Robotic-assisted surgery for the treatment of deep infiltrating bowel endometriosis appears to be feasible, effective, and safe.
Keywords: Endometriosis, Rectum, Robotics, Segmental resection, Bowel endometriosis
Abstract
Background and Objective:
Deep infiltrating pelvic endometriosis with bowel involvement is one of the most aggressive forms of endometriosis. Nowadays, robotic technology and telemanipulation systems represent the latest developments in minimally invasive surgery. The aim of this study is to present our preliminary results and evaluate the feasibility of robotic-assisted laparoscopic colorectal resection for severe endometriosis.
Methods:
Between September 2009 and December 2011, 10 women with colorectal endometriosis underwent surgery with the da Vinci robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA). We evaluated the following parameters: short-term complications, clinical outcomes and long-term follow-up, pain relief recurrence rate, and fertility outcomes.
Results:
Extensive ureterolysis was required in 8 women (80%). Ovarian cystectomy with removal of the cystic wall was performed in 7 women (70%). Torus resection was performed in all women, with unilateral and bilateral uterosacral ligament resection in 1 woman (10%) and 8 women (80%), respectively. In addition to segmental colorectal resection in all cases, partial vaginal resection was necessary in 2 women (20%). An appendectomy was performed in 2 patients (20%). The mean operative time with the robot was 157 minutes (range, 90–190 minutes). The mean hospital stay was 3 days. Six patients had infertility before surgery, with a mean infertility time of 2 years. After a 12-month follow-up period, 4 women (67%) conceived naturally and 2 (33%) underwent in vitro fertilization.
Conclusion:
We show that robotic-assisted laparoscopic surgery for the treatment of deep infiltrating bowel endometriosis is feasible, effective, and safe.
INTRODUCTION
Endometriosis is a gynecologic disorder defined by the presence of the endometrial gland and stroma outside the uterus. Deep infiltrating pelvic endometriosis with bowel involvement is one of its most aggressive forms and can lead to infertility, chronic pelvic pain, dyschezia, and altered quality of life. Bowel endometriosis involvement is estimated to occur in 5.3% to 12% of women with endometriosis,1,2 and the rectum and rectosigmoid junction together account for 70% to 93% of all intestinal endometriotic sites.3,4 Women can be asymptomatic, but most have dysmenorrhea, infertility, dyspareunia, dysuria, dyschezia, tenesmus, and pain on defecation.3,5
Currently available medical approaches are sometimes effective in the treatment of endometriosis-associated pain with temporary relief of symptoms. No treatment has yet been shown to achieve a long-term cure. For these reasons, surgery in these cases should be considered the first-line treatment of choice.6–9 The management of intestinal endometriosis depends on the depth of the bowel wall invasion (superficial, partial, or full-thickness invasion), leading to different surgical options (from disc excision to segmental resection). It has been reported that the best results in terms of recurrence rates and improvement of symptoms are achieved by intestinal resection. Recent studies suggest that complete excision of deep endometriosis with bowel resection leads to a reliable and persistent relief of pain symptoms and improvement of quality of life.9–15
Nowadays, robotic technology and telemanipulation systems represent the latest developments in minimally invasive surgery. They offer an improved ergonomic position to the surgeon, 3-dimensional visualization of the operating field, fine instrumentation, and increased maneuverability of the instruments with 6 df. These key features allow complex minimally invasive procedures to be performed more easily than with conventional laparoscopic surgery.16,17 In addition, the effects of surgery should be minimized in productive-aged women and during the reproductive age. These accomplishments are related to efficacy, fast return to activity, and less pain. In this context, robotic surgery can be seen as an alternative approach because the literature shows significant results regarding safety and efficacy of the method.18 On the basis of these findings, we have started to use robotics as an assistance tool for laparoscopic resection of deep infiltrating endometriotic colorectal lesions. Therefore the aim of this study is to present our preliminary results and evaluate the feasibility of robotic-assisted laparoscopic colorectal resection for severe endometriosis.
MATERIALS AND METHODS
Patients
Between September 2009 and December 2011, we have selected 10 women with colorectal endometriosis, referred to our private clinic (Centro de Endometriose São Paulo, São Paulo, Brazil), to undergo the robotic approach. All patients had a clinical and imaging diagnosis of deep infiltrating colorectal endometriosis.
The mean age was 37 years (range, 29–48 years), and the mean body mass index was 23.5 (range, 20–26). Among all patients, 50% had had a previous pelvic surgery: ovarian cystectomy, oophorectomy, or adhesiolysis.
Our routine evaluation included complete anamnesis and survey of complaints, physical examination, and topography of lesions identified on transvaginal ultrasonography with bowel preparation performed by a unique radiologist.
For pain, bowel symptoms, and infertility, we established a score for each question. Pain was scored as follows: 1, no pain; 2, mild pain; 3, moderate pain; 4, severe pain; and 5, crippling pain. Bowel symptoms were scored as follows: 1, no symptoms; 2, mild pain with evacuation; 3, moderate pain; 4, severe pain; and 5, obstruction. Infertility was scored as present or absent, and the duration of infertility in months was recorded (Table 1). These scores are the basis for assessing the short- and medium-term effectiveness of surgery.
Table 1.
Clinical Symptoms Before Surgery
| Patient No. | Pain | Bowel Symptoms | Infertility | Duration of Infertility, mo |
|---|---|---|---|---|
| 1 | 4 | 3 | Yes | 60 |
| 2 | 4 | 2 | Yes | 36 |
| 3 | 3 | 3 | Yes | 24 |
| 4 | 5 | 4 | Yes | 36 |
| 5 | 5 | 5 | No | 0 |
| 6 | 4 | 2 | No | 0 |
| 7 | 4 | 3 | Yes | 36 |
| 8 | 4 | 5 | Yes | 36 |
| 9 | 5 | 4 | No | 0 |
| 10 | 5 | 5 | No | 0 |
Imaging Diagnosis
Transvaginal ultrasonography scans (Figure 1) were carried out by a single examiner. The scanner used was a GE Voluson 730 Pro (GE Healthcare, Buckinghamshire, England) connected to a 3.7- to 9.2-MHz microconvex endocavitary transducer (anterior and posterior pelvic compartment evaluation—uterus and ovaries, vesicouterine recess, pouch of Douglas, rectum, sigmoid, retrocervical area, uterosacral ligaments, torus uterinus, and posterior vaginal fornix), a 3- to 6-MHz multi-frequency convex transducer (abdominal, urinary tract, and pelvic evaluation), and a 5- to 12-MHz wide-band linear transducer (evaluation of right iliac fossa—ileum, cecum, appendix, small intestine, and abdominal wall).
Figure 1.
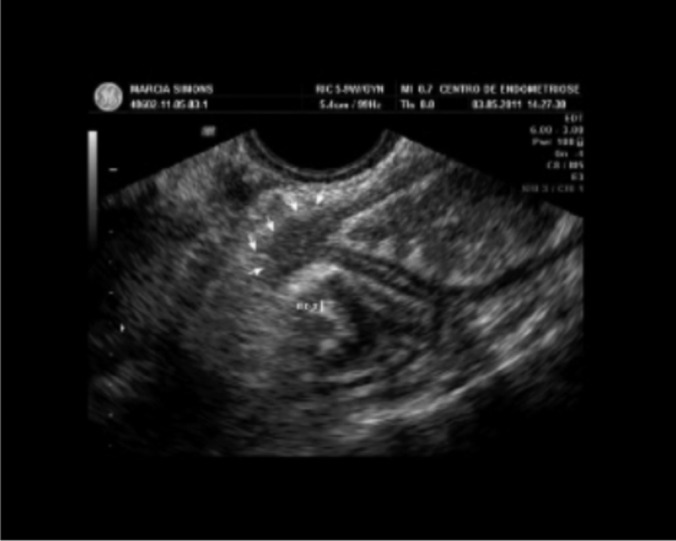
Transvaginal ultrasonography scan of rectosigmoid lesion.
All patients received a rectal enema 1 hour before the examination to eliminate all fecal residue in the rectosigmoid.
Surveillance of all the sites and the extent of endometriosis was performed. Regarding intestinal evaluation, the report included the number of lesions, site of the lesion or lesions (rectum or sigmoid), distance from the anal verge, extension, thickness, affected layer, and circumference of the bowel. Imaging findings in the intestines of all patients are shown in Table 2.
Table 2.
Transvaginal Ultrasound Scan Evaluations Before Surgery
| Patient No. | Length of Lesion, cm | Site of Lesion | Thickness of Lesion, cm | Circumference of Lesion, % | Distance From Anal Verge, cm | Synchronous Lesion | Layer of Bowel Wall |
|---|---|---|---|---|---|---|---|
| 1 | 5.8 | Rectosigmoid | 1.9 | 65 | 8 | Yes | Submucosa/muscularis |
| 2 | 1.6 | Rectosigmoid | 1 | 20 | 9 | No | Internal muscularis |
| 3 | 1.7 | Rectosigmoid | 0.5 | 20 | 8 | No | Internal muscularis |
| 4 | 1.8 | Rectosigmoid | 1.6 | 17 | 8 | Yes | Submucosa/muscularis |
| 5 | 2.8 | Rectosigmoid | 1.5 | 18 | 8 | No | Internal muscularis |
| 6 | 3.3 | Sigmoid | 1.6 | 40 | 11 | No | Submucosa/muscularis |
| 7 | 4 | Rectum | 3 | 25 | 6 | Yes | Submucosa/muscularis |
| 8 | 2.2 | Rectum | 0.7 | 20 | 7 | No | Submucosa/muscularis |
| 9 | 1.5 | Sigmoid | 1.2 | 24 | 11 | No | Internal muscularis |
| 10 | 2.4 | Sigmoid | 1.4 | 20 | 10 | No | Internal muscularis |
Inclusion Criteria
The inclusion criteria were as follows: (1) ≥1 lesion in the rectum and/or sigmoid involving the deep muscle layer of any size, (2) transvaginal ultrasonography with bowel preparation and diagnosis of intestinal lesion with involvement of the deep muscle layer or mucosal layer, (3) no change in symptoms with hormonal treatment (for ≥6 months) or previous surgery, (4) diagnosis of infertility, and (5) able to bear the costs of the procedure (robotic).
Validation of Robotics for Rectosigmoidectomy
To validate the robotic procedure, patients were evaluated for length of stay, postoperative pain, and turnaround time for activities, as well as the existence of complications related to surgery and the postoperative period.
All patients were followed up for ≥12 months, and the questionnaires about pain, bowel symptoms, and infertility were administered again.
Surgical Technique
The da Vinci robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA) was used in all cases. All patients were placed in the dorsal lithotomy position. A uterine manipulator and a Foley catheter were placed.
After pneumoperitoneum induction, a 12-mm trocar in the umbilical incision, two 8-mm trocars in the right and left iliac fossa, a 15-mm trocar in the median suprapubic area, and a 5-mm trocar in the upper right iliac fossa were introduced (Figure 2).
Figure 2.
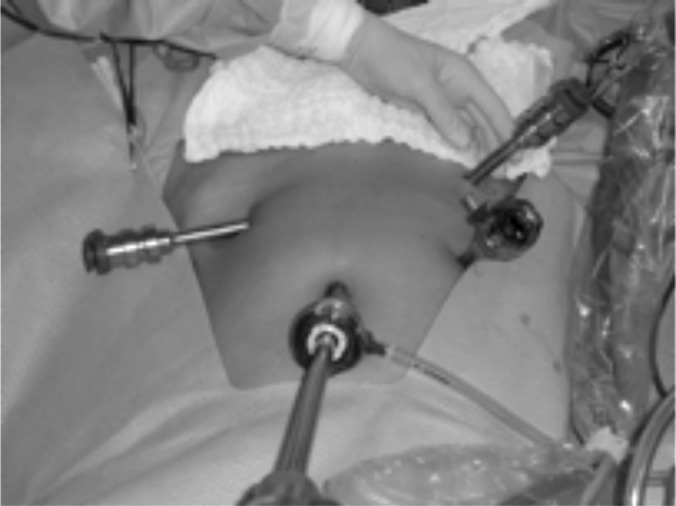
Trocar placement.
At this time, robotic-assisted laparoscopic surgery was initiated. After the extension of endometriosis in the pelvis was determined, the pararectal spaces were opened to obtain mobilization of the bowel. The diseased tissue was removed en bloc; no attempt was made to dissect the endometriotic nodule from the rectosigmoid. The surgeon performed separation of the fibrofatty tissue attached to the bowel with a robotic Harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA), trying to preserve the superior rectal artery irrigation to the distal stump. Total mesorectal excision was performed when the endometriotic lesion was present also in the mesenteric root. The mesentery was dissected no more than 2 cm past the nodular mass deforming the bowel wall. During the procedures, the exposed bowel was transected caudal to the endometriotic lesions with one or two Echelon golden charges (Ethicon Endo-Surgery).
At this point, all other sites of endometriosis were excised (vagina, ovary, uterosacral ligaments, bladder) robotically. After that, laparoscopic mobilization of the rectum allowed extraction of its cephalic portion through a small suprapubic incision (4 cm), which was obtained by enlarging the midline trocar incision site (12 mm). The surgeon created a purse for the anvil before placing the colon in the pelvic cavity with No. 2-0 Prolene (Ethicon, Somerville, NJ, USA) and closing the suprapubic abdominal incision with absorbable suture. An end-to-end laparoscopic anastomosis was performed with a 33-mm rectally introduced circular stapler (Ethicon Endo-Surgery) or with a 29-mm stapler if use of the 33-mm stapler was not possible. Methylene blue was injected through the rectum to confirm the continuity of the suture. Two or three simple stitches (No. 2.0 PDS; Ethicon) were also placed. A liquid diet was started on the second postoperative day if flatus was present. Patients were normally discharged on the third postoperative day.
RESULTS
The mean operative time with the robot was 157 minutes (range, 90–190 minutes). Docking the robot averaged 12 minutes (range, 8–19 minutes) and included time for troubleshooting. Robot disassembly averaged 3 minutes.
Regarding complications, blood loss was insignificant (near 0 mL) in all cases, and there were no intraoperative or postoperative complications (eg, pneumonia, anastomotic or rectovaginal fistula, abdominal collections, long-term ileus, or intestinal adhesions). None of the patients had ileostomy or colostomy. The length of stay was 3 days in all cases.
Surgical Findings
All women were found to have severe adhesions with involvement of the Douglas pouch. Extensive ureterolysis was required in 8 women (80%). Ovarian cystectomy with removal of the cystic wall was performed in 7 women (70%). Torus resection was performed in all women, with unilateral and bilateral uterosacral ligament resection in 1 woman (10%) and 8 women (80%), respectively.
All cases (as described in the “Materials and Methods” section) were robotic-assisted laparoscopic bowel resections (Figures 3–6). Partial vaginal resection was also necessary in two women (20%). An appendectomy was performed in two patients (20%). No blood transfusion was necessary in any case.
Figure 3.
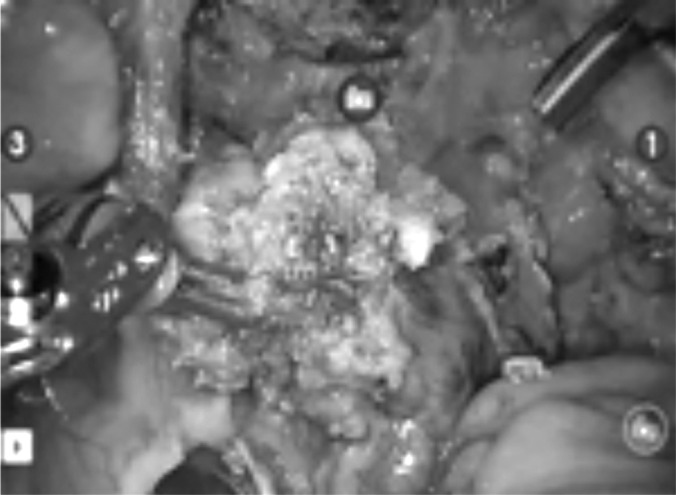
Bowel resection.
Figure 4.

Bowel resection.
Figure 5.

Nerve preservation.
Figure 6.
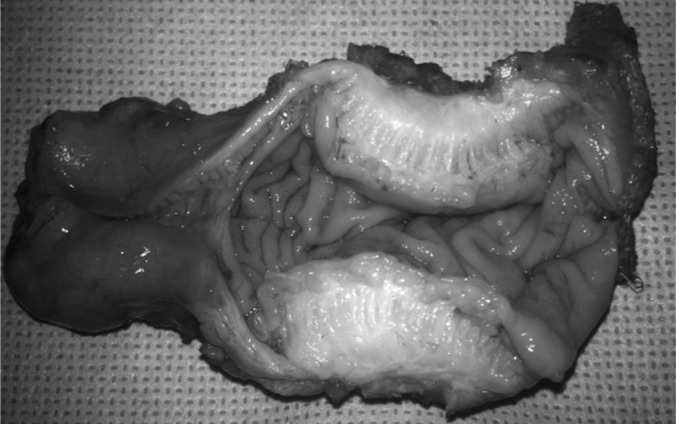
Endometriosis lesion.
Histology
All patients' histologic examination findings showed stromal and glandular endometriosis (Table 3).
Table 3.
Morphologic and Histopathologic Findings After Surgery
| Patient No. | Length of Lesion, cm | Thickness of Lesion, cm | Circumference of Lesion, % | Length of Resected Bowel, cm | Layer of Bowel Wall |
|---|---|---|---|---|---|
| 1 | 5 | 1.7 | 60 | 9 | Submucosa/muscularis |
| 2 | 1.4 | 1 | 27 | 10 | Submucosa/muscularis |
| 3 | 1.8 | 1 | 30 | 9 | Internal muscularis |
| 4 | 1.2 | 0.8 | 20 | 17 | Submucosa/muscularis |
| 5 | 2.8 | 1 | 25 | 12 | External muscularis |
| 6 | 3.6 | 1 | 45 | 18 | Submucosa/muscularis |
| 7 | 3.6 | 3 | 25 | 11 | Submucosa/muscularis |
| 8 | 2.6 | 2 | 30 | 9 | Submucosa/muscularis |
| 9 | 1.2 | 0.9 | 15 | 10 | Internal muscularis |
| 10 | 2.2 | 1.2 | 18 | 14 | Internal muscularis |
Evolution of Symptoms and Quality of Life After Robotic-Assisted Laparoscopic Colorectal Resection for Endometriosis
Symptoms including dysmenorrhea, dyspareunia, dyschezia, intestinal cramping, diarrhea, and constipation disappeared in all women after colorectal resection after 12 months' follow-up. Six patients had infertility before surgery, with a mean infertility time of 2 years. Regarding infertility, after a 12-month follow-up period, 4 women (67%) conceived naturally and 2 (33%) underwent in vitro fertilization (Table 4).
Table 4.
Clinical Symptoms After Surgery
| Patient No. | Pain | Bowel Symptoms | Pregnancy |
|---|---|---|---|
| 1 | 1 | 1 | IVFa |
| 2 | 1 | 1 | Natural |
| 3 | 1 | 1 | Natural |
| 4 | 1 | 1 | — |
| 5 | 1 | 1 | Natural |
| 6 | 1 | 1 | — |
| 7 | 1 | 1 | IVF |
| 8 | 1 | 2 | Natural |
| 9 | 1 | 1 | — |
| 10 | 1 | 1 | — |
IVF = in vitro fertilization.
DISCUSSION
Deep infiltrating pelvic endometriosis with bowel involvement is one of the most invasive forms of endometriosis. These lesions can be removed by either full-thickness disc or segmental resection.10–12,19 For lesions producing partial obstruction and/or in cases of extensive lesions, authors have indicated that bowel resection should be performed.1,2,12,20,21 Most studies have suggested that the incidence of endometriosis recurrence was higher if the bowel endometriotic lesion was not excised.20–22 Remorgida et al.23 showed that at least one-third of patients with bowel endometriosis treated by full-thickness disc resection have persistent disease and suggest that segmental colorectal resection is the best surgical option in this setting.
Currently, preoperative diagnostic resources permit confirmation of rectosigmoid involvement with an accuracy of 99% (sensitivity of 98.1% and specificity of 100%) when transvaginal sonography with bowel preparation is used.24 Preoperatively, among our cases, the endometriotic lesion had reached the submucosal layer of the bowel wall in 3 women, and lesions had reached at least the internal inner muscular layer in all cases.
Robotic-assisted surgery is a topic of interest in the medical community. Since Nezhat and colleagues25–28 worked with the da Vinci robot in the 1990s, other authors have successfully applied this technology. One of the major benefits of robotics is its 3-dimensional technology. In comparison with the traditional 2-dimensional flat view of the surgical field, it provides an ergonomic advantage to the surgeon sitting in the console during surgery, working as a filter to the surgeon's tremor and also improving intuitive movements.27,29 In practice, the 6 df and 3-dimensional visual image permit easier handling to perform suturing. Disadvantages include the cost of implementation and equipment maintenance; the lack of tactile feedback to the surgeon; the presence of bulky robotic arms, as well as long and thick cords; and the inability to move the surgical table once the robot arms are attached and operate in different quadrants at the same time.
Nezhat and colleagues29,30 indicated that robotic technology has no added value for the treatment of early-stage endometriosis, but in severe cases it was a successful tool to avoid conversions to laparotomy. As far as we know, this is the largest reported series regarding robotic assistance for colorectal resection for deep infiltrating endometriosis.
In our initial series, colorectal robotic-assisted laparoscopic bowel resection resulted in a significant improvement in gynecologic and digestive symptoms, as well as the quality of life, in our patients, as was similarly shown by other authors using a laparoscopic approach.5,13 We have obtained a 43% rate of natural pregnancy in our sample only 6 months after surgery, suggesting that the presence of bowel endometriosis negatively influences fecundity. These data are comparable with those reported by other authors.5,29
As we know, the risk of complications depends on clinical conditions, vascular preservation, nerve preservation, the extension of endometriosis infiltration, and the surgeon's experience. The use of robotic assistance provided a very precise dissection of the pelvic area, allowing good visualization of the pelvic plexus nerves, thus providing resection without nerve injury. The stable camera and the freedom of movement allow a very delicate and accurate dissection, as well as identification and preservation of the superior hemorrhoidal artery, providing good irrigation to the rectal stump and diminishing the incidence of rectal fistula. We did not have any complications in this series, such as fistula, local pain, nerve injury, or fecal or urinary incontinence, due to our previous large series in laparoscopic treatment for endometriosis and the association of the robotic technology in these cases.
Because of the high cost of the use of robotics in Brazil, our sample only accounts for a small number of patients. We have selected low-risk patients (young, with low body mass index) as our initial cases to avoid complications in the beginning of our learning curve. We believe that this robotic-assisted laparoscopic approach could represent an alternative technique yielding a more precise dissection, smaller risk of complications, and better patient outcome when compared with conventional laparoscopy, despite the costs of the robotic approach.
Because our center is a referral center for endometriosis treatment, with extensive experience in colorectal laparoscopic surgery, we found that robotic-assisted laparoscopic bowel resection was a feasible alternative approach for the surgeon. In addition, our data are of relevance in clinical practice and show very good results in patients with bowel endometriosis regarding endometriosis treatment, especially with regard to pain relief; bowel symptoms, which showed significant improvement; and the desire to conceive. Data from this study might be important when a decision about bowel resection using a new technology is discussed, especially in cases of deep endometriosis, where precision and safety are even more important because of reports of pelvic nerve injury in complex cases. Larger series with longer-term follow-up and comparison with pure laparoscopic surgery are needed to confirm our initial results and to better understand the role of robotics in deep infiltrating endometriosis. Considering that this kind of surgery requires surgical skills and anatomic knowledge, we think that it should be performed only in selected reference centers where endometriosis surgery is very common.
CONCLUSIONS
Robotic-assisted laparoscopic bowel resection surgery for the treatment of deep infiltrating bowel endometriosis is feasible effective and safe. Robotics can be used as an alternative to the laparoscopic approach. However, other studies are necessary to evaluate the benefits of robotic-assisted laparoscopic bowel resection surgery compared with conventional laparoscopy in the treatment of deep infiltrating colorectal endometriosis.
Contributor Information
Rosa Maria Neme, Faculdade de Medicina, Hospital das Clinicas, Universidade de São Paulo, São Paulo, Brazil.; Centro de Endometriose São Paulo, São Paulo, Brazil. Albert Einstein Hospital, São Paulo, Brazil. Universidade Federal de São Paulo, São Paulo, Brazil.
Vladimir Schraibman, Albert Einstein Hospital, São Paulo, Brazil.; Universidade Federal de São Paulo, São Paulo, Brazil.
Samuel Okazaki, Albert Einstein Hospital, São Paulo, Brazil.; Universidade Federal de São Paulo, São Paulo, Brazil.
Gabriel Maccapani, Albert Einstein Hospital, São Paulo, Brazil.; Universidade Federal de São Paulo, São Paulo, Brazil.
Winston Jenning Chen, Faculdade de Medicina, Hospital das Clinicas, Universidade de São Paulo, São Paulo, Brazil.; Albert Einstein Hospital, São Paulo, Brazil.
Cassia Danielle Domit, Radiology Brazilian College, Digimagem Medicina Diagnóstica, São Paulo, Brazil..
Oskar Grau Kaufmann, Centro de Endometriose São Paulo, São Paulo, Brazil.; Albert Einstein Hospital, São Paulo, Brazil. Universidade Federal de São Paulo, São Paulo, Brazil.
Arnold P. Advincula, Department of Obstetrics and Gynecology, University of Central Florida, Orlando, FL, USA.; Florida Hospital Celebration Health, Celebration, FL, USA.
References:
- 1. Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69(5):727–730 [PubMed] [Google Scholar]
- 2. Chapron C, Dubuisson JB, Chopin N, et al. Deep pelvic endometriosis: management and proposal for a “surgical classification.” Gynecol Obstet Fertil. 2003;31(3):197–206 [DOI] [PubMed] [Google Scholar]
- 3. Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53(3):411–416 [DOI] [PubMed] [Google Scholar]
- 4. Bailey HR, Ott MT, Hartendorp P. Aggressive surgical management for advanced colorectal endometriosis. Dis Colon Rectum. 1994;37(8):747–753 [DOI] [PubMed] [Google Scholar]
- 5. Daraï E, Marpeau O, Thomassin I, et al. Fertility after laparoscopic colorectal resection for endometriosis: preliminary results. Fertil Steril. 2005;84(4):945–950 [DOI] [PubMed] [Google Scholar]
- 6. Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8(6):682–685 [DOI] [PubMed] [Google Scholar]
- 7. Azzena A, Litta P, Ferrara A, et al. Rectosigmoid endometriosis: diagnosis and surgical management. Clin Exp Obstet Gynecol. 1998;25(3):94–96 [PubMed] [Google Scholar]
- 8. Weizman DA, Sullivan P. A laparoscopic approach to small bowel obstruction secondary to endometriosis. Surg Endosc. 2003;17(10):1678–1679 [DOI] [PubMed] [Google Scholar]
- 9. Kumar D. Irritable bowel syndrome, chronic pelvic inflammatory disease and endometriosis. Eur J Gastroenterol Hepatol. 2004;16(12):1251–1252 [DOI] [PubMed] [Google Scholar]
- 10. Redwine DB, Wright JT. Laparoscopic treatment of complete obliteration of the cul-de-sac associated with endometriosis: long-term follow-up of en bloc resection. Fertil Steril. 2001;76(2):358–365 [DOI] [PubMed] [Google Scholar]
- 11. Abbott JA, Hawe J, Clayton RD, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow-up. Hum Reprod. 2003;18(9):1922–1927 [DOI] [PubMed] [Google Scholar]
- 12. Fleisch MC, Xafis D, De Bruyne F, et al. Radical resection of invasive endometriosis with bowel or bladder involvement—long-term results. Eur J Obstet Gynecol Reprod Biol. 2005;123(2):224–229 [DOI] [PubMed] [Google Scholar]
- 13. Dubernard G, Piketty M, Rouzier R, et al. Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod. 2006;21(5):1243–1247 [DOI] [PubMed] [Google Scholar]
- 14. Landi S, Ceccaroni M, Perutelli A, et al. Laparoscopic nerve-sparing complete excision of deep endometriosis: is it feasible? Hum Reprod. 2006;21(3):774–781 [DOI] [PubMed] [Google Scholar]
- 15. Ferrero S, Anserini P, Abbamonte LH, et al. Fertility after bowel resection for endometriosis. Fertil Steril. 2009;92(1):41–46 [DOI] [PubMed] [Google Scholar]
- 16. Advincula AP, Song A. The role of robotic surgery in gynecology. Curr Opin Obstet Gynecol. 2007;19(4):331–336 [DOI] [PubMed] [Google Scholar]
- 17. Holloway RW, Patel SD, Ahmad S. Robotic surgery in gynecology. Scand J Surg. 2009;98(2):96–109 [DOI] [PubMed] [Google Scholar]
- 18. Cho JE, Shamshirsaz AH, Nezhat C, et al. New technologies for reproductive medicine: laparoscopy, endoscopy, robotic surgery and gynecology. A review of the literature Minerva Ginecol. 2010;62(2):137–167 [PubMed] [Google Scholar]
- 19. Verspyck E, Lefranc JP, Guyard B, Blondon J. Treatment of bowel endometriosis: a report of six cases of colorectal endometriosis and a survey of the literature. Eur J Obstet Gynecol Reprod Biol. 1997;71(1):81–84 [DOI] [PubMed] [Google Scholar]
- 20. Anaf V, El Nakadi I, Simon P, et al. Sigmoid endometriosis and ovarian stimulation. Hum Reprod. 2000;15(4):790–794 [DOI] [PubMed] [Google Scholar]
- 21. Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15(4):431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Hooghe T, Hummelshoj L. Multi-disciplinary centres/networks of excellence for endometriosis management and research: a proposal. Hum Reprod. 2006;21(11):2743–2748 [DOI] [PubMed] [Google Scholar]
- 23. Remorgida V, Ragni N, Ferrero S, et al. The involvement of the interstitial Cajal cells and the enteric nervous system in bowel endometriosis. Hum Reprod. 2005;20(1):264–271 [DOI] [PubMed] [Google Scholar]
- 24. Chamié LP, Pereira RM, Zanatta A, Serafini PC. Transvaginal US after bowel preparation for deeply infiltrating endometriosis: protocol, imaging appearances, and laparoscopic correlation. Radiographics. 2010;30(5):1235–1249 [DOI] [PubMed] [Google Scholar]
- 25. Liu C, Perisic D, Samadi D, Nezhat F. Robotic assisted laparoscopic partial bladder resection for the treatment of infiltrating endometriosis. J Minim Invasive Gynecol. 2008;15(6):745–748 [DOI] [PubMed] [Google Scholar]
- 26. Cho JE, Nezhat FR. Robotics and gynecologic oncology: review of the literature. J Minim Invasive Gynecol. 2009;16(6):669–681 [DOI] [PubMed] [Google Scholar]
- 27. Nezhat C, Saberi NS, Shahmohamady B, Nezhat F. Robotic assisted laparoscopy in gynecological surgery. JSLS. 2006;10(3):317–320 [PMC free article] [PubMed] [Google Scholar]
- 28. Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94(7):2758–2760 [DOI] [PubMed] [Google Scholar]
- 29. Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15(3):387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9(1):16–24 [PMC free article] [PubMed] [Google Scholar]


