Total laparoscopic pancreaticoduodenectomy may be a viable alternative to the standard Whipple procedure, offering the potential of a decreased length of stay, quick recovery, and improved quality of life.
Keywords: Laparoscopic, Pancreaticoduodenectomy, Whipple
Abstract
Introduction:
Total laparoscopic pancreaticoduodenectomy (TLPD) remains one of the most advanced laparoscopic procedures. Owing to the evolution in laparoscopic technology and instrumentation within the past decade, laparoscopic pancreaticoduodenectomy is beginning to gain wider acceptance.
Methods:
Data were collected for all patients who underwent a TLPD at our institution. Preoperative evaluation consisted of computed tomography scan with pancreatic protocol and selective use of magnetic resonance imaging and/or endoscopic ultrasonography. The TLPD was done with 6 ports on 3 patients and 5 ports in 2 patients and included a celiac, periportal, peripancreatic, and periduodenal lymphadenectomy. Pancreatic stents were used in all 5 cases, and intestinal continuity was re-established by intracorporeal anastomoses.
Results:
Five patients underwent a TLPD for suspicion of a periampullary tumor. There were 3 women and 2 men with a mean age of 60 years and a mean body mass index of 32.8. Intraoperatively, the mean operative time was 9 hours 48 minutes, with a mean blood loss of 136 mL. Postoperatively, there were no complications and a mean length of stay of 6.6 days. There was no lymph node involvement in 4 out of 5 specimens. The pathological results included intraductal papillary mucinous neoplasm in 2 patients, pancreatic adenocarcinoma in 1 patient (R0 resection), benign 4-cm periampullary adenoma in 1 patient, and a somatostatin neuroendocrine carcinoma in 1 patient (R0, N1).
Conclusion:
TLPD is a viable alternative to the standard Whipple procedure. Our early experience suggests decreased length of stay, quicker recovery, and improved quality of life. Complication rates appear to be improved or equivalent.
INTRODUCTION
Laparoscopic pancreatectomy has recently emerged as one of the most advanced applications of surgery,1 and total laparoscopic pancreaticoduodenectomy (TLPD) has proven to be among the most advanced laparoscopic procedures.2 Gagner and Pomp were the first to describe the laparoscopic Whipple procedure in 1994.3 The long operative times and technical difficulties coupled with the need to develop advanced laparoscopic skills1,2 were valid reasons that supported the initial reluctance to pursue these sophisticated minimally invasive operations. Recently, laparoscopic pancreaticoduodenectomy has started to gain wider acceptance as surgeons become more comfortable with laparoscopic technology. As a result, TLPDs have been reported with an increased frequency from institutions internationally.1–14 The aim of this article was to describe our early experience with TLPD and compare the outcomes with those of other published series, particularly from the standpoint of a community teaching hospital.
METHODS
The data were reviewed from 4 patients who underwent a TLPD by a single surgeon at Providence Hospital in Southfield, Michigan from March 2011 until December 2012. Demographic data, operative time, blood loss, postoperative length of stay, readmission, and final pathologic results were examined.
Preoperative Evaluation
All patients underwent a complete clinical evaluation, with a focus on performance status and optimization of comorbidities. Standard relevant laboratory blood work was performed and included serum tumor markers. Preoperative computerized tomography (CT) scans with pancreas protocol were performed in all patients. Magnetic resonance imaging and/or endoscopic ultrasonography were selectively performed at the discretion of the surgeon.
Appropriate patient selection was important and deemed appropriate by the surgeon. Inclusion criteria included tumors confined to the pancreatic head or periampullary region and favorable vascular anatomy, as demonstrated on preoperative imaging. Exclusion criteria included multiple prior abdominal surgeries, anticipated hostile abdomen, body mass index >40, locally advanced tumors, and inability to withstand prolonged anesthesia.
Technique of Totally Laparoscopic Pancreaticoduodenectomy
The procedures were performed with patients supine with mild reverse Trendelenberg positioning. Between 5 and 6 trocars were required (Figure 1). A diagnostic survey and intraoperative ultrasonography were performed to assess for resectability and other pathologic conditions. The fundus of the gallbladder was retracted anteriosuperiorly for exposure. A 5-mm blunt-tip Ligasure (Tyco Healthcare Group, Dublin, UK) device was used for vessel ligation and dissection where appropriate.
Figure 1.
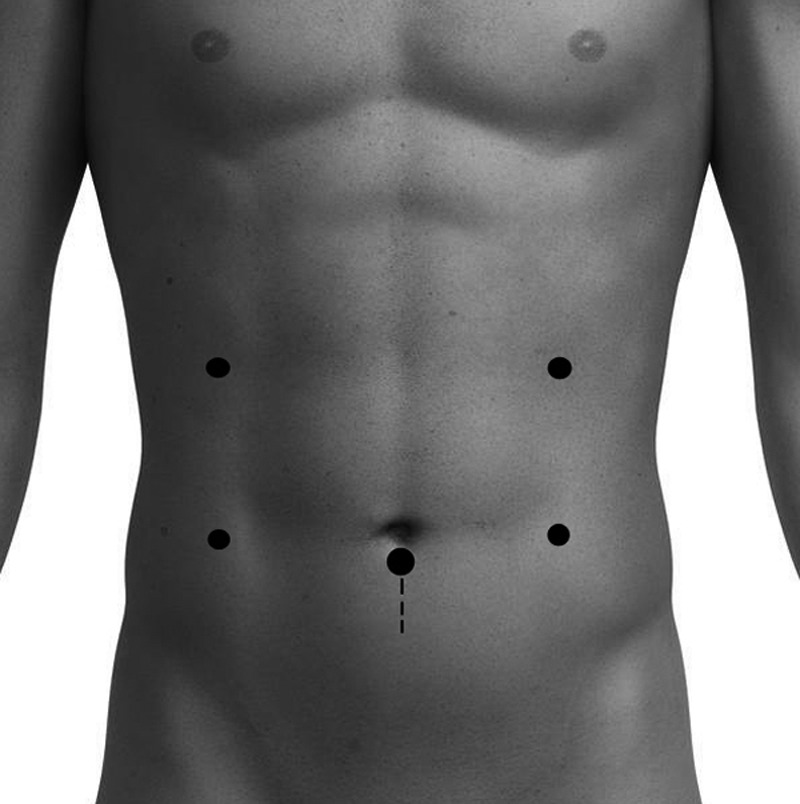
Trocar placement.
The first portion of the duodenum was mobilized and divided using a 3.5-mm Endo GIA stapling cartridge (Covidien, Mansfield, MA, USA). A Kocher maneuver was widely performed from the bile duct through the ligament of Treitz. The jejunum was divided distal to the ligament of Treitz and its mesentery divided down to its origin. A regional lymphadenectomy was performed. The gastroduodenal artery was isolated from the hepatic artery and doubly ligated with 0 silk sutures and metallic clips.
The pancreas was then dissected from the portal vein posteriorly, and a portal vein tunnel was created from an inferior to a superior aspect. A Penrose drain was used to retract the pancreas anteriorly, and the pancreas was subsequently divided using electrocautery. Both the superior and inferior pancreaticoduodenal veins were ligated. The posterior uncinate process were freed. Similarly, the pancreaticoduodenal arteries are ligated and the pancreas was freed from the mesenteric artery vasculature.
The bile duct was divided above the cystic duct when feasible, completing the laparoscopic dissection. The specimen was placed in a large EndoCatch bag (Covidien, Mansfield, MA, USA) and sent for pathological diagnosis. The proximal jejunum was brought through a right mesocolic rent that was later secured. The biliary anastomosis was performed in an end-to-side fashion using radially oriented 3–0 interrupted absorbable sutures in a single layer (Figure 2).
Figure 2.
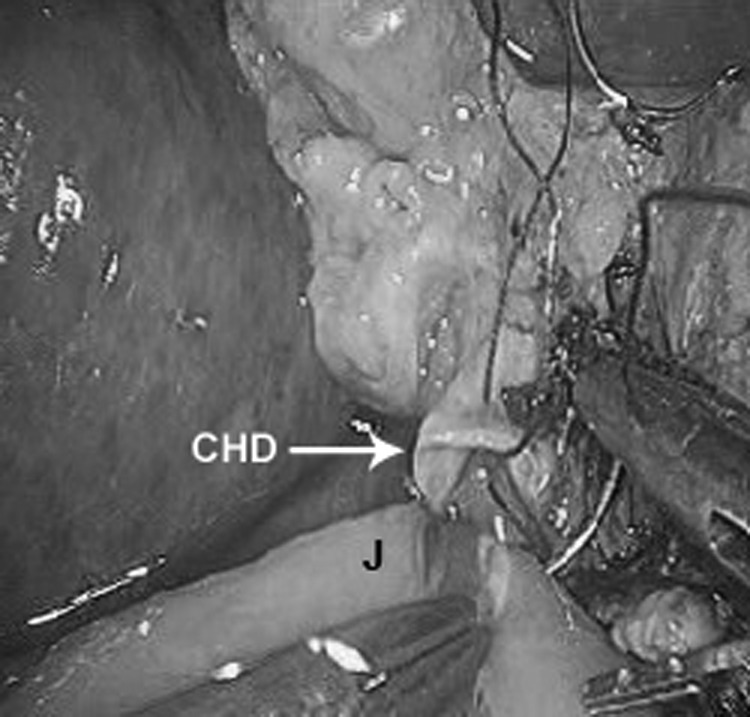
Hepaticojejunostomy. CHD = common hepatic duct, J = jejunal limb.
A 5-Fr pediatric feeding tube was used as an indwelling pancreatic stent before performing the pancreaticojejunostomy. The jejunum was first sutured to the posterior wall of the pancreas with interrupted 3–0 silk sutures. A duct-to-mucosal, end-to-side pancreaticojejunostomy was then performed in all cases over the stent in a radially oriented interrupted fashion with 4–0 Polysorb sutures (Syneture/Covidien, Mansfield, MA) (Figure 3). The anastomosis was completed with interrupted 3–0 silk sutures placed from the pancreatic parenchyma to the seromuscular layer of the jejunum.
Figure 3.
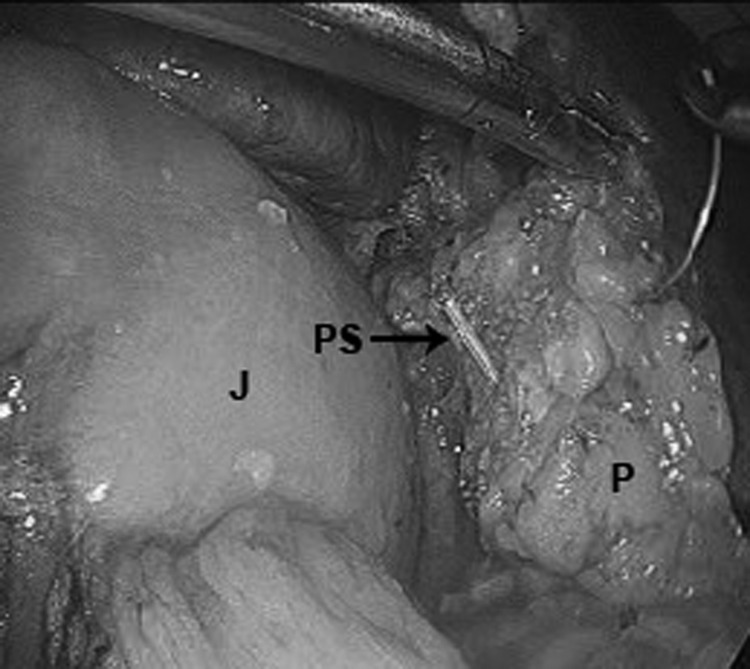
Pancreaticojejunostomy. J = jejunal limb, PS = pancreatic stent, P = pancreas.
An anteocolic, isoperistaltic, end-to-side duodenojejunostomy or a side-to-side gastrojejunostomy was performed with 2 layers. All anastomoses were performed intracorporeally. Finally, a cholecystectomy was performed to complete the operation. A 19-Fr channel drain was placed near the pancreatic head dissection posterior to the pancreaticojejunostomy.
Postoperative Care
After completion of the procedure, the patients were admitted to the intensive care unit and subsequently to the hepatobiliary and pancreas unit. Prophylactic antibiotics were continued perioperatively, and prophylactic subcutaneous unfractionated heparin continued every 8 hours during hospitalization. The nasogastric tube was removed at approximately postoperative day 3, and patients were started on a clear liquid diet. Postoperative analgesia consisted of intravenous ketorolac tromethamine and morphine with conversion to oral analgesia on tolerance of an oral diet. Patients were discharged when they could tolerate a soft diet and there were no signs of complications.
RESULTS
Five patients underwent a TLPD from March 2011 to December 2012 for suspicion of a periampullary tumor (Table 1); there were 3 women and 2 men, with a mean age of 61 years and a mean body mass index of 32.8. The mean operative time was 9 hours 48 minutes with a mean blood loss of 136 mL. There were no postoperative complications related to the technical aspects and a mean length of stay of 6.6 days. One patient was readmitted for bleeding while receiving anticoagulation therapy for history of pre-existing condition but did not require any additional procedural therapy, and the issue resolved. There were no mortalities, and none of the patients have had delayed gastric emptying, pancreatic leak, or other morbidity to date. None of the cases required conversion to an open operation. In all cases, TLPD was done with curative intent and was performed totally laparoscopically, including intracorporeal resection and reconstruction of the anastomoses.
Table 1.
Patient Demographics, Operative Data, and Final Diagnoses
| Age/Sex | BMI (kg/m2) | OR Time (min) | EBL (mL) | LOS (days) | Histology | Tumor Size (cm) | ||
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 56/F | 35 | 555 | 250 | 6 | Periampullary adenoma | R0 | 1.9 |
| Patient 2 | 73/M | 27 | 588 | 120 | 7 | IPMN | R0 | 3 |
| Patient 3 | 65/F | 39 | 575 | 60 | 6 | IPMN | R0 | 3.2 |
| Patient 4 | 66/F | 26 | 566 | 50 | 7 | Periampullary adenocarcinoma | R0 | 4.5 |
| Patient 5 | 43/M | 37 | 660 | 200 | 7 | Somatostatin neuroendocrine Ca | R0 | 1.6 |
| Mean | 60.6 | 32.8 | 588.8 | 136 | 6.6 | 2.8 |
BMI = body mass index, OR = operating room, EBL = estimated blood loss, LOS = length of stay, Ca = carcinoma.
The lymphatic tissue retrieved included celiac, periportal, peripancreatic, and periduodenal nodes. On pathological evaluation, there was no lymph node involvement in 4 out of the 5 specimens. One patient with a somatostatin neuroendocrine carcinoma had 1/18 nodes positive. The pathologic findings included intraductal papillary mucinous neoplasm (IPMN) in 2 patients (R0), pancreatic adenocarcinoma in 1 patient (R0 resection), a benign 1.9-cm periampullary adenoma in 1 patient, and a somatostatin neuroendocrine carcinoma in 1 patient (R0, N1). The intraoperative pancreatic duct diameter ranged from 2 to 5 mm, and the mean tumor size was 3.2 cm. All of the patients in our series had a soft pancreas, except the patient with adenocarcinoma.
DISCUSSION
Minimally invasive surgery has become the standard of care for many procedures because of the advantages that it offers, including decreased pain, decreased length of stay, and quicker recovery. The associated cosmesis is also preferred by most patients. Minimally invasive approaches to pancreatic resections are well-known for laparoscopic distal pancreatectomies, with evidence of reduced blood loss, shorter length of stay, and quicker recovery.2,14 Acceptance of TLPD has not been as widespread, with few surgeons having performed this demanding procedure. This has largely been attributable to the time-consuming complexity of the operation.
A thorough literature review of all laparoscopic pancreaticoduodenectomies was reported in 2009 by Gagner and Palermo and is perhaps the most cited article with regards to laparoscopic pancreaticoduodenectomies.1 This review, however, also included cases that were hand-assisted or hybrid laparoscopic laparotomy approaches. It also included publications that involved the same cohort of patients (Dulucq et al 2005, 2006; Gagner et al. 1997; Milone et al. 2004). We limited our review to cases that were entirely laparoscopic and excluded publications that involved previously published cases. A more recent article by Kendrick and Cusati2 contained a review of published TLPDs and appropriately included only those performed entirely laparoscopically, but it only included case series of 10 patients or more. We therefore present the most complete literature review of total laparoscopic pancreaticoduodenectomies performed to date (Table 2).
Table 2.
Published TLPD Cases and Series
| Author | Year of Publication | n | Age (mean) | OR Time (min), Mean | LOS (d), Mean |
|---|---|---|---|---|---|
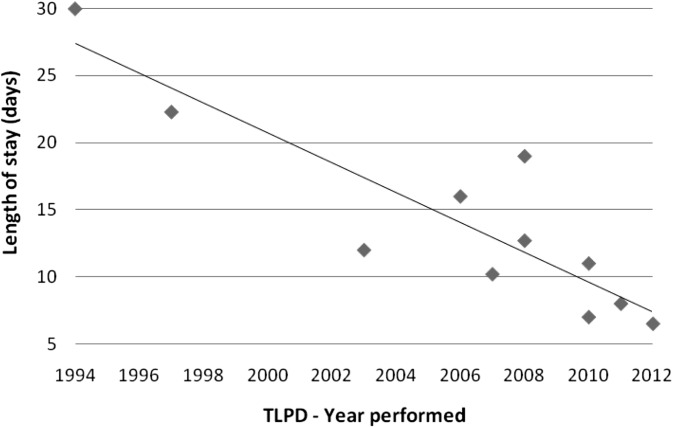
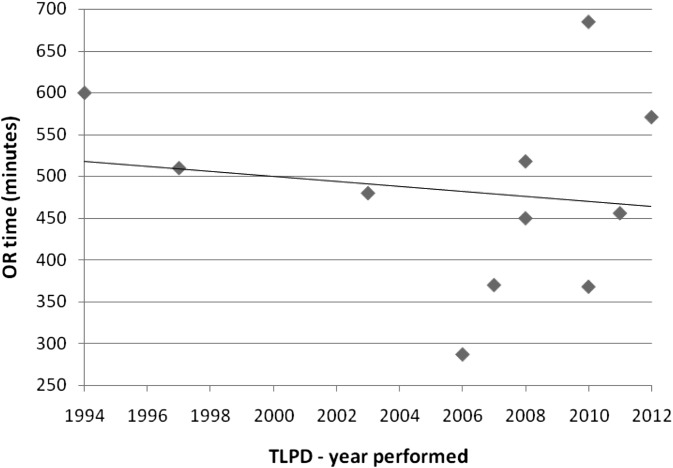
| Gagner and Pomp | 1994 | 1 | 30 | 600 | 30 |
| Gagner and Pomp | 1997 | 6 | 71 | 510 | 22.3 |
| Masson et al. | 2003 | 1 | 37 | 480 | 12 |
| Dulucq et al. | 2006 | 13 | 62 | 287 | 16 |
| Palanivelu et al. | 2007 | 42 | 61 | 370 | 10.2 |
| Pugliese et al. | 2008 | 6 | 64 | 518 | 19 |
| Sa Cunha et al. | 2008 | 1 | 49 | 450 | 12.7 |
| Kendrick and Cusati | 2010 | 62 | 66 | 368 | 7 |
| Khaimook et al. | 2010 | 1 | 40 | 685 | 11 |
| Zureikat et al. | 2011 | 12 | 70 | 456 | 8 |
| Jacobs* | 2012 | 5 | 60 | 589 | 6.6 |
| Total/mean | 150 | 55.5 | 483 | 14.1 |
Current series.
There have been 150 published TLPD cases by 10 different authors. Although there has been great variation in the outcomes reported, most cases report results comparable with the standard open Whipple procedure with regard to mortality and complication rates. One article reported a pancreatic fistula rate of 18% (n = 11/62), whereby 9 of the 11 patients had a soft pancreas with a pancreatic duct 3 mm or smaller. It is suspected, but not known, whether patients who undergo TLPD have similar risks for leak compared with open surgery. In our series, duct size did not have an impact on leak rate. Perhaps excellent intraoperative magnified visualization of the anatomy afforded by the laparoscope facilitates the exposure.
As our experience increased with the TLPD, we determined that the procedure can be performed just as safely and efficiently with 5 ports, thereby eliminating the need for a sixth port. This decrease in the number of trocars occurred in part from our experiences performing laparoscopic distal pancreatectomies, where we routinely use only 3 ports.18
To arrive at the current status of performing TLPDs, the surgeon was fortunate enough to have trained in an era highlighted by tremendous advancements in laparoscopic approaches and techniques, combined with a solid experience in performing open hepatopancreatobiliary surgery backed by advanced hepatopancreatobiliary fellowship training. Furthermore, before attempting the TLPD, the surgeon had acquired a large experience performing totally laparoscopic distal pancreatectomies, hepatectomies, and other foregut operations. Hand-assisted or a hybrid approach was therefore not believed to be necessary and was never performed.
The advantages of the TLPD have shown promise with decreased blood loss and reduced length of stay—without compromising patient satisfaction. Anecdotally, the TLPD patients seemed to recover at an overall faster rate and required fewer narcotics. Interestingly, these same patients were able to tolerate an oral diet sooner than their open-surgery counterparts, which is a likely cause for their earlier discharge. Also, there has been a clear decrease in the published postoperative length of stay since TLPD's inception in 1994 (Figure 4). The mean operative time, however, has not significantly decreased over the years (Figure 5) and still remains longer than the open approach, perhaps because of the complexity of the procedure and learning curves that are associated with most developing techniques.
Figure 4.
Published TLPDs' mean length of stay.
Figure 5.
Published TLPDs' mean operating room time.
The role of residents in TLPDs has been similar to all other minimally invasive surgical cases performed at our institution. Residents and students were intimately involved in all aspects of patient care, including preoperative office consultations, first-assisting in the operating room, and the postoperative care. The residents gain invaluable training in the workup of patients with suspected pancreatic head neoplasms, operative management of pancreatic tumors, and postoperative patient treatment. Finally, anatomical education was facilitated by the use of the laparoscopic approach, providing outstanding visualization of the complex anatomy.
There does not seem to be any negative impact on patient safety, such as reports of technical mishaps. Furthermore, standard oncological principles have been maintained. Our early experience does not show increased complication rates with the laparoscopic approach or need for conversion to an open technique. The TLPD has therefore emerged as a feasible alternative in skilled hands. The disadvantages of a TLPD include the long operative times, although we have not seen any untoward effects of this length on patient outcome. Furthermore, the steep learning curve has enabled only a few surgeons in the past 17 years to attempt the procedure, further limiting additional surgeon education.
CONCLUSION
In our initial experience, TLPD has proven to be a viable alternative to the standard open Whipple procedure. Although most published reports of TLPD are from large-volume tertiary referral centers, we present our early single-institution experience at a community-based teaching hospital. Our early experience suggests the benefit of a decreased length of hospital stay, decreased blood loss, and quicker recovery. Technical complication rates appear to be at least equivalent to the open techniques. Extended experience with hepatopancreaticobiliary surgery and advanced laparoscopic skills are crucial requirements.
Contributor Information
Michael J. Jacobs, Department of Surgery, Providence Hospital, St. John Providence Health, Southfield, MI, USA..
Armin Kamyab, Department of Surgery, Providence Hospital, St. John Providence Health, Southfield, MI, USA..
References:
- 1. Gagner M, Palermo M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg. 2009;16:726–730 [DOI] [PubMed] [Google Scholar]
- 2. Kendrick M, Cusati D. Total laparoscopic pancreaticoduodenectomy. Arch Surg. 2010;145(1):19–23 [DOI] [PubMed] [Google Scholar]
- 3. Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreaticoduodenectomy. Surg Endosc. 1994;8(5):408–410 [DOI] [PubMed] [Google Scholar]
- 4. Gagner M, Pomp A. Laparoscopic pancreatic resection: is it a worthwhile? J Gastrointest Surg. 1997;1(1):20–25, discussion 25–26 [DOI] [PubMed] [Google Scholar]
- 5. Masson B, Sa-Cunha A, Laurent C, et al. Laparoscopic pancreatectomy: report of 22 cases. Ann Chir. 2003;128(7):452–456 [DOI] [PubMed] [Google Scholar]
- 6. Dulucq JL, Wintringer P, Stabilini C, et al. Are major laparoscopic pancreatic resections worthwhile? A prospective study of 32 patients in a single institution. Surg Endosc. 2005;19(8):1028–1034 [DOI] [PubMed] [Google Scholar]
- 7. Staudacher C, Orsenigo E, Baccari P, et al. Laparoscopic assisted duodenopancreatectomy. Surg Endosc. 2005;19(3)352–356 [DOI] [PubMed] [Google Scholar]
- 8. Dulucq JL, Winteringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20(7):1045–1050 [DOI] [PubMed] [Google Scholar]
- 9. Palanivelu C, Jani K, Senthilnatha P, et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007;205(2)222–230 [DOI] [PubMed] [Google Scholar]
- 10. Pugliese R, Scandroglio I, Sansonna F. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech. 2008;18(1):13–18 [DOI] [PubMed] [Google Scholar]
- 11. Sa Cunha A, Rault A, Beau C, et al. A single institution prospective study of laparoscopic pancreatic reserction. Arch Surg. 2008;143(3):289–295, discussion 295 [DOI] [PubMed] [Google Scholar]
- 12. Khaimook A, Borkird J, Alapach S. The first successful laparoscopic Whipple procedure at Hat Yai hospital: surgical technique and a case report. J Med Assoc Thai. 2010;93(9):1098–1102 [PubMed] [Google Scholar]
- 13. Zureikat A, Breaux J, Steel J, et al. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg. 2011;15:1151–1157 [DOI] [PubMed] [Google Scholar]
- 14. Subhas S, Gupta N, Mittal V, Jacobs M. Laparoscopic three-port distal pancreatectomy. HPB. 2011;13(5):361–363 [DOI] [PMC free article] [PubMed] [Google Scholar]