Abstract
Optical pacing has been demonstrated to be a viable alternative to electrical pacing in embryonic hearts. In this study, the feasibility of optically pacing an adult rabbit heart was explored. Hearts from adult New Zealand White rabbits (n = 9) were excised, cannulated and perfused on a modified Langendorff apparatus. Pulsed laser light (λ = 1851 nm) was directed to either the left or right atrium through a multimode optical fiber. An ECG signal from the left ventricle and a trigger pulse from the laser were recorded simultaneously to determine when capture was achieved. Successful optical pacing was demonstrated by obtaining pacing capture, stopping, then recapturing as well as by varying the pacing frequency. Stimulation thresholds measured at various pulse durations suggested that longer pulses (8 ms) had a lower energy capture threshold. To determine whether optical pacing caused damage, two hearts were perfused with 30 µM of propidium iodide and analyzed histologically. A small number of cells near the stimulation site had compromised cell membranes, which probably limited the time duration over which pacing was maintained. Here, short-term optical pacing (few minutes duration) is demonstrated in the adult rabbit heart for the first time. Future studies will be directed to optimize optical pacing parameters to decrease stimulation thresholds and may enable longer-term pacing.
OCIS codes: (140.3460) Lasers, (170.3890) Medical optics instrumentation
1. Introduction
Although cardiac electrophysiology and pacing are routinely used to treat and study arrhythmias, electrical pacing suffers from several drawbacks including low spatial precision [1], stimulation artifacts in electrode recordings [2], interference from magnetic fields [3] and the requirement of tissue contact [4]. Improving the capability or cost of cardiac pacing through alternative approaches may improve existing technology or allow new applications. One alternative approach is to implement high-intensity ultrasound pulses to produce premature beats in a rat heart [5]. Other recent studies have demonstrated the use of optogenetics to pace mouse and zebrafish hearts by genetically modifying the tissue [6, 7]. Additionally, we have recently demonstrated that infrared optical pulses can noninvasively pace early-stage quail embryo hearts without the use of any exogenous agents or genetic modifications [4]. We were able to reliably pace embryonic hearts over a range of developmental stages. At light exposure levels above threshold, we were able to successfully pace 100% of the embryos in a consistent manner. Furthermore, pacing near the threshold level produced no evidence of ultrastructural or functional damage to the tissue. This technique is enabling new methods to study the developing heart. However, because the study was limited to early-stage embryonic quail hearts that were still tubular, semi-translucent and less than a millimeter in length, it did not make clear whether adult hearts, which are much larger, denser and more structurally complex, can be effectively paced with an infrared pulsed laser.
Here, we demonstrate for the first time optical pacing (OP) of an adult rabbit heart. We define OP as using a pulsed light source to pace a heart at a rate above the sinus rhythm with 1:1 capture, where each light pulse initiates a heartbeat. To conclusively demonstrate OP, pacing was captured, stopped, and then recaptured. We also modulated the stimulation pulse frequency to test whether the heart rate would follow the laser pulses 1:1. As a first step toward minimizing energy deposition in tissue, stimulation thresholds were computed for varying laser pulse widths. Histological examination of hearts perfused with propidium iodide was utilized to assess damage to the myocardium.
2. Methods
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. All animals used in this study received humane care in compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, revised 1996).
2.1 Animal model
This study used 9 adult male New Zealand White rabbits weighing 2.9-4.0 kg as outlined in Table 1 . Experiments were performed on an ex vivo heart preparation as previously described [8, 9]. Briefly, the heart was excised from a euthanized rabbit before cannulating and perfusing the heart on a modified Langendorff apparatus. Temperature and pH-controlled oxygenated modified Tyrode's solution at 37 °C was retrogradely perfused through the intact heart. After a period of acclimatization, the heart was removed from the warm bath and suspended in air for ease of positioning the pacing fiber. Electrodes were sutured on to the epicardium of the left ventricle at the anterior apex and the lateral base to obtain a ventricular electrocardiogram (ECG) signal. Hearts survived for 4-6 hours in this configuration.
Table 1. Experiments performed on each heart.
| Capture/ Recapture | Frequency Variation | Long-Term Pacing | Stimulation Threshold | Propidium iodide Assessment | |
|---|---|---|---|---|---|
| Heart 1 | • | ||||
| Heart 2 | • | ||||
| Heart 3 | • | • | |||
| Heart 4 | • | • | • | ||
| Heart 5 | • | • | • | ||
| Heart 6 | • | ||||
| Heart 7 | • | ||||
| Heart 8 | • | • | • | ||
| Heart 9 | • |
2.2 Optical pacing
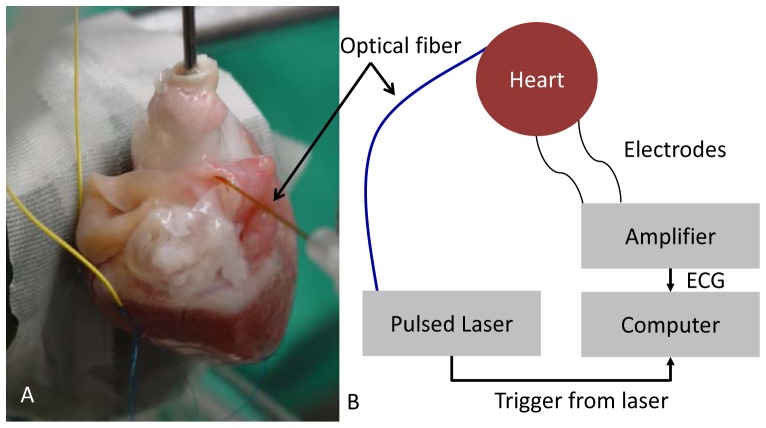
Figure 1 shows the experimental setup. A tunable diode laser (Capella; Lockheed-Martin-Aculight, Bothell, WA) provided short-wavelength infrared stimulation pulses of 1851 nm at varying pulse rates and pulse widths. Water absorption determined the choice of wavelength as optical pacing is themally mediated [10, 11]. Light from the laser was coupled into a 400 ± 8 µm-diameter flat-polished multimode fiber (Ocean Optics, Dunedin, FL) with a numerical aperture of 0.22 ± 0.02. Multimode fiber was utilized to ensure adequate coupling from the laser. An articulating arm held the fiber tip in contact with the atrium and allowed for easy adjustment of position and angle. While fiber contact with tissue is not necessary for stimulation, in this case placing the fiber in contact ensured that the excitation site did not change as the heart moved while beating. To verify 1:1 pacing, both the transistor-transistor logic (TTL) trigger signal from the laser and the ECG from the electrodes were captured simultaneously by a computer. Both signals were recorded and displayed in real-time.
Fig. 1.
Experimental setup. Hearts of male New Zealand White rabbits weighing 2.9-4 kg were extracted, cannulated, and perfused on a modified Langendorff apparatus. A: Photograph of heart 1. Electrodes (yellow wires) are attached to the left ventricle of the heart. Pulsed laser light (λ = 1.851 μm) was directed toward the epicardial surface of the left or right atrium through a multimode optical fiber. The black arrow indicates the optical fiber. A pad directly behind the heart diminishes swinging motion. B: Diagram of the setup. A computer simultaneously recorded both the trigger pulse from the laser and an ECG signal to determine when capture was achieved.
2.3 Radiant exposure calculations
Radiant exposure (J/cm2) was calculated by dividing the pulse energy at the tip of the fiber by the area of illumination on the myocardium. Pulse energy was measured using a pyroelectric energy meter (Nova II, Ophir; PE50BB-VR-ROHS, Ophir) . The area of illumination was estimated using the fiber diameter, the numerical aperture of the fiber, and the thickness of the epicardium between the fiber and myocardium. The fiber had a nominal diameter of 400 µm and a numerical aperture of 0.22 and was in perpendicular contact with the surface of the heart. The thickness of the epicardium at the pacing site was approximated as 75 µm based on histology of hearts used in this study. The calculated illumination area for our experiments was 0.0015 cm2.
2.4 Feasibility of optical pacing
The feasibility of OP in adult rabbit hearts was demonstrated with 2 tests. First, the ability to capture and then recapture was shown (n = 5). The heart and laser were prepared as described above and the heart was paced on either the right or left atrium by the laser at 2.5 Hz, a rate faster than the sinus rhythm in all hearts. After 1:1 capture of at least 10 beats was observed with no skipped beats, the laser pulses were stopped. The heart was allowed to rest without stimulus and beat at its sinus rate for at least 5 s before the laser pulses were restarted without changing the position of the fiber on the heart. Recapture was considered successful if the heart was again paced 1:1 for at least 10 beats with no skipped beats.
Next, to demonstrate that the laser pulses were pacing the heart and not just modulating the frequency of the sinus rhythm, the laser pulse frequency was varied (n = 2). The hearts were paced on either the right or left atrium at 2.5 Hz. Once 1:1 capture with no skipped beats was established for at least 10 beats, the frequency of the laser pulses were increased 4-10% and continued 1:1 pacing was verified by the ECG and laser trigger signals. After at least 10 paced beats at the higher frequency, the laser pulses were returned to 2.5 Hz and the heart rate was again verified to have followed the stimulation rate.
2.5 Stimulation threshold measurements
The stimulation threshold is the lowest energy laser pulse that successfully achieves 1:1 capture. The stimulation threshold was measured in five experiments using four hearts. The heart and laser were prepared and arranged as described above. The hearts were paced at either a cycle length of 300 ms (200 BPM) or 400 ms (150 BPM). Typically Langendorff perfused hearts are paced at physiological rates around 180 to 210 BPM [12, 13]. Laser pulse frequencies were set 15-25% faster than sinus rhythm, while the laser pulse width was varied from 2.5 to 12 ms. For a given pulse width, the power of the laser was varied until the minimum power necessary to pace the heart for at least 10 beats with no skipped beats was found. For each power level, the ECG and laser trigger recordings were observed for 10 s to determine if capture was achieved, and OP was otherwise deemed to be unsuccessful.
2.6 Tissue damage assessment
Propidium iodide (PI) is routinely used to assess whether cardiomyocytes have been compromised by electrical stimulation, where positive staining in the nucleus indicates disruption of the cell membrane [14]. Here, we applied PI staining to determine if OP disrupted the membranes of the adult rabbit cardiac tissues at the site of pacing. We tested PI staining after exposing hearts to 8 ms pulses (the optimal pulse width to minimize pacing pulse energy as determined in our stimulation threshold experiments). Two hearts were employed for these experiments. We directed laser light to a region on the left atrium and exposed the region to light pulses for one minute before moving to an adjacent location and repeating the procedure at a different radiant exposure. The experiment was repeated five times for each heart and included a negative control where the laser was left off, a positive control with radiant exposure set well above the damage threshold (65.8 J/cm2) and three trials within the range of threshold radiant exposures (6.3, 7.9 and 9.7 J/cm2). A surgical marker was used to mark the location of each trial. Following laser exposure, the heart was perfused with 30 µM of PI solution for 20 minutes, washed with Tyrode's solution for 40 minutes, removed from the Langendorff apparatus, and placed in cold Tyrode's solution to stop contractions. The left atrium was excised and each marked region was located and dissected as ~1 mm3 cubes. The five samples from each heart were embedded in O.C.T. (Sakura Finetek, Torrance, CA), frozen on dry ice, and stored at −80°C. The tissue samples were subsequently cryosectioned with 10 µm sections taken every 100 µm and examined under a fluorescence microscope (DIAPHOT 200, Nikon) using either a 4X or 10X objective. Images were recorded with a Q-Imaging Retiga EXi FAST 1394 digital camera using Q-capture software (Q-Imaging Burnaby, BC, Canada).
3. Results
3.1 Optical pacing demonstration
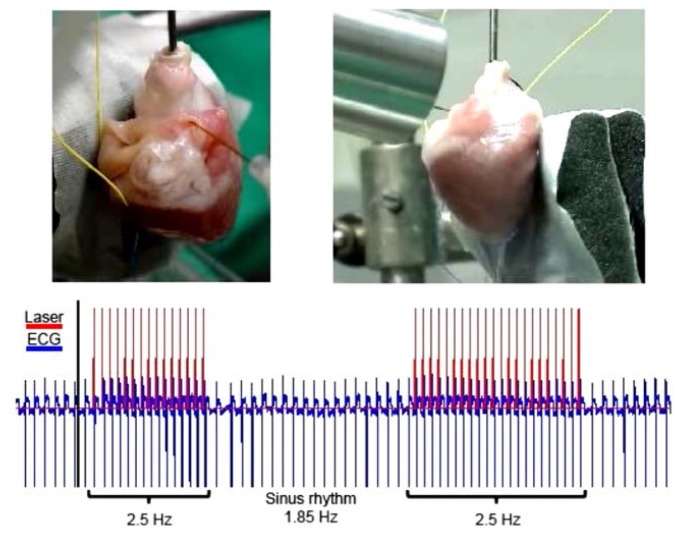
Capture/recapture OP succeeded in all five of the hearts tested. Figure 2 shows representative data from a capture/recapture trial from heart 1. Here, a pulse frequency of 2.5 Hz, well above the sinus rhythm (1.85 Hz) was chosen. After onset of the laser pulses, 1:1 capture of the heart was quickly achieved. In every heart that was optically paced, the QRS complex in the electrode recording closely followed the TTL trigger pulse from the laser (see Figs. 2, 3 and 5). The trigger pulse consistently occurred at the beginning of the cardiac cycle indicating that each laser pulse was initiating the beat and not only modulating the heart rate. The timing between the trigger and QRS complex was 84.2 ± 26.3 ms (N = 4). This was similar to the AV delay of 81 ± 19 ms previously reported in the rabbit, indicating that conduction of the optically paced action potentials propagated through the AV node [15]. Immediately following OP, the heart rate was slightly decreased before quickly returning to sinus heart rate.
Fig. 2.
Optical pacing of an adult rabbit heart (Media 1 (4.6MB, MOV) ). Pacing was captured, stopped, then recaptured to demonstrate 1:1 pacing of the heart. Top left panel: A photograph of heart 1, identical to Fig. 1(a), indicating the position of the optical fiber and electrodes. Top right panel: a video recording of heart 1 beating, while optical pacing is captured, stopped and recaptured. Bottom panel: The blue (lower) trace represents the ECG recording, while the red (upper) trace is the trigger output from the laser.
Fig. 3.
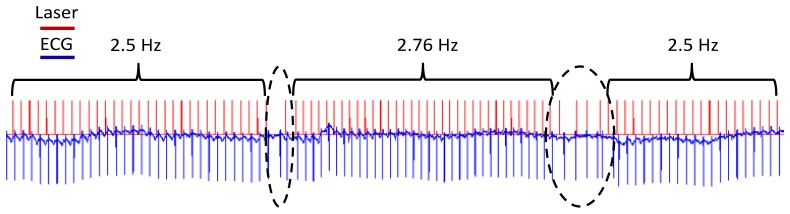
Frequency modulation. The heart rate was modulated from 2.5 to 2.76 to 2.5 Hz using OP. When the laser pulse rate was changed, the heart rate quickly tracked the new pacing frequency. The trigger pulse from the laser is shown in red (upper trace) and the ECG recording in blue (lower trace). The laser did not smoothly transition between frequencies and pacing lapsed during these short periods (dotted ovals).
To further demonstrate that pulsed laser stimulation led to 1:1 OP rather than a general modulation of the heart rate in the adult rabbit heart, the pulse frequency of the laser was varied to establish that the heart rate would follow the laser pulse frequency. In Fig. 3, the laser pulse frequency was varied from 2.5 Hz to 2.76 Hz to 2.5 Hz. The heart rate immediately changed to follow the new frequency set by the laser. When the laser transitioned from one pulse rate to another, there were several pulses at uneven intervals before establishing the new frequency. During this brief transition period the laser and heart rate were not always synchronized. This result was observed in both of the two hearts tested.
3.2 Stimulation threshold
The minimum energy threshold for achieving optical pacing (stimulation threshold), quantified as radiant exposure, was measured for five pulse durations and two pulse rates. The stimulation threshold was measured in five trials using four hearts as shown in Table 2 . Five pulse widths were chosen (2.5, 3, 5, 8, 12 ms) and two pulse rates (2.5 Hz and 3.3 Hz). Obtaining results at threshold radiant exposures for 2.5 and 3 ms pulses was achieved in only 3 out of 10 trials due to inconsistent pacing, which most likely was the result of higher stimulation thresholds. In four out of five experiments, 8 ms pulses resulted in the lowest stimulation threshold. Also, the stimulation threshold was found to be lower pacing at 2.5 Hz compared to 3.3 Hz.
Table 2. Stimulation threshold (radiant exposure) for various laser pulse durations and rates.
| 2.5 ms | 3 ms | 5 ms | 8 ms | 12 ms | |
|---|---|---|---|---|---|
| Heart 4 @ 2.5 Hz (J/cm2) | 7.8 | 7.5 | 7.2 | 6.3 | 6.4 |
| Heart 5 @ 2.5 Hz (J/cm2) | - | - | 8.2 | 7.8 | 7.4 |
| Heart 6 @ 3.3 Hz (J/cm2) | - | - | 11.4 | 10.1 | 11.8 |
| Heart 7 @ 3.3 Hz (J/cm2) | - | - | 10.2 | 9.7 | 10.2 |
| Heart 7 @ 3.3 Hz (J/cm2) | - | 9.7 | 9.8 | 9.5 | 9.7 |
3.3 Damage assessment
To determine whether OP caused membrane disruption, five locations were illuminated on the left atrium of two hearts as described above. The experiment included a positive control exposure well above the damage threshold, a negative control where the laser was left off, and three exposures at radiant exposure levels of 6.3, 7.9 and 9.7 J/cm2 (see Table 3 ). 6.3 J/cm2 represents the lowest stimulation threshold that was measured, while 9.7 J/cm2 is slightly below the highest stimulation threshold measured at 8 ms. 7.9 J/cm2 was chosen as a midway point where approximately half of the hearts would be paced. In the positive controls, there was distinct PI staining, whereas the negative controls showed no staining. Neither of the two hearts showed staining at the lowest radiant exposure (6.3 J/cm2). One out of two hearts exhibited low levels of PI staining at the middle level (7.9 J/cm2) and highest level of exposure (9.7 J/cm2). Figure 4 shows three PI fluorescence images from heart 9. Figure 4(a) shows significant PI staining, indicating cell damage in the positive control, Fig. 4(b) shows light PI staining at the middle radiant exposure level, indicating some disruption of the cell membranes while Fig. 4(c) displays no staining indicating no disruption at the low radiant exposure level.
Table 3. Propidium iodide staining (X indicates positive staining).
| Neg. Ctl. | 6.3 J/cm2 | 7.9 J/cm2 | 9.7 J/cm2 | Pos. Ctl. | |
|---|---|---|---|---|---|
| Heart 8 | - | - | - | X | X |
| Heart 9 | - | - | X | - | X |
Fig. 4.
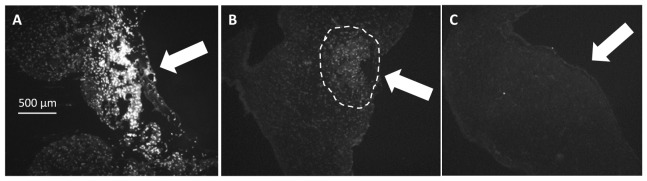
Propidium iodide (PI) staining. To assay for cell membrane disruption caused by OP, the heart was perfused with 30 µM of PI solution. Positive staining indicated permeation Out of the two hearts tested neither showed PI staining at the lowest stimulation threshold (6.3 J/cm2). One out of two hearts at the mid (7.9 J/cm2) and highest thresholds (9.7 J/cm2) exhibited a low level of PI staining. PI staining was found on the epicardial side of the atrium near the position of the optical fiber. The white arrows indicate the stimulation fiber direction. A: Positive control. B: Light staining was evident in one of the hearts when 8 ms pulses containing 7.9 J/cm2 per pulse were directed to the heart for one minute (dashed circle). C: Pulses containing 6.3 J/cm2 under similar conditions resulted in no apparent damage.
4. Discussion
Acute pacing of an adult four-chambered heart with a pulsed infrared laser is demonstrated here for the first time. These results indicate that OP may have the potential to be an alternative to electrical pacing in some basic research and/or clinical applications. While electrical pacing is an invaluable tool and is currently the only way to pace the heart, there are some limitations that OP might overcome. Electric currents from stimulating electrodes can spread for millimeters in cardiac tissue leading to recording artifacts and difficulty in stimulating precise locations [1]. The spatial specificity offered by OP may potentially enable complex spatial stimulation patterns not feasible with electrical pacing and avoid artifacts in electrical recordings. Because OP does not require contact, complications due to lead tissue perforation could potentially be avoided [16–18]. Additionally, an optical pacing lead could be fabricated with no magnetic material and be compatible with magnetic resonance imaging (MRI). New electrical pacing devices have been designed to be conditionally compatible with MRI (e.g. Revo MRI pacing system, Medtronic) but are only utilized under specified conditions [19].
The locations on the atria which could be stimulated optically were limited compared to electrical pacing. During the experiments, we performed OP in both the left and right atria. Our experiments suggested no differences in stimulation threshold between the atria. Multiple locations on the atria were able to be stimulated, but these locations were not consistent between hearts. These differences may be related to the unique structure of each heart. For example, a thinner epicardial layer at a particular location may lead to a lower stimulation threshold. We also attempted to optically pace at several sites on the ventricles. Although we sometimes stimulated beats, consistent pacing from the ventricle was not achieved. The stimulation threshold for pacing the ventricles is likely higher than the atria, preventing more consistent OP. Further optimization of laser, pulse, and delivery parameters may reduce the pacing thresholds and enable OP in the ventricles.
Our data indicate that the stimulation threshold is affected by the pacing frequency. The two hearts paced at 2.5 Hz exhibited lower stimulation thresholds at all pulse widths. An increase in the stimulation threshold with frequency was expected and is also seen with electrical pacing [20]. Further studies are needed to investigate the relationship between pulse frequency and stimulation threshold. It will be important to decrease stimulation threshold below damage thresholds to assure this relationship is not distorted by damage to the tissue.
The experiments presented in this work were limited to short pacing durations, as defined above, as the first demonstration of optical pacing of adult hearts. However, extended pacing (1-5 minutes) was achieved in three hearts. In Fig. 5 , the heart was paced at 2.5 Hz with 5 ms pulses for over 90 seconds without skipping a beat. Extended pacing periods lasting longer than 5 minutes were not achieved. Short-term OP at the experimentally-determined stimulation thresholds compromised the cell membranes in the cells closest to the epicardial exposure site as assessed by PI staining. It is not clear whether this membrane poration is reversible. It is possible that the damage to the membrane may be a factor limiting long-term OP in these experiments. PI staining of the site illuminated at the low range of stimulation threshold for 1 minute revealed no apparent membrane poration. This means that if the stimulation thresholds are decreased moderately, this effect could be avoided. Under the described experimental conditions, we showed minimum stimulation thresholds at a pulse width of 8 ms. At this point, it is unclear why the 8 ms pulses produced lower stimulation thresholds. Several other system parameters may be optimized in order to decrease stimulation threshold. For example, the wavelength of the laser, the shape of the pulse, and how light is delivered to the tissue (e.g. size of spot, angle of illumination, etc.) may influence the exposure level needed for stimulation. This is an important direction of future investigation because decreasing the stimulation threshold is likely to enable long-term OP. In illustration of this point, we observed that heart 4, which was paced at the lowest stimulation threshold (see Table 2), captured more consistently than any other heart.
Fig. 5.
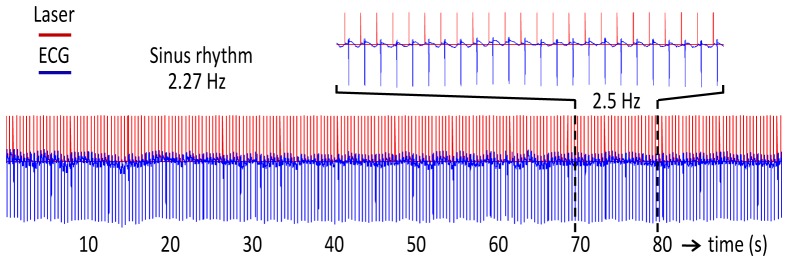
Extended pacing. The heart was paced for extended periods (1-5 minutes) in three hearts. The TTL trigger signal from the laser (red) and the ECG recording (blue) show OP at 2.5 Hz for over 90 seconds without skipping a beat. The dotted lines delineate a region that was expanded and shown directly above the traces to clearly visualize the synchronization of the laser pulses and ECG traces. Extended pacing beyond 5 minutes was not be reliably maintained.
Understanding the mechanisms behind OP will be crucial for optimizing its performance. It is probable that the mechanisms driving infrared neural stimulation (INS) and OP are very similar [4, 21]. INS was first demonstrated in 2005 where infrared laser pulses successfully elicited compound nerve and muscle potentials from the sciatic nerve of a frog [22] and has since been demonstrated in peripheral and cranial motor nerves [23, 24], cavernous nerves in the prostate [25], vestibular hair cells [26] and the cochlear nerve [27, 28]. In 2007, Wells et al concluded that a photothermal effect, where the laser pulse causes transient thermal gradient in the tissue, leads to action potential activation [10]. More recently, Shapiro et al conclusively demonstrated that infrared stimulation alters the electrical capacitance of the plasma membrane causing depolarization of the cell [29]. Although this appears to be the leading explanation for how infrared stimulation operates, other effects may induce contraction including modified mitochondrial Ca2+ cycling [21], tropomyosin dissociation with F-actin [30] and TRPV4 channel activation [31]. We have shown that when combining electrical and optical stimulation in the buccal nerve of the Aplysia californica, both excitation and inhibition of action potentials could be elicited depending on the energy of the light pulses [32]. This suggests that infrared light may have multiple effects and that it might require substantial research to determine how light interacts with activatable tissue. Discerning the mechanisms of OP is likely to enable improved application, and make this new technology more robust.
5. Conclusions
We have demonstrated for the first time that optical pacing of the adult rabbit heart is feasible. Five out of five Langendorff-perfused hearts were successfully synchronized with laser stimulation pulses where pacing was captured, stopped, then recaptured. Also, the heart rate repeatably followed the pacing frequency as it was modulated. Cell membrane permeation detected by PI staining at the stimulation site may have impeded OP for extended time periods. Future studies will explore laser pulse and light delivery parameters (wavelength, illumination area, etc.) to reduce OP thresholds that may in turn reduce membrane poration. If the stimulation threshold for the adult heart can be decreased, OP may provide an interesting alternative to electrical pacing that may enable new investigational and clinical applications.
Acknowledgments
The authors thank Dr. Igor Efimov and Dr. Shi Gu for providing insight into the experimental design and analysis. The project described was supported by the Case-Coulter Translational Research Partnership, the American Heart Association (Wang: 0815500D; Cheng: 0655154B) and the National Heart, Blood and Lung Institute by Grant Number (Rollins: HL095717, HL083048, Jenkins: HL115373). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Blood, and Lung Institute or the National Institutes of Health.
References and links
- 1.Akar F. G., Roth B. J., Rosenbaum D. S., “Optical measurement of cell-to-cell coupling in intact heart using subthreshold electrical stimulation,” Am. J. Physiol. Heart Circ. Physiol. 281(2), H533–H542 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Heffer L. F., Fallon J. B., “A novel stimulus artifact removal technique for high-rate electrical stimulation,” J. Neurosci. Methods 170(2), 277–284 (2008). 10.1016/j.jneumeth.2008.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazarian S., Hansford R., Roguin A., Goldsher D., Zviman M. M., Lardo A. C., Caffo B. S., Frick K. D., Kraut M. A., Kamel I. R., Calkins H., Berger R. D., Bluemke D. A., Halperin H. R., “A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices,” Ann. Intern. Med. 155(7), 415–424 (2011). 10.7326/0003-4819-155-7-201110040-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins M. W., Duke A. R., Gu S., Chiel H. J., Fujioka H., Watanabe M., Jansen E. D., Rollins A. M., “Optical pacing of the embryonic heart,” Nat. Photonics 4(9), 623–626 (2010). 10.1038/nphoton.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hersch A., Adam D., “Premature cardiac contractions produced efficiently by external high-intensity focused ultrasound,” Ultrasound Med. Biol. 37(7), 1101–1110 (2011). 10.1016/j.ultrasmedbio.2011.04.016 [DOI] [PubMed] [Google Scholar]
- 6.Arrenberg A. B., Stainier D. Y., Baier H., Huisken J., “Optogenetic control of cardiac function,” Science 330(6006), 971–974 (2010). 10.1126/science.1195929 [DOI] [PubMed] [Google Scholar]
- 7.Bruegmann T., Malan D., Hesse M., Beiert T., Fuegemann C. J., Fleischmann B. K., Sasse P., “Optogenetic control of heart muscle in vitro and in vivo,” Nat. Methods 7(11), 897–900 (2010). 10.1038/nmeth.1512 [DOI] [PubMed] [Google Scholar]
- 8.Kim S. C., Vasanji A., Efimov I. R., Cheng Y., “Spatial distribution and extent of electroporation by strong internal shock in intact structurally normal and chronically infarcted rabbit hearts,” J. Cardiovasc. Electrophysiol. 19(10), 1080–1089 (2008). 10.1111/j.1540-8167.2008.01201.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L., Nikolski V., Wallick D. W., Efimov I. R., Cheng Y., “Mechanisms of enhanced shock-induced arrhythmogenesis in the rabbit heart with healed myocardial infarction,” Am. J. Physiol. Heart Circ. Physiol. 289(3), H1054–H1068 (2005). 10.1152/ajpheart.01253.2004 [DOI] [PubMed] [Google Scholar]
- 10.Wells J., Kao C., Konrad P., Milner T., Kim J., Mahadevan-Jansen A., Jansen E. D., “Biophysical mechanisms of transient optical stimulation of peripheral nerve,” Biophys. J. 93(7), 2567–2580 (2007). 10.1529/biophysj.107.104786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro M. G., Homma K., Villarreal S., Richter C. P., Bezanilla F., “Infrared light excites cells by changing their electrical capacitance,” Nat Commun 3, 736 (2012). 10.1038/ncomms1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pak H. N., Oh Y. S., Liu Y. B., Wu T. J., Karagueuzian H. S., Lin S. F., Chen P. S., “Catheter ablation of ventricular fibrillation in rabbit ventricles treated with beta-blockers,” Circulation 108(25), 3149–3156 (2003). 10.1161/01.CIR.0000104563.12408.12 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y. T., Efimov I. R., Cheng Y., “Electroporation induced by internal defibrillation shock with and without recovery in intact rabbit hearts,” Am. J. Physiol. Heart Circ. Physiol. 303(4), H439–H449 (2012). 10.1152/ajpheart.01121.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishan A., “Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining,” J. Cell Biol. 66(1), 188–193 (1975). 10.1083/jcb.66.1.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hucker W. J., Sharma V., Nikolski V. P., Efimov I. R., “Atrioventricular conduction with and without AV nodal delay: two pathways to the bundle of His in the rabbit heart,” Am. J. Physiol. Heart Circ. Physiol. 293(2), H1122–H1130 (2007). 10.1152/ajpheart.00115.2007 [DOI] [PubMed] [Google Scholar]
- 16.Henrikson C. A., Leng C. T., Yuh D. D., Brinker J. A., “Computed tomography to assess possible cardiac lead perforation,” Pacing Clin. Electrophysiol. 29(5), 509–511 (2006). 10.1111/j.1540-8159.2006.00385.x [DOI] [PubMed] [Google Scholar]
- 17.Laborderie J., Barandon L., Ploux S., Deplagne A., Mokrani B., Reuter S., Le Gal F., Jais P., Haissaguerre M., Clementy J., Bordachar P., “Management of subacute and delayed right ventricular perforation with a pacing or an implantable cardioverter-defibrillator lead,” Am. J. Cardiol. 102(10), 1352–1355 (2008). 10.1016/j.amjcard.2008.07.025 [DOI] [PubMed] [Google Scholar]
- 18.Yavari A., Khawaja Z. O., Krishnamoorthy S., McWilliams E. T., “Perforation of right ventricular free wall by pacemaker lead detected by multidetector computed tomography,” Europace 11(2), 252–254 (2008). 10.1093/europace/eun381 [DOI] [PubMed] [Google Scholar]
- 19.Wilkoff B. L., Bello D., Taborsky M., Vymazal J., Kanal E., Heuer H., Hecking K., Johnson W. B., Young W., Ramza B., Akhtar N., Kuepper B., Hunold P., Luechinger R., Puererfellner H., Duru F., Gotte M. J., Sutton R., Sommer T., EnRhythm MRI SureScan Pacing System Study Investigators , “Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment,” Heart Rhythm 8(1), 65–73 (2011). 10.1016/j.hrthm.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 20.Hook B. G., Perlman R. L., Callans D. J., Hanna M. S., Kleiman R. B., Flores B. T., Marchlinski F. E., “Acute and chronic cycle length dependent increase in ventricular pacing threshold,” Pacing Clin. Electrophysiol. 15(10), 1437–1444 (1992). 10.1111/j.1540-8159.1992.tb02916.x [DOI] [PubMed] [Google Scholar]
- 21.Dittami G. M., Rajguru S. M., Lasher R. A., Hitchcock R. W., Rabbitt R. D., “Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes,” J. Physiol. 589(6), 1295–1306 (2011). 10.1113/jphysiol.2010.198804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells J., Kao C., Mariappan K., Albea J., Jansen E. D., Konrad P., Mahadevan-Jansen A., “Optical stimulation of neural tissue in vivo,” Opt. Lett. 30(5), 504–506 (2005). 10.1364/OL.30.000504 [DOI] [PubMed] [Google Scholar]
- 23.Wells J., Konrad P., Kao C., Jansen E. D., Mahadevan-Jansen A., “Pulsed laser versus electrical energy for peripheral nerve stimulation,” J. Neurosci. Methods 163(2), 326–337 (2007). 10.1016/j.jneumeth.2007.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teudt I. U., Nevel A. E., Izzo A. D., Walsh J. T., Jr, Richter C. P., “Optical stimulation of the facial nerve: a new monitoring technique?” Laryngoscope 117(9), 1641–1647 (2007). 10.1097/MLG.0b013e318074ec00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried N. M., Lagoda G. A., Scott N. J., Su L. M., Burnett A. L., “Laser stimulation of the cavernous nerves in the rat prostate, in vivo: optimization of wavelength, pulse energy, and pulse repetition rate,” Conf. Proc. IEEE Eng. Med. Biol. Soc. 2008, 2777–2780 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Rajguru S. M., Richter C. P., Matic A. I., Holstein G. R., Highstein S. M., Dittami G. M., Rabbitt R. D., “Infrared photostimulation of the crista ampullaris,” J. Physiol. 589(6), 1283–1294 (2011). 10.1113/jphysiol.2010.198333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izzo A. D., Walsh J. T., Jr, Ralph H., Webb J., Bendett M., Wells J., Richter C. P., “Laser stimulation of auditory neurons: effect of shorter pulse duration and penetration depth,” Biophys. J. 94(8), 3159–3166 (2008). 10.1529/biophysj.107.117150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matic A. I., Robinson A. M., Young H. K., Badofsky B., Rajguru S. M., Stock S., Richter C. P., “Behavioral and electrophysiological responses evoked by chronic infrared neural stimulation of the cochlea,” PLoS ONE 8(3), e58189 (2013). 10.1371/journal.pone.0058189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro M. G., Homma K., Villarreal S., Richter C. P., Bezanilla F., “Infrared light excites cells by changing their electrical capacitance,” Nat Commun 3(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyama K., Mizuno A., Shintani S. A., Itoh H., Serizawa T., Fukuda N., Suzuki M., Ishiwata S., “Microscopic heat pulses induce contraction of cardiomyocytes without calcium transients,” Biochem. Biophys. Res. Commun. 417(1), 607–612 (2012). 10.1016/j.bbrc.2011.12.015 [DOI] [PubMed] [Google Scholar]
- 31.Albert E. S., Bec J. M., Desmadryl G., Chekroud K., Travo C., Gaboyard S., Bardin F., Marc I., Dumas M., Lenaers G., Hamel C., Muller A., Chabbert C., “TRPV4 channels mediate the infrared laser-evoked response in sensory neurons,” J. Neurophysiol. 107(12), 3227–3234 (2012). 10.1152/jn.00424.2011 [DOI] [PubMed] [Google Scholar]
- 32.Duke A. R., Lu H., Jenkins M. W., Chiel H. J., Jansen E. D., “Spatial and temporal variability in response to hybrid electro-optical stimulation,” J. Neural Eng. 9(3), 036003 (2012). 10.1088/1741-2560/9/3/036003 [DOI] [PMC free article] [PubMed] [Google Scholar]