Abstract
Astrocytic energetics of excitatory neurotransmission is controversial due to discrepant findings in different experimental systems in vitro and in vivo. The energy requirements of glutamate uptake are believed by some researchers to be satisfied by glycolysis coupled with shuttling of lactate to neurons for oxidation. However, astrocytes increase glycogenolysis and oxidative metabolism during sensory stimulation in vivo, indicating that other sources of energy are used by astrocytes during brain activation. Furthermore, glutamate uptake into cultured astrocytes stimulates glutamate oxidation and oxygen consumption, and glutamate maintains respiration as well as glucose. The neurotransmitter pool of glutamate is associated with the faster component of total glutamate turnover in vivo, and use of neurotransmitter glutamate to fuel its own uptake by oxidation-competent perisynaptic processes has two advantages, substrate is supplied concomitant with demand, and glutamate spares glucose for use by neurons and astrocytes. Some, but not all, perisynaptic processes of astrocytes in adult rodent brain contain mitochondria, and oxidation of only a small fraction of the neurotransmitter glutamate taken up into these structures would be sufficient to supply the ATP required for sodium extrusion and conversion of glutamate to glutamine. Glycolysis would, however, be required in perisynaptic processes lacking oxidative capacity. Three lines of evidence indicate that critical cornerstones of the astrocyte-to-neuron lactate shuttle model are not established and normal brain does not need lactate as supplemental fuel: (i) rapid onset of hemodynamic responses to activation delivers oxygen and glucose in excess of demand, (ii) total glucose utilization greatly exceeds glucose oxidation in awake rodents during activation, indicating that the lactate generated is released, not locally oxidized, and (iii) glutamate-induced glycolysis is not a robust phenotype of all astrocyte cultures. Various metabolic pathways, including glutamate oxidation and glycolysis with lactate release, contribute to cellular energy demands of excitatory neurotransmission.
Keywords: astrocyte, brain activation, glucose, glutamate, lactate, neuron
1. Introduction
One of the hallmark characteristics of the brain is its regional, cellular, and subcellular heterogeneity, with compartmentation of function and metabolism. The glutamate-glutamine cycle is a classic example of astrocyte-neuron interactions that involve glycolytic, oxidative, biosynthetic, and neurotransmitter fluxes (Peng et al., 1993; Hertz et al., 1999; Hertz et al., 2000). In brief, the typical description glutamate-glutamine cycle portrays release of neurotransmitter glutamate from neurons, Na+-dependent uptake of glutamate from the synaptic cleft by astrocytes and its conversion to glutamine, followed by glutamine release and uptake by neurons where glutamate is regenerated by the action of glutaminase and packaged into synaptic vesicles for release during neurotransmission. However, because the blood-brain barrier restricts glutamate uptake into brain from blood, the glutamate-glutamine cycle must be extended to include glutamate synthesis and degradation in brain. Anaplerosis is the de novo synthesis of glutamate from glucose in astrocytes. This process involves glycolysis and CO2 fixation to generate the precursors (pyruvate and oxaloacetate) that condense to form citrate that is oxidized via the TCA cycle to form α-ketoglutarate, the precursor of glutamate and glutamine. Oxidative degradation of glutamate occurs mainly in astrocyte and involves entry of α-ketoglutarate into the TCA cycle, exit of malate from the cycle and conversion of malate to pyruvate in the cytoplasm, followed by oxidative degradation of pyruvate in the TCA cycle. This process is called pyruvate re-cycling because the glutamate carbon skeleton was originally derived from two pyruvate molecules, and the 4-carbon backbone of the TCA cycle intermediates derived from α-ketoglutarate must exit the cycle for conversion to pyruvate to enable complete degradation of the molecule to CO2. In spite of many studies of the pathways and fluxes that contribute to glutamate turnover and glutamatergic neurotransmission, the energetics of astrocytes has been a long-standing controversial issue because models describing astrocyte-neuron interactions and metabolite trafficking during excitatory neurotransmission predict that different pathways supply the ATP required by astrocytes and neurons. This review will briefly highlight studies of glutamate metabolism and evidence for glutamate oxidation as an astrocytic energy source, then contrast these findings with those of lactate shuttle models.
2. Glutamate is an important astrocytic energy source
2.1. Compartmentation of glutamate synthesis and degradation in astrocytes
Investigation of the in vivo labeling patterns of amino acids derived from various labeled precursors in the 1960–1970s led to the discovery that there is compartmentation of oxidative metabolism in brain, characterized by a large, ‘energy’ TCA cycle, and a smaller, ‘synthetic’ TCA cycle. Data obtained from many studies led to the conclusion that (i) the anaplerotic pathway for de novo synthesis of glutamate and glutamine from glucose resided in astrocytes and (ii) interconversion of labeled glutamate and glutamine reflects, in large part, excitatory neurotransmission (Balázs and Cremer, 1972; Berl et al., 1975). Assignment of the small, synthetic TCA cycle to astrocytes was confirmed by their high enrichment with the enzymes required for glutamine synthesis and de novo synthesis of glutamate from glucose, i.e., glutamine synthetase (Martinez-Hernandez et al., 1977) and pyruvate carboxylase (Yu et al., 1983; Shank et al., 1985), respectively. GABA turnover also involves these anaplerotic reactions and oxidative degradation in the astrocytic TCA cycle (Hertz, 1979; Schousboe et al., 1992; Schousboe and Waagepetersen, 2007), but these reactions are not included in the present discussion.
Many laboratories have demonstrated that exogenous glutamate had two major fates after its uptake into astrocytes, oxidation or conversion to glutamine, with the proportion metabolized by each pathway being concentration dependent. Uptake and metabolism of exogenous [14C, 13C, or 15N]glutamate by brain slices and cultured astrocytes is associated with label incorporation into CO2, aspartate, glutamine, and other compounds (Benjamin and Quastel, 1972, 1974; Schousboe et al., 1977; Yu et al., 1982; Waniewski and Martin, 1986; Yudkoff et al., 1986; Farinelli and Nicklas, 1992; Sonnewald et al., 1993). The higher the extracellular glutamate level the greater the fraction oxidized, with about half being oxidized at 0.5 mmol/L glutamate (McKenna et al., 1996). Because astrocytes have much greater glutamate oxidative rates than GABAergic neurons and the corresponding rates in glutamatergic neurons were negligible, glutamate degradation is predominantly astrocytic (Hertz et al., 1988; Waagepetersen et al., 2002). The conclusion that glutamate is an important energy substrate for astrocytes is strongly supported by (i) the glucose-sparing actions of extracellular glutamate in cultured astrocytes (Swanson et al., 1990; Yu et al., 1992; Peng et al., 2001; Qu et al., 2001) and in isolated, intact hippocampus from adult mice (Dunlop et al., 1984), (ii) robust stimulation of astrocytic respiration by glutamate (Eriksson et al., 1995), and (iii) similar rates of astrocytic oxygen consumption with either glucose or glutamate as sole substrate (Hertz and Hertz, 2003). Under steady state conditions, oxidation of glutamate and GABA approximates the anaplerotic rate, which is ~15% of the total pyruvate oxidation rate (Hertz, 2011; Rothman et al., 2011), and glutamate synthesis and degradation in astrocytes produces nearly as much ATP as direct oxidation of glucose (Hertz et al., 1999; Hertz et al., 2007).
2.2. Glutamate oxidation can fuel glutamate uptake in astrocytes
Peng et al. (2001) tested the hypothesis that oxidation of exogenous glutamate provides the ATP required for Na+ extrusion and demonstrated that (i) extracellular glutamate did not increase glucose utilization even though it inhibited glucose oxidation, (ii) treatment of astrocytes with D-aspartate, a transportable but non-metabolizable glutamate analog, did stimulate glucose utilization, and (iii) monensin, an ionophore that stimulates Na+-K+-ATPase activity, increased glucose utilization. These findings are consistent with studies in isolated, intact hippocampus from adult mice showing that extracellular glutamate was oxidized in greater amounts with increasing concentration, and glutamate reduced oxidation of glucose (Dunlop et al., 1984). Recently, the glutamate transporter was shown to form multi-enzyme complexes with glycolytic enzymes and mitochondria that facilitate oxidation of very low levels (8 μmol/L) of glutamate as it is transported into the astrocytes (Genda et al., 2011; Bauer et al., 2012).
Some, but not all, perisynaptic astrocytic processes in adult rodent brain contain mitochondria (Lovatt et al., 2007; Lavialle et al., 2011; Pardo et al., 2011) and these oxidation-competent filopodial structures are capable of metabolism of glutamate to support the energetics of its uptake. On the other hand, perisynaptic processes without mitochondria would depend on glycolysis. Because glutamate concentration in the synaptic cleft reaches millimolar levels (Bergles et al., 1999; Matsui et al., 2005) it is likely that a substantial fraction of the transmitter glutamate may be oxidized (McKenna et al., 1996) and provide ATP to help fuel glutamate uptake in oxidation-competent filopodia. Mitochondria are essential for both de novo glutamate synthesis from glucose and for its oxidative degradation, and it would, therefore, be of great interest to determine if mitochondria-containing perisynaptic filopodia preferentially surround synapses utilizing glutamate and GABA as neurotransmitters. The proportion and localization of perisynaptic filopodia endowed with mitochondria is a central issue in understanding astrocytic energetics of neurotransmission.
2.3. Does glutamate oxidation support dynamic mobility of astrocytic processes during neurotransmission?
Because astrocytic glycogenolysis and oxidative metabolism, not just glycolysis, rise during brain activation in vivo (Hertz et al., 2007; Dienel, 2012b), other unidentified energy-requiring processes may also be stimulated by excitatory neuronal signaling, not only the expense of Na+ extrusion and glutamine synthesis. For example, filopodial and lamellipodial processes are specialized astrocytic structures that are enriched with specific proteins and are highly mobile; they spontaneously advance towards and retract from active synaptic terminals in brain slices (Hirrlinger et al., 2004; Reichenbach et al., 2010; Derouiche et al., 2012). Although the mechanisms and energetics of filopodial movements are poorly understood, glutamate induces formation of actin-containing filopodia in cultured astrocytes (Cornell-Bell et al., 1990), and actin is present in the fine peripheral astrocytic processes (Derouiche and Frotscher, 2001). These observations raise the question whether the ATP demands associated with actin dynamics (Chen et al., 2000; Bernstein and Bamburg, 2003; Carlier et al., 2003) contribute to the energetics of glutamatergic signaling and mobility of astrocytic processes in vivo. This issue underscores the importance of better knowledge of filopodial mitochondrial localization and their association with mobility.
2.4. Differential turnover of glutamate pools in brain in vivo
Berl and Frigyesi (1969) labeled brain amino acid pools in cat brain with [1-14C]acetate, and measured clearance of label from glutamate, glutamine, aspartate and GABA. They observed similar biphasic decay curves for all four amino acids, with fast components having half-lives of 11–18 min and slow components with half lives of 2.5–4h. The similar decay rates were interpreted to imply a close association for the overall metabolism of the four amino acids via the TCA cycle (Berl and Frigyesi, 1969). These results are in agreement with an earlier study in cats showing that one pool of glutamate had a half life of minutes, the other hours (Berl et al., 1962), and with subsequent studies in rat brain in vivo and in brain slices that identified pools of glutamate with very different sizes and turnover rates (Badar-Goffer et al., 1992; Shank et al., 1993; Kauppinen et al., 1994; Lukkarinen et al., 1997). The rapidly-turning over components of the TCA cycle-derived amino acid pools may be associated with neurotransmission, whereas the long half-lived components may perhaps contribute to glutamate and glutamine dilutions observed in magnetic resonance spectroscopic studies. The fractional enrichment of glutamate and glutamine C4 never reaches the theoretical maximum of half that of [1-13C- or 6-13C]glucose, and dilution of these pools is ascribed to lactate and glutamine exchange, respectively (Rothman et al., 2011). Lactate exchange would reduce brain lactate specific activity due to efflux of labeled lactate to blood coupled with influx of unlabeled lactate to maintain the steady state mass of lactate in brain. However, with relatively short (5–30 min) labeling assays, the lactate specific activity was close to theoretical maxim in awake rat brain during rest, sensory stimulation, and recovery from stimulation and during spreading depression (Dienel and Cruz, 2009), and lack of label dilution is in good agreement with findings in awake mice (Van den Berg et al., 1969) and in brain slices (Badar-Goffer et al., 1992; Kauppinen et al., 1994). These data contrast the finding of lactate dilution at the end of 300 min infusion assays in anesthetized rats (Duarte et al., 2011). The anatomical sites of rapid amino acid turnover and actual causes of glutamate and glutamine dilutions remain to be directly established, but degradation of the short half-lived fraction of the transmitter-related amino acids may be linked to astrocytic energetics of neurotransmission.
2.5. Selective inhibition of the astrocytic TCA cycle
Fluoroacetate and fluorocitrate selectively block the astrocytic TCA cycle at the aconitase step (Fonnum et al., 1997), and these gliotoxins are useful tools for analysis of astrocytic metabolism and energetics. For example, fluoroacetate and fluorocitrate (1 mmol/L in slices or 2 mg/kg, i.p. in vivo) strongly inhibited incorporation of label from [14C]glutamate, [14C]aspartate, [14C]acetate, and [14C]GABA into glutamine in brain slices and mouse brain in vivo (Clarke et al., 1970). Because these drugs did not inhibit purified glutamine synthetase and the slices were incubated in high glucose, the authors suggested that the ATP required for glutamine synthesis from [14C]glutamate is probably provided by the astrocytic TCA cycle, consistent with conclusions drawn from metabolism of exogenous glutamate discussed above, i.e., glutamate oxidation may help pay for its uptake.
Treatment of mice with a higher dose of fluoroacetate (100 mg/kg, i.p.) blocked labeling of glutamine by [1,2-13C]acetate, reduced labeling of glutamine C4 from [1-13C]glucose, and increased the C3/C4 ratio (a measure of TCA cycling) in glutamine compared with glutamate (Hassel et al., 1997). These findings were explained by inhibition of the astrocytic TCA cycle, exchange-mediated labeling of [13C]glutamate from [1-13C]glucose in neurons, and neuronal release of [13C]glutamate, followed by its astrocytic uptake and conversion to [13C]glutamine. The high cycling ratio of labeled glutamine was interpreted in terms of association of the [13C]glutamate with a (neuronal) TCA cycle that turned over faster than the overall brain TCA cycle, in agreement with faster turnover of transmitter glutamate compared with the metabolic pool of glutamate (see (Hassel et al., 1997) and references cited therein). Faster turnover of the neurotransmitter glutamate pool supports the conclusion that oxidation of some glutamate taken up by astrocytes provides energy to fuel its uptake in vivo.
2.6. Glutamate oxidation via a truncated TCA cycle
Incomplete astrocytic oxidation of glutamate was revealed by incorporation of label from exogenous glutamate into aspartate (Benjamin and Quastel, 1972, 1974; Schousboe et al., 1977; Yu et al., 1982; Sonnewald et al., 1993), i.e., there is sequential conversion of the 5-carbon glutamate to α-ketoglutarate, oxaloacetate, and aspartate, with preservation of the 4-carbon skeleton. Qu et al. (2001) extended these findings by showing that addition of 0.5 mmol/L glutamate to the incubation medium of cerebellar astrocytes reduced the amount of [U-13C]glucose consumed, decreased the flux through the pyruvate carboxylase step, and increased the amount of aspartate. Unlabeled glutamate also increased synthesis of labeled glutamate derived from the first and second turns of the TCA cycle. Thus, glutamate substituted for glucose as an energy substrate, it spared glucose use by the anaplerotic pathway, it increased synthesis of glutamate and aspartate, and it revealed compartmentation of intracellular oxaloacetate pools in astrocytes (Qu et al., 2001). Conversion of glutamate to oxaloacetate increases the capacity of the TCA cycle (i.e., the amount of TCA cycle intermediates) without requiring pyruvate carboxylase, and shunting of glutamate through the TCA cycle with regeneration of glutamate produces substantial quantities of ATP.
2.7. Astrocytic energetics and glutamate oxidation
Depolarization-evoked release of neuronal K+ to extracellular fluid is followed by synaptic vesicle fusion and release of glutamate from excitatory neurons into the synaptic cleft. Increases in extracellular [K+] within the range of 3–12 mmol/L stimulate glycogenolysis in brain slices in a concentration-dependent manner (Hof et al., 1988), and K+ uptake into cultured astrocytes mediated by both Na+,K+-ATPase and Na+,K+,Cl− cotransporter1 (NKCC1) is blocked by inhibition of glycogen phosphorylase even in the presence of glucose (Xu et al., 2012). K+ transport is fueled by glycogen, whereas glutamate uptake is not (Magistretti et al., 1981). Astrocytic K+ uptake also causes intracellular pH and glutamine content to increase (Brookes and Turner, 1993, 1994), and alkalinization will stimulate glycolysis due to the high pH sensitivity of phosphofructokinase (Trivedi and Danforth, 1966; Halperin et al., 1969), the major rate-limiting enzyme of the glycolytic pathway (Passonneau and Lowry, 1964). Small increases in extracellular [K+] in hippocampal slice preparations also stimulate glycolysis-dependent protein synthesis which does not occur when pyruvate replaces glucose and it is not related to differences in ATP concentration (Lipton and Robacker, 1983). Thus, both K+ and glutamate are cleared from extracellular fluid by uptake into astrocytes and they cause metabolic activation of different pathways in astrocytes.
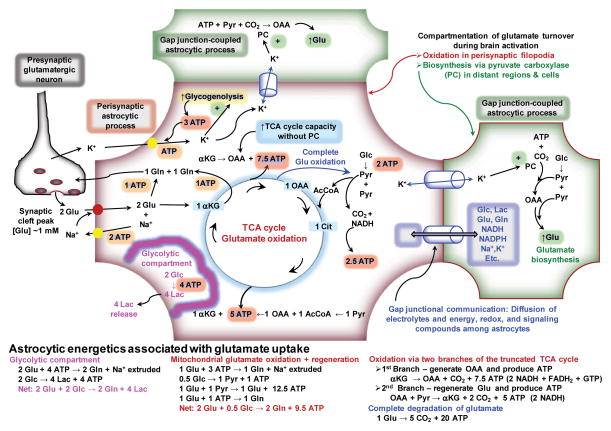
Glutamate taken up into an astrocyte can be either directly converted to glutamine or it can enter into the TCA cycle. For simplicity, the energetic balance sheet illustrated in Fig. 1 is based on uptake of 2 glutamate molecules, one entering each of these pathways. The carbon skeleton of glutamate entering the TCA cycle can also have two fates, shunting through the TCA cycle (i.e., entry, oxidation, and regeneration) or complete degradation. Entry of one glutamate into the truncated TCA cycle generates 7.5 ATP when it is converted to oxaloacetate (this is lower than theoretical ATP yield is due to proton leakage, see (Hertz et al., 2007)), thereby providing an excess of energy compared with the 3 ATP needed to synthesize glutamine from the second glutamate and extrude the Na+ taken up with the 2 glutamate molecules (Fig. 1). Regeneration of glutamate from this oxaloacetate requires oxidation of one pyruvate without the need for pyruvate carboxylase activity, and passage of the molecule through the rest of the TCA cycle generates an additional 5 ATP (Fig. 1) and completes the process of shunting of glutamate through the TCA cycle. The net ATP yield when two glutamate are converted to two glutamine (one directly and one indirectly via shunting through the TCA cycle) is 9.5 ATP, with consumption of 0.5 molecules of glucose (Fig. 1). When glutamate is completely oxidized via the pyruvate re-cycling pathway (Cerdan et al., 1990), malate exits from the TCA cycle (arrow from TCA cycle to pyruvate, Fig. 1) and is converted to pyruvate in the cytoplasm, followed by pyruvate oxidation, for a yield of 20 ATP per glutamate molecule (Fig. 1; for details, see (Hertz et al., 2007)). Due to the large amount of ATP produced by glutamate metabolism, oxidation of only ~10–20% (depending on shunting or complete oxidation) of one incoming neurotransmitter glutamate molecule would match the energy demands of 2 ATP for Na+ extrusion and conversion to glutamine. Furthermore, glutamate oxidation is glucose-sparing, as is glycogenolysis (Dinuzzo et al., 2012), thereby freeing up endogenous glucose for use by nearby neurons, for astrocytic glycogen turnover, or other astrocytic activities. Increasing the flux through the glutamate shunt or complete glutamate degradation when glutamate levels are high can generate surplus of ATP for use by astrocytic activities that are upregulated during strong excitatory neurotransmission, such as dynamic movements of perisynaptic processes.
Figure 1. Energetics of astrocytic glutamate uptake.
K+ released from neurons is taken up by astrocytes and fueled by glycogenolysis. Glutamate (Glu) uptake into perisynaptic processes of astrocytes that contain mitochondria has two fates, conversion to glutamine (Gln) or oxidation. When partially oxidized, the glutamate is converted to α-ketoglutarate (αKG), then oxaloacetate (OAA). This OAA can be converted to aspartate (not shown) and it can serve to increase the catalytic capacity of the TCA cycle without increased pyruvate (Pyr) carboxylase (PC) activity. Glu can be regenerated after condensation of OAA with acetyl CoA (AcCoA) and passage through the TCA cycle. Alternatively, Glu can be completely oxidized by exit of malate from the TCA cycle (blue arrow from TCA cycle to Pyr), its conversion to pyruvate in the cytoplasm, and reentry of pyruvate into the TCA cycle (See text). Energy derived from glutamate oxidation is obtained from its shunting through the TCA cycle and by complete oxidative degradation. Because electrolytes, metabolites, and signaling compounds can diffuse within the astrocytic syncytium via gap junctions, local K+ concentrations may increase in regions of the astrocyte distant from the perivascular zone or in gap junction-coupled astrocytes, stimulate pyruvate carboxylase and increase de novo glutamate synthesis. Compartmentation of glutamate oxidation in perisynaptic regions and glutamate synthesis in more distant structures is denoted by the red and green borders, respectively. A glycolytic compartment is included for structures that do not contain mitochondria.
2.8. Compartmentation of glutamate-glutamine turnover arising from astrocytic gap junctional communication
After uptake into astrocytes from the synaptic cleft or generation within activated astrocytic processes, K+, Na+, NADH, NADPH, glucose, lactate, glutamate, glutamine, and other compounds (see discussion in (Gandhi et al., 2009b) and (Dienel, 2012b)) can diffuse via gap junctions to other regions of the same astrocyte and to other astrocytes (Fig. 1). Gap junctional trafficking can spread the workload of astrocytic activation within the large syncytium and raises the possibility that glutamate oxidation and glutamate biosynthesis may occur in different regions of a single astrocyte or in different astrocytes (Fig. 1). Thus, glutamate oxidation may take place in mitochondria-containing perisynaptic processes and de novo synthesis taking place in more distant processes of the same astrocyte or in different astrocytes. K+ stimulates pyruvate carboxylase (Kaufman and Driscoll, 1992) and increases anaplerotic flux, which rises with brain activity (Öz et al., 2004). Depending on the extent of activation of CO2 fixation and glutamate biosynthesis by K+, the concentrations of glutamate and glutamine may increase in other regions of the cell and compensate for net oxidation of glutamate in perisynaptic processes. Diffusion of metabolites and compounds related to neurotransmitter cycling among coupled astrocytes increases the complexity of modeling and measuring interactions between immediately-adjacent processes of astrocytes and neurons.
A net change in total tissue glutamate level need not, however, arise solely from its turnover; it could also be due to interconversion of amino acids via transaminase reactions. For example, total glutamate concentration increases and aspartate level falls during sensory stimulation of awake rats (Dienel et al., 2002) and humans (Mangia et al., 2007; Lin et al., 2012). Mangia et al. (2007) attributed the concentration changes to increased malate-aspartate shuttle activity, assuming that the mitochondrial glutamate-aspartate antiporter is rate limiting. The malate-aspartate shuttle transfers reducing equivalents from cytoplasmic NADH to mitochondrial NADH and it is required for the formation of pyruvate as an oxidative substrate. When this redox shuttle becomes limiting cytoplasmic NAD+ is regenerated by the action of lactate dehydrogenase, and lactate will be produced in increased amounts. Determination of the capacity and regulation of the malate-aspartate shuttle is very important because it can help explain the rise in lactate production during brain activation. The malate-aspartate shuttle is stimulated by low cytoplasmic calcium levels and inhibited by greater influx of calcium into mitochondria, indicating that neuronal calcium dynamics during brain activation may be involved in regulation of neuronal lactate production (Contreras et al., 2007; Satrustegui et al., 2007; Bak et al., 2009; Contreras and Satrustegui, 2009; Bak et al., 2012).
2.9. Changes in glutamate metabolism may contribute to the CNS pathophysiology of diabetes
Elucidation of the role of glutamate oxidation in energetics of brain activation is not only essential for understanding neuron-astrocyte interactions during normal brain function, it is also critical for evaluation of the pathophysiology of complex diseases that affect the eye and brain. For example, retinopathy is common in diabetic subjects who can also develop progressive cognitive and sensory-processing deficits (Kodl and Seaquist, 2008; Reijmer et al., 2010). The underlying causes of these acquired abnormalities remain to be established but they may, in part, involve glutamate turnover. In retina of diabetic rats, glutamate levels are elevated and conversion of glutamate to glutamine is reduced (Lieth et al., 1998) due to (i) impaired glutamate oxidation, probably arising from inhibition of glutamate transamination by branched chain amino acids and (ii) decreased activity and content of glutamine synthetase, suggesting that abnormal glutamate metabolism in retinal Müller cells may contribute to retinopathy via glutamate excitotoxicity (Lieth et al., 2000; Gowda et al., 2011). The glutamate transporter GLAST is also dysfunctional in retinal Müller cells, presumably due to oxidative damage (Li and Puro, 2002), and glutamate uptake is increased in glial plasmalemmal vesicles isolated from diabetic brain regions, whereas glutamate transporter levels are not altered in various brain structures (Coleman et al., 2004; Coleman et al., 2010). Magnetic resonance spectroscopic (MRS) studies have also identified changes in metabolic fluxes in brain of diabetic rats. Incorporation of label from [1-13C]glucose into glutamate, glutamine, and GABA is reduced, whereas incorporation of 13C from [1,2-13C2]acetate into these compounds increased (Garcia-Espinosa et al., 2003). Regional abnormalities in glycogen and amino acid homeostasis and reduced glutamate-glutamine cycling are also evident in diabetic rat brain (Sickmann et al., 2010; Sickmann et al., 2012). Diabetes and exposure to amyloid-beta, which is sometimes elevated in experimental diabetes, also impair astrocytic gap junctional communication with a time course that lags onset of oxidative stress without involvement of endoplasmic reticulum stress (Cruz et al., 2010; Gandhi et al., 2010; Ball et al., 2011; Lind et al., 2013). Thus, diabetes causes metabolic disturbances in neuronal and astrocytic pathways related to energy metabolism, glucose storage, and excitatory and inhibitory neurotransmission.
In addition to abnormal metabolism during diabetes and chronic hyperglycemia, recurrent bouts of acute, insulin-induced hypoglycemia disrupt the metabolic and functional integrity of brain cells in diabetic subjects (Amaral, 2012). Oxidation of endogenous metabolites is a compensatory mechanism for an inadequate supply of glucose, and insulin-induced hypoglycemia depletes brain levels of glycolytic and TCA cycle intermediates, glutamate, glutamine, GABA, alanine, and phospholipids, and increases levels of aspartate and ammonia (Siesjö, 1978). These findings are consistent with the faster decline in the rate of glucose utilization compared with oxygen consumption after onset of hypoglycemia and the slower recovery of oxygen utilization rate after glucose levels are restored because glucose is used for energy and also for de novo synthesis of the compounds consumed during the hypoglycemic episode (Ghajar et al., 1982). Thus, both hyperglycemia and hypoglycemia have a high impact on many pathways involved in glutamatergic and GABAergic neurotransmission in diabetes.
2.10. Summary
Glutamate synthesis and degradation are critical functions of astrocytes required for neurotransmission. Exogenous glutamate can be completely degraded or shunted through the TCA cycle with regeneration of glutamate. The high ATP yield of glutamate oxidation, the glucose-sparing effects of glutamate, and lack of stimulation of glucose utilization by extracellular glutamate in many studies support the conclusion that astrocytic energetics are supported, at least in part, by oxidation of neurotransmitter glutamate. The advantage of glutamate as an energy source is that it is supplied to activated perisynaptic domains of astrocytes at the site where fuel is needed simultaneously with the onset of increased ATP demand. Glutamate oxidation can buffer glucose use in astrocytes and make more glucose available for neurons. Diabetes and other disease states that affect glutamate-glutamine turnover can disrupt brain function.
2.11. Outstanding questions for future work
Critical issues include localization of mitochondria in perisynaptic filopodia that surround glutamatergic synapses, energetic contributions to astrocytic functions during brain activation of glutamate oxidation compared with glycolysis, glycogenolysis, and glucose oxidation, filopodial mobility related to excitatory synaptic activity, compartmentation of glutamate-glutamine turnover within and among astrocytes, roles of the malate-aspartate shuttle in lactate formation, and involvement of glutamate metabolism pathways in the pathophysiology of diabetes.
3. Astrocytic energetics, glycolysis, and lactate shuttling
3.1. Models for energetics of excitatory neurotransmission
Oxidation of glutamate by astrocytes has been firmly established by work carried out during the past four decades in many laboratories. However, glutamate oxidation and its ATP yield have not yet been explicitly incorporated into models for astrocytic energetics and glutamate-glutamine cycling based on tissue culture experiments, in vivo 13C-MRS metabolic studies, and lactate-loading assays (i.e., experiments in which the lactate concentration greatly exceeds normal levels observed during brain activation, i.e., about 2 μmol/g tissue, by either putting large amounts of lactate into the incubation medium or infusing large amounts of lactate into subjects to raise blood and brain lactate levels) (Magistretti et al., 1999; Hyder et al., 2006; Rothman et al., 2011; Pellerin and Magistretti, 2012). Instead, strong emphasis has been placed on ATP derived from astrocytic glycolysis accompanied by transfer of lactate from astrocytes to neurons as a major oxidative fuel (Jolivet et al., 2010; Pellerin and Magistretti, 2012). The notion of lactate trafficking has diverted attention from experimental examination of important issues related to oxidative metabolism in astrocytes (see section 2, above) and of neuronal glucose utilization, and strengths and limitations of evidence for metabolic models need to be re-evaluated.
3.2. Astrocyte-to-neuron lactate (ANL) transfer-oxidation model
Based on their observation that treatment of cultured astrocytes with glutamate stimulated glycolysis and release of lactate, Pellerin and Magistretti (1994) proposed a model for coupling of glucose utilization with neuronal activity. The model assigns the 2 ATP generated by glutamate-evoked glycolysis to satisfy the astrocytic energetic demands of glutamate-glutamine cycling, i.e., one to fuel Na+,K+-ATPase to extrude the Na+ taken up along with glutamate and one to convert glutamate to glutamine. In addition, the lactate released to the culture medium was stated to be taken up and oxidized by nearby neurons in vivo, serving as a major fuel during excitatory neurotransmission. This model mandates glycolytic glucose consumption in perisynaptic astrocytic processes and lactate oxidation in nearby neurons, in sharp contrast with astrocytic glutamate oxidation to fuel Na+ extrusion and glutamine synthesis. The astrocytic energy balance arising from uptake of 2 glutamate and their conversion to glutamine consumes 2 glucose and produces 4 ATP, with release of 4 lactate (glycolytic compartment, Fig. 1).
In recent reviews, a small number of selected studies were cited in support of the ANL transport-oxidation model (Jolivet et al., 2010; Pellerin and Magistretti, 2012). However, studies in many laboratories during the past 40 years that were not cited in the above reviews clearly demonstrate that cultured neurons and synaptosomes isolated from adult brain are capable of substantially increasing glucose uptake, glycolysis, and glucose oxidation (Dienel, 2012a). Furthermore, critical aspects of ANL transport, including the cellular origin of lactate produced during activation and the direction and magnitude of lactate shuttling have not been directly established in brain of normal awake subjects. An alternative model, the redox shuttle proposed by Cerdan and colleagues, provides a different mechanism to have high rates of glycolysis in astrocytes without net transfer of lactate to neurons; in this model astrocyte-derived lactate is oxidized by neurons to generate NADH that is oxidized by the neurons, and the resulting pyruvate is released to extracellular fluid where it can cycle back to astrocytes for oxidation (Cerdan et al., 2006). Lactate production during activation is generally assumed to be astrocytic, but this remains to be proven in vivo; it could be astrocytic, neuronal, or both.
3.3. Neuron-to-astrocyte lactate transfer
Predicted transport and pathway flux rates and directions depend on model assumptions, and a model that uses different assumptions and accounts for different kinetics of the neuronal and astrocytic glucose transporters predicts that neurons metabolize most of the glucose consumed during brain activation and that lactate is generated in neurons and transferred to astrocytes (Mangia et al., 2011). Experimental evidence that considerable lactate production during activation may be neuronal (Ueda and Ikemoto, 2007; Caesar et al., 2008; Contreras and Satrustegui, 2009; Ivannikov et al., 2010; Bak et al., 2012) and that neurons and astrocytes can oxidize both glucose and lactate (Zielke et al., 2007, 2009) has led to serious challenges of the validity of the ANL transport-oxidation model, a synopsis of which is presented below.
3.4. Glutamate-stimulated glycolysis is not a robust phenotype of astrocyte cultures
Glutamate-stimulated glucose utilization and lactate release occurs in astrocyte preparations of the Pellerin-Magistretti group (Pellerin and Magistretti, 1994; Debernardi et al., 1999; Chatton et al., 2003) and Takahashi et al. (1995). However, more laboratories have reported that glutamate treatment caused either no change or reduced glucose consumption (Dunlop et al., 1984; Swanson et al., 1990; Hertz et al., 1998; Peng et al., 2001; Qu et al., 2001; Liao and Chen, 2003; Dienel and Cruz, 2004) and no change or diminished lactate release (Dunlop et al., 1984; Gramsbergen et al., 2003; Liao and Chen, 2003; Dienel and Cruz, 2004). The basis for these phenotypic differences has never been established, but it probably lies within the culturing procedures (Hertz et al., 1998). The fact that some astrocyte preparations exhibit glutamate-induced glycolysis whereas others do not demonstrates that the cornerstone of the ANL shuttle is not established.
3.5. Astrocytes upregulate oxidative pathways fluxes during brain activation
Assignment of glycolytic ATP to fuel sodium extrusion and glutamine synthesis is reasonable if no other energy-providing fluxes are increased. However, in vivo studies in awake subjects under activating conditions have shown that astrocytes upregulate oxidative metabolism (Dienel et al., 2001; Dienel et al., 2003; Cruz et al., 2005; Dienel et al., 2007b; Wyss et al., 2009; Wyss et al., 2011) and glycogenolysis (Swanson et al., 1992; Madsen et al., 1999; Cruz and Dienel, 2002; Dienel et al., 2007a). Thus, astrocytic ATP is generated by metabolism of endogenous (glutamate and glycogen) and exogenous (glucose and acetate) substrates, and the source(s) of ATP actually used to extrude Na+ and synthesize glutamine remain to be directly identified. By 1994 when the ANL transport-oxidation model was first proposed, there was already a large literature demonstrating oxidation of exogenous glutamate by astrocytes, and studies since 1994 support the conclusion that glutamate oxidation can help fuel its uptake, as proposed by Peng et al. (2001).
3.6. Assays of the oxidative pathways greatly underestimate total glucose utilization
Astrocytic lactate production coupled with lactate oxidation in nearby neurons requires proportionate increases in total glucose utilization and oxidative metabolism of glucose and oxygen consumption. However, many studies carried out during the past 30 years reported that the magnitude of upregulation of the rates of the oxidative pathways assayed with [1- or 6-14C]glucose is ~50% or less of the rate of total glucose utilization assayed in parallel with [14C]deoxyglucose (DG, which measures the hexokinase reaction and total glucose utilization) in awake rodents during normal and pathophysiological states (Dienel, 2012b). These findings indicate that substantial quantities of products of [14C]glucose metabolism are not retained in activated tissue, even during brief 5–10 min assays. Any release of labeled lactate from activated tissue causes a proportionate underestimation of CMRglc when assayed with [14C]glucose. Similar findings would be anticipated for MRS studies of activation using [13C]glucose.
3.7. Lactate release model: lactate release from activated brain is fast and facilitated by astrocytes
Direct assays of lactate release from brain during spreading cortical depression showed that labeled lactate was detected in cerebral venous blood within 2 min after pulse labeling with [6-14C]glucose and by 4–8 min, an approximate steady state was attained in which efflux of both labeled and unlabeled lactate accounted for ~20% of the labeled or unlabeled glucose entering the brain (Cruz et al., 1999). Because this accounted for only about half of the magnitude of underestimation of CMRglc with labeled glucose our laboratory undertook a series of in vivo studies to establish the routes for release of 14C-labeled metabolites during activation. Major findings reviewed in (Dienel, 2012b) include the following: (i) significant label is lost from [1-14C]glucose via the pentose phosphate shunt pathway (e.g., 25% of glucose oxidized in the inferior colliculus during acoustic stimulation; calculated from data of (Cruz et al., 2007) and illustrated in Fig. 6C,c-1 of (Dienel, 2012b)), (ii) most locally-generated lactate is not locally oxidized (Ball et al., 2010), (iii) label dispersal within activated tissue is reduced by inhibition of lactate transporters and gap junctions (Cruz et al., 2007), (iv) astrocytes have a 2–4-fold higher rate and capacity for uptake of lactate from extracellular fluid and for shuttling among gap junction-coupled astrocytes compared with neuronal lactate uptake and lactate shuttling to neurons (i.e., astrocytes are poised to take up, disperse, and discharge lactate over a wide range of lactate levels, ranging from 2–40 mmol/L) (Gandhi et al., 2009a), and (v) label derived from microinfused 14C-labeled-lactate or glucose is recovered in meninges, indicating that labeled metabolites are also released via lymphatic drainage systems in addition to blood (Ball et al., 2010) and that assays of arteriovenous differences underestimate metabolite release from brain. Furthermore, labeled metabolites of [6-14C]glucose are retained in greater quantities if the polarized localization of aquaporin 4 at astrocytic endfeet is disrupted, consistent with lactate release from endfeet in association with endfoot water movement (Cruz et al., 2013). Continuous lactate release into the perivascular-lymphatic drainage system may, in fact, be important for widespread stimulation of blood flow (Laptook et al., 1988; Hein et al., 2006; Yamanishi et al., 2006; Gordon et al., 2008). Together, the above studies demonstrate that most lactate generated from blood-borne glucose within brain is quickly released, not locally oxidized. These in vivo findings in normal awake rats strongly support the predominant lactate release model (Gandhi et al., 2009a; Dienel, 2012b) and demonstrate that the second cornerstone of the ANL transport-oxidation model, local oxidation of lactate within the brain as major neuronal fuel, is not established.
3.8. Astrocytic gap junctional communication reduces the focal energetic burden of glutamate uptake
Pictorial representations of astrocyte-neuron interactions related to the ANL transport-oxidation model (Pellerin and Magistretti, 2012) portray a tight linkage between glutamate cycling, Na+ extrusion, and glutamine synthesis within immediately-adjacent neuronal and astrocytic structures, with the implication that these cellular elements form a ‘closed’ temporal-spatial domain. However, it is well established that astrocytes are highly coupled to each other, and extracellular glutamate and K+ increase gap junctional communication (Enkvist and McCarthy, 1994; De Pina-Benabou et al., 2001), thereby enhancing trans-astrocytic fluxes of Na+ (Langer et al., 2012), glucose, lactate, NADH, and NADPH (Gandhi et al., 2009b), and probably glutamate and glutamine, during excitatory neurotransmission (Fig. 1). The astrocytic syncytium that can be comprised of as many as ten thousand coupled cells (Ball et al., 2007) is an ‘open system’, and the energetic burden of glutamate uptake, Na+ extrusion, and glutamine synthesis is, therefore, not restricted to perisynaptic processes. Glutamate, K+, and Na+ can be dispersed among coupled cells to minimize their accumulation at active sites, and the workload can be shared.
3.9. Discordant contributions of lactate to total oxidation in vitro and in vivo
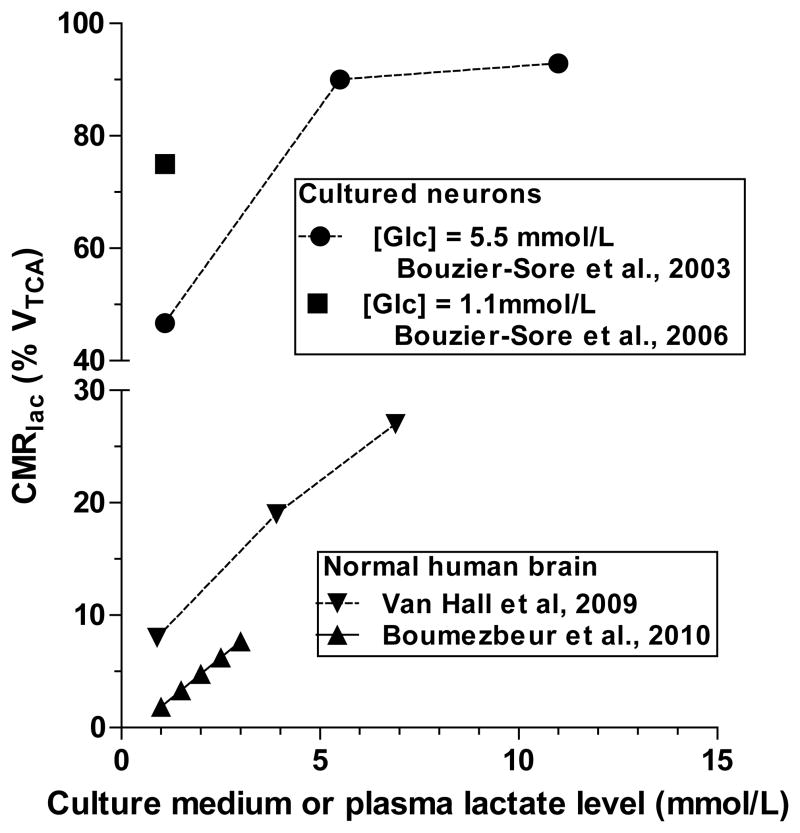
Based on results of tissue culture and lactate loading studies, lactate is claimed to be the ‘preferred’ neuronal substrate compared with glucose (Jolivet et al., 2010; Pellerin and Magistretti, 2012). However, lactate oxidation by cultured neurons in studies by the Pellerin-Magistretti group greatly exceeds that in normal adult human brain, particularly in ‘lactate-loading’ assays. At levels of lactate achieved in brain during activation (<2–3 mmol/L), lactate contributes a small fraction (<8%) to total oxidation in human brain, sharply contrasting the 45–75% in neurons cultured from embryonic rats (Fig 2). Lactate metabolism in these cultured neurons is not equivalent to that in adult brain, even at low lactate levels. Lactate is a supplemental brain fuel that spares glucose when its blood concentrations are high (Fig. 2), but the physiology of lactate utilization during lactate loading and muscular work is not the same as in sedentary subjects with low plasma and brain lactate levels (Dienel, 2012a, b). Lactate transport is passive and follows concentration gradients. Lactate influx into cells with a proton can cause intracellular acidification and depletion of NAD+, thereby inhibiting glycolysis by biochemical regulatory mechanisms expected for a glucose-sparing alternative substrate.
Figure 2. Lactate oxidation in cultured neurons greatly exceeds that in adult human brain.
Lactate oxidation (CMRlac) is expressed as percent of the TCA cycle rate (VTCA). Lactate oxidation in cultured neurons was assayed in the presence of glucose, either 5.5 or 1.1 mmol/L, and different levels of lactate in the medium. Lactate oxidation in human brain was assayed when blood and brain lactate levels were increased by moderate exercise (van Hall et al., 2009) or lactate infusion (Boumezbeur et al., 2010).
3.10. Glycogenolysis rates that sustain neuronal function in the absence of glucose are very low
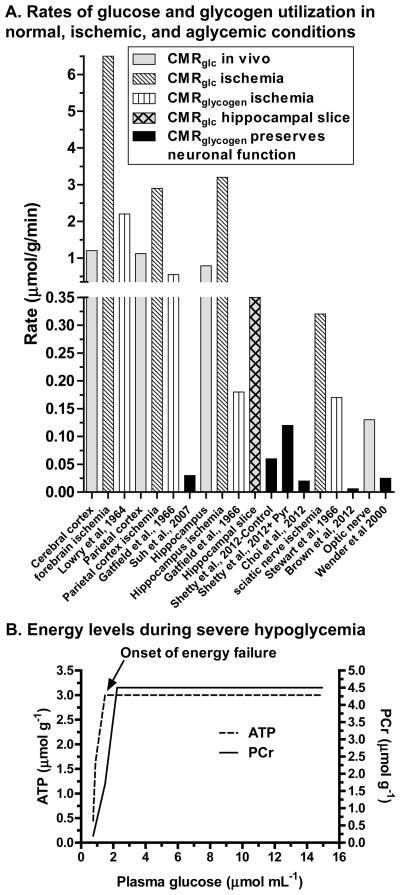
Astrocytic glycogen maintains neuronal function during severe hypoglycemia or aglycemia, and some investigators (Wender et al., 2000; Brown et al., 2005; Brown et al., 2012) claim that these findings provide proof of principle that lactate shuttling is important for normal neuronal function. This notion was evaluated by calculating overall rates of glycogenolysis during glucose deprivation as the difference in glycogen content (expressed as μmol glucosyl units/g) at the onset and endpoint of the experimental interval divided by experimental duration. These values are compared to in vivo CMRglc and CMRglycogen in normal or ischemic tissue (Fig. 3A). CMRglc varies regionally (Sokoloff et al., 1977) (Fig. 3A, gray bars), and it is much lower in brain slices due to deafferentation and postischemic recovery (gray hatched bar, hippocampus). Glycogenolysis rate in resting rodent cerebral cortex is 0.01 μmol/g/min (Watanabe and Passonneau, 1973), or about ~1% of cerebral cortical CMRglc (Fig. 3A). During forebrain ischemia, glucose utilization increases by as much as 6-fold (diagonal-lined bars), and glycogenolysis rises as much as 200-fold, reaching 2 μmol/g/min (vertical-lined bars); ischemic parietal cortex and hippocampus also have large increases in CMRglc and CMRglycogen. These utilization rates during ischemia were calculated from substrate disappearance during the initial 30–60 seconds of ischemia when endogenous compounds were consumed at high rates.
Figure 3. Low rates of glycogen utilization preserve neuronal function during glucose depletion.
(A) Regional rates of glucose utilization (CMRglc) in normal awake resting rat brain are from (Sokoloff et al., 1977) and CMRglc for hippocampal slices is from (Newman et al., 1996). Pairs of values for CMRglc and CMRglycogen during ischemia in situ were determined in cerebral cortex (Lowry et al., 1964), parietal cortex (Gatfield et al., 1966), and hippocampus (Gatfield et al., 1966); data from these references are plotted by denoting the respective brain structure on the abscissa for the CMRglc values and the respective literature reference for the CMRglycogen values. Glycogenolysis rate in cerebral cortex during severe insulin-induced hypoglycemia was calculated from the change in glycogen concentration during the interval in which cortical EEG was maintained (Suh et al., 2007). Glycogenolysis was also calculated from net changes in glycogen concentration in aglycemic hippocampal slices (i) during the interval in which field excitatory postsynaptic potentials fell to 50% of control for samples with initial glycogen levels that were generated by pre-incubation of the slices with glucose or with glucose plus pyruvate (+Pyr) that raised the initial glycogen level above that with glucose and gave a higher glycogenolysis rate (Shetty et al., 2012), and (ii) during the interval with preservation of synaptic function (Choi et al., 2012); the author thanks Drs. Choi and Macvicar for providing the information needed to convert the percent changes to glycogen amount for these calculations. CMRglc and CMRglycogen were assayed in sciatic nerve during ischemia (Stewart et al., 1965), and CMRglc in the intact optic nerve is from Table 3 in (Dienel, 2012b). Glycogenolysis rates in isolated optic and sciatic nerves perfused without glucose were based on net concentration changes from onset of electrical stimulation until maximally-evoked compound action potentials fell by 50% (Wender et al., 2000; Brown et al., 2012). (B) Phosphocreatine (PCr) and ATP levels are maintained at normal levels during progressive insulin-induced hypoglycemia until a critical point is reached. PCr levels fall prior to the decline of ATP concentration, with energy failure occurring when both compounds are depleted. Plotted from data of (Lewis et al., 1974).
In sharp contrast to responses to ischemia, glycogenolysis rates that preserve brain or nerve function during severe hypoglycemia or aglycemia are extremely low, ranging from about 0.01 to 0.06 μmol/g/min (black bars, Fig. 3A), corresponding to about <1–10% of resting CMRglc in the respective structures. Glycogen use rates in isolated optic or sciatic nerve given electrical stimulation to evoke maximal compound action potentials in the absence of glucose (Wender et al., 2000; Brown et al., 2012) are much lower than CMRglycogen during ischemia in situ and they are also considerably less than CMRglc in normal or ischemic nerve (Fig. 3A). This is also true for preservation of evoked potentials in hippocampal slices, even when pre-stimulation glycogen levels were elevated by supplementation of the pre-incubation medium with pyruvate to increase glycogen level over the control value (Shetty et al., 2012). K+-evoked glycogenolysis is linked to lactate shuttling and preservation of synaptic function (Choi et al., 2012), but here, too, the rate is very low (Fig. 3A). These severe glucose-deprivation paradigms are not likely to reflect the actual magnitude of glycogenolysis to functional energetics when glucose is available, and the contributions of neuronal metabolism of glucose to these functions remains to be established. Interpretation of glucose deprivation studies in terms of normal metabolism must be made with special care because survival of the cell or organism now becomes an issue. As an extreme example, cutting the arm off of a right-handed person will increase use of the left arm to carry out normal right-arm activities even though the left arm was normally rarely used for these tasks.
The specific roles of glycogen during brain activation are not well understood, but the magnitude of its turnover appears to be quite high. For example, when glycogen phosphorylase is inhibited during sensory stimulation of awake rats there are large, region-specific compensatory increases in utilization of blood-borne glucose. The net increase in CMRglc during blockade of glycogenolysis in the most-activated structures (sensory cortex and layer 4 of sensory and parietal cortex) is about 0.4–0.5 μmol/g/min (Dienel et al., 2007a), which is more than 7–50 times greater than CMRglycogen in the above experiments in the absence of glucose (Fig 3A). Presumably, the compensatory rise in glucose utilization reflects glycogen turnover and glycogen consumption. In a separate study, we found that less than 1% of the [6-14C]glucose-derived label recovered in metabolites was in glycogen during rest, sensory stimulation, and recovery from stimulation (Dienel et al., 2002). This suggests that glucose label that passes through glycogen to the glycolytic pathway is probably released from brain, since most of the activation-evoked increase in metabolite labeling by [14C]glucose is not retained in brain by trapping in the large amino acid pools derived from the oxidative pathway (Dienel, 2012b). Thus, little, if any, glycogen-derived label is shuttled to neurons, in agreement with the very low glycogenolysis rates that preserve brain function in the absence of glucose.
The supplemental energy provided by glycogenolysis to help maintain EEG, synaptic potentials, and axonal conduction in the absence of glucose is functionally very important, particularly for diabetic patients. However, its low magnitude suggests that either these process may not require much ATP or that other endogenous substrates (e.g., amino acids) are also oxidized to provide most of the ATP required to maintain overall function. When interpreting the role of glycogen in sustaining neuronal function it is important to consider the following points. (i) Severe hypoglycemia and aglycemia do not occur in normal brain because compensatory mechanisms maintain plasma glucose levels and glucose delivery to brain, even during prolonged starvation (Owen et al., 1967). Glucose depletion does occur in diabetic patients who receive too much insulin, but this is abnormal. Nevertheless, the fact that glycogenolysis prolongs EEG (Fig. 3A) and reduces neuronal death during severe hypoglycemia (Suh et al., 2007) is very important for diabetic patients. (ii) ATP levels are maintained near-normal during graded hypoglycemia (Fig. 3B) due to oxidation of available endogenous substrates, including amino acids and TCA cycle intermediates (see section 2.9 and (Siesjö, 1978)), until a critical point at which energy failure abruptly ensues (Fig. 3B). Thus, even a very small energy source can prolong brain functions by helping maintain ATP production just above the point of collapse. (iii) The cells that use most of the glycogen-derived energy are not established. Use of glycogen by astrocytes to support K+ homeostasis (Hof et al., 1988; Xu et al., 2012) would help maintain neuronal function, as would substrate oxidation by glia surrounding axons (Hargittai and Lieberman, 1991). Small fluxes can maintain function and prevent energy failure without making major contributions to energetics under normal conditions.
3.11. Summary
Two essential components of the ANL transfer system, glutamate-induced glycolysis and local oxidation of a substantial fraction of the lactate generated within activated tissue, are not sufficiently documented in vivo to validate the foundation of this model. Lactate may be produced by neurons, astrocytes, or both cell types. Energetics of astrocyte-neuron and astrocyte-astrocyte interactions are much more complex than portrayed by the model. Strong in vivo evidence supports rapid, substantial release of lactate from activated brain, suggesting that maintenance of high glycolytic flux is far more important than lactate shuttling coupled with its oxidation. These findings and the physiological responses to brain activation described in section 4 support the conclusion that lactate release predominates during brain activation and that normal brain does not need lactate as a supplemental fuel.
3.12. Outstanding questions for future work
Key issues related to lactate production and release during brain activation include identification of the processes that preferentially upregulate glycolysis, the cellular basis of lactate production, determination of the magnitude, direction, and time courses of lactate fluxes, identification of roles and metabolic fate of glycogen, and metabolic contributions of the astrocytic syncytium to local synaptic activity.
4. Glucose and oxygen supply exceed demand during brain activation
4.1. Fast onset of hemodynamic responses and surplus oxygen delivery
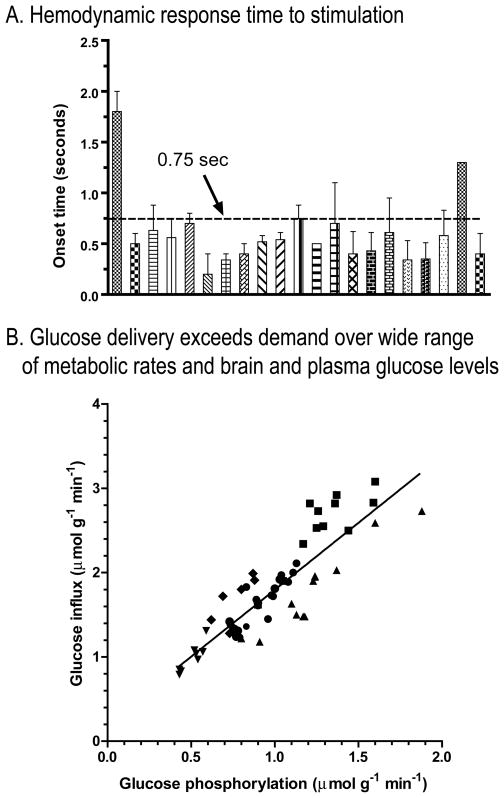
If glucose delivery to brain via arterial blood were limiting for brain metabolism during activation, it would be critical for brain cells to consume all available fuel. However, regulation of cerebral blood flow is described as a ‘feed-forward’ process because signals derived from neuronal activity also stimulate vasodilation and blood flow (Attwell et al., 2010). Measures of hemodynamic responses include vascular volume, blood flow rate, and a positive blood oxygen level-dependent (BOLD) signal that reflects increased oxygen content of venous blood, i.e., oxygen delivery exceeds its utilization (Ogawa et al., 1990; Ogawa, 2012). The onset of the hemodynamic response in anesthetized subjects is very fast, occurring within 0.75s in most studies (Fig. 4A). Fuel delivery to brain is not limited by a slow response of blood flow to activation.
Figure 4. Fuel delivery to activated brain is quickly upregulated and exceeds demand. (A).
Hemodynamic responses include blood volume, blood flow, and blood oxygen level-dependent (BOLD) signal. Data obtained in studies in anesthetized subjects are plotted from studies summarized in Table 1 of (Masamoto and Kanno, 2012). Note the onset time is <0.75s in most studies. (B) Comparison of paired glucose delivery and phosphorylation rates determined in the same tissue sample. Samples were derived from rats assayed under different experimental conditions designed to vary blood and brain glucose levels and brain glucose utilization rates over a wide range. Data are from (Cremer et al., 1983; Hargreaves et al., 1986). The regression line is y = 1.59x + 0.21 (r2 = 0.77). Modified from Fig. 3 of (Dienel, 2012b) with permission of the author.
4.2. Glucose delivery exceeds demand by a wide margin over a large range of glucose levels
Glucose influx into brain tissue exceeds its phosphorylation by ~60% over a 5-fold range of CMRglc when plasma and brain glucose levels range from 4.9–12.6 μmol/mL and 1.8–4.8 μmol/g, respectively (Fig. 4B). Because the Km of hexokinase for glucose is ~0.05 μmol/mL (Wilson, 2003), the enzyme is saturated and can operate at maximal velocity until brain glucose levels fall below ~0.5 μmol/g (note: for simplicity, the water content (~80%) of brain tissue is ignored, and small concentration differences arise when converting in vitro concentrations to those per gram tissue). Rat brain glucose level is ~20% of that in arterial plasma and ranges from ~2–3 μmol/g (Dienel et al., 1991). If CMRglc abruptly doubles and rises from 1 to 2 μmol/g/min, then 1 μmol/g would be consumed in one min. This leaves 1–2 μmol/g in the brain precursor pool of unmetabolized glucose, even without the continuous supply of glucose from blood at a rate that matches the resting CMRglc. Rapid blood flow upregulation and excess delivery of oxygen and glucose indicate that normal brain does not ‘need’ lactate as a supplemental fuel. Some extracellular lactate is probably consumed at stimulus onset, but analysis of available data indicates that this lactate makes a minor contribution to CMRglc (Dienel, 2012a).
4.3. Stimulus-induced increases in glycolysis are highest in awake subjects
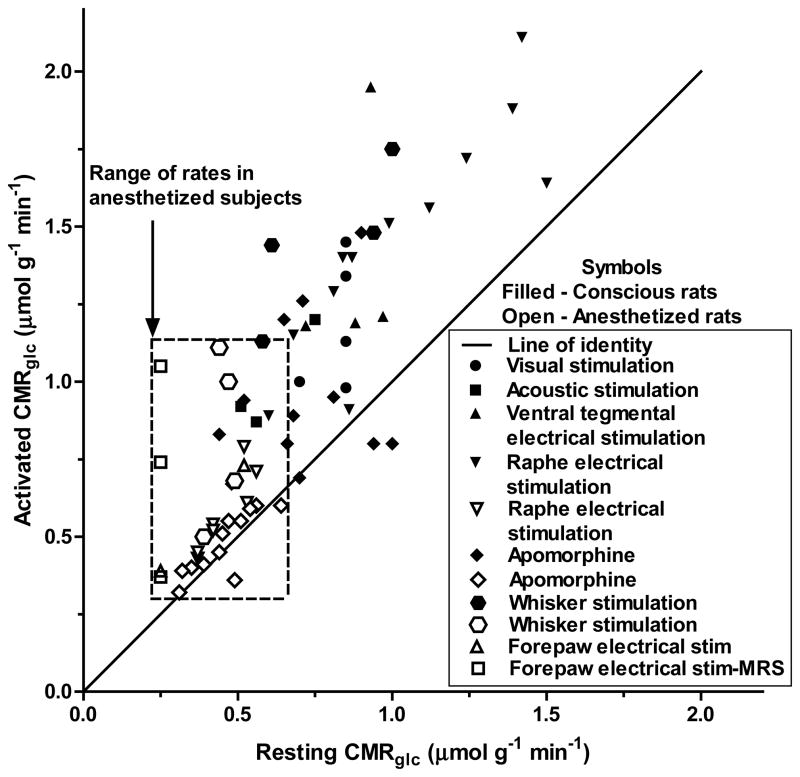
Anesthesia is required to avoid movement artifacts during some types (e.g., positron emission tomographic and MRS) of metabolic assays. However, stimulatory effects of activation are not equivalent in awake and anesthetized subjects, and pathways of fuel use may differ in the two conditions. Parallel studies in which glucose utilization was assayed during rest and activation using [14C]DG or labeled glucose reveal that the resting basal rates are higher in awake subjects and the stimulus-induced rise in CMRglc is generally much greater in awake compared with anesthetized subjects (Fig. 5). Furthermore, when assayed with [14C]DG, the increases usually exceeded those determined with [13C- or 14C]glucose, consistent with disproportionate upregulation of glycolysis compared with glucose oxidation, particularly in awake subjects (Fig. 5). These findings suggest that suppression of brain functions by anesthesia simultaneously blunts the magnitude of cellular activities that activate the glycolytic pathway.
Figure 5. Upregulation of glucose utilization in awake rats exceeds that in anesthetized rats.
Data are plotted from studies summarized in Table 4 of (Dienel, 2012b). Values are pairs of rates obtained in the same brain structure during rest and activation, and they represent different stimuli (sensory, chemical, or electrical) to different brain regions. Values that fall above the line of identity (solid line) indicate upregulation of CMRglc during activation compared with rest. Individual points cannot be directly compared with each other because the responses vary with brain region and stimulus paradigm. Nevertheless, increases in CMRglc in awake subjects are greater and have a wider range compared with anesthetized subjects that are outlined by the dashed rectangle.
4.4. Increase in CMRO2 sets an upper limit for the ANL transport-oxidation model
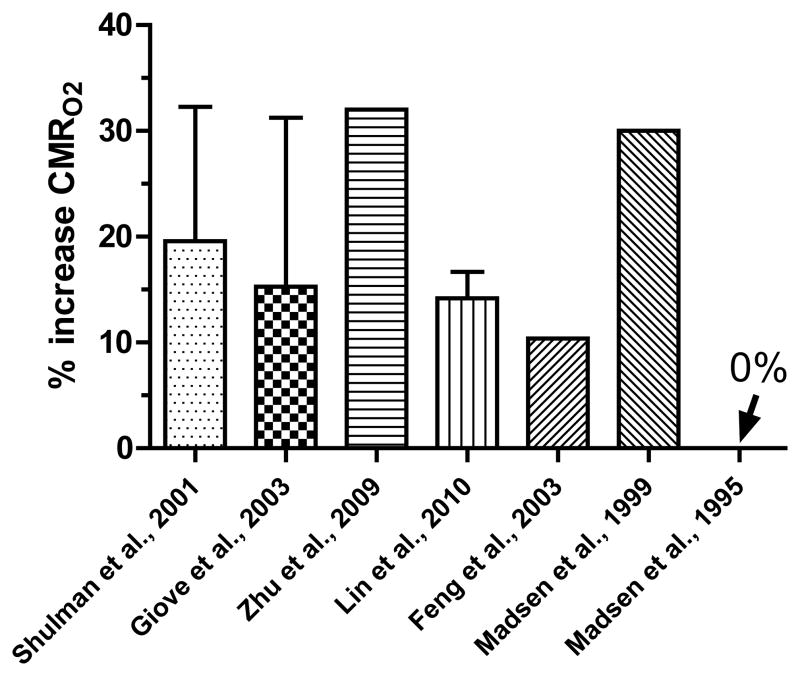
If all of the lactate produced in brain were locally oxidized, the rates of respiration or TCA cycle would rise in parallel with total glucose utilization. However, this is not the case because the percent rise in CMRO2 during brain activation is relatively small, generally ~20% (Fig. 6). The increase in CMRO2 establishes the upper limit for any lactate trafficking linked to its local oxidation because if shuttled lactate were the sole respiratory substrate for neurons, then all oxygen consumption could be ascribed to lactate oxidation. However, neurons and astrocytes each phosphorylate ~50% of the glucose under resting conditions (Nehlig et al., 2004). If neurons directly oxidize the pyruvate generated from glucose they phosphorylate during activation (i.e., accounting for half of the rise in CMRO2, or 10%) and astrocytes account for 20–30% of total oxidation (Hertz, 2011; Rothman et al., 2011), the maximal contribution of lactate shuttling to total oxidation is, on average, <10%. This fraction does not constitute a major fuel.
Figure 6. Increase in CMRO2 during brain activation.
Values are means and SD of tabulated results of CMRO2 in two reviews (n=17 studies (Shulman et al., 2001); n = 15 studies (Giove et al., 2003)) or mean values (with SD for responses to different visual stimulus rates) from representative studies in human or rat brain (Madsen et al., 1995; Madsen et al., 1999; Feng et al., 2003; Zhu et al., 2009; Lin et al., 2010).
4.5. Summary
The conclusion that glucose is the major fuel for normal brain during activation of relatively sedentary subjects is strongly supported by the rapid increase in blood flow, glucose influx in excess of demand, disproportionate increases in glycolysis compared with oxidative metabolism, a small rise in CMRO2, lack of substantial lactate accumulation in brain, and rapid, continuous lactate release that can contribute to upregulation of blood flow. Lactate is a supplemental fuel when blood levels rise during muscular activity and lactate infusions and during pathophysiological conditions. Use of lactate as a supplemental fuel varies with the physiology of the subject. Use of alternative substrates under special conditions does not mean that these compounds are normally major fuels.
4.6. Outstanding questions for future work
Major issues are how glucose and lactate are distributed within the brain during rest, activation, and intense physical activity, identification of processes dependent on glycolysis and those that cause increased production, elucidation of processes and pathways that are down regulated by anesthesia, functions fulfilled by oxidative metabolism and glycolysis, and the subcellular basis for glycolytic and oxidative metabolism in neurons and astrocytes.
5. Concluding comments
5.1. Glutamate vs. glycolysis to fuel astrocytic filopodial activities
Glutamate oxidation provides a simple mechanism to satisfy the temporal-spatial energy demands of mitochondria-containing perisynaptic astrocytes during excitatory neurotransmission. Exogenous glutamate is taken up and oxidized by cultured astrocytes immediately, and turnover of the neurotransmitter glutamate pool in vivo is faster than that of the metabolic pool. Glutamate can either be shunted through the TCA cycle and regenerated by consumption of 0.5 glucose, or it can be completely oxidized by the pyruvate re-cycling pathway. Oxidation of <20% of the transmitter glutamate taken up by either pathway is sufficient to satisfy the energy demands of its uptake, and oxidation of a larger fraction of the glutamate can support increased ATP demand for other activities during strong excitatory neurotransmission. Glutamate oxidation is glucose-sparing, freeing up glucose for use by neurons and by other processes in astrocytes, including glycogen turnover, and it does not rule out glycolytic upregulation in astrocytes and neurons. The conclusion that glutamate helps fuel the energetics of its uptake is consistent with results of many in vitro and in vivo studies, but more work is required to establish the quantitative contributions of neurotransmitter oxidation to astrocytic energetics in vivo. Labeling of neurotransmitter glutamate C4 in neurons by C1- or C6-labeled glucose followed by glutamate shunting through the astrocytic TCA cycle will cause formation of C2,4 and C2,3 isotopomers of glutamate and glutamine, and in vivo studies in the presence and absence of fluoroacetate may be useful to evaluate contributions of glutamate shunting to formation of these isotopomers.
5.2. Refine models for metabolic activation and astrocyte-neuron interactions
The two cornerstones of the ANL transport-oxidation model, i.e., glutamate-induced glycolysis and substantial local neuronal oxidation of lactate during brain activation, have never been validated in vivo. Normal brain does not need lactate as a supplemental fuel because compensatory responses to brain activation increase oxygen and glucose delivery within seconds, and fuel supplies exceed demand by a wide margin. Glycolysis is preferentially upregulated by unidentified cells during brain activation, and the fractional change is much lower in anesthetized subjects, suggesting that suppression of brain function by anesthesia also reduces demand for glycolysis. Fast lactate washout from activated tissue supplied with excess glucose implies that maintenance of elevated glycolytic rates is more important than local lactate utilization. Maximal contributions of lactate to total oxidation are estimated to be <10%, consistent with its being a minor fuel in human brain at low levels of lactate during exercise and lactate flooding that correspond to lactate levels during brain activation of sedentary subjects (less than about 2 mmol/L). Astrocytic glycogen helps maintain brain function during severe glucose depletion, but the rates that preserve function are very low, and the cells that use the glycogen-derived energy remain to be identified in most studies.
5.3. ‘Raise the bar’ for critical analysis and interpretation of translational research
Lactate trafficking coupled with substantial neuronal oxidation has been widely accepted in some scientific circles, but it is intensely debated in others because it has been perpetuated with little direct in vivo evidence and without critical evaluation within the broad scope of the brain energy metabolism literature over the past 30–40 years. It is not sufficient to provide supporting correlative evidence from a few selected studies, from modulation of monocarboxylic acid transporter levels, from the use of high lactate loads, or from assays carried out in the absence of glucose. Three issues must be addressed to generate a more accurate metabolic model of astrocyte-neuron interactions associated with excitatory neurotransmission, (i) identification of the processes and cells that generate lactate during activation in vivo, (ii) quantification of the direction, magnitude, and rate of lactate shuttling coupled with the rate of local lactate oxidation in vivo, and (iii) incorporation of substantial lactate release from activated brain in vivo. Although astrocytic glutamate oxidation is well established, the filopodia surrounding glutamatergic synapses that contain mitochondria in vivo need to be identified and quantified and the contribution of glutamate oxidation must be evaluated. Glutamate oxidation is an efficient, economical process that can help satisfy astrocytic energetics of excitatory neurotransmission. Advantages of glutamate oxidation include the following: fuel is supplied with commensurate with demand, no metabolite trafficking is required, critical fuel is not subject to washout, and glucose is spared for use by neurons and astrocytes for different functions. In addition, glutamate oxidation enables increased glycolysis with continuous lactate release to perivascular fluid, facilitating stimulation of blood flow to activated tissue. These important issues have been neglected by current models of excitatory neurotransmission, and new experimental approaches must be developed to advance the field.
Highlights.
Glutamate oxidation by astrocytes can fuel glutamate uptake and spare glucose
Glutamate-evoked glycolysis and neuronal lactate oxidation in vivo are not proven
Glucose and oxygen supply exceed demand during brain activation]
Very low rates of glycogenolysis can support neuronal functions
Models for astrocyte-neuron interactions are incomplete
Acknowledgments
This work was supported by National Institutes of Health grant DK081936. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or NIH. The funding source had no role in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the article for publication.
Abbreviations
- ANL
astrocyte-neuron-lactate
- BOLD
blood oxygen level-dependent
- CMRglc
cerebral metabolic rate for glucose
- CMRO2
cerebral metabolic rate for oxygen
- DG
2-deoxy-D-glucose
- MRS
magnetic resonance spectroscopy
- TCA cycle
tricarboxylic acid cycle
Footnotes
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral A. Effects of hypoglycaemia on neuronal metabolism in the adult brain: role of alternative substrates to glucose. Journal of Inherited Metabolic Disease. 2012:1–14. doi: 10.1007/s10545-012-9553-3. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar-Goffer RS, Ben-Yoseph O, Bachelard HS, Morris PG. Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. The Biochemical journal. 1992;282 (Pt 1):225–230. doi: 10.1042/bj2820225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Obel LF, Walls AB, Schousboe A, Faek SA, Jajo FS, Waagepetersen HS. Novel model of neuronal bioenergetics: Post-synaptic utilization of glucose but not lactate correlates positively with Ca2+ signaling in cultured mouse glutamatergic neurons. ASN neuro. 2012 doi: 10.1042/AN20120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagepetersen HS. Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. Journal of neurochemistry. 2009;109(Suppl 1):87–93. doi: 10.1111/j.1471-4159.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- Balázs R, Cremer JE. Metabolic compartmentation in the brain. John Wiley & Sons; New York: 1972. [Google Scholar]
- Ball KK, Cruz NF, Mrak RE, Dienel GA. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. Journal of cerebral blood flow and metabolism. 2010;30:162–176. doi: 10.1038/jcbfm.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Gandhi GK, Thrash J, Cruz NF, Dienel GA. Astrocytic connexin distributions and rapid, extensive dye transfer via gap junctions in the inferior colliculus: implications for [(14)C]glucose metabolite trafficking. Journal of neuroscience research. 2007;85:3267–3283. doi: 10.1002/jnr.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Harik L, Gandhi GK, Cruz NF, Dienel GA. Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications. Journal of neuroscience research. 2011;89:2052–2067. doi: 10.1002/jnr.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Jackson JG, Genda EN, Montoya MM, Yudkoff M, Robinson MB. The glutamate transporter, GLAST, participates in a macromolecular complex that supports glutamate metabolism. Neurochemistry international. 2012;61:566–574. doi: 10.1016/j.neuint.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin AM, Quastel JH. Locations of amino acids in brain slices from the rat. Tetrodotoxin-sensitive release of amino acids. The Biochemical journal. 1972;128:631–646. doi: 10.1042/bj1280631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin AM, Quastel JH. Fate of L-glutamate in the brain. Journal of neurochemistry. 1974;23:457–464. doi: 10.1111/j.1471-4159.1974.tb06046.x. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9:293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Berl S, Clarke DD, Schneider D. Metabolic compartmentation and neurotransmission: Relation to brain structure and function. Plenum Press; New York: 1975. [Google Scholar]
- Berl S, Frigyesi TL. The turnover of glutamate, glutamine, aspartate and GABA labeled with [1-14C]acetate in caudate nucleus, thalamus and motor cortex (cat) Brain research. 1969;12:444–455. doi: 10.1016/0006-8993(69)90012-2. [DOI] [PubMed] [Google Scholar]
- Berl S, Takagaki G, Clarke DD, Waelsch H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. The Journal of biological chemistry. 1962;237:2562–2569. [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N, Turner RJ. Extracellular potassium regulates the glutamine content of astrocytes: mediation by intracellular pH. Neuroscience letters. 1993;160:73–76. doi: 10.1016/0304-3940(93)9002-7. [DOI] [PubMed] [Google Scholar]
- Brookes N, Turner RJ. K(+)-induced alkalinization in mouse cerebral astrocytes mediated by reversal of electrogenic Na(+)-HCO3- cotransport. The American journal of physiology. 1994;267:C1633–1640. doi: 10.1152/ajpcell.1994.267.6.C1633. [DOI] [PubMed] [Google Scholar]
- Brown AM, Evans RD, Black J, Ransom BR. Schwann cell glycogen selectively supports myelinated axon function. Annals of neurology. 2012;72:406–418. doi: 10.1002/ana.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. Journal of neuroscience research. 2005;79:74–80. doi: 10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Le Clainche C, Wiesner S, Pantaloni D. Actin-based motility: from molecules to movement. Bioessays. 2003;25:336–345. doi: 10.1002/bies.10257. [DOI] [PubMed] [Google Scholar]
- Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. The Journal of biological chemistry. 1990;265:12916–12926. [PubMed] [Google Scholar]
- Cerdan S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, Garcia-Martin ML. The redox switch/redox coupling hypothesis. Neurochemistry international. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci. 2000;25:19–23. doi: 10.1016/s0968-0004(99)01511-x. [DOI] [PubMed] [Google Scholar]
- Choi HB, Gordon GR, Zhou N, Tai C, Rungta RL, Martinez J, Milner TA, Ryu JK, McLarnon JG, Tresguerres M, Levin LR, Buck J, Macvicar BA. Metabolic Communication between Astrocytes and Neurons via Bicarbonate-Responsive Soluble Adenylyl Cyclase. Neuron. 2012;75:1094–1104. doi: 10.1016/j.neuron.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DD, Nicklas WJ, Berl S. Tricarboxylic acid-cycle metabolism in brain. Effect of fluoroacetate and fluorocitrate on the labelling of glutamate, aspartate, glutamine and gamma-aminobutyrate. The Biochemical journal. 1970;120:345–351. doi: 10.1042/bj1200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman E, Judd R, Hoe L, Dennis J, Posner P. Effects of diabetes mellitus on astrocyte GFAP and glutamate transporters in the CNS. Glia. 2004;48:166–178. doi: 10.1002/glia.20068. [DOI] [PubMed] [Google Scholar]
- Coleman ES, Dennis JC, Braden TD, Judd RL, Posner P. Insulin treatment prevents diabetes-induced alterations in astrocyte glutamate uptake and GFAP content in rats at 4 and 8 weeks of diabetes duration. Brain research. 2010;1306:131–141. doi: 10.1016/j.brainres.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L, Gomez-Puertas P, Iijima M, Kobayashi K, Saheki T, Satrustegui J. Ca2+ Activation kinetics of the two aspartate-glutamate mitochondrial carriers, aralar and citrin: role in the heart malate-aspartate NADH shuttle. The Journal of biological chemistry. 2007;282:7098–7106. doi: 10.1074/jbc.M610491200. [DOI] [PubMed] [Google Scholar]
- Contreras L, Satrustegui J. Calcium signaling in brain mitochondria: interplay of malate aspartate NADH shuttle and calcium uniporter/mitochondrial dehydrogenase pathways. The Journal of biological chemistry. 2009;284:7091–7099. doi: 10.1074/jbc.M808066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Thomas PG, Smith SJ. The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia. 1990;3:322–334. doi: 10.1002/glia.440030503. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Cunningham VJ, Seville MP. Relationships between extraction and metabolism of glucose, blood flow, and tissue blood volume in regions of rat brain. Journal of cerebral blood flow and metabolism. 1983;3:291–302. doi: 10.1038/jcbfm.1983.44. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Adachi K, Dienel GA. Rapid efflux of lactate from cerebral cortex during K+-induced spreading cortical depression. Journal of cerebral blood flow and metabolism. 1999;19:380–392. doi: 10.1097/00004647-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Ball KK, Dienel GA. Functional imaging of focal brain activation in conscious rats: impact of [(14)C]glucose metabolite spreading and release. Journal of neuroscience research. 2007;85:3254–3266. doi: 10.1002/jnr.21193. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Ball KK, Dienel GA. Astrocytic gap junctional communication is reduced in amyloid-beta-treated cultured astrocytes, but not in Alzheimer’s disease transgenic mice. ASN neuro. 2010;2:e00041. doi: 10.1042/AN20100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Ball KK, Froehner SC, Adams ME, Dienel GA. Regional registration of [6-14C]glucose metabolism during brain activation of α-syntrophin knockout mice. Journal of neurochemistry. 2013;125:247–259. doi: 10.1111/jnc.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. Journal of cerebral blood flow and metabolism. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. Journal of neurochemistry. 2005;92:934–947. doi: 10.1111/j.1471-4159.2004.02935.x. [DOI] [PubMed] [Google Scholar]
- De Pina-Benabou MH, Srinivas M, Spray DC, Scemes E. Calmodulin kinase pathway mediates the K+-induced increase in Gap junctional communication between mouse spinal cord astrocytes. J Neurosci. 2001;21:6635–6643. doi: 10.1523/JNEUROSCI.21-17-06635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi R, Magistretti PJ, Pellerin L. Trans-inhibition of glutamate transport prevents excitatory amino acid-induced glycolysis in astrocytes. Brain research. 1999;850:39–46. doi: 10.1016/s0006-8993(99)02022-3. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Frotscher M. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia. 2001;36:330–341. doi: 10.1002/glia.1120. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Pannicke T, Haseleu J, Blaess S, Grosche J, Reichenbach A. Beyond polarity: functional membrane domains in astrocytes and muller cells. Neurochemical research. 2012;37:2513–2523. doi: 10.1007/s11064-012-0824-z. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012a;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Fueling and imaging brain activation. ASN neuro. 2012b;4(5):art:e00093. doi: 10.1042/AN20120021. 00010.01042/AN20120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Ball KK, Cruz NF. A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. Journal of neurochemistry. 2007a;102:466–478. doi: 10.1111/j.1471-4159.2007.04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochemistry international. 2004;45:321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Exchange-mediated dilution of brain lactate specific activity: implications for the origin of glutamate dilution and the contributions of glutamine dilution and other pathways. Journal of neurochemistry. 2009;109(Suppl 1):30–37. doi: 10.1111/j.1471-4159.2009.05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Ball K, Popp D, Gokden M, Baron S, Wright D, Wenger GR. Behavioral training increases local astrocytic metabolic activity but does not alter outcome of mild transient ischemia. Brain research. 2003;961:201–212. doi: 10.1016/s0006-8993(02)03945-8. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Mori K, Holden JE, Sokoloff L. Direct measurement of the lambda of the lumped constant of the deoxyglucose method in rat brain: determination of lambda and lumped constant from tissue glucose concentration or equilibrium brain/plasma distribution ratio for methylglucose. Journal of cerebral blood flow and metabolism. 1991;11:25–34. doi: 10.1038/jcbfm.1991.3. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Liu K, Cruz NF. Local uptake of (14)C-labeled acetate and butyrate in rat brain in vivo during spreading cortical depression. Journal of neuroscience research. 2001;66:812–820. doi: 10.1002/jnr.10063. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Schmidt KC, Cruz NF. Astrocyte activation in vivo during graded photic stimulation. Journal of neurochemistry. 2007b;103:1506–1522. doi: 10.1111/j.1471-4159.2007.04859.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. Journal of cerebral blood flow and metabolism. 2002;22:1490–1502. doi: 10.1097/01.WCB.0000034363.37277.89. [DOI] [PubMed] [Google Scholar]
- Dinuzzo M, Mangia S, Maraviglia B, Giove F. Neurochemical research. 2012. The Role of Astrocytic Glycogen in Supporting the Energetics of Neuronal Activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JM, Lanz B, Gruetter R. Compartmentalized Cerebral Metabolism of [1,6-C]Glucose Determined by in vivoC NMR Spectroscopy at 14.1 T. Front Neuroenergetics. 2011;3:3. doi: 10.3389/fnene.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop DS, Kaufman H, Zanchin G, Lajtha A. Protein synthesis rates in rats with portacaval shunts. Journal of neurochemistry. 1984;43:1487–1489. doi: 10.1111/j.1471-4159.1984.tb05413.x. [DOI] [PubMed] [Google Scholar]
- Enkvist MO, McCarthy KD. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. Journal of neurochemistry. 1994;62:489–495. doi: 10.1046/j.1471-4159.1994.62020489.x. [DOI] [PubMed] [Google Scholar]
- Eriksson G, Peterson A, Iverfeldt K, Walum E. Sodium-dependent glutamate uptake as an activator of oxidative metabolism in primary astrocyte cultures from newborn rat. Glia. 1995;15:152–156. doi: 10.1002/glia.440150207. [DOI] [PubMed] [Google Scholar]
- Farinelli SE, Nicklas WJ. Glutamate metabolism in rat cortical astrocyte cultures. Journal of neurochemistry. 1992;58:1905–1915. doi: 10.1111/j.1471-4159.1992.tb10068.x. [DOI] [PubMed] [Google Scholar]
- Feng CM, Liu Ho-L, Fox PT, Gao JH. Dynamic changes in the cerebral metabolic rate of o2 and oxygen extraction ratio in event-related functional MRI. Neuroimage. 2003;18:257–262. doi: 10.1016/s1053-8119(02)00023-x. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 1997;21:106–113. [PubMed] [Google Scholar]
- Gandhi GK, Ball KK, Cruz NF, Dienel GA. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN neuro. 2010;2:e00030. doi: 10.1042/AN20090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. Journal of neurochemistry. 2009a;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi GK, Cruz NF, Ball KK, Theus SA, Dienel GA. Selective astrocytic gap junctional trafficking of molecules involved in the glycolytic pathway: impact on cellular brain imaging. Journal of neurochemistry. 2009b;110:857–869. doi: 10.1111/j.1471-4159.2009.06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Espinosa MA, Garcia-Martin ML, Cerdan S. Role of glial metabolism in diabetic encephalopathy as detected by high resolution 13C NMR. NMR Biomed. 2003;16:440–449. doi: 10.1002/nbm.843. [DOI] [PubMed] [Google Scholar]
- Gatfield PD, Lowry OH, Schulz DW, Passonneau JV. Regional energy reserves in mouse brain and changes with ischaemia and anaesthesia. Journal of neurochemistry. 1966;13:185–195. doi: 10.1111/j.1471-4159.1966.tb07512.x. [DOI] [PubMed] [Google Scholar]
- Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci. 2011;31:18275–18288. doi: 10.1523/JNEUROSCI.3305-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar JB, Plum F, Duffy TE. Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. Journal of neurochemistry. 1982;38:397–409. doi: 10.1111/j.1471-4159.1982.tb08643.x. [DOI] [PubMed] [Google Scholar]
- Giove F, Mangia S, Bianciardi M, Garreffa G, Di Salle F, Morrone R, Maraviglia B. The physiology and metabolism of neuronal activation: in vivo studies by NMR and other methods. Magnetic resonance imaging. 2003;21:1283–1293. doi: 10.1016/j.mri.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda K, Zinnanti WJ, LaNoue KF. The influence of diabetes on glutamate metabolism in retinas. Journal of neurochemistry. 2011;117:309–320. doi: 10.1111/j.1471-4159.2011.07206.x. [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Leegsma-Vogt G, Venema K, Noraberg J, Korf J. Quantitative on-line monitoring of hippocampus glucose and lactate metabolism in organotypic cultures using biosensor technology. Journal of neurochemistry. 2003;85:399–408. doi: 10.1046/j.1471-4159.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- Halperin ML, Connors HP, Relman AS, Karnovsky ML. Factors that control the effect of pH on glycolysis in leukocytes. The Journal of biological chemistry. 1969;244:384–390. [PubMed] [Google Scholar]
- Hargittai PT, Lieberman EM. Axon-glia interactions in the crayfish: glial cell oxygen consumption is tightly coupled to axon metabolism. Glia. 1991;4:417–423. doi: 10.1002/glia.440040410. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Planas AM, Cremer JE, Cunningham VJ. Studies on the relationship between cerebral glucose transport and phosphorylation using 2-deoxyglucose. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1986;6:708–716. doi: 10.1038/jcbfm.1986.127. [DOI] [PubMed] [Google Scholar]
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1997;17:1230–1238. doi: 10.1097/00004647-199711000-00012. [DOI] [PubMed] [Google Scholar]
- Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47:693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Progress in neurobiology. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Hertz L. Astrocytic energy metabolism and glutamate formation--relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magnetic resonance imaging. 2011;29:1319–1329. doi: 10.1016/j.mri.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Hertz L, Drejer J, Schousboe A. Energy metabolism in glutamatergic neurons, GABAergic neurons and astrocytes in primary cultures. Neurochemical research. 1988;13:605–610. doi: 10.1007/BF00973275. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. Journal of neuroscience research. 1999;57:417–428. [PubMed] [Google Scholar]
- Hertz L, Hertz E. Cataplerotic TCA cycle flux determined as glutamate-sustained oxygen consumption in primary cultures of astrocytes. Neurochemistry international. 2003;43:355–361. doi: 10.1016/s0197-0186(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hertz L, Swanson RA, Newman GC, Marrif H, Juurlink BH, Peng L. Can experimental conditions explain the discrepancy over glutamate stimulation of aerobic glycolysis? Developmental neuroscience. 1998;20:339–347. doi: 10.1159/000017329. [DOI] [PubMed] [Google Scholar]
- Hertz L, Yu AC, Kala G, Schousboe A. Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochemistry international. 2000;37:83–102. doi: 10.1016/s0197-0186(00)00012-7. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- Hof PR, Pascale E, Magistretti PJ. K+ at concentrations reached in the extracellular space during neuronal activity promotes a Ca2+-dependent glycogen hydrolysis in mouse cerebral cortex. J Neurosci. 1988;8:1922–1928. doi: 10.1523/JNEUROSCI.08-06-01922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Ivannikov MV, Sugimori M, Llinas RR. Calcium clearance and its energy requirements in cerebellar neurons. Cell calcium. 2010;47:507–513. doi: 10.1016/j.ceca.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet R, Allaman I, Pellerin L, Magistretti PJ, Weber B. Comment on recent modeling studies of astrocyte-neuron metabolic interactions. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1982–1986. doi: 10.1038/jcbfm.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman EE, Driscoll BF. Carbon dioxide fixation in neuronal and astroglial cells in culture. Journal of neurochemistry. 1992;58:258–262. doi: 10.1111/j.1471-4159.1992.tb09304.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Pirttila TR, Auriola SO, Williams SR. Compartmentation of cerebral glutamate in situ as detected by 1H/13C n.m.r. The Biochemical journal. 1994;298 (Pt 1):121–127. doi: 10.1042/bj2980121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer J, Stephan J, Theis M, Rose CR. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia. 2012;60:239–252. doi: 10.1002/glia.21259. [DOI] [PubMed] [Google Scholar]
- Laptook AR, Peterson J, Porter AM. Effects of lactic acid infusions and pH on cerebral blood flow and metabolism. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1988;8:193–200. doi: 10.1038/jcbfm.1988.49. [DOI] [PubMed] [Google Scholar]
- Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, Derouiche A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LD, Ljunggren B, Ratcheson RA, Siesjo BK. Cerebral energy state in insulin-induced hypoglycemia, related to blood glucose and to EEG. Journal of neurochemistry. 1974;23:673–679. doi: 10.1111/j.1471-4159.1974.tb04390.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Puro DG. Diabetes-Induced Dysfunction of the Glutamate Transporter in Retinal Müller Cells. Investigative Ophthalmology & Visual Science. 2002;43:3109–3116. [PubMed] [Google Scholar]
- Liao SL, Chen CJ. l-Glutamate decreases glucose utilization by rat cortical astrocytes. Neuroscience letters. 2003;348:81–84. doi: 10.1016/s0304-3940(03)00721-3. [DOI] [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- Lieth E, LaNoue KF, Antonetti DA, Ratz M. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. The Penn State Retina Research Group. Exp Eye Res. 2000;70:723–730. doi: 10.1006/exer.2000.0840. [DOI] [PubMed] [Google Scholar]
- Lin AL, Fox PT, Hardies J, Duong TQ, Gao JH. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG. Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1484–1495. doi: 10.1038/jcbfm.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind KR, Ball KK, Cruz NF, Dienel GA. The unfolded protein response to endoplasmic reticulum stress in cultured astrocytes and rat brain during experimental diabetes. Neurochemistry international. 2013;62:784–795. doi: 10.1016/j.neuint.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P, Robacker K. Glycolysis and brain function: [K+]o stimulation of protein synthesis and K+ uptake require glycolysis. Fed Proc. 1983;42:2875–2880. [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV, Hasselberger FX, Schulz DW. Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. The Journal of biological chemistry. 1964;239:18–30. [PubMed] [Google Scholar]
- Lukkarinen J, Oja JM, Turunen M, Kauppinen RA. Quantitative determination of glutamate turnover by 1H-observed, 13C-edited nuclear magnetic resonance spectroscopy in the cerebral cortex ex vivo: interrelationships with oxygen consumption. Neurochemistry international. 1997;31:95–104. doi: 10.1016/s0197-0186(96)00120-9. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bulow J, Holm S, Wildschiodtz G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1995;15:485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, Bloom FE. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:6535–6539. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Mangia S, DiNuzzo M, Giove F, Carruthers A, Simpson IA, Vannucci SJ. Response to ‘comment on recent modeling studies of astrocyte-neuron metabolic interactions’: much ado about nothing. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1346–1353. doi: 10.1038/jcbfm.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kanno I. Anesthesia and the quantitative evaluation of neurovascular coupling. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1233–1247. doi: 10.1038/jcbfm.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, Rubio ME. High-Concentration Rapid Transients of Glutamate Mediate Neural-Glial Communication via Ectopic Release. The Journal of Neuroscience. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. Journal of neurochemistry. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: high-resolution microautoradiographic imaging by cellular 14C-trajectography combined with immunohistochemistry. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:1004–1014. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- Newman GC, Hospod FE, Maghsoudlou B, Patlak CS. Simplified brain slice glucose utilization. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:864–880. doi: 10.1097/00004647-199609000-00011. [DOI] [PubMed] [Google Scholar]
- Ogawa S. Finding the BOLD effect in brain images. Neuroimage. 2012;62:608–609. doi: 10.1016/j.neuroimage.2012.01.091. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain Metabolism during Fasting*. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo B, Rodrigues TB, Contreras L, Garzon M, Llorente-Folch I, Kobayashi K, Saheki T, Cerdan S, Satrustegui J. Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:90–101. doi: 10.1038/jcbfm.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Hertz L, Huang R, Sonnewald U, Petersen SB, Westergaard N, Larsson O, Schousboe A. Utilization of glutamine and of TCA cycle constituents as precursors for transmitter glutamate and GABA. Developmental neuroscience. 1993;15:367–377. doi: 10.1159/000111357. [DOI] [PubMed] [Google Scholar]
- Peng L, Swanson RA, Hertz L. Effects of L-glutamate, D-aspartate, and monensin on glycolytic and oxidative glucose metabolism in mouse astrocyte cultures: further evidence that glutamate uptake is metabolically driven by oxidative metabolism. Neurochemistry international. 2001;38:437–443. doi: 10.1016/s0197-0186(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Qu H, Eloqayli H, Unsgard G, Sonnewald U. Glutamate decreases pyruvate carboxylase activity and spares glucose as energy substrate in cultured cerebellar astrocytes. Journal of neuroscience research. 2001;66:1127–1132. doi: 10.1002/jnr.10032. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res Rev. 2010;63:11–25. doi: 10.1016/j.brainresrev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satrustegui J, Pardo B, Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87:29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Svenneby G, Hertz L. Uptake and metabolism of glutamate in astrocytes cultured from dissociated mouse brain hemispheres. Journal of neurochemistry. 1977;29:999–1005. doi: 10.1111/j.1471-4159.1977.tb06503.x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Waagepetersen HS. GABA: Homeostatic and pharmacological aspects. In: James M, Tepper EDA, Bolam JP, editors. Progress in brain research. Vol. 160. Elsevier; 2007. pp. 9–19. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Yu AC, Hertz L. Regulatory role of astrocytes for neuronal biosynthesis and homeostasis of glutamate and GABA. Progress in brain research. 1992;94:199–211. doi: 10.1016/s0079-6123(08)61751-3. [DOI] [PubMed] [Google Scholar]
- Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain research. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- Shank RP, Leo GC, Zielke HR. Cerebral metabolic compartmentation as revealed by nuclear magnetic resonance analysis of D-[1-13C]glucose metabolism. Journal of neurochemistry. 1993;61:315–323. doi: 10.1111/j.1471-4159.1993.tb03570.x. [DOI] [PubMed] [Google Scholar]
- Shetty PK, Sadgrove MP, Galeffi F, Turner DA. Pyruvate incubation enhances glycogen stores and sustains neuronal function during subsequent glucose deprivation. Neurobiology of disease. 2012;45:177–187. doi: 10.1016/j.nbd.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and aminoacid homeostasis. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1527–1537. doi: 10.1038/jcbfm.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD. Brain glycogen and its role in supporting glutamate and GABA homeostasis in a type 2 diabetes rat model. Neurochemistry international. 2012;60:267–275. doi: 10.1016/j.neuint.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Siesjö BK. Brain Energy Metabolism. John Wiley & Sons; Chichester: 1978. [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. Journal of neurochemistry. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Petersen SB, Unsgard G, Schousboe A. Metabolism of [U-13C]glutamate in astrocytes studied by 13C NMR spectroscopy: incorporation of more label into lactate than into glutamine demonstrates the importance of the tricarboxylic acid cycle. Journal of neurochemistry. 1993;61:1179–1182. doi: 10.1111/j.1471-4159.1993.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Stewart MA, Passonneau JV, Lowry OH. Substrate changes in peripheral nerve during ischaemia and Wallerian degeneration. Journal of neurochemistry. 1965;12:719–727. doi: 10.1111/j.1471-4159.1965.tb06786.x. [DOI] [PubMed] [Google Scholar]
- Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)pro pyl]-1H-indole-2-carboxamide) J Pharmacol Exp Ther. 2007;321:45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Yu AC, Chan PH, Sharp FR. Glutamate increases glycogen content and reduces glucose utilization in primary astrocyte culture. Journal of neurochemistry. 1990;54:490–496. doi: 10.1111/j.1471-4159.1990.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4616–4620. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. The Journal of biological chemistry. 1966;241:4110–4112. [PubMed] [Google Scholar]
- Ueda T, Ikemoto A. Cytoplasmic glycolytic enzymes. Synaptic vesicle-associated glycolytic ATP-generating enzymes: coupling to neurotransmitter accumulation. In: Gibson GE, Dienel GA, editors. Brain Energetics. Integration of Molecular and Cellular Processes. 3. Springer-Verlag; Berlin: 2007. pp. 241–259. [Google Scholar]
- Van den Berg CJ, Krzalic L, Mela P, Waelsch H. Compartmentation of glutamate metabolism in brain. Evidence for the existence of two different tricarboxylic acid cycles in brain. The Biochemical journal. 1969;113:281–290. doi: 10.1042/bj1130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. Blood lactate is an important energy source for the human brain. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Qu H, Hertz L, Sonnewald U, Schousboe A. Demonstration of pyruvate recycling in primary cultures of neocortical astrocytes but not in neurons. Neurochemical research. 2002;27:1431–1437. doi: 10.1023/a:1021636102735. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Exogenous glutamate is metabolized to glutamine and exported by rat primary astrocyte cultures. Journal of neurochemistry. 1986;47:304–313. doi: 10.1111/j.1471-4159.1986.tb02863.x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Passonneau JV. Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. Journal of neurochemistry. 1973;20:1543–1554. doi: 10.1111/j.1471-4159.1973.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- Wyss MT, Magistretti PJ, Buck A, Weber B. Labeled acetate as a marker of astrocytic metabolism. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1668–1674. doi: 10.1038/jcbfm.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss MT, Weber B, Treyer V, Heer S, Pellerin L, Magistretti PJ, Buck A. Stimulation-induced increases of astrocytic oxidative metabolism in rats and humans investigated with 1-11C-acetate. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:44–56. doi: 10.1038/jcbfm.2008.86. [DOI] [PubMed] [Google Scholar]
- Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L. Requirement of Glycogenolysis for Uptake of Increased Extracellular K+ in Astrocytes: Potential Implications for K+ Homeostasis and Glycogen Usage in Brain. Neurochemical research. 2012:1–14. doi: 10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]
- Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H925–934. doi: 10.1152/ajpheart.01012.2005. [DOI] [PubMed] [Google Scholar]
- Yu AC, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. Journal of neurochemistry. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Yu AC, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. Journal of neurochemistry. 1982;39:954–960. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- Yu ACH, LLY, Eng LE. Glutamate as an energy substrate for neuronal-astrocytic interactions. Progress in Brain Research. 1992;94:251–259. doi: 10.1016/s0079-6123(08)61755-0. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nissim I, Hummeler K, Medow M, Pleasure D. Utilization of [15N]glutamate by cultured astrocytes. The Biochemical journal. 1986;234:185–192. doi: 10.1042/bj2340185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Oxidation of (14)C-labeled compounds perfused by microdialysis in the brains of free-moving rats. Journal of neuroscience research. 2007;85:3145–3149. doi: 10.1002/jnr.21424. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. Journal of neurochemistry. 2009;109(Suppl 1):24–29. doi: 10.1111/j.1471-4159.2009.05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]