Abstract
Arginine deprivation is a promising strategy for treating ASS-negative malignant tumors including melanoma. However, autophagy can potentially counteract the effectiveness of this treatment by acting as a pro-survival pathway. By combining TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) with arginine deprivation using ADI-PEG20 (pegylated arginine deiminase), we achieved enhanced apoptosis and accelerated cell death in melanoma cell lines. This implies a switch from autophagy to apoptosis. In our current investigation, we found that TRAIL could induce the cleavage of two key autophagic proteins, Beclin-1 and Atg5, in the combination treatment. Using specific inhibitors for individual caspases, we found caspase-8 inhibitor could completely abolish the cleavage. Furthermore, caspase-8 inhibitor was able to fully reverse the enhanced cytotoxicity induced by TRAIL. Inhibitors for caspase-3, 6, 9, and 10 were able to block the cleavage of these two autophagic proteins to some extent and correspondingly rescue cells from the cytotoxicity of the combination of TRAIL and arginine deprivation. In contrast, calpain inhibitor could not prevent the cleavage of either Beclin-1 or Atg5, and was unable to prevent cell death. Overall, our data indicate that the cleavage of Beclin-1 and Atg5 by TRAIL-initiated caspase activation is one of the mechanisms that lead to the enhancement of the cytotoxicity in the combination treatment.
Keywords: TRAIL, ADI-PEG20, Autophagy, Caspase, Beclin-1, Atg5
Introduction
Melanoma is very recalcitrant to available treatment once metastasis has taken place[1]. A new treatment using arginine deprivation has been investigated to treat melanoma [2,3]. The rationale for this treatment is based on the fact that some melanomas do not express argininosuccinate synthetase (ASS), a key enzyme for arginine biosynthesis [4-6] and hence is auxotrophic for arginine. The normal cells, on the other hand, usually express this enzyme and are prototrophic for arginine. Thus, arginine deprivation using arginine degrading enzyme (arginine deiminase or arginase) has been shown to have targeted antitumor activity against melanoma both in vitro and in vivo[5]. Clinical trial using pegylated arginine deiminase (ADI-PEG20) has shown antitumor activity in melanomas which do not express ASS [2]. Nonetheless, not all ASS-negative patients showed tumor shrinkage [7]. In this regard, we and others have found that arginine deprivation through ADI-PEG20 results in autophagy before apoptosis. The apoptotic process usually does not occur until 3-4 days in arginine depleted media. Importantly, the remaining non-apoptotic cells can resume growth after arginine repletion. It is known that autophagy is a pro-survival pathway to cope with the nutritional stress such as arginine deprivation as a means to provide arginine from the degraded non essential proteins. Consequently, the cell death caused by ADI-PEG20 is delayed. During this delay we have found that some tumor cells can re-express ASS and become resistant to this treatment [8]. In order to improve the therapeutic efficacy of ADI-PEG20 treatment, an additional agent is needed to disrupt autophagy and re-direct the cells toward apoptosis.
We have previously found that by combining tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) with ADI-PEG20, we could effectively increase the apoptosis in melanoma cells in culture [9]. This combination treatment can produce appreciably more cell death than can either agent alone. We also found that the increased cell death in the combination treatment was accompanied by an elevation in caspase activity which was primarily due to increased level of truncated Bid (tBid), a pro-apoptotic protein that links the extrinsic apoptosis pathway triggered by TRAIL and the intrinsic apoptosis pathway that can be initiated by nutritional stress [9]. The question remains whether the addition of TRAIL also affects other pro-survival pathways like autophagy, besides the pro-apoptotic pathway. In our present study, we hypothesize that TRAIL will somehow attenuate the autophagy pathway in arginine deprivation treatment. We investigate this possibility using two melanoma cells lines, A375 and A2058.
Materials and Methods
Cell culture
Melanoma cell lines A375 and A2058 were purchased from ATCC (American Type Culture Collection) and maintained with MEM (Invitrogen) containing 10% FBS (Atlanta Biologicals) and streptomycin/penicillin (Invitrogen) in a humidified incubator with 5% CO2 at 37°C. Both cell lines do not express ASS; however, A2058 can be induced to re-express ASS in arginine-depleted medium while A375 cannot [10,11]. A375 cell line stably expressing GFP-LC3 was created by transfecting plasmid encoding GFP-LC3 fusion protein into the parental A375 cell line with FuGENE 6 transfection reagent (Roch Applied Science). The transfectants were selected by 500 g/ml of G418 (Sigma-Aldrich) and sorted by a FACSAria I (BD Biosciences) cell sorter based on the GFP fluorescence. The resulting cells were kept in complete MEM plus 500 g/ml of G418.
Arginine-free medium preparation
ADI-PEG20 was provided by Polaris, Inc. Complete MEM medium was incubated with 0.1 g/ml of ADI-PEG20 for 48 h to simulate the arginine-depletion condition. We have previously shown that there is no detectable arginine level in this medium since all arginine available was converted to citrulline [11].
Drug Treatment
Soluble recombinant human TRAIL stock solution in PBS was obtained from National Cancer Institute. Inhibitors for caspase-3 (Z-DEVD-FMK), 6 (Z-VEID-FMK), 8 (Z-IETD-FMK), 9 (Z-LEHD-FMK), 10 (Z-AEVD-FMK) and general caspase inhibitor (Z-VAD-FMK) were purchased from R&D Systems, and the inhibitor for calpain (ALLN) was from Calbiochem. All the inhibitors were dissolved in DMSO (dimethyl sulfoxide). Arginine-free medium was used for cell culture with or without TRAIL at 100 ng/ml for specified period of time. All the caspase inhibitors were used at 25 M and added right before TRAIL. The same volume of DMSO was added to controls as vehicle. In all wells, the final concentration for DMSO was 0.125% (v/v).
Cell viability assay
Cells were seeded at 5000 cells/well in a 96-well plate and incubated overnight, and then treated for 24 h. The percentage of viable cells was determined with MTS assay with modified procedure [12]. Briefly, after treatment, the medium with drug was removed and replaced with 100 l of 4.5 g/L glucose in DPBS. Then, 20 l of the CellTiter 96 AQueous One Solution (Promega) was added to each well. After mixing, the plate was returned to cell culture incubator and incubated for 2 h. The plate was read in a Bio-Rad 680 microplate reader at 490 nm.
Detection of apoptosis and GFP-LC3 by flow cytometry
The Annexin V:FITC Assay Kit (AbD SeroTec) was used to label cells which have been treated with drugs. Briefly, the adherent cells (180000 cells/well seeded overnight) were treated in a 12-well plate. The medium was collected; cells were washed once with DPBS. The wash was combined with the collected medium. The cells were detached with 0.25% Typsin/EDTA (Invitrogen) and combined with the collected medium and DPBS. After centrifugation and removal of the supernatant, the cells were washed once with DPBS. Then the cells were processed according to the instruction of the staining kit. The data were collected in an Accuri C6 flow cytometer and analyzed with its bundled CFlow Plus software. For measuring the GFP fluorescence of GFP-LC3 fusion protein stably expressed in A375 cells, the cells were collected using trypsinization as above and washed twice with DPBS. The cells were then fixed with 1% formaldehyde in DPBS and analyzed similarly as above.
Cell lysate preparation
All cells were collected in DPBS containing protease (P8340) and phosphatase (P5726) inhibitors (Sigma-Aldrich) at 1:1000 dilution. The cell pellet was resuspended with 1X lysis buffer (Cell Signaling Technology) plus 1:1000 dilution of protease and phosphatase inhibitors (same as above) and sonicated briefly on ice. The total protein concentration of the lysate was determined with the Micro BCA Protein Assay Kit from Pierce. The prepared lysate then was mixed with SDS-PAGE sample buffer and heated at 100°C for 10 min. The processed lysate can be loaded to SDS-PAGE gel or stored at -80°C.
Immunoblotting
Polyclonal antibody for Atg5 was from Abgent (1:200), and Beclin-1 monoclonal antibody (1:1000) was from BD Biosciences. Polyclonal antibody for LC3B was purchased from Cell Signaling Technology and used at 1:1000. Horseradish peroxidase-conjugated anti-rabbit IgG (H+L) and anti-mouse IgG (H+L) (1:5000) were from Promega. Cell lysate with equal amount of total protein was resolved by SDS-PAGE (4% for stacking gel and 10% or 12.5% for separating gel) and electrotransferred at 250 mA for 2 h to a piece of PVDF membrane in transfer buffer (25 mM Tris, 192 mM glycine, 15% (v/v) methanol). This membrane was then blocked with 5% milk (Bio-Rad) in TBS buffer. After incubation sequentially with primary antibody and horseradish peroxidase-conjuaged secondary antibody, the target protein bands were visualized with SuperSignal West Femto (Thermo-Fisher). The image of bands was captured with a Bio-Rad ChemDoc system. Quantity One (Bio-Rad) was used to measure the optical density of protein bands detected by western blot, and ImageJ was used to estimate the molecular weight of specific protein bands
Statistical Analysis
Data in bar graphs were represented as mean ± SD. Student's t-test was used to compare the two sets of data with p < 0.05 as statistically significant.
Results
Combination of TRAIL and arginine deprivation accelerated cell death in melanoma cell lines
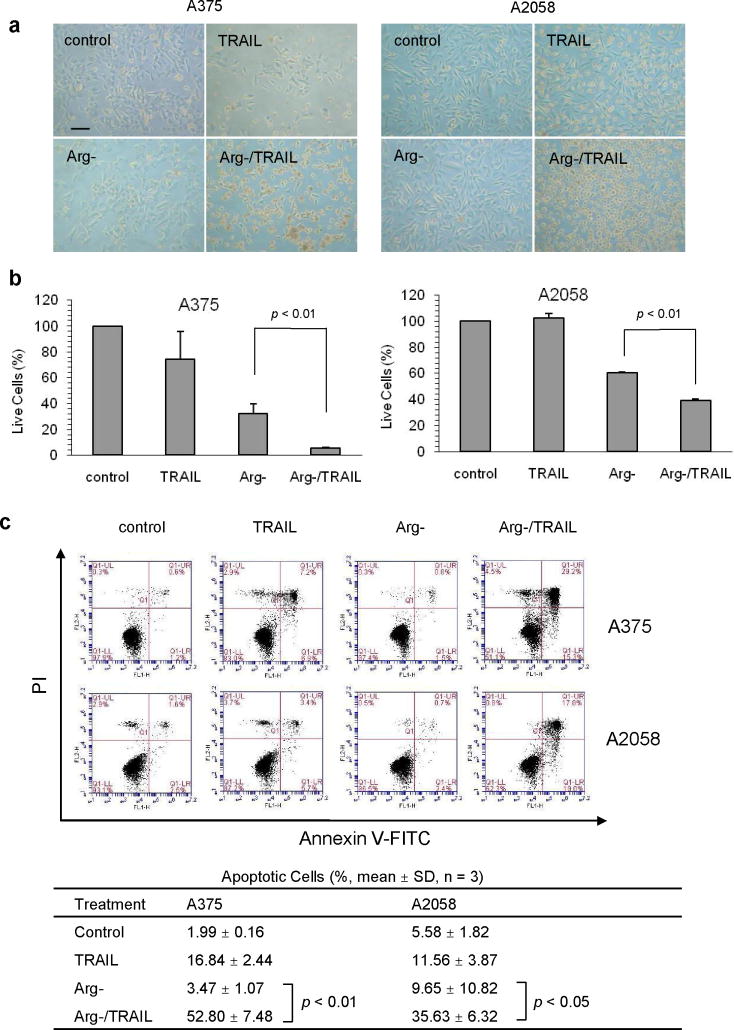
We have shown previously that ASS-negative melanoma cells are sensitive to the combination of ADI-PEG20 and TRAIL treatment [9]. In this study, we use ADI-PEG20-treated medium to simulate the condition of arginine deprivation (see Materials and Methods). As can be seen from Fig. 1a, almost no cell death was detected with this medium alone after 24 h of incubation. While some cell death could be observed in TRAIL-only treatment, it was much more pronounced in the combination treatment. Fig. 1b shows that arginine-free medium could effectively inhibit the proliferation of melanoma cells in both A375 and A2058 at 24 h to 32 ± 7% and 60 ± 0.2%, respectively, while TRAIL alone had almost no effect on A2058 (102 ± 3% ) and with some reduction of cell viability in A375 (74 ± 21%). The combination of both drugs significantly reduced live cells in A375 to less than 10% and less than 40% in A2058. We further determined the apoptotic cell fractions in different treatments by flow cytometry. As shown in Fig. 1c, the total apoptotic cells (Annexin V-positive) increased from 3.47 ± 0.07% in arginine-free media to 52.80 ± 7.48% in combination treatment for A375 (p < 0.01) and from 9.65 ± 10.82% to 35.63 ± 6.32% in A2058 (p < 0.05). TRAIL-alone treatment resulted in about 12-17% apoptotic cells in both cell lines (Fig. 1c). In each treatment, the observed necrotic cell fraction (PI-positive and Annexin V-negative) was less than 7% (see dot plots in Fig. 1c). This suggests that apoptosis did play an important role in the cell death process in the combined treatment. Overall, these data clearly show that TRAIL and arginine deprivation together can promote cell death in melanoma. In addition, these findings are consistent with our previous results using ADI-PEG20 for treatment instead of arginine-free medium. However, it takes longer exposure (72 h) with ADI-PEG20 to yield similar results [9].
Fig. 1.
Combination of TRAIL and arginine deprivation (Arg-) enhanced cell death in melanoma cells. Arginine-free medium (Arg-) and TRAIL at 100 ng/ml were used for treatment of cell lines for 24 h. a) Phase contrast microscopic view of cells. The scale bar = 10 m. b) Live cells were determined with MTS assay with the live cells of the control for each cell line set to 100%. The error bar stands for SD (n = 3). c) The enhanced cell death (positive PI staining) was accompanied by increased apoptosis seen as positive Annexin V-FITC staining detected with flow cytometry. The percentage of apoptotic cells from three independent experiments is summarized in the table below the dot plots
TRAIL attenuated the autophagic process which occured during arginine deprivation
We have previously shown that arginine deprivation was able to induce autophagy in melanoma cells [11]. Indeed, autophagy is pro-survival under this stressful condition. Here we used LC3 which is one of the Atg proteins needed for autophagosome membrane expansion to quantify the extent of autophagy. During autophagy, LC3 is conjugated to phosphoethanolamine in the phagophore membrane (precursor of autophagosome) to form LC3-II and serves as a marker for autophagy [13]. It is degraded after the fusion of autophagosomes and lysosomes, leading to the decrease of this protein. In our study, we have transfected A375 with GFP-LC3, and the stable transfectants were used to determine the levels of autophagy before and after treatment using flow cytometry (Fig. 2). As autophagy continues, the GFP fluorescence intensity decreases as a result of the degradation of GFP-LC3-II that has been incorporated in the autophagosome membrane [14]. Fig. 2 shows that after cells were deprived for arginine for 12 h, there was a decrease of fluorescence compared with control (from 100% to 54.23 ± 4.83%) while TRAIL-alone treatment had an increase in fluorescence over control (data not shown). The fluorescence was restored partially when TRAIL was combined with arginine deprivation (71.00 ± 7.32%), indicating the attenuation of autophagy. When Z-VAD (Z-VAD-FMK), a pan-caspase inhibitor was added in the treatment, the restoration of fluorescence intensity was inhibited (60 ± 7.63%). These results also indicate that the attenuation of autophagy by TRAIL may be mediated via caspase.
Fig. 2.
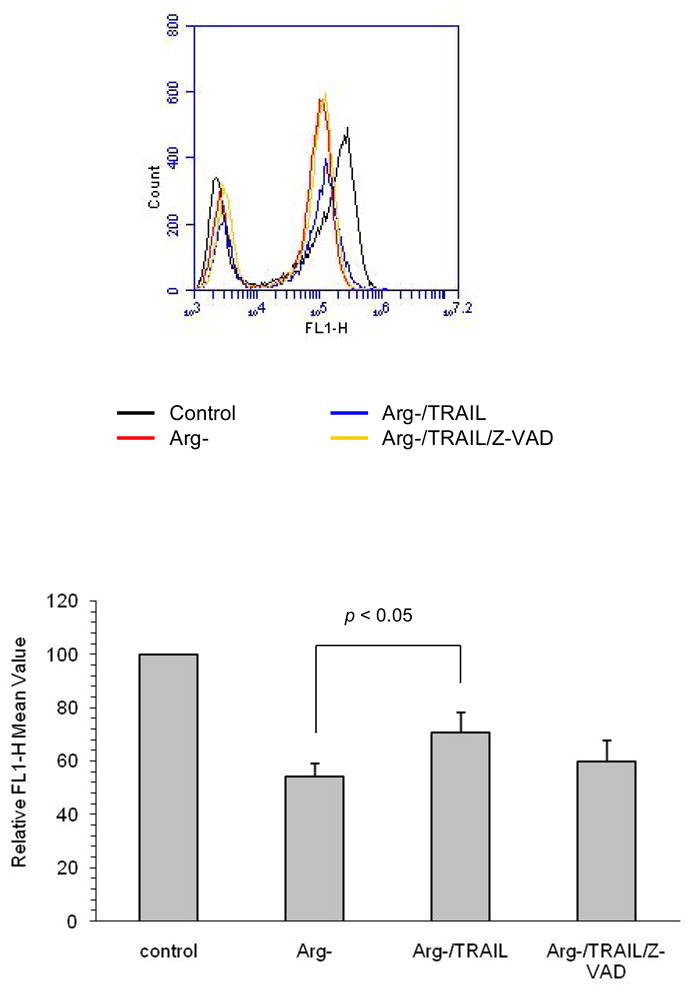
Attenuated autophagy during combination treatment. A375 cell line stably expressing GFP-LC3 was treated with arginine-free medium (Arg-) or combined with TRAIL at 100 ng/ml for 12 h. Z-VAD was used at 25 M and added at the beginning of the treatment. The intensity of GFP-LC3 was detected in the FL1-H channel with flow cytometry. The mean intensities of GFP-LC3 for each treatment from three independent experiments are tabulated below the histogram
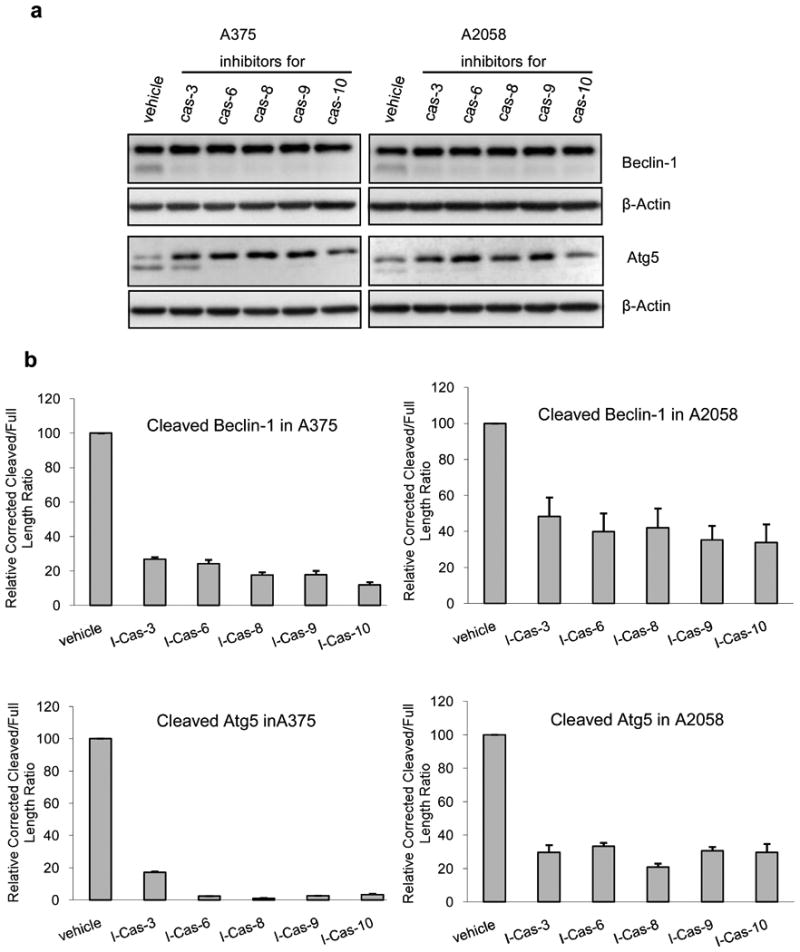
Beclin-1 and Atg5 cleavage upon TRAIL and arginine deprivation
Here we investigated the fate of two of the major proteins in the autophagic pathway. Beclin-1 and Atg5 are essential for autophagosome formation. It has also been reported that these proteins were affected during autophagy to apoptosis switch [15-21]. In our experiments, these two proteins showed a slight but discernable increase in response to arginine deprivation (Fig. 3a). However, in the combination treatment, there was cleavage of these proteins, and their full-length form was reduced. The relative corrected cleave/full length ratio of Beclin-1 increased from around 1.7 in arginine deprivation-alone and TRAIL-alone to about 7.3 (combination) in A375 suggesting the cause of the attenuated autophagy. Similar results were also obtained with A2058 (Fig 3b). For Atg5, the relative corrected cleaved/full length ratio also increased from about 2.0 (Arg-) to 45.8 ± 13.7 (combination) in A375 and from 1.7 (Arg-) to 8.9 ± 2.2 (combination) in A2058. Using ImageJ, we estimated the major cleavage product of Beclin-1 is about 48 kDa, and the one for Atg5 is approximately 30 kDa. While the pattern of Beclin-1 cleavage was similar to that reported by some laboratories [17,20], the size of the major cleavage product is different from those reported by others, which is around 37 kDa in their cases [15,21]. The size of Atg5 cleavage product also differed from the 24 kDa fragment generated by calpain [16,19,22]. Since the addition of TRAIL increases tBid and apoptosis [9], it is reasonable to postulate that the caspase activation also occured. Indeed, we have found activation of caspase-3, 8 and 9 as indicated by the production of their cleaved products (Fig. 3c). At 24 h post treatment in these two cell lines, arginine deprivation alone did not cause appreciable caspase activation, and TRAIL-alone treatment induced weaker activation for caspase-8 and 9 compared to the combination treatment (Fig. 3d). Only for caspase-3, the activation by TRAIL-alone was higher than that of the other two caspases. Nevertheless, the activation was still not as high, at around 57% and 70% of that triggered by the combination in A375 and A2058, respectively. These data indicate a strong correlation between caspase activation and the cleavage of Atg5 and Beclin-1.
Fig. 3.
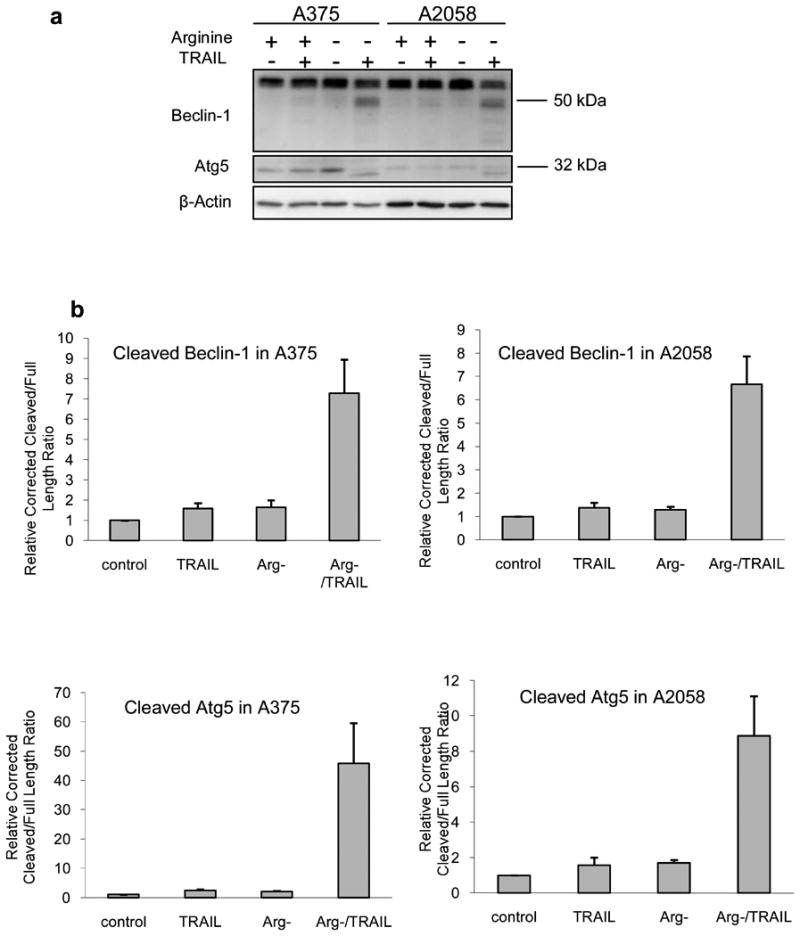
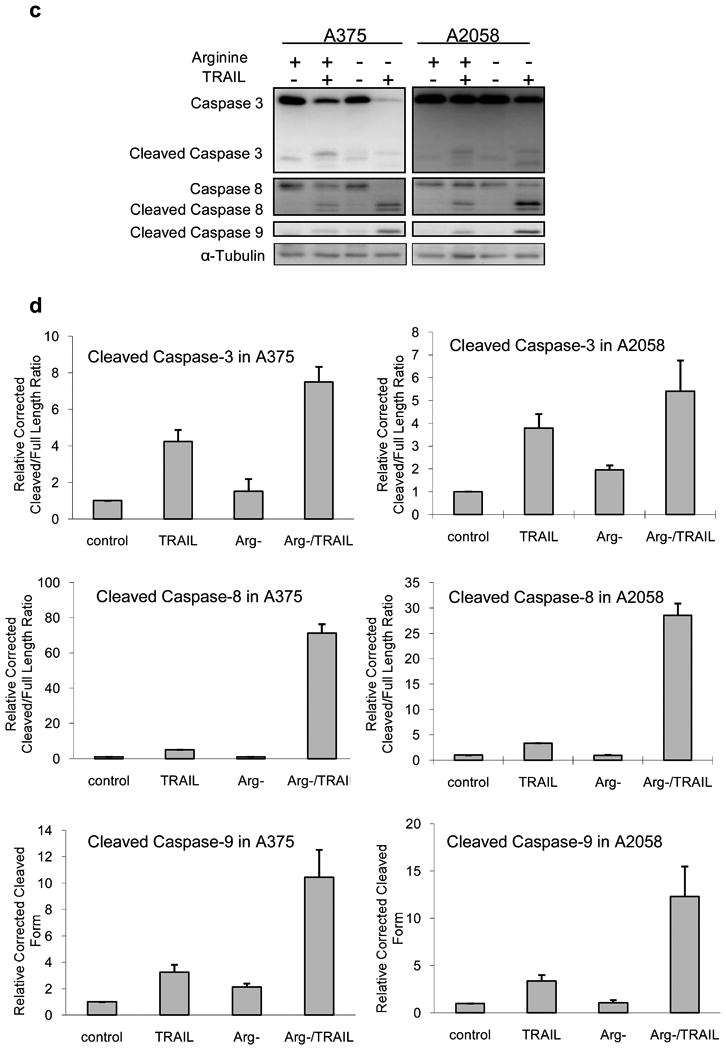
Cleavage of Beclin-1 and Atg5 in the combination treatment was correlated with caspase activation. Melanoma cells were cultured in normal or arginine-free medium (Arg-) with or without 100 ng/ml of TRAIL for 24 h. Cell lysate was analyzed with western blot. a) Cleavage of Beclin-1 and Atg5. b) Relative densitometric ratio of the major cleavage product of Beclin-1 or Atg5 to its corresponding full length form corrected by loading control (β-actin). The ratio for the corrected control was set to 1 (n = 3). c) The activation of caspase-3, 8, and 9 as detected by the processed (cleaved) products accompanying the autophagic protein cleavage. d) Relative densitometric ratio of the cleavage product(s) of caspase-3 or 8 to the corresponding full length form. For caspase-9, only the cleaved product was measured. All the measurement was corrected with the density of the loading control (α-tubulin), and the corrected value for the control was set as 1 (n = 3). One blot from three independent experiment with similar results was shown in panel a or c
Inhibition of caspases could prevent the cleavage of Beclin-1 and Atg5 and rescue melanoma cells from cell death caused by the combination treatment
To further prove that caspases are responsible for the cleavage of Beclin-1 and Atg5, we have used different specific caspase inhibitors to probe this possibility. As can be seen from Fig. 4a and 4b, all the inhibitors were able to prevent the cleavage of Beclin-1. The inhibition is more pronounced in A375 compared to A2058. For Atg5, caspase-8 inhibitor is the most potent one to inhibit the cleavage of Atg5 while caspase-3 inhibitor is the weakest (see Fig. 4a and 4b). Importantly, these inhibitors were able to rescue the cells from death and nullify the effect of TRAIL (Fig. 4c), thus making the combination treatment similar to that of arginine deprivation-only treatment. It is noteworthy that caspase-8 inhibitor which is the most potent in inhibiting the Atg5 cleavage appears to have the greatest effect in reversing the apoptotic process with the percentage of live cells by MTS assay as 97% for A375 and 96% for A2058 (arginine free media was set at 100%). In contrast, caspase-3 inhibitor which can only reverse the cleavage of Atg5 to a much lesser extent in A375 also achieved limited reversal of cell death. Inhibitors for caspase-6, 9, and 10 showed varying ability to prevent the cleavage of Atg5 and reduce cell death, which was not fully as potent as caspase-8 inhibitor in both cell lines. Taken together, our data suggest that multiple caspases can participate in the cleavage of autophagic proteins and halt this process. Interestingly, the inhibitor of calpain, ALLN, was unable to inhibit the cleavage of these two autophagic proteins as shown in Fig. 4d, while the pan-caspase inhibitor, Z-VAD, could completely block the cleavage. In contrast to caspase inhibitors, combination treatment with ALLN resulted in massive cell death observed for both cell lines (Fig. 4e). In general, our data suggest that the cleavage of Beclin-1 and Atg5 was due to caspase but not calpain activity.
Fig. 4.
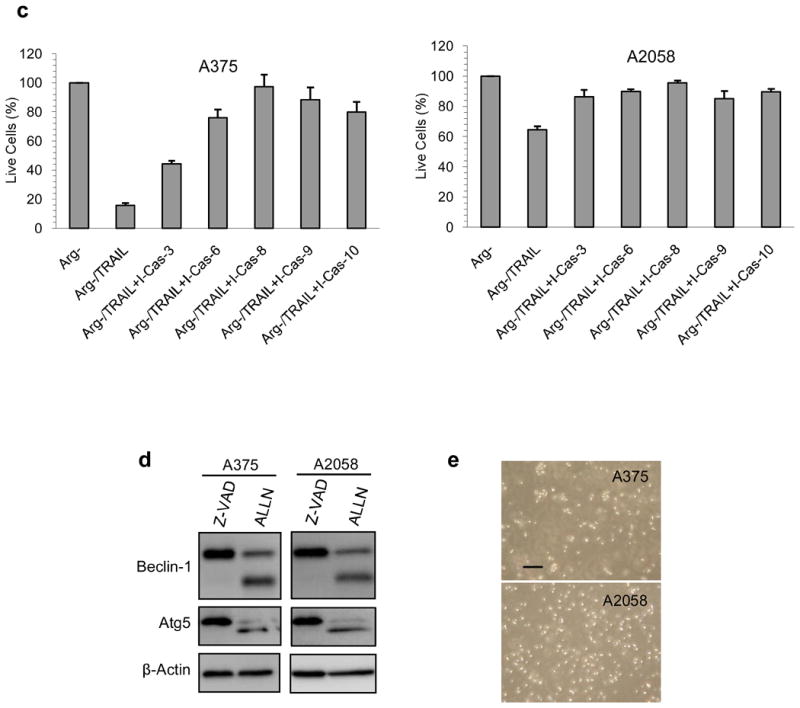
The effect of caspase inhibitors on the cleavage of Atg5 and Beclin-1 in the combination treatment and cell death. A375 and A2058 cells were treated with arginine-free medium and TRAIL at 100 ng/ml. Inhibitors were added at the beginning of the treatment at 25 M and incubated for 24 h before the cells were processed. a) Inhibition of different caspases could inhibit the cleavage of Beclin-1 and Atg5 to different extent as detected by western blot. b) Relative densitometric ratio of cleaved Beclin-1 or Atg5 versus the respective full length form. The ratio was corrected with the density of the loading control (β-actin), and the corrected value for the control was set as 100% (n = 3). “I-Cas” stands for inhibitor for caspase. c) The corresponding rescue of melanoma cells from cell death when different caspase inhibitors were added was detected with MTS assay. The live cells in arginine-free medium (Arg-) are set to 100%. The error bar stands for SD (n = 3). d) Z-VAD inhibits the cleavage of Beclin-1 and Atg5, but ALLN the calpain inhibitor was unable to do so after the cells were treated with the combination of TRAIL (100 ng/ml) and arginine-free medium for 24 h. Z-VAD and ALLN were added at the beginning of the treatment at 25 M. e) Phase contrast microscopy for cells treated as in panel e. Scale bar = 10 m. Every experiment was carried out independently for three times with similar results
Overall, we have found that by inhibiting caspase activities during the combination treatment, we were able to prevent the cleavage of key autophagic proteins and simultaneously block the cells from undergoing apoptosis. This indicates that caspase activation not only induces apoptosis but also attenuates autophagy.
Discussion
Melanoma is known to be resistant to chemotherapy, and targeted therapy is needed to improve treatment of this disease. Recent development of BRAF inhibitor to target V600E mutation has been successful but resistance is almost inevitable and usually occurs in 6-10 months. Another targeted approach is to use arginine degrading enzyme in melanomas which do not express ASS and hence cannot synthesize arginine. Using this approach to treat melanoma, we have found that while some ASS negative patients responded to treatment, others showed only stable disease [2]. In our laboratory, we have found that cells can undergo autophagy as a mean to survive during arginine deprivation which could explain stable disease seen clinically. In this report, we have found a way to circumvent this process by using TRAIL to disrupt autophagy and accelerate apoptosis.
Many investigations have shown the pro-survival nature of autophagy for cancer cells under different kinds of stress induced by chemotherapeutic drugs or nutrition deprivation, oxidative or ER stress [16,23-25]. Autophagy is a catabolic process in which cellular components such as organelles and proteins are enclosed by a double-membrane structure called autophagosome and delivered through fusion to late endosomes or lysosomes for degradation [26]. From its initiation to completion, autophagy requires a series of proteins termed as Atg proteins. Among all these proteins, Beclin-1 (Atg6) [27] and Atg5 [28] have been under rigorous investigation in many laboratories. They are both essential for the formation of autophagosomes and hence critical for the autophagic process. Autophagy occurring as a result of arginine depletion in ASS-negative cells serves as a means to evade apoptosis and an attempt to survive during stress. Thus, the efficacy of this form of treatment will not only rely on the ASS expression but also on the ability of these cells to undergo autophagy. Although complete arginine deprivation will eventually lead to apoptosis, cell death may take a long time (3-7 days depending on cell lines) to occur and may not be achievable in the clinical setting. Importantly, during this autophagic process, certain melanoma cells can re-express ASS to overcome arginine deprivation treatment [11]. Thus, to improve the therapeutic efficacy of arginine deprivation treatment, it is essential to stop the autophagic process. In fact, we have previously silenced Beclin-1 using siRNA combined with arginine deprivation for melanoma cells and we did observe increased cell death [29].
Many compounds have been shown to inhibit the autophagic process; however, the majority of them are non-specific and require high drug concentrations. Among them, hydroxychloroquine has been studied and entered into clinical trial [30]. However, since the drug concentration required to inhibit autophagy is high, it can result in toxicity. Other compounds are under clinical development [31]. Recently, it has been shown that proteases such as activated caspases and calpain can cleave Beclin-1 and Atg5 and impede the autophagic process [15-22].
In this communication, we have shown that the addition of TRAIL resulted in the cleavage of Beclin-1 and Atg5, which led to decreased autophagy and produced an increase in apoptosis. The process is also enhanced by increased DR4/5 upon arginine deprivation [9], and binding of TRAIL to these death receptor resulted in the activation of caspase-8 or 10 and subsequently other downstream caspases such as caspase-9 and then caspase-6 or caspase-3. We noted that caspase-8 inhibition could virtually abolish the cleavage of Beclin-1 and Atg5 and fully reverse the accelerated death induced by TRAIL combination. Other inhibitors were not as potent in this regard. This is consistent with the pivotal role of caspase-8 in initiating the TRAIL-associated extrinsic apoptosis pathway. The likelihood of caspase-8 to be the main caspase to cleave Beclin-1 is corroborated by the recent report that activated caspase-8 can cleave Beclin-1 in colon cancer cell line treated with DNA damaging agents [20]. It is possible that caspase-8 and other caspases can also cleave Atg5. From our data and previous work [9], we postulate that the addition of TRAIL activates caspase-8, and to a lesser extent, caspase-10 which then cleaves Bid and links the intrinsic pathway (activated by arginine deprivation) with the extrinsic pathway. This leads to further activation of caspases-8, 10 and 9 which in turn activates downstream caspase-6 and 3. The activation of these caspases also result in cleavage of autophagic proteins which halted the autophagic process and re-directing ASS-negative melanoma cells toward apoptosis.
At present, our data indicate that calpain is not responsible for the cleavage of Beclin-1 and Atg5 in our treatment, Instead, inhibition of calpain activity appeared to increase cell death in the combination treatment. It is possible that when calpain activity was blocked in this treatment, there was a compensatory increase in caspase activation which led to increased cleavage of Beclin-1 and Atg5. Thus the autophagic process was attenuated, and apoptosis pathway was chosen.
It has been noted that the cleavage product of Beclin-1 by caspase [16] and Atg5 by calpain [19] would translocate to mitochondria to facilitate the release of cytochrome c, thus enhancing the apoptosis. However, we still do not know the cleavage sites in Beclin-1 and Atg5 in our case. Further investigation is needed to answer these questions and to find out whether the cleavage products also have apoptosis-promoting functions.
Overall, our present and previous data showed that the addition of TRAIL improves the therapeutic efficacy of ADI-PEG20 treatment through both halting the autophagic process by cleavage of key autophagic proteins and accelerating the apoptosis process. This combination should be explored for future combination treatment for ASS-negative melanoma patients. TRAIL has been in the clinical trial with some activity; however, because of its short half-life, continuous infusion is needed, which is quite cumbersome. To overcome this, monoclonal antibody agonists to DR5 and DR4 have developed and are undergoing clinical trial. Thus, it is possible to incorporate these monoclonal antibodies with ADI-PEG20 for the treatment of ASS negative melanoma. Since there are other tumor types which do not express ASS such as mesothelioma [32,33], hepatocellular carcinoma [34], prostate carcinoma [32,35], and ovarian cancer [36], this approach can also be applied to treat other ASS negative tumors.
Acknowledgments
We thank Polaris, Inc. for providing ADI-PEG20 for our investigation. This work was supported by grants from the National Institutes of Health R01CA109578 and R01CA152197.
Abbreviations
- ADI
arginine deiminase
- ASS
argininosuccinate synthetase
- DMSO
dimethyl sulfoxide
- DPBS
Dulbecco's phosphate-buffered saline
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HIF1&alpha
hypoxia-inducible factor 1 alpha
- kDa
kilodalton
- MEM
minimum essential media
- PEG
polyethylene glycol
- PI
propidium iodide
- PVDF
polyvinylidene fluoride
- SD
standard deviation
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- siRNA
small interfering ribonucleic acid
- TBS
tris (hydroxymethyl)aminomethane-buffered saline
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
References
- 1.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23(6):488–496. [PMC free article] [PubMed] [Google Scholar]
- 2.Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci MT, Ensor CM, Prestayko AW, Holtsberg FW, Bomalaski JS, Clark MA, Savaraj N, Feun LG, Logan TF. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23(30):7660–7668. doi: 10.1200/JCO.2005.02.0933. [DOI] [PubMed] [Google Scholar]
- 3.Feun L, Savaraj N. Pegylated arginine deiminase: a novel anticancer enzyme agent. Expert Opin Investig Drugs. 2006;15(7):815–822. doi: 10.1517/13543784.15.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takaku H, Takase M, Abe S, Hayashi H, Miyazaki K. In vivo anti-tumor activity of arginine deiminase purified from Mycoplasma arginini. Int J Cancer. 1992;51(2):244–249. doi: 10.1002/ijc.2910510213. [DOI] [PubMed] [Google Scholar]
- 5.Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62(19):5443–5450. [PubMed] [Google Scholar]
- 6.Wheatley DN, Campbell E. Arginine deprivation, growth inhibition and tumour cell death: 3. Deficient utilisation of citrulline by malignant cells. Br J Cancer. 2003;89(3):573–576. doi: 10.1038/sj.bjc.6601134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feun LG, Marini A, Walker G, Elgart G, Moffat F, Rodgers SE, Wu CJ, You M, Wangpaichitr M, Kuo MT, Sisson W, Jungbluth AA, Bomalaski J, Savaraj N. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer. 2012;106:1481–1485. doi: 10.1038/bjc.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des. 2008;14(11):1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You M, Savaraj N, Wangpaichitr M, Wu C, Kuo MT, Varona-Santos J, Nguyen DM, Feun L. The combination of ADI-PEG20 and TRAIL effectively increases cell death in melanoma cell lines. Biochem Biophys Res Commun. 2010;394(3):760–766. doi: 10.1016/j.bbrc.2010.03.066. doi:S0006-291X(10)00517-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai WB, Aiba I, Lee SY, Feun L, Savaraj N, Kuo MT. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther. 2009;8(12):3223–3233. doi: 10.1158/1535-7163.MCT-09-0794. doi:1535-7163.MCT-09-0794 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savaraj N, Wu C, Kuo M, You M, Wangpaichitr M, Robles C, Spector S, Feun L. The relationship of arginine deprivation, argininosuccinate synthetase and cell death in melanoma. Drug Target Insights. 2007;2:119–128. [PMC free article] [PubMed] [Google Scholar]
- 12.Huang KT, Chen YH, Walker AM. Inaccuracies in MTS assays: major distorting effects of medium, serum albumin, and fatty acids. Biotechniques. 2004;37(3):406, 408, 410–402. doi: 10.2144/04373ST05. [DOI] [PubMed] [Google Scholar]
- 13.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 14.Shvets E, Fass E, Elazar Z. Utilizing flow cytometry to monitor autophagy in living mammalian cells. Autophagy. 2008;4(5):621–628. doi: 10.4161/auto.5939. [DOI] [PubMed] [Google Scholar]
- 15.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17(2):268–277. doi: 10.1038/cdd.2009.121. doi:cdd2009121 [pii] 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70(9):3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. doi:0008-5472.CAN-09-3647 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, Agostinis P, Vanden Berghe T, Lippens S, Vandenabeele P. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1(1):e18. doi: 10.1038/cddis.2009.16. doi:cddis200916 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009;274(1):95–100. doi: 10.1016/j.canlet.2008.09.004. doi:S0304-3835(08)00712-X [pii] [DOI] [PubMed] [Google Scholar]
- 19.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8(10):1124–1132. doi: 10.1038/ncb1482. doi:ncb1482 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, Stolz DB, Yu J, Zhang L. Following Cytochrome c Release, Autophagy Is Inhibited during Chemotherapy-Induced Apoptosis by Caspase 8-Mediated Cleavage of Beclin 1. Cancer Res. 2011;71(10):3625–3634. doi: 10.1158/0008-5472.CAN-10-4475. doi:0008-5472.CAN-10-4475 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang X, Jin H, Xu H, Chen Q. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell. 2010;1(5):468–477. doi: 10.1007/s13238-010-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia HG, Zhang L, Chen G, Zhang T, Liu J, Jin M, Ma X, Ma D, Yuan J. Control of basal autophagy by calpain1 mediated cleavage of ATG5. Autophagy. 2010;6(1):61–66. doi: 10.4161/auto.6.1.10326. 10326[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signal. 2011;14(11):2215–2231. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- 25.O'Donovan TR, O'Sullivan GC, McKenna SL. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy. 7(5):509–524. doi: 10.4161/auto.7.6.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115(10):2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995. doi: 10.1126/science.1099993. doi:306/5698/990 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17(10):839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 29.Savaraj N, You M, Wu C, Wangpaichitr M, Kuo MT, Feun LG. Arginine deprivation, autophagy, apoptosis (AAA) for the treatment of melanoma. Curr Mol Med. 2010;10(4):405–412. doi: 10.2174/156652410791316995. CMM#47a[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, Howell A, Pestell RG, Lisanti MP, Sotgia F. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9(12):2423–2433. doi: 10.4161/cc.9.12.12048. 12048[pii] [DOI] [PubMed] [Google Scholar]
- 32.Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100(4):826–833. doi: 10.1002/cncr.20057. [DOI] [PubMed] [Google Scholar]
- 33.Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T, Grimshaw MJ, Steele JP, Rudd RM, Balkwill FR, Fennell DA. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12(23):7126–7131. doi: 10.1158/1078-0432.CCR-06-1101. doi:12/23/7126 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Curley SA, Bomalaski JS, Ensor CM, Holtsberg FW, Clark MA. Regression of hepatocellular cancer in a patient treated with arginine deiminase. Hepatogastroenterology. 2003;50(53):1214–1216. [PubMed] [Google Scholar]
- 35.Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ, Kung HJ. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009;69(2):700–708. doi: 10.1158/0008-5472.CAN-08-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson LJ, Smith PR, Hiller L, Szlosarek PW, Kimberley C, Sehouli J, Koensgen D, Mustea A, Schmid P, Crook T. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009 doi: 10.1002/ijc.24546. [DOI] [PubMed] [Google Scholar]