Abstract
Epithelial cells have an apical–basolateral axis of polarity, which is required for epithelial functions including barrier formation, vectorial ion transport and sensory perception. Here we review what is known about the sorting signals, machineries and pathways that maintain this asymmetry, and how polarity proteins interface with membrane-trafficking pathways to generate membrane domains de novo. It is becoming apparent that membrane traffic does not simply reinforce polarity, but is critical for the generation of cortical epithelial cell asymmetry.
Epithelial cells have a pronounced apical–basolateral axis of asymmetry that is composed of functionally and morphologically distinct membrane domains (Fig. 1). The establishment of this polarity is important for barrier function, sensory perception and vectorial transport, and the failure to generate or maintain cell-surface polarity leads to altered epithelial function and to pathologies such as polycystic kidney disease, hypercholesterolaemia and cancer1. The apical surface forms a critical interface between the extracellular milieu and underlying tissues and faces the lumen of sac- and tube-shaped organs and glands, or lines the inner surfaces of the body cavities. Its outer leaflet is rich in glycosphingolipids, whereas its inner leaflet is enriched in the regulatory lipid At the phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) (refs 2, 3). apicolateral junction of the vertebrate epithelial cell lies the junctional complex, which is composed of the apical-most tight junction, followed by the immediately basal adherens junction and desmosomal junctions (Fig. 1). Although the lateral domain is critical for cell–cell interactions between adjacent cells, and is rich in the lipid phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) (ref. 4), the contiguous basal membrane facilitates interaction with subjacent cells or with the components of the basement membrane through dystroglycans and integrin receptors. Here we review our present understanding of how these distinct membrane domains are formed and maintained, how epithelial-cell asymmetry is generated, and how membrane traffic and polarity proteins act in concert to promote these processes.
Figure 1.
Membrane-trafficking pathways in polarized epithelial cells. Proteins, lipids and fluid are internalized from the apical (step 1a) or basolateral (1b) surfaces, and delivered to apical early endosomes (AEEs) or basolateral early endosomes (BEEs). Cargoes can recycle to the cell surface (2a/b), be delivered to multivesicular bodies (MVBs) (3a/b), from which they will be delivered to lysosomes (not shown), or they can be delivered to the common recycling endosome (CRE; 4a/b). There may be a direct pathway from the AEE to the apical recycling endosome (ARE; 4c). CRE cargoes are shuttled to the basolateral cell surface (5), or delivered to ARE (6), from where they gain access to the apical cell surface (7a). Apical-to-basolateral transcytosis may involve cargo transit from the ARE to CRE (7b). On synthesis in the endoplasmic reticulum (not shown), proteins are delivered by way of the Golgi stacks to the trans-Golgi network (TGN), to take either direct routes to the apical (8) or basolateral (10) surfaces, or indirect routes through the ARE (9) or CRE (11). Endocytic pathways are shown with black arrows; biosynthetic ones with red arrows. The inset shows a magnified view of the apical junctional complex (JC), which is comprised of the zonula occludens (ZO), the zonula adherens (ZA) and the macula adherens (MA).
Membrane-trafficking pathways in polarized epithelial cells
We begin by providing an overview of the pathways that are used for polarized membrane transport in epithelial cells. In the biosynthetic pathway, newly synthesized membrane proteins are trafficked from the endoplasmic reticulum, to the Golgi, and through the trans-Golgi network (TGN), where they are sorted into distinct carriers before their delivery to the appropriate cell-surface domain (Fig. 1)1. The full spectrum of TGN-derived carrier types in epithelial cells is unknown; recent studies, however, indicate that multiple, distinct carriers for apical and basolateral membrane cargoes exist5–7. In addition, epithelial cells with a regulated secretory pathway (for example, pancreatic acinar cells or bladder umbrella cells) form secretory granules at the TGN, which are distinct from constitutive cargo carriers8. Finally, some proteins avoid the Golgi entirely and are secreted by a non-canonical bypass pathway9.
The route from the TGN to the cell surface is not always direct (Fig. 1). For some apical and basolateral proteins, passage through Rab8a and Rab11a GTPase-positive recycling endosomes has been described10–12, with Rab4 additionally involved in apical transport12. Why proteins use an indirect pathway is unknown, but for some (such as the polymeric immunoglobulin receptor (pIgR)) it may modulate their functions by allowing them to bind internalized ligand(s). Alternatively, it may promote their ‘maturation’ by altering their conformation as they encounter the relatively low pH of the endosomes en route to the cell surface. Additionally, the decoding machinery that recognizes some sorting signals is localized to endosomes (see later discussion) and transit through these compartments would ensure polarized delivery to the cell surface. Finally, for some cargo (such as the transferrin receptor, which is the classic basolateral recycling receptor) the choice of a direct or indirect pathway depends on the degree to which polarity has been established13, suggesting that in addition to cortical asymmetry, intracellular trafficking pathways are re-organized during development.
After surface delivery, proteins and lipids can then be internalized (Fig. 1). The best-understood mechanism for internalization involves the vesicle coat clathrin, the membrane-deforming GTPase dynamin, and the heterotetrameric adaptor complex AP2, which binds to endocytic motifs in cargo molecules14. Additional, clathrin-independent endocytic pathways exist, including a RhoA-, dynamin- and integrin-regulated apical endocytic pathway in bladder umbrella cells15. However, the full identity, prevalence and extent of use of different endocytic pathways in polarized tissue remain to be elucidated.
Upon endocytosis, apical and basolateral cargo enter spatially distinct, peripherally localized apical or basolateral early endosomes (AEE or BEE); these are typically Rab5 positive16–18 (Fig. 1). The cargo then has several alternative fates. First, cargo can undergo rapid recycling to the cell surface, generally via Rab4-positive compartments19. Second, cargo may be partitioned into multivesicular bodies, which fuse to generate Rab7-positive late endosomes containing lysosome-directed cargo internalized from both apical and basolateral surfaces16. The third fate involves routing of a considerable fraction of cargo to the Rab8- and Rab10-positive common recycling endosome (CRE) (Fig. 1), which contains perinuclear and supranuclear elements20–22. The name ‘common recycling endosome’ derives from the intermixing of apical and basolateral cargo, both endocytic and from the indirect biosynthetic pathways. Within the CRE, apical and basolateral cargoes are again sorted and directed towards their appropriate cell surface (Fig. 1). Apically destined proteins, including basolaterally internalized proteins (such as the pIgR), may be further routed through the Rab11a-positive apical recycling endosome (ARE) before arriving at the cell surface20,23, although whether the ARE and CRE are separate entities or merely subdomains of the same structure is controversial19,24,25. In addition to the well-studied basolateral-to-apical transcytotic pathway, an apical-to-basolateral pathway exists, involving Rab25, but considerably less is known about its mechanics24,26.
The epithelial sorting and transport machinery
Although trafficking pathways provide a mechanism for delivery to a particular domain, vectorial transit of proteins require sorting determinants that are present in their cytoplasmic, transmembrane or extracellular domains. In turn, these sorting signals are decoded by a corresponding sorting and transport machinery that ensures correct membrane delivery1. Although lipids are also sorted in epithelial cells, their recognition and sorting is less well understood27.
Sorting of basolateral membrane proteins
Our knowledge of sorting signals derives mainly from the cytoplasmic domains of basolateral membrane proteins, which often bear a superficial similarity to endocytosis signals that include YXXØ-like or dileucine (LL/IL) motifs28. Moreover, like endocytosis, the machinery responsible for decoding many basolateral signals involves clathrin and heterotetrameric adaptor protein complexes28–32. The AP1, AP3 and AP4 complexes have all been implicated in basolateral sorting28,30–32, yet most of our understanding comes from the AP1 complex. Although the AP1A complex is ubiquitous, a subset of epithelial cells also express an AP1B variant, wherein μ1b adaptin is substituted for μ1a30,33. Both AP1A and AP1B are thought to promote basolateral traffic, but, in the absence of AP1B, AP1A may be a substitute for some cargoes34. Furthermore, Ap1m2 (also known as μ1b)-knockout mice develop normally and survive for several weeks after birth35. Compensation is incomplete, however, and mice spontaneously develop colitis, possibly due to apical mistargeting of the pIgR and other basolateral cytokine receptors to colon epithelia apical domains35. Similarly, the normal absence of AP1B in mouse kidney proximal tubules confers a unique apical localization to a number of otherwise basolateral membrane proteins (when in AP1m2-expressing cells)36. Thus, the cortical polarity of some proteins is determined by the differential repertoire of membrane-trafficking machinery.
Basolateral sorting adaptors probably function in a manner analogous to the plasma membrane AP2 complex. Like the μ2 subunit of AP2, AP1m2 is thought to interact with YXXØ-like basolateral sorting motifs in proteins such as the vesicular stomatitis virus glycoprotein (VSV-G)37,38. Less is understood about the detection of LL-based basolateral sorting signals but, by analogy to AP2, these may be recognized by the σ1/subunits of AP139. Interestingly, AP1m2 also recognizes cargoes with non-canonical motifs such as the GDNS motif in the transferrin receptor or the proximal tyrosine-based basolateral sorting motif in the low-density lipoprotein receptor (LDLR)13,40. These observations indicate that either AP1m2 has multiple interaction surfaces and/or its interactions depend on accessory proteins. Evidence for the latter includes the PIP5K1γ lipid kinase, a protein that binds AP1m2 via a cytoplasmic YXXØ motif (YSPL), and bridges AP1B to E-cadherin and the Exo70 (also known as Exoc7) subunit of the exocyst41,42. The latter is an octomeric complex that apparently acts by tethering apical- and basolateral-directed vesicles before fusion43–45. There is conflicting evidence as to whether the AP1B–Exo70–PIP5K1γ complex facilitates basolateral E-cadherin targeting, however, as E-cadherin also possesses a LL motif required for basolateral targeting46, which apparently occurs via an AP1B-independent mechanism13. An additional example is the LDLR, an AP1B-dependent cargo that contains two tyrosine-based basolateral sorting determinants; however, neither motif interacts directly with AP1B47. Instead, its basolateral sorting may depend on ARH — a clathrin-associated adaptor protein previously implicated in LDLR endocytosis48 — that may act by recruiting AP1B. Interestingly, this interaction is only required when the distal tyrosine-based motif is removed47, indicating that there may be multiple sorting signals in the cytoplasmic domain of basolateral cargo, some of which can be dominant, or masked by accessory proteins. The sum of these sorting signals would determine the surface polarity of a cargo molecule.
Interestingly, AP1B functions at the CRE, where it sorts both biosynthetic and endocytic recycling cargoes13. AP1B may be recruited to the CRE by interactions with phosphoinositides, the GTPase Arf6, and the Sec10 (also known as Exoc5) exocyst subunit49–51. AP1B-dependent basolateral transport apparently requires Rab8a, its effectors optineurin and Myo6, as well as Cdc42 and cellubrevin (Vamp3), but neither a direct interaction between these proteins and AP1B nor a functional hierarchy in these proteins has been established52–54. Furthermore, the involvement of Rab8a in these processes is unclear, as recent studies demonstrate defects in apical, not basolateral, transport when Rab8a and Cdc42 are depleted in cultured cells, or in Rab8a-null mice55–60. Thus, further experimentation is required to define the role of these GTPases in cargo transport.
AP1B is not expressed by all epithelia28, and there are basolateral proteins such as CD147 and E-cadherin that require clathrin, but not AP1B, for their proper sorting13,29,61. Thus, additional mechanisms for basolateral sorting must exist. One example is naked 2, which binds to LL and HCCQVRKH motifs in the cytoplasmic domain of the epidermal growth factor receptor (EGFR) ligand transforming growth factor α (TGF-α) and is required for AP1B-independent TGF-α basolateral delivery62. Ankyrin G and its binding protein β2-spectrin may also have a role in basolateral sorting63. Finally, although no sorting signals are known for secreted basolateral proteins, mutations in the Drosophila melanogaster protein Scarface, a secreted serine proteinase-like protein, causes ectopic apical laminin A accumulation64. Furthermore, mutated Crag, a Drosophila homologue of the mammalian DENND4 family of Rab GTPase exchange factors (GEFs), results in a non-polarized distribution of the extracellular matrix proteins perlecan, laminin and collagen IV65. Precise details of how these proteins work, and their effect on membrane protein traffic, however, is unknown.
Sorting of apical membrane proteins
Whereas basolateral signals are often cytoplasmic, apical sorting determinants are varied and can be localized to cytoplasmic, transmembrane or extracellular domains66. Furthermore, attachment to the membrane by a glycosylphophatidylinositol (GPI) anchor can specify apical sorting, although this is cell-type and molecule specific66. Moreover, some apical proteins, such as the influenza virus haemagglutinin protein and many GPI-anchored proteins, associate with lipid rafts67, which are specialized lipid domains (described later). In the case of those linear sorting determinants identified in the cytoplasmic or transmembrane domains of apical proteins, we do not know the mechanism(s) of signal recognition, nor do we have insights into where recognition occurs. In contrast, more is understood about those sorting determinants associated with the extracellular domain, and with N- and O-linked glycosylation, in particular. Although it remains an open question whether glycans function, in part, by ensuring proper folding of the protein backbone, work in the past decade points to the existence of apical sorting machinery that recognizes carbohydrate determinants66,68.
The latter involves a family of endogenous sugar-binding proteins called galectins that recognize β-galactosides, and can cross-link proteins into clusters, arrays or lattices by way of protein–carbohydrate interactions69. Depletion of galectin-3 in MDCK cells leads to mistargeting of several lipid-raft-independent apical membrane proteins to the basolateral cell surface, a phenotype also observed in the enterocytes of galectin-3-knockout mice70,71. Galectin-3 function may be specific to trafficking of membrane proteins, as depletion of galectin-3 (and galectin-4) has no effect on transport of glycosylated apical secretory cargoes72. Other galectins implicated in apical targeting include galectin-4, which associates with sulphatides, and galectin-9, which binds Forssman glycosphingolipids73–75. Downregulation of the latter results in a marked loss of epithelial polarity. The intracellular site of galectin function has not been determined. However, after their release by a non-conventional secretory pathway, they can bind apical proteins and, on internalization, they can concentrate in endosomes or progress to the Golgi, where they apparently cluster their associated cargoes74–76.
Beyond the sorting motifs present in peptide backbones and associated with glycans, some apical protein traffic is also governed by annexins, the myelin and lymphocyte proteolipids MAL (as known as VIP17) and MAL2, BAR-domain containing proteins such as sorting nexin 18 (Snx18), and lipid rafts77–81. The latter are nanometre-sized, highly dynamic microdomains of cholesterol, glycosphingolipids and proteins82. At sites of apical transport carrier formation, such as the TGN, the rafts and their cargoes are selected by clustering agents, possibly the galectins75. The lipid raft hypothesis is supported by work in yeast that shows that those TGN-derived vesicles containing lipid-raft-associated membrane proteins are enriched in sphingolipids and ergosterol83, the yeast equivalent of cholesterol. Furthermore, glycosphingolipids are critical for mediating apical sorting in the gut of Caenorhabditis elegans84. Although much has been learned in the past decade, further work is needed to understand raft component synthesis and assembly, the protein determinants and features that specify raft inclusion, the regulation of raft clustering, the site of raft action, and the machinery that is incorporated into the lipid-raft-containing vesicles and ensures apical targeting and fusion.
Transport mechanisms and membrane fusion
During vectorial transport, cargoes must pass through multiple compartments en route to the cell surface. These events are regulated by Rab proteins (44 subfamilies in humans), which modulate cargo selection and the uncoating, movement, maturation, tethering and fusion of vesicles with their target membranes85. Other than Rab3, Rab13 and Rab27 (refs 8, 86, 87), surprisingly few Rab proteins have been identified that specifically regulate the polarized traffic of cargo from the TGN of epithelial cells. In contrast, a number of Rab proteins, discussed earlier, are associated with the endosomes of polarized epithelial cells, including some (for example, Rab17) that are exclusive to epithelial cells88. Polarized traffic is also dependent on the cytoskeleton. Transport along microtubules is driven by multiple kinesin family proteins including Kifc3, Kif5B and Kif17, primarily for apical cargoes89–91, whereas the minus-end-directed microtubule motor dynein promotes exogenously expressed rhodopsin transport to the apical domain92. Actin-based motors have also been implicated in polarized membrane traffic, including the minus-end-directed motor Myo6, which is implicated in apical endocytosis and basolateral delivery53,93, and Myo5A and Myo5B, which are critical for apical exocytosis94–96. Finally, vesicle fusion is mediated by the soluble N-ethylmaleimide-sensitive factor attachment receptors associated with vesicles (v-SNAREs) or target membranes (t-SNAREs). The t-SNAREs syntaxin-1, -2 and -3 have all been implicated in apical transport97–100, supporting the idea that multiple routes exist to the plasma membrane. In contrast, syntaxin-4 is localized exclusively to the basolateral cell surface, and its delivery requires AP1B, where Vamp3 is the likely v-SNARE54,101,102. Disruption to the polarized localization of either syntaxin-3 or- 4 results in the abolition of apical–basal polarity102,103, indicating that membrane trafficking is part of the core polarity-generating machinery of the cell.
Polarity establishment and vectorial membrane traffic
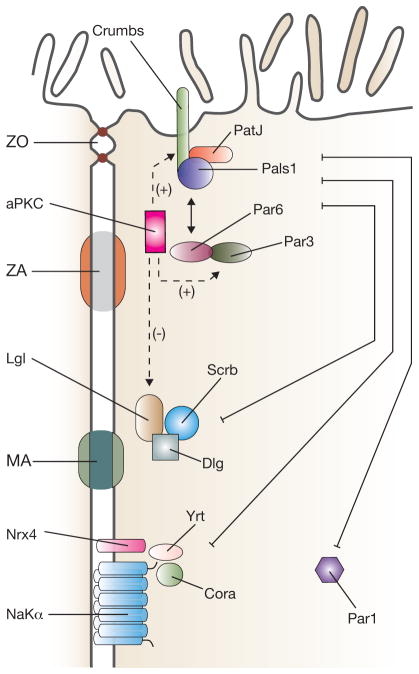
We have explored membrane sorting signals and targeting machinery. Now we examine how epithelial cells develop distinct apical and basolateral membrane domains separated by junctional complexes, and how trafficking contributes to this process. These processes have been studied in model organisms and two-dimensional and three-dimensional cultures of epithelial cells (Fig. 2). The epithelial program is initiated in response to extracellular cues (for example, by cell–cell contact), and is reinforced by polarity complexes that promote the formation of asymmetric membrane domains1,104. These highly conserved assemblages of proteins (Fig. 3) include: the Crumbs complex (composed of crumbs (Crb), Pals1 (known as Stardust in Drosophila) and Patj (known as Discs lost in Drosophila), which in vertebrates is localized to the apical side of the junctional complex and required for its formation; and the Par complex, composed of aPKC (atypical protein kinase C; it has PKC-ι/λ and PKC-ζ isoforms), Par3 and Par6, as well as the Rho family GTPase Cdc42. This complex is localized near the junctional complex and, like the Crumbs complex, modulates the assembly and function of the junctional complex; and the Scribble complex (Scrib, Dlg and Lgl), which is found below the tight junctional complex, along the lateral membrane domain105,106. It is important to note that although the components of each complex are functionally linked, they do not always work as one. For example, Cdc42–Par6–aPKC is associated with the apical surface whereas Par3–aPKC is associated with the tight junction104.
Figure 2.
Use of two-dimensional versus three-dimensional models to study epithelial polarity. Epithelial cells can be grown under a number of conditions including on plastic dishes or coverslips (a), or on permeable filter supports such as Transwells (b). The latter, two-dimensional culture system is popular because it allows the epithelial cells to feed from their basolateral surfaces, they establish polarity within days, and the arrangement allows for access to both the apical and basolateral surfaces of the culture. An alternative to traditional two-dimensional culture involves growing epithelial tissues, cells or progenitors in a three-dimensional environment comprising extracellular matrix proteins, which allows for some aspects of tissue morphogenesis, such as lumen formation, to be recapitulated (c). For example, Lgr5-positive intestinal stem cells grown in three-dimensional cultures form ‘mini-guts’ lined by intestinal epithelial cells organized in the expected manner133. Furthermore, when fragments of mammary glands, kidney and other organs are grown in such matrices they can recapitulate developmental processes such as branching morphogenesis, a phenotype not observed in two-dimensional cultures134,135. Moreover, when MDCK, MCF-10A or a number of other epithelial cell lines are cultured in a three-dimensional matrix, they form spheroid structures, variably referred to as cysts or acini, depending on the epithelial lineage of origin134–136. When mature, these spheroids comprise a single layer of epithelial cells with their apical surfaces lining an inner lumen, and their basolateral surface in contact with the extracellular matrix. When treated with morphogens some spheroids are able to undergo tubulogenetic programs, another process that cannot be modelled in two dimensions137,138.
Figure 3.
Polarity complexes in polarized vertebrate epithelial cells. The apical-most Crumbs complex includes the transmembrane protein crumbs and the cytoplasmic proteins Pals1 and PatJ. It interacts with the Par complex (aPKC, Par3 and Par6). aPKC phosphorylates crumbs and Par3, promoting apical identity. In contrast, aPKC-dependent phosphorylation of Lgl promotes its degradation, suppressing basolateral identity. Basolateral polarity is promoted by the Scribble complex (Scribble (Scrb), Discs large (Dlg) and Lethal giant larvae (Lgl)), Par1 and the Yurt (Yrt)–coracle (Cora) complex (Yrt, Cora, neurexin IV (Nrx4) and Na+/K+-ATPase α-subunit (NaKα)). The basolateral and apical polarity complexes act antagonistically to promote apical and basolateral membrane asymmetry. Components of the junctional complex are described in the legend to Fig. 1.
The asymmetric distribution of polarity complexes facilitates the expansion of the membrane domain with which they associate. The Crumbs complex promotes apical membrane formation, whereas in organisms such as Drosophila the Scribble complex promotes basolateral surface expansion104. Whether Scribble proteins act in a similar manner in mammalian cells is less clear105. Furthermore, there are additional polarity proteins involved in basolateral sorting including Par1b (also known as MARK2)107; Dlg5, which binds to syntaxin-4 and regulates basolateral trafficking of cadherins108; and the recently described Drosophila Yrt–Coracle complex, composed of Yurt (Yrt), coracle (Cora), the Na+/K+-ATPase and neurexin IV109. The asymmetrically located polarity complexes can be mutually antagonistic, an example of which is the phosphorylation of lethal giant larvae (Lgl) by aPKC, which triggers the dissociation of Lgl from the membrane, reinforcing domain identity110,111. These complexes do not act alone: their function depends on the concerted action of lipid modifying enzymes, signalling molecules, the cytoskeleton and its associated proteins104. Furthermore, there is a reciprocal interplay between polarity complexes and membrane traffic involving bidirectional influence on their localization and function112. For instance, Rab11a/exocyst-dependent apical transport pathways reinforce the localization of the apical Par complex56. Concomitantly, Par5, acting upstream of basolateral Par1 and apical Par3, controls the subapical positioning of Rab11-positive recycling endosomes in C. elegans intestine in vivo113. Thus, epithelial polarization involves not only asymmetry of the cell cortex, but also rearrangement of intracellular trafficking pathways.
Membrane traffic during the formation of polarized membrane domains
An instructive system to explore the interface between polarity proteins, membrane traffic and epithelial asymmetry is the formation of lumens, a structure found in the tube- and sac-shaped organs of the body. Lumens can be generated by folding pre-existing epithelial sheets, removal of an inner mass of cells by apoptosis, or by exocytosis of vesicles at specialized sites of cell–cell contact, resulting in the de novo lumen generation104. An example of the latter occurs soon after the first cell division of MDCK cells in three-dimensional cultures114. The process begins when apical proteins and the Crumbs polarity complex are delivered to a specialized zone of cell–cell contact called the apical membrane initiation site (AMIS) (Fig. 4a, b)115. The AMIS is characterized by the transient accumulation of a number of apical polarity and trafficking proteins at the surface and subjacent vesicles115, and it precedes the formation of a tight-junction-delimited lumen. Ultimately, the AMIS gives rise to a pre-apical patch, which is formed once a tight-junction-delimited lumen is established containing Crb3a and podocalyxin (Fig. 4c)116. Analogous AMIS structures have been observed in vivo in various tissues undergoing de novo lumen formation117,118. Further cell divisions, events that reinforce polarity, and ion and water flow result in a cyst with a central lumen bound by polarized epithelial cells (Fig. 4d)116,119.
Figure 4.
Early steps in lumen formation during MDCK cyst development. Before lumen formation, individual MDCK cells undergo mitosis. (a) At the two-cell stage polarization begins: E-cadherin, occludin and the exocyst subunit Sec10 are at the cell–cell adhesion, whereas Par3 and the exocyst subunit Sec8 are enriched at the periphery. The apical protein podocalyxin is found at the extracellular-matrix-abutting surface at this time. (b) During formation of the apical membrane initiation site (AMIS), part of the cell–cell contact is converted into an apical (luminal) surface. AMIS formation requires a series of trafficking events that include: endocytosis of podocalyxin and the crumbs complex from the periphery, and Rab11a-dependent transport to, and exocytosis at, the AMIS. Rab11a recruits a Fip5–Snx18 complex to promote vesicle formation from the apical recycling endosome (ARE). Rab11a also activates Rab8a/b by the GTP exchange factor (GEF) Rabin8 (opposed by the Rab8 GTPase activating protein (GAP) Tbc1d30). Rab8a and 11a recruit Myo5b and the Sec15a exocyst subunit to transport vesicles to the AMIS, and interact with Sec10 at the AMIS to promote vesicle docking. A Rab27a/b–Slp2-a complex clusters these vesicles at the AMIS. Slp4-a, with Rab3b/Rab8a/Rab27a/b and the t-SNARE syntaxin-3 (Stx3), promotes vesicle fusion at the AMIS. Exocytosis also functions in initial recruitment of the Par3–aPKC complex to the AMIS: Rab8 stimulates Cdc42 loading onto exocytic carriers, possibly through the Cdc42 GEF Tuba. The Cdc42–Par6–aPKC–Par3 complex thus forms at the AMIS, in a step that may co-scaffold the exocyst complex at the AMIS. (c) The AMIS gives rise to the pre-apical patch, where the cell’s apical and basolateral surfaces are distinct, but the apical surfaces are still closely apposed between the two (or more) neighbouring cells. (d) Opening of the lumen results from ion and water transport, and further polarization of the membranes, junctional complexes, cytoskeleton and organelles gives rise to the mature cyst. Figure is modified with permission from ref. 139.
AMIS formation is a transcytotic pathway that is initiated by endocytosis of apical proteins such as podocalyxin and the Crb3a complex from the extracellular-matrix-contacting surface, accumulation of these proteins in Rab11-positive vesicles, and finally their Rab11a-dependent exocytosis (Fig. 4b)56. Although it is known that this delivery system ensures that the polarity complexes are present at the site of lumen formation and at the correct time, the sorting machinery involved in these early events is only recently becoming clear. Rab11a initiates a cascade of GTPase action, recruiting the GEF Rabin8α, which in turn activates Rab8a/b (a process opposed by the Rab8-specific GTPase-activating protein TBC1D30). Rab11a also recruits another effector, Rab11Fip5, which acts in concert with the aforementioned Snx18, presumably to facilitate formation of apically destined vesicles and/or tubules to be transported to the nascent lumen from the ARE120. Concomitantly, Rab11a recruits the Myo5b actin-based motor to the ARE, where it additionally interacts with Rab8a to facilitate transport of these vesicles to the AMIS95. Rab11a, and probably Rab8, further recruit the exocyst subunit Sec15a (also known as Exoc6)56,57, which facilitates exocytosis by promoting binding of the exocytic carrier to the Sec10 exocyst subunit localized to the emerging AMIS. A homologous complex to Rab11–Rabin8–Rab8–Myo5b–Sec15 in budding yeast controls exocytosis to newly forming daughter buds, suggesting that this is an ancient polarity-generating module121. Exocytosis is also important for the initial recruitment of the Par3–aPKC complex to the AMIS56. Active Rab8 stimulates Cdc42 interaction with the exocytic carriers, which is probably the result of Rab8-dependent recruitment of the Cdc42 GEF Tuba. The AMIS is formed at a previously basolateral cell–cell contact, through conversion of the basolateral PtdIns(3,4,5)P3 to PtdIns(4,5)P2 via the lipid phosphatase PTEN3. PtdIns(4,5)P2, newly enriched at this site, binds annexin 2, which in turn scaffolds activated Cdc42 at the AMIS3. Finally, in a step that probably requires the Cdc42–Par6–aPKC complex122, AMIS formation probably reinforces exocytosis by colocalizing a Sec10–Sec8–Par3 complex to the emerging lumen56.
Studies in the C. elegans intestine also demonstrate a crucial function for Rab11 in the promotion of apical polarization123,124. Somewhat surprisingly, depletion of the clathrin heavy chain and subunits of the AP1 complex affect both apical and basal protein localization, and there is formation of ectopic lateral lumens between cell contacts. AP1 depletion causes loss of apical Rab11 vesicles and consequently — similar to MDCK cysts56 — the loss of apical enrichment of Cdc42 and its effector Par6. Similar to mammals, C. elegans possess two AP1 median subunits. Although from sequence alignment these cannot be discriminated between Ap1m1 or Ap1m2, a single subunit, apm-1, causes this phenotype, suggesting it may functionally correspond to AP1A. These data emphasize an unanticipated function for AP1 in apical sorting processes at the Rab11 endosome, controlling apical transport and Cdc42–Par6 polarity complex localization.
A fundamental question related to tissue development is how cargo is directed to the nascent domain(s) during domain biogenesis; that is, when that domain has not yet been established. Recent analysis shows the involvement of a novel Rab3b–Rab27a/b-dependent pathway, co-reliant on the synaptotagmin-like proteins Slp2-a and 4-a87. Slp2-a binds to PtdIns(4,5)P2 at the forming lumen, and clusters apically destined vesicles to the AMIS in a Rab27a/b-dependent fashion (Fig. 4b). Concomitantly, Slp4-a bridges Rab27–Rab3b–Rab8 and the apical SNARE syntaxin 3 to promote vesicle tethering and fusion exclusively at the AMIS, to create a singular apical surface and lumen. Thus, a network, and in some instances a cascade, of Rab proteins control the specificity of vesicle transport, targeting, docking and fusion with the cell surface to ensure de novo membrane biogenesis, and thus cortical asymmetry, in epithelial cells.
The biogenesis of the basolateral surface is probably a dance between the activity of polarity complexes and membrane-trafficking events. However, this interplay is not well understood. In mammalian cells, a widely used model to explore the mesenchymal-to-epithelial transition is to examine the events that occur when migratory cells coalesce to form an epithelium after cell touching. This cell–cell interaction is mediated by E-cadherin, as well as nectins and the junctional adhesion membrane protein125. The latter two interact with, and may position, the Par complex at the site of touching and may later promote the recruitment of the Crumbs and Scribble complex at the forming junction126. E-cadherin interacts with the exocyst at the site of cell–cell contact127, and it is tempting to speculate that this may, by analogy with the AMIS, create a ‘basolateral membrane initiation site’ to reinforce delivery of basolateral proteins to the nascent cell–cell contact. Although this process is assumed to depend on the cytoskeleton128, Rab11a129 and the t-SNARE syntaxin-4–exocyst complex127,130, further work is needed to understand how the basolateral membrane domain is generated in vertebrate cells125.
Final words
Much of our understanding of membrane-trafficking pathways, machineries and sorting signals comes from non- or poorly polarized cells. Such studies have been instrumental in the identification of the types of machineries and compartments involved and the definition of the principles of membrane transport. However, not all cells are created equal: a fact often overlooked is that different cell types in vivo possess differentially expressed cargoes, according to physiological need, and they must reorganize trafficking pathways to facilitate such needs. Furthermore, epithelial morphogenesis often includes the formation of specialized apical membrane domains, such as cilia and microvilli, a process dependent on polarity proteins and membrane traffic131,132. A continuing challenge for both the fields of epithelial polarity and membrane traffic therefore is to identify how the sorting and transport machineries described earlier are coordinated to generate epithelial-cell form and function. Three-dimensional models, studies in C. elegans and in vivo RNA interference studies in mice in particular have begun to illuminate such pathways4,18,87,95,113,123,124. Moreover, although we know the existence of multiple regulatory machineries, such as Rab GTPases and polarity complexes that can control membrane transport, a further challenge is to understand how these operate as a network to generate polarized, vectorial membrane transport — a process fundamental for the formation and maintenance of epithelial asymmetry.
Acknowledgments
This work was supported by National Institutes of Health grants R37DK54425 and RO1DK077777 (to G.A.) and K99CA163535 (to D.M.B.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Gerard Apodaca, Email: gla6@pitt.edu, Departments of Medicine and Cell Biology, University of Pittsburgh, Pittsburgh 15261, Pennsylvania, USA.
Luciana I. Gallo, Departments of Medicine and Cell Biology, University of Pittsburgh, Pittsburgh 15261, Pennsylvania, USA
David M. Bryant, Department of Anatomy, University of California, San Francisco 94158, California, USA
References
- 1.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X, Surma MA, Simons K. Polarized sorting and trafficking in epithelial cells. Cell Res. 2012;22:793–805. doi: 10.1038/cr.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Belmonte F, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gassama-Diagne A, et al. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol. 2006;8:963–970. doi: 10.1038/ncb1461. [DOI] [PubMed] [Google Scholar]
- 5.Jacob R, Naim HY. Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol. 2001;11:1444–1450. doi: 10.1016/s0960-9822(01)00446-8. [DOI] [PubMed] [Google Scholar]
- 6.Kreitzer G, et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- 7.Farr GA, Hull M, Mellman I, Caplan MJ. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol. 2009;186:269–282. doi: 10.1083/jcb.200901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tveit H, Akslen LK, Fagereng GL, Tranulis MA, Prydz K. A secretory Golgi bypass route to the apical surface domain of epithelial MDCK cells. Traffic. 2009;10:1685–1695. doi: 10.1111/j.1600-0854.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- 10.Ang AL, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Bio. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresawn KO, et al. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cramm-Behrens CI, Dienst M, Jacob R. Apical cargo traverses endosomal compartments on the passage to the cell surface. Traffic. 2008;9:2206–2220. doi: 10.1111/j.1600-0854.2008.00829.x. [DOI] [PubMed] [Google Scholar]
- 13.Gravotta D, et al. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 15.Khandelwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J. 2010;29:1961–1975. doi: 10.1038/emboj.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parton RG, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucci C, et al. Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc Natl Acad Sci USA. 1994;91:5061–5065. doi: 10.1073/pnas.91.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeigerer A, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465–470. doi: 10.1038/nature11133. [DOI] [PubMed] [Google Scholar]
- 19.Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang E, et al. Apical and basolateral pathways of MDCK cells meet in acidic common endosomes distinct from a nearly-neutral apical recycling endosome. Traffic. 2000;1:480–493. doi: 10.1034/j.1600-0854.2000.010606.x. [DOI] [PubMed] [Google Scholar]
- 21.Babbey CM, et al. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell. 2008;19:2059–2068. doi: 10.1091/mbc.E07-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung SM, Ruiz WG, Apodaca G. Sorting of membrane and fluid at the apical pole of polarized MDCK cells. Mol Biol Cell. 2000;11:2131–2150. doi: 10.1091/mbc.11.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzaban S, et al. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson A, et al. Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains. Mol Biol Cell. 2007;18:2687–2697. doi: 10.1091/mbc.E05-09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerdeva GV, et al. Comparison of FcRn- and pIgR-mediated transport in MDCK cells by fluorescence confocal microscopy. Traffic. 2010;11:1205–1220. doi: 10.1111/j.1600-0854.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ait Slimane T, Hoekstra D. Sphingolipid trafficking and protein sorting in epithelial cells. FEBS Lett. 2002;529:54–59. doi: 10.1016/s0014-5793(02)03183-6. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez A, Rodriguez-Boulan E. Clathrin and AP1B: key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 2009;583:3784–3795. doi: 10.1016/j.febslet.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deborde S, et al. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fölsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 31.Simmen T, Höning S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nature Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura N, Plutner H, Hahn K, Balch WE. The delta subunit of AP-3 is required for efficient transport of VSV-G from the trans-Golgi network to the cell surface. Proc Natl Acad Sci USA. 2002;99:6755–6760. doi: 10.1073/pnas.092150699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno H, et al. μ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- 34.Gravotta D, et al. The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell. 2012;22:811–823. doi: 10.1016/j.devcel.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi D, et al. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology. 2011;141:621–632. doi: 10.1053/j.gastro.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 36.Schreiner R, et al. The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int. 2010;78:382–388. doi: 10.1038/ki.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fölsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugimoto H, et al. Differential recognition of tyrosine-based basolateral signals by AP-1B subunit μ1B in polarized epithelial cells. Mol Biol Cell. 2002;13:2374–2382. doi: 10.1091/mbc.E01-10-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly BT, et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvajal-Gonzalez JM, et al. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci USA. 2012;109:3820–3825. doi: 10.1073/pnas.1117949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong X, et al. An association between type Iγ PI4P 5-kinase and Exo70 directs E-cadherin clustering and epithelial polarization. Mol Biol Cell. 2012;23:87–98. doi: 10.1091/mbc.E11-05-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Ling K, Wagoner MP, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase is required for EGF-stimulated directional cell migration. J Cell Biol. 2007;178:297–308. doi: 10.1083/jcb.200701078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oztan A, et al. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell. 2007;18:3978–3992. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grindstaff KK, et al. Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 45.Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miranda KC, et al. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 2001;276:22565–22572. doi: 10.1074/jbc.M101907200. [DOI] [PubMed] [Google Scholar]
- 47.Kang RS, Folsch H. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J Cell Biol. 2011;193:51–60. doi: 10.1083/jcb.201012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishra SK, Watkins SC, Traub LM. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with the clathrin-coat machinery. Proc Natl Acad Sci USA. 2002;99:16099–16104. doi: 10.1073/pnas.252630799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ang AL, Folsch H, Koivisto UM, Pypaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol. 2003;163:339–350. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fields IC, King SM, Shteyn E, Kang RS, Folsch H. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol Biol Cell. 2010;21:95–105. doi: 10.1091/mbc.E09-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shteyn E, Pigati L, Folsch H. Arf6 regulates AP-1B-dependent sorting in polarized epithelial cells. J Cell Biol. 2011;194:873–887. doi: 10.1083/jcb.201106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ang AL, Folsch H, Koivisto UM, Pypaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol. 2003;163:339–350. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F. Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J Cell Biol. 2007;177:103–114. doi: 10.1083/jcb.200608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fields IC, et al. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J Cell Biol. 2007;177:477–488. doi: 10.1083/jcb.200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato T, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366–369. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- 56.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng S, et al. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem. 2012;287:15602–15609. doi: 10.1074/jbc.M111.333245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knodler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Omori Y, et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 61.Deora AA, et al. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol Biol Cell. 2004;15:4148–4165. doi: 10.1091/mbc.E04-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C, et al. Naked2 acts as a cargo recognition and targeting protein to ensure proper delivery and fusion of TGF-α containing exocytic vesicles at the lower lateral membrane of polarized MDCK cells. Mol Biol Cell. 2007;18:3081–3093. doi: 10.1091/mbc.E07-02-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kizhatil K, et al. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem. 2007;282:26552–26561. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 64.Sorrosal G, Perez L, Herranz H, Milan M. Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo. EMBO Rep. 2010;11:373–379. doi: 10.1038/embor.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Denef N, Chen Y, Weeks SD, Barcelo G, Schupbach T. Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev Cell. 2008;14:354–364. doi: 10.1016/j.devcel.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez-Boulan E, Gonzalez A. Glycans in post-Golgi apical targeting: sorting signals or structural props? Trends Cell Biol. 1999;9:291–294. doi: 10.1016/s0962-8924(99)01595-0. [DOI] [PubMed] [Google Scholar]
- 69.Boscher C, Dennis JW, Nabi IR. Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol. 2011;23:383–392. doi: 10.1016/j.ceb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Delacour D, et al. Requirement for galectin-3 in apical protein sorting. Curr Biol. 2006;16:408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 71.Delacour D, et al. Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo. J Cell Sci. 2008;121:458–465. doi: 10.1242/jcs.020800. [DOI] [PubMed] [Google Scholar]
- 72.Mattila PE, et al. Multiple biosynthetic trafficking routes for apically secreted proteins in MDCK cells. Traffic. 2012;13:433–442. doi: 10.1111/j.1600-0854.2011.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delacour D, et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stechly L, et al. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic. 2009;10:438–450. doi: 10.1111/j.1600-0854.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 75.Mishra R, Grzybek M, Niki T, Hirashima M, Simons K. Galectin-9 trafficking regulates apical-basal polarity in Madin–Darby canine kidney epithelial cells. Proc Natl Acad Sci USA. 2010;107:17633–17638. doi: 10.1073/pnas.1012424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delacour D, et al. Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic. 2007;8:379–388. doi: 10.1111/j.1600-0854.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- 77.Astanina K, Delebinski CI, Delacour D, Jacob R. Annexin XIIIb guides raft-dependent and -independent apical traffic in MDCK cells. Eur J Cell Biol. 2010;89:799–806. doi: 10.1016/j.ejcb.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 78.Jacob R, et al. Annexin II is required for apical transport in polarized epithelial cells. J Biol Chem. 2004;279:3680–3684. doi: 10.1074/jbc.C300503200. [DOI] [PubMed] [Google Scholar]
- 79.Magal LG, et al. Clustering and lateral concentration of raft lipids by the MAL protein. Mol Biol Cell. 2009;20:3751–3762. doi: 10.1091/mbc.E09-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou G, et al. MAL facilitates the incorporation of exocytic uroplakin-delivering vesicles into the apical membrane of urothelial umbrella cells. Mol Biol Cell. 2012;23:1354–1366. doi: 10.1091/mbc.E11-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Marco MC, et al. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J Cell Biol. 2002;159:37–44. doi: 10.1083/jcb.200206033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 83.Klemm RW, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang H, et al. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Biol. 2011;13:1189–1201. doi: 10.1038/ncb2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nokes RL, Fields IC, Collins RN, Folsch H. Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells. J Cell Biol. 2008;182:845–853. doi: 10.1083/jcb.200802176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galvez-Santisteban M, et al. Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells. Nat Cell Biol. 2012;14:838–849. doi: 10.1038/ncb2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunziker W, Peters PJ. Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. 1998;273:15734–15741. doi: 10.1074/jbc.273.25.15734. [DOI] [PubMed] [Google Scholar]
- 89.Jaulin F, Kreitzer G. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol. 2010;190:443–460. doi: 10.1083/jcb.201006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jaulin F, Xue X, Rodriguez-Boulan E, Kreitzer G. Polarization-dependent selective transport to the apical membrane by KIF5B in MDCK cells. Dev Cell. 2007;13:511–522. doi: 10.1016/j.devcel.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noda Y, et al. KIFC3, a microtubule minus end-directed motor for the apical transport of annexin XIIIb-associated Triton-insoluble membranes. J Cell Biol. 2001;155:77–88. doi: 10.1083/jcb.200108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeh TY, Peretti D, Chuang JZ, Rodriguez-Boulan E, Sung CH. Regulatory dissociation of Tctex-1 light chain from dynein complex is essential for the apical delivery of rhodopsin. Traffic. 2006;7:1495–1502. doi: 10.1111/j.1600-0854.2006.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic. 2007;8:998–1006. doi: 10.1111/j.1600-0854.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 94.Eichler TW, Kogel T, Bukoreshtliev NV, Gerdes HH. The role of myosin Va in secretory granule trafficking and exocytosis. Biochem Soc Trans. 2006;34:671–674. doi: 10.1042/BST0340671. [DOI] [PubMed] [Google Scholar]
- 95.Roland JT, et al. Rab GTPase–Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci USA. 2011;108:2789–2794. doi: 10.1073/pnas.1010754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bond LM, Brandstaetter H, Sellers JR, Kendrick-Jones J, Buss F. Myosin motor proteins are involved in the final stages of the secretory pathways. Biochem Soc Trans. 2011;39:1115–1119. doi: 10.1042/BST0391115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Born M, Pahner I, Ahnert-Hilger G, Jons T. The maintenance of the permeability barrier of bladder facet cells requires a continuous fusion of discoid vesicles with the apical plasma membrane. Eur J Cell Biol. 2003;82:343–350. doi: 10.1078/0171-9335-00326. [DOI] [PubMed] [Google Scholar]
- 98.Nielsen S, et al. Expression of VAMP-2-like protein in kidney collecting duct intracellular vesicles. Colocalization with Aquaporin-2 water channels. J Clin Invest. 1995;96:1834–1844. doi: 10.1172/JCI118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Procino G, et al. AQP2 exocytosis in the renal collecting duct — involvement of SNARE isoforms and the regulatory role of Munc18b. J Cell Sci. 2008;121:2097–2106. doi: 10.1242/jcs.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karvar S, Yao X, Crothers JM, Jr, Liu Y, Forte JG. Localization and function of soluble N-ethylmaleimide-sensitive factor attachment protein-25 and vesicle-associated membrane protein-2 in functioning gastric parietal cells. J Biol Chem. 2002;277:50030–50035. doi: 10.1074/jbc.M207694200. [DOI] [PubMed] [Google Scholar]
- 101.Low SH, et al. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reales E, Sharma N, Low SH, Folsch H, Weimbs T. Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity. PLoS ONE. 2011;6:e21181. doi: 10.1371/journal.pone.0021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma N, Low SH, Misra S, Pallavi B, Weimbs T. Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J Cell Biol. 2006;173:937–948. doi: 10.1083/jcb.200603132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schluter MA, Margolis B. Apicobasal polarity in the kidney. Exp Cell Res. 2012;318:1033–1039. doi: 10.1016/j.yexcr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol. 2012;28:655–685. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- 107.Cohen D, Rodriguez-Boulan E, Musch A. Par-1 promotes a hepatic mode of apical protein trafficking in MDCK cells. Proc Natl Acad Sci USA. 2004;101:13792–13797. doi: 10.1073/pnas.0403684101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nechiporuk T, Fernandez TE, Vasioukhin V. Failure of epithelial tube maintenance causes hydrocephalus and renal cysts in Dlg5−/− mice. Dev Cell. 2007;13:338–350. doi: 10.1016/j.devcel.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laprise P, et al. Yurt, Coracle, Neurexin IV and the Na+, K+-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- 110.Plant PJ, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- 111.Laprise P, Tepass U. Novel insights into epithelial polarity proteins in Drosophila. Trends Cell Biol. 2011;21:401–408. doi: 10.1016/j.tcb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 112.Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winter JF, et al. Caenorhabditis elegans screen reveals role of PAR-5 in RAB-11-recycling endosome positioning and apicobasal cell polarity. Nat Cell Biol. 2012;14:666–676. doi: 10.1038/ncb2508. [DOI] [PubMed] [Google Scholar]
- 114.Schluter MA, et al. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–4663. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferrari A, Veligodskiy A, Berge U, Lucas MS, Kroschewski R. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J Cell Sci. 2008;121:3649–3663. doi: 10.1242/jcs.018648. [DOI] [PubMed] [Google Scholar]
- 117.Xu K, et al. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell. 2011;20:526–539. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herwig L, et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21:1942–1948. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 119.Schluter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol. 2009;20:1444–1452. doi: 10.1681/ASN.2008090949. [DOI] [PubMed] [Google Scholar]
- 120.Willenborg C, et al. Interaction between FIP5 and SNX18 regulates epithelial lumen formation. J Cell Biol. 2011;195:71–86. doi: 10.1083/jcb.201011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin Y, et al. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell. 2011;21:1156–1170. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Horikoshi Y, et al. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci. 2009;122:1595–1606. doi: 10.1242/jcs.043174. [DOI] [PubMed] [Google Scholar]
- 123.Zhang H, et al. Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development. 2012;139:2071–2083. doi: 10.1242/dev.077347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shafaq-Zadah M, Brocard L, Solari F, Michaux G. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development. 2012;139:2061–2070. doi: 10.1242/dev.076711. [DOI] [PubMed] [Google Scholar]
- 125.Nelson WJ. Remodeling epithelial cell organization: transitions between front–rear and apical–basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Q, Chen XW, Margolis B. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol Biol Cell. 2007;18:874–885. doi: 10.1091/mbc.E06-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007;178:323–335. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaw RM, et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J Cell Biol. 2012;197:697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.ten Klooster JP, et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell. 2009;16:551–562. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 133.Sato T, et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 134.O’Brien LE, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 135.O’Brien LE, et al. Morphological and biochemical analysis of Rac1 in three-dimensional epithelial cell cultures. Methods Enzymol. 2006;406:676–691. doi: 10.1016/S0076-6879(06)06053-8. [DOI] [PubMed] [Google Scholar]
- 136.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 137.Zegers MM, O’Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 138.Yu W, et al. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell. 2003;14:748–763. doi: 10.1091/mbc.E02-06-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Apodaca G. Opening ahead: early steps in lumen formation revealed. Nat Cell Biol. 2010;12:1026–1028. doi: 10.1038/ncb1110-1026. [DOI] [PubMed] [Google Scholar]