Abstract
The aim of this study was to characterize the pathways of basolateral secretion of common dietary tocopherols from polarized Caco-2 monolayers, a model of intestinal absorption. Given differences in structure and physical properties, we hypothesized that secretion may differ between different forms of vitamin E, thus potentially contribute to the selectivity seen in vivo. Monolayers were incubated apically and simultaneously with 10 μmol/L α-, γ-, and δ-tocopherol (1:1:1) in lipid micelles. Treatment with the microsomal triglyceride transfer protein inhibitor BMS201038 revealed that the triglyceride-rich particle secretory pathway (apolipoprotein B–dependent pathway) accounted for ∼80% of total tocopherol secretion, without selectivity among the three forms of vitamin E. Apolipoprotein B–independent secretion of tocopherols (and cholesterol) was greatly enhanced by the liver X receptor agonist T0901317. T0901317 induced ATP-binding cassette transporter A1 (ABCA1) protein expression and basolateral secretion of tocopherols to apolipoprotein A1. ABCA1-dependent secretion demonstrated vitamer selectivity such that efficiency of secretion of α- and γ-tocopherols exceeded that of δ-tocopherol. Basal addition of HDL stimulated vitamin E secretion but without selectivity among the three forms, whereas LDL had no effect. Basal addition of scavenger receptor class B member I (SR-BI) blocking antibody, which inhibits the interaction between SR-BI and HDL, increased basal accumulation of all tocopherols, demonstrating a role for SR-BI in cellular re-uptake of secreted vitamin E. These findings demonstrated that vitamin E and cholesterol utilize common pathways of secretion and that secretion via the ABCA1 pathway favors certain forms of vitamin E.
Introduction
Vitamin E and cholesterol share several features, including physical properties, localization, and transport. However, the extent to which they share pathways of cellular secretion, particularly those involving membrane ATP-binding cassette (ABC)3 lipid transport proteins, has not been systematically investigated. The best characterized mechanisms of vitamin E secretion are those involving triglyceride-rich lipoprotein particles such as chylomicrons from enterocytes and very low density lipoproteins from the liver. The physiological relevance of these mechanisms is reflected by the severe vitamin E deficiency seen in individuals unable to synthesize the microsomal triglyceride transfer protein (MTP) (1). MTP is necessary for the transfer of lipids to the nascent apolipoprotein (APO) B particle in the endoplasmic reticulum. However, the vitamin E deficiency associated with the resulting familial abetalipoproteinemia can be overcome by increasing the amount of vitamin E in the diet (2), suggesting alternative pathways of vitamin E secretion from intestine and liver.
It is now known that there are several pathways of cellular cholesterol secretion or uptake that involve membrane-spanning proteins such as the scavenger receptor class B member I (SR-BI) and the ABC transporters. Several members of the latter category, including ABCA1, ABCG1, ABCB4, and ABCG5/G8 are known to be central to the reverse cholesterol transport of cholesterol from peripheral tissues to the liver or intestine for excretion. ABCA1 facilitates the efflux of cholesterol and phospholipids to APOA1, and this specific APOA1-dependent stimulation of secretion is a signature marker for ABCA1 activity (3, 4). ABCA1 expression can be induced by activation of the nuclear receptors liver X receptor (LXR) and retinoid X receptor (5, 6). ABCG1 facilitates the secretion of cholesterol to pre-HDL, resulting in the formation of mature HDL particles, but not to lipid-free APOA1 (7, 8). One of the final steps in reverse cholesterol transport involves liver SR-BI, which takes up cholesterol from HDL. However, in macrophages, SR-BI is a bidirectional transporter, which can efflux or uptake cholesterol (9, 10). Pathways of cholesterol secretion involving these transporters have been studied in Caco-2 monolayers.
Caco-2 is a cell line derived from a human colon adenocarcinoma, which when grown on a porous membrane spontaneously differentiates, forming a polarized monolayer with tight junctions separating the apical and basolateral membranes (11). Caco-2 monolayers develop microvilli and express most of the brush border hydrolases on the apical membrane. These monolayers express, or can be induced to express, several pathways of cholesterol secretion from the basolateral membrane. Differentiated Caco-2 monolayers express MTP (12) and secrete apolipoproteins B-100, B-48, E, A-I, A-IV and C-III from the basolateral membrane (11). Cholesterol is secreted basally through either an APOB-dependent or -independent pathway (13). Lipoproteins of the chylomicron size (APOB-dependent) are formed and secreted basolaterally when oleic acid is administered apically in mixed micelles (14). Treatment of Caco-2 monolayers with 0.1μmol/L of the MTP inhibitor BMS201038 decreases basolateral secretion of APOB and triacylglycerol by 95% and 98%, respectively (15). A similar MTP inhibitor (BMS200150) does not affect APOB-independent cholesterol secretion pathways (13), indicating specificity of MTP inhibition. When confluent this cell line expresses ABCA1 and ABCG1 on the basolateral membrane (15–17), which are likely involved in the secretion of APOA1-containing HDL-like particles (APOB independent) (13, 18). In Caco-2 monolayers ABCA1 is induced by LXR agonists such as T0901317 (T), resulting in increased basolateral cholesterol efflux (15). SR-BI is expressed on both the apical and basal membranes (19, 20). Apical SR-BI is involved in cholesterol efflux to mixed micelles (20), but the role of basal SR-BI in cholesterol efflux or uptake has not been investigated.
The role(s) that these specific proteins play in cellular tocopherol trafficking have recently raised interest. One of the few reports on this topic demonstrated that α-tocopherol (α-TOH) secretion was enhanced from human fibroblasts overexpressing ABCA1 and was reduced in human fibroblasts lacking functional ABCA1 (21). Using Caco-2 monolayers, Anwar et al. (18) reported that α-TOH could be secreted through both large APOB particles and HDL particles, and Reboul et al. (22) demonstrated that α-TOH and γ-tocopherol (γ-TOH) could be secreted to APOA1, suggesting a role for ABCA1. Finally, SR-BI has been implicated in apical uptake and apical efflux of α- and γ-TOH from Caco-2 monolayers (23), but the potential role of this protein in basolateral tocopherol trafficking has not been investigated. Therefore, several cholesterol transporters appear to play roles in membrane transport of vitamin E, but little is known about the selectivity for the different forms of vitamin E and the possible involvement of other cholesterol transporters. Because of the many advantages offered by the Caco-2 Transwell model, we used this system to investigate APOB-dependent and -independent pathways by which vitamin E is secreted from the basolateral surface of these cells and whether such pathways exhibit selectivity among the common dietary tocopherols.
Materials and Methods
Materials.
Caco-2 cells (BBE1 subclone) were purchased from ATCC and grown on Transwells (0.4-μm pores, 4.7 cm2, Corning). RRR-α-TOH (natural stereoisomer) was obtained from ACROS Organics, and RRR-γ-TOH and -δ-tocopherol (δ-TOH) from Fluka Biochemicals. Deuterated RRR-α-TOH (d6α-TOH) was produced from d6α-tocopheryl acetate by potassium carbonate hydrolysis. The acetate form was a gift from Graham Burton (Occell). We custom synthesized d9α-TOH, used as an internal standard, in our laboratory. All tocopherols had a purity >98%. FBS, penicillin, streptomycin, amphotericin B, and DMEM were from Cellgro (Corning). PBS was from Hyclone. Oleic acid, monoolein (2-oleoylglycerol), sodium taurocholate, BSA, human APOA1, and human HDL were from Sigma-Aldrich. Human LDL was from Biomedical Technologies. The microsomal triglyceride inhibitor, BMS201038, was a gift from Bristol-Myers Squibb; stock solution was 200 μmol/L in DMSO. The LXR agonist, T0901317, was from Cayman; stock solution was 500 μmol/L in ethanol. Probucol was from EMD Biosciences. N,O-bis(trimethylsilyl)trifluoroacetamide + 1% trimethylchlorosilane and pyridine were from Pierce. Antibodies against ABCG1 and SR-BI were purchased from Novus and that against ABCA1 was from Abcam.
Cell culture.
Caco-2 cells were grown on 100-mm plates and subcultured onto porous membranes inserted into Transwells. Cells were grown with DMEM supplemented with 10% FBS, l-glutamine, penicillin (100 IU), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/mL). Media in the Transwell cultures was replaced every other day. Confluency was observed at least 5 d after seeding, and cultures were used for experimentation 10 d postconfluency because it had been demonstrated that at this time point Caco-2 monolayers secreted APOB-containing lipoprotein and HDL particles with typical characteristics (24). Because only the basal compartment contained phenol red, monolayer integrity was assessed by measuring the appearance of phenol red in the apical compartment. The concentration of phenol red was determined spectrophotometrically at 560 nm in aliquots of apical media containing 5% NaOH 1N. The maximum acceptable transfer rate was 3%/24 h; 5%/h was reported to correspond to a transepithelial electrical resistance >150 Ω (25), a common minimum for monolayer integrity assessed by electrical impedance. We confirmed the phenol red method in experiments by comparing to transepithelial electrical resistance measurements and thereafter assessed monolayer integrity using the phenol red method.
Basal secretion of tocopherols.
The three vitamers α-, γ-, and δ-TOH were combined in a 1:1:1 ratio in mixed lipid micelles and added to the apical compartment containing PBS and 1% BSA. The final lipid concentrations in the apical compartment were 25 μmol/L monoolein, 50 μmol/L oleic acid, 500 μmol/L taurocholate, and 10 μmol/L of each of the 3 tocopherols. Mixed micelles were made by evaporating ethanolic solutions of lipids under nitrogen and swirling the resulting film in PBS, resulting in a clear solution. The apical compartment contained PBS because micelles precipitate in DMEM as a result of the presence of calcium (26). The basal compartment of the Transwells were rinsed with DMEM without serum for 3 h before the apical addition of tocopherol micelles and then fresh DMEM was added. As needed, BMS201038 (0.1 μmol/L), T0901317 (1 μmol/L), or probucol (50 μmol/L) was added apically, and the corresponding amounts of DMSO or ethanol were added to control cultures, which did not exceed 0.05% and 0.2% respectively. Monolayers were preincubated with BMS201038 and probucol for 3 h before administration of tocopherol micelles; T0901317 was pre-incubated overnight. APOA1, HDL, and LDL were added as needed into the basolateral compartment. When HDL or LDL was used as an acceptor, micelles were made with d6α-TOH because of the presence of (unlabeled) endogenous α-TOH in the lipoproteins. Antibody against SR-BI was added to the basolateral compartment (1:1000 dilution) 1 h before apical administration of tocopherols.
Analysis of tocopherols and cholesterol.
Tocopherols and unesterified cholesterol were extracted from cells, apical media, and basal media. Cells were washed twice with cold 0.9% NaCl, scraped off the porous membrane, and sonicated in 0.9% NaCl:ethanol (1:2, v:v). Two volumes of ethanol were added to basal media. The internal standard, d9-αTOH, was added to cells and media, and lipids were extracted with methyl tert-butyl ether:hexane (1:6, v:v). Tocopherols and cholesterol were extracted and analyzed simultaneously by GC-MS using selective ion monitoring, as described previously (27).
Determination of ABCA1, ABCG1, and SR-BI protein.
Scraped monolayers were sonicated in buffer A containing 10 mmol/L HEPES, 1.5 mmol/L MgCl, 10 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1.1 mmol/L dithiothreitol, and the following protease inhibitors: leupeptin (10 μg/mL), chymostatin (2 μg/mL), antipain (5 μg/mL), aprotinin (10 μg/mL), calpeptin (1 μmol/L), and pepstatin (1 μg/mL). After centrifugation, the pellet was resuspended in buffer A and sonicated again. Western blots were performed according to manufacturers’ instructions, with the following modifications: for ABCA1, 100 μg protein was analyzed by SDS-PAGE (7% polyacrylamide gel) without previous boiling (ABCA1 was undetectable when samples were boiled); for ABCG1, 20 μg protein was boiled; and for SR-BI, 10 μg protein was boiled. Actin was used as the loading control. Total protein was determined by the Bradford method using Bio-Rad reagents according to manufacturer’s instructions.
Statistical analysis.
Data are expressed as means ± SDs of independent observations, and homogeneity of the variances was tested. Statistical analysis was performed using 1-factor ANOVA with post-hoc paired Student’s t test to determine the significance of differences among vitamers and using 2-factor ANOVA with post-hoc Student’s t test to determine whether treatment effects were different among vitamers. JMP 7 software (SAS) was used to perform the analysis. Differences were considered statistically significant at P < 0.05. Within a typical experiment, each treatment involved 3 Transwell cultures. Data from each Transwell culture were considered an independent observation because the cultures were treated and analyzed independently. In cases where n > 3, independent observations from multiple similar experiments were used. In all cases, results are representative of at least 3 independent experiments.
Results
Cellular and basal accumulation of tocopherols by untreated Caco-2 monolayers in Transwell cultures.
α-, γ-, and δ-TOHs administered in the apical compartment to untreated (control) Caco-2 monolayers in a 1:1:1 ratio (10 μmol/L each) as a single micelle preparation for 24 h were taken up by the cells to similar extents (Fig. 1A, control data). Cell-associated tocopherol was similar among cultures treated simultaneously and those treated with individual tocopherols (results not shown); therefore, the 3 vitamers were administered simultaneously in all subsequent experiments. Caco-2 monolayers typically took up 30–40% of added tocopherols (results not shown).
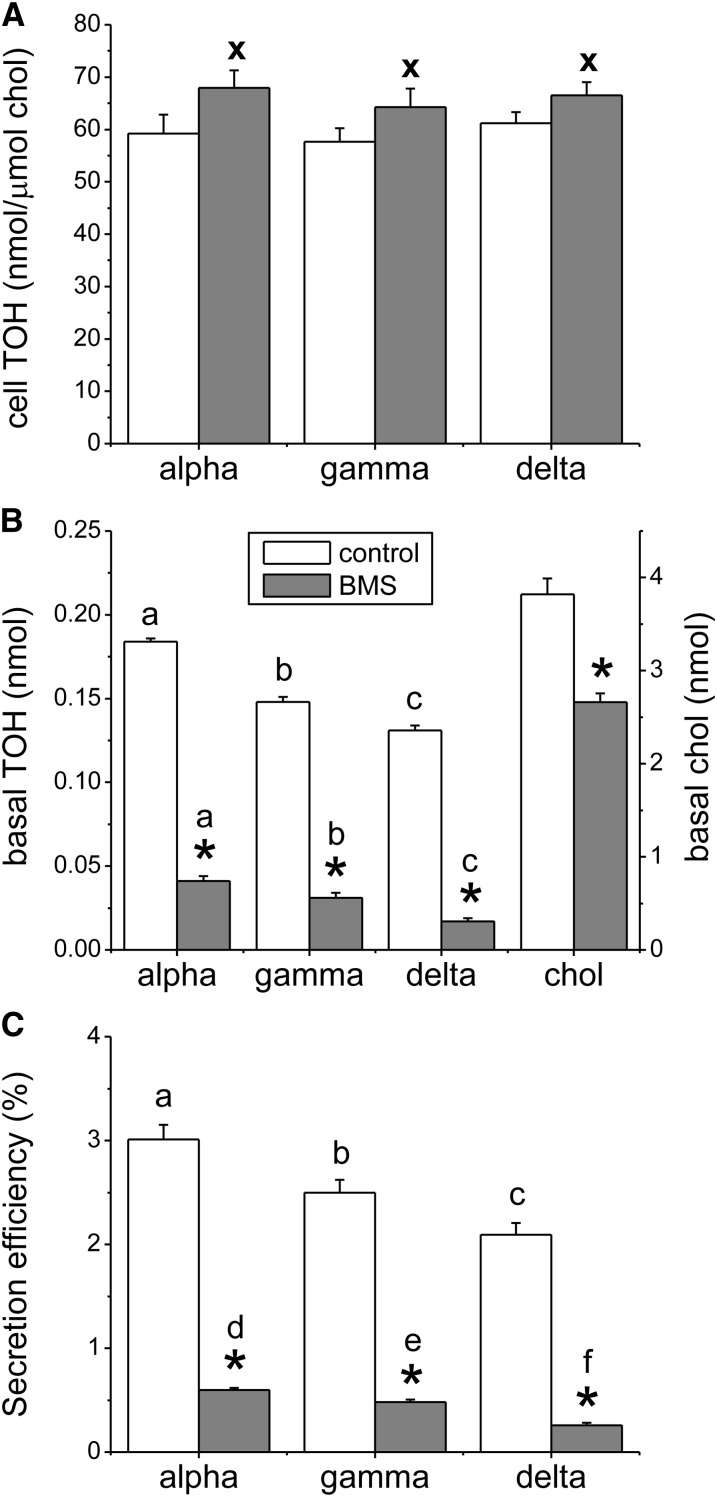
FIGURE 1.
Effect of MTP inhibition on cell-associated tocopherols and basal tocopherol secretion in Caco-2 monolayers. Monolayers were preincubated for 3 h with BMS201038 or with the equivalent volume of DMSO vehicle. Mixed micelles providing 10 μmol/L α-, γ-, and δ-TOH were added apically. (A) Cell-associated tocopherols were determined after 24 h. (B) Basal tocopherol and cholesterol secretion into DMEM was determined after 24 h. (C) Secretion efficiency after 24 h, where secretion efficiency = mass secreted relative to the total mass taken up by the cells (cell plus secreted tocopherol). Results are expressed as means ± SDs, n = 3. *P < 0.001 vs. control; x, P < 0.05 vs. control; labeled means in the same treatment without a common letter differ: a–c, P < 0.01; d–f, P < 0.05; BMS, BMS201038; chol, unesterified cholesterol; MTP, microsomal triglyceride transfer protein; TOH, tocopherol.
Tocopherol accumulation in the basolateral compartment by untreated cultures was assessed 24 h after micelle addition to the apical compartment (Fig. 1B, control data). Basolateral accumulation of the 3 vitamers was significantly different, in the order α > γ > δ (P < 0.001). Secretion efficiencies, that is, the mass secreted relative to the total mass taken up by the cells (the total mass taken up by the cells corresponds to the mass recovered in cells plus that in basal medium after 24 h), were in the same order (P < 0.001), ranging from a mean of 3.5% for α-TOH to 2.4% for δ-TOH (Fig. 1C, control data).
Effect of MTP inhibition on basal tocopherol secretion by Caco-2 monolayers.
BMS201038, a synthetic MTP inhibitor known to completely block lipidation of APOB and thus chylomicron assembly and secretion in Caco-2 cultures (15), substantially reduced basolateral secretion of all 3 tocopherols as well as that of unesterified cholesterol (Fig. 1B). The percent inhibition of tocopherol secretion by BMS201038 was similar among the 3 tocopherols, suggesting that the APOB pathway did not discriminate between vitamers. About 20% of total tocopherol secretion persisted after MTP inhibition. This APOB-independent secretion differed among the 3 vitamers when expressed either in absolute terms (Fig. 1B, P < 0.001) or as secretion efficiency (Fig. 1C, P < 0.01) in the order α > γ > δ. Consistent with its inhibition of TOH secretion, BMS201038 significantly increased cell-associated tocopherols of all 3 vitamers (Fig. 1A, P < 0.05). Whereas BMS201038 decreased basal unesterified cholesterol, cellular unesterified cholesterol remained unchanged, probably because of homeostatic regulation of cellular unesterified cholesterol through esterification and synthesis. Therefore, cell-associated TOH was expressed relative to cellular unesterified cholesterol.
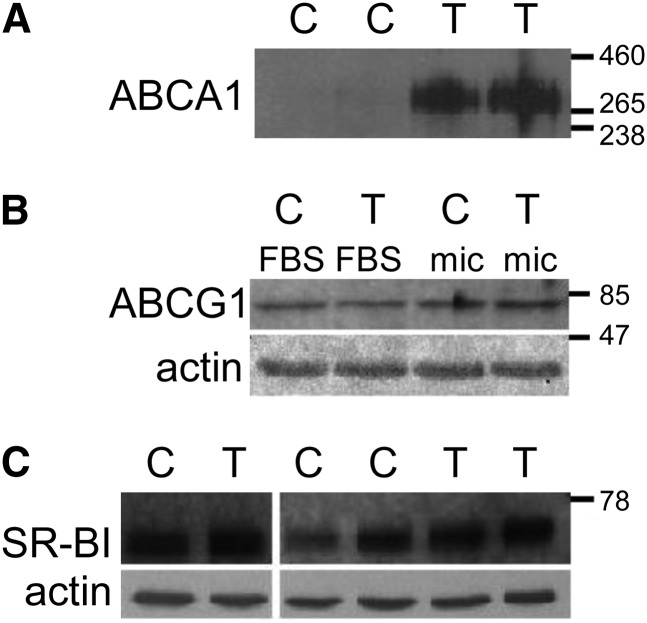
LXR induction increases ABCA1 protein expression and basal tocopherol accumulation in Caco-2 monolayers.
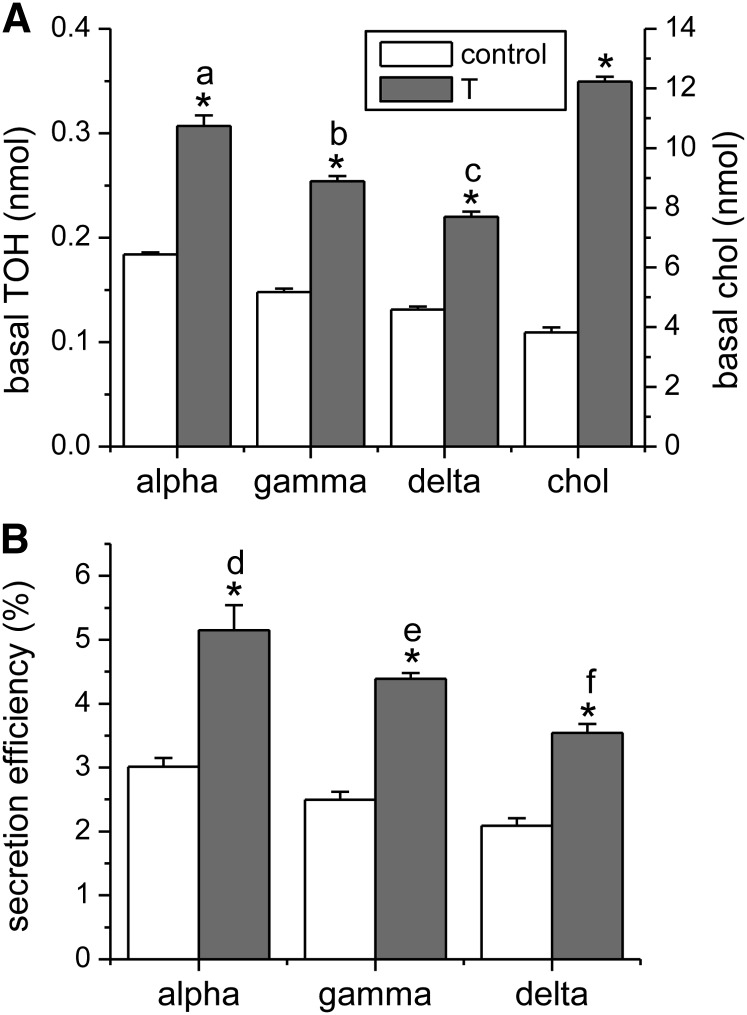
Treatment of Caco-2 monolayers with the LXR agonist T0901317 increased ABCA1 protein but did not affect expression of ABCG1 or SR-BI as determined by Western blot (). For the determination of ABCA1, we loaded 100 μg protein without boiling, which affected actin determination (signal from lanes overlapped). LXR activation is known to also induce expression of ABCG5 and ABCG8 (28), which are apical cholesterol transporters. However, no change occurred in apical cholesterol secretion from monolayers treated with the LXR agonist T0901317 (data not shown), suggesting that expression of ABCG5 and ABCG8 proteins was not affected. LXR activation significantly increased total basolateral tocopherol accumulation of all 3 vitamers (Fig. 3A, P < 0.001) without affecting tocopherol uptake. The percent increase in basal tocopherol secretion (Fig. 3A) and in secretion efficiencies (Fig. 3B) was similar for all 3 vitamers, ∼70%. Basal tocopherol accumulation in the LXR-induced state differed among the 3 vitamers when expressed either in absolute terms (Fig. 3A, P < 0.001) or as secretion efficiency (Fig. 3B, P < 0.01) in the order α > γ > δ. The stimulatory effect of T0901317 on basal accumulation of unesterified cholesterol (∼300% increase, Fig. 3A, P < 0.001) was greater than that on the tocopherols.
FIGURE 3.
Effect of LXR activation on basal tocopherol secretion in Caco-2 monolayers. Monolayers were treated as in Fig. 2. (A) Basal tocopherol and cholesterol secretion were determined after 24 h. (B) Secretion efficiency. Results are expressed as means ± SDs, n = 3 independent observations. *P < 0.001 vs. control; labeled means in the same treatment without a common letter differ: a–c, P < 0.001; d–f, P < 0.01. chol, unesterified cholesterol; LXR, liver X receptor; T, T0901317; TOH, tocopherol.
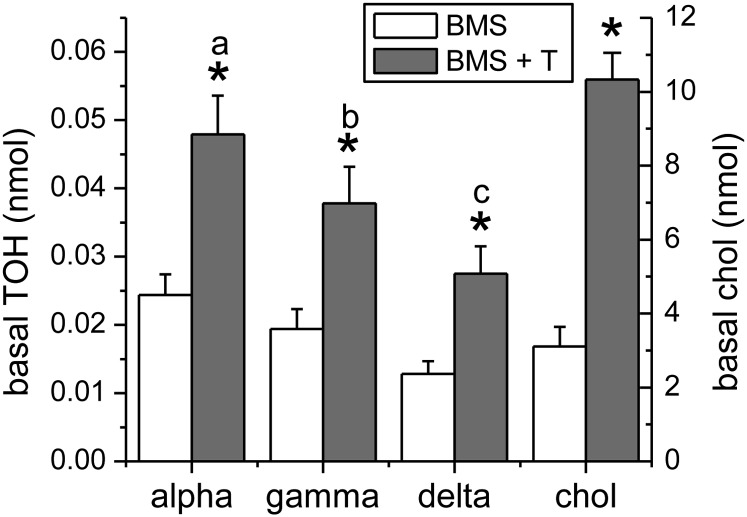
To assess whether the observed stimulation of tocopherol secretion upon LXR induction could involve an APOB-independent pathway, we treated Caco-2 monolayers simultaneously with the MTP inhibitor BMS201038 and the LXR agonist T0901317. Treatment with both agents resulted in a significantly greater basal accumulation of unesterified cholesterol and of all 3 tocopherols relative to treatment with the MTP inhibitor alone (Fig. 4, P < 0.001). Basal tocopherol secretion differed among the 3 vitamers (P < 0.001) when cells were treated simultaneously with BMS201038 and T0901317. The magnitude of the effect of LXR activation in the MTP-inhibited state was similar for all 3 tocopherols and again greater for cholesterol.
FIGURE 4.
Influence of LXR activation on chylomicron-independent basal secretion of tocopherols by Caco-2 monolayers. Monolayers were treated with T0901317, BMS201038, and mixed micelles as in Fig. 1 and 2. Basal tocopherol and unesterified cholesterol secretion were determined after 24 h. Results are expressed as means ± SDs, n = 9 independent observations. *P < 0.001 vs. BMS; labeled means without a common letter differ: a–c, P < 0.01. BMS, BMS201038; chol, unesterified cholesterol; LXR, liver X receptor; T, T0901317; TOH, tocopherol.
Implication of ABCA1 in tocopherol secretion by Caco-2 monolayers.
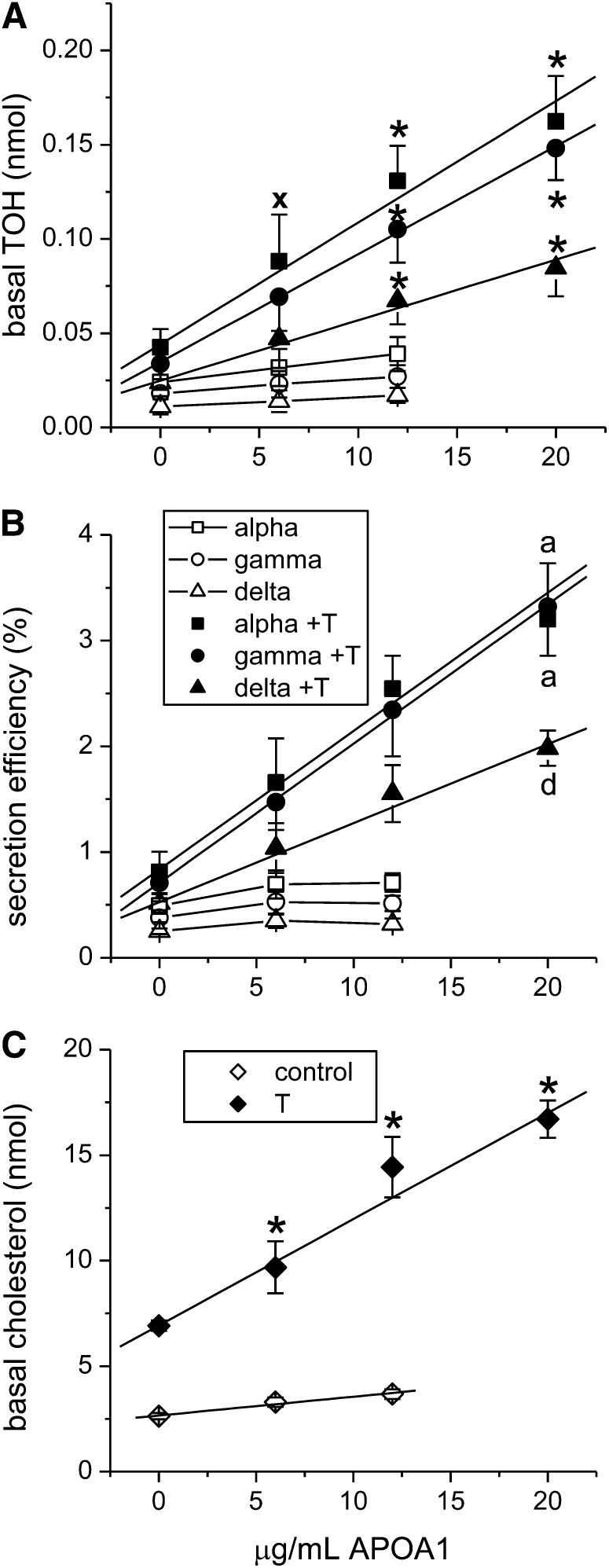
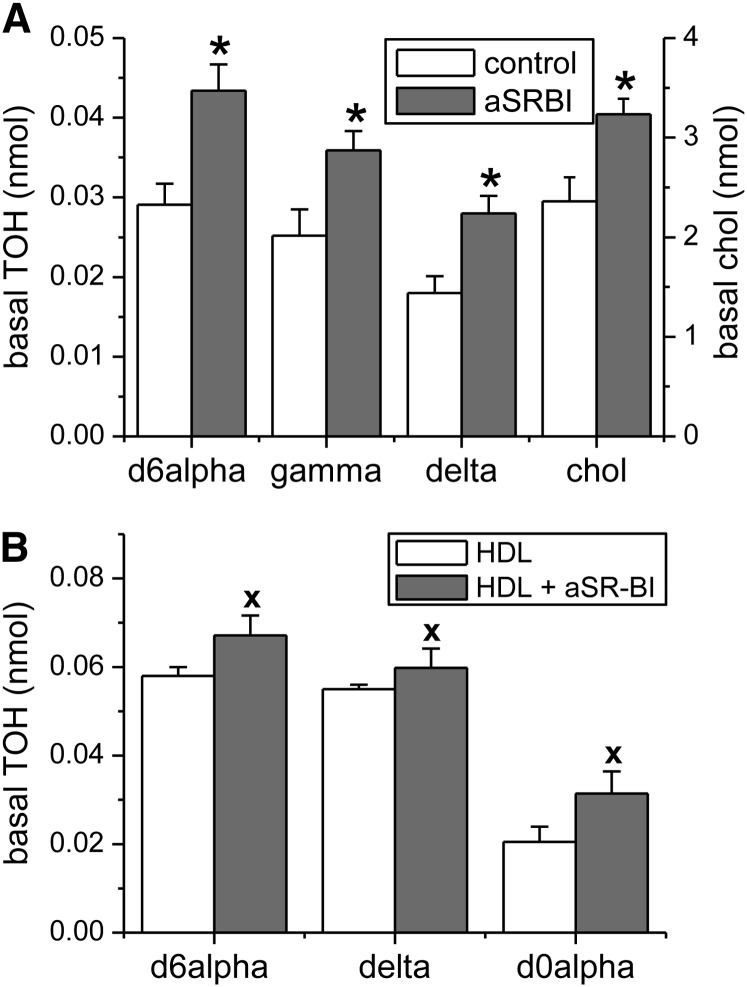
To ascertain a role for the ABCA1 transporter in basal tocopherol secretion, we determined the effect of added APOA1 on tocopherol secretion in monolayers treated with the MTP inhibitor BMS201038 and either with or without LXR activation by T0901317. In control cultures, not treated with T0901317, addition of APOA1 to the basal compartment had no effect on basal accumulation of any of the 3 tocopherols (Fig. 5A) or cholesterol ( . In contrast, in monolayers treated with T0901317, addition of APOA1 significantly increased basal accumulation of tocopherols (Fig. 5A) and cholesterol (Fig. 5C, P < 0.001). Tocopherol and cholesterol secretion were linearly related to the amount of APOA1 added over the range of APOA1 concentrations tested. Saturation of tocopherol secretion was not observed, probably because the robust induction of ABCA1 consequent to LXR activation by T0901317 rendered APOA1 the limiting factor in secretion over the concentration range used. APOA1-dependent secretion efficiency was consistently lower for δ-TOH than for α- and γ-TOHs (Fig. 5B, P < 0.001). Tocopherol uptake was not affected by basal addition of APOA1. Treatment with 50 μmol/L probucol, a known ABCA1 inhibitor (29, 30), significantly reduced basal secretion of tocopherols and unesterified cholesterol, but its effect was confounded by reduced cell-associated tocopherols and unesterified cholesterol (results not shown). Tocopherol secretion was also inhibited by glyburide, but at an extremely high concentration (2 mmol/L) at which monolayer integrity was compromised (data not shown).
FIGURE 5.
Effect of APOA1 on basal tocopherol secretion in control and LXR-induced Caco-2 monolayers. Monolayers were treated with T0901317, BMS201038, and mixed micelles as in Fig. 1 and 2. APOA1 (0, 6, 12, or 20 μg/mL media) was added to the basolateral compartment and basal tocopherol (A) and unesterified cholesterol (C) secretion were determined after 24 h. (B) Secretion efficiency. Results are expressed as means ± SDs (A and B, n = 9; C, n = 3). *P < 0.001 vs. 0 μg/mL; x, P < 0.02 vs. 0 μg/mL; linear fits without a common letter differ: a, d, P < 0.001. APOA1, apolipoprotein A1; T, T0901317; TOH, tocopherol.
Implication of ABCG1 in tocopherol secretion by Caco-2 monolayers.
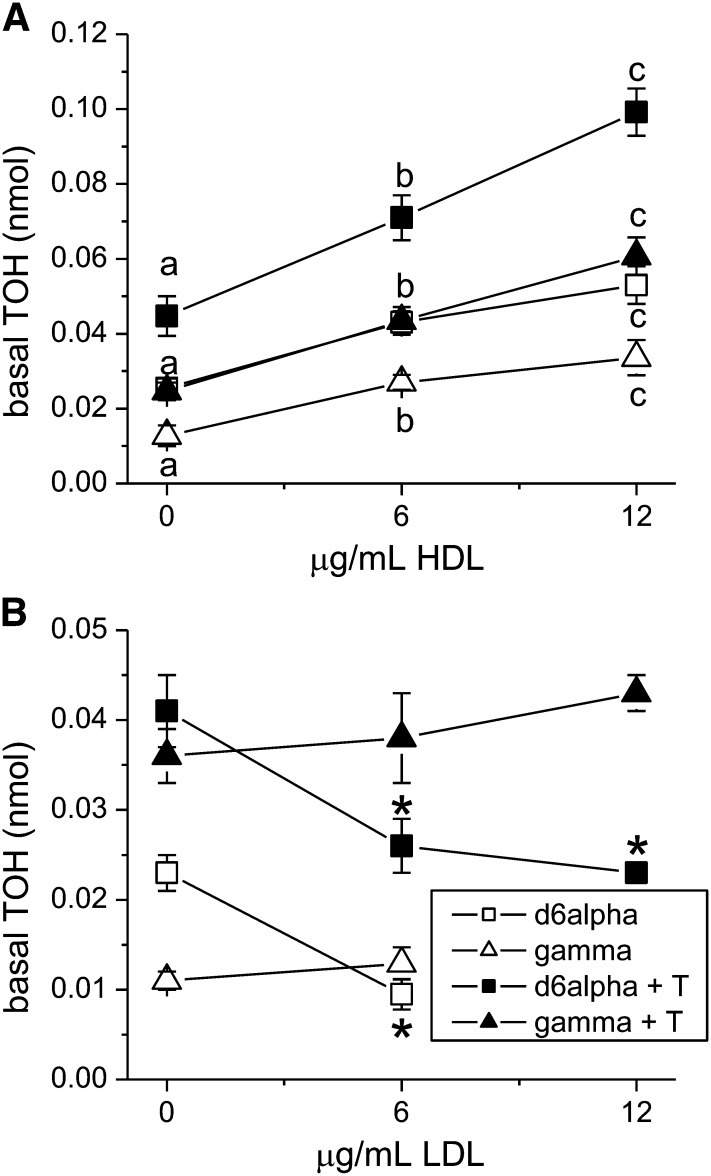
ABCG1 is known to secrete cholesterol to HDL or to partially lipidated APOA1 (pre-HDL) particles produced by the action of ABCA1 (8, 31). Because Western blotting demonstrated constitutive expression of ABCG1 by these Caco-2 monolayers, we investigated the role of this transporter in tocopherol secretion using HDL-stimulated secretion as a marker for its activity in cultures treated with the MTP inhibitor BMS201038. Addition of HDL to the basolateral compartment significantly induced the basal accumulation of d6α- and δ-TOHs (Fig. 6A, P < 0.001). Increasing the amount of added HDL from 6 to 12 μg/mL further increased basolateral tocopherol accumulation of both d6α- and δ-TOH to the same extent. It was not possible to accurately assess γ-TOH accumulation because the amount secreted was comparable to the amount of γ-TOH endogenously present in the added HDL. To rule out an effect of increased secretion to HDL in the LXR-activated state, we measured tocopherol secretion to added HDL in the presence of T0901317. Addition of HDL significantly induced the basal accumulation of d6α- and δ-TOH (Fig. 6A). The increase caused by the addition of HDL (difference between HDL and no HDL treatments) was similar in the presence and absence of T0901317 (parallel linear fits, Fig. 6A). To verify that secretion to HDL was specific to HDL, we also investigated the effect of adding LDL. Basal addition of LDL had no effect on basal accumulation of δ-TOH, but accumulation of d6α-TOH was reduced (Fig. 6B). The latter may have resulted from dilution of the intracellular d6α-TOH pool with unlabeled α-TOH endogenous to the added LDL. Additionally, in the presence of T0901317, addition of 6 or 12 μg/mL LDL to the basolateral compartment resulted in similar basal accumulation of d6-αTOH and δ-TOH (Fig. 6B). The absence of a stimulatory effect of LDL on tocopherol secretion suggests specificity of the observed HDL effect, perhaps involving ABCG1. Basal addition of lipoproteins did not affect tocopherol uptake or unesterified cell cholesterol. We undertook to suppress expression of ABCG1 by small interfering RNA but were unsuccessful in transfecting confluent Caco-2 monolayers.
FIGURE 6.
HDL, but not LDL, stimulates basal tocopherol secretion by Caco-2 monolayers. Monolayers were treated with BMS201038 and mixed micelles, with or without T0901317 as in Fig. 1 and 2. Either HDL (0, 6 or 12 μg/mL)(A) or LDL (0, 6 or 12 μg/mL) (B) were added to the basolateral compartment at 0 h, and basal tocopherol was determined after 24 h. Results are expressed as means ± SDs (HDL, n = 9; LDL, n = 3). *P < 0.005 vs. 0 μg/mL; given a vitamer and treatment, labeled means without a common letter differ, P < 0.01. HDL, high density lipoprotein; LDL, low density lipoprotein; TOH, tocopherol.
Evidence that SR-BI is involved in basolateral uptake of secreted tocopherols in Caco-2 monolayers.
Caco-2 monolayers expressed SR-BI as assessed by Western blot (Fig. 2), and SR-BI is known to bind HDL (32). SR-BI could therefore be involved in the stimulation of tocopherol secretion upon addition of HDL to the basolateral compartment. We used an SR-BI blocking antibody (Novus, NB400–100) to investigate the involvement of SR-BI in tocopherol secretion in the absence or presence of added HDL. The SR-BI blocking antibody (anti-SR-BI) binds to the extracellular domain of SR-BI, preventing its interaction with HDL (20, 33). The basal addition of anti-SR-BI had no effect on tocopherol uptake or unesterified cell cholesterol. In the absence of added HDL, anti-SR-BI addition resulted in increased tocopherol and cholesterol accumulation in the basolateral compartment (Fig. 7A), suggesting that SR-BI facilitated the uptake of secreted tocopherols rather than their (net) secretion. The anti-SR-BI effect was similar among the 3 tocopherols, suggesting that cellular tocopherol uptake via this pathway occurs without selectivity. Importantly, when HDL was added basally, tocopherol also significantly accumulated basally in the presence of the blocking antibody relative to the absence of anti-SR-BI (Fig. 7B). The concentration of unlabeled α-TOH (endogenous to the added HDL) was also higher in the presence of the antibody, likely reflecting inhibition of its uptake by the cells in a fashion similar to that of the secreted tocopherols originating from the micelles added to the apical compartment. This finding suggests that the enhancement of basal accumulation of tocopherols by added HDL more likely involved ABCG1 than SR-BI.
FIGURE 2.
Effect of LXR activation on protein expression in Caco-2 monolayers. Monolayers were preincubated overnight with T0901317 or with the equivalent volume of ethanol vehicle. Mixed micelles providing 10 μmol/L α-, γ- and δ-TOH were then added apically. At 24 h, cells were collected and ABCA1 protein (A) and SR-BI protein (C) were determined by Western blot. (B) After overnight treatment with T, cells were administered normal media (FBS) or micelles (mic) and ABCG1 protein was assesed by western blot. ABC, ATP-binding cassette; C, control; SR-BI, scavenger receptor class B member I; T, T0901317.
FIGURE 7.
Role of SR-BI in basal tocopherol accumulation from Caco-2 monolayers. Monolayers were treated with BMS201038 and mixed micelles as in Fig. 1. Anti-SR-BI was added basolaterally where indicated one hour prior to addition of mixed micelles. (A) Basal accumulation of TOH after 24 h, in the presence or absence of anti-SR-BI (1:1000). (B) Basal accumulation of TOH after 24 h, when HDL (6 μg/mL) was included in the basolateral compartment in the presence or absence of anti-SR-BI as indicated. Results are expressed as means ± SDs, n = 6 independent observations. *, P < 0.005 vs. control; x, P < 0.05 vs. HDL. aSR-BI, anti-scavenger receptor class B member I (SR-BI) blocking antibody against the extracellular domain of SR-BI; chol, unesterified cholesterol; HDL, high density lipoprotein; TOH, tocopherol.
Discussion
Vitamin E is presumed to function primarily as a lipophilic radical-trapping antioxidant, whose efficacy may therefore be related to its cellular concentration. This concentration is determined by the net processes of cellular uptake and secretion. Because tocopherols are transported by plasma lipoproteins, it is not surprising that tissue uptake has implicated LDL, HDL, and chylomicrons (34, 35). The processes responsible for tocopherol secretion are less well understood. Because of the similarities in structure and membrane localization of vitamin E and cholesterol, the numerous pathways of cholesterol secretion that exist in Caco-2 monolayers, and advantages offered by the Transwell system, we used differentiated Caco-2 monolayers grown on porous filters to investigate specific pathways of tocopherol secretion from these cells. In one of the few reported investigations using this model to study vitamin E secretion, Anwar et al. (18) found that Caco-2 monolayers, incubated apically with α-TOH in Tween 40(Sigma), secreted α-TOH associated with HDL and chylomicrons. BMS197636, an inhibitor of MTP, blocked α-TOH secretion via chylomicrons but not via HDL. Because glyburide, an agent whose effects include inhibition of ABCA1, reduced secretion of α-TOH, the authors speculated that ABCA1 may be involved in a APOB-independent pathway of vitamin E secretion from these cells. ABCA1−/− mice were found to have a lower postprandial response of plasma γ-TOH than wild-type mice, but this difference disappeared when plasma γ-TOH values were corrected for plasma lipids (22).
The concentration of tocopherols (10 μmol/L) chosen in this study corresponded to those that could be expected to exist in the intestine given typical total meal volumes and tocopherol intakes (e.g., 5 mg tocopherol/L of the sum of meal volume plus fasting stomach fluid). We found that at these concentrations the cellular accumulation and basal secretion of α-, γ-, and δ-TOHs in Caco-2 monolayers were similar when administered apically in mixed lipid micelles for 24 h, regardless of whether they were administered together or separately. Reboul et al. (23) reported that at high concentrations (40 μmol/L) administered in Tween, basal α-TOH secretion decreased in the presence of γ-TOH, suggesting a common transporter for the 2 vitamers. Our lack of observation of competition between vitamers could be related to the difference in concentrations or means of delivery.
Caco-2 monolayers secreted tocopherols basolaterally with a small but consistent difference between vitamers, favoring α-TOH, that could not be accounted for by differences in cell-associated tocopherol. When used at a concentration shown to completely block the basal secretion of APOB from Caco-2 monolayers (15), the MTP inhibitor BMS201038 inhibited the basal secretion of α-, γ-, and δ-TOHs similarly by ∼80%. This extent of inhibition was approximately twice the 40% inhibition of α-TOH secretion by BMS197636 observed by Anwar et al. (18). This difference may have resulted from the use of different inhibitors; the presence of oleic acid in our system, which induces chylomicron secretion; or the physical nature of the Transwell systems. In preliminary studies we found that Caco-2 monolayers grown on membranes with 3-μm pores (used by Anwar et al.) consistently yielded lower rates of MTP-inhibitable tocopherol secretion than monolayers grown on filters with 0.4-μm pores (results not shown).
A primary objective of ours was to characterize the pathway(s) of APOB-independent basolateral tocopherol secretion and to determine whether such pathways exhibited selectivity among the common dietary tocopherols. Building on the finding of Murthy et al. that LXR agonists induced expression of ABCA1 in Caco-2 monolayers (15), we treated cultures with T0901317 to determine whether its effects on ABCA1 protein expression would be reflected in its effect on basolateral tocopherol secretion. Consistent with this hypothesis, treatment with T0901317 induced ABCA1 protein expression and significantly stimulated tocopherol secretion. This stimulatory effect occurred in cultures treated with the MTP inhibitor BMS201038, demonstrating its independence from APOB secretion. Although these data were suggestive of a role for ABCA1 in tocopherol secretion, a more definitive approach involving APOA1-stimulated secretion was taken. ABCA1 binds and secretes cholesterol and phospholipids specifically to APOA1 (3, 4, 36) but not to HDL (37), indicating that APOA1-dependent lipid secretion is a marker of ABCA1 activity. Addition of APOA1 to the basolateral compartment statistically increased tocopherol (and cholesterol) secretion in cultures treated with the LXR agonist T0901317 but not in its absence. Moreover, the magnitude of increase in basal tocopherol secretion resulting from the addition of HDL (difference between HDL and no HDL treatments, Fig. 6A) was similar in the presence or absence of T0901317, indicating that increased secretion in the LXR-activated state most likely resulted from increased expression of ABCA1. Although ABCA1 protein was undetectable in non-LXR-induced Caco-2 cultures by Western blot, it is likely that small amounts of the transporter are responsible for the APOB-independent tocopherol secretion observed in the non-induced state. Caco-2 monolayers secrete APOA1 basolaterally (11), but in the absence of LXR activation the limiting factor for tocopherol secretion is likely to be ABCA1 expression, because we found that added APOA1 had no effect. Upon LXR activation, ABCA1 protein expression is enhanced and the limiting factor becomes APOA1 availability. The addition of APOA1 had a greater effect on α- and γ-TOH secretion than on δ-TOH secretion. These results demonstrate that ABCA1 facilitates tocopherol secretion in a vitamer-selective fashion. The reason for this selectivity is unclear but may reflect differences in the subcellular localization of different forms of vitamin E, such that some may be more accessible by the ABCA1-mediated lipid export pathway than others. ABCA1 is known to traffic cholesterol from late endosomes/lysosomes to the plasma membrane in macrophages (4).
Caco-2 monolayers also express ABCG1, which facilitates secretion of cholesterol to pre-HDL but not APOA1 (7, 8). We observed that addition of HDL, but not LDL, to the basolateral compartment induced tocopherol secretion and that the stimulatory effect was similar for α- and δ-TOH. Although these data suggested a role for ABCG1 in basolateral secretion of tocopherols from Caco-2 monolayers, HDL is also known to interact with SR-BI, another membrane protein expressed basally by these cells (19, 20). SR-BI is thought to function primarily in cellular uptake of HDL cholesterol, but this receptor additionally mediates cholesterol efflux from macrophages to HDL (9, 10) and has been implicated in the apical efflux of α-TOH to mixed micelles from Caco-2 monolayers (23). Therefore, SR-BI expressed at the basolateral membrane could have promoted tocopherol efflux to HDL in our system. However, basolateral treatment of monolayers with anti-SR-BI resulted in increased accumulation of tocopherols in the basal compartment in a nonselective fashion. Although it is possible that SR-BI facilitates tocopherol movement both into and out of these cells, our results indicated that the net flux was into the cells from the basolateral compartment. Thus, the observed stimulatory effect of HDL on tocopherol secretion was most likely due to the action of ABCG1.
In a previous study, T0901317 induced ABCG1 mRNA expression, but protein was not assessed (15). Although we treated our Caco-2 monolayers with the same LXR agonist, we did not observe an increase in ABCG1 protein. We hypothesize that this discrepancy is due to posttranslational degradation of ABCG1. Indeed, ABCA1 has been shown to be highly regulated at the posttranslational level and the same could be true for ABCG1. In accordance, a recent study demonstrated that degradation of both ABCG1 and ABCA1 protein is promoted by inhibition of MAPK Erk1/2 (38). Because LXR activation induced ABCA1, but not ABCG1, protein, the stimulation of tocopherol secretion by LXR activation most probably resulted from induction of ABCA1-mediated secretion to APOA1, forming a pre-HDL particle, which was then lipidated by ABCG1, and finally resulting in a nonselective secretion of the vitamers. Vitamer selectivity is seen only when the ABCA1 substrate, APOA1, is no longer the limiting factor. Additionally, SR-BI has been implicated in uptake of HDL α-TOH by brain capillary endothelial cells (39) and may promote uptake of other HDL-borne lipids such as carotenoids. Measurements of basolateral secretion rates of such lipids by Caco-2 monolayers (40) therefore might be more accurately assessed by blocking SR-BI activity as in the present study.
In summary, these studies strongly implicated the membrane lipid transporter ABCA1 in APOB-independent basolateral secretion of 3 common dietary forms of vitamin E from differentiated Caco-2 monolayers after their apical administration in mixed lipid micelles. APOB-mediated secretion exhibited no selectivity among the 3 tocopherols. In contrast, ABCA1-mediated secretion was selective in that δ-TOH was less efficiently secreted via this pathway. Indirect evidence suggested a role for ABCG1 in nonselective tocopherol secretion in this model. The concerted actions of ABCA1 and AGCG1 may represent an alternative, low-capacity pathway of intestinal tocopherol absorption that becomes important in individuals with impaired chylomicron secretion. SR-BI was involved in the uptake of secreted tocopherols from the basolateral side of the monolayer, without selectivity among the tocopherols. ABCA1, ABCG1, and SR-BI play key roles in the elimination of cholesterol via reverse transport. Although all 3 proteins have now been implicated in tocopherol transport, it is unclear what affect such a process has on vitamin E status, but it raises the possibility that attempts to increase cholesterol elimination via these transporters may also promote loss of tocopherols by an analogous reverse tocopherol transport.
Acknowledgments
R.S.P. and N.N. designed the research, N.N. conducted the research and analyzed data, and R.S.P. and N.N. wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ABC, ATP-binding cassette transporter; anti-SR-BI, antibody against scavenger receptor class B member; APO, apolipoprotein; LXR, liver X receptor; MTP, microsomal triglyceride transfer protein; SR-BI, scavenger receptor class B member; TOH, tocopherol.
Literature Cited
- 1.Kayden HJ, Traber MG. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res. 1993;34:343–58. [PubMed] [Google Scholar]
- 2.Kayden HJ, Hatam LJ, Traber MG. The measurement of nanograms of tocopherol from needle aspiration biopsies of adipose tissue: normal and abetalipoproteinemic subjects. J Lipid Res. 1983;24:652–6. [PubMed] [Google Scholar]
- 3.Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–8. [DOI] [PubMed] [Google Scholar]
- 4.Orsó E, Broccardo C, Kaminski WE, Bottcher A, Liebisch G, Drobnik W, Gotz A, Chambenoit O, Diederich W, Langmann T, et al. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat Genet. 2000;24:192–6. [DOI] [PubMed] [Google Scholar]
- 5.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–5. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz K, Lawn RM, Wade DP. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem Biophys Res Commun. 2000;274:794–802. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem. 2005;280:30150–7. [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA. 2004;101:9774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jian B, de la Llera-Moya M, Ji Y, Wang N, Phillips MC, Swaney JB, Tall AR, Rothblat GH. Scavenger receptor class B type I as a mediator of cellular cholesterol efflux to lipoproteins and phospholipid acceptors. J Biol Chem. 1998;273:5599–606. [DOI] [PubMed] [Google Scholar]
- 10.Huang ZH, Gu D, Lange Y, Mazzone T. Expression of scavenger receptor BI facilitates sterol movement between the plasma membrane and the endoplasmic reticulum in macrophages. Biochemistry. 2003;42:3949–55. [DOI] [PubMed] [Google Scholar]
- 11.Traber MG, Kayden HJ, Rindler MJ. Polarized secretion of newly synthesized lipoproteins by the Caco-2 human intestinal cell line. J Lipid Res. 1987;28:1350–63. [PubMed] [Google Scholar]
- 12.Mathur SN, Born E, Murthy S, Field FJ. Microsomal triglyceride transfer protein in CaCo-2 cells: characterization and regulation. J Lipid Res. 1997;38:61–7. [PubMed] [Google Scholar]
- 13.Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J Biol Chem. 2003;278:31610–20. [DOI] [PubMed] [Google Scholar]
- 14.Luchoomun J, Hussain MM. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J Biol Chem. 1999;274:19565–72. [DOI] [PubMed] [Google Scholar]
- 15.Murthy S, Born E, Mathur SN, Field FJ. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J Lipid Res. 2002;43:1054–64. [DOI] [PubMed] [Google Scholar]
- 16.Field FJ, Born E, Mathur SN. LXR/RXR ligand activation enhances basolateral efflux of beta-sitosterol in CaCo-2 cells. J Lipid Res. 2004;45:905–13. [DOI] [PubMed] [Google Scholar]
- 17.Ohama T, Hirano K, Zhang Z, Aoki R, Tsujii K, Nakagawa-Toyama Y, Tsukamoto K, Ikegami C, Matsuyama A, Ishigami M, et al. Dominant expression of ATP-binding cassette transporter-1 on basolateral surface of Caco-2 cells stimulated by LXR/RXR ligands. Biochem Biophys Res Commun. 2002;296:625–30. [DOI] [PubMed] [Google Scholar]
- 18.Anwar K, Kayden HJ, Hussain MM. Transport of vitamin E by differentiated Caco-2 cells. J Lipid Res. 2006;47:1261–73. [DOI] [PubMed] [Google Scholar]
- 19.Cai SF, Kirby RJ, Howles PN, Hui DY. Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J Lipid Res. 2001;42:902–9. [PubMed] [Google Scholar]
- 20.Cai L, Eckhardt ER, Shi W, Zhao Z, Nasser M, de Villiers WJ, van der Westhuyzen DR. Scavenger receptor class B type I reduces cholesterol absorption in cultured enterocyte CaCo-2 cells. J Lipid Res. 2004;45:253–62. [DOI] [PubMed] [Google Scholar]
- 21.Oram JF, Vaughan AM, Stocker R. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J Biol Chem. 2001;276:39898–902. [DOI] [PubMed] [Google Scholar]
- 22.Reboul E, Trompier D, Moussa M, Klein A, Landrier JF, Chimini G, Borel P. ATP-binding cassette transporter A1 is significantly involved in the intestinal absorption of alpha- and gamma-tocopherol but not in that of retinyl palmitate in mice. Am J Clin Nutr. 2009;89:177–84. [DOI] [PubMed] [Google Scholar]
- 23.Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem. 2006;281:4739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy S, Born E, Mathur SN, Field FJ. Liver-X-receptor-mediated increase in ATP-binding cassette transporter A1 expression is attenuated by fatty acids in CaCo-2 cells: effect on cholesterol efflux to high-density lipoprotein. Biochem J. 2004;377:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). J Pharmacol Exp Ther. 2003;306:355–62. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenberg D, Werker E, Bor A, Almog S, Nir S. Precipitation of calcium palmitate from bile salt-containing dispersions. Chem Phys Lipids. 1988;48:231–43. [DOI] [PubMed] [Google Scholar]
- 27.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–6. [DOI] [PubMed] [Google Scholar]
- 28.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–800. [DOI] [PubMed] [Google Scholar]
- 29.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345–50. [DOI] [PubMed] [Google Scholar]
- 30.Wu CA, Tsujita M, Hayashi M, Yokoyama S. Probucol inactivates ABCA1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem. 2004;279:30168–74. [DOI] [PubMed] [Google Scholar]
- 31.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–40. [DOI] [PubMed] [Google Scholar]
- 32.Xu S, Laccotripe M, Huang X, Rigotti A, Zannis VI, Krieger M. Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J Lipid Res. 1997;38:1289–98. [PubMed] [Google Scholar]
- 33.Gantman A, Fuhrman B, Aviram M, Hayek T. High glucose stimulates macrophage SR-BI expression and induces a switch in its activity from cholesterol efflux to cholesterol influx. Biochem Biophys Res Commun. 2010;391:523–8. [DOI] [PubMed] [Google Scholar]
- 34.Hacquebard M, Carpentier YA. Vitamin E: absorption, plasma transport and cell uptake. Curr Opin Clin Nutr Metab Care. 2005;8:133–8. [DOI] [PubMed] [Google Scholar]
- 35.Rigotti A. Absorption, transport, and tissue delivery of vitamin E. Mol Aspects Med. 2007;28:423–36. [DOI] [PubMed] [Google Scholar]
- 36.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–11. [DOI] [PubMed] [Google Scholar]
- 37.Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr Opin Lipidol. 2000;11:253–60. [DOI] [PubMed] [Google Scholar]
- 38.Mulay V, Wood P, Manetsch M, Darabi M, Cairns R, Hoque M, Chan KC, Reverter M, Alvarez-Guaita A, Rye KA, et al. Inhibition of mitogen-activated protein kinase Erk1/2 promotes protein degradation of ATP binding cassette transporters A1 and G1 in CHO and HuH7 cells. PLoS ONE. 2013;8:e62667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, Malle E, Sattler W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem. 2004;89:939–50. [DOI] [PubMed] [Google Scholar]
- 40.During A, Harrison EH. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J Lipid Res. 2007;48:2283–94. [DOI] [PubMed] [Google Scholar]