Abstract
Identifying neural mechanisms associated with addiction has substantially improved the overall understanding of addictive processes. Indeed, research suggests that drug-associated cues may take advantage of neural mechanisms originally intended for emotional processing of stimuli relevant to survival. In this study, we investigated cortical responses to several categories of emotional cues (erotic, romance, pleasant objects, mutilation, sadness, unpleasant objects) as well as two types of smoking-related cues (people smoking and cigarette-related objects). We recorded ERPs from 180 smokers prior to their participation in a smoking cessation clinical trial and assessed emotional salience by measuring the amplitude of the late positive potential (LPP; 400 to 600 ms after picture onset). As expected, emotional and cigarette-related pictures prompted a significantly larger LPP than neutral pictures. The amplitude of the LPP increased as a function of picture arousal level, with high-arousing erotic and mutilation pictures showing the largest response in contrast to low-arousing pleasant and unpleasant objects, which showed the smallest response (other than neutral). Compared to females, male participants showed larger LPPs for high-arousing erotic and mutilation pictures. However, unlike emotional pictures, no difference was noted for the LPP between cigarette stimuli containing people versus those containing only objects, suggesting that in contrast to emotional objects, cigarette-related objects are highly relevant for smokers. We also compared the smokers to a small (N=40), convenience sample of never-smokers. We found that never-smokers had significantly smaller LPPs in response to erotic and cigarette stimuli containing only objects compared to smokers.
Keywords: Event related potentials, ERP, emotion, nicotine dependence, smoking, LPP
Introduction
Nearly 35 million American smokers express a desire to quit every year. Unfortunately, more than 85 % of those who actually try to quit on their own will relapse, often within the first week following the cessation attempt (NIDA, 2009). The adverse health risks of smoking increase significantly with duration and amount smoked per day (USDHHS, 1988), and it is precisely these heavier and more nicotine dependent smokers that are most refractory to treatment (USDHHS, 1990). Several theories of addiction highlight the pivotal role that emotion plays in the development of nicotine dependence as well as the subsequent difficulty many smokers experience in the process of quitting. Negative affect following a quit attempt has been related to treatment failure and relapse across a variety of treatment modalities (Borrelli et al., 1996; Kenford et al., 2002; Burgess et al., 2002), characterizing over 50% of all smoking lapses (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). Both affective experiences and the presence of cigarette cues have been associated with relapse in smokers (Shiffman et al. 2007). Another theory describes complex processes including emotional states, environmental cues, and physiological processes that result in the consolidation of an ‘addiction memory’ (Wolffgramm & Heyne, 1995), likely involving the interaction of several neural networks (Fehr, Wiedenmann, & Herrmann, 2006; Fehr, Wiedenmann, & Herrmann, 2007) Continuing to delineate the neural mechanisms and adaptations associated with nicotine dependence, emotion, and drug-related cues can meaningfully improve the overall understanding of the addictive process.
Several studies have demonstrated that the presentation of emotional stimuli lead to specific cortical electrophysiological patterns that are reliably different from neutral stimuli (Hajcak, MacNamara, & Olvet, 2010); (Lang & Bradley, 2009; Olofsson, Nordin, Sequeira, & Polich, 2008). These cortical patterns, called event related potentials (ERPs), represent the coordinated activation of large populations of neurons in response to a stimulus. More specifically, the late positive potential (LPP) is a slow centro-parietal positive ERP that typically occurs between 300 and 700 milliseconds (ms) after the onset of a stimulus (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Schupp et al., 2000). The LPP is thought to reflect increased attention to or facilitated processing of motivationally relevant (or emotional) stimuli. (Schupp et al., 2000; Lang, Bradley, & Cuthbert, 1997). Both pleasant and unpleasant stimuli reliably increase the amplitude of the LPP (compared to neutral) (Cuthbert et al., 2000; Keil et al., 2002; Schupp et al., 2000) and the effect is particularly pronounced for highly arousing emotional pictures (e.g., erotica or mutilations; (Schupp et al., 2004a), suggesting that the LPP tracks the arousal level (but not valence) of emotional stimuli.
The majority of studies investigating the effect of emotional stimuli on the LPP have used broad, heterogeneous categories of picture types that might include a variety of semantic categories of pleasant (e.g., erotica, romantic couples, landscapes, food), unpleasant (e.g., mutilated bodies, threat-related images, animal attacks, disgusting objects), and neutral (e.g., household objects, abstract art, and scenes with or without people) contents (Schupp et al., 2000; Hajcak & Olvet, 2008). Interestingly, four studies that divided the emotional pictures into sub-categories based on their specific semantic contents found that the LPP was largest for erotic and mutilation pictures compared to all other categories, which is consistent with self-reported arousal ratings in response to the pictures (Briggs & Martin, 2009; Schupp, Junghofer, Weike, & Hamm, 2004b; Weinberg & Hajcak, 2010d; De & Codispoti, 2011). Additionally, pleasant, unpleasant, and neutral stimuli that contain people elicit higher arousal ratings and larger LPP amplitudes compared to those that depict objects or scenes without people (Schupp et al., 2004b). These results suggest that breaking down heterogeneous emotional categories into specific semantic content may allow for elucidation of meaningful individual differences in cortical activity for a variety of research questions, particularly in the case of smoking cues that are quite ubiquitous in our culture.
Research shows that in drug users, drug-related cues may also receive preferential processing in ways similar to emotional stimuli. In fact, several studies have demonstrated that drug-associated cues may take advantage of existing neural mechanisms originally intended for emotional processing of stimuli relevant to survival (Everitt, Dickinson, & Robbins, 2001; Hyman, 2005; Robinson & Berridge, 2003). Sensitization theory proposes that through conditioning, cues predicting drug delivery also became imbued of motivational significance and are able to trigger compulsive drug consumption (Robinson & Berridge, 1993). In drug users, drug related stimuli have been shown to produce electrophysiological patterns similar to those produced by emotional stimuli (Cinciripini et al., 2006; Versace et al., 2010; Gilbert et al., 2004).
A recent study from our lab with current smokers reported that cigarette-related stimuli evoked an LPP that was significantly higher than that to neutral stimuli, but did not differ from pleasant or unpleasant stimuli (Versace et al., 2011). A similar pattern was noted in cocaine users, who responded to cocaine-related and emotional stimuli similarly (Dunning et al., 2011). Other studies have demonstrated that drug-related stimuli evoke ERPs similar to emotional pictures in smokers (Gilbert et al., 2004; Versace et al., 2011), marijuana users (Wolfling, Flor, & Grusser, 2008), and opiate users (Lubman, Allen, Peters, & Deakin, 2008). These studies suggest that drug related stimuli acquire motivational salience in chronic drug users that rivals the significance of inherently motivational emotional stimuli, though it is not clear if drug cues vary by specific semantic content in a manner similar to that of emotional cues because most of the previously described studies reported results over heterogeneous groups of emotional pictures. Because these drug cues have acquired substantial significance, they would be expected to produce activation similar to the most arousing emotional pictures (erotic couples and mutilation pictures).
The current study tested the hypothesis that visual stimuli related to cigarette smoking would evoke cortical ERPs similar to those produced by emotional stimuli. Initially, we attempted to replicate previous findings that cigarette pictures evoke cortical responses similar to emotional pictures in smokers. Additionally, we compared several semantic categories of emotional, neutral, and smoking-related stimuli, including unpleasant content: mutilation (MUT; high arousal), sadness/grief (SAD; low arousal), and objects (UNPo; accidents, pollution), pleasant content: erotic couples (ERO; high arousal), romantic couples (ROM; low arousal), and objects (PLEo; food, landscapes), neutral content: people (NEUp) and objects (NEUo; household objects), and cigarette content: people smoking (CIGp) and objects (CIGo; ashtrays, cigarettes). We hypothesized that cigarette-related images involving people would not differ significantly from high arousing pleasant and unpleasant images (erotic couples and mutilation). We specifically focused on the cigarette stimuli involving people because emotional images containing people evoke larger LPPs than those containing only objects (Weinberg & Hajcak, 2010c). We also took advantage of the relatively large sample size to examine potential gender and racial differences in the amplitude of the LPP as an exploratory analysis. In order to obtain preliminary evidence about the specificity of our potential results to a non-smoking population, we also included an exploratory analysis of a small convenience sample of 40 never-smokers.
Method
Participants
Participants were recruited via local advertisements requesting volunteers who wanted to quit smoking and were willing to participate in a clinical trial of smoking cessation medications. The trial was a double-blinded, placebo-controlled investigation of the effects of bupropion or varenicline on smoking cessation. To participate smokers had to be aged 18-65 years, smoke 5 or more cigarettes per day, have a baseline expired carbon monoxide (CO) level greater than 6 parts per million (ppm), be fluent in English, have a working telephone, be not currently taking psychotropic medication, not have a current psychiatric disorder (assessed by MINI International Neuropsychiatric Interview(Sheehan et al., 1998), not be involved in any smoking cessation activities, have no contraindications for bupropion or varenicline, and have no uncontrolled medical illness. The treatment included 10 weeks of medication or placebo treatment as well as behavioral counseling. After screening for basic trial-related inclusion and exclusion criteria but before beginning treatment, eligible participants completed a baseline laboratory session, which was the source of these data. A total of 208 eligible participants completed a baseline laboratory session. Because of poor recording quality (24 participants) or technical errors (4 participants), laboratory data from 28 participants were discarded, yielding a total of 180 participants in this study.
Non-smoking participants (n=40) were also recruited via local advertisements requesting volunteers who were smokers, ex-smokers, or never-smokers. To be consistent with our previous study, all participants were aged 18-65 years, fluent in English, had a working telephone, had not taken psychotropic, anticonvulsive, or narcotic medication in the past 30 days, were not involved in any smoking cessation activities, had not used a nicotine product in the past year, had not used marijuana or other illicit drugs within the week preceding the screening, and did not have current visual or auditory problems that in the opinion of the investigator would interfere with the completion of study assessments. To be eligible for the never smokers group, participants must have smoked less than 100 cigarettes in their lifetime and have a baseline expired CO less than 4 ppm. All participants provided written, informed consent before being subjected to any study procedure, and the research was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
Measures
We measured nicotine dependence using the Fagerström Test for Nicotine Dependence (FTND), a 6-item questionnaire that assesses various components of smoking behavior such as daily intake and time to first cigarette after waking. (Fagerström, 1982; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Additionally, the Wisconsin Inventory of Smoking Dependence Motives (Piper et al., 2004) was administered as a multidimensional assessment of nicotine dependence. We assessed affect using the Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item self-report measure developed to assess depressive symptoms in community (non-clinical) populations (Ross & Mirowsky, 1984) as well the Positive and Negative Affect Scale (Watson, Clark, & Tellegen, 1988), comprised of two 10-item mood scales, Positive Affect (PA) and Negative Affect (NA), rated on a scale of 1-5.
Design
Three equivalent (based on content, arousal and valence ratings, and luminosity) picture sets were created by selecting pictures from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005) and from cigarette-related picture collections previously used in our (Carter et al., 2006) and other (Gilbert & Rabinovich, 1999) laboratories. The following semantic categories were represented: unpleasant content: mutilation (MUT; high arousal), sadness/grief (SAD; low arousal), and objects (UNPo; accidents, pollution), pleasant content: erotic couples (ERO; high arousal), romantic couples (ROM; low arousal), and objects (PLEo; food, landscapes), neutral content: people (NEUp) and objects (NEUo; household objects), and cigarette content: people smoking (CIGp) and objects (CIGo; ashtrays, cigarettes). The average IAPS normative ratings for valence and arousal (respectively) in each of our emotional categories are as follows: MUT: 1.79, 6.36; SAD: 2.91, 4.82; UNPo: 3.01, 5.45; ERO: 6.63, 6.29; ROM: 7.40, 4.90; PLEo: 6.92, 4.67; NEUp: 5.30, 3.55; NEUo: 4.94, 2.76. To avoid possible confounds due to sequence effects, each participant saw the pictures in a pseudo-random sequence with no more than two pictures of the same valence presented consecutively. To evaluate the effectiveness of our randomization scheme, for each picture that was presented in each sequence, we checked the category of the preceding picture. On average, for each picture presented in a sequence, the preceding picture was: a picture of the same category 0.4% of the time; a picture of the same valence, but of one of the other two subcategories, 2.44% of the time; a picture belonging to one of the 9 remaining categories 10% of the time. The remaining 4.2% of the time, the picture was presented at the beginning of a block. Each picture was presented for 4 seconds and was followed by a random inter-trial interval of 3-5 s, during which the screen had a black background with a white fixation cross. The entire picture presentation lasted approximately 30 min (the pictures were presented twice during the session, for a total of 192 pictures). Participants were simply asked to view the pictures on the screen and to remain as still as possible. Stimuli were presented with a Pentium 4 computer using Psychology Tools’ E-prime software (version 1.4; Pittsburgh, PA) on a plasma screen placed approximately 1.5 m from the participant’s eyes. The pictures subtended a horizontal viewing angle of approximately 24°. All pictures had the same canvas size (1024 × 768) and were presented in BMP format.
Procedure
The ERP laboratory session occurred prior to treatment randomization. Participants were instructed to smoke normally before the laboratory session so as to be in a non-deprived state and they provided an expired carbon monoxide (CO) sample upon arrival. During the slide presentation, the electroencephalogram (EEG) was recorded using a 129-channel Geodesic Sensor Net, amplified with an AC-coupled high input impedance (200 MΩ) amplifier (Geodesic EEG System 250; Electrical Geodesics Inc., Eugene, OR), and referenced to Cz. The sampling rate was 250 Hz, and data were filtered online by using 0.1 Hz high-pass and 100 Hz low-pass filters. Scalp impedance of each sensor was kept below 50 KΩ, as suggested by the manufacturer.
Data Reduction and Statistical Analyses
After data collection, a 30-Hz low-pass filter was applied off-line. Data were visually inspected, and channels contaminated by artifacts for more than 50% of the recording were interpolated with use of spherical splines. On average, approximately 2% of the channels met this criterion and were interpolated. Eye blinks were then corrected by using a spatial filtering method as implemented in BESA ver. 5.1.8.10 (MEGIS Software GmbH, Gräfelfing, Germany). After eye blink correction, the EEG data were transformed to the average reference, which was necessary for accurate topographic mapping and topographic waveform plots, and segmented into 900-ms segments starting 100 ms before onset of the picture. Baseline was defined as the 100-ms interval preceding the picture. Using the segmented data, artifacts affecting sensors within specific trials were identified. Artifacts were defined by the following criteria: EEG amplitude above 100 or below −100 μV; absolute voltage difference between any two data points within the segment larger than 100 μV; voltage difference between two contiguous data points above 25 μV; and less than 0.5 μV variation for more than 100 ms. A segment was excluded from the subsequent averages if more than 10% of the sensors within the segment were contaminated by artifacts. Overall, fewer than 5% of the segments were excluded. At the end of this process, the average ERPs were calculated at each scalp site for each category (i.e., pleasant, unpleasant, neutral, and cigarette-related) using Brain Vision Analyzer software (Brain Products GmbH, Munich, Germany).
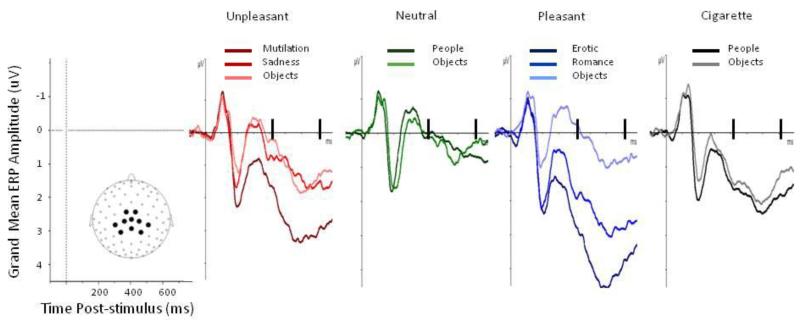
Visual inspection of grand-averaged ERPs confirmed results from previous studies (Cuthbert et al., 2000; Keil et al., 2002; Schupp et al., 2000; Weinberg & Hajcak, 2010b). Presentation of motivationally relevant pictures (including cigarette-related ones) increased the amplitude of the LPP over central and parietal sensors. The largest difference between neutral and motivationally relevant pictures was observed at about 600 ms after picture onset. Voltages from 10 sensors covering the area with the largest LPP differences between neutral and motivationally relevant pictures were averaged (Figure 1), and the mean LPP amplitude between 400 and 700 ms after picture onset was calculated for each category for each participant (Versace et al., 2011). The same procedures described above were also used to obtain LPP amplitude associated with the various semantic contents (i.e., MUT, SAD, UNPo, NEUp, NEUo, ERO, ROM, PLEo, CIGp, CIGo).
Figure 1.
Event-related potentials to specific semantic categories of unpleasant, neutral, pleasant, and cigarette-related pictures within smokers. The waveforms represent grand-averages from 10 electrodes (see inset for electrode location).
Statistical Analyses
The primary unit of analysis was the mean LPP amplitude computed for each participant for each condition (both the super-ordinate emotional categories as well as the semantic categories). A mixed models regression approach (Littell, Milliken, Stroup, Wolfinger, & Schabenberfer, 2006) was the primary analytic strategy for estimating the effects of stimuli content, gender, and their interaction terms on the amplitude of the LPP. Subject was included as a random effect. Where appropriate, significant interactions were further evaluated using least-square means and Bonferroni univariate error corrected t-tests for all relevant pair-wise comparisons. Likelihood ratio tests were conducted to evaluate model fit after the inclusion of additional variables and only models with significant increases were retained. Subsequent iterations of the model involved including other potential variables of interest such as age, race, time since last cigarette, CO, nicotine dependence, and negative affect and testing for interactions, though none were significant and will not be reported here. These measures are included Table 1.
Table 1.
Demographics and Baseline Characteristics
| Characteristic | Male Smokers | Female Smokers | Total Smokers | Never-smokers |
|---|---|---|---|---|
| (N=117) | (N=63) | (N=180) | (N=40) | |
| N (%) | N (%) | N (%) | N (%) | |
| Race/ethnicity* | ||||
| African American, non-Hispanic | 29 (24.8) | 20 (31.8) | 49 (27.2) | 28 (70.0) |
| White, non-Hispanic | 71 (60.7) | 33 (52.4) | 104 (57.8) | 7 (17.5) |
| Other | 17 (14.5) | 10 (15.9) | 27 (15.0) | 5 (12.5) |
| Gender | ||||
| Female | 20 (50) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 44.8 (11.3) | 45.7 (9.1) | 45.1 (10.6) | 46.2 (11.0) |
| Expired carbon monoxide (CO) | 26.1 (15.3) | 23.5 (10.7) | 25.7 (13.9) | |
| Cigarettes/day** | 16.3 (7.5) | 20.6 (8.4) | 19.1 (8.3) | |
| Years of Smoking | 24.3 (12.1) | 25.2 (10.8) | 24.6 (11.6) | |
| Time Since Last Cigarette (hrs) | 0.9 (0.8) | 1.2 (2.1) | 1.0 (1.4) | |
| FTND Scorea | 4.6 (2.1) | 4.4 (2.3) | 4.6 (2.1) | |
| WISDM Total Scoreb | 4.0 (0.9) | 4.2 (1.0) | 4.1 (1.8) | |
| CESD Total Scorec | 7.3 (6.4) | 8.4 (7.9) | 7.7 (7.0) | 8.6 (8.0) |
| PANAS Positive Affectd | 36.1 (6.4) | 34.9 (7.0) | 35.7 (6.6) | |
| PANAS Negative Affectd | 15.4 (5.3) | 16.2 (5.3) | 15.7 (5.7) |
c2(2, N = 220) = 31.04, p <.0001 between smokers and never smokers;
p<001 between male and female smokers;
Fagerstrom Test for Nicotine Dependence;
Wisconsin Inventory of Smoking Dependence Motives;
Center for Epidemiologic Studies Depression Scale;
Positive and Negative Affect Scale
Lastly, in order to assess the specificity of the results to smokers, we also evaluated a similar model in a small convenience sample of 40 never-smokers (20 female). The primary unit of analysis in never-smokers was the mean LPP amplitude computed for each participant for each condition (both the super-ordinate emotional categories as well as the semantic categories, as described above). Again, a mixed models regression approach was the primary analytic strategy for estimating the effects of stimuli content on the amplitude of the LPP. Subject was included as a random effect and race was included as a covariate since the two groups had significantly different racial compositions. This final model included both smokers and never-smokers and evaluated the effects of group, picture category, and the interaction of these factors on the amplitude of the LPP.
Results
Participant Characteristics
Participant characteristics for both smokers and never-smokers are presented in Table 1. Within the smoker sample, there were no significant differences between males and females on any of the measures of smoking behavior, nicotine dependence, time since last cigarette, or affect with one exception: males reported smoking 4 fewer cigarettes a day on average than females (t=−3.34, p<.001). None of our questionnaire or behavioral data (such as CO and time since last cigarette) were related to the amplitude of the LPP in any of our analyses, and will not be further discussed here (see Table 2. for correlations between these measures). Never-smokers did not differ from smokers with regard to age, gender, or depressive symptomatology (measured by the CESD), though they had a significantly different racial composition than the smoker sample.
Table 2.
Correlations of Demographics and Baseline Questionnaires in Smokers
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | 1.00 | |||||||||||
| 2. RaceyEthnicity | 0.06 | 1.00 | ||||||||||
| 3. Age | 0.05 | −0.02 | 1.00 | |||||||||
| 4. Expired Carbon Monoxide | −0.11 | −0.01 | 0.10 | 1.00 | ||||||||
| 5. CigarettesVday | −0.24 | −0.27 | 0.12 | 0.15 | 1.00 | |||||||
| 6. Years of Smoking | 0.03 | −0.12 | 0.79 | 0.13 | 0.24 | 1.00 | ||||||
| 7. Time since last cigarette (hrs) | 0.14 | 0.03 | −0.06 | −0.06 | −0.20 | −0.17 | 1.00 | |||||
| 8. FTHD Score | −0.07 | −0.19 | 0.19 | 0.21 | 0.63 | 0.31 | −0.17 | 1.00 | ||||
| 9. WISDM Total Score | 0.12 | −0.13 | −0.04 | 0.09 | 0.21 | 0.06 | −0.09 | 0.41 | 1.00 | |||
| 10. CESD Total Score | 0.03 | −0.04 | −0.16 | 0.14 | −0.07 | −0.16 | 0.00 | 0.06 | 0.34 | 1.00 | ||
| 11. PANAS Positive Affect Scale | −005 | 0.11 | −0.03 | −0.14 | −0.04 | −0.01 | 0.07 | −0.10 | −0.31 | −0.58 | 1.00 | |
| 12. PANAS Negative Affect Scale | 0.04 | −0.01 | −0.24 | 0.09 | −0.13 | −0.20 | −0.07 | −0.01 | 0.32 | 0.69 | −0.36 | 1.00 |
Note: Bold text indicates a significant correlation (p<05)
The Late Positive Potential in Smokers
Initial results revealed that the super-ordinate categories of emotional valence (pleasant, unpleasant, neutral, and cigarette) significantly predicted LPP amplitude (F (3,534) = 73.05, p < 0.0001). More specifically, the amplitude of the LPP in response to pleasant, unpleasant, and cigarette stimuli were significantly larger than the amplitude of the LPP in response to neutral stimuli (all p values <.0001), but did not differ from one another (Figure 1). Gender also significantly predicted LPP amplitude (F (1,178) = 11.74, p = 0.0008), indicating that males had significantly larger LPP’s than females. Lastly, a significant valence by gender interaction was observed (F (3,534) = 5.02, p = 0.0019) on the amplitude of the LPP. Subsequent error-corrected pair-wise comparisons revealed that females had significantly smaller LPP’s to pleasant (p = 0.0004) stimuli than males and marginally smaller LPP’s to unpleasant (p = 0.056) stimuli, though both were still significantly greater than neutral.
We also examined the effects of gender and specific semantic stimuli categories on the amplitude of the LPP. Results revealed that semantic category (F (9,1602) = 65.83, p < 0.0001) significantly predicted the amplitude of the LPP (see Figure 1.). Subsequent pair-wise error corrected comparisons revealed that erotic (ERO) and mutilation (MUT) categories evidenced the highest LPP amplitude, though cigarette people (CIGp), cigarette objects (CIGo), and romance (ROM) categories were also significantly higher than neutral people (NEUp) and neutral objects (NEUo) (p’s < .002). Neutral people did not significantly differ from neutral objects. Additionally, the following lower-arousing emotional categories did not differ from neutral: sadness/disgust (SAD), pleasant objects (PLEo), and unpleasant objects (UNPo). There was no significant difference between the erotic and mutilation categories or any difference between cigarette people and cigarette objects. However, both cigarette objects and cigarette people resulted in significantly smaller LPP amplitudes than both erotic and mutilation stimuli (p’s < .001).
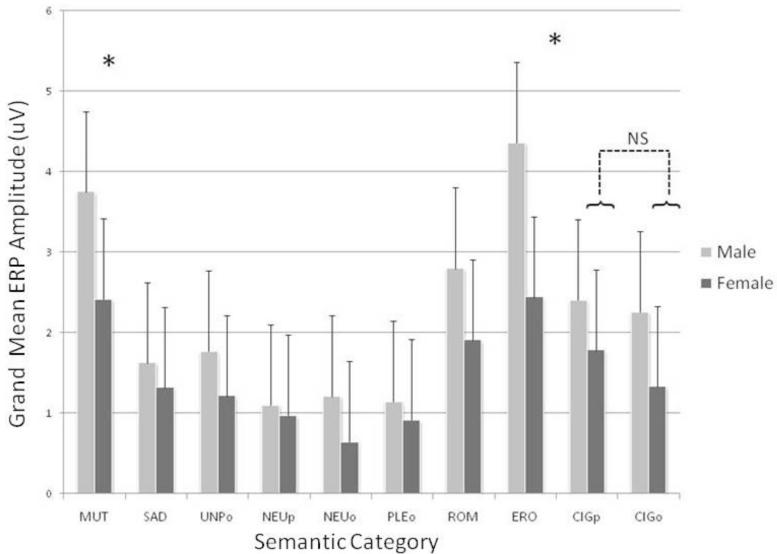
There was also a main effect of gender (F (1,178) = 13.49, p = 0.0003) on the amplitude of the LPP, in which males evidenced a significantly larger LPP than females. Additionally, we observed a significant semantic category by gender interaction on the amplitude of the LPP (F (9,1602) = 6.60, p < 0.0001). Subsequent error-corrected pair-wise comparisons revealed that males had significantly higher LPP amplitudes than females for erotic (p < .0001) and mutilation (p = .0007) stimuli. No other semantic category evidenced significant differences between males and females (Figure 2).
Figure 2.
Mean LPPs from centro-parietal sensors evoked by the different semantic contents in female and male smokers. Unpleasant contents: mutilations (MUT; high emotional arousal), sad (SAD; low emotional arousal; e.g., grief, disease), and objects (UNPo; e.g., pollution, accidents). Pleasant contents: erotic couples (ERO; high emotional arousal), romantic couples (ROM; low emotional arousal), and objects (PLEo; e.g., food, landscapes). Neutral contents: people (NEUp) and objects (NEUo; e.g., household objects). Cigarette-related contents: people smoking (CIGp) and cigarette-related objects (CIGo; e.g., ashtrays, cigarettes). Cigarette stimuli with people did not differ from cigarette-related objects. Note: * = p < 0.001.
The Late Positive Potential in Smokers Compared to Never-Smokers
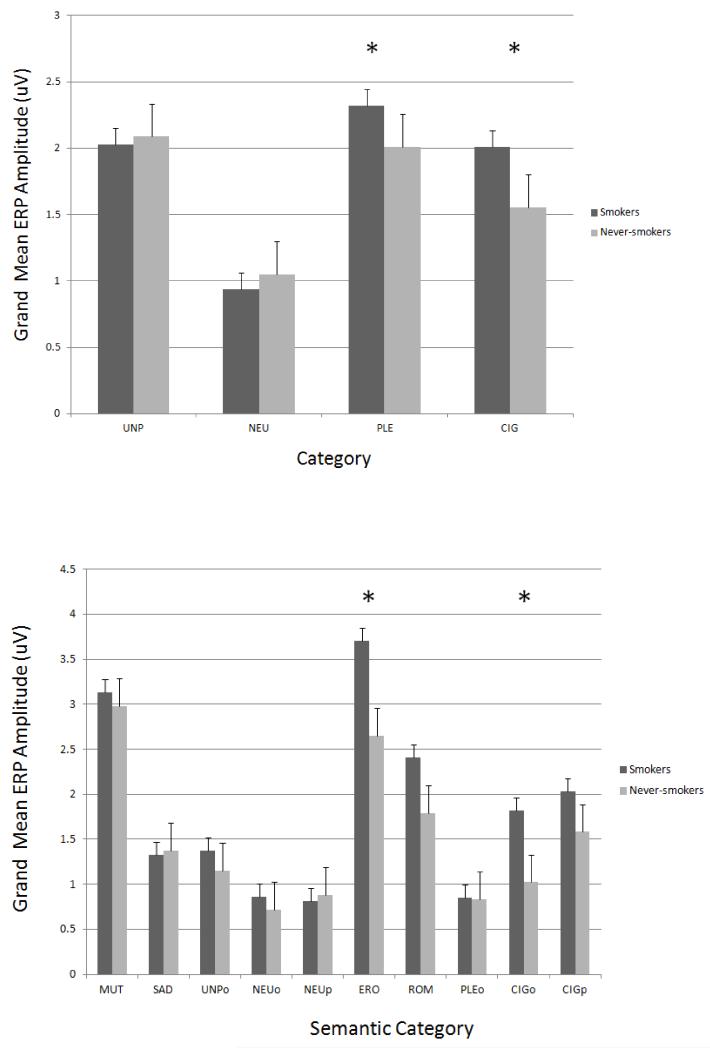
As expected, the results revealed that the super-ordinate categories of emotional valence (pleasant, unpleasant, neutral, and cigarette) significantly predicted LPP amplitude (F (3,654) = 50.84, p < 0.0001) in both smokers and never-smokers. There was also a significant group by category interaction (F (3,654) = 3.51, p = 0.02). Within the never-smokers, the amplitude of the LPP in response to pleasant (p < 0.0001), unpleasant (p < 0.0001), and cigarette stimuli (p = 0.008) were significantly larger than the amplitude of the LPP in response to neutral stimuli, though cigarette stimuli had significantly smaller LPPs than both pleasant (p = 0.02) and unpleasant (p = 0.005) stimuli. Additionally, smokers evidenced significantly larger LPP amplitudes in response to both pleasant (p < 0.01) and cigarette (p < 0.002) stimuli compared to never-smokers (Figure 3).
Figure 3.
Mean LPPs from centro-parietal sensors evoked by super-ordinate categories (unpleasant, UNP; neutral, NEU; pleasant, PLE, and cigarette, CIG) as well as semantic contents in smokers and never-smokers. Unpleasant contents: mutilations (MUT; high emotional arousal), sad (SAD; low emotional arousal; e.g., grief, disease), and objects (UNPo; e.g., pollution, accidents). Pleasant contents: erotic couples (ERO; high emotional arousal), romantic couples (ROM; low emotional arousal), and objects (PLEo; e.g., food, landscapes). Neutral contents: people (NEUp) and objects (NEUo; e.g., household objects). Cigarette-related contents: people smoking (CIGp) and cigarette-related objects (CIGo; e.g., ashtrays, cigarettes). Smokers compared to never-smokers had higher LPP amplitudes to PLE and CIG categories as well as ERO and CIGo semantic categories. Note: * = p < 0.05.
We also examined the effects of group and specific semantic stimuli categories on the amplitude of the LPP. Results revealed a significant group by semantic category interaction (F (9,1962) = 2.27, p = 0.02; see Figure 3.). Within never-smokers, pair-wise comparisons revealed that erotic (ERO) and mutilation (MUT) categories evidenced the highest LPP amplitude, though cigarette people (CIGp), and romance (ROM) categories were also significantly higher than neutral people (NEUp) and neutral objects (NEUo) (p’s < .05). Neutral people did not significantly differ from neutral objects. Additionally, the following did not differ from neutral: sadness/disgust (SAD), pleasant objects (PLEo), unpleasant objects (UNPo), and cigarette objects (CIGo). There was no significant difference between the erotic and mutilation categories and cigarette people (CIGp) resulted in significantly smaller LPP amplitudes than both erotic and mutilation stimuli (p’s < .001). Smokers evidenced significantly larger LPP amplitudes than non-smokers for erotic stimuli (p’s = .002) and cigarette objects (p’s = .02), though surprisingly not for cigarette people.
Discussion
Our results replicated previous studies that reported that the presence of cigarette cues employed cortical processing similar to that of intrinsically motivating emotional stimuli in smokers. More specifically, viewing cigarette-related stimuli modulated the amplitude of the late positive potential (LPP) to the same level as both unpleasant and pleasant stimuli. These cigarette stimuli produced significantly smaller increases in LPP amplitude in the never-smokers, though they were still not completely neutral. Increases in the LPP are thought to represent the mobilization of resources needed to facilitate rapid and appropriate responses to motivationally salient stimuli holding evolutionary significance (Lang et al., 1997; Bradley, 2009). While cigarette-related stimuli would not be expected to possess intrinsic significance in the average individual, they may acquire significance over time through the repeated pairing with the presence of nicotine in the brain (Dunning et al., 2011; Versace et al., 2011). A recent study reported that direct stimulation of the prefrontal cortex produces a reduction in the LPP (Hajcak et al., 2010), suggesting that the prefrontal cortex may directly modulate parietal attentional networks involved in the automatic processing of emotionally salient stimuli. This is particularly interesting in light of the evidence that the prefrontal cortex plays a direct role in the addiction cycle. Abnormalities in frontal cortical activity have been repeatedly linked with the inability to control drug-seeking behavior and increased salience attribution to drug cues (Goldstein & Volkow, 2002). These concepts are highly consistent with the notion that addiction memory networks, comprised of functional associations between emotional cues, cognitive processes, and perceptual qualities of drug cues, especially in the frontal brain regions, might increase the amount of effort needed to avoid drug consumption (Fehr et al., 2007). Underlying deficits in frontal activity may inherently increase the automatic, motivated attention to drug-related cues that is indexed by the LPP.
Upon examination of the specific semantic categories, we revealed several findings of interest. While cigarette-stimuli with and without people prompted significantly larger LPPs than neutral stimuli, they were significantly smaller in amplitude compared to the most arousing emotional pictures (erotic and mutilation categories). Therefore, it appears that while the cigarette-related stimuli are indeed relevant, the level of electrical activity engendered by such stimuli is similar to the lower arousing pleasant stimuli (i.e., romance). This seems counter-intuitive given that cigarette cues are able to trigger compulsive drug use that is highly resistant to change (Robinson & Berridge, 1993);(Robinson & Berridge, 2000). In line with the usual interpretation of the LPP as a marker of motivational significance, it is possible that erotic and mutilation pictures convey information more directly relevant to survival and other biological imperatives (Briggs & Martin, 2009) than a cigarette cue is able to acquire. So, while cigarette cues do appear to engage the motivated attentional system, they may be unable to command the degree of resources afforded to the high-arousing emotional stimuli. However, the level of engagement may be sufficient enough to activate reward circuitry, subjective cravings, and approach behavior.
Additionally, cigarette pictures with people did not significantly differ from cigarette-related objects, unlike the emotional pictures, in which the objects elicited LPP’s lower than those with people. It is unlikely that this lack of difference can be attributed to lack of power, as we estimated that we have greater than 95% power to detect an effect size of .45 or greater (equivalent to the effect sizes noted in the pleasant and unpleasant categories). In fact, the pleasant and unpleasant objects did not significantly differ from neutral stimuli. Whereas the emotional objects do not appear salient to smokers (or never-smokers), smoking-related objects are highly salient. Perhaps the distinctive perceptual properties of a cigarette (i.e. shape) help to facilitate the automatic salience attribution to the stimulus, regardless of whether the image contains people. This may increase the probability that drug users will encounter “captivating” cues, which on the surface may seem mundane or irrelevant, such as the butt of a cigarette on the ground. However, even the mere presence of cigarette cues might command substantial cortical resources that can stimulate drug-seeking behavior and craving. Further study into the impact of different types of drug cues may continue to enhance the overall understanding of cortical processing in drug users. For example, a recent study investigated the impact of cigarette cues displaying either the beginning or ending phases of a smoking ritual on neural activity and reported that these stimulus categories resulted in activation of different brain structures (Stippekohl et al., 2010). More specifically, the authors noted that the “begin” stimuli tended to activate structures thought to be related to the addiction network, such as the ventral striatum, orbitofrontal cortex, and anterior cingulate cortex, while “end” stimuli were associated with de-activation of some of these structures, possibly suggesting inhibitory effects.
Unexpectedly, smokers and never-smokers did not differ in their responses to cigarette-related stimuli involving people, though never-smokers had significantly smaller responses than smokers to smoking-related objects (they were no different than neutral stimuli). This result is somewhat puzzling and needs to be replicated in future studies. Perhaps the presence of the people in the cigarette-people stimuli set was arousing in itself. Another possibility is that the demand characteristics of participation in the study itself resulted in the priming of these cues in never-smokers, as they were coming to a cancer hospital to participate in a study that included former smokers and non-smokers, i.e., they were aware that the study had something to do with smokers. Lastly, as smoking has been socially perceived in a more negative way over the past 5 to 10 years, perhaps these never-smokers are aroused by stimuli depicting people who are smoking, though in an unpleasant, rather than pleasant way. These possibilities are ultimately speculative at this point and can be tested directly in future studies designed to examine differences in the LPP specifically between smokers and non-smokers. Additionally, we are unable to make any conclusions about the motivational relevance of cigarette-related cues in former smokers. This relevance might decrease over time or even disappear. Future studies investigating the potential changes in cortical responses to drug cues in former drug users at varying stages following cessation (i.e., weeks, years, etc.) would substantially contribute to the addiction literature.
Our results also resulted in a significant interaction of stimulus type with gender, indicating that the LPP in males was significantly larger than females for the super-ordinate category of pleasant stimuli. Within the subordinate semantic categories, we found that males had significantly larger LPP amplitudes to erotic and mutilation images than females. As such, it seems that the true differences between males and females in this sample are specifically confined to the high-arousing emotional stimuli. There were no differences between male and female responses to neutral or cigarette-related stimuli. The differences in this study could not be attributed to age, nicotine dependence level, smoking behaviors, or self-reported affect. Other studies have reported marginal differences between the reactivity of men and women on the LPP (Weinberg & Hajcak, 2010a) as well as several other measures of cortical and peripheral activation (Bradley, Codispoti, Sabatinelli, & Lang, 2001). Future studies are needed in order to replicate these findings and explore potential individual difference factors between males and females that might further explain their cortical responses to emotional stimuli.
The current study provides data that replicates previous findings indicating that drug-related cues exploit cortical mechanisms intended for the motivated processing of intrinsically emotional stimuli. Moreover, we demonstrated that unlike emotional objects, cigarette-related stimuli are highly salient to smokers. With such a large sample size, we were able to explore gender differences in cortical processing and believe that these data provide a reliable examination of the late positive potential to varying types of emotional and cigarette-related stimuli. Continued research in this and related areas may ultimately contribute to more refined and individualized treatment strategies and more in-depth understanding of the addictive process.
Highlights.
emotional and cigarette-related pictures prompted a larger LPP than neutral
amplitude of the LPP increased as a function of picture arousal level
male participants showed larger LPPs for erotic and mutilation pictures
no difference for cigarette stimuli containing people versus those without people
Acknowledgements
This work was supported in part by the National Institute on Drug Abuse through grant 1R01DA017073-01 to Paul Cinciripini and training grant 1K99DA025181-01 to Jennifer Minnix.
Dr. Cinciripini has served on the scientific advisory board of Pfizer Pharmaceuticals, received grant support and has conducted educational talks sponsored by Pfizer on smoking cessation for physicians from 2006-2008.
Footnotes
Declaration of Interest: The other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, DePue JD, Murphy C, et al. Development of major depressive disorder during smoking-cessation treatment. Journal of Clinical Psychiatry. 1996;57:534–538. doi: 10.4088/jcp.v57n1106. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- Briggs KE, Martin FH. Affective picture processing and motivational relevance: arousal and valence effects on ERPs in an oddball task. Int.J Psychophysiol. 2009;72:299–306. doi: 10.1016/j.ijpsycho.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Burgess ES, Kahler CW, Niaura R, Abrams DB, Goldstein MG, Miller IW. Patterns of change in depressive symptoms during smoking cessation: Who’s at risk for relapse? Journal of Consulting and Clinical Psychology. 2002;70:356–361. doi: 10.1037//0022-006X.70.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, et al. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, De Moor CA, et al. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8:379–392. doi: 10.1080/14622200600670272. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- De CA, Codispoti M. Affective modulation of the LPP and alpha-ERD during picture viewing. Psychophysiology. 2011;1:1–8. doi: 10.1111/j.1469-8986.2011.01204.x. doi: 10.1111/j.1469-8986.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, ia-Klein N, Woicik PA, et al. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users - an ERP study. Eur.J Neurosci. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. Journal of Behavioral Medicine. 1982;5:343–351. doi: 10.1007/BF00846161. [DOI] [PubMed] [Google Scholar]
- Fehr T, Wiedenmann P, Herrmann M. Nicotine Stroop and addiction memory--an ERP study. Int.J Psychophysiol. 2006;62:224–232. doi: 10.1016/j.ijpsycho.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Fehr T, Wiedenmann P, Herrmann M. Differences in ERP topographies during color matching of smoking-related and neutral pictures in smokers and non-smokers. Int.J Psychophysiol. 2007;65:284–293. doi: 10.1016/j.ijpsycho.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. The international smoking image series (with neutral counterparts), v. 1.2. Department of Psychology, Southern Illinois University; Carbondale, IL: 1999. [Google Scholar]
- Gilbert DG, Sugai C, Zuo Y, Eau Claire N, McClernon FJ, Rabinovich NE, et al. Effects of nicotine on brain responses to emotional pictures. Nicotine & Tobacco Research. 2004;6:985–996. doi: 10.1080/14622200412331324947. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Anderson BS, Arana A, Borckardt J, Takacs I, George MS, et al. Dorsolateral prefrontal cortex stimulation modulates electrocortical measures of visual attention: evidence from direct bilateral epidural cortical stimulation in treatment-resistant mood disorder. Neuroscience. 2010;170:281–288. doi: 10.1016/j.neuroscience.2010.04.069. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev.Neuropsychol. 2010;35:129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi: 10.1016/j.addbeh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am.J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. doi: 10.1111/1469-8986.3950641. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: Contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70:216–227. doi: doi: 10.1037/0022-006X.70.1.216. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2009;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Lawrence Erlbaum; Mahwah, NJ: 1997. pp. 97–136. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report no. A-6. University of Florida; Gainesville, FL: 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberfer O. SAS for mixed models. SAS Institute Inc.; Cary, NC: 2006. [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of Psychophysiology. 2008;22:836–842. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- NIDA Tobacco Addiction. 2009 (Research Report Series Rep. No. NIH Publication Number 09-4342) [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol Psychol. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, et al. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. doi: 10.1037/a0013298. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction. 2000;95(Suppl. 2):91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Components of depressed mood in married men and women. The Center for Epidemiologic Studies’ Depression Scale. American Journal of Epidemiology. 1984;119:997–1004. doi: 10.1093/oxfordjournals.aje.a113819. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. doi: 10.1111/1469-8986.3720257. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition and Emotion. 2004a;18:593–611. [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004b;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Shiffman S, Paty J, Gnys M, Kassel J, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. doi: 10.1037/0022-006X.64.2.366. [DOI] [PubMed] [Google Scholar]
- Stippekohl B, Winkler M, Mucha RF, Pauli P, Walter B, Vaitl D, et al. Neural Responses to BEGIN- and END-Stimuli of the Smoking Ritual in Nonsmokers, Nondeprived Smokers, and Deprived Smokers. Neuropsychopharmacology. 2010;35:1209–1225. doi: 10.1038/npp.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS . The Health Consequences of Smoking: Nicotine Addiction - - a report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Centers for Health Promotion and Education, Office on Smoking and Health; Rockville, MD: 1988. Rep. No. Publication No. (CDC) 88-8406) [Google Scholar]
- USDHHS . The Health Benefits of Smoking Cessation: a report of the Surgeon General. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Centers for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Rockville, MD: 1990. Rep. No. Publication No. (CDC) 90-8416) [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addiction Biology. 2011;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. doi: 10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, et al. Cigarette cues capture smokers’ attention: Evidence from event-related potentials. Psychophysiology. 2010;47:435–441. doi: 10.1111/j.1469-8986.2009.00946.x. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010a;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010b;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010c;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010d;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. European Journal of Neuroscience. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]