SUMMARY
Purpose
In simultaneous electroencephalography (EEG) and functional magnetic resonance imaging (fMRI), increased neuronal activity from epileptiform spikes commonly elicits positive blood oxygenation level–dependent (BOLD) responses. Negative responses are also occasionally seen and have not been explained. Recent studies describe BOLD signal changes before focal EEG spikes. We aimed to systematically study if the undershoot of a preceding positive response might explain the negative BOLD seen in the focus.
Methods
Eighty-two patients with focal epilepsy who underwent EEG-fMRI at 3T were retrospectively studied. Studies with a focal negative BOLD response in the region of the spike field were reanalyzed using models with hemodynamic response functions (HRFs) peaking from −9 to +9 s around the spike.
Results
Eight patients met the inclusion criteria, showing negative BOLD responses in the spike field on standard analysis. None had positive BOLD responses immediately adjacent to the areas of deactivation. Regions of deactivation were found to have congruent preceding positive responses in two cases. These early activations were seen at the combined maps of −5 to −9 s.
Discussion
This study indicates that in a small proportion of patients with focal epilepsy in whom the standard analysis reveals focal negative responses, an earlier positive BOLD response is probably the cause. The origin of negative BOLD signal changes in the focus as a result of an epileptic event remains, however, unexplained in most of the patients in whom it occurs.
Keywords: fMRI, Focal epilepsy, Spike, Deactivation
Functional magnetic resonance imaging (fMRI) allows the dynamic study of neuronal activity linked to a particular event using a change in blood oxygenation–level dependent (BOLD) effects as a surrogate marker (Ogawa et al.,1992). Interictal discharges as seen on electroencephalography (EEG) are a result of summated membrane events from abnormally hypersynchronous neurons in epileptic tissue manifesting as a deviation from a baseline “resting state” (Matsumoto & Ajmonemarsan, 1964). In epilepsy patients, BOLD signal changes can be used to detect neuronal activity linked to spikes seen on the scalp EEG in simultaneous EEG-fMRI recording, thereby allowing the noninvasive study of epileptogenic networks (for review see Gotman et al., 2006; Laufs & Duncan, 2007). The BOLD response reflects the synchronized neuronal activity that occurs as a result of an epileptiform discharge. A delay is observed between the EEG spike and corresponding BOLD signal change, which usually peaks 4–6 s later (Krakow et al., 2001; Bénar et al., 2002; Kobayashi et al., 2006; Raichle & Mintun, 2006). However, the quantitative measure of this response, known as the hemodynamic response function (HRF) may vary between subjects, for different brain regions or for different types of spikes (Aguirre et al., 1998; Bénar et al., 2002; Kang et al., 2003; Handwerker et al., 2004; Menz et al., 2006). The sensitivity of EEG-fMRI could be improved with the use of HRF models peaking at 3, 5, 7, and 9 s from the time of the EEG discharge to maximize the capture of statistically significant BOLD changes (Bagshaw et al., 2004). Studies of the BOLD responses in focal and generalized epilepsies have uncovered two curious phenomena for which a definite explanation remains elusive.
Firstly, changes in BOLD can be positive or negative, alternatively termed activations and deactivations (Archer et al., 2003; Gotman et al., 2006; Laufs & Duncan, 2007). They may occur within the region of the spike field or at regions of the brain distant from the epileptic discharge seen on the EEG (Kobayashi et al., 2006; Laufs et al., 2007). Based on the assumption that activation in a region concordant with EEG spikes reflects the epileptic discharge, the significance of a deactivation in this region is uncertain. (Al-Asmi et al., 2003; Gotman, 2008). Various hypotheses have been proposed to explain this phenomenon, such as a vascular “steal” phenomenon, disruption of neurovascular coupling, reduced synaptic activity, and γ-aminobutyric acid (GABA)ergic inhibition (Shmuel et al., 2002; Gotman, 2008; Mangia et al., 2009). Deactivations in the “default pattern” were commonly found in generalized discharges but also reported for focal discharges (Aghakhani et al., 2004; Hamandi et al., 2006; Laufs et al., 2007) and believed to be a result of a suspension of the baseline state during attentiveness (Raichle et al., 2001; Gotman et al., 2005; Laufs et al., 2007). Focal deactivation within the region of the spike field appears less commonly and is not easily explained. The true incidence of an isolated and concordant deactivation in focal epilepsy remains uncertain.
Secondly, both in focal and generalized epilepsies, patients have been described in whom BOLD signal changes preceded the spikes seen on the scalp EEG and were not detected by the standard analysis (Hawco et al., 2007; Moeller et al., 2008; Jacobs et al., 2009). The findings of preceding hemodynamic changes prior to epileptic activity seen on the scalp EEG are supported by near-infrared spectroscopy (NIRS) studies in humans (Roche-Labarbe et al., 2008) as well as by animal studies, which showed BOLD signal changes several seconds before induced discharges (Mäkiranta et al., 2005; Brevard et al., 2006). By studying the modeled HRFs prior to the spike, Jacobs et al. (2009) demonstrated that a negative BOLD response in the spike field was preceded by a positive response in the same region in 3 of 11 children with focal epilepsy. There was no evidence that the prespike appearances were secondary to any subtle EEG changes. They proposed that these deactivations merely reflect the undershoot following earlier activations. This explanation could shed new light on the understanding of focal negative BOLD responses observed in some patients.
The aim of our study was to determine systematically the incidence of deactivations within the spike fields in a larger group of patients with focal epilepsy. In such patients, we studied the prespike BOLD response to determine if the deactivations were due to a preceding activation in the same region.
Methods
Eighty-two adult patients with focal epilepsy who underwent 3T EEG-fMRI between October 2006 and April 2009 at the Montreal Neurological Hospital and Institute were identified from a database. Patients with clear interictal EEG discharges and corresponding focal negative BOLD responses within the spike field were included in the study. The spike field (the anatomic region that generated the spike) was estimated at the sublobar level by visual inspection of the EEG. Visual comparison was then made between the estimated spike field and the localization of the BOLD response. Concordance between EEG and fMRI was presumed if a negative BOLD response was noted in a region of the brain that corresponded with the estimated spike field. For example, temporal spikes involving the anterior temporal EEG channels suggested that the spike field was localized to this particular region and that a BOLD response isolated to the posterior temporal lobe was not considered concordant. The following exclusion criteria were applied:
Generalized or very widespread discharges
Prolonged bursts of interictal discharges (in order to avoid overlap between consecutive HRFs)
Deactivations in the “default mode” pattern
Concomitant areas of activation immediately adjacent to the deactivation
Patients provided informed consent in accordance with the requirements of the ethics board at the Montreal Neurological Institute and Hospital.
Data acquisition
EEG was continuously acquired inside the 3T magnetic resonance imaging (MRI) scanner (Siemens Magnetom Trio, Erlangen, Germany) using 27 MRI-compatible Ag/AgCl electrodes. EEG data were collected using a BrainAmp amplifier (Brain Products, Munich, Germany). A 1 × 1 × 1 mm T1-weighted scan was obtained for anatomic localization of the functional data. Functional MRI images of 62 patients were acquired using echo-planar imaging (5 × 5 × 5 mm voxels, 25 slices, 64 × 64 matrix, TR/TE = 1,750/30 ms, flip angle = 90°) in runs of 200 frames lasting 6 min. An alternate acquisition parameter was used in 20 patients using a different head-coil (3.7 mm isotropic voxels, 33 slices, 64 × 64 matrix, TR/TE = 1,900/25 ms, flip angle = 70°).
EEG analysis
The EEG signal was of good quality and processed offline using Brain Vision Analyser software (Brain Products) with correction of the gradient artifact and filtering of the EEG signal (Allen et al., 2000). A 50-Hz low-pass filter was applied to remove the remaining artifact. Independent component analysis was used to extract the ballistocardiogram artifact (Bénar et al., 2003). Epileptiform discharges observed on EEG performed inside and outside the scanner were identical. An experienced neurophysiologist marked and categorized these according to their morphology, location, and duration. The relationship between spike frequency (number of spikes per min of acquisition) and preceding fMRI activation was analyzed with analysis of variance (ANOVA).
Standard fMRI analysis
The echo planar imaging (EPI) images were motion corrected by linear six-parameter rigid-body transformations (three translations and three rotations) and smoothed (6-mm full width at half maximum) using the software package from the Brain Imaging Center of the Montreal Neurological Hospital and Institute (http://www.bic.mni.mcgill.ca/ServicesSoftware/HomePage).
In this standard analysis, maps of the t statistic (t maps) were created using the timing of each event onset in an event-related design (Worsley et al., 2002). At each voxel, the maximum |t| value was taken from four individual t maps created using a general linear model with one of four gamma-shaped (HRFs) with peaks at 3, 5, 7, and 9 s, which were convolved with the duration of the EEG discharge (Bagshaw et al., 2005). Slice timing was taken into account by sampling the modeled BOLD responses at the acquisition times of each slice. EEG-fMRI responses were then classified into positive (activation) and negative (deactivation) BOLD changes. For a response to be considered significant it required a minimum of five contiguous voxels with a |t| > 3.1, corresponding to p = 0.05 corrected for the multiple comparisons resulting from the number of voxels in the brain and the four HRFs. Six motion correction regressors representing the three translations and three rotations were included in the analysis. Runs with more than 2 mm of motion were excluded from analysis. Anatomic and functional MRIs were coregistered using a rigid transformation (three rotations + three translations).
fMRI analysis for early BOLD signal changes
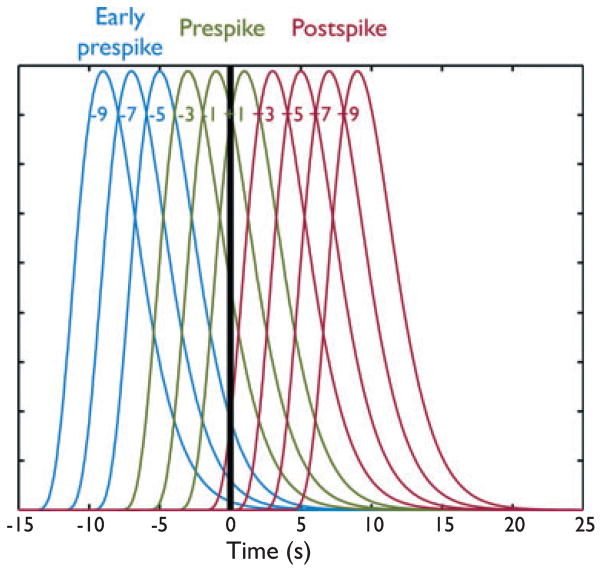
Studies that met the inclusion criteria for the focal deactivations in the spike field were reanalyzed using models with gamma-shaped HRFs peaking every 2 s from −9 to +9 s around the spike to investigate possible earlier BOLD responses (see Fig. 1). It was thus possible to investigate the variability in HRF peak latency by computing the response to each of the 10 HRFs in each patient. Peaks were then grouped into early prespike [(−9) to (−5)], prespike [(−3) to (+1)], and postspike [(+3) to (+9)] clusters as described by Jacobs et al. (2009). The t map in each category was generated using the maximum |t| values of all HRFs involved within that group. The (+1) HRF is considered “prespike,” although its peak occurs 1 s after the spike because the HRF starts to rise before the spike (the rise time is approximately 5.4 s) (Glover, 1999). The (+3) HRF was included in the “postspike” group for consistency with previous analyses, and also because it shows very little variation preceding the spike (see Fig. 1). The remaining six HRFs were thus not only earlier than the canonical HRF, but also had their onsets unambiguously earlier than the EEG events. These six HRFs were partitioned into the two subgroups “early prespike” [(−9) to (−5)] and “prespike” [(−3) to (+1)] to facilitate the interpretation of the results. The t-map results were represented using red–yellow scale corresponding to positive BOLD changes (activation) and blue–white scale corresponding to negative BOLD changes (deactivation).
Figure 1.
Plot showing the 10 HRFs used in the analysis, in relation to the spike timing (represented by the vertical black bar). HRFs are partitioned into “early prespike” (blue), “prespike” (green), and “postspike” (red) groups.
Epilepsia © ILAE
HRF calculation
In order to confirm the findings obtained with the gamma analysis, an HRF was calculated in the negative BOLD response most concordant with the spike field in the posts-pike map. For this purpose a region of interest (ROI) was defined in the postspike maps, which was then used to select the voxels for the HRF calculation. The shape of the HRF was determined by investigating the voxel with the highest t-value and the surrounding six significantly activated voxels. The average time course in those seven voxels was then fitted by a Fourier basis set of 10 sine–cosine waves over a 50 s time window (from 20 s prior to the EEG spike to 30 s after the spike) (Josephs et al., 1997; Kang et al., 2003).
Results
Eighty-four EEG-fMRI scans from 82 patients with focal epilepsy were reviewed. Interictal activity was localized to the frontal region in 17 patients, temporal in 44, and extra-frontotemporal in 14. In seven patients, the spikes were either multifocal or had a field that involved more than one of the regions described above.
Eight individual patient studies (9.5%) with one spike type each met the inclusion criteria, with focal negative BOLD signal changes concordant with the spike field. Patient details are presented in Table 1. The mean age was 30.25 years (range 20–50 years). The distribution according to the origin of the interictal discharges was as follows: three frontal (22.2% of frontal patients studied), four temporal (6.8%), and one extrafrontotemporal (14.2%). Mean spike rate was 2.9 per min (range 0.22–7.78 min). Seven patients had identifiable structural abnormalities on the anatomic MRI scans.
Table 1.
Clinical information of the patients in whom focal deactivations had been observed using the standard analysis (postspike)
| Patient | Age | Epilepsy | Structural MRI | EEG spikes | Early prespike (−9) to (−5) | Prespike (−3) to (+1) | Postspike (+3) to (+9) |
|---|---|---|---|---|---|---|---|
| 1 | 40 | Temporal | Right hippocampal sclerosis | Right anterior and midtemporal region | None | None | Deactivation over right mesial temporal region |
| 2 | 29 | Extra-FTLE | Right temporal atrophy | Right parietal region | None | None | Deactivation over right parietal region |
| 3 | 20 | Frontal | T2 signal abnormality over the left frontal lobe | Left frontotemporal region | None | Preceding deactivation inferior left frontal lobe | Focal deactivation inferior left frontal lobe |
| 4 | 23 | Frontal | Focal cortical dysplasia at right prerolandic region | Right frontocentral region | None | Preceding deactivation over posterior aspect of the right frontal lobe | Deactivation over posterior aspect of the right frontal lobe (perilesional) |
| 5 | 50 | Frontal | Low grade glioma at left frontal lobe | Left frontal region | Area of activation partially concordant to the deactivation | Preceding deactivation involving part of inferior region of the left fontal lobe | Focal deactivation seen inferior region of the left frontal lobe |
| 6 | 24 | Temporal | Normal | Left anterior temporal region | Preceding deactivation over left anterior temporal region | Preceding deactivation over the left anterior temporal region | Focal deactivation over the left anterior temporal region |
| 7 | 26 | Temporal | Periventricular band heterotopia | Left posterior temporal region | Focal activation over the left posterior temporal region (concordant) | None | Left posterior temporal deactivation |
| 8 | 30 | Frontal | Left hemimegalencephaly | Left frontocentral spikes | None | None | Focal deactivation over left parasagittal frontal region |
The distribution of the prespike and early prespike BOLD in these regions is shown.
FTLE, frontotemporal lobe epilepsy.
Patients 5 and 7 had a preceding activation in the congruent region of focal negative BOLD seen on the standard analysis (see Figs 2 and 3). In both cases these were seen in the early prespike maps. Patient 2 had a preceding activation, but this was not in a congruent region. Three patients (3, 4, and 6) had negative responses prior to the spikes (see Fig. 4). Three patients (1, 2, and 8) did not have any preceding responses in the region of spike field. There was no association between the spike frequencies to particular patterns of preceding BOLD responses (ANOVA, p > 0.05).
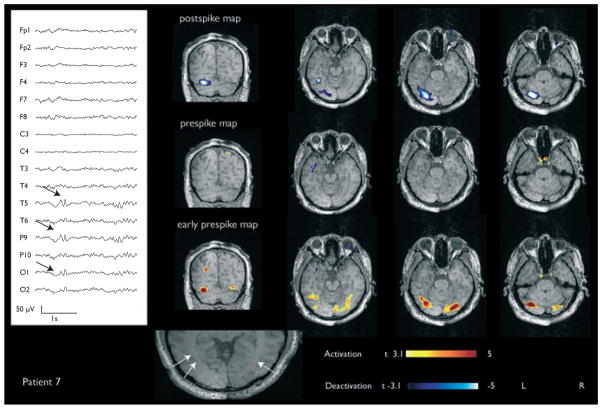
Figure 2.
Patient 7 with a left posterior temporal deactivation and congruous activation in the early prespike map. Periventricular band heterotopia was noted on structural MRI shown at the bottom indicated by white arrows.
Epilepsia © ILAE
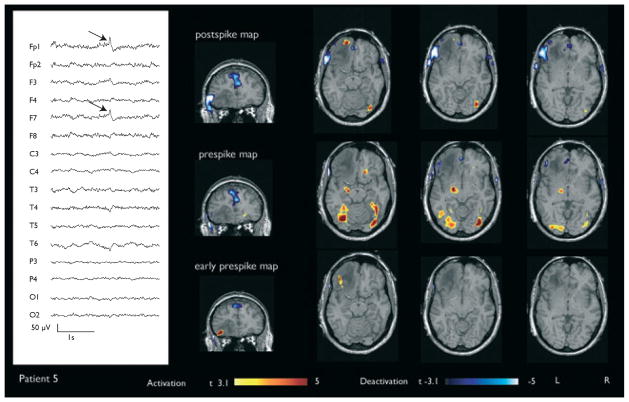
Figure 3.
Patient 5 with a focal left frontal deactivation (red arrows) and a preceding partially congruous activation in the early prespike map, visible notably in the coronal plane. Structural MRI revealed a lesion involving the left frontal lobe that was pathologically diagnosed to be a low grade glioma.
Epilepsia © ILAE
Figure 4.
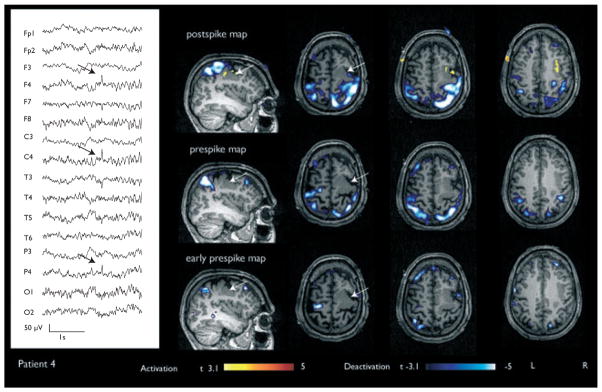
Patient 4 with a right frontal deactivation. A preceding deactivation was seen in the prespike map. Focal cortical dysplasia at the right frontal lobe was observed on MRI (indicated in white arrows).
Epilepsia © ILAE
In Patient 7, the t-statistics for deactivation and preceding activation were −5.6 and 5.0, respectively. Both were in congruous areas. In Patient 5, the maximum t-statistic for deactivation in the postspike analysis was −5.6 and for activation in the early prespike analysis, 5.3. The areas of maximum activation and deactivation were seen perilesionally and immediately adjacent to each other. No significant BOLD response was seen in the prespike map of Patient 7, whereas a small region of residual deactivation was seen in Patient 5.
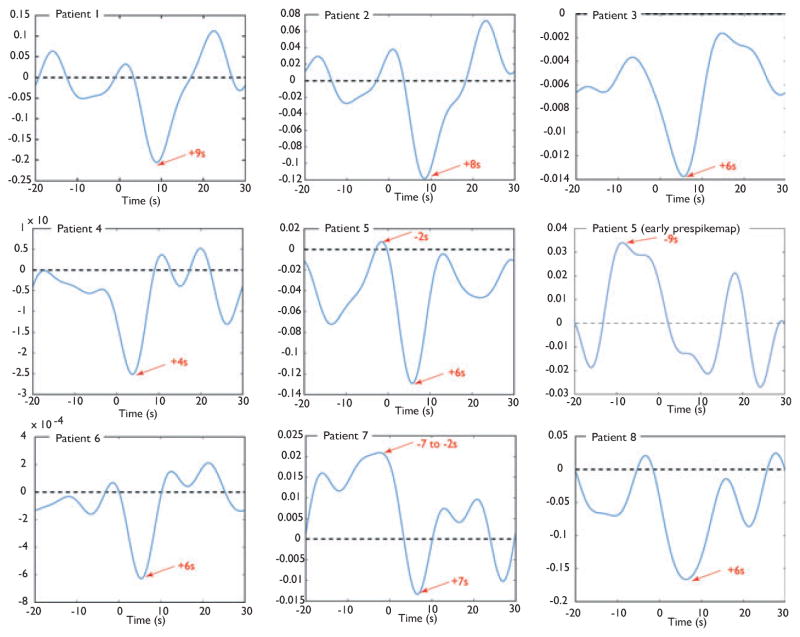
The plotted HRF confirmed the results seen in the maps: All HRFs plotted in the negative BOLD responses most concordant with the spike field in the postspike map showed a clear negative peak varying from 4 to 9 s after the spike (see Fig. 5). In patients in whom only deactivations in the early maps were found (Patients 3, 4, and 6) the decrease started before the onset of the spike. In Patient 7 the HRF showed a broad positive peak from −9 to −2 s, followed by a negative peak 7 s after the spike. This is in line with the early activation seen in the prespike map in the area of the negative BOLD response of the postspike map. For Patient 5, the HRF plotted in the negative BOLD response of the posts-pike map showed a positive peak 2 s before the onset, followed by a negative peak 6 s after the spike. Because the areas of activation in the early prespike map and the deactivation in the postspike map were adjacent to each other, the HRF of the early activation was plotted separately. This showed a broad positive peak starting 9 s before the spike followed by a negative peak 12 s after the spike.
Figure 5.
HRF plots of negative BOLD responses most concordant with the spike field in the postspike map. An HRF in the early prespike activation was also plotted in Patient 5 in whom the activation seen in the early prespike map was immediately adjacent to the deactivation in the postspike map. Peak times are indicated by red arrows. Please note that the amplitude scales of BOLD signal changes were adjusted to the HRF and vary from patient to patient.
Epilepsia © ILAE
Discussion
This study of 82 patients demonstrates that the occurrence of a focal deactivation in the region of the spike field in adults with focal epilepsies is an uncommon (9.5% of cases) yet consistent phenomenon. This is in accordance with other EEG-fMRI studies in focal epilepsies (Al-Asmi et al., 2003; Bagshaw et al., 2004; Kobayashi et al., 2005, 2006). An EEG-fMRI study at 3T in 11 children with symptomatic and idiopathic focal epilepsy revealed 27.2% incidence of focal deactivations in the spike fields (Jacobs et al., 2009). In another 3T study, similar deactivations were found in 36% of children with only symptomatic focal epilepsy (Jacobs et al., 2007). A clear reason for the higher incidence in younger patient populations has not been elucidated. The effects of age and the circumstances under which such scans are performed (usually under sedation and with predominant sleep activity) might play a contributory role (Jacobs et al., 2007).
The issues of negative BOLD responses and of changes prior to the interictal events are intertwined. Hemodynamic changes prior to interictal discharges on the scalp have been previously described both in animal studies (Mäkiranta et al., 2005; Brevard et al., 2006) and humans in fMRI and NIRS studies (Hawco et al., 2007; Moeller et al., 2008; Roche-Labarbe et al., 2008; Jacobs et al., 2009). However, the pathophysiologic mechanisms of this phenomenon are still insufficiently understood. It was postulated that the early BOLD responses might reflect preliminary processes occurring at the neuronal level that are invisible at the time of the scalp EEG (Mäkiranta et al., 2005). This hypothesis is plausible, as not all spike activity is visible on the scalp, especially if it originated in deep structures or involves only a small cortical area (Tao et al., 2005). In our study, there was partial overlap between the concordant areas of deactivation and preceding activation. It is possible that a metabolic change occurred before the epileptiform activity was detected on the surface. Jacobs et al. (2009) showed that the BOLD changes seen prior to the spike are not the result of subtle EEG changes that occurred in the seconds preceding the spike. Simultaneous EEG-fMRI using intracranial electrodes might be able to answer this question, however, this method is currently in preliminary stages (Cunningham et al., 2008).
Our study of BOLD responses prior to the spikes suggests that 25% of focal deactivations are preceded by earlier activations. We limited our analysis to HRFs peaking 9 s prior to the spike, since earlier studies have shown BOLD signal changes in this time window prior to the discharges (Hawco et al., 2007; Moeller et al., 2008; Jacobs et al., 2009). However, it cannot be excluded that BOLD signal changes even earlier than 9 s prior to the discharges might occur. In this study, the HRF plots using the Fourier method were used to confirm our findings based on analysis with gamma functions. Indeed, using the Fourier fits, positive peaks occurring earlier than −9 s were seen in Patients 1 and 2 (see Fig. 5). The significance of these is uncertain and merits further investigation.
Jacobs et al. (2009) demonstrated a preceding activation in all three of their cases that had a focal deactivation in the spike field. They proposed that the negative response may represent an undershoot of the earlier positive response occurring before the spike. This is an explanation for only a minority of cases in our study. There were no distinguishing clinical or EEG features between these patients and others in whom a preceding positive response was not observed. The disparity between the studies may be related to the different patient populations: Jacobs et al. studied children with idiopathic and symptomatic focal epilepsy. In adults with symptomatic and cryptogenic focal epilepsy as per our study, the question remains: What causes a focal deactivation in the epileptogenic area when it is not the undershoot of an earlier activation?
The areas of focal deactivations we observed were in the region of the spike field and did not contain any activation. In physiologic studies, a negative BOLD response has been correlated with neuronal inhibition and a decrease in neuronal activity. (Chatton et al., 2003; Shmuel et al., 2006). Extending this observation to our study, it would suggest that in some cases, decreases in neuronal activity occur within epileptogenic tissue. Whereas inhibition could be the synchronizing factor that gives rise to the discharge, the discharge itself consists of actively firing neurons resulting from synchronized excitation. It is, therefore, less likely that deactivations are the direct result of neuronal inhibition.
Structural changes may result in compromised local cerebral blood flow and possibly cause a decreased BOLD signal (Sakatani et al., 2007). The extent of the role of structural abnormalities contributing to focal spike-relevant deactivations in epilepsy is uncertain, although it does not appear to be the primary cause. In our study, focal regions of deactivation were observed despite relatively diffuse structural abnormalities (Patients 7 and 8). One patient had a normal anatomic scan (Patient 6). Furthermore, this phenomenon was also observed in idiopathic focal epilepsy, where characteristically there is no demonstrable anatomic lesion (Jacobs et al., 2009).
Another possible explanation involves the interpretation of the fMRI signal. A positive BOLD represents increased oxyhemoglobin content (Ogawa et al., 1992). This is a paradoxical result of a disproportionate increase in regional cerebral blood flow and volume relative to the oxygen demand (Raichle & Mintun, 2006). The opposite holds true for negative responses (Shmuel et al., 2006). Various hypotheses have been cited to explain negative BOLD responses, which include a dysregulated neurovascular coupling in abnormal tissue, a vascular steal phenomenon, or a primarily neuronal mechanism via a decrease in neuronal activity (Harel et al., 2002; Shmuel et al., 2006; Schridde et al., 2008). However, Schridde et al. (2008) demonstrated the occurrence of a sustained negative BOLD signal during increased neuronal activity. This is caused by a lesser degree of mismatch between cerebral blood flow and oxygen consumption, emphasizing that BOLD involves complex interactions among metabolic, hemodynamic, and neuronal parameters (Raichle & Mintun, 2006; Mangia et al., 2009). In addition, a true baseline in fMRI has not been identified, as BOLD does not remain constant (Raichle & Mintun, 2006; Shulman et al., 2007). What is visually observed as a focal change in BOLD is merely the deviation from an arbitrary “resting state” (Shulman et al., 2007). For these reasons, the physiologic correlate of a focal deactivation in epileptogenic tissue remains uncertain and caution should be exercised when interpreting such signals.
Conclusion
Focal EEG-fMRI deactivations concordant to the spike fields are an uncommon but consistent phenomenon in adults with focal epilepsy. These are explained by a preceding positive response in only a minority of cases. For the remainder, the explanation remains uncertain. However, recent discoveries related to neuronal metabolism and energetics suggest that these negative BOLD responses are more complex and should not be assumed to be simply an indicator of neuronal suppression. Further studies incorporating blood flow measurements and intracranial EEG within these regions may further our understanding of this phenomenon.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) grant MOP-38079. Pierre LeVan was supported by the Canadian National Science and Engineering Research Council (NSERC). We thank Natalja Zazubovits and Francesca Pittau for helping to collect and analyze the data.
Footnotes
Disclosure
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflict of interest to disclose.
References
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Aguirre G, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Al-Asmi A, Bénar CG, Gross DW, Khani YA, Andermann F, Pike B, Dubeau F, Gotman J. fMRI activation in continuous and spike-triggered EEG-fMRI studies of epileptic spikes. Epilepsia. 2003;44:1328–1339. doi: 10.1046/j.1528-1157.2003.01003.x. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12:230–239. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Aghakhani Y, Bénar CG, Kobayashi E, Hawco C, Dubeau F, Pike GB, Gotman J. EEG-fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolinium-enhanced MR angiograms. Hum Brain Mapp. 2004;22:179–192. doi: 10.1002/hbm.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw AP, Hawco C, Bénar CG, Kobayashi E, Aghakhani Y, Dubeau F, Pike GB, Gotman J. Analysis of the EEG-fMRI response to prolonged bursts of interictal epileptiform activity. Neuroimage. 2005;24:1099–1112. doi: 10.1016/j.neuroimage.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Bénar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J. The BOLD response to interictal epileptiform discharges. Neuroimage. 2002;17:1182–1192. doi: 10.1006/nimg.2002.1164. [DOI] [PubMed] [Google Scholar]
- Bénar C, Aghakhani Y, Wang Y, Izenberg A, Al-Asmi A, Dubeau F, Gotman J. Quality of EEG in simultaneous EEG-fMRI for epilepsy. Clin Neurophysiol. 2003;114:569–580. doi: 10.1016/s1388-2457(02)00383-8. [DOI] [PubMed] [Google Scholar]
- Brevard ME, Kulkarni P, King JA, Craig FF. Imaging the neuronal substrates involved in the genesis of Pentylenetetrazol-induced seizures. Epilepsia. 2006;47:745–754. doi: 10.1111/j.1528-1167.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci USA. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CJ, Badawy R, Zaamout MF, Jensen EJ, Pittman DJ, Good-year BG, Federico P. Successful integration of intracranial EEG and functional MRI at 3 Tesla. Epilepsia. 2008;49(suppl 7):390. [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Kobayashi E, Bagshaw AP, Bénar CG, Dubeau F. Combining EEG and fMRI: a multimodal tool for epilepsy research. J Magn Reson Imaging. 2006;23:906–920. doi: 10.1002/jmri.20577. [DOI] [PubMed] [Google Scholar]
- Gotman J. Epileptic networks studied with EEG-fMRI. Epilepsia. 2008;49(suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, Duncan JS, Lemieux L. EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31:1700–1710. doi: 10.1016/j.neuroimage.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22:908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Hawco CS, Bagshaw AP, Lu Y, Dubeau F, Gotman J. BOLD changes occur prior to epileptic spikes seen on scalp EEG. Neuroimage. 2007;35:1450–1458. doi: 10.1016/j.neuroimage.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kobayashi E, Boor R, Muhle H, Stephan W, Hawco C, Dubeau F, Jansen O, Stephani U, Gotman J, Siniatchkin M. Hemodynamic responses to interictal epileptiform discharges in children with symptomatic epilepsy. Epilepsia. 2007;48:2068–2078. doi: 10.1111/j.1528-1167.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Moeller F, Boor R, Stephani U, Gotman J, Siniatchkin M. Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG-fMRI. Neuroimage. 2009;45:1220–1231. doi: 10.1016/j.neuroimage.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston KJ. Event-related fMRI. Hum Brain Mapp. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kang JK, Bénar C, Al-Asmi A, Khani YA, Pike GB, Dubeau F, Gotman J. Using patient-specific hemodynamic response functions in combined EEG-fMRI studies in epilepsy. Neuroimage. 2003;20:1162–1170. doi: 10.1016/s1053-8119(03)00290-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Jansen A, Andermann F, Andermann E, Gotman J, Dubeau F. Intrinsic epileptogenicity in polymicrogyric cortex suggested by EEG-fMRI BOLD responses. Neurology. 2005;64:1263–1266. doi: 10.1212/01.WNL.0000154640.23656.A3. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp. 2006;27:488–497. doi: 10.1002/hbm.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow K, Lemieux L, Messina D, Scott CA, Symms MR, Duncan JS, Fish DR. Spatio-temporal imaging of focal interictal epileptiform activity using EEG-triggered functional MRI. Epileptic Disord. 2001;3:67–74. [PubMed] [Google Scholar]
- Laufs H, Duncan JS. Electroencephalography/functional MRI in human epilepsy: what it currently can and cannot do? Curr Opin Neurol. 2007;20:417–423. doi: 10.1097/WCO.0b013e3282202b92. [DOI] [PubMed] [Google Scholar]
- Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp. 2007;28:1023–1032. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkiranta M, Ruohonen J, Suominen K, Niinimäki J, Sonkajärvi E, Kiviniemi V, Seppänen T, Alahuhta S, Jäntti V, Tervonen O. BOLD signal increase precedes EEG spike activity – a dynamic penicillin induced focal epilepsy in deep anesthesia. Neuroimage. 2005;27:715–724. doi: 10.1016/j.neuroimage.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Mangia S, Giove F, TkQc I, Logothetis NK, Henry PG, Olman CA, Maraviglia B, Di Salle F, Ug urbil K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab. 2009;29:441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Ajmonemarsan C. Cellular mechanisms in experimental epileptic seizures. Science. 1964;10:193–194. doi: 10.1126/science.144.3615.193. [DOI] [PubMed] [Google Scholar]
- Menz M, Neumann J, Muller K, Zysset S. Variability of the BOLD response over time: an examination of within-session differences. Neuroimage. 2006;32:1185–1194. doi: 10.1016/j.neuroimage.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2008;39:1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Roche-Labarbe N, Zaaimi B, Berquin P, Nehlig A, Grebe R, Wallois F. NIRS-measured oxy- and deoxyhemoglobin changes associated with EEG spike-and-wave discharges in children. Epilepsia. 2008;49:1871–1880. doi: 10.1111/j.1528-1167.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Murata Y, Fujiwara N, Hoshino T, Nakamura S, Kano T, Katayama Y. Comparison of blood-oxygen-level-dependent functional magnetic resonance imaging and near-infrared spectroscopy recording during functional brain activation in patients with stroke and brain tumors. J Biomed Opt. 2007;12:062110. doi: 10.1117/1.2823036. [DOI] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex. 2008;18:1814–1827. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. Neuroimage. 2007;36:277–281. doi: 10.1016/j.neuroimage.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–676. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]