Abstract
Macrophages play a pivotal role in host defense against multiple foreign materials such as bacteria, parasites and artificial devices. Some macrophage lineage cells, namely osteoclasts and foreign body giant cells (FBGCs), form multi-nuclear giant cells by the cell–cell fusion of mono-nuclear cells. Osteoclasts are bone-resorbing cells, and are formed in the presence of RANKL on the surface of bones, while FBGCs are formed in the presence of IL-4 or IL-13 on foreign materials such as artificial joints, catheters and parasites. Recently, fusiogenic mechanisms and the molecules required for the cell–cell fusion of these macrophage lineage cells were, at least in part, clarified. Dendritic cell specific transmembrane protein (DC-STAMP) and osteoclast stimulatory transmembrane protein (OC-STAMP), both of which comprise seven transmembrane domains, are required for both osteoclast and FBGC cell–cell fusion. STAT6 was demonstrated to be required for the cell–cell fusion of FBGCs but not osteoclasts. In this review, advances in macrophage cell–cell fusion are discussed.
Keywords: DC-STAMP, FBGCs, IL-4, JAK, OC-STAMP, STAT1, STAT6, cell–cell fusion, macrophages, osteoclasts
Macrophage Giant Cells
Multinuclear macrophage giant cells were described by Evans et al. in 1914 as giant cells associated with tuberculosis.1 Multinuclear giant cells were also observed in association with tumors and foreign materials,2,3 and their multi-nucleation was considered to be the result of the cell–cell fusion of mono-nuclear cells rather than abnormal cell division with a lack of cytokinesis.3 The formation of multi-nuclear giant macrophages by cell–cell fusion was experimentally proved by Aronson and Elberg in 1962.4 Osteoclast cell–cell fusion was described by Jee, Nolan, and Tonna in 1963.5,6
Macrophage Fusion and Cytokines
Macrophage giant cell formation in vitro was attempted by Galindo in 1973.7 Galindo treated normal rabbit alveolar macrophages with supernatants of Bacillus Calmette–Guerin (BCG)-sensitized lymph node cells to promote multi-nuclear macrophage formation,7 and stated that macrophage fusion factor (MFF) was released from sensitized T cells upon stimulation with a specific antigen.8 In 1988, McInnes and Rennick reported that multi-nuclear macrophages were formed by IL-4,9 and that MFF was likely to be IL-4.
From then, multi-nuclear giant macrophage formation has been promoted by IL-4 or other cytokines such as IL-13 and their combination, such as IL-4,9 IL-13,10 GM-CSF plus IL-4, IL-3 plus IL-4, M-CSF plus IL-4, and M-CSF + IL-13.11-15
RANKL and Osteoclastogenesis
In vitro osteoclast formation was established by a co-culture system of osteoblastic and osteoclast progenitor cells.16 Osteoclasts were formed on the osteoblastic cells in the presence of osteotropic factors such as 1,25(OH)2D3, and the direct interaction of osteoblastic and osteoclast progenitor cells was reportedly required for osteoclast formation.16 Thus, some membrane-bound factors expressed in osteoblasts were considered to be required for osteoclast formation.
Osteoclast formation was reportedly negatively regulated by osteoclastogenesis inhibitory factor (OCIF)/osteoprotegerin (OPG), a soluble receptor belonging to the TNFα receptor superfamily.17,18 Since OCIF/OPG inhibited osteoclast formation by binding to 1,25(OH)2D3-treated osteoblastic cells, the membrane-bound osteoclastogenesis inducing factor expressed in osteoblastic cells was considered to be the ligand of OCIF/OPG.19 Indeed, the cytokine, which was required for osteoclastogenesis and was identified as the OCIF/OPG binding ligand: osteoclast differentiation factor, ODF, and osteoprotegerin ligand, OPGL,20,21 was a membrane-bound ligand belonging to the TNF superfamily, and the expression was stimulated upon 1,25(OH)2D3 treatment. ODF/OPGL was now called receptor activator of nuclear factor kappa B ligand (RANKL) since ODF/OPGL was identical to RANKL, which was identified before the cloning of ODF/OPGL.22 RANKL was found to be expressed in T cells and activate dendritic cells through its receptor, RANK, expressed in dendritic cells.23 Although RANKL was a membrane-bound ligand, as expected, the soluble form of RANKL also actively induced osteoclast differentiation, and osteoclasts were formed in the presence of the soluble form of RANKL and macrophage colony stimulating factor (M-CSF), which is also an essential cytokine for osteoclastogenesis,23 without osteoblastic cells.20
Identification of DC-STAMP: An Essential Cell–Cell Fusion Regulator of Macrophages and Osteoclasts
The identification of RANKL enabled us to screen for osteoclast-specific genes since osteoclasts were formed without osteoblastic cells. We further established a pure osteoclast culture system by culturing purified osteoclast progenitor cells in the presence of M-CSF and a soluble form of RANKL (hereafter termed RANKL).24-26 We found that osteoclast cell–cell fusion was promoted by RANKL stimulation,27 and thus, we tried to isolate osteoclast fusion molecules by subtractive screening between M-CSF + RANKL-induced multi-nuclear osteoclasts and M-CSF-induced mono-nuclear macrophages.13 We identified dendritic cell specific transmembrane protein (DC-STAMP), a seven transmembrane protein, as a highly expressed molecule in osteoclasts with this screening, and DC-STAMP was not expressed in M-CSF-treated mono-nuclear macrophages but was strongly upregulated by stimulation with RANKL.13 DC-STAMP was originally identified in dendritic cells as DC-STAMP and IL-4-stimulated macrophages as IL-4-induced (FIND), respectively.28,29 DC-STAMP was also identified in osteoclasts and implicated in osteoclast differentiation.30 We generated DC-STAMP-deficient mice, and found that they exhibited complete abrogation of multi-nuclear osteoclast formation in vivo and in vitro.13 Since tartrate resistance acid phosphatase (TRAP), a marker of osteoclasts, or other osteoclast differentiation markers such as Cathepsin K were equally expressed in DC-STAMP-deficient mono-nuclear osteoclasts as multi-nuclear wild-type osteoclasts, DC-STAMP was considered specifically required for osteoclast cell–cell fusion rather than differentiation.13 Osteoclast cell–cell fusion was reportedly promoted in heterogeneous osteoclast precursors expressing low and high levels of DC-STAMP.31 DC-STAMP expression in osteoclasts was promoted by nuclear factor of activated T cells 1 (NFATc1),13,32 an essential transcription factor for osteoclastogenesis.33 DC-STAMP was also demonstrated to be promoted by vitamin E-induced MITF, or tal1-PU.1/MITF pathway in osteoclasts.34,35 Similar to osteoclasts, multi-nuclear FBGC formation was also completely inhibited in DC-STAMP-deficient mice in vivo and in vitro; thus demonstrating that DC-STAMP was required for both osteoclast and FBGC cell–cell fusion.13 DC-STAMP expression in FBGCs was regulated by NFκB.13
Identification of OC-STAMP
Since DC-STAMP was identified as specifically expressed in RANKL-induced multi-nuclear osteoclasts but not in M-CSF-induced mono-nuclear macrophages, we overexpressed DC-STAMP in M-CSF-induced macrophages and analyzed whether cell–cell fusion was induced in DC-STAMP-overexpressed macrophages without RANKL.36 However, cell–cell fusion was not induced in DC-STAMP-overexpressed macrophages without RANKL, suggesting that some molecules other than DC-STAMP were required for osteoclast cell–cell fusion.36 Then, we tried to isolate the next molecules for osteoclast cell–cell fusion, and identified osteoclast stimulatory transmembrane protein (OC-STAMP) in osteoclasts.14 Similarly to DC-STAMP, OC-STAMP was a seven transmembrane protein, and was not expressed in M-CSF-treated macrophages but was strongly upregulated with RANKL stimulation.14 We generated OC-STAMP-deficient mice, and found that they exhibited complete abrogation of osteoclast cell–cell fusion in vivo and in vitro.14 Since osteoclast differentiation marker expression in OC-STAMP-deficient mono-nuclear osteoclasts was equivalent to those with wild-type multi-nuclear osteoclasts, OC-STAMP was considered specifically required for osteoclast cell–cell fusion rather than differentiation as DC-STAMP.14 OC-STAMP was also demonstrated to be required for FBGC cell–cell fusion.14 Recently, OC-STAMP antibody was demonstrated to inhibit osteoclast and FBGC cell–cell fusion.37 Thus, DC-STAMP and OC-STAMP were both required for osteoclast and FBGC cell–cell fusion.
STATs and Macrophage Cell–Cell Fusion
Signal transducer and activator of transcription (STAT) family molecules were found to be required for the transduction of cytokine signals. STATs consist of seven family members, STAT1–4, 5A, 5B, and 6, and each STAT has its own cytokines to transduce their signals. Among them, STAT6 plays a pivotal role in transducing signals of IL-4 and IL-13, both of which promote FBGC formation.9-15 Thus, STAT6 was suggested to play a role in macrophage cell–cell fusion. Indeed, Moreno et al. reported that STAT6 was required for FBGC cell–cell fusion, and that STAT6-deficient mice showed the marked inhibition of FBGC multi-nucleation.38 They demonstrated that expressions of DC-STAMP and E-cadherin were significantly inhibited in STAT6-deficient FBGCs.38 We also found that STAT6-deficient mice exhibited significant inhibition of multi-nuclear FBGC formation in vivo and in vitro, and the expressions of DC-STAMP and OC-STAMP were both significantly inhibited in STAT6-deficient FBGCs.15 Thus, DC-STAMP and OC-STAMP were considered the targets of IL-4-STAT6 signals.15 In addition, since FBGC formation was promoted in the presence of GM-CSF plus IL-4, we searched for activated molecules under stimulation of GM-CSF, and found that STAT1 was activated by GM-CSF.15 STAT1-deficient cells showed accelerated cell–cell fusion in FBGCs, suggesting that STAT1 was considered an inhibitor of FBGC multi-nucleation.15 STAT1 and STAT6 reportedly reciprocally regulate each other in T cells.39 Interestingly, STAT1 was strongly activated in STAT6-deficient FBGCs in the presence of GM-CSF + IL-4, indicating that STAT6 was the inhibitor of STAT1 in FBGCs, and that IL-4 induced STAT6 activation followed by STAT1 suppression was required for FBGC formation (Fig. 1).15 Indeed, STAT1-deficiency was sufficient to promote cell–cell fusion in FBGCs without IL-4.15 In contrast, neither STAT1 nor STAT6 were required for osteoclast cell–cell fusion.15
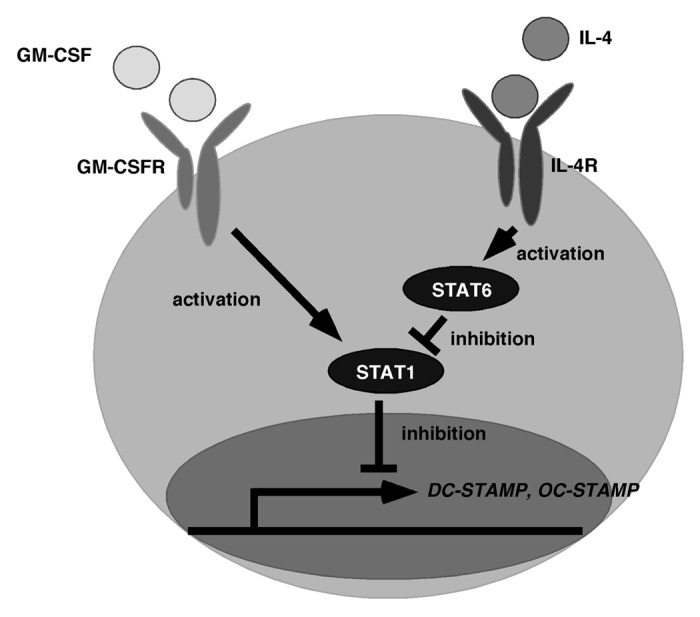
Figure 1. Schematic signaling for macrophage cell–cell fusion. GM-CSF activates STAT1 through the GM-CSF receptor (GM-CSFR), while IL-4 activates STAT6 through IL-4 receptor (IL-4R). Activation of STAT6 followed by STAT1 inhibition is required for the expression of DC-STAMP and OC-STAMP, both of which are essential molecules for macrophage and osteoclast cell–cell fusion. STAT6 is not involved in osteoclast cell–cell fusion.
Future Perspectives Regarding JAK-STAT and Macrophage Fusion
Various factors discussed below were identified and demonstrated to play a role in cell–cell fusion of osteoclasts or macrophages or both; however, their regulation by STATs was not fully demonstrated. Since IL-4/STAT6-STAT1 signals are specifically required for macrophage cell–cell fusion and DC-STAMP/OC-STAMP expression, the other molecules required for macrophage cell–cell fusion are likely to be regulated by STAT6-STAT1.
STAT6 is activated by IL-4 and IL-13, both of which promote macrophage fusion, via JAK1 and JAK3.40 Meanwhile, STAT1 is activated by interferon gamma via JAK1 and JAK2.40 JAK1-deficient mice exhibited impaired lymphoid development,41 while JAK2-deificient mice presented with no definitive erythropoiesis.42,43 JAK3-deficient mice exhibited defective lymphoid development and dysregulated myelopoiesis.44-46 However, the roles of JAKs in macrophage fusion were not demonstrated. The molecules demonstrated to play a role in osteoclast or macrophage cell–cell fusion are listed in Tables 1, 2, and 3. It is still possible that other STATs contribute to osteoclast and macrophage cell–cell fusion, and further studies will uncover the molecular mechanisms of osteoclast and macrophage cell–cell fusion by STATs.
Table 1. Putative regulators for both osteoclast and macrophage cell–cell fusion.
| Molecule | Osteoclast fusion | Macrophage fusion | Materials used | References |
|---|---|---|---|---|
| DC-STAMP |
Complete inhibition |
Complete inhibition |
DC-STAMP KO |
13 |
| OC-STAMP |
Complete inhibition |
Complete inhibition |
OC-STAMP KO |
14 |
| Meltin α (ADAM12) |
70% inhibition |
50% inhibition |
Anti-sense oligo |
34 |
| Atp6v0d2 | Defective | Severely impaired | Atp6v0d2 KO | 35 |
Table 2. Putative regulators for both osteoclast cell–cell fusion.
Table 3. Putative regulators for both osteoclast and macrophage cell–cell fusion.
| Molecule | Macrophage fusion | Materials used | References |
|---|---|---|---|
| MCP1 (CCL2) |
Reduction |
CCL2 KO CCL2 inhibitory peptide Anti-CCL2 Ab |
36 |
| LFA1 |
Inhibited (IFNγ induced macrophage fusion) |
Monoclonal Ab against LFA1 |
45 |
| ICAM1 |
Inhibited (IFNγ induced macrophage fusion) |
Monoclonal Ab against ICAM1 |
45 |
| MFR (SHPS1) |
Inhibit Prevent |
Monoclonal Ab against MFR Extracellular domain of MFR |
46 47 |
| CD47 |
Prevent |
GST-extracellular domain of CD47 Monoclonal Ab against CD47 |
48 |
| DAP12 |
Impaired |
DAP12 DAP12 KI (loss of function) DAP12 RNAi |
49 |
| TREM2 |
Severely impaired |
TREAM2 siRNA |
49 |
| Syk |
Reduced |
Syk KO |
49 |
| MMP9 |
Reduced Delayed shape change and abnormal morphology |
Function-blocking Ab MMP9-null |
50 |
| CD36 |
Severely impaired (CD36 is not involved in osteoclast fusion) |
CD36 KO |
51 |
| Rac1 | Attenuated | Inhibitor of Rac activation (NSC23766) Rac1 siRNA |
52 |
Molecules for both osteoclast and macrophage cell–cell fusion
Meltrin-α, also called A disintegrin and metalloprotease 12 (ADAM12), was demonstrated to play a role in both osteoclast and macrophage cell–cell fusion by using anti-sense oligo.47 The d2 isoform of vacuolar (H[+]) ATPase (v-ATPase) V(0) domain (Atp6v0d2) was demonstrated to play a role in cell–cell fusion of both osteoclasts and FBGCs, and Atp6v0d2-deficient cells showed marked inhibition of osteoclast and FBGC cell–cell fusion.48 Monocyte chemoattractant protein 1 (MCP1, also called chemokine C–C motif ligand 2, CCL2) and its receptor chemokine C–C motif receptor 2 (CCR2) were implicated in macrophage and osteoclast cell–cell fusion.49 Meanwhile, MCP1/CCR2 was demonstrated to play a role in osteoclast differentiation rather than cell–cell fusion.50-52 ADAM8 was reportedly highly expressed in osteoclasts, and overexpression of ADAM8 in transgenic mice under a control of TRAP-promoter resulted in increased multi-nucleation of osteoclasts and bone loss.53
Molecules for osteoclast cell–cell fusion
E-cadherin, intercellular adhesion molecule-1 (ITAM1), and leukocyte function-associated antigen-1 (LFA1) were demonstrated to be involved in osteoclast cell–cell fusion and maturation by using a neutralizing antibody.54-56 CD200-deficient mice exhibited inhibition of osteoclast cell–cell fusion and an increased bone mass.57 SH3 and PX domains 2A (SH3PXD2A, also called Tks5), a substrate of c-Src, was demonstrated to play a role in osteoclast cell–cell fusion downstream of phosphoinositide 3-kinase and Src.58
Molecules for macrophage cell–cell fusion
Interferon-gamma (IFNγ) and intercellular adhesion molecule-1 (ICAM1) were reportedly involved in macrophage cell–cell fusion, and monoclonal antibodies against LFA1 or ICAM-1 inhibited the multi-nuclear macrophage formation.59 Macrophage fusion receptor (MFR: also called SHPS-1), belonging to the immunoglobulin (Ig) superfamily, was highly expressed in macrophages at the stage of cell–cell fusion, and monoclonal antibodies against MFR or the soluble form of the extracellular domain of MFR blocked the cell–cell fusion of macrophages.60,61 CD47 is a ligand of MFR, and it was also implicated in macrophage cell–cell fusion.62 DNAX activating protein of 12 kD (DAP12), DAP12 associated receptor triggering receptor expressed by myeloid cells 2 (TREM2), and the downstream signaling molecule Syk were shown to be required for IL-4-induced macrophage cell–cell fusion by using DAP12-knockin and knockout mice, Syk-knockout mice and RNAi against DAP12 and TREM2.63 Matrix metalloprotease 9 (MMP9) and scavenger receptor CD36 were shown to play a role in macrophage cell–cell fusion by using their blocking antibodies and MMP9- or CD36-deficient mice.64,65 Rac inhibitor NSC23766 or Rac1 knockdown by siRNA resulted in attenuation of macrophage cell–cell fusion.66
Acknowledgment
The author declares no competing financial interests.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/24777
References
- 1.Evans HM, Bowman FB, Winternitz MC. An experimental study of the histogenesis of the military tubercle in vitally stained rabbits. J Exp Med. 1914;19:283–302. doi: 10.1084/jem.19.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto T, Suda T. Molecules regulating macrophage fusions. Cell Fusions: Regulation and control. Springer, 2010; 233-48. [Google Scholar]
- 3.Forkner CE. The origin and fate of two types of multi-nucleated giant cell in the circulating blood. J Exp Med. 1930;52:279–97. doi: 10.1084/jem.52.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson M, Elberg SS. Fusion of peritoneal histocytes with formation of giant cells. Nature. 1962;193:399–400. doi: 10.1038/193399a0. [DOI] [PubMed] [Google Scholar]
- 5.Jee WS, Nolan PD. Origin of osteoclasts from the fusion of phagocytes. Nature. 1963;200:225–6. doi: 10.1038/200225a0. [DOI] [PubMed] [Google Scholar]
- 6.Tonna EA. Origin of osteoclasts from the fusion of phagocytes. Nature. 1963;200:226–7. doi: 10.1038/200226a0. [DOI] [PubMed] [Google Scholar]
- 7.Galindo BJ. Antigen-mediated fusion of specifically sensitized rabbit alveolar macrophages. Infect Immun. 1972;5:583–94. doi: 10.1128/iai.5.4.583-594.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galindo B, Lazdins J, Castillo R. Fusion of normal rabbit alveolar macrophages induced by supernatant fluids from BCG-sensitized lymph node cells after elicitation by antigen. Infect Immun. 1974;9:212–6. doi: 10.1128/iai.9.2.212-216.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McInnes A, Rennick DM. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988;167:598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158:3385–90. [PubMed] [Google Scholar]
- 11.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147:1487–99. [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda T, Ikeda K, Sasaki K, Kawakami K, Hatake K, Kaji Y, et al. IL-13 as well as IL-4 induces monocytes/macrophages and a monoblastic cell line (UG3) to differentiate into multinucleated giant cells in the presence of M-CSF. Biochem Biophys Res Commun. 1998;253:265–72. doi: 10.1006/bbrc.1998.9702. [DOI] [PubMed] [Google Scholar]
- 13.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005;202:345–51. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto H, Suzuki T, Miyauchi Y, Iwasaki R, Kobayashi T, Sato Y, et al. Osteoclast stimulatory transmembrane protein and dendritic cell–specific transmembrane protein cooperatively modulate cell–cell fusion to form osteoclasts and foreign body giant cells. J Bone Miner Res. 2012;27:1289–97. doi: 10.1002/jbmr.1575. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto H, Katsuyama E, Miyauchi Y, Hoshi H, Miyamoto K, Sato Y, et al. An essential role for STAT6-STAT1 protein signaling in promoting macrophage cell-cell fusion. J Biol Chem. 2012;287:32479–84. doi: 10.1074/jbc.M112.358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, et al. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, et al. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–42. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 18.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–37. doi: 10.1210/en.139.3.1329. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 22.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 24.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto T, Arai F, Ohneda O, Takagi K, Anderson DM, Suda T. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand. Blood. 2000;96:4335–43. [PubMed] [Google Scholar]
- 26.Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, et al. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001;98:2544–54. doi: 10.1182/blood.V98.8.2544. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto K, Miyamoto T, Sawatani Y, Hosogane N, Hamaguchi I, Takami M, et al. Dimer formation of receptor activator of nuclear factor kappaB induces incomplete osteoclast formation. Biochem Biophys Res Commun. 2004;325:229–34. doi: 10.1016/j.bbrc.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Hartgers FC, Vissers JL, Looman MW, van Zoelen C, Huffine C, Figdor CG, et al. DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur J Immunol. 2000;30:3585–90. doi: 10.1002/1521-4141(200012)30:12<3585::AID-IMMU3585>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Staege H, Brauchlin A, Schoedon G, Schaffner A. Two novel genes FIND and LIND differentially expressed in deactivated and Listeria-infected human macrophages. Immunogenetics. 2001;53:105–13. doi: 10.1007/s002510100306. [DOI] [PubMed] [Google Scholar]
- 30.Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004;200:941–6. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mensah KA, Ritchlin CT, Schwarz EM. RANKL induces heterogeneous DC-STAMP(lo) and DC-STAMP(hi) osteoclast precursors of which the DC-STAMP(lo) precursors are the master fusogens. J Cell Physiol. 2010;223:76–83. doi: 10.1002/jcp.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP) Mol Endocrinol. 2008;22:176–85. doi: 10.1210/me.2007-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 34.Courtial N, Smink JJ, Kuvardina ON, Leutz A, Göthert JR, Lausen J. Tal1 regulates osteoclast differentiation through suppression of the master regulator of cell fusion DC-STAMP. FASEB J. 2012;26:523–32. doi: 10.1096/fj.11-190850. [DOI] [PubMed] [Google Scholar]
- 35.Fujita K, Iwasaki M, Ochi H, Fukuda T, Ma C, Miyamoto T, et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat Med. 2012;18:589–94. doi: 10.1038/nm.2659. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki R, Ninomiya K, Miyamoto K, Suzuki T, Sato Y, Kawana H, et al. Cell fusion in osteoclasts plays a critical role in controlling bone mass and osteoblastic activity. Biochem Biophys Res Commun. 2008;377:899–904. doi: 10.1016/j.bbrc.2008.10.076. [DOI] [PubMed] [Google Scholar]
- 37.Khan UA, Hashimi SM, Bakr MS, Forwood MR, Morrison NA. Foreign body giant cells and osteoclasts are TRAP positive, have podosome-belts and both require OC-STAMP for cell fusion. J Cell Biochem. 2013;114:1772–8. doi: 10.1002/jcb.24518. [DOI] [PubMed] [Google Scholar]
- 38.Moreno JL, Mikhailenko I, Tondravi MM, Keegan AD. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J Leukoc Biol. 2007;82:1542–53. doi: 10.1189/jlb.0107058. [DOI] [PubMed] [Google Scholar]
- 39.Yu CR, Mahdi RM, Ebong S, Vistica BP, Chen J, Guo Y, et al. Cell proliferation and STAT6 pathways are negatively regulated in T cells by STAT1 and suppressors of cytokine signaling. J Immunol. 2004;173:737–46. doi: 10.4049/jimmunol.173.2.737. [DOI] [PubMed] [Google Scholar]
- 40.Ward AC, Touw I, Yoshimura A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood. 2000;95:19–29. [PubMed] [Google Scholar]
- 41.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83. doi: 10.1016/S0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 42.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–95. doi: 10.1016/S0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 43.Neubauer H, Cumano A, Müller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/S0092-8674(00)81168-X. [DOI] [PubMed] [Google Scholar]
- 44.Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, et al. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–2. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 45.Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–82. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 46.Grossman WJ, Verbsky JW, Yang L, Berg LJ, Fields LE, Chaplin DD, et al. Dysregulated myelopoiesis in mice lacking Jak3. Blood. 1999;94:932–9. [PubMed] [Google Scholar]
- 47.Abe E, Mocharla H, Yamate T, Taguchi Y, Manolagas SC. Meltrin-alpha, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif Tissue Int. 1999;64:508–15. doi: 10.1007/s002239900641. [DOI] [PubMed] [Google Scholar]
- 48.Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403–9. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- 49.Kyriakides TR, Foster MJ, Keeney GE, Tsai A, Giachelli CM, Clark-Lewis I, et al. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–66. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MS, Day CJ, Selinger CI, Magno CL, Stephens SR, Morrison NA. MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption. J Biol Chem. 2006;281:1274–85. doi: 10.1074/jbc.M510156200. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto K, Ninomiya K, Sonoda KH, Miyauchi Y, Hoshi H, Iwasaki R, et al. MCP-1 expressed by osteoclasts stimulates osteoclastogenesis in an autocrine/paracrine manner. Biochem Biophys Res Commun. 2009;383:373–7. doi: 10.1016/j.bbrc.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, et al. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15:417–24. doi: 10.1038/nm.1945. [DOI] [PubMed] [Google Scholar]
- 53.Ishizuka H, García-Palacios V, Lu G, Subler MA, Zhang H, Boykin CS, et al. ADAM8 enhances osteoclast precursor fusion and osteoclast formation in vitro and in vivo. J Bone Miner Res. 2011;26:169–81. doi: 10.1002/jbmr.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mbalaviele G, Chen H, Boyce BF, Mundy GR, Yoneda T. The role of cadherin in the generation of multinucleated osteoclasts from mononuclear precursors in murine marrow. J Clin Invest. 1995;95:2757–65. doi: 10.1172/JCI117979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okada Y, Morimoto I, Ura K, Watanabe K, Eto S, Kumegawa M, et al. Cell-to-Cell adhesion via intercellular adhesion molecule-1 and leukocyte function-associated antigen-1 pathway is involved in 1alpha,25(OH)2D3, PTH and IL-1alpha-induced osteoclast differentiation and bone resorption. Endocr J. 2002;49:483–95. doi: 10.1507/endocrj.49.483. [DOI] [PubMed] [Google Scholar]
- 56.Möst J, Neumayer HP, Dierich MP. Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-gamma and expression of LFA-1. Eur J Immunol. 1990;20:1661–7. doi: 10.1002/eji.1830200807. [DOI] [PubMed] [Google Scholar]
- 57.Cui W, Cuartas E, Ke J, Zhang Q, Einarsson HB, Sedgwick JD, et al. CD200 and its receptor, CD200R, modulate bone mass via the differentiation of osteoclasts. Proc Natl Acad Sci U S A. 2007;104:14436–41. doi: 10.1073/pnas.0702811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oikawa T, Oyama M, Kozuka-Hata H, Uehara S, Udagawa N, Saya H, et al. Tks5-dependent formation of circumferential podosomes/invadopodia mediates cell-cell fusion. J Cell Biol. 2012;197:553–68. doi: 10.1083/jcb.201111116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fais S, Burgio VL, Silvestri M, Capobianchi MR, Pacchiarotti A, Pallone F. Multinucleated giant cells generation induced by interferon-gamma. Changes in the expression and distribution of the intercellular adhesion molecule-1 during macrophages fusion and multinucleated giant cell formation. Lab Invest. 1994;71:737–44. [PubMed] [Google Scholar]
- 60.Saginario C, Qian HY, Vignery A. Identification of an inducible surface molecule specific to fusing macrophages. Proc Natl Acad Sci U S A. 1995;92:12210–4. doi: 10.1073/pnas.92.26.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E, et al. MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol. 1998;18:6213–23. doi: 10.1128/mcb.18.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, et al. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem. 2000;275:37984–92. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- 63.Helming L, Tomasello E, Kyriakides TR, Martinez FO, Takai T, Gordon S, et al. Essential role of DAP12 signaling in macrophage programming into a fusion-competent state. Sci Signal. 2008;1:ra11. doi: 10.1126/scisignal.1159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacLauchlan S, Skokos EA, Meznarich N, Zhu DH, Raoof S, Shipley JM, et al. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J Leukoc Biol. 2009;85:617–26. doi: 10.1189/jlb.1008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helming L, Winter J, Gordon S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J Cell Sci. 2009;122:453–9. doi: 10.1242/jcs.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jay SM, Skokos E, Laiwalla F, Krady MM, Kyriakides TR. Foreign body giant cell formation is preceded by lamellipodia formation and can be attenuated by inhibition of Rac1 activation. Am J Pathol. 2007;171:632–40. doi: 10.2353/ajpath.2007.061213. [DOI] [PMC free article] [PubMed] [Google Scholar]


