This review revealed a lack of statistical difference for staple line leak with or without staple line reinforcement in laparoscopic sleeve gastrectomy.
Keywords: Sleeve gastrectomy, Staple line, Reinforcement
Abstract
Background and Objectives:
Laparoscopic sleeve gastrectomy is gaining popularity as a bariatric procedure, with outcomes similar to gastric band and gastric bypass. Staple-line disruption is a significant source of morbidity and death. We aim to evaluate the effect of staple-line reinforcement on the gastric leak rate, morbidity, and mortality rate.
Methods:
A systematic review was performed using title key words “sleeve gastrectomy,” and articles were reviewed for description of operative technique and postoperative outcomes including staple-line leak. Rates of leak, bleeding, surgical-site infection, reintervention, readmission, and mortality were analyzed. We calculated pooled event rates and 95% confidence intervals using fixed-effects modeling to determine differences between the reinforcement group (group A) and non-reinforcement group (group B).
Results:
We identified 390 articles, and 30 met the inclusion criteria. Group A had 3293 patients, and group B had 1588 patients. After heterogeneity calculations, 9 variables met the criteria to be analyzed. The leak rate was 3.9% (95% confidence interval, 2.9%–5.5%) in group A and 3.2% (95% confidence interval, 2.8%–4.1%) in group B. The mortality rate was 0.8% (95% confidence interval, 0.4%–1.5%) in group A and 0.7% (95% confidence interval, 0.4%–1.1%) in group B. Our results also showed no statistical difference for any of our other 7 outcome variables.
Conclusion:
Our study shows a lack of statistical difference in leak rate, overall morbidity, or mortality rate in laparoscopic sleeve gastrectomy with or without staple-line reinforcement. Because of study limitations, we propose that prospective trials are needed to determine the effect of staple-line reinforcement on leak rates.
INTRODUCTION
Laparoscopic sleeve gastrectomy (LSG) has gained increasing acceptance among bariatric surgeons and patients because of encouraging excess weight loss and resolution of comorbidities. Initially established as the first stage of a 2-stage bariatric approach, it is now used as a primary bariatric procedure because of documented excellent weight loss and an acceptable risk of complication. Advantages include the avoidance of implantable material, maintenance of gastrointestinal continuity, avoidance of malabsorption, and convertibility to other operations.
The major disadvantage of LSG is the severity of the major postoperative complications of bleeding and staple-line leakage. Staple-line disruption is the most life-threatening complication after LSG, with a mean incidence of 2.7% from 24 studies with 1749 patients.1 Leaks after sleeve gastrectomy (SG) commonly occur at the proximal aspect of the staple line immediately below the gastroesophageal junction because of the creation of a high internal pressure.
Staple-line reinforcement has been advocated by many surgeons but not well studied through prospective or retrospective methodology. Moreover, surgeon practice with respect to staple-line reinforcement varies widely. Options for staple-line reinforcement include non-reinforcement, oversewing, and use of buttressing within the stapler load.
We performed a systematic review of retrospective studies (and one prospective study) to analyze the effect of staple-line reinforcement on leak rate, mortality rate, and overall morbidity of SG.
METHODS
A thorough literature search of the Medline online database was combined with reference checks of articles involving LSG for the past 15 years. Search phrases used were made of combinations of the following key words: “sleeve,” “gastric,” and/or “gastrectomy.” Secondary inclusion criteria used included reporting of (1) a description of the operative technique including whether reinforcement was applied, (2) the type of reinforcement applied, and (3) outcomes including staple-line leak and bleeding.
The selected articles were then thoroughly evaluated for the presence or absence of staple-line reinforcement within the operative technique and associated outcomes, including staple-line leak rate, bleeding, surgical-site infection, operative reintervention, readmission, conversion to laparotomy, abdominal fluid collection, postoperative venous thromboembolic complications, 30-day mortality rate, inpatient length of stay, and excess weight loss. Most included publications were retrospective chart reviews. Articles that included most or all of the desired outcomes reported were included for further analysis and possible inclusion in the study.
The statistical methods used were as follows. We compiled data from all eligible studies and grouped data into 2 groups: those studies or patients where reinforcement was used and those studies or patients where reinforcement was not used. All studies were retrospective, 1-group studies (with the exception of Casella2 et al., Sanchez-Santos3 et al., Ser4 et al., and Dapri5 et al.) that provided details of the outcomes of patients undergoing SG. The aforementioned studies had groups of patients with and without staple-line reinforcement. For the purposes of data analysis, we separated the information for these separate groups and analyzed their data as part of their respective group. Statistical analysis followed recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green)6. To determine heterogeneity between studies, we calculated χ2 values (Q) as well as an inconsistency statistic (I2). Taken together, we used Q and I2 to make judgments about heterogeneity of specific outcome variables and the usefulness of further data analysis. Finally, we used pooled fixed-effects models to determine differences in effect size.
According to Higgins and Green, “A low P value (or a large chi-squared statistic relative to its degree of freedom) provides evidence of heterogeneity of intervention effects (variation in effect estimates beyond chance).” We used a P value less than .10 or a large χ2 value (Q) relative to degrees of freedom to determine evidence of statistical significance against the null hypothesis that the studies are not heterogeneous. In addition, we evaluated the I2 statistic as recommended by Higgins and Green, where 0% to 40% indicates that the inconsistency might not be important; 30% to 60% indicates that the inconsistency may represent moderate heterogeneity, 50% to 90% indicates that the inconsistency may represent substantial heterogeneity, and inconsistency values of 75% to 100% indicate considerable heterogeneity. However, Higgins and Green suggest that “the importance of the observed value of I2 depends on (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity (e.g., P value from the chi-squared test, or a confidence interval for I2).”
After determining heterogeneity of our variables, we calculated pooled event rates and 95% confidence intervals (CIs) using fixed-effects modeling to determine significant differences in rates between our two treatment groups.
RESULTS
Search Results and Demographics
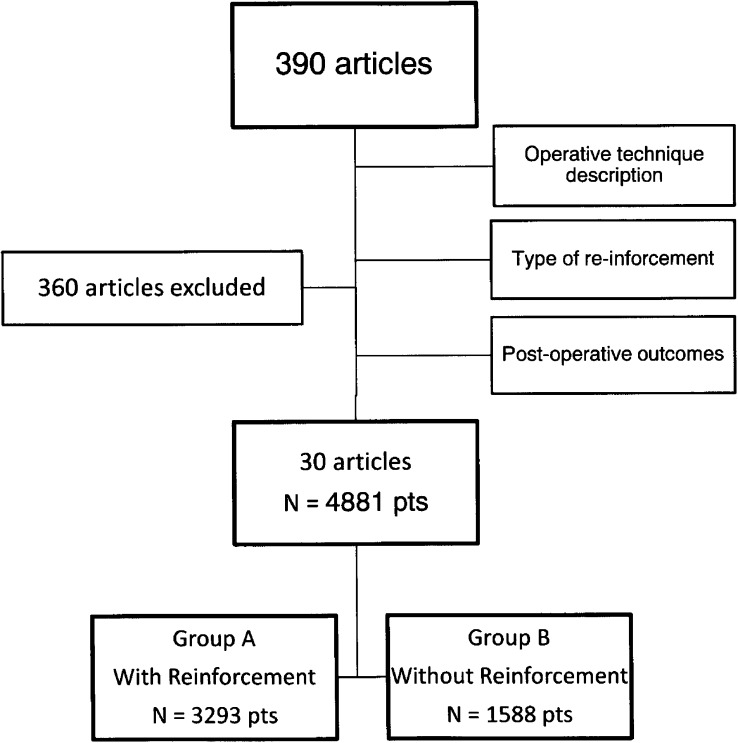
The initial key word search returned 390 articles. After review with inclusion and exclusion criteria (Figure 1), 30 articles were identified for the actual systematic review (Figure 1). Group A (reinforcement) included 3293 patients and group B (non-reinforcement) included 1588 patients, for a total of 4881 patients. There were 25 single-cohort studies (15 reinforcement only and 10 non-reinforcement only) and 5 multiple-cohort studies. In terms of study design, there were 24 retrospective reviews, 5 prospective cohort studies, and 1 randomized controlled trial (Table 1). The overall female-to-male ratio was 3:1. The body mass index ranged from 30 to 85 kg/m2 for group A and 32 to 103 kg/m2 for group B. The ranges for percent of estimated weight loss after 12 months' follow-up were 30.6% to 81.1% for group A and 47.2% to 81.1% in group B.
Figure 1.
Flowchart of study selection methodology.
Table 1.
Characteristics of Included Studies
| Author | Year | Study Type | Group Aa | Group Ba | Group A (Reinforcement) |
Group B (Non-Reinforcement) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leak, % | Bleed, % | Mortality, % | Leak, % | Bleed, % | Mortality, % | ||||||
| No reinforcement | |||||||||||
| 1 | Casella et al.b2 | 2009 | Retrospective | 100 | 2.0 | 0.0 | 0.0 | ||||
| 2 | Sanchez-Santos et al.b3 | 2009 | Retrospective | 159 | 5.3 | 1.0 | 0.3 | ||||
| 3 | Sammour et al6 | 2010 | Retrospective | 100 | 3.0 | 2.0 | 0.0 | ||||
| 4 | Magee et al.7 | 2011 | Retrospective | 68 | 0.0 | 0.0 | 0.0 | ||||
| 5 | Bellanger et al.8 | 2011 | Retrospective | 529 | 0.0 | 0.4 | 0.2 | ||||
| 6 | Sabbagh et al.9 | 2010 | Prospective | 59 | 1.7 | 0.0 | 0.0 | ||||
| 7 | Nienhuijs et al.10 | 2010 | Retrospective | 74 | 5.4 | 1.3 | 0.0 | ||||
| 8 | Armstrong et al.11 | 2010 | Retrospective | 185 | 0.0 | 1.1 | 0.0 | ||||
| 9 | Ser et al.b4 | 2010 | Retrospective | 40 | 1.0 | 0.0 | 0.0 | ||||
| 10 | Triantafyllidis et al.12 | 2011 | Retrospective | 85 | 3.5 | 3.5 | 0.0 | ||||
| 11 | Dapri et al.b5 | 2010 | RCTc | 25 | 4.0 | 0.0 | 0.0 | ||||
| 12 | Rice et al.13 | 2010 | Retrospective | 115 | 3.5 | 0.0 | 0.9 | ||||
| 13 | Kiriakopoulos et al.14 | 2009 | Prospective | 15 | 6.7 | 6.7 | 0.0 | ||||
| 14 | Behrens et al.15 | 2011 | Retrospective | 34 | 2.9 | 2.9 | 0.0 | ||||
| Reinforcement | |||||||||||
| 1 | Chowbey et al.16 | 2010 | Retrospective | 75 | 0.0 | 0.0 | 1.3 | ||||
| 2 | Burgos et al.17 | 2009 | Prospective | 214 | 3.3 | 0.0 | 0.0 | ||||
| 3 | Leyba et al.18 | 2011 | Prospective | 42 | 0.0 | 2.4 | 0.0 | ||||
| 4 | Daskalakis et al.19 | 2011 | Retrospective | 144 | 2.1 | 2.8 | 0.0 | ||||
| 5 | Menenakos et al.20 | 2010 | Retrospective | 261 | 3.8 | 1.9 | 0.3 | ||||
| 6 | Albanopoulos et al.21 | 2010 | Retrospective | 353 | 3.4 | 2.5 | 0.8 | ||||
| 7 | Alley et al.22 | 2010 | Retrospective | 85 | 0.0 | 2.3 | 0.0 | ||||
| 8 | Gluck et al.23 | 2010 | Retrospective | 204 | 0.0 | 1.0 | 0.0 | ||||
| 9 | Ayloo et al.24 | 2011 | Retrospective | 30 | 0.0 | 0.0 | 0.0 | ||||
| 10 | Casella et al.b2 | 2009 | Retrospective | 100 | 4.0 | 0.0 | 0.0 | ||||
| 11 | Arias et al.25 | 2009 | Retrospective | 130 | 0.8 | 0.0 | 0.0 | ||||
| 12 | Diamanitus et al.26 | 2010 | Retrospective | 25 | 0.0 | 0.0 | 0.0 | ||||
| 13 | Jacobs et al.27 | 2010 | Retrospective | 157 | 1.3 | 0.0 | 0.6 | ||||
| 14 | Basso et al.d28 | 2011 | Retrospective | 100 | 4.0 | 8.0 | 1.0 | ||||
| 15 | Basso et al.d | 2011 | Retrospective | 200 | 2.5 | 2.5 | 0.5 | ||||
| 16 | Lakdawala et al.29 | 2010 | Retrospective | 50 | 2.0 | 0.0 | 0.0 | ||||
| 17 | Ser et al.b4 | 2010 | Retrospective | 78 | 0.0 | 2.5 | 0.0 | ||||
| 18 | Dapri et al.b,e5 | 2010 | RCT | 50 | 6.0 | 0.0 | 0.0 | ||||
| 19 | Dapri et al.b,e | 2010 | RCT | 50 | 6.0 | 0.0 | 0.0 | ||||
| 20 | Angrisani et al.30 | 2011 | Retrospective | 121 | 0.0 | 0.0 | 0.0 | ||||
| 21 | Sanchez-Santos et al.b3 | 2009 | Retrospective | 381 | 2.6 | 0.8 | 0.6 | ||||
| 22 | Csendes et al.31 | 2010 | Prospective | 343 | 4.7 | 2.3 | 0.0 | ||||
Group A comprises patients with reinforcement of the staple line, whereas group B comprises patients with non-reinforcement of the staple line.
Studies with reinforced and non-reinforced cohorts.
RCT = randomized controlled trial.
Basso et al. included 300 patients who all underwent staple-line reinforcement: 100 underwent Peri-Strip (Synovis Surgical Innovations, Deerfield, IL USA) reinforcement, whereas 200 underwent suture reinforcement.
Dapri et al. included 3 cohorts: 1 non-reinforced and 2 reinforced (suture and Seamguard [W. L. Gore and Associates, Flagstaff, AZ, USA]).
Heterogeneity Results
Our results indicate that the variables length of stay and excess weight loss are heterogeneous among studies. The P values in both cases were calculated to be <.001, whereas the χ2 values for length of stay and excess weight loss were 135 times and 100 times the degrees of freedom, respectively. In addition, the I2 value for each variable was 99%. Taken together, this is sufficient evidence to exclude these variables from further analysis. The variable reintervention has a Q of 61, with 32 df; a P value of <.0013; and an I2 value of 42%. The variable readmission has a Q of 84, with 34 df; a P value of <.001; and an I2 value of 59%. Although the significant χ2 value indicates heterogeneity, the ratios of Q to degrees of freedom are only 2 and 2.5 times for reintervention and readmission, respectively. In addition, the I2 value for each is 47% and 59%, respectively. Given the low Q–to–degrees of freedom ratio and because the inconsistency statistic indicates that there may be moderate heterogeneity, we decided to continue to analyze these variables further.
In all, we further evaluated the following variables: leak rate, bleed rate, infection rate, reintervention rate, readmission rate, conversion rate, abdominal fluid collection rate, thromboembolic event rate, and mortality rate. In each study the authors reported numbers of patients who had each of these situations. As such, we were able to determine event rates for each study for each variable, broken out by our 2 predetermined groups: reinforcement and no reinforcement. We used fixed-effects model analysis to determine pooled event rates and 95% CIs for each group.
Leak Rates
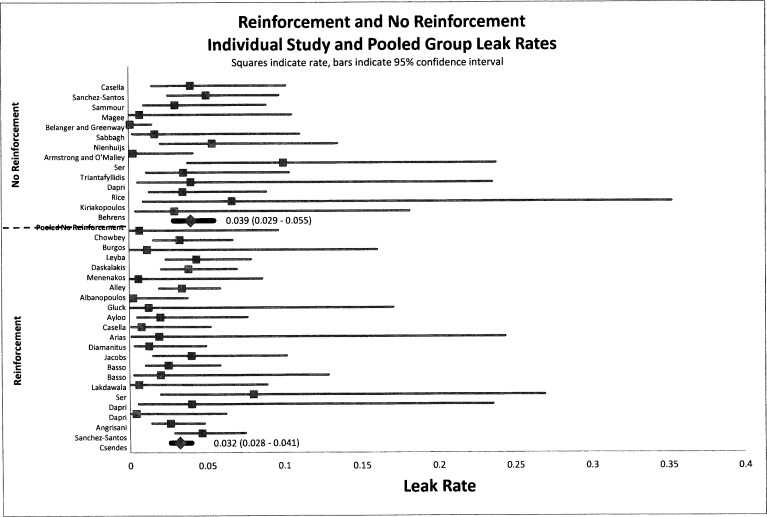
Leak rates were not affected by reinforcement. Figure 2 presents a forest plot for leak rate. Our results indicate that there is no statistical difference in the pooled event rate for leak rate. The statistical leak rates (Figure 2) for group A and group B were 3.2% (95% CI, 0.028–0.041) and 3.9% (95% CI, 0.029–0.055), respectively, indicating no difference. Both leak rates show agreement with currently published studies.
Figure 2.
Forest plot of pooled event rates of odds ratios for gastric leak rates of group A (reinforced) and group B (non-reinforced) for each article. The squares indicate the event rates, and the lines indicate the extent of the 95% CI. Summative pooled event rates are shown with 95% CIs in parentheses.
Mortality, Bleeding, and Reintervention Rates
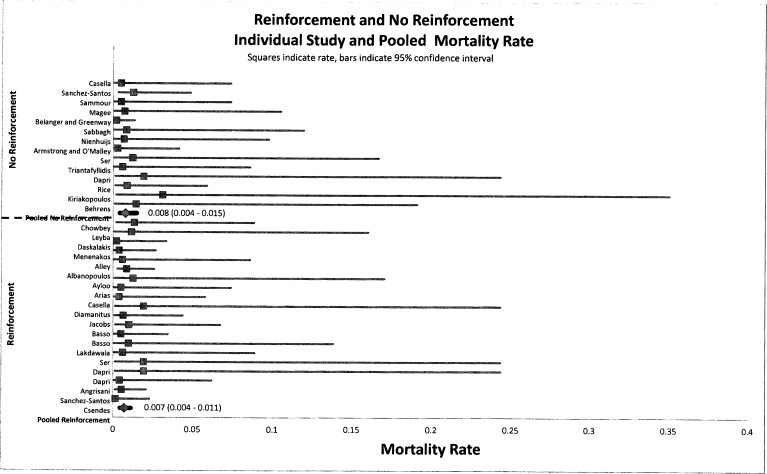
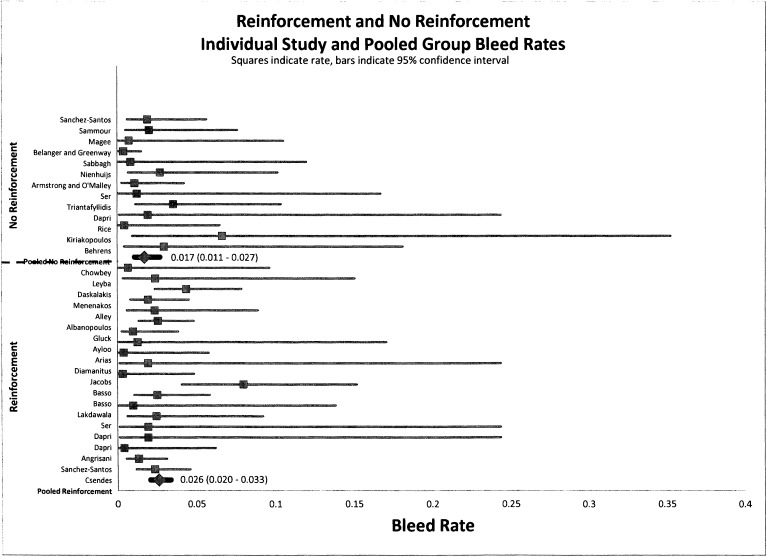
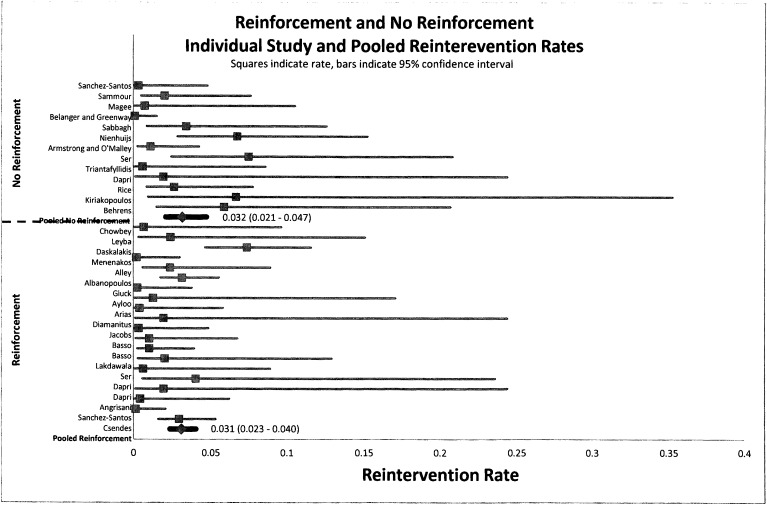
Mortality, bleeding, and reintervention rates were not affected by reinforcement. Figures 3, 4, and 5 indicate that there is no statistical difference in the pooled rate of mortality, bleeding, or reintervention. The statistical mortality rates (Figure 3) for the reinforcement group and non-reinforcement group were 0.7% (95% CI, 0.004–0.011) and 0.8% (95% CI, 0.004–0.015), respectively, indicating no difference. For bleeding and reintervention, the results were similar: 2.6% (95% CI, 0.020–0.033) and 1.7% (95% CI, 0.011–0.027), respectively, for bleeding and 3.1% (95% CI, 0.023–0.040) and 3.2% (95% CI, 0.021–0.047), respectively, for reintervention. Data for mortality, bleeding, and reintervention rates after LSG show agreement with currently published studies.
Figure 3.
Forest plot of pooled event rates of odds ratios for mortality rates of group A (reinforced) and group B (non-reinforced) for each article. The squares indicate the event rates, and the lines indicate the extent of the 95% CI. Summative pooled event rates are shown with 95% CIs in parentheses.
Figure 4.
Forest plot of pooled event rates of odds ratios for perioperative bleeding rates of group A (reinforced) and group B (non-reinforced) for each article. The squares indicate the event rates, and the lines indicate the extent of the 95% CI. Summative pooled event rates are shown with 95% CIs in parentheses.
Figure 5.
Forest plot of pooled event rates of odds ratios for reintervention rates of group A (reinforced) and group B (non-reinforced) for each article. The squares indicate the event rates, and the lines indicate the extent of the 95% CI. Summative pooled event rates are shown with 95% CIs in parentheses.
Similar results for infection, readmission, conversion to open surgery, abdominal fluid collection, and venous thromboembolism were found (data not shown). Summary results of the 9 outcome variables compared between groups A and B are shown in Table 2.
Table 2.
Summary of Comparison of Reinforced and Non-Reinforced Groups Across All 9 Measured Variables
| Variable | Reinforced, % (%)a | Non-Reinforced, % (%)a |
|---|---|---|
| Leak rateb | 3.9 (CI, 2.9–5.5) | 3.3 (CI, 2.8–4.1) |
| Bleed rateb | 1.7 (CI, 1.1–2.7) | 2.6 (CI, 2.0–3.3) |
| Infection rateb | 1.3 (CI, 0.7–2.3) | 1.5 (CI, 0.9–2.3) |
| Reintervention rateb | 3.2 (CI, 2.1–4.7) | 3.1 (CI, 2.3–4.0) |
| Readmission rateb | 3.2 (CI, 2.0–4.8) | 3.5 (CI, 2.7–4.7) |
| Conversion rateb | 1.4 (CI, 0.8–2.5) | 0.7 (CI, 0.4–1.2) |
| Abdominal collection rateb | 1.9 (CI, 1.1–3.2) | 1.7 (CI, 1.2–2.4) |
| Thromboembolic complication rateb | 0.7 (CI, 0.4–1.3) | 0.7 (CI, 0.4–1.1) |
| Mortality rateb | 0.8 (CI, 0.4–1.5) | 0.7 (CI, 0.4–1.1) |
Values represent overall percentage pooled rates (CI = 95% confidence interval).
No statistically significant difference in pooled rate.
DISCUSSION
Sleeve gastrectomy is gaining popularity whether as a primary, staged, or revision operation. In 2009 the American Society for Metabolic and Bariatric Surgery endorsed the SG for its potential value as a first-stage operation for high-risk patients.32 In June 2012 Medicare affirmed to reimburse and recognize SG as an appropriate weight loss procedure for patients who met National Institutes of Health guidelines for weight loss surgery candidacy.33,34 Despite LSG's success, staple-line leakage and bleeding after the procedure continue to be the most serious complications (1%–3% in large published series)8,13,20,28,35 and are the most frequent causes of death after bariatric surgery including LSG.19 Theoretically, staple-line reinforcement should increase its strength and help decrease the incidence of complications associated with staple lines. Though a relatively standardized operation, the reinforcement step in SG is quite often a matter of surgeon preference. We therefore report the largest systematic literature review investigating staple-line reinforcement, subsequent leak rate, and other outcomes in LSG.
The pathophysiology of staple-line leaks after LSG is unclear. Compromise of blood supply, especially at the angle of His near the crura, stapler device failure, poor technique, and postoperative gastroparesis with an intact pylorus causing increasing intragastric pressure have all been implicated.37 Although 3 of 5 bariatric surgeons surveyed at an international conference reported reinforcing the sleeve staple line, many still believe that the aforementioned pathophysiologic factors cannot be overcome with simple staple-line reinforcement.
Our analysis showed an overall leak rate and mortality rate comparable with most published large series. However, we found no statistical difference in 9 different outcome variables between the use of staple-line reinforcement and non-reinforcement. This is in contrast to Choi et al.,38 who reported through a meta-analysis of 8 studies that staple-line reinforcement had the advantages of decreased postoperative leak and overall complications. This may very well be because of differences in study design (systematic review vs meta-analysis) and, therefore, types of articles included in the analysis. Ser et al.,4 in their experience in 118 consecutive patients, found a statistically significant difference between their 2 groups, citing a 0% leak rate in their reinforced group versus 10% in the non-reinforced group, which is one of the greatest differences of any large cohort study performed to date.
Our overall initial article review yielded close to 400 articles, of which 2 were randomized controlled trials, one by Dapri et al.5 and one by Musella et al.39 Dapri et al. showed, through a prospective randomized trial, with 3 treatment arms (non-reinforced, suture reinforced, and stapler-load buttressing), a difference in intraoperative blood loss parameters, but no difference for leak rate, after staple-line reinforcement. They did not report any deaths in their study, and their overall leak rate was 4% to 6%, which is consistent with the overall leak rate of 3% to 4% in our systematic analysis. According to their study, the additional cost and time of staple-line reinforcement may be justified by reduced intraoperative bleeding complications.
In another randomized prospective study published later, Musella et al.39 showed no difference in the rate of leak or bleeding but did show a higher rate of stenosis with staple-line reinforcement. Their study included two arms: non-reinforced and suture reinforced. Compared with the prior randomized controlled trial, there were some minor differences in technique, such as the type of cartridge load and bougie size, and it is unclear whether these differences contributed to the differences in overall outcomes with respect to staple-line bleeding. As before, their overall staple-line leak rate of 2.5% to 5% agrees with the general literature on this topic, as well as with our systematic review results.
Our study possesses a number of limitations. First, our analysis was purely retrospective and based on pooled results of heterogeneously constructed studies. Not all of the studies consistently reported all outcomes that we wanted to analyze. To offset this inconsistency, we performed a detailed heterogeneity analysis to pick the most appropriate outcome variables as reported; however, this methodology is fraught with retrospective bias even in the best-case scenario. In addition, we were unable to stratify our results based on the type of reinforcement used (e.g., stapler-load reinforcement vs suture reinforcement). This was because many of the studies actually did not report the exact type of reinforcement used. We are therefore unable to make any conclusions about the superiority of one reinforcement method or another. Finally, because of the retrospective methodology used, we were not able to perform a multivariate risk factor analysis to determine which variables predispose patients to worse outcomes with and without reinforcement.
Given the previously mentioned limitations, we propose that rigorous, level I, prospective trials are needed to determine the true effect of staple-line reinforcement on leak rates and overall morbidity in LSG. Because of the rarity of the complication, as well as the number of different types of reinforcements available, a large number of patients will be required in each arm of the study for meaningful, clinically relevant results.
CONCLUSION
Our systematic review shows a lack of statistical difference for staple-line leak in LSG with or without staple-line reinforcement, as well as other major complications. Future prospective randomized trials are needed to determine the true effect of staple-line reinforcement on leak rates and overall morbidity in LSG.
Contributor Information
Jean Knapps, Department of Surgery, Central Michigan University College of Medicine, 1000 Houghton Avenue, 2nd Floor Saginaw, MI 48602, USA..
Maher Ghanem, Department of Surgery, Central Michigan University College of Medicine, 1000 Houghton Avenue, 2nd Floor Saginaw, MI 48602, USA..
John Clements, Department of Research and Quality, Central Michigan University College of Medicine, 1000 Houghton Avenue, 2nd Floor Saginaw, MI 48602, USA..
Aziz M. Merchant, Department of Surgery, Central Michigan University College of Medicine, 1000 Houghton Avenue, 2nd Floor Saginaw, MI 48602, USA..
References:
- 1. Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;4:469–475 [DOI] [PubMed] [Google Scholar]
- 2. Casella G, Soricelli E, Rizzello M, et al. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg 2009;19:821–826 [DOI] [PubMed] [Google Scholar]
- 3. Sánchez-Santos R, Masdevall C, Baltasar A, et al. Short- and mid-term outcomes of sleeve gastrectomy for morbid obesity: the experience of the Spanish National Registry. Obes Surg 2009;19:1203–1210 [DOI] [PubMed] [Google Scholar]
- 4. Ser KH, Lee WJ, Lee YC, Chen JC, Su YH, Chen SC. Experience in laparoscopic sleeve gastrectomy for morbidly obese Taiwanese: staple-line reinforcement is important for preventing leakage. Surg Endosc 2010;9:2253–2259 [DOI] [PubMed] [Google Scholar]
- 5. Dapri G, Cadiere GB, Himpens J. Reinforcing the staple line during laparoscopic sleeve gastrectomy: prospective randomized clinical study comparing three different techniques. Obes Surg 2010;4:462–467 [DOI] [PubMed] [Google Scholar]
- 6. Sammour T, Hill AG, Singh P, Ranasinghe A, Babor R, Rahman H. Laparoscopic sleeve gastrectomy as a single-stage bariatric procedure. Obes Surg. 2010;20:271–275 [DOI] [PubMed] [Google Scholar]
- 7. Magee CJ, Barry J, Arumugasamy M, Javed S, Macadam R, Kerrigan DD. Laparoscopic sleeve gastrectomy for high-risk patients: weight loss and comorbidity improvement–short-term results. Obes Surg 2011;21:547–550 [DOI] [PubMed] [Google Scholar]
- 8. Bellanger DE, Greenway FL. Laparoscopic sleeve gastrectomy, 529 cases without a leak: short-term results and technical considerations. Obes Surg 2011;21:146–150 [DOI] [PubMed] [Google Scholar]
- 9. Sabbagh C, Verhaeghe P, Dhahri A, et al. Two-year results on morbidity, weight loss and quality of life of sleeve gastrectomy as first procedure, sleeve gastrectomy after failure of gastric banding and gastric banding. Obes Surg. 2010;20:679–684 [DOI] [PubMed] [Google Scholar]
- 10. Nienhuijs SW, de Zoete JP, Berende CA, de Hingh IH, Smulders JF. Evaluation of laparoscopic sleeve gastrectomy on weight loss and co-morbidity. Int J Surg 2010;8:302–304 [DOI] [PubMed] [Google Scholar]
- 11. Armstrong J, O'Malley SP. Outcomes of sleeve gastrectomy for morbid obesity: a safe and effective procedure? Int J Sur. 2010;8:69–71 [DOI] [PubMed] [Google Scholar]
- 12. Triantafyllidis G, Lazoura O, Sioka E, Tzovaras G, Antoniou A, Vassiou K, Zacharoulis D. Anatomy and complications following laparoscopic sleeve gastrectomy: radiological evaluation and imaging pitfalls. Obes Surg. 2011;21:473–478 [DOI] [PubMed] [Google Scholar]
- 13. Rice RD, Simon TE, Seery JM, Frizzi JD, Husain FA, Choi YU. Laparoscopic sleeve gastrectomy: outcomes at a military training center. Am Surg 2010;8:835–840 [PubMed] [Google Scholar]
- 14. Kiriakopoulos A, Varounis C, Tsakayannis D, Linos D. Laparoscopic sleeve gastrectomy in morbidly obese patients. Technique and short term results. Hormones (Athens) 2009;8:138–143 [DOI] [PubMed] [Google Scholar]
- 15. Behrens C, Tang BQ, Amson BJ. Can J Surg. Early results of a Canadian laparoscopic sleeve gastrectomy experience. 2011;54:138–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chowbey PK, Dhawan K, Khullar R, et al. Laparoscopic sleeve gastrectomy: an Indian experience-surgical technique and early results. Obes Surg 2010;20:1340–1347 [DOI] [PubMed] [Google Scholar]
- 17. Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg 2009;19:1672–1677 [DOI] [PubMed] [Google Scholar]
- 18. Leyba JL, Aulestia SN, Llopis SN. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the treatment of morbid obesity. A prospective study of 117 patients. Obes Surg 2011;21:212–216 [DOI] [PubMed] [Google Scholar]
- 19. Daskalakis M, Berdan Y, Theodoridou S, Weigand G, Weiner RA. Impact of surgeon experience and buttress material on postoperative complications after laparoscopic sleeve gastrectomy. Surg Endosc 2011;1:88–97 [DOI] [PubMed] [Google Scholar]
- 20. Menenakos E, Stamou KM, Albanopoulos K, et al. Laparoscopic sleeve gastrectomy performed with intent to treat morbid obesity: a prospective single-center study of 261 patients with a median follow-up of 1 year. Obes Surg 2010;3:276–282 [DOI] [PubMed] [Google Scholar]
- 21. Albanopoulos K, Alevizos L, Linardoutsos D, et al. Routine abdominal drains after laparoscopic sleeve gastrectomy: a retrospective review of 353 patients. Obes Surg. 2011;21:687–691 [DOI] [PubMed] [Google Scholar]
- 22. Alley JB, Fenton SJ, Harnisch MC, Angeletti MN, Peterson RM. Integrated bioabsorbable tissue reinforcement in laparoscopic sleeve gastrectomy. Obes Surg. 2011;21:1311–1315 [DOI] [PubMed] [Google Scholar]
- 23. Gluck B, Movitz B, Jansma S, Gluck J, Laskowski K. Laparoscopic sleeve gastrectomy is a safe and effective bariatric procedure for the lower BMI (35.0–43.0 kg/m2) population. Obes Surg. 2011;21:1168–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayloo S, Buchs NC, Addeo P, Bianco FM, Giulianotti PC. Robot-assisted sleeve gastrectomy for super-morbidly obese patients. J Laparoendosc Adv Surg Tech A 2011;21:295–299 [DOI] [PubMed] [Google Scholar]
- 25. Arias E, Martínez PR, Ka Ming Li V, Szomstein S, Rosenthal RJ. Mid-term follow-up after sleeve gastrectomy as a final approach for morbid obesity. Obes Surg 2009;19:544–548 [DOI] [PubMed] [Google Scholar]
- 26. Diamantis T, Alexandrou A, Pikoulis E, Diamantis D, Griniatsos J, Felekouras E, Papalambros E. Laparoscopic sleeve gastrectomy for morbid obesity with intra-operative endoscopic guidance. Immediate peri-operative and 1-year results after 25 patients. Obes Surg 2010;20:1164–1170 [DOI] [PubMed] [Google Scholar]
- 27. Jacobs M, Bisland W, Gomez E, et al. Laparoscopic sleeve gastrectomy: a retrospective review of 1- and 2-year results. Surg Endosc 2010;24:781–785 [DOI] [PubMed] [Google Scholar]
- 28. Basso N, Casella G, Rizzello M, et al. Laparoscopic sleeve gastrectomy as first stage or definitive intent in 300 consecutive cases. Surg Endosc 2011;2:444–449 [DOI] [PubMed] [Google Scholar]
- 29. Lakdawala MA, Bhasker A, Mulchandani D, Goel S, Jain S. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1 year study. Obes Surg 2010;20:1–6 [DOI] [PubMed] [Google Scholar]
- 30. Angrisani L, Cutolo PP, Buchwald JN, et al. Laparoscopic reinforced sleeve gastrectomy: early results and complications. Obes Surg 2011;21:783–793 [DOI] [PubMed] [Google Scholar]
- 31. Csendes A, Braghetto I, León P, Burgos AM. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg 2010;14:1343–1348 [DOI] [PubMed] [Google Scholar]
- 32. Clinical Issues Committee of the American Society for Metabolic and Bariatric Surgery Updated Position Statement on Sleeve Gastrectomy as a Bariatric Procedure. Surg Obes Relat Dis. 2010;1:1–5 [DOI] [PubMed] [Google Scholar]
- 33. Jacques L, Jensen TS, Schafer J, Chin J, Ciccanti M. (2012) Decision memo for bariatric surgery for the treatment of morbid obesity. 2012;CAG-00250R2. Available at: http://www.cms.gov Accessed August 20, 2012
- 34. Gastrointestinal Surgery for Severe Obesity NIH Consens Statement Am J Clin Nutr. February 1992;55: 615S–619S [DOI] [PubMed] [Google Scholar]
- 35. Gadiot RP, Biter LU, Zengerink HJ, et al. Laparoscopic sleeve gastrectomy with an extensive posterior mobilization: technique and preliminary results. Obes Surg 2012;2:320–329 [DOI] [PubMed] [Google Scholar]
- 36. Spyropoulos C, Argentou MI, Petsas T, Thomopoulos K, Kehagias I, Kalfarentzos F. Management of gastrointestinal leaks after surgery for clinically severe obesity. Surg Obes Relat Dis. 2012;8:609–615 [DOI] [PubMed] [Google Scholar]
- 37. Chen B, Kiriakopoulos A, Tsakayannis D, et al. Reinforcement does not necessarily reduce the rate of staple line leaks after sleeve gastrectomy. A review of the literature and clinical experiences. Obes Surg. 2009;19:166–172 [DOI] [PubMed] [Google Scholar]
- 38. Choi YY, Bae J, Hur KY, Choi D, Kim YJ. Reinforcing the Staple Line During Laparoscopic Sleeve Gastrectomy: Does It Have Advantages? A Meta-analysis. Obes Surg 2012;22:1206–1213 [DOI] [PubMed] [Google Scholar]
- 39. Musella M, Milone M, Bellini M, Leongito M, Guarino R, Milone F. Laparoscopic sleeve gastrectomy. Do we need to oversew the staple line? Ann Ital Chir 2011;82:273–277 [PubMed] [Google Scholar]