Abstract
We performed cerebellum segmentation and parcellation on magnetic resonance images from right-handed boys, aged 6–13 years, including 22 boys with autism (16 with language impairment (ALI)), 9 boys with Specific Language Impairment (SLI), and 11 normal controls. Language-impaired groups had reversed asymmetry relative to unimpaired groups in posterior-lateral cerebellar lobule VIIIA (right side larger in unimpaired groups, left side larger in ALI and SLI), contralateral to previous findings in inferior frontal cortex language areas. Lobule VIIA Crus I was smaller in SLI than in ALI. Vermis volume, particularly anterior I-V, was decreased in language-impaired groups. Language performance test scores correlated with lobule VIIIA asymmetry and with anterior vermis volume. These findings suggest ALI and SLI subjects show abnormalities in neurodevelopment of fronto-corticocerebellar circuits that manage motor control and the processing of language, cognition, working memory, and attention.
Keywords: autism, specific language impairment, cerebellum, Broca’s area, asymmetry
Autism is a neurodevelopmental disorder displaying deficits in social interaction and communication skills, repetitive behaviors, and stereotyped interests (APA, 1994). Language deficits range from absence of functional language, to impairments in phonological processing, vocabulary, and higher order syntax and semantics (Rapin, 1996; Tager-Flusberg, 2003, 2006; Tager-Flusberg & Caronna, 2007; Tager-Flusberg, Paul, & Lord, 2005). However, some children with autism have normal language skills (Tager-Flusberg & Joseph, 2003). Language-impaired children with autism displayed a similar language profile to non-autistic children with specific language impairment (SLI) (Bishop, 2003; Kjelgaard & Tager-Flusberg, 2001), a disorder of delayed language development in the absence of other cognitive impairments. Furthermore, family and genetic linkage studies have implicated overlap between autism and SLI (Fisher, Lai, & Monaco, 2003; Santangelo & Folstein, 1999).
Neuroimaging studies in autism and SLI have demonstrated brain structure and function abnormalities in inferior frontal gyrus (IFG) language-association cortex (Broca’s area). In typically developing right-handed subjects, Broca’s area regions tend to be larger in the left hemisphere than in the right (Foundas, Eure, Luevano, & Weinberger, 1998; Keller et al., 2007). However, magnetic resonance imaging (MRI) reports demonstrated reversal from normal IFG structural asymmetry (larger in the right hemisphere) in right-handed boys with autism (Herbert et al., 2002), particularly those with autism and language-impairment (ALI), and in non-autistic boys with specific language impairment (SLI) (De Fosse et al., 2004). As early as 1986, researchers identified reversed language dominance in right-handed male autistic children in reports using cortical evoked reponses to language stimuli (Dawson, Finley, Phillips, & Galpert, 1986). Functional imaging studies have also demonstrated reversed or abnormal cerebral blood flow asymmetry in frontal language cortex in children with autism (Burroni et al., 2008; Chiron et al., 1995; Ohnishi et al., 2000), and during performance of a language task in high-functioning adults with autism (Boddaert et al., 2004; Muller et al., 1999). High-functioning adults with autism spectrum disorders (ASD) showed decreased functional MRI (fMRI) activation in Broca’s area in response to syntactic and semantic tasks (Harris et al., 2006; Just, Cherkassky, Keller, & Minshew, 2004) or increased right frontal activation (Takeuchi, Harada, Matsuzaki, Nishitani, & Mori, 2004), further implicating abnormalities in frontal language areas in autism.
Functional neuroimaging studies in right-handed normal control subjects have demonstrated contralateral activation in right posterior lateral cerebellum in concert with left inferior frontal activation during language tasks (Binder et al., 1997; Desmond, Gabrieli, Wagner, Ginier, & Glover, 1997; Harris et al., 2006; Hubrich-Ungureanu, Kaemmerer, Henn, & Braus, 2002; Jansen et al., 2005; Petersen, Fox, Posner, Mintun, & Raichle, 1988; Xiang et al., 2003), including phonological processing (Mathiak, Hertrich, Grodd, & Ackermann, 2002). While normal control subjects displayed lexical-semantic fMRI activation in left inferior frontal gyrus and also in right posterior lateral cerebellum, high-functioning adults with ASD had decreased Broca’s area activation and did not present right cerebellar activation (Harris et al., 2006).
The cerebellum was traditionally associated primarily with sensorimotor function and balance. However, cerebellum has extensive reciprocal connections with cerebral cortex and limbic systems (Schmahmann, 2001; Schmahmann, 1996). The expanded neocerebellar hemispheres evolved in concert with cerebral association areas, in particular prefrontal cortex, facilitating a cerebellar role in language processing (Leiner, Leiner, & Dow, 1986). There are robust interconnections between frontal cortex language regions and contralateral posterior cerebellar hemispheres (Gebhart, Petersen, & Thach, 2002; Roskies, Fiez, Balota, Raichle, & Petersen, 2001; Stoodley & Schmahmann, 2009). These fronto-corticocerebellar circuits could facilitate most areas of cognitive function, including language, executive function, working memory, attention, and emotion (Makris et al., 2005; Schmahmann et al., 2001). Clinical lesion studies and functional neuroimaging link anterior cerebellum with sensorimotor function, while posterior lateral cerebellum (lobules VI-VIII) is associated with language, verbal working memory, cognition, and attention (Stoodley & Schmahmann, 2009; Desmond & Fiez, 1998; Leiner, Leiner, & Dow, 1991; Levisohn, Cronin-Golomb, & Schmahmann, 2000; Neau, Arroyo-Anllo, Bonnaud, Ingrand, & Gil, 2000; Petersen, Fox, Posner, Mintun, & Raichle, 1989; Riva & Giorgi, 2000; Schmahmann & Sherman, 1998; Harris et al., 2006).
Cerebellar neuropathology has been implicated in autism, including enlarged IV ventricle, loss of Purkinje cells in lateral and inferior cerebellar cortex (most severe in posterior lateral hemispheres), and abnormal or reduced numbers of neurons in deep cerebellar nuclei (Bauman & Kemper, 1985; Kemper & Bauman, 1998; Kemper & Bauman, 2002; Kulesza & Mangunay, 2008; Ritvo et al., 1986). Subjects with partial cerebellar agenesis demonstrate autistic-like behaviors, including stereotypical performance, obsessive rituals, difficulty understanding social cues, tactile defensiveness, perseveration, disinhibition, poor working memory, and language deficits such as problems with verbal fluency, expressive language delay, impaired prosody, and overgeneralization of past tense verbs (Bobylova, Petrukhin, Dunaevskaya, Piliya, & Il’ina, 2007; Chheda, Sherman, & Schmahmann, 2002; Schmahmann, 2004). Several prior structural imaging studies implicated cerebellar deficits in autism, although the neuroimaging literature on this issue is mixed (Stanfield et al., 2007; Brambilla et al., 2003). While several studies have demonstrated decreased vermis size in autism (Courchesne et al., 1994; Courchesne, Yeung-Courchesne, Press, Hesselink, & Jernigan, 1988; Hashimoto, Tayama, Miyazaki, Murakawa, & Kuroda, 1993; Kaufmann et al., 2003), other studies have not replicated this (Hardan, Minshew, Harenski, & Keshavan, 2001; Kleiman, Neff, & Rosman, 1992; Piven et al., 1992; Piven, Saliba, Bailey, & Arndt, 1997), or have related it to differences in intelligence (Piven & Arndt, 1995; Stanfield et al., 2007). Thus, there is variability in the structural imaging literature related to cerebellum and autism (Courchesne, 1999; Piven & Arndt, 1995; Rapin, 1999). However, most prior cerebellar imaging studies in autism have focused on the measurement of the cross-sectional area of the vermis.
The current study is the first to systematically assess specific putative cognitive and language-related regions in the cerebellar hemispheres using cerebellar volumetric segmentation and parcellation with MRI in groups of normal control (NC) boys, boys with autism (with and without language impairment; ALI and ALN respectively), and in boys with SLI. Since posterior lateral cerebellar lobules VI-VIII were identified through lesion and functional neuroimaging studies as most closely tied to language, verbal working memory, and cognition (Stoodley & Schmahmann, 2009), our hypotheses were: 1) posterior lateral cerebellar hemisphere lobules VI-VIII would show reversed asymmetry in ALI and SLI relative to ALN and NC, contralateral to that previously reported in inferior frontal language regions; 2) volumetric differences in lobules VI-VIII would be observed between groups; and 3) decreased vermis volume would be observed in ALI and SLI if vermis effects were tied to language deficits, or alternatively in ALI and ALN if vermis effects were more closely tied to autism symptoms.
Methods
Participants
Subjects were identical to those previously described in a study of volumetric asymmetry in language-association cerebral cortex (De Fosse et al., 2004), including 22 boys with autism (16 of whom had language impairment (ALI) and 6 with normal language (ALN)), 9 boys with specific language impairment (SLI), and 11 normal control (NC) boys. This project was approved by the human studies institutional review board (IRB), and all subjects enrolled in this study provided signed assent (for minors) and parental consent.
Subjects included males 6 – 13 years old (Table 1), with no significant group-matching differences in age among the groups, F(3, 38) = 1.3, p = 0.3. All subjects were predominantly right-handed, as determined by the Edinburgh Inventory in NC subjects (Oldfield, 1971) and by the Dean Laterality Preference Schedule in all other groups (Dean, 1978, 1982). Subjects were excluded if they had neurological damage or had been diagnosed with Fragile X, neurofibromatosis, cerebral palsy, tuberous sclerosis, William’s syndrome or Down’s syndrome. Autism spectrum disorders were ruled out in SLI and NC groups. The NC group had no DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, version 4) Axis I diagnoses (APA, 1994) based on consensus agreement between the Schedule for Affective Disorders and Schizophrenia for School-Age Children -- Epidemiologic Version (Orvaschel & Puig-Antich, 1987) and clinical interview with a board-certified child psychiatrist (JAF). None of the children were currently on psychotropic medications.
Table 1.
Characteristics of the subject groups.
| NC | ALN | ALI | SLI | Overall | Post Hoc Contrasts | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n=11 | n=6 | n=16 | n=9 | F | P | NC & ALN vs. ALI & SLI | NC vs. ALN | SLI vs. ALI | |
| Mean ± SD | |||||||||
| Age (years) | 10.4 ± 2.7 | 8.3 ± 0.9 | 9.8 ± 2.1 | 9.9 ± 2.3 | 1.3 | 0.3 | |||
| Full Scale IQ a | 114.5 ± 11.3 | 109.3 ± 24.1 | 78.3 ± 14.7 | 93.4 ± 15.5 | 13.4 | <0.001 | * | * | |
| Verbal IQ | 115.9 ± 12.4 | 97.7 ± 19.3 | 75.1 ± 15.9 | 92.5 ± 15.6 | 14.9 | <0.001 | * | * | * |
| Nonverbal IQ | 110.6 ± 11 | 116.3 ± 24.9 | 87.9 ± 14.3 | 95.7 ± 14.6 | 7.2 | <0.001 | * | ||
| Language Testing | |||||||||
| Nonword Repetition b | - | 8.5 ± 1.9 | 6 ± 2.3 | 8.1 ± 2.9 | 3.1 | 0.06 | |||
| CELF c | - | 101 ± 12.1 | 65.5 ± 9.4 | 85.4 ± 11.2 | 23.5 | <0.001 | * | ||
p < 0.05
SLI and subjects with autism: Differential Ability Scales (Elliot, 1990); control subjects: WISC-III (Wechsler, 1991).
Repetition of Nonsense Words subtest of NEPSY (Korkman et al., 1998).
Clinical Evaluation of Language Fundamentals (Semel et al., 1995).
Abbreviations: NC - normal control; ALN - autism with normal language; ALI - autism with language impairment; SLI - specific language impairment.
Diagnosis of autism was established via the Autism Diagnostic Interview – Revised (Lord, Rutter, & Le Couteur, 1994), Autism Diagnostic Observation Schedule (Lord et al., 2000), and examination by an expert clinician to verify that subjects met DSM-IV criteria for autism (APA, 1994). Language skills were assessed using the Clinical Evaluation of Language Fundamentals (CELF, 3rd edition; Semel, Wiig, & Secord, 1995) and the nonsense word repetition subtest of the NEPSY (Korkman, Kirk, & Kemp, 1998). Criteria for language impairment were a CELF score ≤ 81 or a score ≤6 on the NEPSY nonsense word repetition subtest. These criteria for specific language impairment (SLI) were selected based on current research on language impairments in older children (Tager-Flusberg & Cooper, 1999), and are similar to those used in other research on SLI (e.g., Conti-Ramsden, 2003). Based on these criteria, 16 subjects with autism were classified with language impairment (ALI) and six subjects with autism were classified with normal language (ALN).
Subjects with SLI had a history of significant language delay and had been referred for clinical treatment. Of the nine subjects in the SLI group, five met the above criteria for current language impairment. Those subjects with only a history of language impairment fell within the normal limits on the language measures at the time of testing, which is common in older children with SLI (Conti-Ramsden & Botting, 1999). We included these children because although they no longer met strict criteria for a current SLI diagnosis, they all had documented histories of poor performance on language tests and were all currently still struggling with language-based learning difficulties, which is a common developmental trajectory for children with SLI (Catts et al., 2008). Although subjects in the NC group were not administered the CELF or NEPSY, they all had verbal IQ and reading scores within normal range, no language abnormality as assessed by neurocognitive assessment, and no history of language delay or language-based learning disabilities based on parental interview.
There were significant differences in full scale IQ (Elliot, 1990; Wechsler, 1991) between the groups (FSIQ for the controls was estimated using a short form that included the Vocabulary and Block Design subtests of the Wechsler Intelligence Scale for Children – Third Edition (WISC-III, Wechsler, 1991)), F(3, 38) = 13.4, p < 0.0001: the language impaired groups (ALI and SLI) had significantly lower verbal, t(38) = 4.4, p < 0.0001, and nonverbal IQ, t(38) = 4.2, p < 0.001, than the unimpaired language groups (ALN and NC). The ALI group had significantly lower verbal IQ than the SLI group, t(38) = 2.6, p = 0.01, but there was no significant difference in nonverbal IQ, t(38) = 1.2, p = 0.2. The same pattern was observed with the ALN group, who had significantly lower verbal IQ than the NC group, t(38) = 2.3, p = 0.03, but had comparable nonverbal IQ, t(38) = 0.7, p = 0.5.
Image Acquisition
Full details of image acquisition procedures were presented in our prior report on frontal language asymmetries in these same scans (De Fosse et al., 2004), with a brief summary presented here. Subjects were desensitized to scanner environment and noise through training in a mock scanner equipped with behavioral shaping techniques (Slifer, Cataldo, Cataldo, Llorente, & Gerson, 1993). Images were acquired as single or dual-acquisitions on a General Electric (Milwaukee, WI) 1.5 Tesla Signa MRI system with the following range of parameters: TR = 11.0–13.7 msec, TE = 1.9–2.7 msec, TI = 300 msec, flip angle = 25°, slice thickness = 1.5 mm (contiguous), image matrix = 256 x 256 pixels, field of view (FOV) = 240–300 mm. All scans were free from gross neuroanatomical abnormalities upon review by a staff radiologist and pediatric neurologist. Validation studies were performed to determine the effect of the change in scanning protocol and software upgrade. Four people unrelated to the study agreed to be scanned with both the single and dual protocols. Three additional people volunteered to be scanned before and after the upgrade of the scanning software. We obtained the volumes of the right and left cerebellar cortex for all scans. Not only is cerebellar cortex the basis for the subsequent parcellation and thus includes all the regions of interest in this study, but it also represents two intensity class boundaries (gray matter–extracerebral cerebrospinal fluid and gray matter–white matter) that would be expected to show any global effects of scan parameter changes. Intraclass correlation coefficients (ICCs) were calculated for consistency of the volume measurements. For both the protocol change and for the scanning software upgrade, all ICC > 0.97, and measurements differed by less than 2% on average across the changes. Furthermore, because the principal measures of interest and hypotheses in this study involve asymmetry measures, these changes in scan protocols would not be expected to have an impact on one hemisphere versus the other, and impact on asymmetry would be unlikely.
Morphometric analysis
Scans were reoriented in a common stereotactic space (Talairach & Tournoux, 1988; Worth, Makris, Meyer, Caviness, & Kennedy, 1998), but without rescaling the image size parameters. Images were resliced in position-normalized coronal plane with 1.5 mm slice thickness. Images were magnified to an in-plane pixel resolution of 0.837 mm (except for those where the FOV was 240 mm) and intensity-nonuniformity effects were removed (Arnold et al., 2001; Worth, Makris, Patti et al., 1998).
Cerebellum segmentation and parcellation have been described previously (Makris et al., 2003; Makris et al., 2005). Parcellation divides the cerebellar cortex into 32 Parcellation Units (PUs) per hemicerebellum. Subdivision is based on eleven mediolateral “limiting” fissures forming boundaries between units (Caviness, Meyer, Makris, & Kennedy, 1996), which intersect with the longitudinal paravermian sulcus and two longitudinal sagittal borders (Makris et al., 2005). Fissures divide cortex into lobules, while longitudinal divisions separate vermis from hemisphere, and subdivide hemispheres into medial and lateral zones. To parcellate cerebellum interactively, we used Cardviews (Caviness et al., 1996) for manipulations in the volume domain, and FreeSurfer (Dale, Fischl, & Sereno, 1999) for operations in the surface domain (Figure 1). We segmented the cerebellar cortex and white matter using Cardviews, then used FreeSurfer to create a flattened surface representation of the boundary between cortex and white matter upon which fissures were labeled, and finally completed the parcellation in Cardviews (Makris et al., 2005). The MRI Atlas of the Human Cerebellum (Schmahmann, Doyon, Toga, Petrides, & Evans, 2000) was referenced to identify landmarks and fissures in three cardinal planes, without referring to the specific coordinate space.
Figure 1.
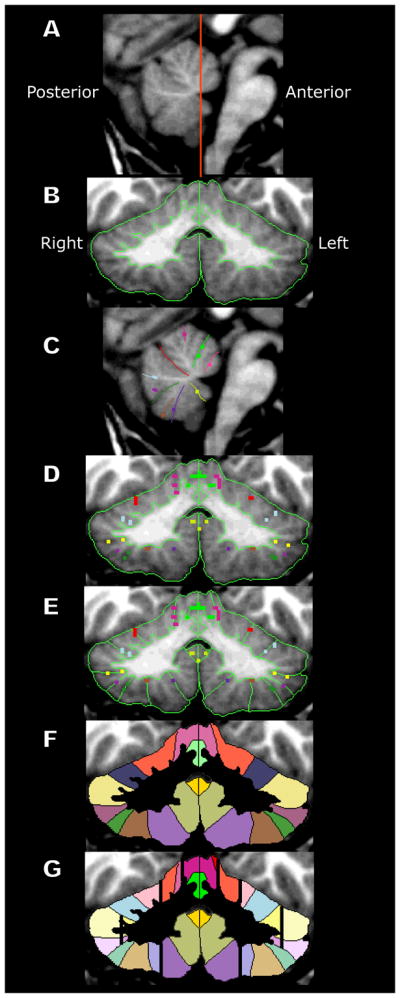
A brief overview of the method of parcellating the cerebellum in MRI images. (A) Midsagittal image of the cerebellum showing the vermis. The line indicates the coronal plane of section for B and D-G. (B) The results of the intensity-based segmentation of the cerebellum exterior and the gray-white matter interface are shown. The gray-white boundary is the basis for the surface assisted parcellation (not shown, see Makris et al., 2003; Makris et al., 2005). (C) Fissures identified by the surface assisted parcellation are mapped back onto the original image and manually extended on sequential slices. (D) Fissures identified in axial and sagittal planes appear in cross section in the coronal plane. (E) Based on these fissures parcellation units are identified, enclosed and (F) labeled. (G) a post-processing algorithm subdivides the cerebellum hemispheres into three zones (medial, Lateral 1, and Lateral 2) and sets the lateral border of the vermis in the anterior lobe.
For comparison to previous studies, we also measured the mid-sagittal area of the vermis in zones I/II – V (anterior), VI – VIIB (superior posterior), and VIII – X (inferior posterior and nodulus), excluding the non-vermal portion of IX (the “tonsils”). The divisions between the zones were based on the primary and prepyramidal-prebiventor fissures. Although there is no actual paravermian sulcus delineating the vermis in lobules I/II – V (Schmahmann, Doyon, Toga, Petrides, & Evans, 2000; Makris, et al., 2005), we include it under the label “vermis” according to historical convention.
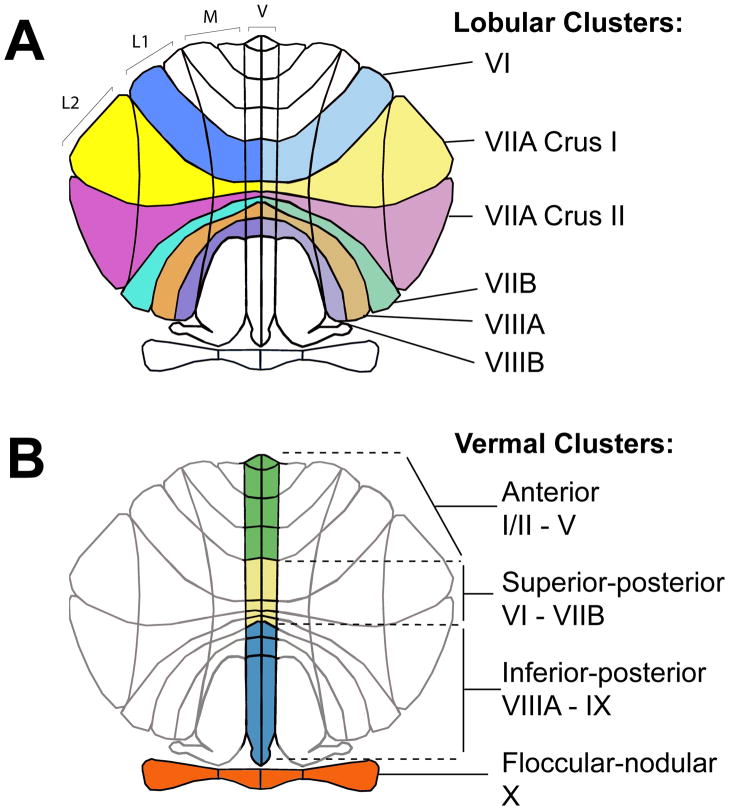
Individual parcellation units were clustered according to two schemes (Figure 2). Principal regions-of-interest (ROIs) included cerebellar hemisphere lobules VI-VIII, regions most closely related to language, cognition, working memory, and attention. Vermal, medial, and hemispheric PUs were summed for these lobules: VI, VIIA Crus I, VIIA Crus II, VIIB, VIIIA, and VIIIB. Secondary volumetric regions-of-interest were in the vermis, including anterior (I-V), superior posterior (VI-VIIB), inferior posterior (VIIIA-IX), and floccular nodular (X) lobules.
Figure 2.
Two representations of the flattened cerebellum cortex, colored to show the clustering schemes. (A) The lobular clusters are comprised of the parcellation units in the vermis (V), medial (M), Lateral 1 (L1) and Lateral 2 (L2) zones of each hemicerebellum. The lobules are bounded by identifiable fissures: VI – primary and superior posterior fissures; VIIA Crus I – superior posterior and horizontal fissures; VIIA Crus II – horizontal and ansoparamedian fissures; VIIB – ansoparamedian and prepyramidal prebiventer fissures; VIIIA - prepyramidal prebiventer and intrabiventer fissures; VIIIB – intrabiventer and secondary fissures. (B) The vermal clusters are comprised of the vermis regions summed across the hemispheric midline and grouped by major fissure locations: anterior (I/II – V) – superior hemispheric margin to primary fissure; superior-posterior (VI – VIIB) – primary fissure to prepryamidal prebiventer fissure; inferior posterior (VIIIA – IX) – prepyramidal prebiventer fissure to posterolateral fissure; Floccular-nodular (X) – posterolateral fissure to the inferior hemispheric margin (Makris et al., 2003).
One Master’s degree neuroimaging specialist (SMH), trained extensively through Center for Morphometric Analysis under the supervision of our neuroanatomist (NM), peformed all image analyses, blind to subjects’ diagnostic group affiliation. A training set of 10 scans were parcellated to establish intra- and interrater reliability. For all cerebellar hemispheric lobule volumes and vermis area measures, intra- and interrater ICCs > 0.89. For the vermal volume clusters, all intra- and interrater ICCs > 0.83, except intrarater for superior-posterior vermis (which is is a small region with mean volume = 2.5cc), with ICC = 0.7, but mean difference of only 3.2%.
The volume of each structure was calculated semi-automatically based on the voxel dimensions (Kennedy, Filipek, & Caviness, 1989). Hemispheric asymmetry was assessed by a symmetry index (Galaburda, Rosen, & Sherman, 1990): (L−R)/[(L+R)/2]. Positive values indicate larger left hemisphere volume.
Data Analysis
Cerebellum, cerebellar white matter, and cerebellar cortex volumes were compared with one-way ANOVAs with pooled estimates of error variance among the four subject groups, with Student’s t-tests for post-hoc pairwise comparisons. One-way ANOVAs with Group (ALI, ALN, SLI, NC) as a between-subjects variable were used to analyze the raw volumes of cerebellar parcellation unit clusters (our primary and secondary ROIs), relative volumes of the ROIs as a percentage of cerebellar cortex volume, and the symmetry index of the ROIs. Planned linear contrasts were used to determine whether (a) the language impaired groups (SLI and ALI) differed from language unimpaired groups (ALN and NC) and (b) subjects with autism differed from their language co-group (ALI versus SLI; ALN versus NC). However, because of the limited amount of power available for post-hoc comparisons, we chose to include only orthogonal pairwise comparisons. In planning these comparisons, our hypotheses were geared toward the initial examination of changes due to language function (NC + ALN vs SLI + ALI). Our secondary comparisons were targeted toward highlighting regions specific to autism, but because of the necessity of independence from the primary comparison, these were restricted to comparisons within each language group (NC vs. ALN, and SLI vs. ALI). This is consistent with our previous publication in frontal language regions (see DeFosse, et al., 2004). Regions with significant group differences were further compared by the inclusion of age and IQ as covariates. Significant regional cerebellar asymmetry was compared to measures of inferior frontal gyrus asymmetry from our prior report on these same subjects (De Fosse et al., 2004) using nonparametric (rank) correlation and chi-square analysis. Regression analyses were applied to examine the relation between regional morphometric effects and cognitive measures (IQ, language scores). Analyses were performed using the JMP statistical software package, version 5.0.1.2 (SAS Institute Inc., Carey, NC). The threshold for statistical significance was set at p < 0.05.
Results
Whole cerebellum and cerebellar cortex volumes did not differ among the four subject groups (Table 2). Cerebellar white matter was significantly larger in the ALI group (28.2 cc) than in the SLI group (25.1 cc), F(3,38) = 3.0, p = 0.04, contrast t(38) = 2.96, p < 0.005. The ratio of cerebellar white matter to cerebellar cortex was similar among groups (approximately 18% white matter and 82% cortex, F(3, 38) = 1.3, p = 0.3). The contrast between ALI (18.4% white matter) and SLI (17.3% white matter) showed a moderate effect, t(38) = 1.96, p = 0.06. In rank order, SLI tended to have proportionally smaller cerebelli (including white matter and cortex), and ALI tended toward larger cerebelli, with NC and ALN falling between the language-impaired groups, but these trends were not significant, similar to our observations of volumes of the cerebrum and cerebral cortex in our prior report on these same subjects (De Fosse et al., 2004). There were no significant group differences in volumetric symmetry of whole cerebellum, cerebellar cortex, or cerebellar white matter.
Table 2.
Gross cerebellar volumes.
| Region | NC | ALN | ALI | SLI | Overall | Post Hoc Contrasts | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n = 11 | n = 6 | n = 16 | n = 9 | F | P | NC & ALN vs. ALI & SLI | NC vs. ALN | SLI vs. ALI | |
| Mean ± SD | |||||||||
| Raw Volume (cc) | |||||||||
| Whole cerebellum | 149.8 ± 10.8 | 148.5 ± 9.3 | 153.5 ± 12.1 | 145.3 ± 10.1 | 1.1 | 0.4 | |||
| Cerebellar White Matter | 26.7 ± 2.9 | 26.5 ± 1.9 | 28.2 ± 2.8 | 25.1 ± 1.9 | 3.0 | 0.04 | * | ||
| Cerebellar Cortex | 123.1 ± 10.0 | 122.0 ± 8.2 | 125.3 ± 10.4 | 120.2 ± 8.8 | 0.6 | 0.7 | |||
| Ratio to whole cerebellum volume (%) | |||||||||
| Cerebellar White Matter | 17.9 ± 1.8 | 17.9 ± 1.0 | 18.4 ± 1.4 | 17.3 ± 0.9 | 1.3 | 0.3 | |||
| Cerebellar Cortex | 82.1 ± 1.8 | 82.1 ± 1.0 | 81.6 ± 1.4 | 82.7 ± 0.9 | 1.3 | 0.3 | |||
| Symmetry Coefficient a | |||||||||
| Whole cerebellum | 0.4 ± 1.1 | −0.9 ± 1.6 | 0.3 ± 1.9 | 0.1 ± 1.4 | 1.0 | 0.4 | |||
| Cerebellar White Matter | 1.2 ± 6.8 | 0.7 ± 6.3 | 3.2 ± 11.8 | −4.9 ± 15.9 | 1.0 | 0.4 | |||
| Cerebellar Cortex | 0.2 ± 1.6 | −1.3 ± 2.3 | −0.4 ± 2.0 | 1.0 ± 2.7 | 1.6 | 0.2 | |||
p < 0.05
Positive symmetry coefficient indicates left hemisphere volume is larger than right hemisphere volume. Negative value indicates right hemisphere volume is larger.
Abbreviations: NC - normal control; ALN - autism with normal language; ALI - autism with language impairment; SLI - specific language impairment.
Among posterior-lateral cerebellum regions (Table 3), only right VIIA Crus I differed significantly between-groups, F(3, 38) = 2.9, p < 0.05, where SLI was 1.9 cc smaller than ALI, t(38) = 2.5, p = 0.02. This effect remained after covarying for cerebellar cortex volume, t(37) = 2.1, p = 0.04. Though moderate, there was a trend toward a similar pattern in the left VIIA Crus I, where SLI was 1.3 cc smaller than ALI (overall F(3, 38) = 2.0, p = 0.1; contrast t(38) = 1.7, p = 0.09), although this comparison remained non-significant after covarying for cerebellar cortex volume (p > 0.2).
Table 3.
Asymmetries and volumes of the lobular (gyral) cerebellar parcellation units in posterior lateral cerebellum (Lobules VI – VIII).
| Region | NC | ALN | ALI | SLI | Overall | Post Hoc Contrasts | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n = 11 | n = 6 | n = 16 | n = 9 | F | P | NC & ALN vs. ALI & SLI | NC vs. ALN | SLI vs. ALI | |
| Mean ± SD | |||||||||
| Symmetry coefficienta | |||||||||
| VI | −0.6 ± 14.0 | −4.0 ± 3.4 | −4.5 ± 12.0 | 5.5 ± 14.6 | 1.3 | 0.3 | |||
| VIIA Crus I | −4.3 ± 6.0 | 0.4 ± 4.1 | −2.7 ± 8.6 | 1.3 ± 8.8 | 1.2 | 0.3 | |||
| VIIA Crus II | −1.5 ± 15.8 | −8.4 ± 19.5 | −13.6 ± 17.5 | −6.1 ± 11.2 | 1.3 | 0.3 | |||
| VIIB | 7.1 ± 25.3 | 10.9 ± 28.8 | 4.3 ± 24.1 | −4.7 ± 17.4 | 0.6 | 0.6 | |||
| VIIIA | −4.2 ± 19.6 | −6.6 ± 19.5 | 10.9 ± 11.5 | 6.3 ± 15.5 | 2.9 | 0.05 | * | ||
| VIIIB | 7.8 ± 10.7 | 12.2 ± 14.8 | 9.3 ± 12.1 | 8.2 ± 17.1 | 0.2 | 0.9 | |||
| Right lobular zone volume | |||||||||
| VI | 9.2 ± 1.5 | 9.1 ± 1.0 | 9.6 ± 1.9 | 8.5 ± 1.2 | 0.8 | 0.5 | |||
| VIIA Crus I | 14.1 ± 1.7 | 13.6 ± 1.3 | 15.4 ± 1.8 | 13.5 ± 2.2 | 2.9 | 0.05 | * | ||
| VIIA Crus II | 8.4 ± 1.9 | 8.5 ± 1.5 | 9.2 ± 2.2 | 9.0 ± 2.0 | 0.4 | 0.7 | |||
| VIIB | 6.5 ± 1.5 | 6.2 ± 1.3 | 6.1 ± 1.5 | 6.2 ± 1.9 | 0.2 | 0.9 | |||
| VIIIA | 6.1 ± 1.6 | 6.5 ± 2.2 | 5.9 ± 1.4 | 5.1 ± 0.7 | 1.3 | 0.3 | |||
| VIIIB | 4.5 ± 0.8 | 4.6 ± 1.1 | 4.7 ± 1.0 | 4.8 ± 0.7 | 0.2 | 0.9 | |||
| Left lobular zone volume | |||||||||
| VI | 9.2 ± 2.0 | 8.7 ± 0.8 | 9.1 ± 1.7 | 9.0 ± 1.2 | 0.1 | 0.9 | |||
| VIIA Crus I | 13.5 ± 1.5 | 13.6 ± 1.1 | 15.0 ± 1.9 | 13.7 ± 2.4 | 2.0 | 0.1 | |||
| VIIA Crus II | 8.2 ± 1.4 | 7.9 ± 2.0 | 8.0 ± 1.8 | 8.5 ± 1.7 | 0.2 | 0.9 | |||
| VIIB | 6.9 ± 1.0 | 6.9 ± 1.5 | 6.4 ± 2.0 | 5.9 ± 1.8 | 0.7 | 0.5 | |||
| VIIIA | 5.8 ± 1.5 | 5.9 ± 1.2 | 6.6 ± 1.8 | 5.6 ± 1.3 | 1.1 | 0.4 | |||
| VIIIB | 4.8 ± 0.7 | 5.3 ± 1.7 | 5.1 ± 0.8 | 5.4 ± 1.6 | 0.4 | 0.7 | |||
p < 0.05.
Positive symmetry coefficient indicates left hemisphere volume is larger than right hemisphere volume. Negative value indicates right hemisphere volume is larger.
Abbreviations: NC - normal control; ALN - autism with normal language; ALI - autism with language impairment; SLI - specific language impairment.
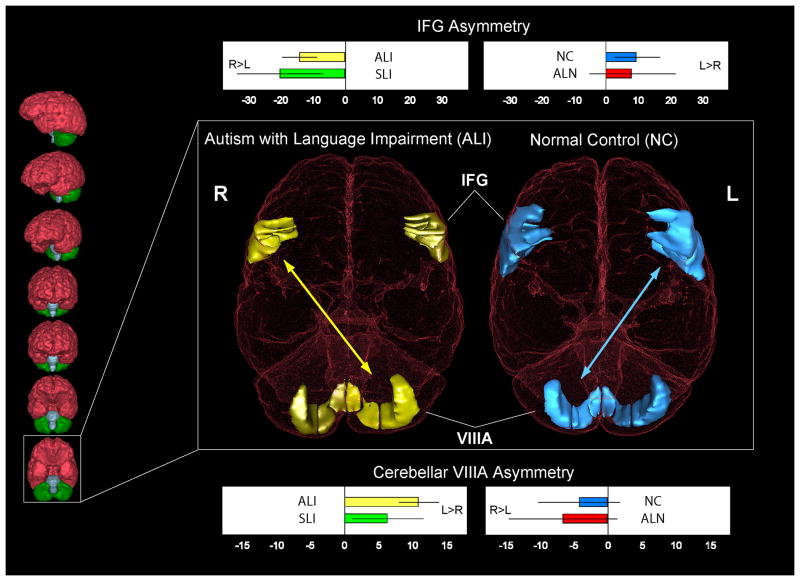
Posterior cerebellar lobule VIIIA showed significant asymmetry differences: VIIIA was larger in right hemisphere for ALN and NC (language-normal) groups (7% and 4% rightward asymmetry respectively), but larger in left hemisphere for ALI and SLI (language-impaired) groups (11% and 6% leftward asymmetry respectively), F(3,38) = 2.9, p < 0.05, contrast t(38) = 2.7, p = 0.01 (Table 3 and Figure 3). VIIIA asymmetry was oriented contralaterally from asymmetry of inferior frontal gyrus (IFG; Broca’s area: pars opercularis and pars triangularis) reported previously in these same subjects (De Fosse et al., 2004): IFG was asymmetrically leftward in ALN and NC groups, but rightward in ALI and SLI groups. Combining the data from both regions, we found a significant negative correlation between symmetry coefficients for cerebellar lobule VIIIA and IFG for each subject, rs (42) = −0.34, p = 0.02. Twenty-nine of 42 subjects (69%) had either lobule VIIIA larger in right hemisphere with IFG larger in left, or lobule VIIIA larger in left hemisphere with IFG larger in right. Among this subset, the ALN and NC groups tended to have the former, while the ALI and SLI subjects tended to have the latter, X2(1, N = 29) = 6.7, p < 0.01. A comparison including all subjects was also significant, X2(3, N = 42) = 8.2, p = 0.04, which includes subjects where both symmetry coefficients were in the same direction.
Figure 3.
Inferior view of the brain of a normal control subject (NC, right) and a language-impaired subject with autism (ALI, left), highlighting left and right inferior frontal gyri (IFG, Broca’s area), and cerebellum lobule VIIIA. In the NC group, the asymmetry is oriented with larger IFG volume in the left hemisphere and larger cerebellar lobule VIIIA in the right hemisphere, while ALI subjects had the opposite asymmetry in these regions (arrows indicate contralateral fronto-corticocerebellar connectivities and reversal of asymmetry orientations). Graphs of the symmetry coefficients of each group are shown for the IFG (top of figure) and VIIIA (bottom of figure). A positive coefficient indicates left hemisphere volume is larger than right hemisphere volume. Negative value indicates right hemisphere volume is larger. Graphs display group means with standard error bars. The scans chosen for display were of subjects who had frontal and cerebellar asymmetry near the mean of their respective subject groups. Abbreviations: R = right, L = left; NC - normal control; ALN - autism with normal language; ALI - autism with language impairment; SLI - specific language impairment.
The volume of the vermis was significantly smaller in ALI and SLI when compared to NC and ALN, overall F(3, 38) = 2.7, p = 0.06, contrast mean difference = 1.6 cc, t(38) = 2.7, p = 0.01 (Table 4). This was maintained when vermis was expressed as ratio to total cerebellar cortex volume, F(3, 38) = 3.2, p = 0.03, contrast t(38) = 2.8, p = 0.007. Among subdivisions, anterior vermis volume (I/IIv through Vv) showed the same pattern, contrast t(38) = 2.7, p = 0.01, even when expressed as ratio to cerebellum cortex volume, t(38) = 2.9, p = 0.007. Volumes and ratios among the other divisions (superior-posterior, inferior-posterior, and floccular-nodular) were always smaller in ALI and SLI than in ALN and NC, but were not significant.
Table 4.
Cerebellar vermis cluster volumes and areas. Volume units are the sum of left and right hemispheres.
| Vermis region | NC | ALN | ALI | SLI | Overall | Post Hoc Contrasts | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n = 11 | n = 6 | n = 16 | n = 9 | F | P | NC & ALN vs. ALI & SLI | NC vs. ALN | SLI vs. ALI | |
| Mean ± SD | |||||||||
| Raw volume (cc) | |||||||||
| Whole vermis | 13.6 ± 2.0 | 13.5 ± 1.7 | 12.0 ± 1.7 | 12.0 ± 1.6 | 2.7 | 0.06 | * | ||
| Anterior, I/II – V | 7.4 ± 1.5 | 7.1 ± 0.8 | 6.1 ± 1.3 | 6.2 ± 1.0 | 3.1 | 0.04 | * | ||
| Superior-posterior, VI – VIIB | 2.7 ± 0.4 | 2.9 ± 0.5 | 2.7 ± 0.4 | 2.6 ± 0.4 | 0.5 | 0.7 | |||
| Inferior-posterior, VIIIA – IX | 3.0 ± 0.5 | 3.1 ± 0.6 | 2.8 ± 0.5 | 2.8 ± 0.5 | 1.4 | 0.3 | |||
| Floccular-nodular, X | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 | 0.8 | |||
| Ratio to cerebellar cortex volume (%) | |||||||||
| Whole vermis | 11.0 ± 1.2 | 11.1 ± 1.2 | 9.6 ± 1.6 | 10.0 ± 1.1 | 3.2 | 0.03 | * | ||
| Anterior, I/II – V | 6.0 ± 1.0 | 5.8 ± 0.5 | 4.9 ± 1.1 | 5.1 ± 0.7 | 3.6 | 0.02 | * | ||
| Superior-posterior, VI – VIIB | 2.2 ± 0.3 | 2.3 ± 0.3 | 2.2 ± 0.4 | 2.2 ± 0.3 | 0.4 | 0.7 | |||
| Inferior-posterior, VIIIA – IX | 2.5 ± 0.3 | 2.6 ± 0.5 | 2.2 ± 0.3 | 2.3 ± 0.4 | 2.0 | 0.1 | |||
| Floccular-nodular, X | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.05 | 0.4 ± 0.1 | 0.7 | 0.6 | |||
| Raw area (mm2) | |||||||||
| Whole vermis | 1121.5 ± 120.5 | 1186.5 ± 150.4 | 1156.8 ± 111.5 | 1070.7 ± 66.4 | 1.7 | 0.2 | |||
| Anterior, I/II – V | 472.2 ± 58.0 | 503.4 ± 72.1 | 476.2 ± 55.0 | 441.6 ± 20.2 | 1.7 | 0.2 | |||
| Superior-posterior VI – VIIB | 276.9 ± 35.3 | 323.9 ± 67.0 | 315.9 ± 43.1 | 285.0 ± 34.6 | 2.7 | 0.06 | * | ||
| Inferior-posterior, VIIIA–X | 372.5 ± 54.1 | 359.1 ± 43.5 | 364.7 ± 56.8 | 344.0 ± 35.7 | 0. 6 | 0.7 | |||
| Ratio to whole vermis area (%) | |||||||||
| Anterior, I/II – V | 42.1 ± 2.3 | 42.4 ± 1.4 | 41.1 ± 2.2 | 41.3 ± 2.1 | 0.8 | 0.5 | |||
| Superior-posterior VI – VIIB | 24.8 ± 2.5 | 27.1 ± 3.1 | 27.4 ± 3.7 | 26.6 ± 2.1 | 1. 8 | 0.2 | |||
| Inferior-posterior, VIIIA–X | 33.1 ± 2.5 | 30.5 ± 3.7 | 31.4 ± 2.8 | 32.1 ± 2.4 | 1.4 | 0.3 | |||
p < 0.05
Abbreviations: NC - normal control; ALN - autism with normal language; ALI - autism with language impairment; SLI - specific language impairment.
The analysis of midsagittal vermis area (mm2) is somewhat at odds with the analysis of vermis volume (cc). Whereas vermis volumes tended to be smaller in both language impaired groups (ALI, SLI) than in language normal groups, vermis areas tended to be larger in both autism groups. ALI and ALN groups had a trend toward larger superior-posterior (VI-VIIB) areas than their language co-groups, overall F(3, 38) = 2.7, p = 0.06 (Table 4). However, when vermis areas were expressed as a ratio to total vermis area, no vermis area ratio differences were observed (this was also true when vermis areas were adjusted for cerebellum volume or overall brain volume). The correlation between each vermis area (mm2) and its respective volume (cc) showed a high correlation (r(42) = 0.68) for the superior-posterior and the inferior-posterior regions, but a lower correlation (r(42) = 0.42) for the anterior vermis. A comparison of slopes along the autism dimension (ALN & ALI versus NC & SLI) showed that anterior vermis area was correlated with volume in the NC & SLI group (r (20) = 0.73, p < 0.001), but not in the ALN & ALI group (r(22) = 0.30, p = 0.2), slope comparison T = 2.5, p = 0.02. This suggests that while the ALI and ALN groups may tend toward larger midsagittal areas, discordantly, language-impaired groups (ALI, SLI) may have a restricted lateral extent of the vermis as indicated by the location of the paravermian sulcus, leading to smaller volumes.
Further exploratory analyses were done to clarify the role of age and IQ in the results reported above. When added as a covariate, neither age nor nonverbal IQ changed the significance of group contrasts for right lobule VIIA Crus I, symmetry of lobule VIIIA, total vermis, or anterior vermis. When verbal IQ (which is significantly correlated with anterior vermis, r(40) = 0.39, p = 0.01) was covaried, group contrasts for total and anterior vermis were inconclusive, but there was no change in significant group contrasts for the volume of right lobule VIIA Crus I or symmetry of lobule VIIIA. Examining the correlation of language performance and structural measures further demonstrated a relationship between posterior-lateral cerebellar asymmetry and language: CELF total language scores were significantly correlated with the symmetry of lobule VIIIA, r(27) = −0.43, p = 0.02. That is, higher scores were related to increased rightward asymmetry. In addition, anterior vermis volumes were significantly correlated with nonverbal IQ (r(40) = 0.39, p = 0.03), the NEPSY nonsense word repetition score (r(27) = 0.41, p = 0.03), and a composite measure of vocabulary based on the Expressive Vocabulary Test (Williams, 1997) and the Peapody Picture Vocabulary Test (Dunn & Dunn, 1997) (r(27) = 0.38, p = 0.03).
Discussion
In the current study, we investigated cerebellar regional structural volume and asymmetry in the same subjects as our previous report on Broca’s area asymmetry (De Fosse et al., 2004). We applied quantitative analysis of discrete cerebellar regions to determine whether previously reported asymmetry patterns in Broca’s area would be reflected contralaterally in posterior lateral cerebellum. In lobule VIIIA, we found that language-normal groups (ALN and NC) had larger right-sided volume, while the language-impaired groups (ALI and SLI) had larger volume on the left. Furthermore, most subjects with language impairment also showed asymmetry reversal of inferior frontal gyrus (IFG). While the majority of normal control boys had larger left-sided IFG coupled with larger right-sided cerebellar lobule VIIIA, language-impaired groups (ALI and SLI) showed the opposite pattern with larger right-sided IFG and left-sided VIIIA. Language-normal boys with autism were more similar to controls. While prior functional neuroimaging studies in normal controls demonstrated contralateral connectivity and correspondence between left frontal language regions and right posterior lateral cerebellum (Desmond & Fiez, 1998; Harris et al., 2006; Metter et al., 1987; Roskies et al., 2001), the current study is the first to identify correspondence between structural asymmetries in both Broca’s area and contralateral posterior lateral cerebellum regions. Furthermore, the regional cerebellar asymmetries reported in the current study were observed in the presence of global cerebellar symmetry in all subject groups, indicating that the asymmetry was regionally specific. In contrast to this localized observation in boys, adult controls displayed rightward cerebellar cortex asymmetry, whereas dyslexic adults had cerebellar symmetry (Rae et al., 2003). Thus, the development of cerebellar cortex asymmetry may occur later during adolescence, as it was not observed in the NC boys in the current study.
In our prior reports of language-related frontal lobe regions (De Fosse et al., 2004; Herbert et al., 2002), right-handed control subjects displayed typical Broca’s area asymmetry, oriented with larger gray matter volumes in left hemisphere, while this asymmetry pattern was reversed in language-impaired subjects with autism and SLI. In studies of cerebellar asymmetry in developmental dyslexia, rightward cerebellar asymmetries were reported in normal controls, with reversal in dyslexic subjects (Leonard et al., 2001; Eckert et al., 2003; Kibby, Fancher, Markanen, & Hynd., 2008). However, asymmetry of respective language and cognition-associated regions of posterior lateral cerebellum has not been previously reported in subjects with autism or SLI. Posterior lateral cerebellar regions have robust interconnections with contralateral frontal cerebral cortex and are integral to language and cognitive functions, as shown in cerebellar lesion (Gebhart et al., 2002; Schmahmann, 2004) and functional imaging studies (Stoodley & Schmahmann, 2009; Benson et al., 1999; Desmond & Fiez, 1998; Harris et al., 2006; Hubrich-Ungureanu et al., 2002; Jansen et al., 2005; Petersen et al., 1989). Evidence for these fronto-corticocerebellar connections also includes the development of crossed cerebellar diaschisis (hypometabolism in a cerebellar hemisphere contralateral to a cerebral hemispheric lesion) following lesions in contralateral frontal language areas (Metter et al., 1987), and reversed cerebellar diaschisis (hypometabolism in a cerebral hemisphere contralateral to a cerebellar hemispheric lesion) leading to decreased activation in contralateral cerebral cortex following cerebellar lesions (Botez-Marquard & Botez, 1997; Schmahmann, 1991; Schmahmann & Sherman, 1998). Furthermore, posterior lateral cerebellar lesions are frequently associated with language deficits (Leggio, Silveri, Petrosini, & Molinari, 2000; Pollack, Polinko, Albright, Towbin, & Fitz, 1995), including agrammatism, decreased verbal fluency, inability to detect one’s own errors on a verb generation task, dysprosodia, mild anomia, impaired initiation of language, and retrieval of words and stories (Silveri et al., 1994; Molinari et al, 1997; Fiez et al., 1992; Schmahmann et al., 2004), particularly with lesions in right posterior lateral cerebellum (Molinari et al., 1997; Gebhart et al., 2002). Thus, in association with its direct corticocerebellar connections with left inferior frontal language regions, right posterior lateral cerebellum has been directly implicated in language functioning (Gebhart et al., 2002).
The focus of our study was posterior lateral cerebellum, particularly lobules VI, VII, and VIII, since these regions have been identified as most closely tied with cognitive function, language, verbal working memory, and attention (Stoodley & Schmahmann, 2009; Desmond & Fiez, 1998; Leiner, Leiner, & Dow, 1991; Levisohn, Cronin-Golomb, & Schmahmann, 2000; Neau, Arroyo-Anllo, Bonnaud, Ingrand, & Gil, 2000; Petersen, Fox, Posner, Mintun, & Raichle, 1989; Riva & Giorgi, 2000; Schmahmann & Sherman, 1998). Posterior lateral cerebellum also displayed the most marked decrease in number of Purkinje cells in autism (Kemper & Bauman, 2002). In primate studies, lobule VIII is the site of the second sensorimotor homunculus and receives inputs from motor areas, while area 46 projects to lobule VII/Crus I/II (Kelly & Strick, 2003). However, in human neuroimaging studies, while prior research has suggested that language is more closely identified with lobules VI and VIIA Crus I (Stoodley & Schmahmann, 2009; Ackermann, Wildgruber, Daum, & Grodd, 1998; Fiez & Raichle, 1997), VIIIA (as well as VI, VIIB, and VIIA Crus I) is generally associated with working memory, with VIIIA particularly identified with motoric rehearsal and working memory (Desmond et al., 1997). Thus, VIIIA may play a substantial role in language as well, perhaps through mediation of verbal working memory (Stoodley & Schmahmann, 2009). Furthermore, Broca’s area is related to language production, and thus, the relationship between IFG and VIIIA asymmetry differences in language impaired groups could be related to a potential motoric role of VIIIA in language production and/or verbal rehearsal. Children with autism often show deficits in verbal mediation and verbal encoding strategies that foster working memory (Joseph, Steele, Meyer, & Tager-Flusberg, 2005). Furthermore, we observed a correlation between VIIIA asymmetry and language scale scores on the CELF, further supporting a relationship between language function and VIIIA asymmetry.
While we observed similarities between ALI and SLI in reversal of VIIIA asymmetry and reduced anterior vermis volume measures, we also observed specific differences between these language-impaired groups. Cerebellar white matter was significantly larger in ALI than in SLI, with language-normal groups’ (ALN and NC) measures falling between. Increased cerebellar white matter volume in ALI may result from overgrowth and lack of pruning, and is also consistent with earlier reports of increased cerebral white matter volume in boys with autism (Herbert et al., 2003, 2004). Furthermore, we observed regional volume differences between ALI and SLI groups in right hemisphere lobule VIIA Crus I, a cerebellar region consistently associated with language processing, suggesting that, while there are structural imaging and language similarities between these disorders, there may be important language-related developmental differences as well. For example, the ALI group had significantly worse CELF scores than the SLI group; thus, the larger VIIA Crus I volume in ALI relative to SLI in this case is likely an abnormal finding, despite smaller volumes in right lobule VIIIA being related to language deficit and lower CELF scores.
To date, cerebellar structural neuroimaging findings in autism have largely focused on vermis sagittal cross-sectional area, yet results have been mixed among research groups, with some disagreement of results (Stanfield et al., 2007; Brambilla et al., 2003). We observed differences in anterior vermis (I/II – V) volumes, with smaller vermis volume in ALI and SLI groups than in the language normal groups, and we observed a correlation between anterior vermis volume and language test scores (verbal IQ, NEPSY non-word repetition, and vocabulary tests), an interesting observation given that anterior vermis is traditionally associated with motor function. However, we did not observe decreased vermis cross-sectional areas in autism or SLI, and in fact, vermis area had a trend toward being larger, particularly in superior-posterior vermis area, in both autism groups (ALI, ALN). The discrepancy between vermis volume and area results in the current study, particularly in anterior vermis, may result from abmormalities in lateral width of the anterior vermis, as suggested by the low correlation between anterior vermis area and volume in the autism groups. Thus, to some extent, our data supports a vermis deficit in autism observed by some groups (Courchesne et al., 1994; Courchesne et al., 1988; Hashimoto et al., 1993; Kaufmann et al., 2003; Rojas et al., 2006), at least in language-impaired subjects. However, we localized the volumetric deficit primarily in anterior vermis (I/II-V), whereas several other studies have reported autism vermis area differences in VI-VII (Stanfield et al., 2007; Courchesne et al., 1994; Courchesne et al., 1988; Kaufmann et al., 2003) or VIII-X (Hashimoto et al., 1993). Our observation of correlation between anterior vermis volumes and IQ measures is consistent with earlier studies suggesting that the vermis effect may be IQ related, particularly with respect to verbal IQ (Piven et al., 1995; Piven et al., 1992), as demonstrated in a large meta-analysis of autism MRI studies that demonstrated that the area of vermis VI-VII showed modifying effects of age and IQ based on meta-regression analysis (Stanfield et al., 2007). Thus, in relation to the controversy surrounding vermis size in autism, our findings are consistent with a vermis effect more closely related to language and IQ effects than to autism specifically. However, there is reason to consider that vermis deficits may also be related to autism. For example, the vermis is thought to be involved with affective function through interconnections with the limbic system (Schmahmann, 2004; Schmahmann & Sherman, 1998). Cerebellar lesions or tumors in vermis lead to irritability and autistic-like features (Levisohn et al., 2000; Riva & Giorgi, 2000; Schmahmann, 2004). Thus, vermis is considered to be limbic cerebellum (Heath, 1977; Schmahmann, 1991, 2000, 2004), and may play a role in affective aspects of autism.
In the current study, ALI and SLI shared similar abnormalities in both VIIIA asymmetry and in decreased anterior vermis volume, while ALN was more similar with normal controls. This pairing of structural brain abnormalities among ALI and SLI subjects (also observed in Broca’s asymmetry reversal in our prior report (De Fosse et al., 2004)) suggests these anomalies are more closely related to language and cognitive deficits than to autism effects per se. However, SLI and ALI share a similar genetic predisposition to language and communication deficits (Fisher, Lai, & Monaco, 2003; Santangelo & Folstein, 1999), and these shared genotype or phenotypic traits may manifest in abnormal structural brain development. For example, family and twin studies found that first degree relatives of probands with autism more frequently display deficits in language skills than occurr in the general population (Bailey et al., 1995; Folstein et al., 1999; Fombonne, Bolton, Prior, Jordan, & Rutter, 1997; Ruser et al., 2007). Furthermore, siblings of children with SLI have an elevated risk for autism (Tomblin, Hafeman, & O’Brien, 2003). Genetic linkage studies in these two developmental disorders point to overlapping regions on chromosome 7q (Barrett et al., 1999; IMGSAC, 1998; O’Brien, Zhang, Nishimura, Tomblin, & Murray, 2003) and chromosome 13q (Barrett et al., 1999; Bartlett et al., 2002). In genetic studies of autism, signals on both 7q and 13q increase significantly when linkage studies restrict their analyses to autism families with clear signs of language impairment (Alarcon, Cantor, Liu, Gilliam, & Geschwind, 2002; Bradford et al., 2001) or language delay (Alarcon et al., 2008). These findings suggest that genetic abnormalities leading to the phenotype of developmental language disorders (Fisher et al., 2003) may overlap with genetic alterations that are liability factors for autism. Furthermore, decreased volumes combined with reversed asymmetry in these frontal and cerebellar regions may represent a preexisting marker of illness susceptibility or endophenotype (Almasy & Blangero, 2001; Breiter & Gasic, 2004). Asymmetry patterns are developmental in nature (Giedd, 2004; Giedd et al., 1996; Makris et al., 2004), and alterations in such patterns are genetically influenced (Galloway, 1990; Hyatt & Yost, 1998; Lowe et al., 1996; Piedra, Icardo, Albajar, Rodriguez-Rey, & Ros, 1998). Genetic transcription factors may lead to atypical asymmetry patterns that emerge very early in fetal development. For example, Sun et al. (2005) identified genes that were differentially expressed in left and right perisylvian regions in cortices from 14-week-old fetuses. These findings suggest genetic factors may contribute to development of cerebral asymmetry, and may influence the atypical asymmetry patterns reported in autism and SLI.
There were several limitations in the current study. First, there were relatively small subject groups and modest effect sizes. Second, we did not perform multiple comparison corrections: however, the approach was focused on hypothesis-driven analyses of a discrete, limited number of anatomically-specific regions, and the current approach was specifically selected to expand upon prior observations in language-related cerebral cortex regions in these groups, and link asymmetry reversals in cortex to those in contralateral regions of posterior-lateral cerebellum. While the modest effect and sample sizes, combined with a limited number of uncorrected multiple comparisons, may detract from the robustness and impact of the findings, prior studies of cerebellum differences in autism tend to be moderate as well. The fact that the current study does demonstrate significant cerebellar regional asymmetry reversal and volume differences despite the modest sample and effect sizes is promising and noteworthy, particularly since the differences reported in the present and previous studies may be constrained by the extensive heterogeneity inherent in autism. Thus, as noted in the current report, differences were observed between autism language subgroups, supporting the need for care in subject selection and inclusion criteria in the presence of heterogeneous populations in autism studies. Finally, our study would benefit from further confirmation in larger subject samples, and could be expanded to assess girls with autism and specific language impairment, as well as older and higher-functioning subjects with autism.
In conclusion, posterior lateral cerebellum is highly interconnected with frontal cortex, and involved in cognitive function, language, working memory, and attention (Schmahmann, 2004; Schmahmann & Sherman, 1998). Right posterior lateral cerebellum is closely linked with left frontal language areas, including Broca’s area. We observed reversed asymmetry in posterior cerebellar lobule VIIIA in language-impaired boys, with and without autism, but not in language normal boys with or without autism. This cerebellar asymmetry reversal was contralateral to that previously reported in Broca’s area in these same subjects. Combined with our earlier report of reversed asymmetry in Broca’s area, the current study supports abnormal development of the typical asymmetry of fronto-corticocerebellar language circuits in subjects with language-impairment, with or without autism.
Acknowledgments
This work was supported in part by a grant from NIDCD to Drs. Tager-Flusberg and Harris, U19/PO1 DC 03610, which is part of the NICHD/NIDCD funded Collaborative Programs of Excellence in Autism; a grant from the NIMH to Dr. Jean Frazier, K08 MH01573; and by a Young Investigator Award funded by NARSAD and a grant from ALSA to Dr. Nikos Makris. The authors also thank Trinity Urban and Joyce Miller for proofreading comments on the manuscript.
References
- Ackermann H, Wildgruber D, Daum I, Grodd W. Does the cerebellum contribute to cognitive aspects of speech production? A functional magnetic resonance imaging (fMRI) study in humans. Neuroscience Letters. 1998;247:187–190. doi: 10.1016/s0304-3940(98)00328-0. [DOI] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American Journal of Human Genetics. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. American Journal of Human Genetics. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. American Journal of Medical Genetics. 2001;105:42–44. [PubMed] [Google Scholar]
- APA. DSM-IV: Diagnostic and statistic manual of mental disorders. 4. Washington, D.C: American Psychiatric Press; 1994. [Google Scholar]
- Arnold JB, Liow JS, Schaper KA, Stern JJ, Sled JG, Shattuck DW, et al. Qualitative and quantitative evaluation of six algorithms for correcting intensity nonuniformity effects. Neuroimage. 2001;13:931–943. doi: 10.1006/nimg.2001.0756. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Barrett S, Beck JC, Bernier R, Bisson E, Braun TA, Casavant TL, et al. An autosomal genomic screen for autism. Collaborative linkage study of autism. American Journal of Medical Genetics. 1999;88:609–615. doi: 10.1002/(sici)1096-8628(19991215)88:6<609::aid-ajmg7>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bartlett CW, Flax JF, Logue MW, Vieland VJ, Bassett AS, Tallal P, et al. A major susceptibility locus for specific language impairment is located on 13q21. American Journal of Human Genetics. 2002;71:45–55. doi: 10.1086/341095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LeSueur LL, Kennedy DN, Kwong KK, Buchbinder BR, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DV. Autism and specific language impairment: categorical distinction or continuum? Novartis Foundation Symposium. 2003;251:213–226. discussion 226–234, 281–297. [PubMed] [Google Scholar]
- Bobylova MY, Petrukhin AS, Dunaevskaya GN, Piliya SV, Il’ina ES. Clinical-psychological characteristics of children with dysgenesis of the cerebellar vermis. Neuroscience and Behavioral Physiology. 2007;37:755–759. doi: 10.1007/s11055-007-0078-4. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Belin P, Bourgeois M, Royer V, Barthelemy C, et al. Perception of complex sounds in autism: abnormal auditory cortical processing in children. American Journal of Psychiatry. 2004;161:2117–2120. doi: 10.1176/appi.ajp.161.11.2117. [DOI] [PubMed] [Google Scholar]
- Botez-Marquard T, Botez MI. Olivopontocerebellar atrophy and Friedreich’s ataxia: neuropsychological consequences of bilateral versus unilateral cerebellar lesions. In: Bradly RJ, Harris RA, Jenner P, Schmahmann JD, editors. International review of neurobiology: Vol. 41. The cerebellum and cognition. San Diego: Academic Press; 1997. pp. 387–410. [DOI] [PubMed] [Google Scholar]
- Bradford Y, Haines J, Hutcheson H, Gardiner M, Braun T, Sheffield V, et al. Incorporating language phenotypes strengthens evidence of linkage to autism. American Journal of Medical Genetics. 2001;105:539–547. [PubMed] [Google Scholar]
- Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Research Bulletin. 2003;61:557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gasic GP. A general circuitry processing reward/aversion information and its implications for neuropsychiatric illness. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge: MIT Press; 2004. pp. 1043–1065. [Google Scholar]
- Burroni L, Orsi A, Monti L, Hayek Y, Rocchi R, Vattimo AG. Regional cerebral blood flow in childhood autism: a SPET study with SPM evaluation. Nuclear Medicine Communications. 2008;29:150–156. doi: 10.1097/MNM.0b013e3282f1bb8e. [DOI] [PubMed] [Google Scholar]
- Catts H, Bridges M, Little T, Tomblin JB. Reading achievement growth in children with language impairments. Journal of Speech Language and Hearing Research. 2008;51:1569–79. doi: 10.1044/1092-4388(2008/07-0259). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Meyer JW, Makris N, Kennedy DN. MRI-based topographic parcellation of the human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Chheda M, Sherman J, Schmahmann JD. Neurologic, psychiatric and cognitive manifestations in cerebellar agenesis. Neurology. 2002;58:356. [Google Scholar]
- Chiron C, Leboyer M, Leon F, Jambaque I, Nuttin C, Syrota A. SPECT of the brain in childhood autism: evidence for a lack of normal hemispheric asymmetry. Developmental Medicine and Child Neurology. 1995;37:849–860. doi: 10.1111/j.1469-8749.1995.tb11938.x. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G. Processing and linguistic markers in young children with specific language impairment (sli) Journal of Speech, Language, and Hearing Research. 2003;46(5):1029–1037. doi: 10.1044/1092-4388(2003/082). [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, Botting N. Classification of children with specific language impairment: longitudinal considerations. Journal of Speech, Language, and Hearing Research. 1999;42:1195–1204. doi: 10.1044/jslhr.4205.1195. [DOI] [PubMed] [Google Scholar]
- Courchesne E. An MRI study of autism: the cerebellum revisited. Neurology. 1999;52:1106–1107. doi: 10.1212/wnl.52.5.1106. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, et al. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR American Journal of Roentgenology. 1994;162:123–130. doi: 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. New England Journal of Medicine. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dawson G, Finley C, Phillips S, Galpert L. Hemispheric specialization and the language abilities of autistic children. Child Development. 1986;57:1440–1453. [PubMed] [Google Scholar]
- De Fosse L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, McGrath L, et al. Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- Dean RS. Cerebral laterality and reading comprehension. Neuropsychologia. 1978;16:633–636. doi: 10.1016/0028-3932(78)90092-1. [DOI] [PubMed] [Google Scholar]
- Dean RS. Assessing patterns of lateral preference. Journal of Clinical Neuropsychology. 1982;4:124–128. [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends in Cognitive Science. 1998;2:355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. Journal of Neuroscience. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Elliot CD. Differential ability scales. San Antonio, TX: The Psychological Corporation, Harcourt Brace and Co; 1990. [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115(Pt 1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME. Linguistic processing. In: Bradly RJ, Harris RA, Jenner P, Schmahmann JD, editors. International review of neurobiology: Vol. 41. The cerebellum and cognition. San Diego: Academic Press; 1997. pp. 233–254. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Lai CS, Monaco AP. Deciphering the genetic basis of speech and language disorders. Annual Review of Neuroscience. 2003;26:57–80. doi: 10.1146/annurev.neuro.26.041002.131144. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Santangelo SL, Gilman SE, Piven J, Landa R, Lainhart J, et al. Predictors of cognitive test patterns in autism families. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:1117–1128. [PubMed] [Google Scholar]
- Fombonne E, Bolton P, Prior J, Jordan H, Rutter M. A family study of autism: cognitive patterns and levels in parents and siblings. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1997;38:667–683. doi: 10.1111/j.1469-7610.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca’s area: the pars triangularis and pars opercularis. Brain and Language. 1998;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- Galloway J. Developmental biology. A handle on handedness. Nature. 1990;346:223–224. doi: 10.1038/346223a0. [DOI] [PubMed] [Google Scholar]
- Gebhart AL, Petersen SE, Thach WT. Role of the posterolateral cerebellum in language. Annals of the New York Academy of Sciences. 2002;978:318–333. doi: 10.1111/j.1749-6632.2002.tb07577.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Harenski K, Keshavan MS. Posterior fossa magnetic resonance imaging in autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:666–672. doi: 10.1097/00004583-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain and Cognition. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Miyazaki M, Murakawa K, Kuroda Y. Brainstem and cerebellar vermis involvement in autistic children. Journal of Child Neurology. 1993;8:149–153. doi: 10.1177/088307389300800207. [DOI] [PubMed] [Google Scholar]
- Heath RG. Modulation of emotion with a brain pacemamer. Treatment for intractable psychiatric illness. Journal of Nervous and Mental Disease. 1977;165:300–317. [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, et al. Abnormal asymmetry in language association cortex in autism. Annals of Neurology. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hubrich-Ungureanu P, Kaemmerer N, Henn FA, Braus DF. Lateralized organization of the cerebellum in a silent verbal fluency task: a functional magnetic resonance imaging study in healthy volunteers. Neuroscience Letters. 2002;319:91–94. doi: 10.1016/s0304-3940(01)02566-6. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Yost HJ. The left-right coordinator: the role of Vg1 in organizing left-right axis formation. Cell. 1998;93:37–46. doi: 10.1016/s0092-8674(00)81144-7. [DOI] [PubMed] [Google Scholar]
- IMGSAC. A full genome screen for autism with evidence for linkage to a region on chromosome 7q. International Molecular Genetic Study of Autism Consortium. Human Molecular Genetics. 1998;7:571–578. doi: 10.1093/hmg/7.3.571. [DOI] [PubMed] [Google Scholar]
- Jansen A, Floel A, Van Randenborgh J, Konrad C, Rotte M, Forster AF, et al. Crossed cerebro-cerebellar language dominance. Human Brain Mapping. 2005;24:165–172. doi: 10.1002/hbm.20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Steele SD, Meyer E, Tager-Flusberg H. Self-ordered pointing in children with autism: failure to use verbal mediation in the service of working memory? Neuropsychologia. 2005;43:1400–1411. doi: 10.1016/j.neuropsychologia.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cooper KL, Mostofsky SH, Capone GT, Kates WR, Newschaffer CJ, et al. Specificity of cerebellar vermian abnormalities in autism: a quantitative magnetic resonance imaging study. Journal of Child Neurology. 2003;18:463–470. doi: 10.1177/08830738030180070501. [DOI] [PubMed] [Google Scholar]
- Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N. Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR images. Journal of Anatomy. 2007;211:534–555. doi: 10.1111/j.1469-7580.2007.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. Journal of Neuropathology and Experimental Neurology. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. Neuropathology of infantile autism. Molecular Psychiatry. 2002;7(Suppl 2):S12–13. doi: 10.1038/sj.mp.4001165. [DOI] [PubMed] [Google Scholar]
- Kennedy DN, Filipek PA, Caviness VS. Autonomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Transactions on Medical Imaging. 1989;8:1–7. doi: 10.1109/42.20356. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Fancher JB, Markanen R, Hynd GW. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J Child Neurol. 2008;23:368–380. doi: 10.1177/0883073807309235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman MD, Neff S, Rosman NP. The brain in infantile autism: are posterior fossa structures abnormal? Neurology. 1992;42:753–760. doi: 10.1212/wnl.42.4.753. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. San Antonio, TX: The Pyschological Corporation, Harcourt Brace and Co; 1998. [Google Scholar]
- Kulesza RJ, Mangunay K. Morphological features of the medial superior olive in autism. Brain Research. 2008;1200:132–137. doi: 10.1016/j.brainres.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Silveri MC, Petrosini L, Molinari M. Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. Journal of Neurology, Neurosurgery and Psychiatry. 2000;69:102–106. doi: 10.1136/jnnp.69.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behavioral Neuroscience. 1986;100:443–454. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behavioral Brain Research. 1991;44:113–128. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, et al. Anatomical risk factors for phonological dyslexia. Cereb Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, et al. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature. 1996;381:158–161. doi: 10.1038/381158a0. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Makris N, Hodge SM, Haselgrove C, Kennedy DN, Dale A, Fischl B, et al. Human cerebellum: surface-assisted cortical parcellation and volumetry with magnetic resonance imaging. Journal of Cognitive Neuroscience. 2003;15:584–599. doi: 10.1162/089892903321662967. [DOI] [PubMed] [Google Scholar]
- Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, Seidman LJ, et al. MRI-based surface-assisted parcellation of human cerebellar cortex: an anatomically specified method with estimate of reliability. Neuroimage. 2005;25:1146–1160. doi: 10.1016/j.neuroimage.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Hertrich I, Grodd W, Ackermann H. Cerebellum and speech perception: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2002;14:902–912. doi: 10.1162/089892902760191126. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Kempler D, Jackson CA, Hanson WR, Riege WH, Camras LR, et al. Cerebellar glucose metabolism in chronic aphasia. Neurology. 1987;37:1599–1606. doi: 10.1212/wnl.37.10.1599. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Silveri MC. Verbal fluency and agrammatism. In: Bradly RJ, Harris RA, Jenner P, Schmahmann JD, editors. International Review of Neurobiology: Vol. 41. The Cerebellum and Cognition. San Diego: Academic Press; 1997. pp. 325–339. [DOI] [PubMed] [Google Scholar]
- Muller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, et al. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. Journal of Autism and Developmental Disorders. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Neau JP, Arroyo-Anllo E, Bonnaud V, Ingrand P, Gil R. Neuropsychological disturbances in cerebellar infarcts. Acta Neurologica Scandinavica. 2000;102:363–370. doi: 10.1034/j.1600-0404.2000.102006363.x. [DOI] [PubMed] [Google Scholar]
- O’Brien EK, Zhang X, Nishimura C, Tomblin JB, Murray JC. Association of specific language impairment (SLI) to the region of 7q31. American Journal of Human Genetics. 2003;72:1536–1543. doi: 10.1086/375403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, et al. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123(Pt 9):1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J. Schedule for affective disorders and schizophrenia for school-age children -- epidemiologic version (K-SADS-E) 4. Fort Lauderdale, FL: Nova University, Center for Psychological Studies; 1987. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of single-words. Journal of Cognitive Neuroscience. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodriguez-Rey JC, Ros MA. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 1998;94:319–324. doi: 10.1016/s0092-8674(00)81475-0. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S. The cerebellum and autism. Neurology. 1995;45:398–402. doi: 10.1212/wnl.45.2.398-a. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. American Journal of Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Piven J, Nehme E, Simon J, Barta P, Pearlson G, Folstein SE. Magnetic resonance imaging in autism: measurement of the cerebellum, pons, and fourth ventricle. Biological Psychiatry. 1992;31:491–504. doi: 10.1016/0006-3223(92)90260-7. [DOI] [PubMed] [Google Scholar]
- Piven J, Saliba K, Bailey J, Arndt S. An MRI study of autism: the cerebellum revisited. Neurology. 1997;49:546–551. doi: 10.1212/wnl.49.2.546. [DOI] [PubMed] [Google Scholar]
- Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37:885–893. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, et al. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40:1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autism in search of a home in the brain. Neurology. 1999;52:902–904. doi: 10.1212/wnl.52.5.902. [DOI] [PubMed] [Google Scholar]
- Rapin I. Clinics in developmental medicine:Vol. 139. Preschool children with inadequate communication. London: Mac Keith Press; 1996. [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. American Journal of Psychiatry. 1986;143:862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(Pt 5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Ruser TF, Arin D, Dowd M, Putnam S, Winklosky B, Rosen-Sheidley B, et al. Communicative competence in parents of children with autism and parents of children with specific language impairment. Journal of Autism and Developmental Disorders. 2007;37:1323–1336. doi: 10.1007/s10803-006-0274-z. [DOI] [PubMed] [Google Scholar]
- Santangelo SL, Folstein SE. Autism: A genetic perspective. In: Tager-Flusberg H, editor. Neurodevelopmental disorders. Cambridge, MA: MIT Press; 1999. pp. 431–447. [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Archives of Neurology. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Schmahmann JD. The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. International Review of Psychiatry. 2001;13:247–260. [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga A, Petrides M, Evans A. MRI atlas of the human cerebellum. San Diego: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 3. San Antonio, TX: The Psychological Corporation, Harcourt Brace and Co; 1995. [Google Scholar]
- Silveri MC, Leggio MG, Molinari M. The cerebellum contributes to linguistic production: a case of agrammatic speech following a right cerebellar lesion. Neurology. 1994;44:2047–2050. doi: 10.1212/wnl.44.11.2047. [DOI] [PubMed] [Google Scholar]
- Slifer KJ, Cataldo MF, Cataldo MD, Llorente AM, Gerson AC. Behavior analysis of motion control for pediatric neuroimaging. Journal of Applied Behavior Analysis. 1993;26:469–470. doi: 10.1901/jaba.1993.26-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. European Psychiatry. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. Language impairments in children with complex neurodevelopmental disorders: The case of autism. In: Levy Y, Schaeffer JC, editors. Language competence across populations: Toward a definition of specific language impairment. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 297–321. [Google Scholar]
- Tager-Flusberg H. Defining language phenotypes in autism. Clinical Neuroscience Research. 2006;6:219–224. [Google Scholar]
- Tager-Flusberg H, Caronna E. Language disorders: autism and other pervasive developmental disorders. Pediatric Clinics of North America. 2007;54:469–481. vi. doi: 10.1016/j.pcl.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Cooper J. Present and future possibilities for defining a phenotype for specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42(5):1275–1278. doi: 10.1044/jslhr.4205.1275. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2003;358:303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Paul R, Lord CE. Language and communication in autism. In: Volkmar F, Paul R, Klin A, Cohen DJ, editors. Handbook of autism and pervasive developmental disorders: Vol. 1. Diagnosis, development, neurobiology, and behavior. 3. New York: Wiley; 2005. pp. 335–364. [Google Scholar]
- Takeuchi M, Harada M, Matsuzaki K, Nishitani H, Mori K. Difference of signal change by a language task on autistic patients using functional MRI. The Journal of Medical Investigation : JMI. 2004;51:59–62. doi: 10.2152/jmi.51.59. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Tomblin JB, Hafeman LL, O’Brien M. Autism and autism risk in siblings of children with specific language impairment. International Journal of Language and Communication Disorders. 2003;38:235–250. doi: 10.1080/1368282031000086363. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The wechsler intelligence scale for children - third edition. San Antonio, TX: The Psychological Corporation, Harcourt Brace and Co; 1991. [Google Scholar]
- Williams KT. Expressive vocabulary test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Worth AJ, Makris N, Meyer JW, Caviness VS, Jr, Kennedy DN. Semiautomatic segmentation of brain exterior in magnetic resonance images driven by empirical procedures and anatomical knowledge. Medical Image Analysis. 1998;2:315–324. doi: 10.1016/s1361-8415(98)80013-3. [DOI] [PubMed] [Google Scholar]
- Worth AJ, Makris N, Patti MR, Goodman JM, Hoge EA, Caviness VS, Jr, et al. Precise segmentation of the lateral ventricles and caudate nucleus in MR brain images using anatomically driven histograms [letter] IEEE Transactions on Medical Imaging. 1998;17:303–310. doi: 10.1109/42.700743. [DOI] [PubMed] [Google Scholar]
- Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, et al. Involvement of the cerebellum in semantic discrimination: an fMRI study. Human Brain Mapping. 2003;18:208–214. doi: 10.1002/hbm.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]