Abstract
Background
Fear of weight gain is a significant obstacle to smoking cessation, preventing some smokers from attempting to quit. Several previous studies of naltrexone yielded promising results for minimization of post-quit weight gain. Given these encouraging findings, we endeavored to test whether minimization of weight gain might translate to better quit outcomes for a population that is particularly concerned about gaining weight upon quitting.
Methods
Smokers (N = 172) in this investigation were prospectively randomized to receive either 25 mg naltrexone or placebo for 27 weeks (1 week pre-, 26 weeks post-quit) for minimization of post-quit weight gain and smoking cessation. All participants received open label therapy with the nicotine patch for the first 8 weeks post-quit and behavioral counseling over the 27 week treatment. The 2 pre-specified primary outcomes were change in weight for continuously abstinent participants and biologically verified end-of-treatment 7-day point prevalence abstinence at 26 weeks after the quit date.
Results
The difference in weight at 26 weeks post-quit between the naltrexone and placebo groups (naltrexone: 6.8 lbs ± 8.94 vs placebo: 9.7 lbs ± 9.19, p = .45) was not statistically different. Seven-day point prevalence smoking abstinence rates at 26 weeks post-quit was not significantly different between the 2 groups (naltrexone: 22% vs placebo: 27%, p = .43).
Conclusions
For smokers high in weight concern, the relatively small reduction in weight gain with low dose naltrexone is not worth the potential for somewhat lower rates of smoking abstinence.
Keywords: Smoking cessation, tobacco, naltrexone, weight gain, nicotine patch
1. Introduction
Fear of weight gain is a significant obstacle to smoking cessation, preventing some smokers from attempting to quit (Pomerleau et al., 2001). The effects of pharmacotherapy on minimizing post-quit weight gain have been mixed (Fiore et al., 2008). For instance, nicotine replacement therapy (NRT) is fairly effective in enhancing smoking cessation success rates (Silagy et al., 2004). However, transdermal nicotine therapy does not reduce hunger or weight gain significantly (Abelin et al., 1989b; Cooper et al., 2005; Rose et al., 1990). The effects of nicotine gum on delaying weight gain are stronger than nicotine patch, but the size of this difference, although significant, is modest (Doherty et al., 1996). Sustained-release (SR) bupropion hydrochloride has proven effective in increasing smoking cessation success rates (Ahluwalia et al., 2002; Hurt et al., 1997; Jorenby et al., 1999; Swan et al., 2003), and this treatment is moderately successful in helping participants reduce weight gain upon quitting. Varenicline is also an efficacious smoking cessation medication, but it does not significantly reduce post-quit weight gain (Gonzales et al., 2006; Jorenby et al., 2006). Thus, current pharmacological treatments for smoking cessation are modestly successful in assisting smokers to quit, but only nicotine gum and bupropion significantly attenuate post-cessation weight gain. A medication associated with less weight gain upon quitting could address one of the most important barriers to smoking cessation (Ahluwalia et al., 2002; Hurt et al., 1997).
Clearly, further research is needed to develop medications that address concern about gaining weight after smoking cessation (Pomerleau et al., 2001). Not only does concern about weight gain prevent smokers from trying to quit, weight-concerned smokers may also be less successful in achieving abstinence from smoking because the effort required to control food intake may undermine efforts to avoid smoking (Hall et al., 1986). In fact, attempts to integrate weight control and smoking cessation efforts have sometimes resulted in poorer smoking cessation outcomes (Hall et al., 1992). Thus, a successful intervention that minimizes post-quit weight gain may be attractive to people who are reluctant to quit or find it difficult to maintain abstinence due to weight gain.
Naltrexone hydrochloride is a medication that has shown promise in reducing post-cessation weight gain and may therefore address weight concerns (King et al., 2006; Krishnan-Sarin et al., 2003; O’Malley et al., 2006; Toll et al., 2008). Several reasons have been suggested for why naltrexone may be effective in minimizing post-quit weight gain. Animal models have implicated opioid antagonists in decreased body weight and food intake (Bodnar et al., 2003), and μ opioid receptor knockout mice have shown resistance to obesity (Tabarin et al., 2005).
Several randomized controlled studies have shown that naltrexone significantly reduces post-quit weight gain. A small preliminary study in 32 smokers found that naltrexone in combination with nicotine patch suppressed weight gain compared to placebo alone (Krishnan-Sarin, Meandzija and O’Malley, 2003). Subsequently, King et al. (King et al., 2006) conducted an 8-week placebo controlled study of naltrexone (50 mg/day) combined with the nicotine patch with 110 subjects and found that participants in the naltrexone group gained significantly less weight (1.5 pounds) as compared to those in the placebo + nicotine patch group (4.2 pounds). The largest clinical trial conducted to date was a dose ranging study of naltrexone (placebo, 25 mg, 50 mg, or 100 mg – taken daily) in combination with transdermal nicotine patch in 400 participants (O’Malley et al., 2006). The highest dose showed promise for promoting smoking abstinence, but effects on weight were not significant. In contrast, low dose (25 mg/day) naltrexone significantly reduced post-cessation weight gain over 6 weeks, with participants showing an average weight gain of 1.5 pounds on this dose compared to 4.2 pounds for those taking placebo, although it did not increase smoking abstinence. Based on the weight gain findings, Toll et al. treated 20 weight-concerned smokers combining 25 mg naltrexone with 300 mg bupropion SR, and showed that continuously abstinent participants in the naltrexone + bupropion group gained less weight (1.67 pounds) than those in a matched group of patients who received bupropion only (3.17 pounds; p = .35; Cohen’s d = 0.56) (Toll et al., 2008). Consistent with these findings, a recent review concluded that naltrexone showed promise as a potential drug treatment for preventing post smoking cessation weight gain (Parsons et al., 2009).
Although naltrexone appears to reduce weight gain after quitting, effects on smoking cessation have been inconclusive. Several studies showed that naltrexone did not help participants quit smoking or were mixed (Ahmadi et al., 2003; King et al., 2006; Toll et al., 2008; Wong et al., 1999), whereas other studies showed that naltrexone may be beneficial for smoking cessation (Covey et al., 1999; Krishnan-Sarin et al., 2003; O’Malley et al., 2006). Only the small pilot study by Toll and colleagues (Toll et al., 2008) selected weight concerned smokers. Prior studies tested short-term treatment from 4–8 weeks; whereas most smokers continue to gain weight over the first 6-months following smoking cessation (Hall et al., 1986; Klesges et al., 1997; Pirie et al., 1992).
In the present study, we tested the hypothesis that minimization of weight gain with low dose naltrexone might translate to better quit outcomes for a population of weight concerned smokers who believe that smoking helps control their weight. The study design was a double blind placebo controlled trial of 25 mg of naltrexone or placebo administered for 1 week pre-quit and for 26 weeks post-quit, the major period of risk for weight gain following smoking cessation. In addition, all subjects received transdermal nicotine replacement for 8 weeks following their quit date and brief smoking cessation counseling throughout the entire treatment period. We hypothesized that participants who received naltrexone would report higher rates of abstinence from cigarette smoking and lower post-quit weight gain compared to participants who received placebo.
2. Methods
2.1 Participants
One hundred seventy-two cigarette smokers were enrolled. Recruitment was via advertisements placed in local media outlets, mailings (to past participants, potential participants, and health care professionals), fliers, fax referrals from healthcare providers, press releases, and websites.
To be eligible, all smokers needed to be classified as weight concerned smokers based on 2 criteria. Concern about gaining weight after quitting was assessed using the questions Perkins and colleagues (Perkins et al., 2001) used to define weight concern in their clinical trial of CBT. These included “How concerned are you about gaining weight after quitting?” and “How concerned would you be if quitting smoking caused you to permanently gain 10 lbs?” Consistent with their criteria, a rating of 50 or higher on a 100 mm scale on either question qualified the subject on this criterion. Smoking to manage weight was assessed with the weight control subscale of the Smoking Consequences Questionnaire [SCQ] (Copeland et al., 1995) on which participants rate their expectations about the consequences of smoking a cigarette on a scale of 0–9 with 1 being “completely unlikely” and 9 being “completely likely”. Five items make up this subscale (alpha = .96) and include “smoking keeps my weight down”, “cigarettes keep me from eating more than I should”, “smoking helps me control my weight’, “cigarettes keep me from overeating”, and “smoking controls my appetite”. A mean rating of 6 or above (“somewhat likely”) qualified participants on this criterion.
Other inclusion and exclusion criteria were age 18 and older, willingness and ability to give written consent, smoking greater than 10 cigarettes per day for at least 1 year, at least 1 prior attempt to stop smoking, baseline expired carbon monoxide (CO) level of at least 10 ppm, weight of at least 100 lbs, English speaking, and only 1 participant per household. Exclusion criteria included pregnant or nursing women or women attempting to conceive, unstable cardiac disease, history of dermatoses, current alcohol or drug dependence other than nicotine dependence, serious current neurologic, psychiatric, suicidal risk or medical illness, chronic pain conditions necessitating opioid treatment, history of cirrhosis or of significant hepatocellular injury, current use of smokeless tobacco, pipes, cigars, nicotine gum, patch, lozenge, inhaler, or nasal spray, patients requiring concomitant therapy with any psychotropic drug or on any drug with a psychotropic component [except those who were on a stable dose of an Selective Serotonin Reuptake Inhibitor for at least 2 months for the indications of Major Depressive Disorder, Premenstrual Syndrome or Premenstrual Dysphoric Disorder], subjects with a positive opioid urine drug screen, current use of opioids, or currently on a medically prescribed diet. The institutional review board of the Yale University School of Medicine approved this study.
2.2 Procedures
Following written informed consent, patients completed baseline assessments, a physical examination, and laboratory testing. Eligible participants were randomized to conditions, with blocked stratified (for gender) randomization due to the fact that weight concerned samples are usually mostly female (Perkins et al., 2001). Random sequence was provided by one of the authors (RW) to the pharmacist who assigned participants; all others were blind to treatment assignment. All participants were seen at a community mental health center. Participants were randomized between February 3, 2005 and September 25, 2008, and the last treatment appointment was completed on April 27, 2009.
2.3 Medication Conditions
Participants received placebo or 25-mg naltrexone daily beginning the week before quitting. Naltrexone (Depade, Mallinckrodt Pharmaceuticals) was titrated for the first 2 days (i.e., 12.5-mg for 1 day, then 25-mg thereafter) then taken for a total of 27 weeks (1 week pre-and 26 weeks post-quit). Naltrexone medication in opaque capsules was dispensed in bottles, with the first dose in an individual glassine envelope within the bottle. Participants received 21 mg transdermal nicotine patches (Nicoderm CQ, GlaxoSmithKline) for 6 weeks, then 14 mg patches for 2 weeks, beginning on their quit date. Participants were instructed to take their naltrexone and replace their patch at the same time. Based on tolerability, dose reductions or discontinuation were permitted with the option to continue the nicotine patch and counseling.
2.4 Counseling
The counseling was adapted from the CBT protocol for weight concerned smokers created by Perkins and colleagues (Perkins et al., 2001) and the treatment manual was developed in collaboration with Dr. Michele Levine, who assisted in implementation and development of the source CBT protocol. The first session with the nurse lasted 45 minutes and subsequent weekly sessions with a research assistant supervised by an investigator (BAT) lasted 5–15 minutes, with longer sessions occurring in earlier meetings. Counseling occurred weekly for the first 4 weeks, bi-weekly twice, then monthly. Handouts described the benefits of quitting smoking and addressed aspects of quitting for this population (e.g., relative risk of weight gain, tips to eat a balanced diet, drink water, and exercise). However, following the model of Perkins et al. (Perkins et al., 2001) participants were asked to not diet and accept a modest amount of weight gain while they were engaged in active treatment. The recommendation to resist dieting during treatment was based on research suggesting that interventions that advocate dieting for weight loss may be counterproductive to smoking cessation. One hypothesis is that those who restrain themselves from eating in order to avoid weight gain make themselves vulnerable to relapse by making smoking more reinforcing. The preclinical literature provides substantial evidence demonstrating that food deprivation enhances an animal’s responding for drugs of abuse (Alsene et al., 2003; Carroll et al., 1979; Carroll and Meisch, 1981; De La Garza and Johanson, 1987), including nicotine (Lang et al., 1977). Withdrawal may be more pronounced with food restriction, an idea supported by data demonstrating that carbohydrate consumption or glucose tablets reduced nicotine withdrawal or the urge to smoke (Bowen et al., 1991; West et al., 1990; West and Willis, 1998) and that dextrose tablets compared to placebo improved smoking abstinence rates over 4 weeks in 1 study (West et al., 1999).
2.5 Assessments
Participants completed a core battery with questions about demographics, smoking variables, mood, alcohol use, and other areas of functioning. Diagnostic information was obtained with the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991), an alcohol screening questionnaire (AUDIT) (Babor et al., 1992), and the Structured Clinical Interview for DSM-IV Axis I Disorders eating disorder, alcohol, and depression modules (First et al., 1996). At each weekly appointment, weight, CO levels and reports of daily tobacco and alcohol consumption were obtained, the latter using the Timeline Follow-back Interview (TLFB) beginning 30 days prior to screening (Brown et al., 1988; Sobell and Sobell, 2003). Participant weight (in street clothes, without shoes) was measured using a calibrated balance beam scale. If a participant dropped out of treatment, we attempted to obtain their smoking data by phone. If they reported abstinence, they were only coded as abstinent when an in-person breath CO measurement was obtain biologically verifying their self-report. Serum cotinine was measured at intake and post-treatment follow-ups. Other weekly self-reports included the Questionnaire on Smoking Urges-Brief (QSU-Brief) (Cox et al., 2001) and the Minnesota Nicotine Withdrawal Scale (MNWS) (Hughes, 1992; Toll et al., 2007).
A checklist of common adverse events for naltrexone and for nicotine patch was administered weekly with other concerns elicited with questioning. Liver function tests (LFTs) were obtained at intake, 4, 14, and 26 weeks post-randomization.
2.6 Data Analysis
All patients who were randomized comprised the primary ITT population. The 2 pre-specified primary outcomes were change in weight for continuously abstinent participants and biologically verified end-of-treatment 7-day point prevalence abstinence at 26 weeks after the quit date. Change in weight from baseline was analyzed with 1-way ANOVA GLM for abstainers who completed treatment. A sensitivity analysis was performed using a linear mixed effects model with weight as the response, and time, treatment and time*treatment entered as fixed effects with baseline weight entered as a covariate. The primary smoking cessation outcome was point-prevalence abstinence over the past 7 days at 26 weeks after the quit date and the secondary smoking cessation outcome was point-prevalence abstinence over the past 7 days at 6 weeks after the quit date to allow comparisons to our earlier 6-week study (O’Malley et al., 2006). Self-reported abstinence (not even a puff) was verified by exhaled CO level ≤ 10ppm. Participants who dropped out or missed multiple appointments were considered failures. A single missed appointment was coded abstinent only if abstinence was verified at the appointments before and after the missed session. For baseline group comparisons, chi-square tests and GLM were used for categorical and continuous variables respectively. Smoking abstinence outcomes (yes/no) were initially analyzed using a logistic regression model including treatment condition (naltrexone versus placebo), gender (male versus female), and condition*gender. After this, if we found that the interaction was not significant, we tested a reduced, main effects only model including only treatment condition (naltrexone versus placebo) and gender (male versus female).
Secondary analyses of cigarettes smoked per day, craving (QSU-Brief scores), and withdrawal (MNWS scores) were analyzed using linear mixed effects models from 1 week to 26 weeks post-quit including gender as a covariate. Baseline (intake) was also treated as a covariate in the smoked per day analysis.
3. Results
3.1 Patient characteristics and disposition
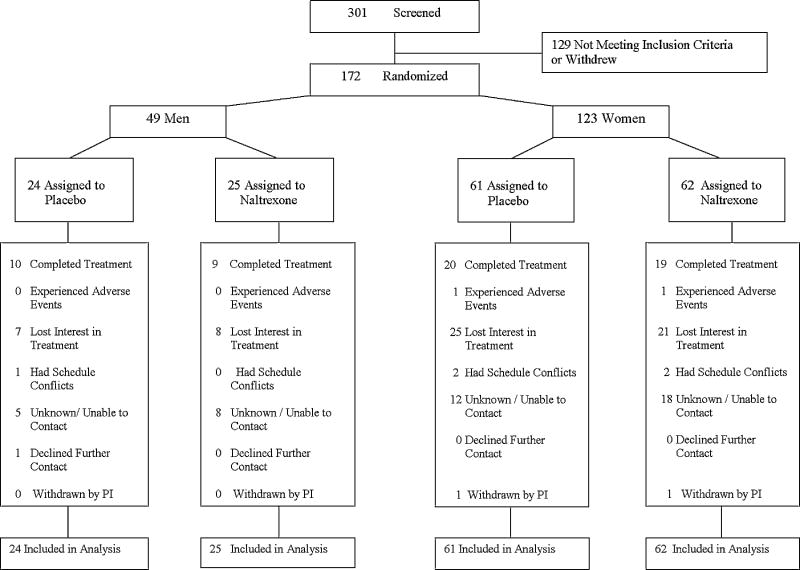
Of the 301 participants who were screened, 172 were randomized to the naltrexone or placebo condition. For the intent-to-treat population, Table 1 shows the between-group distribution of baseline demographic and other patient characteristics. The two treatment groups are well-balanced on all factors, and no variables differ by group at p < .05.
Table 1.
Baseline characteristics
| Naltrexone | Placebo | Overall | P-values | |
|---|---|---|---|---|
| (N = 87) | (N = 85) | (N = 172) | ||
| Age (yr) | 43.2 ± 10.0 | 45.5 ± 11.25 | 44.4 ± 10.67 | 0.152 |
| Male (%) | 28.7 | 28.2 | 28.5 | 0.942 |
| White (%) | 85.1 | 83.5 | 84.3 | 0.783 |
| Body Mass Index | 28.5 ± 6.36 | 28.3 ± 6.01 | 28.4 ± 6.16 | 0.872 |
| Education (%) | 0.587 | |||
| High-school graduate or less | 32.2 | 34.9 | 33.5 | |
| Some education after high school | 43.7 | 36.1 | 40 | |
| College graduate or more | 24.1 | 28.9 | 26.5 | |
| Marital status (%) | 0.86 | |||
| Married or cohabitating | 43.7 | 41.7 | 42.7 | |
| Divorced or separated | 26.4 | 32.1 | 29.2 | |
| Never married | 27.6 | 23.8 | 25.7 | |
| Widowed | 2.3 | 2.4 | 2.3 | |
| Full-time employment (%) | 75.3 | 61.5 | 68.5 | 0.054 |
| No. of cigarettes smoked per day | 22.1 ± 10.27 | 22.2 ± 8.62 | 22.2 ± 9.46 | 0.946 |
| Years of smoking cigarettes | 26.4 ± 10.20 | 27.6 ± 11.47 | 27.0 ± 10.82 | 0.466 |
| Expired carbon monoxide (ppm) | 25.6 ± 11.14 | 25.3 ± 10.42 | 25.5 ± 10.76 | 0.839 |
| Serum cotinine (ng/ml) | ||||
| Fagerstrom score | 5.5 ± 1.96 | 5.9 ± 2.11 | 5.7 ± 2.04 | 0.185 |
| Other smokers in household (%) | 29.1 | 36.9 | 32.9 | 0.277 |
| CES-D score | 8.4 ± 5.89 | 8.2 ± 6.68 | 8.3 ± 6.27 | 0.846 |
| Perkins 1 | 89.9 ± 12.75 | 89.3 ± 11.88 | 89.6 ± 12.29 | 0.742 |
Of the 172 subjects randomized, there were 87 subjects in the active treatment arm and 85 subjects in the control group. Figure 1 presents patient disposition data. Of the 87 active group participants, 28 completed treatment. Similarly, for the control group, of the 85 participants, 29 completed treatment. Of note, this study was initially powered based on a total sample size of 270 smokers. However, based on an interim analysis, it was decided to end the study after recruitment of 172 participants.
Figure 1.
Study flowchart.
3.2 Weight Gain
We studied the change in weight over time, beginning at 1 week post-quit until the study end at week 26, among those who achieved total smoking abstinence. As presented in Table 2a, on average, there was a weight increase of 6.8 pounds (SD = 8.94) in the active group compared to an increase of 9.7 pounds (SD = 9.19) in the control group. Thus, both treatment groups had a weight increase that was not statistically different (p = 0.45). When this group of subjects was evaluated at the intermediate time point of 6 weeks, there was a weight increase from week 1 to week 6 of 2.5 pounds (SD = 4.58) in the active treatment group and 2.2 pounds (SD = 4.20) in the control group (see Table 2a). Again, this difference was not statistically significant (p = 0.79).
Table 2a.
Weight gain over 26- and 6-weeks post-quita
| Outcome | Placebo M (SD) |
25mg M (SD) |
F | P |
|---|---|---|---|---|
| Weight Gain Over 26 Weeks |
9.71 ± 9.19 | 6.75 ± 8.94 | 0.58 | 0.45 |
| Weight Gain Over 6 Weeks |
2.19 ± 4.20 | 2.53 ± 4.58 | 0.07 | 0.79 |
3.3 Smoking Abstinence
As displayed in Table 2b, for the primary study endpoint of point prevalence abstinence at week 26, the ITT population had 19 of 87 (22%) active treatment subjects belonging to this category compared to 23 of 85 (27%) of the placebo subjects (p = 0.43). Of 87 active treatment ITT subjects, 33 of 87 (38%) subjects in the naltrexone condition achieved point prevalence abstinence at week 6 compared to 43 of 85 (51%) ITT subjects in the placebo arm (p = 0.10; see Table 2b).
Table 2b.
Odds of 26- and 6-week point prevalence abstinencea
| Outcome | Placebo % (n) |
25mg % (n) |
Odds Ratio (95% CI)b | P |
|---|---|---|---|---|
| 26-Week Point Prevalence Abstinence |
27.06 (23) | 21.84 (19) | 0.75 (0.37–1.51) | 0.43 |
| 6-Week Point Prevalence Abstinence |
50.59 (43) | 37.93 (33) | 0.60 (0.33–1.09) | 0.10 |
aAll groups also received 21 mg transdermal nicotine replacement for the first 8 weeks post-quit.
Odds ratios comparing 25 mg dose to placebo are reported along with 2-sided 95% confidence intervals (95% CI)
3.4 Cigarettes Smoked Per Week and Symptoms of Withdrawal and Craving
A secondary endpoint that was evaluated was amount smoked per occasion from 1 week to 26 weeks post-quit. As shown in Figure 2, there was a non-significant interaction of condition-by-week [p = .05], such that the naltrexone group (n = 67; M = 7.10, SD = 8.43) smoked slightly fewer cigarettes per occasion over time than those in the placebo group (n = 67; M = 7.85, SD = 8.35) at 26 weeks post-quit. Neither craving nor withdrawal scores were significantly different for scores averaged over time.
Figure 2.
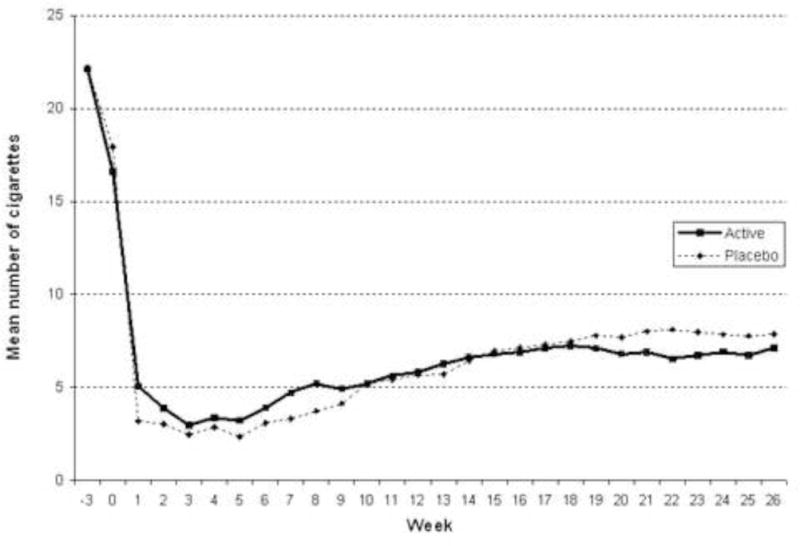
Number of cigarettes smoked per day over time.
3.5 Safety
Four serious adverse events (SAEs; 3 requiring an overnight hospitalization and 1 cancer diagnosis) occurred during the study. Two of these SAEs (anxiety, abnormal EKG) were in the naltrexone condition, and two (cut fingers with saw, diagnosis of thyroid cancer) were in the placebo condition. All of these SAEs were deemed unlikely to be related to study participation. Two participants were withdrawn by the PI including a subject who reported a blood clot before starting the study medication and a participant who initially denied opioid use at screening but later had a positive opioid drug test. Consistent with the principle of ITT, these two participants were included in study analyses. Excluding them did not alter the primary study outcome analyses of weight and smoking.
LFT values were evaluated using cutoff values of 3 times the upper limit for ALT and AST and over 10% of the upper limit for total bilirubin during treatment. No subjects were found to be above these cutoff values at any time during the study. The percentage of unique participants reporting non-serious adverse events rated moderate or severe with a prevalence of ≥ 5% differed by treatment group for depression and decreased appetite [in each case there were 4 (5%) naltrexone subjects versus 0 (0%) placebo subjects, X2 = 4.21, p = .04).
4. Discussion
This was a placebo-controlled double-blind investigation of low-dose naltrexone for smoking cessation with minimized post-quit weight gain. Although there was a small numerical difference in weight at 6 months after quitting smoking that favored the naltrexone group, this difference was not statistically significant. Furthermore, rates of smoking cessation, although also statistically non-significant, numerically favored the placebo group. Thus, we conclude that the relatively small benefits on weight do not offset the potentially higher rates of relapse to smoking for highly weight concerned smokers.
The average weight gain at 6 weeks post-quit in the placebo group was 2.5 pounds. This value is lower than the mean weight gain of 4.2 pounds at 6 weeks post-quit in the placebo group in our dose ranging study of naltrexone (O’Malley et al., 2006), and 4.2 pounds at 4 weeks post-quit in King et al.’s study of 50 mg naltrexone (King et al., 2006). It is also lower than the 3.17 pound weight gain 6 weeks after quitting that we found in smokers taking bupropion SR only in our pilot study of naltrexone plus bupropion SR (Toll et al., 2008). Indeed, other investigations noting that bupropion SR significantly reduces weight gain over 6 to 8 weeks post-quit have found weight gain in the range of 3.3 – 3.7 pounds (Hurt et al., 1997; Jorenby et al., 1999) that is still higher than the mere 2.5 pounds found in the present sample for the placebo group. Weight gain at 26 weeks post-quit is generally not reported. However, among the few studies that have reported this variable, the weight gain of 9.7 pounds in the placebo group in the present study is comparable to or less than weight gain reported in other investigations that have used bupropion SR [9.9 – 10.6 pounds (Hurt et al., 1997; Tønnesen et al., 2003)] or no medications [12.0 pounds (Klesges et al., 1997)] for smoking cessation. Thus, in the short-term, the population of smokers evaluated in this study appears to gain considerably less weight post-quit compared to smokers in prior studies taking placebo naltrexone or bupropion SR, a drug known to suppress weight gain. In the long-term, this population of smokers still appears to gain less than or equal to the weight gain found in other treatment studies.
The most likely reason for the overall low weight gain in this sample relates to the study population (i.e., weight-concerned smokers). Indeed, at 4 weeks post-quit, Perkins et al. found an average weight gain of 2.2 pounds in their control group of weight concerned smokers. Another related plausible explanation is the counseling protocol implemented in conjunction with the medications regimen. This protocol was adapted from the CBT manual employed by Perkins et al. (Perkins et al., 2001). Importantly, Perkins and colleagues found evidence that a CBT intervention to reduce weight concerns that specifically discouraged dieting resulted in superior quit rates compared to both weight control and standard counseling interventions (Perkins et al., 2001). Our adaptation was designed to be less time-intensive (i.e., 5–15 minute individual sessions versus 90-minute group sessions). Even so, the same overall theoretical rationale was employed, in which dieting was explicitly discouraged, and this may have led to less weight gain for both study groups. Although this is a hypothesis that would need to be validated in another study, if found to be true, the much briefer smoking protocol used in the present study could be used by primary care providers to address smoking cessation in weight concerned smokers.
Even though naltrexone does not appear to reduce weight gain in the specific subpopulation of smokers tested in the current study (i.e., highly weight concerned smokers who report that smoking helps manage their weight), most studies find that naltrexone reduces post-cessation weight gain (King et al., 2006; Krishnan-Sarin et al., 2003; O’Malley et al., 2006; Toll et al., 2008). There are other sub-populations of smokers for whom minimization of post-quit weight gain may be valued. For instance, overweight smokers who binge eat have important reasons to appreciate less weight gain upon quitting smoking (White et al., 2010). Similarly, the combination of naltrexone and other pharmacological treatments may be effective for some smokers [e.g., overweight and obese (Wilcox et al., 2010)]. In addition, greater smoking cessation weight gain has been associated with disinhibited eating and eating in response to negative affect (Hudmon et al., 1999). Whereas the current study tested individuals with the cognitive features of eating disturbance (i.e., elevated weight concerns), future studies should consider testing naltrexone in populations with the behavioral features of eating disturbance (such as emotional eating, excessive overeating, and binge eating).
The evidence regarding smoking cessation outcomes with naltrexone has been inconsistent with multiple negative (Ahmadi et al., 2003; King et al., 2006; Toll et al., 2008; Wong et al., 1999), mixed (King et al., 2006) and positive (Covey et al., 1999; Krishnan-Sarin et al., 2003; O’Malley et al., 2006) studies. The present study which used 25 mg daily adds to accumulating evidence that naltrexone either does not aid in smoking cessation or has a weak effect (David et al., 2001). The most promising results in prior studies were found for higher dose naltrexone (100 mg daily), and future studies should be conducted to replicate this finding (O’Malley et al., 2006). Research might also evaluate whether naltrexone augmentation of first-line smoking cessation therapies will improve smoking cessation outcomes for heavy drinking smokers (Leeman et al., 2008) given the documented efficacy of naltrexone for reducing heavy drinking (O’Malley et al., 2009; Pettinati et al., 2006).
The only adverse events to show medication effects were decreased appetite and depression, with higher rates in the naltrexone group. Although these differences were statistically significant, the numerical frequency was relatively low. Moreover, decreased appetite was expected in the naltrexone group.
There are several limitations to this investigation. Our sample was comprised primarily of Caucasian smokers, and recruitment via media outlets (e.g., the internet, television, and newspapers) potentially yielded a highly motivated group of smokers. Moreover, the population enrolled is one with high weight concern who reported smoking to manage their weight. We also tested only 1 dose of naltrexone (i.e., 25 mg naltrexone vs placebo), and testing higher doses might have yielded different results. The long study treatment length (i.e., 26 weeks) may have contributed to high rates of drop out. Although participants were instructed not to diet, adherence to these instructions was not measured, and participants might have engaged in weight control practices that could possibly alter study outcomes.
In summary, treatment with low dose naltrexone does not significantly reduce weight gain or improve smoking cessation in highly weight-concerned smokers. Given that this population gained relatively little weight even on placebo, cognitive interventions to reduce weight concerns (Perkins et al., 2001) in combination with approved smoking cessation pharmacotherapy are preferable. Nevertheless, there may be other sub-populations of smokers at risk of substantial weight gain following smoking cessation for whom the weight suppressing effects of naltrexone might be of benefit.
Supplementary Material
Appendix A
CONSORT Checklist
Footnotes
A CONSORT checkslist is available as supplementary material with the online version of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelin T, Ehrsam R, Buhler-Reichert A, Imhof P, Muller P, Thommen A, Vesanen K. Effectiveness of a transdermal nicotine system in smoking cessation studies. Methods & Find in Exp & Clin Pharmacol. 1989b;11:205–214. [PubMed] [Google Scholar]
- Ahluwalia J, Harris K, Catley D, Okuyemi K, Mayo M. Sustained-release bupropion for smoking cessation in African Americans: A randomized controlled trial. JAMA. 2002;288:468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- Ahmadi J, Ashkani H, Ahmadi M, Ahmadi N. Twenty-four week maintenance treatment of cigarette smoking with nicotine gum, clonidine and naltrexone. J Subst Abuse Treat. 2003;24:251–255. doi: 10.1016/s0740-5472(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Alsene K, Li Y, Chaverneff F, de Wit H. Role of abstinence and visual cues on food and smoking craving. Behav Pharmacol. 2003;14:145–151. doi: 10.1097/00008877-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Babor T, de la Fuente J, Saunders J, Grant M. AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care. World Health Organization; Geneva, Switzerland: 1992. [Google Scholar]
- Bodnar R, Hadjimarkou M, Krzanowska E, Silva R, Stein J. Differential dose-dependent effects of central morphine treatment upon food intake in male and female rats receiving neonatal hormone manipulations. Nutr Neurosci. 2003;6:53–57. doi: 10.1080/1028415021000042848. [DOI] [PubMed] [Google Scholar]
- Bowen D, Spring B, Fox E. Tryptophan and high-carbohydrate diets as adjuncts to smoking cessation therapy. J Behav Med. 1991;14:97–110. doi: 10.1007/BF00846173. [DOI] [PubMed] [Google Scholar]
- Brown R, Burgess S, Sales S, Whiteley J, Evans D, Miller I. Reliability and validity of a smoking Timeline Followback interview. Psychology of Addictive Behaviors. 1988;12:101–112. [Google Scholar]
- Carroll M, France C, Meisch R. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Carroll M, Meisch R. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology (Berl) 1981;74:197–200. doi: 10.1007/BF00427092. [DOI] [PubMed] [Google Scholar]
- Cooper T, Klesges R, DeBon M, Zbikowski S, Johnson K, Clemens L. A placebo controlled randomized trial of the effects of phenylpropanolamine and nicotine gum on cessation rates and postcessation weight gain in women. Addict Behav. 2005;30:61–75. doi: 10.1016/j.addbeh.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Copeland A, Brandon T, Tiffany S, Baker T. The Smoking Consequences Questionnaire – Adult: measurement of smoking outcome expectancies of experienced smokers. Psychol Assess. 1995;7:484–494. [Google Scholar]
- Covey L, Glassman A, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis. 1999;18:31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- Cox L, Tiffany S, Christen A. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- David S, Lancaster T, Stead L. Opiod antagonists for smoking cessation. Cochrane Database of Systematic Reviews. 2001;3 doi: 10.1002/14651858.CD003086. Art No.: CD 003086. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Johanson C. The effects of food deprivation on the self-administration of psychoactive drugs. Drug Alcohol Depend. 1987;19:17–27. doi: 10.1016/0376-8716(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Doherty K, Militello F, Kinnunen T, Garvey A. Nicotine gum dose and weight gain after smoking cessation. J Consult Clin Psychol. 1996;64:799–807. [PubMed] [Google Scholar]
- Fiore M, Jaén C, Baker T, Bailey W, Benowitz N, Curry S, et al. Treating Tobacco Use and Dependence 2008 Update: Clinical Practice Guideline. USDHHS; Rockville, MD: 2008. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, research version, patient edition. Biometrics Research, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Gonzales D, Rennard S, Nides M, Oncken C, Azoulay S, Billing C, Watsky EJ, Gong J, Reeves KR, Varenicline Phase 3 Study Group Varenicline, an alpha-4-beta-2 nicotinic acetylcholine receptor partial agonist, vs. sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hall S, Ginsberg D, Jones R. Smoking cessation and weight gain. J Consult Clin Psychol. 1986;54:342–346. doi: 10.1037//0022-006x.54.3.342. [DOI] [PubMed] [Google Scholar]
- Hall S, Tunstall C, Vila K, Duffy J. Weight gain prevention and smoking cessation: Cautionary findings. Am J Public Health. 1992;82:799–803. doi: 10.2105/ajph.82.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerström K. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hudmon K, Gritz E, Clayton S, Nisenbaum R. Eating orientation, postcessation weight gain, and continued abstinence among female smokers receiving an unsolicited smoking cessation intervention. Health Psychol. 1999;18:29–36. doi: 10.1037//0278-6133.18.1.29. [DOI] [PubMed] [Google Scholar]
- Hughes J. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hurt R, Sachs D, Glover E, Offord K, Johnston J, Dale L, Khayrallah M, Schroeder D, Glover P, Sullivan C, Croghan I, Sullivan P. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337 doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jorenby D, Hays J, Rigotti N, Azoulay S, Watsky E, Williams K, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study Group Efficacy of varenicline, an alpha-4-beta-2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Jorenby D, Leischow S, Nides M, Rennard S, Johnston J, Hughes A, Smith S, Muramoto M, Daughton D, Doan K, Fiore M, Baker T. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- King A, de Wit H, Riley R, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: A preliminary study and an examination of sex differences. Nicotine Tob Res. 2006;8:671–682. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- Klesges R, Winders S, Meyers A, Eck L, Ward K, Hultquist C, Ray J, Shadish W. How much weight gain occurs following smoking cessation: A comparison of weight gain using both continuous and point prevalence abstinence. J Consult Clin Psychol. 1997;65:286–291. doi: 10.1037//0022-006x.65.2.286. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Meandzija B, O’Malley S. Naltrexone and nicotine patch in smoking cessation: A preliminary study. Nicotine Tob Res. 2003;5:851–857. doi: 10.1080/14622200310001614601. [DOI] [PubMed] [Google Scholar]
- Lang W, Latiff A, McQueen A, Singer G. Self administration of nicotine with and without a food delivery schedule. Pharmacol Biochem Behav. 1977;7:65–70. doi: 10.1016/0091-3057(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Leeman R, McKee S, Toll B, Krishnan-Sarin S, Cooney J, Makuch R, O’Malley S. Risk factors for treatment failure in smokers: Relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine Tob Res. 2008;10:1793–1809. doi: 10.1080/14622200802443742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley S, Cooney J, Krishnan-Sarin S, Dubin J, McKee S, Cooney N, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P. Controlled trial of naltrexone augmentation of nicotine replacement for smoking cessation. Arch Intern Med. 2006;166:667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Krishnan-Sarin S, McKee S, Leeman R, Cooney N, Meandzija B, Wu R, Makuch R. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropharmacol. 2009;12:589–597. doi: 10.1017/S146114570800936X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 2009;1 doi: 10.1002/14651858.CD006219.pub2. Art No.: CD 006219. [DOI] [PubMed] [Google Scholar]
- Perkins K, Marcus M, Levine M, D’Amico D, Miller A, Broge M, Ashcom J, Shiffman S. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- Pettinati H, O’Brien C, Rabinowitz A, Wortman S, Oslin D, KM K, CA D. The status of naltrexone in the treatment of alcohol dependence: Specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Pirie P, McBride C, Hellerstedt W, Jeffery R, Hatsukami D, Allen S, Lando H. Smoking cessation in women concerned about weight. Am J Public Health. 1992;82:1238–1243. doi: 10.2105/ajph.82.9.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau C, Zucker A, Stewart A. Characterizing concerns about post-cessation weight gain: Results from a national survey of women smokers. Nicotine Tob Res. 2001;3:51–60. doi: 10.1080/14622200020032105. [DOI] [PubMed] [Google Scholar]
- Rose J, Levin E, Behm F, Adivi C, Schur C. Transdermal nicotine facilitates smoking cessation. Clin Pharmacol Ther. 1990;47:323–330. doi: 10.1038/clpt.1990.35. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. The Cochrane Database of Systematic Reviews. 2004:3. doi: 10.1002/14651858.CD000146.pub2. Art. No.: CD000146.pub000142. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Alcohol consumption measures. In: Allen J, Wilson V, editors. Assessing Alcohol Problems: A Guide for Clinicians and Researchers. 2. National Institute on Alcohol Abuse & Alcoholism; Bethesda, MD: 2003. pp. 75–99. [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, Bergman K. Effectiveness of bupropion sustained release for smoking cessation in a health care setting. Arch Intern Med. 2003;163:2337–2344. doi: 10.1001/archinte.163.19.2337. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts A, Coscina DV, Rousset S, Redonnet A, Parker GC, Inoue K, Ricquier D, Pénicaud L, Kieffer BL, Koob GF. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene. Diabetes. 2005;54:3510–3516. doi: 10.2337/diabetes.54.12.3510. [DOI] [PubMed] [Google Scholar]
- Toll B, Leary V, Wu R, Salovey P, Meandzija B, O’Malley S. A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addict Behav. 2008;33:173–179. doi: 10.1016/j.addbeh.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll B, O’Malley S, McKee S, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol Addict Behav. 2007;21:216–225. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tønnesen P, Tonstad S, Hjalmarson A, Lebargy F, Van Spiegel P, Hider A, Sweet R, Townsend J. A multicentre, randomized, double-blind, placebo-controlled, 1-year study of bupropion SR for smoking cessation. J Intern Med. 2003;254:184–192. doi: 10.1046/j.1365-2796.2003.01185.x. [DOI] [PubMed] [Google Scholar]
- West R, Courts S, Beharry S, May S, Hajek P. Acute effect of glucose tablets on desire to smoke. Psychopharmacology. 1999;147:319–321. doi: 10.1007/s002130051174. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Burrows S. Effect of glucose tablets on craving for cigarettes. Psychopharmacology. 1990;101:555–559. doi: 10.1007/BF02244237. [DOI] [PubMed] [Google Scholar]
- West R, Willis N. Double-blind placebo controlled trial of dextrose tablets and nicotine patch in smoking cessation. Psychopharmacology. 1998;136:201–204. doi: 10.1007/s002130050557. [DOI] [PubMed] [Google Scholar]
- White M, Grilo C, O’Malley S, Potenza M. Binge eating disorder, obesity, and tobacco smoking. J Addict Med. 2010;4:11–19. doi: 10.1097/ADM.0b013e3181ce38c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C, Oskooilar N, Erickson J, Billes S, Katz B, Tollefson G, Dunayevich E. An open-label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addict Behav. 2010;35:229–234. doi: 10.1016/j.addbeh.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Wong G, Wolter T, Croghan G, Croghan I, Offord K, Hurt R. A randomized trial of naltrexone for smoking cessation. Addiction. 1999;94:1227–1237. doi: 10.1046/j.1360-0443.1999.948122713.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.