Abstract
The aim of this study was to investigate the interaction of cadmium chloride with mineral elements in rat nephrocytes in terms of the biosynthesis of nanocomplexes. The results show that selenium supplementation enhanced cadmium accumulation in kidneys. Analysis of the fluorescence revealed an increase in red fluorescence in the kidneys of rats co-exposed to cadmium and selenium. Interestingly, X-ray diffraction measurements carried out on kidney fractions of co-exposed rats point to the biosynthesis of cadmium selenide and/or sulfide nanoparticles (about 62 nm in size). Oxidative stress assays showed the ability of selenium to reduce lipid peroxidation and to restore glutathione peroxidase and superoxide dismutase activity in kidneys. Hence, cadmium complexation with selenium and sulfur at a nanoscale level could reduce oxidative stress induced by cadmium in kidneys.
Keywords: nanoparticles, detoxification, oxidative stress, X-ray diffraction, fluorescence microscopy, kidneys
Introduction
Cadmium (Cd) is a well-recognized environmental pollutant with numerous adverse health effects. Sources of exposure to this metal include food, water, and alcoholic beverages.1–3 Many studies revealed that Cd accumulates preferentially in hepatic and renal tissues.3–5 Previous studies point out that metallothionein (MT) biosynthesis – a family of cysteine-rich low molecular weight proteins – sequestrates the metal.3,6,7 In fact, lesions of the proximal tubule in the kidney cortex were observed after Cd exposure.8 Various studies point out that Cd toxicity is related to oxidative stress, since this metal can alter the antioxidant defense system in several animal tissues. Studies on mammals have shown that Cd stimulated formation of reactive oxygen species.2,3 Moreover, Cd has been suggested to practice some of its toxic effects by disturbing the metabolism of essential metals, such as zinc (Zn)9–11 and selenium (Se).12,13 Treatment with Se during Cd exposure has been demonstrated to have beneficial effects on Cd toxicity.12,14–16 However, based on the available published literature, the effect of Se on Cd toxicity is not yet well studied.
In fact, the main target organs for Cd accumulation are the liver, kidneys, and other tissues.2,3 However, Trabelsi et al showed that the subacute toxicity of Cd may be related to its eventual potential to generate Cd sulfide (CdS) and/or Cd selenide (CdSe) nanomaterials at the cellular level.17 In addition, to the authors’ knowledge, there are no studies investigating whether the protective effect of Se is related to its eventual potential to bind Cd, leading to nanocomplexes in living systems.
The aim of this investigation was to study the reduction of Cd-induced nephrotoxicity in terms of the biosynthesis of nanocomplexes.
Material and methods
Chemicals
Cd chloride (CdCl2) and sodium selenite (Na2SeO3) were purchased from Sigma-Aldrich (St Louis, MO, USA). All other chemicals were of analytical grade and were purchased from standard commercial suppliers.
Animals
Adult Wistar male rats (SIPHAT, Bin Arous, Tunisia) weighing 200–220 g at the time of the experiments were randomly divided into the following groups: control rats (n = 6), Cd-exposed rats (n = 6), and rats co-exposed to cadmium and selenium (Cd + Se) (n = 6). Animals were housed at 25°C, under a 12-hour/12-hour light/dark cycle, with free access to water and commercial mash. Animals were cared for under the Tunisian code of practice for the Care and Use of Animals for Scientific Purposes. The experimental protocols were approved by the Faculty Ethics Committee (Faculté des Sciences de Bizerte, Jarzouna, Tunisia).
Animal treatment
The control group was intraperitoneally injected with 0.10 mL of 0.90% saline solution for 14 consecutive days. The Cd-treated group was intraperitoneally injected with a sublethal dose of Cd (1.50 mg Cd/kg of body weight) for 14 days.3 The co-exposed rats were treated with Cd (1.50 mg Cd/kg of body weight) and Se (0.20 mg/L per os [by mouth]) for 14 days.13
Cd determination
Renal slices for Cd analyses were oven dried (60°C) to a constant weight. The dried tissues (100 mg from each sample) were digested with 3 mL trace pure nitric acid at 120°C. The volume was then adjusted to 10 mL with deionized water.18
Cd concentrations in the acid solutions were measured by atomic absorption spectrophotometry using a PerkinElmer 306 spectrometer equipped with a PerkinElmer Intensitron® lamp (Waltham, MA, USA). Cd concentration is expressed in μg/g dry tissue weight.19
Fluorescence microscopy
Kidney fractions were fixed with 10% formaldehyde and were evaluated by fluorescence microscopy using a DM-IRBE microscope (Leica Microsystems, Wetzlar, Germany) coupled with a digital charge-coupled device camera (CoolSNAP™ FX; Princeton Instruments, Trenton, NJ, USA).
Powder samples preparation and X-ray diffraction (XRD) measurements
Fourteen days after intraperitoneal injection, the control and treated groups were sacrificed and their kidneys were harvested. The tissues were weighed rinsed with ice-cold deionized water, and dried with filter paper. Kidney fractions were dried for 5 days at 50°C. Fractions were mixed and sieved in order to obtain powder. XRD measurements were carried out on a Bruker D8 ADVANCE powder X-ray diffractometer (Bruker Corporation, Billerica, MA, USA), using copper (Cu) Kα (λ = 1:5402 Å) incident radiation with a scan range of 20< 2θ <70.17
Tissue preparation
The control and treated groups were sacrificed and their kidneys were immediately harvested. The tissues were weighed rinsed with ice-cold deionized water, and dried with filter paper. Fractions of tissues (500 mg) were homogenized in buffer (tris[hydroxymethyl]aminomethane 10 mmol/L, ethylenediaminetetraacetic acid 1 mmol/L, phenylmethylsulfonyl fluoride 1 mmol/L; pH 7.4). The homogenates were centrifuged at 600 g for 10 minutes and centrifuged again at 13,000 g for 20 minutes at 4°C to obtain a postnuclear homogenate and postmitochondrial supernatant fractions.20
Antioxidant enzyme assays
Lipid peroxidation in tissues was measured by thiobarbituric acid reactive substances and was expressed in terms of malondialdehyde (MDA) content.21 Catalase (CAT) activity was assayed by ultraviolet spectrophotometry22 Glutathione peroxidase (GPx) activity was measured according to Gunzler et al.23 Superoxide dismutase (SOD) activity was determined by measuring the inhibition of the auto-oxidant of pyrogallol by spectrophotometry (Jenway 6505 UV/Visible; Bibby Scientific Limited Stone, UK) at 420 nm, using Marklund and Marklund’s modified method.24
Data presentation and statistical analysis
A one-way analysis of variance followed by Tukey’s multiple comparisons test was performed using GraphPad Prism® version 6.00 for Windows (GraphPad Software, Inc, La Jolla, CA, USA). Data are reported as the mean ± standard deviation. The level of significance was set at P < 0.05.
Results
Cd accumulation in kidneys
Figure 1 shows an accumulation of Cd in rat kidneys in comparison to the control group. Moreover, Se supplementation (0.20 mg/L per os) facilitates Cd accumulation compared to values found with Cd-exposed rats (1827 ± 172.72 μg/g versus 1065 ± 217.80 μg/g; P < 0.01).
Figure 1.
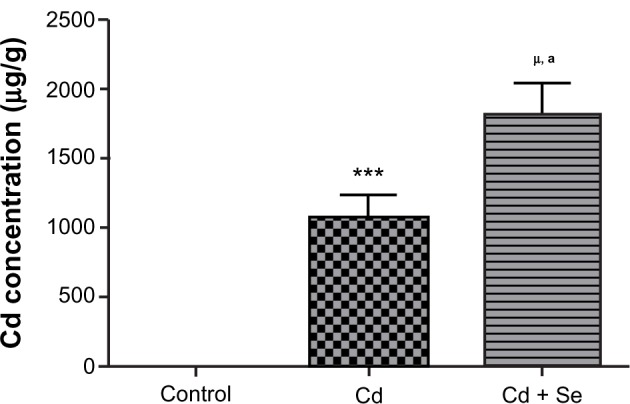
Cadmium (Cd) concentration in the kidneys of control rats, cadmium-treated rats (1.50 mg/kg intraperitoneally), and rats co-exposed to cadmium and selenium (cadmium: 1.50 mg/kg intraperitoneally and selenium [Se]: 0.20 mg/L per os).
Notes: Data represent the mean ± standard deviation of six animals per group. *** P < 0.001; μP < 0.0001 compared with the control group. aP < 0.01 compared with the cadmium group.
Fluorescence microscopy
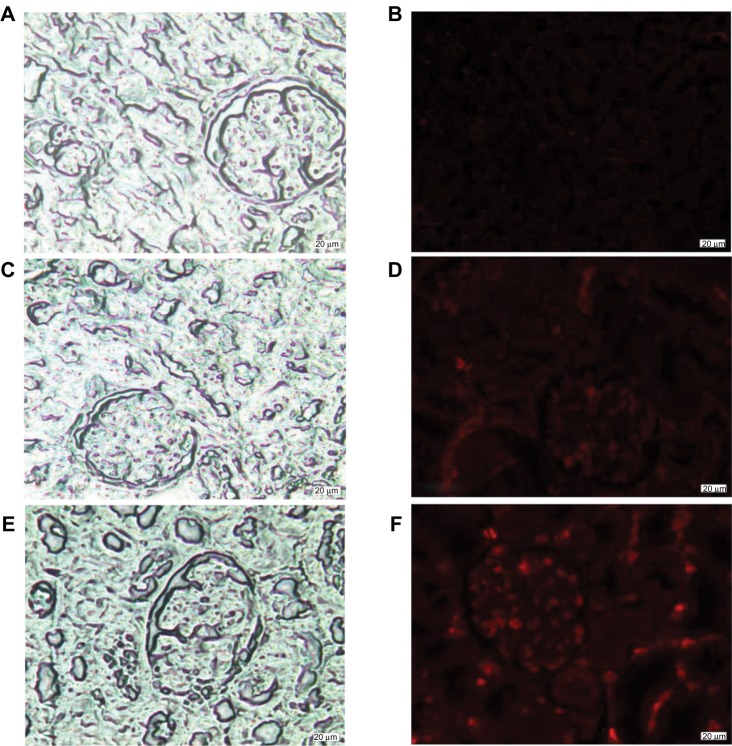
Fluorescence microscopy images show no fluorescence signals in the control kidneys (Figure 2A–B). However, red fluorescence was detected in the glomeruli and renal tubular of Cd-treated kidneys (Figure 2C–D). Interestingly, the intensity of the fluorescence signal was higher after exposure to Cd and Se supplementation in comparison with the Cd-treated group (Figure 2E–F).
Figure 2.
Light microscopy (A) and fluorescence microscopy images (B) of the kidney cortex of control rats. Light microscopy (C) and fluorescence microscopy images (D) of the kidney cortex of cadmium-treated rats (1.50 mg/kg intraperitoneally). Light microscopy (E) and fluorescence microscopy images (F) of the kidney cortex of rats co-exposed to cadmium and selenium (cadmium: 1.50 mg/kg intraperitoneally and selenium: 0.20 mg/L per os).
XRD pattern
The XRD pattern gives information about crystalline structure, grain size, and strain. Importantly, the XRD pattern of kidney powder harvested from Cd-treated rats revealed three new peaks observed at 2θ = 23.49°, 2θ = 30.55°, and 2θ = 46.41°, referring to diffraction of cubic (Zn blende) CdSe and/or CdS nanoparticles. The XRD pattern of co-treated rats showed three new peaks compared to the control group (Figure 3). New peaks were observed at 2θ = 21.51°, 2θ = 42.96°, and 2θ = 50.06°, referring to diffraction of cubic (Zn blende) CdSe and/or CdS nanoparticles. The average CdSe and/or CdS nanoparticles size was determined to be about 98.65 nm for the Cd-treated group and 62.60 nm for the co-exposed group. The size was determined from the full width at half maximum of the most intense peak using the Scherrer equation,
Figure 3.
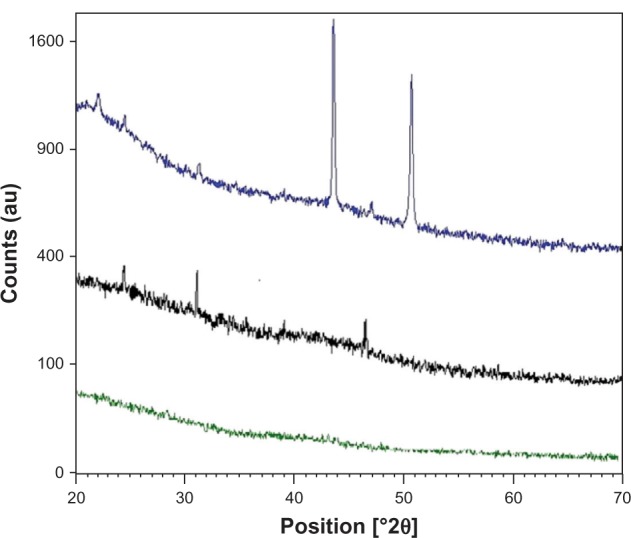
X-ray diffraction pattern of kidney powder of control rats (green pattern), cadmium-treated rats (1.50 mg/kg intraperitoneally) (black pattern), and rats co-exposed to cadmium and selenium (cadmium: 1.50 mg/kg intraperitoneally and selenium: 0.20 mg/L per os) (blue pattern).
| [1[ |
where λ is the wavelength of the X-ray radiation, β is the full width at half maximum in radians of the XRD peak, and θ is the angle of diffraction.
Oxidative stress assays
MDA concentration
Rats exposed to Cd displayed an increase in MDA (0.76 ± 0.03 versus 0.43 ± 0.01 nmol/mg protein; P < 0.01). Simultaneous administration of Se and Cd induced a decrease in MDA level compared to the Cd-treated group (0.21 ± 0.01 versus 0.76 ± 0.03 nmol/mg protein; P < 0.001) (Figure 4).
Figure 4.
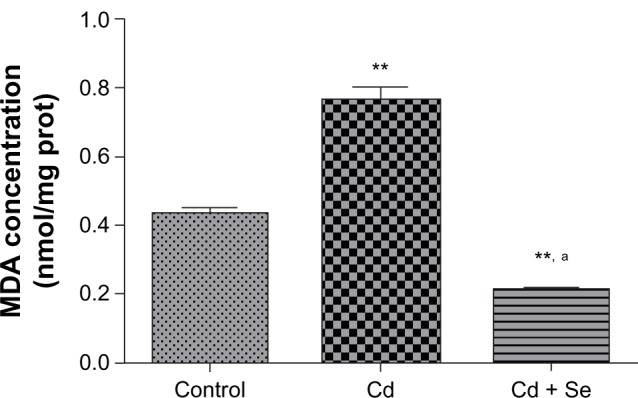
Effects of selenium on renal malondialdehyde level in rats exposed to cadmium.
Notes: Data represent the mean ± standard deviation of six animals per group. **P < 0.01 compared with the control group. aP < 0.001 compared with the cadmium-treated group.
Abbreviations: Cd, cadmium; MDA, malondialdehyde; prot, protein; Se, selenium.
Antioxidant enzymes activities in kidneys
Cd administration showed a decrease in GPx, CAT, CuZn-SOD, and manganese SOD activity. In contrast, Se supplementation induced an increase in GPx (P < 0.01), CuZn-SOD (P < 0.001), and manganese SOD (P < 0.001) activity compared to the Cd-exposed group. However, Se failed to restore CAT activity, which remained lower than the control group (Table 1).
Table 1.
Effects of cadmium and selenium treatments on antioxidant enzymes in rat kidneys
| GPx (U/mg prot) | CAT (U/mg prot) | CuZn-SOD (U/mg prot) | Mn-SOD (U/mg prot) | |
|---|---|---|---|---|
| Control | 1698.96 ± 115.09 | 76.08 ± 8.25 | 8.35 ± 0.96 | 1.91 ± 0.09 |
| Cd | 1120.90 ± 119.83* | 10.85 ± 1.85** | 3.68 ± 0.90* | 1.27 ± 0.16 |
| Cd + Se | 3357.85 ± 288.15**,a | 29.82 ± 7.08* | 29.61 ± 1.04***,b | 15.43 ± 1.22**,b |
Notes: Data represent the mean ± standard deviation of six animals per group.
P < 0.05;
P < 0.01;
P < 0.001 compared with the control group.
P < 0.01;
P < 0.001 compared with the cadmium-treated group.
Abbreviations: CAT, catalase; CuZn-SOD, copper/zinc superoxide dismutase; GPx, glutathione peroxidase; Mn-SOD, manganese superoxide dismutase; prot, protein; Cd, cadmium; Se, selenium.
Discussion
In this study, Wistar male rats were intraperitoneally treated with Cd chloride (1.50 mg/kg of body weight) and received Se supplementation (0.20 mg/L per os) in order to better explain the protective effect of Se against Cd nephrotoxicity in terms of nanotoxicity.
The results indicate an increase in toxic metal level (Cd) in kidneys in agreement with previous studies.18–21 In fact, it has been reported that after its absorption Cd is taken up by the hepatocytes where it induces the biosynthesis of MTs. Cd ions are bound by MTs via thiol groups of cysteine residues leading to Cd–MT complexes;2,3,25–29 then from the liver the Cd ions circulate in blood bound to MTs until reaching the kidneys.7,17,30 The Cd–MT complex is easily filtered through the glomerular membrane and taken up by renal tubular cells. MTs are then catabolized, releasing Cd ions in the cytoplasm where they induce the synthesis of new MT molecules. These, in turn, bind and retain Cd in the kidneys for a long period of time.4,31,32 Previously, Trabelsi et al showed that Cd may induce nanoparticle biosynthesis.17 This biosynthesis was explained by the ability of Cd to react with sulfur in MTs and/or with Se, leading to CdS and/or CdSe nanoparticles in hepatocytes and nephrocytes. Moreover, the current results show that Se increased the accumulation of Cd in kidneys. In contrast, El Heni et al indicated that Se supplementation did not influence Cd accumulation in kidneys.25 Moreover, Jamba et al showed that administration of Se reduced Cd accumulation in kidneys.27 The current data indicate the proportional accumulation of Cd in kidneys with organ Se content.
Trabelsi et al reported that the interaction of Cd with some kidney elements could generate quantum dots like CdS and/or CdSe. Interestingly, fluorescence microscopy images show that co-treatment (Cd + Se group) induced a localized red fluorescence signal.17 The superposition of fluorescence images and light microscopy images showed that fluorescence was localized in the glomeruli and renal tubules as shown in Figure 2E–F. The origin of fluorescence signals can be explained by the biosynthesis of CdSe and/or CdS nanoparticles in nephrocytes. In fact, CdSe and/or CdS nanoparticles are characterized by size-dependent photoluminescence colors which are distributed throughout the visible region of the electromagnetic spectrum.33
In addition, in order to confirm the hypothesis of nanocomplexes synthesis, XRD measurements of kidney powder were performed. In fact, Se reduces the Cd level in blood leading to insoluble complexes.34 Complexation reactions depend on the nature of either mineral or organic Se. The mineral form of Se (selenite) leads to less soluble complexes than organic forms.35 Interestingly, the current results show that 2θ values are almost the same as those observed after chemical or biological synthesis of CdSe and/or CdS nanoparticles, as reported in many studies.17,36–38 Differences observed in 2θ values compared to chemical synthesis may be due to temperature variation, ie, the high temperature during chemical synthesis and rat body temperature (about 37°C). Moreover, the size of nanoparticles found in kidneys (98.65 nm) following subacute exposure (1.50 mg/kg for 14 days) indicates a probable agglomeration of nanoparticles related to the histological properties of nephrocytes.
In order to investigate the interaction of CdSe and/or CdS nanoparticle biosynthesis with oxidative stress, lipid peroxidation and antioxidant enzymes activities were investigated. In fact, the basis of Cd toxicity is its negative influence on the enzymatic systems of cells resulting from the substitution of divalent mineral elements (Zn2+, Cu2+, and calcium [Ca2+]) in metalloenzymes and its very strong affinity to biological structures containing –SH groups, such as proteins, enzymes, and nucleic acids.39,40 Many effects of Cd result from interactions with necessary micro- and macroelements, especially calcium, Zn, Cu, iron, and Se.41,42
The current results have clearly demonstrated the ability of Cd to induce oxidative stress in rat kidneys, as evidenced by increased lipid peroxidation (MDA) after 14 days of Cd treatment. This finding is in agreement with previous studies.2,3,43
The enhanced kidney MDA in rats exposed to Cd is associated with a reduction in SOD, CAT, and GPx tissue activity, indicating oxidative stress. The inhibition of SOD activity is probably a consequence of the interaction between Cd and Zn in SOD molecules. Bauer et al reported that 111Cd was able to occupy the site of Zn in the CuZn-SOD molecule, creating an inactive form of the enzyme (Cu111CdSOD).44 Regarding CAT, as the presence of Cd in the organism decreases the level of iron in the blood, liver, and kidneys and since CAT contains iron in its active center, the decreased activity of the enzyme in rat kidneys exposed to Cd might be a result of iron deficiency.45
There is increasing evidence that Cd interacts with Se and disrupts GPx activity, which proves Se is a co-factor. Galazyn-Sidorczuk et al found that Cd exposure decreased Se concentration and GPx activity in rat serum, liver, and kidneys.46 Due to the formation of Cd–Se complexes – especially CdSe – Se bioavailability and absorption from the gastrointestinal tract decrease with excessive Cd intake.46 Moreover, the ability of Cd to form Cd–Se complexes in the blood stream, liver, and kidneys decreases Se availability for GPx and potentiates the effects of its deficiency in tissues.47,48 The effect of Se on GPx activity may be attributed to an increase in the bioavailability of Se following co-treatment with sodium selenite, which is reflected in the increased GPx activity, as suggested by Jamba et al.15 The observed decrease of MDA concentration in the co-exposed group is in agreement with previous investigations and can be explained by the recovery of biological activity of SOD and GPx.49,50
The results suggest that Cd induced oxidative stress in kidneys. This oxidative stress may be associated with the biosynthesis of nanocomplexes (CdSe and/or CdS). The data report for the first time, to the authors’ knowledge, that Se decreases the oxidative response by enhancing the biosynthesis of nanocomplexes (CdSe and/or CdS) in rat nephrocytes. This biosynthesis may represent a detoxification pathway after Cd treatment. Hence, as CdSe and/or CdS nanoparticle biosynthesis increases in nephrocytes, the bioavailability of Cd decreases, leading to weak oxidative responses. This finding can be explained by the following ratio:
where (X) refers to sulfur and/or selenium. The ratio increase is correlated to the number of biosynthesized nanoparticles associated with a concomitant decrease in Cd bioavailability and MDA level in kidneys. The nanocomplexes could be evaluated by imagery methods based on the evaluation of red fluorescence intensity, which could be used as a health marker.
Conclusion
To the authors’ knowledge, this is the first study that investigates interactions of Cd with sulfur and Se in nephrocytes in terms of CdSe and/or CdS nanoparticle biosynthesis. The results show that Cd may induce the biosynthesis of red fluorescent CdSe and/or CdS nanoparticles in kidneys. This study also shows that Cd induced oxidative stress by disturbing antioxidant enzymes activities. Se supplementation reduced Cd-induced toxicity in rat kidneys, probably due to its ability to bind Cd in nanosized insoluble and fluorescent complexes.
Acknowledgments
The authors would like to thank Dr Ahmed Rejeb (Department of Anatomic Pathology, Ecole Nationale de Médecine Vétérinaire, Sidi Thabet, Tunisia) and Mr Hazem Ben Mabrouk (Institut Pasteur de Tunis, Belvedere, Tunisia) for their help in the fluorescence microscopy work.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure: a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- 2.Amara S, Abdelmelek H, Garrel C, et al. Influence of static magnetic field on cadmium toxicity: study of oxidative stress and DNA damage in rat tissues. J Trace Elem Med Biol. 2006;20(4):263–269. doi: 10.1016/j.jtemb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Chater S, Douki T, Garrel C, Favier A, Sakly M, Abdelmelek H. Cadmium-induced oxidative stress and DNA damage in kidney of pregnant female rats. C R Biol. 2008;331(6):426–432. doi: 10.1016/j.crvi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Nordberg M, Nordberg GF. Distribution of metallothionein-bound cadmium and cadmium chloride in mice: preliminary studies. Environ Health Perspect. 1975;12:103–108. doi: 10.1289/ehp.7512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshikawa H. Accumulation of cadmium in organs of mice by a long-term injection of cadmium and interactions of cadmium with copper, manganese and zinc already present in the animals. Sangyo Igaku. 1979;21(2):171–177. doi: 10.1539/joh1959.21.171. Japanese. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh ZA, Smith JC. The biosynthesis of metallothionein in rat liver and kidney after administration of cadmium. Chem Biol Interact. 1976;15(4):327–336. doi: 10.1016/0009-2797(76)90138-1. [DOI] [PubMed] [Google Scholar]
- 7.Nordberg GF, Nordberg M. Different binding forms of cadmium: implications for distribution and toxicity. J UOEH. 1987;(Suppl 9):153–164. [PubMed] [Google Scholar]
- 8.Jarup L. Cadmium overload and toxicity. Nephrol Dial Transplant. 2002;17(Suppl 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- 9.Petering HG, Choudhury H, Stemmer KL. Some effects of oral ingestion of cadmium on zinc, copper, and iron metabolism. Environ Health Perspect. 1979;28:97–106. doi: 10.1289/ehp.792897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonner FW, King LJ, Parke DV. The effect of dietary cadmium on zinc, copper and iron levels in the bone of rats. Toxicol Lett. 1980;5(2):105–108. doi: 10.1016/0378-4274(80)90157-5. [DOI] [PubMed] [Google Scholar]
- 11.Amara S, Abdelmelek H, Garrel C, et al. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J Reprod Dev. 2008;54(2):129–134. doi: 10.1262/jrd.18110. [DOI] [PubMed] [Google Scholar]
- 12.Chen RW, Whanger PD, Weswig PH. Selenium-induced redistribution of cadmium binding to tissue proteins: a possible mechanism of protection against cadmium toxicity. Bioinorg Chem. 1975;4(2):125–133. doi: 10.1016/s0006-3061(00)81021-2. [DOI] [PubMed] [Google Scholar]
- 13.Ghodbane S, Amara S, Garrel C, et al. Selenium supplementation ameliorates static magnetic field-induced disorders in antioxidant status in rat tissues. Environ Toxicol Pharmacol. 2011;31(1):100–106. doi: 10.1016/j.etap.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Whanger PD, Ridlington JW, Holcomb CL. Interactions of zinc and selenium on the binding of cadmium to rat tissue proteins. Ann N Y Acad Sci. 1980;355:333–346. doi: 10.1111/j.1749-6632.1980.tb21351.x. [DOI] [PubMed] [Google Scholar]
- 15.Jamba L, Nehru B, Bansal MP. Effect of selenium supplementation on the influence of cadmium on glutathione and glutathione peroxidase system in mouse liver. The Journal of Trace Elements in Experimental Medicine. 2000;13(3):299–304. [Google Scholar]
- 16.El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology. 2007;235(3):185–193. doi: 10.1016/j.tox.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Trabelsi H, Azzouz I, Sakly M, Abdelemelek H. Subacute toxicity of cadmium on hepatocytes and nephrocytes in the rat could be considered as a green biosynthesis of nanoparticles. Int J Nanomedicine. 2013;8:1121–1128. doi: 10.2147/IJN.S39426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takashima M, Nishino K, Itokawa Y. Effect of cadmium administration on growth, excretion, and tissue accumulation of cadmium and histological alterations in calcium-sufficient and -deficient rats: an equalized feeding study. Toxicol Appl Pharmacol. 1978;45(2):591–598. doi: 10.1016/0041-008x(78)90120-5. [DOI] [PubMed] [Google Scholar]
- 19.Congui L, Chicca M, Pilastro A, Turchetto M, Tallandini L. Effects of chronic dietary cadmium on hepatic glutathione levels and glutathione peroxidase activity in starlings (Sturnus vulgaris) Arch Environ Contam Toxicol. 2000;38(3):357–361. doi: 10.1007/s002449910047. [DOI] [PubMed] [Google Scholar]
- 20.Beytut E, Aksakal M. The effect of long-term supplemental dietary cadmium on lipid peroxidation and the antioxidant system in the liver and kidneys of rabbits. Turkish Journal of Veterinary and Animal Sciences. 2002;26(5):1055–1060. [Google Scholar]
- 21.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 22.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 23.Gunzler WA, Kremers H, Flohe L. An improved coupled test procedure for glutathione peroxidase (EC 1-11-1-9-) in blood. Z Klin Chem Klin Biochem. 1974;12(10):444–448. doi: 10.1515/cclm.1974.12.10.444. [DOI] [PubMed] [Google Scholar]
- 24.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 25.El Heni J, Messaoudi I, Hamouda F, Kerkeni A. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem Toxicol. 2008;46(11):3522–3527. doi: 10.1016/j.fct.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 26.Aughey E, Fell GS, Scott R, Black M. Histopathology of early effects of oral cadmium in the rat kidney. Environ Health Perspect. 1984;54:153–161. doi: 10.1289/ehp.8454153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamba L, Nehru B, Bansal MP. Redox modulation of selenium binding proteins by cadmium exposures in mice. Mol Cell Biochem. 1997;177(1–2):169–175. doi: 10.1023/a:1006869623864. [DOI] [PubMed] [Google Scholar]
- 28.Mitsumori K, Shibutani M, Sato S, et al. Relationship between the development of hepato–renal toxicity and cadmium accumulation in rats given minimum to large amounts of cadmium chloride in the long term: preliminary study. Arch Toxicol. 1998;72(9):545–552. doi: 10.1007/s002040050541. [DOI] [PubMed] [Google Scholar]
- 29.Rodilla V, Miles AT, Jenner W, Hawksworth GM. Exposure of cultured human proximal tubular cells to cadmium, mercury, zinc and bismuth: toxicity and metallothionein induction. Chem Biol Interact. 1998;115(1):71–83. doi: 10.1016/s0009-2797(98)00059-3. [DOI] [PubMed] [Google Scholar]
- 30.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- 31.Nordberg M. General aspects of cadmium: transport, uptake and metabolism by the kidney. Environ Health Perspect. 1984;54:13–20. doi: 10.1289/ehp.845413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nordberg GF, Jin T, Nordberg M. Subcellular targets of cadmium nephrotoxicity: cadmium binding to renal membrane proteins in animals with or without protective metallothionein synthesis. Environ Health Perspect. 1994;102(Suppl 3):191–194. doi: 10.1289/ehp.94102s3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc. 1993;115(19):8706–8715. [Google Scholar]
- 34.Seppanen K, Laatikainen R, Salonen JT, et al. Mercury-binding capacity of organic and inorganic selenium in rat blood and liver. Biol Trace Elem Res. 1998;65(3):197–210. doi: 10.1007/BF02789096. [DOI] [PubMed] [Google Scholar]
- 35.Feroci G, Badiello R, Fini A. Interactions between different selenium compounds and zinc, cadmium and mercury. J Trace Elem Med Biol. 2005;18(3):227–234. doi: 10.1016/j.jtemb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Chang W, Shen Y, Xie A, Zhan H, Wang J, Lu W. Controlled synthesis of CdSe and CdSe/CdS core/shell nanoparticles using Gemini surfactant Py-16-10-16 and their bioconjugates with BSA. J Colloid Interface Sci. 2009;335(2):257–263. doi: 10.1016/j.jcis.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Zeng R, Zhang T, Liu J, et al. Aqueous synthesis of type-II CdTe/CdSe core–shell quantum dots for fluorescent probe labeling tumor cells. Nanotechnology. 2009;20(9):095102. doi: 10.1088/0957-4484/20/9/095102. [DOI] [PubMed] [Google Scholar]
- 38.Huang F, Lin X, Cheng C, Chen P. Fabrication of chitosan–CdSe/CdS/ZnS multilayer films by electrostatic self-assembly method. Appl Surf Sci. 2012;258(19):7359–7364. [Google Scholar]
- 39.Jacobson KB, Turner JE. The interaction of cadmium and certain other metal ions with proteins and nucleic acids. Toxicology. 1980;16(1):1–37. doi: 10.1016/0300-483x(80)90107-9. [DOI] [PubMed] [Google Scholar]
- 40.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 41.Waalkes MP, Coogan TP, Barter RA. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit Rev Toxicol. 1992;22(3–4):175–201. doi: 10.3109/10408449209145323. [DOI] [PubMed] [Google Scholar]
- 42.Beyersmann D. Interactions in metal carcinogenicity. Toxicol Lett. 1994;72(1–3):333–338. doi: 10.1016/0378-4274(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 43.Hussain T, Shukla GS, Chandra SV. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacol Toxicol. 1987;60(5):355–358. doi: 10.1111/j.1600-0773.1987.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 44.Bauer R, Demeter I, Hasemann V, Johansen JT. Structural properties of the zinc site in Cu, Zn-superoxide dismutase; perturbed angular correlation of gamma ray spectroscopy on the Cu, 111Cd superoxide dismutase derivative. Biochem Biophys Res Commun. 1980;94(4):1296–1302. doi: 10.1016/0006-291x(80)90560-4. [DOI] [PubMed] [Google Scholar]
- 45.Jurczuk M, Brzoska MM, Moniuszko-Jakoniuk J, Gaazyn-Sidorczuk M, Kulikowska-Karpinska E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol. 2004;42(3):429–438. doi: 10.1016/j.fct.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Galazyn-Sidorczuk M, Brzoska MM, Rogalska J, Roszczenko A, Jurczuk M. Effect of zinc supplementation on glutathione peroxidase activity and selenium concentration in the serum, liver and kidney of rats chronically exposed to cadmium. J Trace Elem Med Biol. 2012;26(1):46–52. doi: 10.1016/j.jtemb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Gambhir J, Nath R. Effect of cadmium on tissue glutathione and glutathione peroxidase in rats: influence of selenium supplementation. Indian J Exp Biol. 1992;30(7):597–601. [PubMed] [Google Scholar]
- 48.Sasakura C, Suzuki KT. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem. 1998;71(3–4):159–162. doi: 10.1016/s0162-0134(98)10048-x. [DOI] [PubMed] [Google Scholar]
- 49.Ognjanovic BI, Markovic SD, Pavlovic SZ, Zikic RV, Stajn AS, Saicic ZS. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res. 2008;57(3):403–411. doi: 10.33549/physiolres.931197. [DOI] [PubMed] [Google Scholar]
- 50.Klotz LO, Kroncke KD, Buchczyk DP, Sies H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr. 2003;133(5 Suppl 1):1448S–1451S. doi: 10.1093/jn/133.5.1448S. [DOI] [PubMed] [Google Scholar]