Summary
Purpose
In simultaneous scalp electroencephalography (EEG) and functional magnetic resonance imaging (fMRI), blood oxygen level dependent (BOLD) changes occurring before the spike have been sometimes described but could not be explained. To characterize the origin of this prespike BOLD signal change, we looked for electrographic changes in stereo-EEG (SEEG) possibly preceding the scalp spike in patients that showed early BOLD response in EEG/fMRI.
Methods
We studied four patients with drug-resistant focal epilepsy who underwent EEG/fMRI, showed a pres-pike BOLD response, and were then studied with depth electrodes for presurgical localization of the epileptic generator. Early BOLD responses in the region of the spike field were analyzed using models with hemodynamic response functions (HRFs) peaking from −9 to +9 s around the spike. SEEG recordings in the period and location corresponding to the early HRF responses were analyzed to detect if electrographic changes were present in the SEEG before the scalp abnormality.
Key Findings
One of the four patients presented a SEEG interictal discharge in the period corresponding to the early BOLD response. In the other three, no electrographic changes were detected in the SEEG in the period corresponding to early BOLD changes.
Significance
Although the early BOLD activity may sometimes be explained by a synchronized neural discharge detectable with SEEG but not visible on the scalp EEG, in most cases the early BOLD response reflects a metabolic phenomenon that does not appear to result from a synchronized neuronal discharge. Prespike metabolic responses can result from synchronized or nonsynchronized neuronal activity, or from nonneuronal mechanisms including glia.
Keywords: fMRI, Blood oxygenation level dependent effect, Early BOLD response, Stereo-EEG, Spikes
Functional magnetic resonance imaging (fMRI) uses the blood oxygen level dependent (BOLD) effect to map the neuronal activity linked to a particular event (Ogawa et al., 1992). Simultaneous electroencephalography (EEG) and fMRI, EEG/fMRI, is a noninvasive technique that aims to detect hemodynamic changes in the brain related to interictal epileptic activity identified on scalp EEG. Combining the high temporal resolution of EEG signal with the high spatial resolution of BOLD images, EEG/fMRI has been shown to be useful to characterize various forms of focal and generalized epileptic discharges (Gotman et al., 2006).
The quantitative measure of the BOLD response, the hemodynamic response function (HRF), has been derived from the auditory response in event-related BOLD fMRI measured in healthy subjects (Glover, 1999). It has been demonstrated that the HRF to epileptic spikes is most often not significantly different from that physiologic response (Lemieux et al., 2001). The BOLD changes are thought to result from synchronized neuronal activity arising at the time of the spike and are usually detected with a delay of 4–6 s (Benar et al., 2002; Kobayashi et al., 2006). In some studies, HRFs with different shapes (Benar et al., 2002; Lemieux et al., 2008) and peak times (Gotman et al., 2006; Jacobs et al., 2007) have been observed. Unexpectedly, BOLD changes occurring or at least starting before the spike and not detected by standard analysis have been described in patients with focal (Hawco et al., 2007; Jacobs et al., 2009) and generalized epilepsy (Moeller et al., 2008). Similar early hemodynamic changes preceding the visible neuronal epileptic activity were described in animal models (Makiranta et al., 2005; Brevard et al., 2006) and supported by near infrared spectroscopy studies in humans (Roche-Labarbe et al., 2008) and in rats (Osharina et al., 2010).
We will refer to a BOLD response that occurs at least in part earlier than the corresponding EEG event, and, therefore, cannot result exclusively from it, as an early BOLD response. Although from the first descriptions of this early BOLD response some hypotheses about its origin have been formulated (Hawco et al., 2007), the pathophysiology of this phenomenon is still not understood. It has been first hypothesized that the early BOLD response corresponds to a change in metabolic activity not resulting from a change in neuronal activity. An alternative hypothesis is that the early BOLD response corresponds to a synchronized neuronal event that is not visible in the scalp EEG but occurs a few seconds before the visible epileptic discharge. Quantitative studies demonstrated the absence of a subtle scalp EEG change corresponding to the early BOLD activity (Jacobs et al., 2009).
The aim of this study is to characterize the origin and the electrophysiologic correlate of the early BOLD signal in a group of patients with focal refractory seizures who underwent both EEG/fMRI and stereo-EEG (SEEG). In such patients we looked for electrographic changes in the SEEG recordings preceding the scalp interictal epileptic discharge (that for practical reason we will call spike) to determine if the early BOLD response corresponds to a synchronized neural activity invisible on the scalp EEG, but detectable with intracerebral electrodes.
Methods
We included in this study consecutive patients with drug-resistant focal epilepsy who underwent EEG/fMRI and then were studied with depth electrodes for presurgical evaluation. We selected the subpopulation of patients in whom there were both early and postspike fMRI responses concordant with the scalp EEG focus, and who had electrodes implanted in the same region. Written informed consent approved by the Montreal Neurological Institute and Hospital Research Ethics Committee was obtained from each subject.
EEG–fMRI acquisition
EEG was continuously recorded inside a 3T MRI scanner (Siemens, Trio, Germany). No sedation was given. The EEG acquisition was performed with 25 MR compatible electrodes (Ag/AgCl) placed on the scalp using the 10–20 (reference at FCz) and the 10–10 (F9, T9, P9, F10, T10, and P10) placement systems. Two electrodes were placed on the back to record the electrocardiography (ECG). The head of the patient was immobilized with a pillow filled with foam microspheres (Siemens) to minimize movement artifacts and for patient’s comfort. Data were transmitted from a BrainAmp amplifier (Brain Products, Munich, Germany, 5 kHz sampling rate) to the EEG monitor located outside the scanner room via an optic fiber cable.
A T1-weighted anatomic acquisition was first done [1 mm slice thickness, 256 × 256 matrix; echo time (TE) = 7.4 ms and repetition time (TR) = 23 ms; flip angle 30 degrees] and used to superimpose the functional images. The functional data were acquired in runs of 6 min each with the patient in the resting state using a T2*-weighted EPI sequence (64 × 64 matrix; either 25 slices, 5 × 5 × 5 mm, TE = 30 ms, TR = 1.7 s, or 33 slices, 3.7 × 3.7 × 3.7 mm, TE = 25 ms, TR = 1.9 s; flip angle 90 degrees).
EEG-fMRI processing
EEG
Brain Vision Analyser software (Brain Products) was used for offline correction of the gradient artifact (Allen et al., 2000). A 50-Hz low-pass filter was also applied to remove the remaining artifact. The ballistocardiogram artifact was removed by independent component analysis (Benar et al., 2003). A neurologist reviewed the EEG recording and marked the interictal epileptic discharges (burst of spike and wave complexes, paroxysmal slow waves, sharp waves or spikes), according to those observed during clinical monitoring (outside the scanner).
fMRI
The echo planar imaging (EPI) sequences were motion corrected and smoothed (6 mm full width at half maximum) using the software package from the Brain Imaging Center of the Montreal Neurological Institute (http://www.bic.mni.mcgill.ca/ServicesSoftware/HomePage). Data were then analyzed as an event-related design using fMRIstat software (Worsley et al., 2002).
Statistical analysis was performed using the timing of spikes as events, using HRFs consisting of a single gamma function peaking at −9, −7, −5, −3, −1, +1, +3, +5, +7, or +9 s around the event. Three “combined maps” were created to fuse several peak timings: the early prespike map combining the t-statistics of the three HRFs peaking from −9 to −5 s, the prespike map those peaking from −3 to +1 s, and the postspike map those peaking from +3 to +9 s (Fig. S1). Maps were thresholded at |t| > 3.1, which corresponds to a p-value of 0.001 (uncorrected). A spatial extent threshold of five contiguous voxels was then necessary to obtain a p-value of 0.05, corrected for multiple comparisons [family wise error (FEW) rate] due to the number of analyzed voxels (correction based on random field theory) (Friston et al., 1994) and the combination of three statistical maps (Bonferroni correction).
We examined the volumes of activations (positive BOLD response) and deactivations (negative BOLD response) and the maximum t-value of the three maps separately, for each patient. The t map results were represented using red-yellow scale corresponding to activations and blue-white scale corresponding to deactivations. BOLD responses were compared with the EEG to verify that the responses were focal and concordant with the assumed area of spike onset.
HRF calculation
For each spike type, the HRF shape was calculated for the voxel with the highest t-statistic value in the map with the earliest BOLD response, along with surrounding 10 most significantly activated voxels. If fewer than 10 voxels around the peak voxel showed a significant BOLD response, the HRF was projected only over these significant voxels. The HRF was calculated by fitting the average time-course of the significant voxels over a 50 s time window (from 20 s prior to the EEG spike to 30 s after the spike) using a Fourier basis set of 20 sine–cosine waves (Kang et al., 2003). Projected HRFs were sampled at one data point per second.
SEEG recordings
The patients underwent intracranial EEG recordings as part of their presurgical evaluation. To determine the planning of electrode implantation, clinical and diagnostic data (ictal features, surface EEG and structural MRI findings) were considered. Multicontact intracerebral electrodes (contact spacing 5 mm) were inserted orthogonally with a frameless neuronavigation system, the deepest contacts aiming the mesial structures and the most superficial being close or in the lateral cortical mantle. The acquisition parameters of the SEEG were: sampling rate of 200 Hz, low pass filter 0.3 Hz, and high pass filter 70 Hz. Scalp electrodes were not placed under the bandage that covers the head during the period of implantation because of the risk of infection. To infer which intracranial pattern of interictal spiking was the most likely to correspond to the activity visible on the scalp, the recordings were reviewed by three experienced electroencephalographers (J.G, F.D. and F.P.).
The required conditions for the intracranial spikes to correspond to the spikes that had been seen on scalp EEG were:
similar frequency of occurrence;
similar morphology;
compatible spatial distribution;
involvement of several electrodes, including superficial intracerebral contacts or epidural electrodes;
detectable in the same state (wake, sleep);
In addition, a time distance of at least 20 s between events (to exclude the possibility that the early BOLD activity is actually related to a previous spike).
After marking the SEEG recordings, we visually analyzed the period corresponding to the early prespike and to the prespike BOLD maps, to detect if electrographic changes were present in the SEEG before the scalp abnormality. We then selected segments of 4 s length of early prespike, prespike, and baseline in the SEEG recordings. Assuming that the BOLD response takes approximately 5–6 s from onset to peak (Glover, 1999), early prespike segments (−9, −7, −5 s) were centered 13 s before the spike and prespike segments (−3, −1, +1) were centered 7 s before the spike. Baseline segments were far from any spike and when possible about 20 s away from the corresponding spike. The power spectral density (Welch’s method) was computed for these segments for the electrodes of interest. Statistical comparisons between early prespike or prespike and baseline were performed with analysis of variance (ANOVA) for the traditional frequency bands (delta, 0.1–4 Hz; theta, 4–8 Hz; alpha, 8–15 Hz; beta 15–30 Hz; and gamma 30–70 Hz). Significance level was set at p < 0.05.
Cluster volume
For every patient, separate volumes of clusters of interest were calculated for each map (early prespike, prespike, and postspike). For this purpose, the volume of clusters containing the highest t-statistic value, concordant with the spike field, were computed using MNI-Display software (v1.3; MacDonald, 1996), and converted to cubic millimeters.
Coregistration
For the patients who had a postimplantation T1-weighted MRI, the BOLD t-map images were linearly registered to the postimplantation MRI, allowing the visualization of fMRI activation maps in relation to the SEEG electrode position (Figs. 2, 3, and S2).
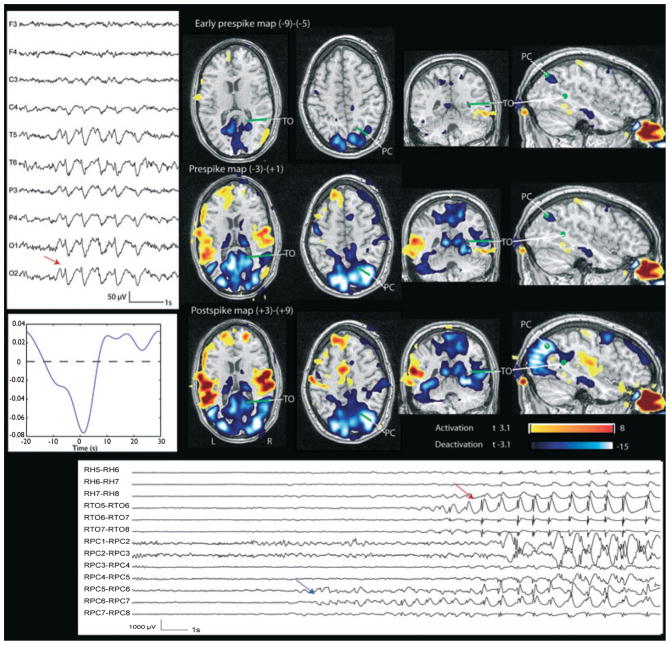
Figure 2.
Patient 2. Right temporal-occipital deactivation and corresponding deactivation in the early prespike and prespike maps. The green points and lines indicate the position of the SEEG contacts visible on this view, obtained by coregistration with the BOLD map. Left: EEG (referential montage) shows burst of sharp waves maximum at O2. Left bottom: HRF. Bottom: SEEG. Red arrow indicates the onset of the interictal event which has a spatial distribution, frequency, and morphology compatible with the scalp EEG events. A discharge involving only the precuneus contacts (RPC6-RPC7, blue arrow) is visible several seconds before it spreads to more superficial and numerous contacts, becoming detectable in the scalp EEG. This focal discharge explains the early BOLD response.
Epilepsia © ILAE
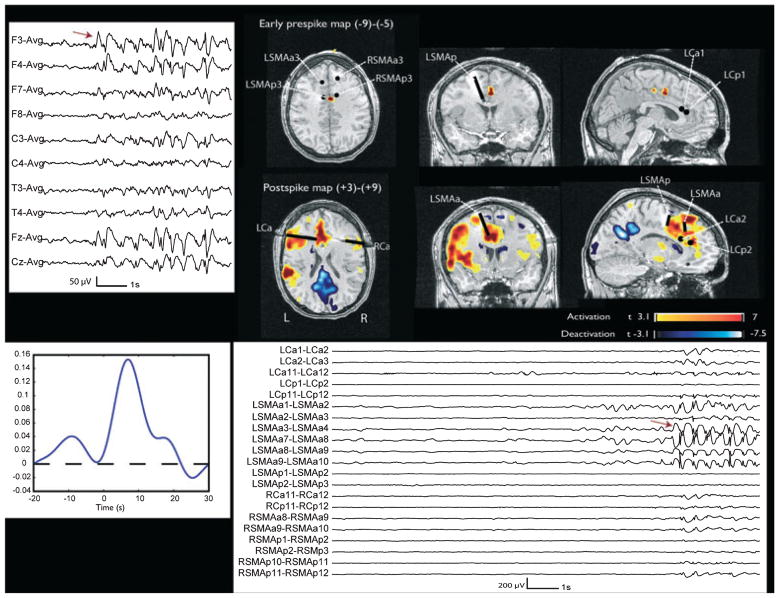
Figure 3.
Patient 3. The early prepsike map shows an activation with maximum between the left and right posterior supplementary motor area (SMA) electrode (deepest contacts). The postspike map shows a left mesial frontal activation, with maximum corresponding to the L SMA anterior electrode and involving also the deepest contacts of the anterior and posterior cingulate electrodes. The black points and lines indicate the electrodes visible in these views and are obtained by coregistration with the BOLD map. Left: EEG (average montage) shows burst of F3, Fz, F4 spikes, maximum on the left. Left bottom: HRF. Bottom: SEEG. Red arrow indicates the onset of the interictal event which has a spatial distribution, frequency and morphology compatible with the scalp EEG events. It is not preceded by any visible change.
Epilepsia © ILAE
Results
Twenty-nine EEG/fMRI studies acquired from 2004 to 2009 from patients who underwent SEEG implantation were reviewed. Three patients were excluded because of movement artifacts during the EEG/fMRI study. In seven patients the EEG was not active or not localizing. Of the 19 remaining patients, the interictal activity was localized to the frontal region in 3, frontotemporal (2), temporal (5), bitemporal (3), parietotemporal (3), occipital (2), and parietal (1).
Eleven of the 19 patients (58%) had an early BOLD response. Of these, 9 (47%) were concordant with the scalp EEG spike. Of these nine patients, four also had a postspike BOLD response concordant with the EEG. Because we decided to include the subpopulation of patients in whom there were both early and postspike fMRI responses concordant with the scalp EEG focus, we studied only four patients (13.7%), the other five not presenting any postspike response. The mean spike rate in these four patients was 1.6 per min (range 0.7–2.2).
Patient details are presented in Table 1. The mean age was 21.2 years (range 17–24). Two patients had cryptogenetic focal epilepsy and two had lesional epilepsy. Only Patient 2 had both early prespike and prespike responses; the others had only one of the early BOLD changes.
Table 1.
Clinical details
| Patient/Sex/Age | Epilepsy | Structural MRI | AEDs | Scalp EEG spikes | Postspike +3/+9 (cluster in mm3) | Prespike −3/+1 (cluster in mm3) | Early Prespike −9/−5 (cluster in mm3) | Depth electrodes |
|---|---|---|---|---|---|---|---|---|
| 1/F/24 | L FL | L F dorsal FCD | Carbamazepine, Clonazepam, Lamotrigine | F3, F7 | Act. over L F2, L ant. C, thalamus (36,363) | _ | Deact. over L ant. C (1,321) | Bilateral OF, ant. and superior C. L Perilesional (LP) |
| 2/F/24 | R OL | Normal | Lamotrigine | Burst of sharp waves max at O2 | Deact. max on R precuneus and TO junction (132,037) | Deact. max on R precuneus and TO junction (104,135) | Deact. max on R precuneus and TO junction (18,929) | R A, ant. H, TO junction, SC and IC structures, precuneus |
| 3/M/17 | L FL | Normal | Lamotrigine, Levetiracetam, Topiramate | F3, F4, Fz, more on the L | Act. max over L mesial F region (71,427) | _ | Act. over post C (2,281) | Bilateral OF, ant. and post. SMA, ant. and post. C |
| 4/F/20 | R TP | T-P-O heterotopic area | Carbamazepine, Lamotrigine, Levetiracetam | T4–T10 | Act. over R mesial T structures (7,671) | Act. over R mesial T structures (1,986) | _ | RA, RH, R post. H (RC), R Perilesional (RP), R inferior P (RS), RO |
AEDs, antiepileptic drugs; A, amygdala; Act, activation; Ant, anterior; C, cingulate gyrus; Deact, deactivation; F, frontal; FCD, focal cortical dysplasia; H, hippocampus; IC, infracalcarine; L, left; LV, lateral ventricle; O, occipital; OF, orbitofrontal; P, parietal; Post, posterior; R, right; SC, supracalcarine; SMA, supplementary motor area; T, temporal; TO, temporooccipital.
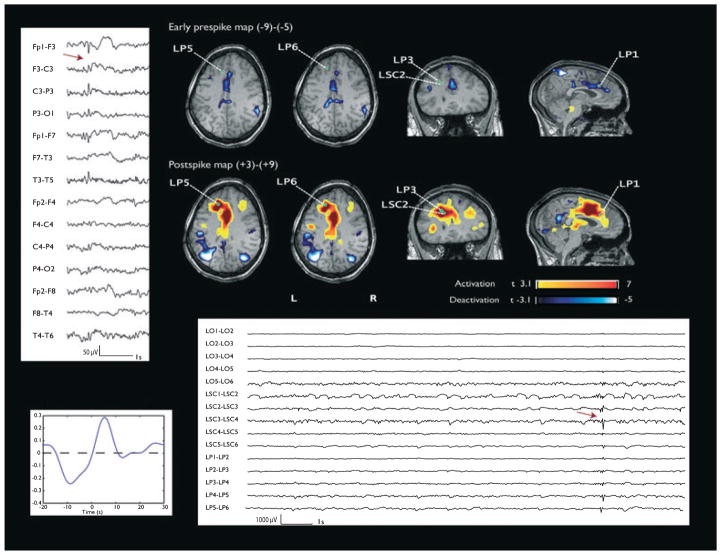
Patient 1 (interictal events: F3-F7 spikes; 70 spikes analyzed: spike rate 1.45/min) had a focal cortical dysplasia in the left dorsofrontal region. The standard BOLD analysis (postspike map) showed an activation over the perilesional region in the second frontal convolution (max t-value 10.8), in the left anterior cingulate gyrus and thalamus, and a less significant deactivation in bilateral parietal regions. An early prespike (−9 to −5 s) deactivation was present over the left anterior cingulate gyrus (max t-value −4.5), concordant with the focal positive BOLD seen on standard analysis (Fig. 1). Electrodes were placed symmetrically in both hemispheres in the orbitofrontal region (OF) and the anterior and superior aspects of the cingulate gyrus (LAC and LSC), and one electrode was placed close or in the lesion (LP). The SEEG recordings showed a subcontinuous sharp activity in the deepest contacts of the perilesional and superior cingulate electrodes (LP and LSC). The interictal SEEG events (equivalent to the scalp F3-F7 spikes) were marked on LP and LSC electrodes. The visual analysis did not show any changes of the SEEG recording in the early prespike period. The spectral analysis did not show any significant differences between the early prespike and baseline segments. Patient 1 did not have a postimplantation MRI but only a postresection one. The resection included the area of the most active electrodes (deepest contacts of LP and LSC), and it is, therefore, not possible for us to display the tracks of these electrodes.
Figure 1.
Patient 1. Left mesial frontal activation and corresponding deactivation in the early prespike map. The green points indicate the estimated position of the SEEG contacts visible on this view. Left: EEG (bipolar montage) shows F3-F7 spikes. Left bottom: HRF. Right bottom: SEEG. Arrow indicates the interictal event thought to correspond to the scalp spike. It is not preceded by any visible change.
Epilepsia © ILAE
Patient 2 [interictal events: bursts of sharp waves maximum at O2; 160 bursts of sharp wave analyzed (marked as SW start-end): the rate of the bursts was 2/min] had a normal MRI. An early prespike (−9 to −5 s, max t-value −9.2) and a prespike (−3 to +1 s, max t-value −20) deactivation were present in the congruent region of focal negative BOLD (max t-value −24) seen on the postspike map (right precuneus and temporooccipital junction, Fig. 2). Other less significant (max t-value 8.9) areas of activation were found in the standard analysis in the insulae bilaterally. Electrodes were implanted in the right hemisphere only in the amygdala, anterior hippocampus, temporooccipital junction, and precuneus, and in the supracalcarine and infracalcarine structures. The interictal events marked on SEEG (equivalent of the scalp bursts of sharp waves maximum at O2) were bursts of sharp waves involving at the same time primarily the temporooccipital junction and precuneus electrodes and, less clearly, the mesial temporal structures. In this case, the SEEG shows a discharge involving only the contacts of the precuneus electrode in the period corresponding to the early BOLD responses. When the discharge spreads from the pre-cuneus to the temporooccipital junction and mesial temporal structures, involving more superficial and numerous contacts, it most likely becomes detectable in the scalp EEG. The spectral analysis showed a significant increase in all bands in the early prespike and prespike period when compared to the baseline.
Patient 3 [interictal events: F3, Fz, F4 spikes, very often in bursts (marked as SW start-end), maximum left; 158 bursts analyzed: burst rate 2.2/min] had a normal MRI. An early prespike activation (−9 to −5 s, max t-value 5.19) was detectable in the region of focal positive BOLD (max t-value 13.11) seen on the postspike map (left anterior mesial frontal area, Fig. 3). Electrodes were placed in the orbitofrontal regions, anterior and superior aspects of the cingulate gyri, and in the anterior and posterior supplementary motor area (SMA). The SEEG showed that the most epileptogenic area was located in the left frontal lobe with focal accentuation of the ictal and interictal discharges seen in the superficial contacts of depth left cingulate electrodes, and in all the contacts of the left anterior SMA electrode. The interictal events marked on SEEG (equivalent of the scalp F3, Fz, and F4 bursts, maximum left) were bursts of spikes involving the most external contacts of the left anterior cingulate and SMA electrodes (lateral and parasagittal cortex of the superior and middle frontal gyri), and to a lesser extent the contralateral homologous regions. In this patient no electrographic changes were present in the SEEG recording in the period corresponding to early BOLD changes. The spectral analysis did not show any significant differences between the early prespike and baseline segments.
Patient 4 (interictal events: spikes maximum at T4-T10; 101 spikes analyzed: spike rate 0.7/min) had an area of subependymal and subcortical nodular heterotopia with cortical dysplasia within the temporal lobe (extending to the occipital lobe). The prespike analysis showed an activation (−3 to +1 s, max t-value 4.8) concordant with the focal positive BOLD (max t-value 8.1) seen on the postspike map (right temporoparietal lobe, in the area of heterotopia, Fig. S2). Electrodes were placed in the right amygdala, anterior (RH) and posterior (RC) hippocampus, inferior parietal (RS), and occipital regions (RO). Another electrode was placed in the heterotopic area (RP). SEEG investigation showed that the epileptic generator was located in a large area covering the posterior temporal, occipital, or parietooccipital region where the maximum of the developmental anomaly was described (depth electrodes RS and RP and to a lesser extent at RO). The interictal SEEG events (equivalent of the scalp maximum at T4-T10) were marked on the RP, RC, and RS electrodes. In this patient no electrographic changes were detectable by visual and spectral analysis in the SEEG recording in the period corresponding to early BOLD changes.
HRF projections
Four HRFs, one for each patient, were analyzed. Every HRF was calculated for the voxel with the highest t-statistic value in the earliest BOLD response map, concordant with the spike field (anterior cingulate gyrus for Patient 1, right precuneus for Patient 2, left anterior mesial frontal area for Patient 3, and right temporal heterotopic cortex for Patient 4). Those HRFs calculated in the spike field showed peak times between 10 s before and 6 s after the event. The shape of the HRFs and their peak times explained the early BOLD responses observed (see HRFs for Patients 1, 2, 3, and 4 in Figs 1, 2, 3, and S2).
Cluster volumes
Volumes were calculated for clusters containing the highest t-statistic value, concordant with the spike field in each early map and in the standard analysis BOLD map. Results are reported in Table 1. In every case the early map cluster has a smaller volume than the one in the postspike map. BOLD changes in the early maps were always more focal than in later maps, and a development over time from more to less focal could be observed. This increasing extent of the response could consist of larger clusters, of several small clusters within the spike field, or of more clusters outside the spike field in the late map, as demonstrated in Figs 1, 2, and S2 for the different response types. This resulted in later maps being less specific than earlier maps in regard to the spike field.
Discussion
In our study we found an early BOLD response concordant with the spike field and with the postspike map in four patients and we tried to investigate the meaning of this unexpected phenomenon. As shown by the HRF peak times, changes in metabolic activity occur some seconds before the spike is visible on the scalp EEG. Previous studies demonstrated HRFs starting or peaking before the epileptic discharge. In a population of patients with generalized polyspike-wave discharges, subcortical BOLD changes were found with an HRF onset 9 s prior to the discharge (Moeller et al., 2008). In patients with focal discharges, a cortical early BOLD response concordant with the spike field was found in 84% of cases (Jacobs et al., 2009). The general variability in BOLD responses to epileptic spikes has been emphasized in several studies, including those of Benar et al. (2002) and the recent study of Grouiller et al. (2010). In this paper, we have addressed the following question: can it be that some of the variability in the measured HRF is caused by the fact that the neuronal event is not just a time point but may be more complex and may not start at the time at which we originally thought it started.
The early BOLD response was an activation (two patients) or a deactivation (two patients). An activation in a region concordant with EEG spikes has been assumed to reflect the epileptic discharge, but the significance of a deactivation in this region is uncertain (Gotman, 2008). A recent study (Rathakrishnan et al., 2010) has demonstrated that the occurrence of a focal deactivation in the region of the spike field in adults with focal epilepsies is an uncommon yet consistent phenomenon, and has suggested that these negative BOLD responses are complex and should not be assumed to be simply an indicator of neuronal suppression.
In the four patients with both early and postspike BOLD responses, we found that the early response was more focal and restricted to the spike field than the late response, suggesting a diffusion of the metabolic activity. The finding of a more focal and restricted early BOLD response is in agreement with an earlier study of Jacobs et al. (2009), where an increase in number of voxels from the early to late maps was seen in almost all the cases analyzed. Early BOLD change may represent the initial metabolic response of the epileptiform activity, while the later response includes propagated activity. For this reason the early BOLD response could have a better localizing value than the later BOLD changes. The propagation discussed here is obviously not the electrical propagation, which usually takes place within milliseconds (Emerson et al., 1995).
Because the early BOLD response was described for the first time, we hypothesized (Hawco et al., 2007) that it corresponds to a change in metabolic activity not resulting from a change in synchronized neuronal activity, explaining why the EEG would not detect any paroxysmal activity. The EEG and the BOLD signal come from different neurophysiologic and cellular mechanisms (one electrical and the other based on a venous response to metabolic changes, as BOLD results from complex interactions between blood flow, blood volume, and O2 consumption), so the occurrence of events observed with only one modality is possible (Nunez & Silberstein, 2000). The second postulated hypothesis is that the early BOLD response corresponds to a neural event that is not seen in the scalp EEG but occurs a few seconds before a visible epileptic discharge. Jacobs et al. (2009) have shown that the BOLD changes seen prior to the spike do not correspond to any subtle changes visible in the scalp EEG in the seconds preceding the spike. It could be argued that not all spike activity is visible on the scalp, especially if it originated in deep structures or involves only a small cortical area (Tao et al., 2005). A study comparing scalp and intracranial EEG has shown that the visibility of spikes on surface EEGs is dependent on the cortical area that generates them, with mesial temporal spikes being unlikely visible on scalp EEG (Marks et al., 1992).
This is the first study that aims to understand the pathogenesis of the early BOLD response by comparing EEG/fMRI with intracranial electrode recordings. We included in our analysis only events that were separated from each other by at least 20 s, to exclude the possibility that the early BOLD activity is actually related to a previous spike [an event occurring 14 s prior to the EEG spike would be required to explain an HRF peaking at −9 s, assuming that the BOLD response takes approximately 5 s from onset to peak (Glover, 1999)]. Considering the period included between −9 and −1 s before the spike, in one of our four cases a clear very focal SEEG discharge was detectable in correspondence with the early BOLD response; in the other three patients no SEEG changes were evident prior to the scalp EEG abnormality. This suggests that in some cases the early BOLD activity is explained by a synchronized neural discharge detectable with SEEG and not visible in the scalp EEG, whereas in other cases the early BOLD response may reflect a metabolic phenomenon that does not result from a synchronized neuronal discharge. The early BOLD response suggests the presence of metabolic changes preceding the epileptic spike and not resulting from an electrical phenomenon. This is in agreement with a recent study (Osharina et al., 2010) that has demonstrated, by using electrocorticography and near-infrared spectroscopy (ECoG/NIRS) in rats, hemodynamic changes (an oxyhemoglobin decrease and a deoxyhemoglobin increase) starting about 6 s before the spike activity, without any other electrical change before the spike detected by ECoG.
Even if a possible influence of antiepileptic drugs on the hemodynamic response could underlie an abnormal neurovascular coupling in the epileptic brain, we cannot infer about the effect of the drugs on the early BOLD response given the heterogeneity of AEDs in our patients and the small sample.
The mechanisms that cause neurons to synchronize in epileptic spikes are still subject to debate. Different mechanisms including synaptic and nonsynaptic communication among neurons and glial cells can be involved. In the last years, several studies have demonstrated the role of astrocytes both in neurovascular coupling (and consequently in the origin of the BOLD changes) and in neuronal synchronization (Haydon & Carmignoto, 2006). Astrocytes are electrically nonexcitable cells that express in the synaptic cleft receptors for most neurotransmitters [glutamate, γ-aminobutyric acid (GABA), norepinephrine, and acetylcholine]. These receptors link to a second messenger system that leads to a dynamic increase in cytosolic Ca++ and a release of vasoactive agents at the astrocytic/vasculature interface. Depending on which biochemical messengers are released, astrocytes are able to induce either localized vasodilation or vasoconstriction (Wang et al., 2009). Bennett et al. (2008) have shown changes in the BOLD and in vascular deoxyhemoglobin after the action of synaptically released glutamate on astrocytes (an index of neuronal synaptic activity): this causes a release of lipids involved in signaling pathways from astrocyte endfeet onto arteriolar smooth muscle cells, resulting in a change of blood volume and blood flow. The presence of transporters for glutamate and GABA in astrocytes suggests an important role in the regulation of neurotransmitters in the synaptic cleft (Schousboe, 2003; Haydon & Carmignoto, 2006) and, therefore, for the implication of astrocytes in neuronal synchronization as well as in blood flow and volume.
The correlation between early BOLD response and high frequency oscillations (HFOs) has not been investigated yet, so the role of the HFOs in the pathogenesis of this phenomenon could not be excluded.
One limitation of our study is that we could not record simultaneously the scalp EEG and SEEG; we have considered the SEEG abnormalities that were most probably the equivalent of the scalp discharges recorded in the scanner. Simultaneous EEG/fMRI using intracranial electrodes, currently in early stages (Vulliemoz et al., 2010), might be able to provide more accurate answers to this question. Considering the different types of interictal discharges in the four patients (isolated interictal spike in Patients 1 and 4, bursts of spike and wave complexes in Patients 2 and 3), it could be argued that the generation of these dissimilar events may be due to different mechanisms and that a synchronized neural discharge detectable with SEEG is present only before the burst of spike and wave complexes (Patient 2) and not before the single interictal spike. Nevertheless for Patient 3, as shown by the spectral analysis, no discharge is detected in the SEEG before the burst of spike and wave complexes. However, it could be that mechanisms generating interictal discharges are different among different types of epilepsy and are specific for each patient.
Overall our results suggest that metabolic events that start before the appearance of scalp epileptic spikes may result from both neuronal and nonneuronal mechanisms. Studies on the role of unsynchronized local neurons, interneurons, glial, or distant subcortical structures might provide additional information to explain their contribution in the pathogenesis of epileptic spike.
Conclusions
A BOLD response preceding the spike is a common phenomenon in adults with focal epilepsy. This early response is more focal and for this reason it could have a better localizing value than the later BOLD changes. Recent discoveries related to the neuronal synchronization mechanisms suggest that early BOLD responses can be the result of unsynchronized local neurons or glial structures involved in the pathogenesis of epileptic spikes. The identification of early BOLD changes may be used as an additional tool to understand BOLD patterns in EEG/fMRI studies and the pathogenesis of interictal epileptic discharge.
Supplementary Material
Acknowledgments
The authors wish to thank Natalja Zazubovits for her help with data collection and analysis. This work was supported by grant MOP-38079 of the Canadian Institutes of Health Research.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional supporting information may be found in the online version of this article (http://doi.org/10.1111/j.1528-1167.2011.03072.x)
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12:230–239. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Benar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J. The BOLD response to interictal epileptiform discharges. Neuroimage. 2002;17:1182–1192. doi: 10.1006/nimg.2002.1164. [DOI] [PubMed] [Google Scholar]
- Benar C, Aghakhani Y, Wang Y, Izenberg A, Al-Asmi A, Dubeau F, Gotman J. Quality of EEG in simultaneous EEG-fMRI for epilepsy. Clin Neurophysiol. 2003;114:569–580. doi: 10.1016/s1388-2457(02)00383-8. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Farnell L, Gibson WG. Origins of the BOLD changes due to synaptic activity at astrocytes abutting arteriolar smooth muscle. J Theor Biol. 2008;252:123–130. doi: 10.1016/j.jtbi.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Brevard ME, Kulkarni P, King JA, Ferris CF. Imaging the neural substrates involved in the genesis of pentylenetetrazol-induced seizures. Epilepsia. 2006;47:745–754. doi: 10.1111/j.1528-1167.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- Emerson RG, Turner CA, Pedley TA, Walczak TS, Forgione M. Propagation patterns of temporal spikes. Electroencephalogr Clin Neurophysiol. 1995;94:338–348. doi: 10.1016/0013-4694(94)00316-d. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Maziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49(suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Kobayashi E, Bagshaw AP, Benar CG, Dubeau F. Combining EEG and fMRI: a multimodal tool for epilepsy research. J Magn Reson Imaging. 2006;23:906–920. doi: 10.1002/jmri.20577. [DOI] [PubMed] [Google Scholar]
- Grouiller F, Vercueil L, Krainik A, Segebarth C, Kahane P, David O. Characterization of the hemodynamic modes associated with interictal epileptic activity using a deformable model-based analysis of combined EEG and functional MRI recordings. Hum Brain Mapp. 2010;31:1157–1173. doi: 10.1002/hbm.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawco CS, Bagshaw AP, Lu Y, Dubeau F, Gotman J. BOLD changes occur prior to epileptic spikes seen on scalp EEG. Neuroimage. 2007;35:1450–1458. doi: 10.1016/j.neuroimage.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kobayashi E, Boor R, Muhle H, Stephan W, Hawco C, Dubeau F, Jansen O, Stephani U, Gotman J, Siniatchkin M. Hemodynamic responses to interictal epileptiform discharges in children with symptomatic epilepsy. Epilepsia. 2007;48:2068–2078. doi: 10.1111/j.1528-1167.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Moeller F, Boor R, Stephani U, Gotman J, Siniatchkin M. Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG-fMRI. Neuroimage. 2009;45:1220–1231. doi: 10.1016/j.neuroimage.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Kang JK, Benar C, Al-Asmi A, Khani YA, Pike GB, Dubeau F, Gotman J. Using patient-specific hemodynamic response functions in combined EEG-fMRI studies in epilepsy. Neuroimage. 2003;20:1162–1170. doi: 10.1016/s1053-8119(03)00290-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp. 2006;27:488–497. doi: 10.1002/hbm.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L, Krakow K, Fish DR. Comparison of spike-triggered functional MRI BOLD activation and EEG dipole model localization. Neuroimage. 2001;14:1097–1104. doi: 10.1006/nimg.2001.0896. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Laufs H, Carmichael D, Paul JS, Walker MC, Duncan JS. Noncanonical spike-related BOLD responses in focal epilepsy. Hum Brain Mapp. 2008;29:329–345. doi: 10.1002/hbm.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D. MNI-Display: program for display and segmentation of surfaces and volumes. McConnell Brain Imaging Center, Montreal Neurological Institute; Montreal, Canada: 1996. Available at: http://www.bic.mni.mcgill.ca/ServicesSoftware/HomePage. [Google Scholar]
- Makiranta M, Ruohonen J, Suominen K, Niinimaki J, Sonkajarvi E, Kiviniemi V, Seppanen T, Alahuhta S, Jantti V, Tervonen O. BOLD signal increase preceeds EEG spike activity – a dynamic penicillin induced focal epilepsy in deep anesthesia. Neuroimage. 2005;27:715–724. doi: 10.1016/j.neuroimage.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Marks DA, Katz A, Booke J, Spencer DD, Spencer SS. Comparison and correlation of surface and sphenoidal electrodes with simultaneous intracranial recording: an interictal study. Electroencephalogr Clin Neurophysiol. 1992;82:23–29. doi: 10.1016/0013-4694(92)90178-k. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2008;39:1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB. On the relationship of synaptic activity to macroscopic measurements: does co-registration of EEG with fMRI make sense? Brain Topogr. 2000;13:79–96. doi: 10.1023/a:1026683200895. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osharina V, Ponchel E, Aarabi A, Grebe R, Wallois F. Local haemodynamic changes preceding interictal spikes: a simultaneous electrocorticography (ECoG) and near-infrared spectroscopy (NIRS) analysis in rats. Neuroimage. 2010;50:600–607. doi: 10.1016/j.neuroimage.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Rathakrishnan R, Moeller F, Levan P, Dubeau F, Gotman J. BOLD signal changes preceding negative responses in EEG-fMRI in patients with focal epilepsy. Epilepsia. 2010;51:1837–1845. doi: 10.1111/j.1528-1167.2010.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche-Labarbe N, Zaaimi B, Berquin P, Nehlig A, Grebe R, Wallois F. NIRS-measured oxy- and deoxyhemoglobin changes associated with EEG spike-and-wave discharges in children. Epilepsia. 2008;49:1871–1880. doi: 10.1111/j.1528-1167.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Schousboe A. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem Res. 2003;28:347–352. doi: 10.1023/a:1022397704922. [DOI] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–676. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Carmichael DW, Rosenkranz K, Diehl B, Rodionov R, Walker MC, McEvoy AW, Lemieux L. Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage. 2010;54:182–190. doi: 10.1016/j.neuroimage.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Wang X, Takano T, Nedergaard M. Astrocytic calcium signaling: mechanism and implications for functional brain imaging. Methods Mol Biol. 2009;489:93–109. doi: 10.1007/978-1-59745-543-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.