Laparoscopy combined with transvaginal management appeared to result in less bleeding, shorter operative times, and a lower rate of complication in patients with uterine wound rupture and dense adhesions.
Keywords: Laparoscopy. Cesarean scar pregnancy, Ectopic pregnancy, Uterine artery ligation
Abstract
Objective:
To evaluate the clinical effectiveness of laparoscopic management of cesarean scar pregnancy (CSP) by deep implantation.
Background:
A pregnancy implanting within the scar from a previous cesarean delivery is a rare condition of ectopic pregnancy. There are two different types of CSPs. Type I is caused by implantation of the amniotic sac on the scar with progression toward either the cervicoisthmic space or the uterine cavity. Type II (CSP-II) is caused by deep implantation into a previous CS defect with infiltrating growth into the uterine myometrium and bulging from the uterine serosal surface, which may result in uterine rupture and severe bleeding during the first trimester of pregnancy. Thus, timely management with an early and accurate diagnosis of CSP-II is important. However, laparoscopic management in CSP-II has not yet been evaluated.
Methods:
Eleven patients with CSP-II underwent conservative laparoscopic surgery or laparoscopy combined with transvaginal bilateral uterine artery ligation and resection of the scar with gestational tissue and wound repair to preserve the uterus from March 2008 to November 2011. Patients with CSP-II were diagnosed using color Doppler sonography, and the diagnosis was confirmed by laparoscopy. The operation time, the blood loss during surgery, the levels of β-human chorionic gonadotropin (β-hCG) before surgery, the time taken for serum β-hCG levels to return to <100 mIU/mL postoperatively, and the time for the uterine body to revert to its original state were retrospectively analyzed.
Results:
All 11 operations were successfully performed using laparoscopy with preservation of the uterus. One patient underwent a dilation and curettage after laparoscopic bilateral uterine artery ligation. Eight patients were treated solely by laparoscopic bilateral uterine artery ligation and resection of the scar with gestational tissue and wound repair. The remaining two patients underwent laparoscopic bilateral uterine artery ligation and transvaginal resection of the CS with gestational tissue and wound repair because of dense adhesions and heavy bleeding. The average operation time was 85.5 (±17.5) minutes, and the blood loss was 250.0 (±221.4) mL. The blood serum level of β-hCG returned to <100 mIU/mL in 16.4 (±5.3) days postoperatively. Among the 10 patients who underwent resection of CS and wound repair, the time for the uterus to revert to its original state (judged by ultrasonography) was 10.8 (±3.0) days postoperatively.
Conclusions:
Laparoscopy can remove ectopic gestational tissue and allow subsequent wound repair, as well as provide diagnostic confirmation. Being a minimally invasive procedure, laparoscopic or laparoscopy combined with transvaginal bilateral uterine artery ligation and resection of the scar with gestational tissue and wound repair can become an effective alternative for the treatment of CSP-II.
INTRODUCTION
Cesarean scar pregnancy (CSP) is defined as the gestational sac being implanted into the myometrial scar from a previous cesarean delivery. It is a rare and life-threatening condition of ectopic pregnancy, with an estimated incidence of 1 in 2226 in the study by Seow et al.2 For a pregnancy that develops in a previous CS, the 3 serious complications are massive hemorrhage, uterine rupture, and secondary infection, which often require emergency hysterectomy when hemorrhagic shock occurs and endangers life. Of the many theories for explaining its occurrence, the most reasonable one seems to be that the conceptus enters into the myometrium through a microscopic dehiscent tract of a CS1 and its trophoblasts implant into the myometrium.
According to the classification of CSP by Vial et al,3 there are two different types of CSP. The first type (CSP-I) is caused by implantation of the amniotic sac into the previous CS with progression of pregnancy toward the cervico-isthmic space and the uterine cavity. Such a situation may allow a viable birth, but at an increased risk of massive bleeding from the site of implantation. The second type (CSP-II) is caused by deep implantation into a CS defect with infiltrating growth into the uterine myometrium and bulging from the uterine serosal surface of the uterus. The thickness of uterine myometrium between the sac and the bladder wall is usually <4 mm. Because of the high risk of uterine rupture with life-threatening hemorrhage during the first trimester, CSP-II may result in emergency hysterectomy. Once CSP-II is diagnosed, termination of the pregnancy should be considered. Thus, timely management with early and accurate diagnosis that allows the successful preservation of the uterus is very important.
The main objectives in the clinical management of CSP should be the prevention of massive blood loss and the conservation of the uterus to maintain further fertility, women's health, and quality of life. Although many interventions, including medical or surgical methods, have been reported, there is currently no standardized treatment for CSP, especially for CSP-II. The medical treatment with local and/or systemically administered methotrexate (MTX) carries the risk of heavy bleeding, as reported in a few studies.3,4 Surgical treatment includes excision of the gestational tissues by laparotomy or laparoscopy, or by hysterectomy. Therefore, here we share our experience of the past 4 years in the treatment of 11 patients with CSP-II using laparoscopic surgery or laparoscopy combined with transvaginal management aiming to decrease blood loss and preserve the uterus.
MATERIALS AND METHODS
Clinical Characteristics of Patients
We analyzed the clinical data of 11 patients with CSP-II who were treated with laparoscopic surgery in the Department of Obstetrics and Gynecology at Beijing Anzhen Hospital, Capital Medical University, China, between March 2008 and November 2011. The diagnosis of CSP was confirmed according to the Doppler sonographic criteria proposed by Godin et al. in 1997.1 These criteria are (1) empty uterus and empty cervical canal, (2) development of the gestational sac or mixed-echo mass in the anterior part of the isthmic portion or in the CS, or (3) a very thin myometrium or an absence of healthy myometrium between the bladder wall and the sac or the mass, or a discontinuity in the anterior uterine wall as demonstrated on a sagittal view of the uterus when running through the amniotic sac.
The clinical data of our patients are presented in Table 1. The ages of the patients were from 27 to 44 years. The average time interval between the present CSP to the previous cesarean delivery was 6.3 years (range, 1–14 years). Nine patients had one previous cesarean delivery and two patients had two previous cesarean deliveries. The time of previous suction or dilation and curettage (D&C) ranged from 1 to 4 times. The time interval between the present CSP to the last suction and uterine curettage ranged from 6 months to 5 years. All patients with missed menses had symptoms that ranged from 46 to 78 days. Eight patients presented with irregular vaginal bleeding ranging from 1 to 30 days. The Doppler sonography showed no gestational sac in the uterine cavity and cervical canal, as well as a gestational sac or mixed-echo mass in the anterior part of the isthmic portion or in the CS with a very thin myometrium (1–4 mm) or a discontinuous myometrium in all of the patients in our hospital. Serum β-human chorionic gonadotropin (β-hCG) levels varied from 2104 to 74,000 mIU/mL in the 11 patients.
Table 1.
Characteristics of the 11 Patients with CSP-II
| Patient | Age, y | Time of Previous Cesarean Section, y | Gestational Age at Diagnosis, d | Period of Vaginal Bleeding, d | Treatment Before Hospitalization in Our Department | Initial β-hCG Level, mIU/mL | Hemoglobin, g/L | Cardiac Activity in Gestational Sac (Y/N)a | Diameter of Gestational Sac, cm | Thickness of Myometrium of Anterior Lower Uterine Part, mma |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 1998 | 78 | None | Mifepristone, MTX | 51,520 | 117 | — | 5.0 × 3.0 | — |
| 2 | 32 | 1998, 2002 | 53 | 13 | Mifepristone | 74,000 | 118 | Y | 2.8 | 3 |
| 3 | 30 | 2002, 2009 | 69 | 30 | D&C | 2104 | 60 | N | 4.5 × 4.5 × 3.8 (mass) | Discontinuous myometrium |
| 4 | 35 | 2001 | 55 | 1 | None | >15,000 | 135 | Y | 2.4 | 4 |
| 5 | 33 | 2008 | 58 | 4 | None | 7534 | 117 | — | 1.2 | 4 |
| 6 | 28 | 2000 | 48 | 10 | D&C | 5592 | 99 | N | 8.6 × 6.7 × 6.1 (mass) | — |
| 7 | 27 | 2007 | 60 | None | None | >15,000 | 97 | Y | 6.4 | 3 |
| 8 | 44 | 1997 | 50 | 7 | None | 5949 | 123 | N | 2.4 × 4 (mass) | 2 |
| 9 | 38 | 2003 | 56 | 14 | Mifepristone, D&C | 14,203 | 98 | N | 4.3 × 3.2 (mass) | — |
| 10 | 29 | 2005 | 50 | None | None | >15,000 | 134 | Y | 3.5 | 1 |
| 11 | 34 | 2002 | 46 | 3 | None | 9843 | 121 | — | 2.5 | 2 |
Columns with dashes indicate where there was no information available.
Five patients (1, 2, 3, 6, and 9) had received prior medical treatment or suction and curettage in local hospitals before they were transferred to our hospital for further treatment. Patients 1 and 2 were diagnosed with CSP in the local hospitals. Patient 1 was first treated with 150 mg of mifepristone orally and 600 μg of misoprostol. After 1 week, the medications had not been effective, so 50 mg of MTX was subsequently given intramuscularly. Further, elevated hepatic enzyme levels were noted, and the gestational sac was still alive as determined by ultrasonography. For patient 2, ultrasonography showed the embryo alive after 10 days of 150 mg of oral mifepristone. Patients 3, 6, and 9, who were falsely diagnosed with intrauterine pregnancy in local hospitals, were treated with 150 mg of mifepristone orally, uterine suction (patient 9), and direct uterine suction (patients 3 and 6), to terminate their pregnancies. They were transferred to our hospital immediately because of heavy bleeding (1500 mL, 800 mL, and 500 mL in patients 3, 6, and 9, respectively) during suction. Their prior treatments are presented in Table 1.
Serum β-hCG levels were determined using the Chemiluminescent Microparticle Immunoassay (Architect i-2000 System, Abbott Company, Abbott Park, IL). Pretreatment maternal serum β-hCG levels are presented in Table 1.
Operative Procedure
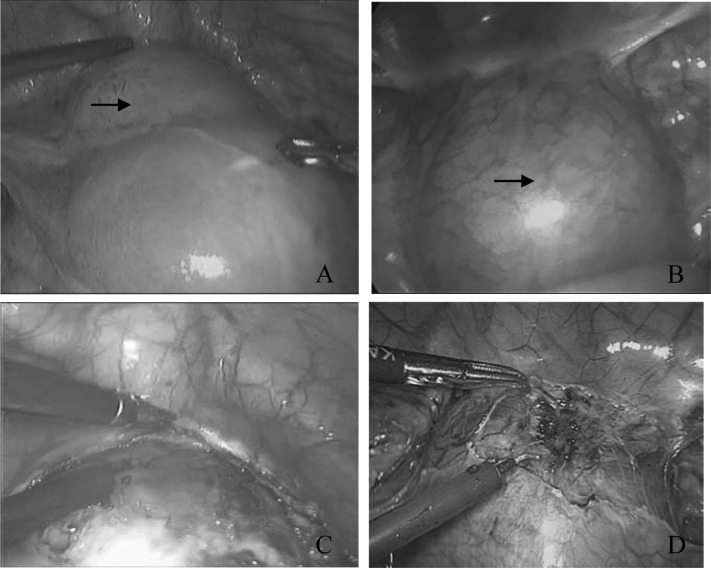
Laparoscopy was performed under general anesthesia with the patient lying in the supine lithotomy position. Laparoscopy revealed a bluish bulging mass over the serosal surface of the lower uterine segment (Figure 1A and B). This confirmed the ultrasonographic diagnosis of CSP-II.
Figure 1.
A bulging mass over the serosal surface of the lower uterus (arrow) in patients 6 (A) and 7 (B) under laparoscopy. A bluish mass made up of gestational tissue, blood clots, fibrin, and myometrial scar, covered by a thin wall of myometrium, when the bladder peritoneum was incised in patients 6 (C) and 7 (D).
All patients received laparoscopic bilateral uterine artery ligation. Patient 1 underwent suction and curettage only, which was monitored under laparoscopy after bilateral uterine artery ligation without the scar resection and the wound repair. In 8 patients, we performed laparoscopic bilateral uterine artery ligation, as well as resection of the scar with its gestational product removed together, followed by closure of the wound. In the other 2 patients, laparoscopic bilateral uterine artery ligation was performed, followed by resection of the CS with its gestational tissue and wound repair transvaginally because of the dense adhesions and heavy bleeding when depressing the bladder during surgery.
Operation I: Laparoscopic Bilateral Uterine Artery Ligation and Resection of the Scar with Gestational Tissue and Wound Repair.
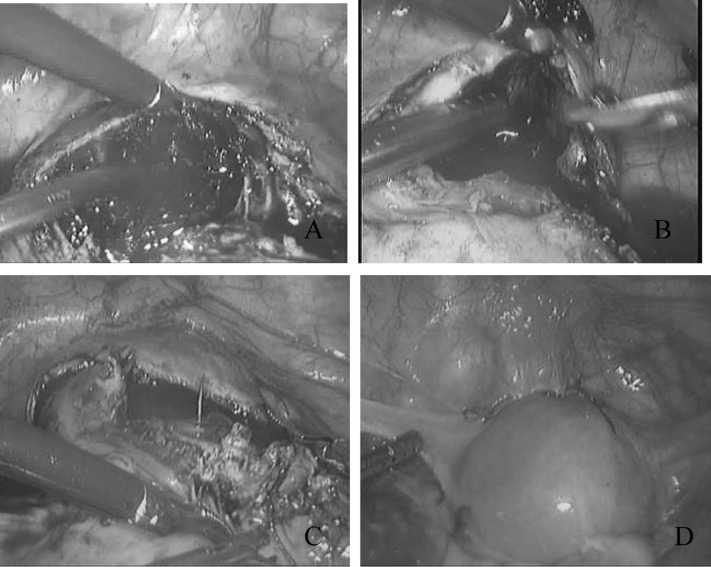
In this procedure, either the anterior or the posterior board ligament was opened to locate both ureters under laparoscopy. The uterine arteries were seen lying over the ureters at the level of the uterine isthmus. Both uterine arteries were ligated using vascular clips. Then 6 IU of dilute vasopressin solution was injected into the myometrium surrounding the scar pregnancy. The bladder peritoneum was then incised, and the bladder was pushed down to allow access to the lower uterine segment and the upper cervical segment. A bluish mass, made up of gestational tissue, blood clots, and myometrial scar, was seen covered by a thin wall of myometrium (Figure 1C and D). Under laparoscopy, uterine suction was performed to remove most of the gestational tissue. A transverse incision was then made over the most prominent area of the mass. Any remnant of the gestational product was removed using grasping forceps. The resulting gap wound in the myometrium was cleaned using suction irrigation, and any dead tissue was further removed using scissors. A continuous suture with Vicryl 0 was used to close the uterine wound laparoscopically (Figure 2).
Figure 2.
(A) Transverse incision over the most prominent area of the mass in patient 6. (B) The mass was removed using grasping forceps, and the resulting space in the myometrium is cleaned using suction irrigation and then clipped using scissors. (C) One layer of continuous sutures along the affected uterine wall was made under laparoscopy. (D) The appearance of the uterus after sutures.
Operation II: Laparoscopic Bilateral Uterine Artery Ligation and Transvaginal Resection of the CS with Gestational Tissue and Wound Repair.
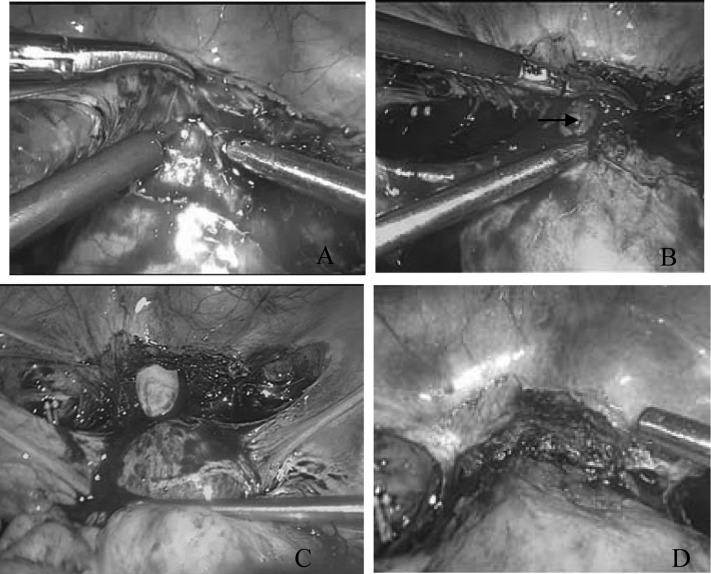
In this procedure, the bilateral uterine arteries were ligated first under laparoscopy. When there were dense adhesions between the bladder and the lower uterine segment, difficult separation and severe bleeding could occur when the bladder was depressed in the attempt to expose the mass (Figure 3A and B). The bleeding could be temporarily stopped by clipping with laparoscopic forceps. Then we immediately incised the anterior fornix and separated the bladder from the cervix using our fingers transvaginally (Figure 3C). The gestational product and its dead tissue were removed, and the wound was cleaned transvaginally. One layer of continuous sutures with Vicryl 0 was used to repair the uterine wound, also transvaginally. Finally, the wound repair was inspected for hemostasis under laparoscopy (Figure 3D).
Figure 3.
(A) In patient 7, severe bleeding occurred when the bladder was pushed down because of the dense adhesion between the bladder and the lower uterine segment. (B) The myometrial scar broken and the conceptus (arrow) was seen. (C) The anterior fornix was incised, and the bladder was separated from the cervix using a finger transvaginally. (D) The closure checked by laparoscopy.
Follow-up
Patients were followed up at 1 month and 3 months after the surgery, and they were also contacted by telephone for information about their menstruation, lower abdominal pain, or any other symptoms. Long-term follow-up was scheduled for the next pregnancy for all the patients.
RESULTS
All 11 operations were successfully performed using laparoscopy with conservative preservation of the uterus without conversion to laparotomy. Patient 1 was an exception who underwent a uterine suction only after laparoscopic bilateral uterine artery ligation without resection of the CS and wound repair. Her intraoperative blood loss was 800 mL. A Foley catheter was inserted transcervically to compress the scar wound for hemostasis. On day 3 postoperatively, ultrasonographic imaging showed a 9.1 × 5.3-cm hyperechoic mass, without active blood flow, bulging to the bladder in the lower part of the uterus. The myometrium of the anterior lower part of the uterus was 7 mm thick. A large hematoma was diagnosed. We chose conservative treatment because the patient's condition was stable and there was not a lot of blood loss from the uterine cavity. The hematoma gradually shrank and finally disappeared 3 months after operation as seen by ultrasonographic monitoring. No other complication occurred during this period. Of the remaining 10 patients, 8 (2, 3, 4, 5, 6, 8, 9, and 11) were treated by laparoscopic bilateral uterine artery ligation and resection of the scar with gestational tissue and wound repair (operation 1) and 2 patients (7 and 10) underwent laparoscopic bilateral uterine artery ligation and transvaginal resection of the CS with gestational tissue and wound repair (operation 2).
The operation time ranged from 60 to 110 minutes (mean, 85.5 ± 17.5). Blood loss during surgery ranged from 50 to 500 mL (average, 250 ± 221.4). The time to serum β-hCG level decrease to <100 mIU/mL after surgery ranged from 9 to 22 days (average, 16.4 ± 5.3). If patient 1, who needed 3 months to recover, is excluded, the time for complete uterine reversion to normalization by ultrasonographic monitoring in the remaining 10 patients ranged from 7 to 14 days (mean, 10.8 ± 3.0). The aforementioned operative data are shown in Table 2.
Table 2.
Operative Data of 11 Patients Who Had Laparoscopic Surgeries for CSP II
| Patient | Initial β-hCG Level, mIU/mL | Blood Loss During Operation, mL | Operation Time, min | Time for β-hCG Level to Reach <100 mIU/mL, d | Time for Uterine Reversion to Original State, da |
|---|---|---|---|---|---|
| 1 | 51,520 | 800 | 110 | 17 | 90 |
| 2 | 74,000 | 150 | 90 | 22 | 10 |
| 3 | 2104 | 100 | 60 | 9 | 14 |
| 4 | >15,000 | 200 | 70 | 16 | 14 |
| 5 | 7534 | 100 | 100 | 10 | 10 |
| 6 | 5592 | 50 | 80 | 9 | 7 |
| 7 | >15,000 | 200 | 90 | 19 | 8 |
| 8 | 5949 | 100 | 60 | 17 | 10 |
| 9 | 14,203 | 300 | 90 | 15 | 7 |
| 10 | >15,000 | 500 | 110 | 22 | 14 |
| 11 | 9843 | 250 | 80 | 24 | 14 |
Time measured by ultrasonography images.
After a follow-up period of 1 to 36 months, no other pregnancy was reported by the 11 patients.
DISCUSSION
CSP is a rare type of ectopic pregnancy. Its risk factors include one or more cesarean deliveries with or without uterine curettage.4 Color Doppler ultrasonography enables earlier and accurate diagnosis of increasing CSP.1,5,6 Treatment methods of CSP, mainly medicine, embolization, surgery, or combinations of those, aim to eradicate the gestational material, reduce bleeding, and preserve the uterus to conserve future fertility. Yet, the standard treatment of choice is still unknown. Management options should be individually based on the type of CSP, the related factors of patients (eg, gestational week, bleeding quality, uterine rupture), the appropriate facilities, and physicians' skills.7,8 Using a combination of methods seems more suitable than using a single method.9
Medical treatment involves systemic or local administration of MTX, potassium chloride, trichosanthis, or mifepristone. The medical approach requires a long therapeutic period, with hospitalization for more than 1 month. It takes about 4 to 16 weeks to reduce the serum level of β-HCG to normal, and it takes 2 months to 1 year for complete reabsorption of the gestational mass. Irregular vaginal bleeding is common and lasts for 2 to 3 months after medication. Therefore, and more important, there have been so many failures in medical treatment. An example of successful medical treatment has been reported in only one limited case report.10 Surgery even by hysterectomy was sometimes performed for profuse bleeding when treatment with medication failed. In addition, systemic MTX may induce side effects of nausea, vomiting, elevated hepatic enzymes, or marrow depression.11,12 In our study, patient 1 had stopped medical treatment when she presented with elevated hepatic enzymes and the embryo alive after systemic administration of mifepristone and MTX. While in case 2 and case 9, the gestational product remained also viable after systemic administration of mifepristone. Patients 2 and 9 were successfully treated with laparoscopic bilateral uterine artery ligation and resection of the scar with gestational tissue and wound repair.
Intra-arterial administration of MTX together with arterial embolization of both uterine arteries had been suggested as an alternative treatment for CSP-II. This was followed by D&C performed 24 to 48 hours after embolization. In this approach, the hospital stay was shorter than it would have been with simple medical MTX administration. The cure rate was reported to reach >90%. However, ultrasonography showed no change in the myometrial thickness of the CS after embolization, which may have resulted in poor healing of the scar and uterine perforation during D&C,12 and even having the risk of the second CSP during the next pregnancy. In patients 7 and 10, small dehiscences were seen on the CS when the pelvic peritoneum was incised under laparoscopy.
The surgical options of CSP include D&C, hysteroscopic resection, laparotomy, or laparoscopic CS gestational product resection and hysterectomy. The gestational product usually could not be totally aspirated by manual vacuum suction or curettage. Intra- or postoperative complications can occur, such as profuse hemorrhage, uterine perforation, shock, and even life-threatening events, especially in CSP-II. Heavy bleeding could happen in approximately 80% of patients with CSP during D&C.5 Three patients with D&C treatment in our series also had severe bleeding during D&C. Therefore, D&C should not be the first and only treatment of choice to treat CSP, especially not CSP-II. D&C monitoring by ultrasonography could be performed 24 to 48 hours after embolization for CSP-I under the condition of preparing for surgery.
Hysteroscopic resection was used to treat CSP-I, but not CSP-II.5 However, there is an increased risk of uterine perforation and failure to resect the gestational product completely at the first attempt. Yang et al. reported that 39 patients with CSP were cured by hysteroscopic removal of conceptive tissues under ultrasonographic guidance after use of mifepristone or MTX and preoperative bilateral uterine artery embolization.13
The important risks of CSP-II include severe bleeding, sometimes during uterine curettage for mistaken diagnosis of intrauterine pregnancy, uterine rupture during the first trimester of pregnancy, severe hemorrhage, hypovolemic shock, emergency hysterectomy, and even life-threatening illnesses. Therefore, surgery should be the first choice of treatment for CSP-II, which may result in complete cure, reduced blood loss, shorter hospital stay, and preserved uterus. Lee et al.14 first reported a case of CSP treated successfully using laparoscopic CS gestational product resection in 1999. Reports of more successful cases followed.15–17 Bilateral uterine artery ligation or diluted vasopressin solution injection into the myometrium was used to decrease blood loss during surgery. In 2002, Fylstra18 reviewed articles on therapeutic management of CSP and concluded that termination of a CS pregnancy by laparotomy and hysterectomy, with repair of the accompanying uterine scar wound, may be the best treatment option.
CS resection with wound repair after uterine artery ligation may avoid severe bleeding. The uterus reverts to its original state much more quickly after scar tissue excision and wound repair. In our series, 10 patients underwent gestational mass resection with wound repair under laparoscopy. However, we did not have a lot of experience when we treated patient 1, during which there was heavy blood loss of nearly 800 mL during uterine suction after laparoscopic ligation of both uterine arteries. We used a Foley catheter to stop the heavy bleeding by pressure tamponade, without performing CS resection and wound repair. The patient had a wound hematoma that disappeared at 3 months postoperatively. We therefore performed our subsequent operations with CS resection and proper wound repair. The blood loss during those surgeries was much less than that in our first surgery. From ultrasonographic monitoring, we found that the uterus reverted to its original state in approximately 2 weeks postoperatively in these 10 patients, which was also a shorter period than that for patient 1.
The key procedures during the operation are to separate the bladder from the lower uterine segment carefully to avoid injury to the bladder and ureters and to completely remove the gestational product and the uterine scar tissue. The approach to repair of the uterine scar wound depended on the size of the gestational mass and the amount of bleeding during the operation. If there is no adhesion and heavy bleeding when depressing the bladder, resection of the CS and wound repair may be performed under laparoscopy. However, if there are dense adhesions, which may cause difficulty in separating the bladder from the lower uterine segment, and heavy bleeding, even damaging the bladder, transvaginal surgery may be a better alternative. These approaches allow us to rapidly separate the bladder from the cervix, resect the gestational mass and the scar, and suture the wound. Patients may benefit from less bleeding, shorter operation time, and fewer complications by undergoing laparoscopy combined with the transvaginal approach rather than laparoscopy alone. We performed the laparoscopic procedure in 8 of our 10 patients. In the other 2 patients (7and 10), we finished the operation by laparoscopy combined with the transvaginal approach. In patient 7, there was a rupture of the lower uterine segment with a small fetal part emerging from the wound, followed by active bleeding when separating the bladder from the lower uterine segment because of dense adhesions. The same condition was also seen in patient 10. Bleeding was controlled by clipping the wound with forceps under laparoscopy. We rapidly separated the bladder from the cervix, resected the gestational mass and the scar, and sutured the wound using the transvaginal approach monitored with laparoscopy.
None of the 11 patients has had another pregnancy, including unwanted pregnancy, because they did not plan to have more children during the follow-up period of 1 to 36 months in our series. Only a few articles about subsequent pregnancy after treatment of CSP were reported. In a study by Ben Nagi et al.,19 29 of 38 women with CSP who were treated by the uterine-conserving surgery were followed. Twenty-four of 29 women attempted to become pregnant; only 1 woman had a recurrent ectopic CSP.
Management strategy, including medication, embolization, surgery, or their combinations, should be selected individually based on the patient's condition and the doctors' skills. From the treatment experience of our 11 patients, we consider that after bilateral uterine artery ligation, laparoscopic CS resection with wound repair is a safe and effective treatment for CSP-II, especially after treatment with medication fails. The operation may be accomplished laparoscopically or by laparoscopy combined with the transvaginal approach. Decreased bleeding, shorter operation time, and fewer complications were seen by using laparoscopy combined with the transvaginal approach than with laparoscopy alone in patients with uterine wound rupture, dense adhesions between the bladder and the lower uterine segment, and active bleeding during operation. Recurrence of CSP has not been reported to our knowledge. It seemed that the resection of the old scar and the new repair closure might minimize the recurrent risk. There should be longer follow-up of these patients and more future studies should be done to assess its safety for subsequent pregnancy.
Contributor Information
Huan-Ying Wang, From the Department of Obstetrics and Gynecology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China..
Jun Zhang, From the Department of Obstetrics and Gynecology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China..
Yan-Na Li, From the Department of Obstetrics and Gynecology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China..
Wei Wei, From the Department of Obstetrics and Gynecology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China..
Da-Wei Zhang, From the Department of Obstetrics and Gynecology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China..
Yu-Qiu Lu, From the Department of Obstetrics and Gynecology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China..
Hao-Feng Zhang, From the Department of Obstetrics and Gynecology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China..
References:
- 1. Godin RA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Feril Steril. 1997;67(2):398–400 [DOI] [PubMed] [Google Scholar]
- 2. Seow KM, Huang LW, Lin YH. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3): 247–253 [DOI] [PubMed] [Google Scholar]
- 3. Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(4):592–593 [DOI] [PubMed] [Google Scholar]
- 4. Maymon R, Halperin R, Mendlovic S, et al. Ectopic pregnancies in a Caesarean scar: review of the medical approach to an iatrogenic complication. Human Reprod Update. 2004;10(6):515–523 [DOI] [PubMed] [Google Scholar]
- 5. Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373–1381 [DOI] [PubMed] [Google Scholar]
- 6. Kirk E, Bourne T. Diagnosis of ectopic pregnancy with ultrasound. Best Pract Res Clin Obstet Gynaecol. 2009;23(4):501–508 [DOI] [PubMed] [Google Scholar]
- 7. Tagore S, Teo SH, Chua SY, Ong CL, Kwek YC. A retrospective review of uterine scar pregnancies: single centre experience. Arch Gynecol Obstet. 2010;282(6):711–715 [DOI] [PubMed] [Google Scholar]
- 8. Michener C, Dickinson JE. Caesarean scar ectopic pregnancy: a single centre case series. Aust N Z J Obstet Gynaecol. 2009;49(5):451–455 [DOI] [PubMed] [Google Scholar]
- 9. Yang XY, Yu H, Li KM, Chu YX, Zheng A. Uterine artery embolisation combined with local methotrexate for treatment of caesarean scar pregnancy. BJOG. 2010;117(8):990–996 [DOI] [PubMed] [Google Scholar]
- 10. Goynumer G, Gokcen C, Senturk B, et al. Treatment of a viable caesarean scar pregnancy with transvaginal methotrexate and potassium chloride injection. Arch Gynecol Obstet. 2009;280(5):869–872 [DOI] [PubMed] [Google Scholar]
- 11. Zhuang YL, Wei LH, Wang W, et al. Treatment of pregnancy in a previous caesarean section scar with uterine artery embolization: analysis of 60 cases. Zhonghua Yi Xue Za Zhi. 2008;88(33):2372–2374 [PubMed] [Google Scholar]
- 12. Wang CJ, Chao AS, Yuen LT, et al. Endoscopic management of cesarean scar pregnancy. Fertil Steril. 2006;85(2):494.e1–4 [DOI] [PubMed] [Google Scholar]
- 13. Yang Q, Piao S, Wang G, Wang Y, Liu C. Hysteroscopic surgery of ectopic pregnancy in the cesarean section scar. J Minim Invasive Gynecol. 2009;16(4):432–436 [DOI] [PubMed] [Google Scholar]
- 14. Lee CL, Wang CJ, Chao A, et al. Laparoscopic management of an ectopic pregnancy in a previous caesarean section scar. Hum Reprod. 1999;14:1234–1236 [DOI] [PubMed] [Google Scholar]
- 15. Wang YL, Su TH, Chen HS. Operative laparoscopy for unruptured ectopic pregnancy in a caesarean scar. BJOG. 2006;113(9):1035–1038 [DOI] [PubMed] [Google Scholar]
- 16. Yan CM. Laparoscopic management of three rare types of ectopic pregnancy. Hong Kong Med J. 2010;16(2):132–136 [PubMed] [Google Scholar]
- 17. Hong SC, Lau MS, Yam PK. Ectopic pregnancy in previous Caesarean section scar. Singapore Med J. 2011;52(6):e115–e117 [PubMed] [Google Scholar]
- 18. Fylstra DL. Ectopic pregnancy within a cesarean scar: a review. Obstet Gynecol Surv. 2002;57:537–543 [DOI] [PubMed] [Google Scholar]
- 19. Ben Nagi J, Helmy S, Ofili-Yebovi D, et al. Reproductive outcomes of women with a previous history of Caesarean scar ectopic pregnancies. Hum Reprod. 2007;22(7):2012–2015 [DOI] [PubMed] [Google Scholar]