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Ferland CE, Pailleux F, Vachon P, Beaudry F. Determination of specific neuropeptides modulation time course in a rat model of osteoarthritis pain by liquid chromatography ion trap mass spectrometry. Neuropeptides. 2011;45:423–429. doi: 10.1016/j.npep.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 83.Ferreira-Gomes J, Adães S, Sarkander J, Castro-Lopes JM. Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum. 2010;62:3677–3685. doi: 10.1002/art.27713. [DOI] [PubMed] [Google Scholar]
- 84.Ferland CE, Beaudry F, Vachon P. Antinociceptive effects of eugenol evaluated in a monoiodoacetate-induced osteoarthritis rat model. Phytother Res. 2012;26:1278–1285. doi: 10.1002/ptr.3725. [DOI] [PubMed] [Google Scholar]
- 85.Ogbonna AC, Clark AK, Gentry C, Hobbs CMM. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur J Pain. 2012 doi: 10.1002/j.1532-2149.2012.00223.x. [DOI] [PubMed] [Google Scholar]
- 86.Ivanavicius SP, Ball AD, Heapy CG, Westwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: Increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128:272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DL. Characterisation of a peripheral neuropathic component of the rat monoiodoacetate model of osteoarthritis. PLoS One. 2012;7:e33730. doi: 10.1371/journal.pone.0033730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234:271–282. doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Li A, Zhang Y, Lao L, Xin J, Ren K, Berman BM, et al. Serotonin Receptor 2A/C Is Involved in Electroacupuncture Inhibition of Pain in an Osteoarthritis Rat Model. Evid Based Complement Alternat Med. 2011;2011:619650. doi: 10.1093/ecam/neq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain. 2009;5:45. doi: 10.1186/1744-8069-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Upadhyay J, Baker SJ, Rajagovindan R, Hart M, Chandran P, Hooker BA, et al. Pharmacological modulation of brain activity in a preclinical model of osteoarthritis. NeuroImage. 2013;64:341–355. doi: 10.1016/j.neuroimage.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gear RW, Levine JD. Nucleus accumbens facilitates nociception. Exp Neurol. 2011;229:502–506. doi: 10.1016/j.expneurol.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]