Hand-assisted laparoscopic nephrectomy for polycystic kidney disease seemed to result in shorter length of hospital stay and reduced need for transfusion compared with patients undergoing the same procedure with an open technique.
Keywords: Nephrectomy, Polycystic kidney disease
Abstract
Background and Objectives:
Historically, nephrectomy for autosomal dominant polycystic kidney disease was performed by an open technique. We performed this study to compare outcomes in hand-assisted laparoscopic nephrectomy with open nephrectomy in this population.
Methods:
Charts of patients with autosomal dominant polycystic kidney disease who underwent nephrectomy by a transplant surgeon from January 1, 2000, to December 31, 2011, were reviewed. The hand-assisted laparoscopic nephrectomy group was compared with the open group. Data collected included unilateral versus bilateral nephrectomy, operative time, complications, transfusion requirement, and length of stay.
Results:
Of the 78 patients identified, 18 underwent open transabdominal nephrectomy, 56 underwent hand-assisted laparoscopic nephrectomy, and 2 underwent hand-assisted laparoscopic nephrectomy that was converted to an open procedure. Two patients were excluded because another major procedure was performed at the same time as the nephrectomy. Operative times were similar. Patients undergoing open bilateral nephrectomy were more likely to receive transfusion (odds ratio, 3.57 [95% confidence interval, 0.74–17.19]; P = .016), and the length of stay was longer in the open groups (5.9 days vs 4.0 days for unilateral [P = .013] and 7.8 days vs 4.6 days for bilateral [P = .001]). Overall complication rates were similar. The most frequent complications associated with hand-assisted laparoscopic nephrectomy were the development of an incisional hernia at the hand-port site and arteriovenous fistula thrombosis.
Conclusion:
Hand-assisted laparoscopic nephrectomy can be safely performed without increased operative times or complications. The hand-assisted laparoscopic nephrectomy group enjoyed a shorter length of stay, and fewer patients in this group received transfusion. For patients considering renal transplantation, avoidance of transfusion is important to prevent sensitization and limiting access to compatible organs.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder that results in the development of multiple cysts of the kidney and, occasionally, extrarenal organs.1,2 Often, these cysts are detected by early adulthood. Over time, kidney function deteriorates as normal kidney tissue is replaced by cysts. Patients may have pain as a consequence of cyst rupture or hemorrhage. In addition, patients may have symptoms of mass effect including constipation and early satiety. Those with symptoms of cyst rupture, hemorrhage, or extremely large kidneys may benefit from nephrectomy.
Although pretransplant nephrectomy may improve graft and patient survival after renal transplantation,3 we reserve nephrectomy for patients with symptoms or extremely large kidneys that do not allow placement of an allograft. For patients considering renal transplantation, it is in their best interest to receive no blood transfusion before transplantation. Transfusion of blood products may result in the production of antihuman leukocyte antigen antibodies.4–6 The sensitized patient can have difficulty locating compatible organs for transplantation.
Historically, nephrectomy was performed as an open procedure, through a flank or transabdominal approach, and was associated with significant morbidity.7 We performed this study to determine whether hand-assisted laparoscopic nephrectomy (HALN) is the better option.
METHODS
After we received approval from the institutional review board, charts of patients who underwent nephrectomy by a transplant surgeon diagnosed with ADPKD from January 1, 2000, to December 31, 2011, were reviewed. The surgical approach was at the discretion of the operating surgeon. All patients had a noncontrast computed tomography scan performed before surgery. HALN was compared with the open group. Patients in whom conversion to an open procedure occurred were included in the HALN group for data analysis. Patients who underwent a major unrelated operative procedure at the same time or during the same hospitalization were excluded from the study. Data collected included indication for surgery, history of abdominal surgery, unilateral versus bilateral nephrectomy, operative time, complications, transfusion requirement, and length of stay (LOS).
Statistical Analysis
Analyses between HALN and open nephrectomy were compared for unilateral and bilateral nephrectomy separately. Continuous variables were summarized as mean ± standard deviation and compared by use of the Student t test. We used the χ2 or Fisher exact test for comparisons of categorical variables. P < .05 was considered statistically significant.
Operative Technique
All patients underwent general anesthesia with nasogastric or orogastric tube decompression and urinary catheter tube drainage. The patients were positioned supine with arms abducted and received perioperative antibiotics.
The open technique was performed through a midline incision as described by Bennett et al.7
For the HALN, the hand-port incision is placed midline periumbilically. A 30° camera is used. All dissections are performed with the Harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA). For a right nephrectomy, we place a right lower quadrant camera port and a subxiphoid working port. For a left nephrectomy, the camera port is placed in the subxiphoid area and the working port is in the left lower abdomen. The working port must be large enough to allow placement of a laparoscopic gastrointestinal anastomosis (GIA) stapling device.
For bilateral nephrectomy, we prefer to address the right kidney first. A gel roll is placed under the patient's right side, and the operating table is turned to the left. The surgeon's right hand is inserted, and we begin with mobilization of the cecum and ascending colon up to the hepatic flexure. With the surgeon and assistant on the left side of the patient, the surgeon's left hand is inserted, and we begin with mobilization of the cecum and ascending colon up to the hepatic flexure. Superior attachments are divided close to the kidney to avoid injury to the adrenal gland. The kidney is rotated medially while the posterior attachments are divided. Next, the medial aspect of the kidney is carefully dissected, with the surgeon working superiorly from the inferior pole. The ureter is identified first and is ligated with vascular clips and divided. Because of the large size of the kidney, it may be difficult to dissect out the renal vessels. Once the renal pedicle is free, a reticulating GIA stapler with a vascular load is applied high up in the renal pelvis to avoid injury to the aorta and inferior vena cava.
The left nephrectomy is performed with the patient rotated to the right. For unilateral left nephrectomy, a gel roll beneath the patient's left side is used. In the case of bilateral nephrectomy, the right-sided bump is removed before the patient is rotated to the right. A bump is not required because the bowels will fall into the void created from the right nephrectomy. With the surgeon and assistant on the right side of the patient, the surgeon's left hand is inserted and the sigmoid, left colon, and splenic flexure are mobilized. The Gerota fascia is entered and the inferior, lateral, and posterior attachments divided. Superiorly, dissection is carried out close to the kidney to avoid injury to the adrenal gland. Splenorenal ligaments are divided to avoid traction injury on the spleen. With only the medial aspect attached, dissection is performed superiorly from the inferior pole. The ureter is identified, ligated, and divided between vascular clips. Finally, the GIA stapler with a vascular load is used to divide the renal hilum high in the renal pelvis.
The kidneys are delivered through the hand-port incision site. Given the large size of the kidneys, cysts are often ruptured to allow their removal without extension of the hand-port incision. After hemostasis is ensured, the colon is returned to its normal anatomic position and the abdomen closed (Figure 1).
Figure 1.
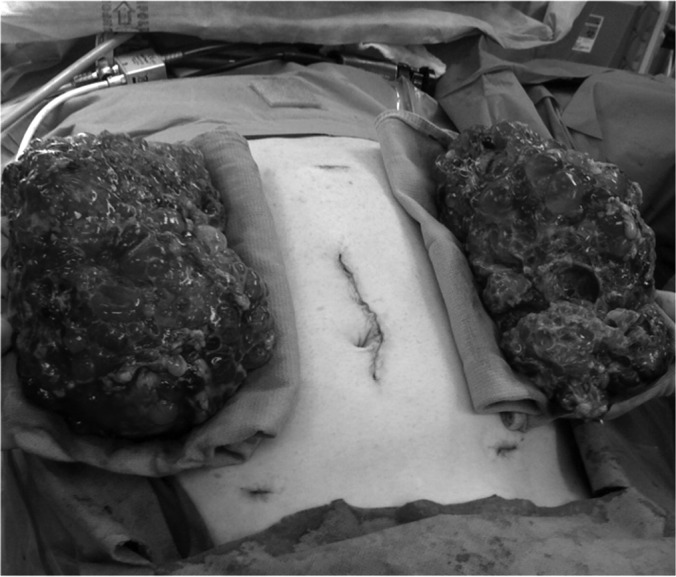
Completed bilateral HALN showing relative size of kidneys in relation to surgical incisions.
RESULTS
Our chart review identified 78 patients who underwent nephrectomy by the transplant surgery service for ADPKD. After we excluded 2 patients undergoing concomitant major abdominal surgery unrelated to the nephrectomy, there were 76 patients in the study. Excluded patients included one patient in the HALN group who underwent concomitant sigmoid colon resection for diverticular disease and one patient who had a splenectomy with splenorenal shunt for portal hypertension due to hepatic fibrosis at the time of his open nephrectomy. Demographics and indications for nephrectomy are shown in Table 1.
Table 1.
Demographics
| HALN (n = 58) | Open (n = 18) | |
|---|---|---|
| Median age, yr | 51.4 ± 8.0 | 49.3 ± 8.3 |
| Male gender | 56.9% | 72.2% |
| Indicationa | ||
| Size | 42 (72.4%) | 16 (88.9%) |
| Pain | 15 (25.9%) | 0 |
| Hematuria/bleeding | 6 (10.3%) | 1 (5.6%) |
| Infection | 2 (3.4%) | 1 (5.6%) |
| Mass | 2 (3.4%) | 0 |
| Prior abdominal surgery | 32 (55.2%) | 7 (38.9%) |
| Simultaneous minor procedure | 24 (41.4%) | 6 (33.3%) |
Some patients had more than one indication for nephrectomy.
Of the 36 patients undergoing unilateral nephrectomy, 24 (66.7%) underwent HALN and 12 (33.3%) underwent the open approach. Of the remaining 40 patients who had a bilateral nephrectomy, 34 (85.0%) had HALN and 6 (15.0%) had an open operation. Two patients in the HALN group underwent conversion to an open procedure but were analyzed in the HALN group by intention to treat. One conversion was because of dense intra-abdominal adhesions from a previous hepatic cyst fenestration, whereas the second patient did not progress laparoscopically because of the inability to dissect adhesions from the kidney to the retroperitoneum, adrenal gland, and liver.
Two patients had a suspicious mass as their indication for nephrectomy. These kidneys were substantially smaller than the cohort (764 g and 379 g) and were removed intact without deliberate rupture of cysts. Pathologic examination confirmed two (1.6-cm and 0.7-cm) papillary renal cell carcinomas: the tumor was limited to the kidney in one specimen, whereas the other was negative for malignancy. There were no unexpected malignancies.
In the unilateral nephrectomy cohort, there were no significant differences between the two groups with regard to history of abdominal surgery, operative time, transfusion requirement, or complications (Table 2). LOS was significantly longer in the open group compared with the HALN group (5.9 days vs 4.0 days, P = .013).
Table 2.
Comparison of HALN Versus Open Unilateral and Bilateral Nephrectomy
| Unilateral Nephrectomy |
Bilateral Nephrectomy |
|||||
|---|---|---|---|---|---|---|
| HALN (n = 24) | Open (n = 12) | P Value | HALN (n = 34) | Open (n = 6) | P Value | |
| Operative times, min | 173.0 ± 82.8 | 147.1 ± 40.2 | .32 | 226.0 ± 59.5 | 234.7 ± 33.7 | .73 |
| Length of stay, d | 4.0 ± 1.9 | 5.9 ± 2.4 | .013 | 4.6 ± 2.0 | 7.8 ± 2.6 | .001 |
| Transfusion | 4 (16.7%) | 5 (41.7%) | .13 | 9 (26.5%) | 5 (83.3%) | .014 |
| Complications | 4 (16.7%) | 2 (16.7%) | >.999 | 11 (32.3%) | 3 (50.0%) | .64 |
| Prior abdominal surgery | 14 (58.3%) | 5 (41.7%) | .48 | 18 (52.9%) | 2 (33.3%) | .66 |
| Specimen weight, g | 1026.1 ± 425.2 | 2381.6 ± 1218.2 | <.0001 | 966.9 ± 369.5 | 2476.6 ± 1120.9 | <.0001 |
In the bilateral nephrectomy cohort, there were no significant differences in history of surgery, operative time, or rate of complications (Table 2). LOS after open bilateral nephrectomy was significantly longer than that after HALN (7.8 days vs 2.6 days, P = .001). Patients undergoing open bilateral nephrectomy were also more likely to require blood transfusion (odds ratio, 3.57 [95% confidence interval, 0.74–17.19]; P = .016).
Complications after nephrectomy are listed in Table 3. The most frequent complications of HALN were wound complications (5 patients), followed by arteriovenous fistula thrombosis (3 patients). Most incisional hernias were identified within the first year after nephrectomy, with one patient presenting 11 days postoperatively with a fascial dehiscence. Arteriovenous fistula thrombosis occurred immediately and required thrombectomy and/or placement of a dialysis catheter. Ascites requiring paracentesis developed in two patients, and both resolved without additional intervention.
Table 3.
Observed Complications of Nephrectomy
| HALN (n = 58) | Open (n = 18) | |
|---|---|---|
| Fascial dehiscence, incisional herniaa | 6 (10.3%) | 1 (5.6%) |
| Arteriovenous fistula thrombosisa,b | 3 (5.2%) | 1 (5.6%) |
| Ascites requiring paracentesisb | 2 (3.4%) | |
| Splenic injury requiring splenectomya | 1 (5.6%) | |
| Bleeding requiring reoperationa | 1 (1.7%) | |
| Vascular injurya | 1 (1.7%) | |
| Postoperative myocardial infarctionc | 1 (1.7%) | |
| Temporary acute mental status changesc | 1 (1.7%) | |
| Pneumoniad | 1 (5.6%) | |
| Prolonged ileuse | 1 (5.6%) |
Clavien classification IIIb, requiring intervention under general anesthesia.
Clavien classification IIIa, requiring intervention without general anesthesia.
Clavien classification IVa, single-organ dysfunction.
Clavien classification II, requiring pharmacologic treatment.
Clavien classification I, not requiring additional intervention.
There were two major surgical complications in the HALN group and one in the open group. One patient had an injury to the right internal iliac artery during dissection of an adherent right lower pole off the iliac vessels. The internal iliac artery was subsequently ligated. The second patient, who was receiving anticoagulation for a prosthetic cardiac valve, returned to the operating suite for postoperative bleeding. At the time of the re-exploration, no active sites of bleeding were identified. The single major complication in the open group was a splenic injury that required splenectomy. This was identified at the time of the open left nephrectomy. Other complications were rare, and there were no in-hospital deaths.
DISCUSSION
Laparoscopic nephrectomy for ADPKD was first described by Elashry et al.8 The kidney dissection was performed through a pure laparoscopy and the enlarged kidney removed by a separate lower abdominal incision. Early studies have concluded that laparoscopic nephrectomy is feasible and safe, with the benefits of decreased pain, shorter hospital LOS, and improved cosmesis but with the disadvantage of significantly longer operative times.9–11
HALN appears to have the benefits of laparoscopic nephrectomy with shorter operative times,12 with the first published report by Nakada et al.13 Use of the hand permitted the surgeon to use tactile sensation, blunt hand dissection, and hand retraction and yielded the ability to apply manual tamponade of bleeding quickly.14,15
Because of the benefits of HALN, the open technique is rarely offered at our center. Unless the patient is known to have significant intra-abdominal adhesions or requires another procedure that requires a laparotomy, most patients will be offered the HALN approach. In contrast to the study of Lipke et al.,16 we did not consider the size of the kidney as a contraindication for HALN.
To our knowledge, this is the largest study comparing HALN with open nephrectomy for ADPKD. Pure laparoscopic nephrectomy for ADPKD has a significantly longer operative time and does not avoid an additional incision to remove the enlarged kidney.8–10 HALN does not incur additional risks compared with the pure laparoscopic technique and offers the same benefits with reduced operative times.11 Despite 41% of patients in the HALN group having an additional procedure at the time of the nephrectomy, as compared with 33% in the open group (Table 1), operative times were not significantly longer. We should mention that many of these additional procedures were of relatively short duration and included placement of dialysis catheters, ventral hernia repair, and cholecystectomy. A prior abdominal surgery did not significantly increase operative times or transfusion or complication rates given that 55% of the HALN group had at least one previous abdominal procedure (Table 1).
On average, LOS was reduced by 2 days in the HALN group. Transfusion requirements were decreased in the HALN group, especially when bilateral nephrectomy was performed. This is particularly important for potential recipients of renal transplantation in which transfusion will expose patients to foreign antigens to which antibodies may form. The development of antihuman leukocyte antigen antibodies (sensitization) may result in difficulty locating a compatible donor kidney in the future.
The primary complications observed were mainly wound complications, which presented within the first year after nephrectomy, and immediate thrombosis of the arteriovenous fistula. The cause of thrombosis is not clear but may be related to arm positioning or transient hypotension during the procedure. Previously reported complications were rare and are similar to those seen in our population.14 Unusual complications include serosal duodenal tear, postoperative pulmonary emboli, and splenic capsular tear.17,18
Interestingly, the specimen size (by weight) was greater in the open group. Through the larger midline incision in the open technique, the kidney can often be removed intact with minimal rupture of cysts. In the HALN group, except in the two patients in whom malignancy was a concern, cysts were deliberately ruptured to allow extraction of the enlarged kidneys through the smaller incision, resulting in lesser weight of the specimen but not necessarily smaller size of the kidneys. Desai et al.18 report concerns of peritonitis-like symptoms or prolonged ileus when cysts are ruptured. This was not observed in our HALN group. Wound infection, likewise, was not evident despite cyst rupture.
As with other procedures, there is a learning curve. A total of 5 surgeons performed HALN during our study period, with 2 surgeons performing most of the HALN procedures. When we compare the operative times for those who performed <5 or >10 procedures, we observed a trend for longer operative times by the less experienced surgeons (276.5 ± 184.6 minutes vs 163.5 ± 69.1 minutes for bilateral [P = .06] and 303.5 ± 31.8 minutes vs 223.5 ± 60.5 minutes for unilateral [P = .07]).
Finally, we compared the patients' charges for their respective procedures. We limited review of the hospital bill to patients who underwent the nephrectomy without additional procedures and only analyzed the operating room charges. We identified 5 open bilateral nephrectomies, 7 open unilateral nephrectomies, and 18 bilateral and 16 unilateral HALNs. For bilateral nephrectomy, the operating room charges were similar ($17,268.13 ± $6484.98 for open vs $22,173.90 ± $7135.45 for HALN, P = .18). However, the operating room charges for unilateral nephrectomy were significantly lower in the open group ($9093.81 ± $2310.98 for open vs $15,845.40 ± $4029.77 for HALN, P = .0005). Because the open group had a 2-day longer LOS on average and therefore may have required an additional inpatient hemodialysis treatment, the difference in overall hospital charge would be less. At our hospital, the current charge for a medical-surgical semiprivate room is $798 per day and inpatient hemodialysis costs $399 per session.
The principal limitation to this study is that it is retrospective. Our department will initially offer the HALN approach. Certainly, it would be difficult to randomize patients to open transabdominal nephrectomy in this era. Patients with known intra-abdominal adhesions are being referred for retroperitoneal nephrectomy (open vs minimally invasive) by our urology colleagues. Although this approach avoids violation of the peritoneal cavity, bilateral nephrectomy will require two incisions, as well as patient repositioning.19,20
CONCLUSION
Compared with open nephrectomy, HALN for ADPKD can be safely performed without increased operative times or complications. Patients who underwent HALN enjoyed a shorter LOS, and fewer patients in this group received transfusion. Extremely large kidney size or history of abdominal surgery is not an absolute contraindication for the HALN approach.
Contributor Information
Mary Eng, Department of Surgery, University of Louisville, Louisville, KY, USA..
Christopher M. Jones, Department of Surgery, University of Louisville, Louisville, KY, USA..
Robert M. Cannon, Department of Surgery, University of Louisville, Louisville, KY, USA..
Michael R. Marvin, Department of Surgery, University of Louisville, Louisville, KY, USA..
References:
- 1. Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342 [DOI] [PubMed] [Google Scholar]
- 2. Parfrey PS, Bear JC, Morgan J, et al. The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1085–1090 [DOI] [PubMed] [Google Scholar]
- 3. Brazda E, Ofner D, Riedmann B, Spechtenhauser B, Margreiter R. The effect of nephrectomy on the outcome of renal transplantation in patients with polycystic kidney disease. Ann Transplant. 1996;1:15–18 [PubMed] [Google Scholar]
- 4. Everett ET, Kao KJ, Scornik JC. Class I HLA molecules on human erythrocytes. Transplantation. 1987;44:123–129 [DOI] [PubMed] [Google Scholar]
- 5. Scornik JC, Ireland JE, Howard RJ, Pfaff WW. Assessment of the risk for broad sensitization by blood transfusion. Transplantation. 1984;37:249–253 [DOI] [PubMed] [Google Scholar]
- 6. Scornik JC, Pfaff WW, Howard RJ, et al. Increased antibody responsiveness to blood transfusion in pediatric patients. Transplantation. 1994;58:1361–1365 [PubMed] [Google Scholar]
- 7. Bennett AH, Stewart W, Lazarus JM. Bilateral nephrectomy in patients with polycystic renal disease. Surg Gynecol Obstet. 1973;137:819–820 [PubMed] [Google Scholar]
- 8. Elashry OM, Nakada SY, Wolf JS, McDougall EM, Clayman RV. Laparoscopy for adult polycystic kidney disease: a promising alternative. Am J Kidney Dis. 1996;27:224–233 [DOI] [PubMed] [Google Scholar]
- 9. Dunn MD, Portis AJ, Elbahnasy AM, et al. Laparoscopic nephrectomy in patients with end-stage renal disease and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:720–725 [DOI] [PubMed] [Google Scholar]
- 10. Seshadri PA, Poulin EC, Pace D, Schlachta CM, Cadeddu MO, Mamazza J. Transperitoneal laparoscopic nephrectomy for giant polycystic kidneys: a case control study. Urology. 2001;58:23–27 [DOI] [PubMed] [Google Scholar]
- 11. Binsaleh S, Luke PP, Nguan C, Kapoor A. Comparison of laparoscopic and open nephrectomy for adult polycystic kidney disease: operative challenges and technique. Can J Urol. 2006;13:3340–3345 [PubMed] [Google Scholar]
- 12. Wolf JS, Moon TD, Nakada SY. Hand assisted laparoscopic nephrectomy: comparison to standard laparoscopic nephrectomy. J Urol. 1998;160:22–27 [PubMed] [Google Scholar]
- 13. Nakada SY, Moon TD, Gist M, Mahvi D. Use of the PneumoSleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997;49:612–613 [DOI] [PubMed] [Google Scholar]
- 14. Jenkins MA, Crane JJ, Munch LC. Bilateral hand-assisted laparoscopic nephrectomy for autosomal dominant polycystic kidney disease using a single midline handport incision. Urology. 2002;59:32–36 [DOI] [PubMed] [Google Scholar]
- 15. Rehman J, Landman J, Andreoni C, et al. Laparoscopic bilateral hand assisted nephrectomy for autosomal dominant polycystic kidney disease: initial experience. J Urol. 2001;166:42–47 [PubMed] [Google Scholar]
- 16. Lipke MC, Bargman V, Milgrom M, et al. Limitations of laparoscopy for bilateral nephrectomy for autosomal dominant polycystic kidney disease. J Urol. 2007;177:627–631 [DOI] [PubMed] [Google Scholar]
- 17. Brown JA, Siddiqi K. Bilateral hand-assisted laparoscopic renal surgery in the supine position: the spleen at risk. JSLS. 2011;15:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai PJ, Castle EP, Daley SM, et al. Bilateral laparoscopic nephrectomy for significantly enlarged polycystic kidneys: a technique to optimize outcome in the largest of specimens. BJU. 2008;101:1019–1023 [DOI] [PubMed] [Google Scholar]
- 19. Gill IS, Kaouk JH, Hobart MG, et al. Laparoscopic bilateral synchronous nephrectomy for autosomal dominant polycystic kidney disease: the initial experience. J Urol. 2001;165:1093–1098 [PubMed] [Google Scholar]
- 20. Wyler SF, Bachmann A, Ruszat R, et al. Retroperitoneoscopic nephrectomy for autosomal dominant polycystic kidney disease: initial experience. Urol Int. 2007;79:137–141 [DOI] [PubMed] [Google Scholar]


