Abstract
The development of clinical therapeutics that interfere with the migration of leukocytes has revolutionized the treatment of multiple sclerosis and holds great promise for the treatment of a wide range of inflammatory diseases. As the molecules essential for the multi-step adhesion cascade that mediates cellular migration have been elucidated, the number of potential targets available to modulate leukocyte trafficking has increased exponentially. In this Viewpoint, we briefly review our current understanding of these molecular targets and how these targets vary by tissue and leukocyte subset with emphasis on T cells. We then describe the two currently approved therapeutics that target cell migration, natalizumab and fingolimod, and discuss how an improved understanding of their function could pave the way for the development of safer and more efficacious therapies for inflammatory and autoimmune diseases.
Introduction
Nearly 50 years ago Gowans and Knight published a seminal study demonstrating that labeled lymphocytes injected into rats migrated from the blood into secondary lymphoid organs (SLOs) and then returned to the circulation via the thoracic duct [1]. In an accompanying paper by Marchesi and Gowans, lymphocytes were observed to adhere to what are now called high endothelial venules (HEVs) and to pass through the endothelial layer in a directed migration into the lymph node [2]. This process was hypothesized to be selective, as only small lymphocytes emigrated from the venules while larger lymphocytes were excluded. In the time since these first observations were made, knowledge of the molecular mechanisms that underpin lymphocyte trafficking has exploded. The selective migration observed by Marchesi and Gowans is now understood to be a tightly orchestrated multi-step adhesion cascade, regulated by selectins, integrins, chemokines and chemoattractant lipids, that specifically directs the trafficking of leukocytes into sites essential for their function. Such an improved understanding of the underlying mechanisms involved has resulted in the identification of an array of potential drug targets aimed at modulating cell migration in order to treat a broad range of autoimmune and inflammatory diseases. Today, two drugs targeting cell migration are approved for clinical use in multiple sclerosis, one of which is also approved for Crohn’s disease; and many more are currently in clinical trial for these and other inflammatory diseases. In this Viewpoint, we will briefly discuss the wide range of molecular targets now recognized to inhibit leukocyte migration and our understanding that some of these targets may be unique to particular leukocyte subsets. We will then discuss two therapeutics that are currently in use for the inhibition of T-cell trafficking and how knowledge about their mechanism will inform the future development of drugs that target pathologic inflammation via the modulation of cell migration.
Sticky targets
The concept of a multi-step adhesion cascade responsible for leukocyte extravasation has been an extremely successful framework for contextualizing the large array of molecules that participate in cell migration [3, 4]. Currently the leukocyte adhesion cascade is understood as a process of four successive steps: leukocyte rolling along the endothelium, leukocyte activation, followed by adhesion onto endothelial cells and subsequent diapedesis into the target tissue [5]. The multi-step adhesion cascade is driven by an overlapping but sequential interaction of a diverse group of adhesion and chemoattractant molecules [6, 7]. The initial rolling step is mediated by the selectins, a three member family of C-type lectins, which bind with a high on/off rate to a wide range of sialylated carbohydrate ligands expressed on endothelial cells and the leukocytes themselves. This association then allows the circulating leukocyte to interact with regionally produced chemoattractant molecules. These chemoattractant molecules act to precisely control access of particular cell types to specific tissues and therefore are composed of a diverse group of lipids and chemokines that function in a combinatorial and likely non-redundant fashion in vivo [8].
Lipid chemoattractants include a relatively small number of eicosanoids, such as leukotriene B4, (LTB4) and prostaglandin D2 (PGD2), and have recently been shown to initiate early inflammatory cell migration via activation of G protein-coupled receptors (GPCRs) [9–11]. However, the most diverse group of chemoattractants is composed of the chemokines, which are a large group of over 50 secreted ligands. These interact with at least 20 members of the seven transmembrane spanning GPCR family to tightly regulate cell motility and adhesion under both resting and inflammatory conditions [12, 13]. During leukocyte rolling, the interaction of chemokines with their coordinate GPCRs then activates the circulating cell via an “inside-out” signal that changes the conformation of the integrins on the leukocyte surface from a low to high affinity state for its ligand [14]. Integrins are a family of heterodimers composed of a small number of α and β subunits that interact with both extracellular matrix molecules, such as fibronectin, and cell surface molecules of the immunoglobulin family, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) that are expressed in a variety of cell types, including endothelial cells, fibroblastic reticular cells, dendritic cells and lymphocytes [13, 15]. The high affinity integrin interaction with its ligands allows for the arrest and adhesion of the leukocyte on the endothelial cell – a process that is necessary for the subsequent trans-migration into the targeted tissue. Once leukocytes gain access to the appropriate tissue, they migrate to their particular targets along chemotactic or hapatotatic gradients [16]. Finally, at their target site, the retention of leukocytes in the tissue is tightly controlled and for T cells and dendritic cells, this process is regulated by the lysophospholipid shingosine 1-phosphate (S1P) and by the chemokine receptor CCR7 and its ligands CCL19 and CCL21 [17–20].
On T cells the differential expression of particular combinations of selectins, chemokine receptors and integrins on leukocytes is highly regulated and results in a directed trafficking of cellular subsets to particular organs and tissue beds. Naive T cells, for example, largely express the chemokine receptor CCR7 and the selectin CD62L, which directs them to circulate through the SLOs where they are more likely to have a productive interaction with antigen and antigen-presenting cells [13]. Once activated by antigen, the activated effector T cells upregulate the expression of chemokine receptors that correspond and can react to the chemokine ligands produced in inflamed tissues. For CD4+ T cells, the combination of chemokine receptors that are upregulated correlates with the cell-differentiation program upon activation. Thus, CXCR3 and CCR5 are preferentially upregulated on Th1 cells while Th2 cells preferentially express CRTH2, CCR4 and CCR8 [21]. The Th17 subset preferentially expresses CCR6 [22], and T follicular helper cells express CXCR5 [23, 24]. Memory T cells can be divided into CCR7+, CD62Lhi central memory T cells that circulate in the SLOs and CCR7−, CD62Llo effector memory T cells, which traffic to peripheral tissues [25]. Interestingly, amongst T effector memory cells there appears to be a difference in the expression of P and E selectins by CD4 and CD8 cells, resulting in further differences of localization and migration of these lymphocyte subsets within the memory population [26].
The site where antigen is encountered by the naive cell also affects the expression of chemokine receptors and integrins, “imprinting” them to return to particular tissue beds. This process has been best characterized for the gut and skin but also may occur in the CNS and lung [27]. In the mesenteric lymph nodes and GALT, for example, DC-produced retinoic acid induces the expression of CCR9 and the integrin α4β7 on effector memory T cells. As the ligands for CCR9 and α4β7 (CCL25 and MAdCAM-1, respectively) are mainly expressed on endothelial cells in the venules of the small intestine, these effector memory T cells then specifically home to the gut [28, 29]. In skin draining peripheral nodes, activated CD4+ T cells upregulate CLA, CCR4 and CCR10 and downregulate CCR9 and α4β7, resulting in preferential homing back to the dermis and epidermis. Interestingly, another vitamin, vitamin D3, has been found to control this homing in part through downregulation of the gut homing α4β7 integrin and upregulation of the epidermis-homing CCR10 [28, 30]. Thus, targeting particular chemokine receptors or integrins for pharmacologic blockade may allow for the selected modulation or inhibition of the migration of specific pathogenic subsets of T cells that traffic to an affected organ and cause disease. Despite some obstacles, this idea is quickly becoming reality as an array of drugs that inhibit or modulate cell migration are actively being studied in clinical trials (Table 1). Furthermore, two drugs, natalizumab and fingolimod, that target different aspects of T-cell migration (Figure 1), have already been approved for use in the clinic.
Table 1.
Status and indication of drugs currently in clinical trials that target molecules involved in cell migration.
| Target | Drugsa) | Company | Indicationb) | Status |
|---|---|---|---|---|
| CCR1 | ||||
| BX471 | Schering AG/Berlex | Pelvic pain | Phase 2 completed | |
| CCX354 | ChemoCentryx | RA | Phase 2 completed | |
| AZD-4818 | AstraZeneca | COPD | Phase 2 completed | |
| CCR2 | ||||
| PF-04634817 | Pfizer | Diabetic Nephropathy | Phase 2 recruiting | |
| PF-04136309 | Pfizer | Osteoarthritis Pancreatic cancer |
Phase 2 completed Phase 1 recruiting |
|
| MK-0812 | Merk | MS RA |
Phase 2 completed Phase 2 failed |
|
| CCX-140 | ChemoCentryx | Diabetic Nephropathy | Phase 2 recruiting | |
| CNTO-888 | Centocor | IPF | Phase 2 completed | |
| CCR3 | ||||
| Bertilimumab | Cambridge Antibody Technology | UC | Phase 2 planned | |
| GW766944 | GlaxoSmithKline | Asthma | Phase 2 completed | |
| CCR4 | ||||
| AMG-761 | Amgen | Asthma | Phase 1 recruiting | |
| CCR5 | ||||
| Maraviroc | Pfizer | HIV, immune reconstitution | Active | |
| GSK706769 | GlaxoSmithKline | RA | Phase 2 withdrawn | |
| HGS1025 | GlaxoSmithKline | UC | Phase 1 withdrawn | |
| CCR9 | ||||
| GSK 1605786A | GlaxoSmithKline | UC | Phase 3 recruiting | |
| CCX282-B | ChemoCentryx | UC Crohn’s Celiac |
Phase 2 completed Phase 2 completed Phase 2 completed |
|
| CXCR2 | ||||
| GSK 1325756 | GlaxoSmithKline | COPD | Phase 1 completed | |
| SB-6569333-AAA | GlaxoSmithKline | COPD CF |
Phase 1 completed Phase 1 completed |
|
| CXCR3 | ||||
| MSX1100 | Bristol-Meyer Squibb | RA UC |
Phase 2 completed Phase 2 completed |
|
| CXCR1/CXCR2 | ||||
| SCH 527123 | Schering-Plough | Psoriasis Asthma COPD |
Phase 2 completed Phase 2 completed Phase 2 completed |
|
| CRTH2 | ||||
| ARRY-502 | Array BioPharma | Asthma | Phase 2 recruiting | |
| QAV680 | Novartis | Allergic Rhinitis | Phase 2 completed | |
| QAW039 | Novartis | Asthma | Phase 2 recruiting | |
| ADC3680B | Pulmagen Therapeutics | Asthma | Phase 2 completed | |
| CRTH2 and DP1 | ||||
| AMG 853 | Amgen | Asthma | Phase 2 completed | |
| LTB4 | ||||
| CP-195543 | Pfizer | RA | Phase 2 terminated | |
| BIIL-284 | Boehringer Ingelheim | CF | Phase 2 terminated | |
| S1P1 | ||||
| GSK 2018682 | GlaxoSmithKline | MS | Phase 1 completed | |
| ACT-128800 | Actelion | MS Psoriasis |
Phase 2 ongoing Phase 2 completed |
|
| Selectins (E,P,L) | ||||
| Bimosiamose | Revotar | COPD Psoriasis |
Phase 2 completed Phase 2 completed |
|
| Integrin α4β7 | ||||
| Vedolizumab | Millennium | Crohn’s UC |
Phase 3 completed Phase 3 ongoing |
|
| AMG 181 | Amgen | UC | Phase 2 planned | |
| Integrin αLβ2 | ||||
| BMS-587101 | Bristol-Meyers Squibb | Psoriasis | Phase 2 terminated | |
| MIRT 2584 | Boehringer Ingelheim | Psoriasis | Phase 2 suspended |
This table does not list all compounds currently in development but instead summarizes drugs targeting the indicated molecules that are listed on www.clinicaltrials.gov.
RA: rheumatoid arthritis, COPD: chronic obstructive pulmonary disease, MS: multiple sclerosis, IPF: idiopathic pulmonary fibrosis, UC: ulcerative colitis, HIV: human immunodeficiency virus, CF: cystic fibrosis, SIP1: sphingosine-1 phosphate receptor subtype 1.
Figure 1.
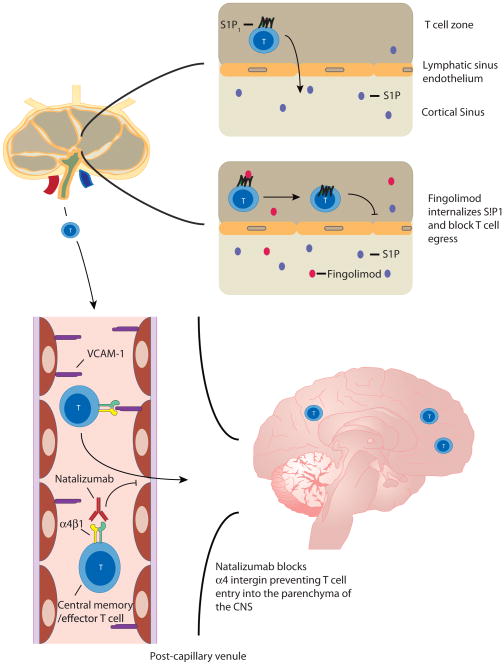
In MS patients, naive T cells are thought to enter the lymph node (LN) where they encounter auto-antigens resulting in differentiation and activation into encephalitogenic effector T cells. During the later phases of activation, T cells upregulate S1P1, which then mediates T-cell egress from the lymph node via migration towards the increased concentration of S1P present in the medullary sinus and efferent lymph (top right). Once these cells gain access to the circulation they then adhere to endothelial cells in the CNS via the interaction of the integrin α4β1 on T cells with VCAM-1 on the endothelial cell (bottom left). T cells then access the brain parenchyma where they become reactivated and secrete inflammatory cytokines and chemokines that recruit other effector cells resulting in the typical MS lesion in the white matter. In this sequence of events, fingolimod (red symbols) is thought to act via agonistic down regulation of the S1P1 receptor thereby blocking lymph node egress (top right) while natalizumab blocks the α4β1 integrin, effectively blocking the multi-step adhesion cascade and T-cell homing to the brain parenchyma (bottom left).
Natalizumab
In 1992, a mere twenty-eight years after Gowans and Knight first observed the trafficking of lymphocytes [1], the group of Steinman and Karin reported that blockade of the integrin α4β1 (VLA-4) with an antibody prevented experimental autoimmune encephalomyelitis (EAE), a rodent model of multiple sclerosis (MS)[31]. Using an in vitro binding assay that allowed for the adhesion of lymphocytes and monocytes to vessels in brain sections to be visualized, this group tested a panel of antibodies directed against various integrins known to participate in the multi-step adhesion cascade on brain sections from Lewis rats with EAE. They found that antibodies directed against the integrin subunits α4 or β1 prevented lymphocyte and monocyte binding. They then demonstrated that the development of paralysis caused by injection of a CD4+ T-cell clone specific for myelin basic protein could be prevented by blockade of α4 integrin (Figure 1) [31].
Based on these observations, a humanized monoclonal IgG4 antibody to α4 integrin called natalizumab (Tysabri, Biogen Idec and Elan Pharmaceuticals) was developed and tested in clinical trials. Phase III clinical trials with relapsing-remitting MS patients demonstrated that, compared with a placebo, natalizumab reduced the risk of sustained progression of disability by 42 percent and the annualized relapse rate by 68% [32], and resulted in a 54 percent reduction in annualized relapse rates when given with interferon-β [33]. After an interim one-year analysis of these trials, the FDA approved natalizumab in 2004 for relapsing forms of MS. Approval was also given for the short-term treatment of Crohn’s disease after it was demonstrated that some Crohn’s disease patients treated with natalizumab had higher remission rates, as compared with those patients given a placebo, an effect presumably driven by natalizumab’s ability to prevent leukocyte homing to the gut by blocking the α4β7 integrin [34]. However, as cases of the rare but deadly disease progressive multifocal encephalopathy (PML) were identified in both MS and Crohn’s patients taking natalizumab, the drug was pulled from the market for all patients in 2005 only three months after approval. PML is a demyelinating disease of the white matter and is caused by opportunistic infection with the JC virus in immunocompromised hosts [35]. A review of all patients who had been treated with natalizumab during clinical trials for MS, Crohns’ disease and rheumatoid arthritis estimated the risk to be 1:1000 for the development of PML while on the drug [36]. Given this low risk and proven benefits, the drug was re-introduced as a monotherapy for relapsing MS and Crohn’s disease in 2006 but the drug carries a black box warning and can only be prescribed in registered centers under the Tysabri Outreach: Unified Commitment to Health (TOUCH®) program [37]. More recently, an analysis of 212 confirmed cases of PML that have occurred in the postmarketing setting have identified the risk for development of PML in MS patients taking natalizumab and have stratified these risks based on seropositivity for JC virus, prior immunosuppressant use and duration of treatment with natalizumab greater than 2 years [38]. Using this risk stratification, the authors estimated that a negative anti-JC virus antibody status had a risk of development of PML at 0.09 per 1000 natalizumab treated patients while patients with all three risk factors had an estimated incidence of 11.1 per 1000. In addition to the infectious complications, there have also been case reports of patients who develop a severe worsening of MS after drug initiation [39]. The cause for this decline is currently unclear, but it is hoped that further study of these side effects will allow for the selection of only those patients who will safely benefit from natalizumab treatment.
Fingolimod
In the 1990s a fungal metabolite with immunosuppressive properties was identified from culture filtrates of the ascomycete Isaria sinclairii [40], and subsequently chemically modified to a less toxic molecule termed FTY720. This molecule was originally thought to be a “classic” immunosuppressant that modulated T and B cell activation as it was found to induce long term graft acceptance in animal transplant models in synergy with calcineurin inhibitors [41]. However the idea that FTY720 was a “classic” immunosuppressant was challenged by observations that FTY720 did not inhibit the activation or proliferation of T and B cells [42] and the lack of therapeutic benefit compared with standard therapy in phase III trials of renal transplant rejection [43, 44] FTY720’s mechanism of action became clear as studies demonstrated that FTY720 was an agonist of four out of the five known GPCRs for S1P, and it blocked lymphocyte egress from lymph nodes via downregulation and degradation of the S1P1 receptor on lymphocytes (Figure 1) [17, 45]. Understanding the function of FTY720 revealed the critical importance of S1P gradients in mediating lymphocyte egress from the lymph node. This concept has been reinforced by studies that have demonstrated that disruption of the S1P gradient by inhibiting either S1P generation or its degradation inhibits lymphocyte egress from the lymph node [46, 47].
As these discoveries came to light, the clinical effectiveness of FTY720 or fingolimod (Gilenya, Novartis) for the treatment of MS was studied in two large phase III clinical trials involving relapsing-remitting MS patients [48] [49]. Compared with a placebo, fingolimod decreased the annualized relapse rate by 54% [48], and when compared with interferon-β, fingolimod decreased the annualized relapse rate from 0.33 to 0.16 [49]. Thus, in September 2010 fingolimod was approved for use in patients with relapsing forms of MS. It should be noted that two deaths were reported in the trials [48], [49] but in patients taking a higher dose than that which is currently clinically approved. In one of these patients, disseminated primary varicella infection occurred during intravenous steroid treatment for relapse; in the other patient, herpes simplex encephalitis developed, also while the patient was on steroids. Other serious reported effects of fingolimod include bradycardia, a slight increase in lower respiratory tract infections, macular edema and a reported increase in the development of skin and breast cancers. More recently, as seen with natalizumab, cases of paradoxical worsening of MS [50], or tumefactive MS [51], have been reported after initiation of fingolimod although the cause of these rare events is still unclear. Furthermore there have been more recent reports of serious herpes infections in patients taking fingolimod at the clinically approved dose [52, 53], reinforcing the need for further surveillance of safety [54]. Thus, patients treated with fingolimod will be followed by a 5 year post-authorization safety study to monitor for adverse events [55].
Learning lessons and making them stick
Although the approval of natalizumab and fingolimod represents the successful targeting of molecules that modulate cell migration, the explosion of knowledge about other cell migration targets, such as the chemokine receptors, has thus far been challenging to translate into new clinical therapeutics. The reasons for these disappointing results are numerous and have been thoroughly reviewed elsewhere recently [8, 56], but likely include ‘redundancy’ of chemokine function, inadequate in vivo dosing, and the improper selection of targets as was suggested to have occurred in the clinical trials for CCR2 inhibition in rheumatoid arthritis [57]. We believe that an improved understanding of the mechanism and side effects of natalizumab and fingolimod will help address some of these obstacles. For instance, both of these drugs have highlighted the subtleties of modulating lymphocyte trafficking, such as only affecting particular subsets, subtleties that were not fully appreciated prior to their clinical approval.
Natalizumab, for instance, has been demonstrated to reduce the number of inflammatory cells in the cerebral spinal fluid (CSF) of patients with MS, suggesting that it may indeed prevent the access of pathogenic T cells to the brain in humans [58]. This reduction in inflammatory cells, however, is not global but appears to be relatively selective for particular leukocyte subsets. For example, the CD4+/CD8+ T-cell ratio is decreased in the CSF [59], DC numbers are decreased in the perivascular spaces [60] and peripheral CD19+ B cell and NK cell numbers are increased [61] in natalizumab-treated MS patients. In addition, recent animal data using the EAE model demonstrated that blockade of α4-integrin is selective for Th1 cells and does not prevent the accumulation of pathogenic Th17 cells in the brain during disease [62, 63]. As suggested by the authors of these studies, if confirmed in humans this finding would imply that the majority of patients who respond to natalizumab therapy likely have a Th1-mediated disease while patients who do not respond may have a predominately Th17-driven disease.
Fingolimod also appears to have differential effects on particular cellular subsets. For example, fingolimod selectively promotes the peripheral retention of naive and central memory cells while having less effect on the homing of effector memory T cells in MS patients [64]. In particular, it has been shown that Th17 cells form a significant part of the central memory pool and numbers of these cells are reduced in the blood of MS patients taking fingolimod [65]. Although there have been conflicting reports about the action of fingolimod on regulatory T (Treg) cells [66, 67], it has been reported in mice that fingolimod differentially effects the trafficking of Treg cells as compared with CD25− CD4+ T cells [68]. In contrast, it appears that natalizumab has minimal effects on Treg cells [69]. Given these differential effects on T-cell subsets, it is tempting to speculate that the paradoxical worsening of MS that can occasionally be seen in patients taking fingolimod or natalizumab may be secondary to an inhibition of trafficking of a beneficial T-cell type such as Treg cells to the MS lesions or to an alteration of the balance of Th1/Th17 cells in MS lesions; however, confirmation of this theory awaits further clinical study.
To sum up, the data obtained from studying the effects of natalizumab and fingolimod suggest that cell migration inhibitors may have very specific and differential effects on lymphocyte subsets that may be difficult to predict without further study. As more drugs that inhibit migration progress through clinical trials for diseases as diverse as COPD, asthma, rheumatoid arthritis, MS and Crohn’s, the reports of devastating infections in patients on natalizumab and fingolimod should also give us pause for thought. Somewhat surprisingly, current reports suggest that natalizumab and fingolimod each increase the risk of a specific but different type of infection - natalizumab increases the risk for PML [35] while fingolimod may be associated with a slightly increased risk for herpes infections, although this risk needs to be confirmed with further postmarketing surveillance [52, 53]. These observations should arouse caution when contemplating the inhibition of similar cellular subsets as those targeted by natalizumab and fingolimod. For instance, if it is confirmed that natalizumab selectively inhibits the accumulation of Th1 cells in the CNS of patients, then other cell migration inhibitors that target Th1 cells, such as inhibitors of CXCR3 and CCR5, should be carefully assessed for the risk of similar infectious complications, including the development of PML. Likewise, as fingolimod appears to selectively inhibit naive and central memory cells, including those cells differentiated into a Th17 subset, vigilance for similar infections to those observed for fingolimod - namely herpes infections - should be high when undertaking clinical trials of migration inhibitors that target these subsets.
Finally, the effects of these drugs beyond their modulation of cell migration add complexity to understanding the clinical response that they induce. For instance, natalizumab induces the release of immature CD34+ leukocytes from the bone marrow [70], impairs the ability of DCs to stimulate antigen-specific T-cell responses [71], and could potentially block VLA-4’s ability to synergize with TCR signaling to augment T-cell stimulation and proliferation [72, 73]. In contrast, fingolimod has effects on vascular permeability, mast cell activation, astrocyte susceptibility to apoptosis and cardiomyocyte function [74]. Teasing apart these effects from those affecting T-cell migration will be challenging but will nonetheless likely improve our understanding of the exact mechanisms of action of cell migration inhibitors proposed for therapeutic use.
Future Prospects
The successful clinical implementation of natalizumab and fingolimod provides proof that modulating cell migration is an effective means to modulate inflammation. The explosion of knowledge about the molecules that mediate the cell migration of leukocytes has resulted in a significant number of new targets that hold promise for new therapies [4, 56, 75]. However, as the drugs natalizumab and fingolimod demonstrate, we still need to refine our understanding of the molecules that are important for the trafficking of specific lymphocyte subsets in humans and how these subpopulations mediate disease and resistance to infection. As more drugs enter the pipeline, this knowledge should allow for a better prediction of clinical benefit and the possible infectious complications of treatment with cell migration inhibitors and allow for strategies to maximize clinical effectiveness while minimizing the risks of this promising class of drugs.
Acknowledgments
JWG was supported by an NHLBI/NIH T32 training grant and ADL was supported by grants from the NIAID and the NCI at the NIH.
Abbreviations
- GPCR
G protein-coupled receptor
- PML
progressive multifocal encephalopathy
- S1P
shingosine 1-phosphate
- SLO
secondary lymphoid organ
Footnotes
The authors declare no financial or commercial conflict of interest.
Literature Cited
- 1.Gowans JL, Knight EJ. Proc R Soc Lond B Biol Sci. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 2.Marchesi VT, Gowans JL. Proc R Soc Lond B Biol Sci. 1964;159:283–290. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 3.Butcher EC. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 4.Mackay CR. Nat Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- 5.Ley K, et al. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 6.Luster AD, et al. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 7.Springer TA. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 8.Schall TJ, Proudfoot AE. Nat Rev Immunol. 2011;11:355–363. doi: 10.1038/nri2972. [DOI] [PubMed] [Google Scholar]
- 9.Sadik CD, Luster AD. J Leukoc Biol. 2012;91:207–215. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tager AM, et al. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 11.Luster AD, Tager AM. Nat Rev Immunol. 2004;4:711–724. doi: 10.1038/nri1438. [DOI] [PubMed] [Google Scholar]
- 12.Schaerli P, Moser B. Immunol Res. 2005;31:57–74. doi: 10.1385/IR:31:1:57. [DOI] [PubMed] [Google Scholar]
- 13.Bromley SK, et al. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 14.Kinashi T. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 15.Springer TA. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 16.Friedl P, Weigelin B. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 17.Matloubian M, et al. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 18.Bromley SK, et al. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 19.Debes GF, et al. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forster R, et al. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 21.Syrbe U, et al. Springer Semin Immunopathol. 1999;21:263–285. doi: 10.1007/BF00812257. [DOI] [PubMed] [Google Scholar]
- 22.Acosta-Rodriguez EV, et al. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 23.Breitfeld D, et al. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaerli P, et al. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallusto F, et al. Nature. 1999;401:708–712. [Google Scholar]
- 26.Gebhardt T, et al. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 27.Agace WW. Nat Rev Immunol. 2006;6:682–692. doi: 10.1038/nri1869. [DOI] [PubMed] [Google Scholar]
- 28.Sigmundsdottir H, Butcher EC. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salmi M, Jalkanen S. Immunol Rev. 2005;206:100–113. doi: 10.1111/j.0105-2896.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 30.Kupper TS, Fuhlbrigge RC. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yednock TA, et al. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 32.Polman CH, et al. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 33.Rudick RA, et al. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, et al. N Engl J Med. 2003;348:24–32. [Google Scholar]
- 35.Major EO. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 36.Yousry TA, et al. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ransohoff RM. N Engl J Med. 2007;356:2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 38.Bloomgren G, et al. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 39.Rinaldi F, et al. Mult Scler. 2009;15:1359–1362. doi: 10.1177/1352458509107011. [DOI] [PubMed] [Google Scholar]
- 40.Fujita T, et al. J Antibiot. 1994;47:208–215. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S, et al. Transplantation. 1996;61:200–205. doi: 10.1097/00007890-199601270-00006. [DOI] [PubMed] [Google Scholar]
- 42.Brinkmann V, et al. Transplant P. 2001;33:530–531. doi: 10.1016/s0041-1345(00)02126-6. [DOI] [PubMed] [Google Scholar]
- 43.Hoitsma AJ, et al. Nephrol Dial Transpl. 2011;26:3802–3805. doi: 10.1093/ndt/gfr503. [DOI] [PubMed] [Google Scholar]
- 44.Tedesco-Silva H, et al. Transplantation. 2006;82:1689–1697. doi: 10.1097/01.tp.0000251718.95622.b3. [DOI] [PubMed] [Google Scholar]
- 45.Mandala S, et al. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 46.Pham TH, et al. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab SR, et al. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 48.Kappos L, et al. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 49.Cohen JA, et al. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 50.Centonze D, et al. Neurology. 2012;79:2004–2005. doi: 10.1212/WNL.0b013e3182735c7a. [DOI] [PubMed] [Google Scholar]
- 51.Visser F, et al. Neurology. 2012;79:2000–2003. doi: 10.1212/WNL.0b013e3182735cb3. [DOI] [PubMed] [Google Scholar]
- 52.Gross CM, et al. Neurology. 2012;79:2006–2007. doi: 10.1212/WNL.0b013e3182735d24. [DOI] [PubMed] [Google Scholar]
- 53.Ratchford JN, et al. Neurology. 2012;79:2002–2004. doi: 10.1212/WNL.0b013e3182735d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourdette D, Gilden D. Neurology. 2012;79:1942–1943. doi: 10.1212/WNL.0b013e3182735edf. [DOI] [PubMed] [Google Scholar]
- 55.Hohlfeld R, et al. Neurology. 2011;76:S28–37. doi: 10.1212/WNL.0b013e31820db40f. [DOI] [PubMed] [Google Scholar]
- 56.Allegretti M, et al. Immunol Lett. 2012;145:68–78. doi: 10.1016/j.imlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Proudfoot AE. Arthritis Rheum. 2008;58:1889–1891. doi: 10.1002/art.23590. [DOI] [PubMed] [Google Scholar]
- 58.Stuve O, et al. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 59.Stuve O, et al. Arch Neurol. 2006;63:1383–1387. doi: 10.1001/archneur.63.10.1383. [DOI] [PubMed] [Google Scholar]
- 60.del Pilar Martin M, et al. Arch Neurol. 2008;65:1596–1603. doi: 10.1001/archneur.65.12.noc80051. [DOI] [PubMed] [Google Scholar]
- 61.Harrer A, et al. PLoS One. 2012;7:e31784. doi: 10.1371/journal.pone.0031784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothhammer V, et al. J Exp Med. 2011;208:2465–2476. doi: 10.1084/jem.20110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glatigny S, et al. J Immunol. 2011;187:6176–6179. doi: 10.4049/jimmunol.1102515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehling M, et al. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 65.Mehling M, et al. Neurology. 2010;75:403–410. doi: 10.1212/WNL.0b013e3181ebdd64. [DOI] [PubMed] [Google Scholar]
- 66.Wolf AM, et al. J Immunol. 2009;183:3751–3760. doi: 10.4049/jimmunol.0901011. [DOI] [PubMed] [Google Scholar]
- 67.Daniel C, et al. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 68.Sawicka E, et al. J Immunol. 2005;175:7973–7980. doi: 10.4049/jimmunol.175.12.7973. [DOI] [PubMed] [Google Scholar]
- 69.Stenner MP, et al. PLoS One. 2008;3:e3319. doi: 10.1371/journal.pone.0003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zohren F, et al. Blood. 2008;111:3893–3895. doi: 10.1182/blood-2007-10-120329. [DOI] [PubMed] [Google Scholar]
- 71.de Andres C, et al. PLoS One. 2012;7:e34103. doi: 10.1371/journal.pone.0034103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dang NH, et al. J Exp Med. 1990;172:649–652. doi: 10.1084/jem.172.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato T, et al. J Immunol. 1995;155:2938–2947. [PubMed] [Google Scholar]
- 74.Rosen H, Goetzl EJ. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 75.Salmi M, Jalkanen S. Eur J Immunol. 2012;42:284–292. doi: 10.1002/eji.201142223. [DOI] [PubMed] [Google Scholar]