Abstract
JAK-STAT signaling is a highly conserved regulator of stem cells and their niches. Aberrant activation in hematopoietic stem cells is the underlying cause of a majority of myeloproliferative diseases. This review will focus on the roles of JAK-STAT activity in three different adult stem cell systems in Drosophila. Tightly controlled levels of JAK-STAT signaling are required for stem cell maintenance and self-renewal, as hyperactivation of the pathway is associated with stem cell overproliferation. JAK-STAT activity is further essential for anchoring the stem cells in their respective niches by regulating different adhesion molecules.
Keywords: JAK-STAT, stem cells, stem cell niche, intestinal stem cells, ovary, testis, midgut
Introduction
The JAK-STAT signaling pathway is highly conserved from flies to mammals, and plays essential roles during development and cellular processes such as segmentation, proliferation and organogenesis. In addition to these roles, the JAK-STAT pathway is also a crucial regulator of stem cells and their niches, with Drosophila having emerged as an exceptional model system to study the relationship between stem cells and their microenvironments. The unique architecture and accessibility of larval and adult stem-cell bearing tissues, combined with the advantage of precise genetic manipulation allow the study of cell–cell interactions at the highest resolution, without losing the context of the whole tissue or organism. A recent example showcasing the exceptional potential of Drosophila for stem cell research provided novel insights into the chicken and egg dilemma of what comes first—a stem cell or a niche? Mathur et al. were able to demonstrate that in the developing midgut, a stem cell progenitor gives rise to both a stem cell and a niche cell, a discovery which provides the first paradigm for the origin of stem cell niches.1
Adult stem cells are essential for tissue homeostasis and regeneration, and are promising candidates for therapeutic approaches for degenerative diseases, myocardial infarction, and hematological malignancies. Their ability to both self-renew as well as to differentiate into a restricted number of lineages depends on their stem cell niche, which provides them with structural support and signaling cues for cell fate decisions. Importantly, it has become clear that cancer stem cells depend on their niches as much as non-pathological stem cells.2 An especially exciting new view has emerged from the understanding that motile cancer cells need a supportive niche for successful metastasis.3 A better understanding of stem cells and their niches is therefore essential for clinical advancement and safety.
This review will focus on three stem cell regions—testis, ovary, and the intestine. We will discuss recent findings of novel mechanisms of JAK-STAT signaling, as well as its interactions with other signaling pathways, an important aspect to understand the wider implications of changes in pathway activity.
JAK-STAT Signaling
In contrast to the mammalian system, Drosophila offers a low complexity version of the JAK-STAT pathway, in which all components downstream of the pathway ligand are present in a single copy. The Drosophila ligands Unpaired (Upd), Upd2 and Upd3 are partially redundant, but also provide functional and tissue specificity, marked by their partly overlapping, partly independent expression patterns.4,5 Once a ligand binds to the receptor Domeless (Dome), signaling is triggered by activation of the associated JAK Hopscotch (Hop), which recruits and phosphorylates the transcription factor STAT92E. Subsequent dimerization of STAT92E allows translocation into the nucleus, where dimers bind to defined STAT92E binding sites and activate target gene expression. All steps of the pathway are tightly regulated, as aberrant pathway activity leads to developmental and hematopoietic defects in flies and mammals, and is associated with cancer and leukemia in humans. In addition, pathway targets such as protein tyrosine phosphatase at 61F (Ptp61F) and suppressor of cytokine signaling at 36E (Socs36E) activate regulatory feedback loops.6-8
Drosophila Stem Cells and Their Niches
Stem cells are unique in their ability to divide symmetrically or asymmetrically. Symmetric division leads to self-renewal, as two new stem cells are produced, which ensures maintenance of the stem cell pool and replenishment if necessary. Asymmetric division produces a stem cell and a daughter cell committed to differentiation, thus ensuring tissue homeostasis. Stem cells therefore have a theoretically limitless capacity to continuously replace cells lost from the tissue, while maintaining their own population, which is essential for regeneration and repair.
Adult stem cells, which are usually more restricted in their potency to produce different lineages, promise great therapeutic potential after initial clinical applications.9 Work in Drosophila has been instrumental to the discovery that many stem cells reside in and rely on a special microenvironment, the stem cell niche. The niche is comprised of a group of somatic cells, which provide structural support and specific cues for recruitment, development and maintenance of stem cells. Moreover, niche cells are in active signaling communication with stem cells, often contributing to cell fate choice after stem cell division. However, whereas the niche is clearly defined in some cases, like the testis hub, its identification and function in other tissues remains unclear. By contrast to the Drosophila system, mammalian stem cell niches appear more complex, diverse, and dispersed than their Drosophila counterparts, making the fly an excellent model to understand the underlying characteristics of the stem cell niches.10,11
The Hub: Stem Cell Meeting Point in the Drosophila Testis
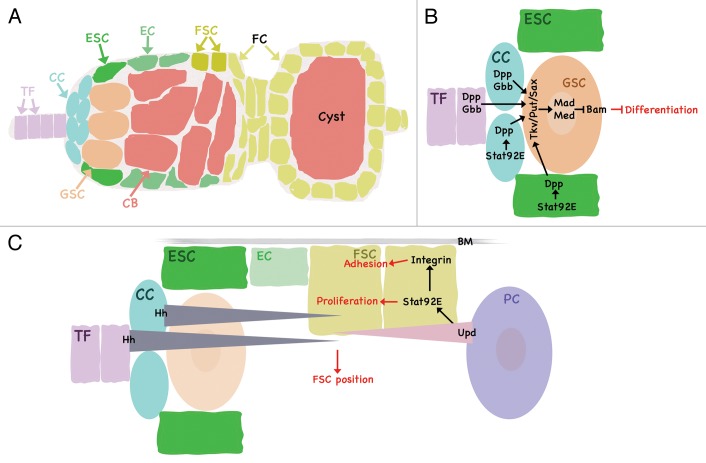
Drosophila males are fertile a few hours after eclosure from the pupal case, and continue to produce sperm throughout their lifetime. Their testis resemble a production line, with the stem cell niche located at the anterior tip of the testis tube, and mature sperm leaving the testis at the posterior end. The testis stem cell niche is one of the least complex versions, and therefore lends itself to very detailed analyses of cellular events, such as cell orientation or differentiation, and even allows for live imaging.12 A tightly packed spherical cluster of about a dozen post-mitotic somatic cells form the hub, a structure that both germline stem cells (GSC) and the somatic cyst stem cells (CySC) attach to. GSCs undergo asymmetrical division, producing a daughter cell destined for differentiation called gonialblast (GB), which then goes through four rounds of mitotic amplification with incomplete cytokinesis, producing a cyst of 16 interconnected spermatogonia (Fig. 1A). These then enter meiosis and form a bundle of 64 spermatids, which finally mature and differentiate into motile sperm. CySCs also divide asymmetrically, but the daughter cyst cell (CyC) does not differentiate any further. A pair of cyst cells surrounds the newly formed GB and envelopes the developing cyst throughout their maturation process until it is finally shed during sperm maturation.
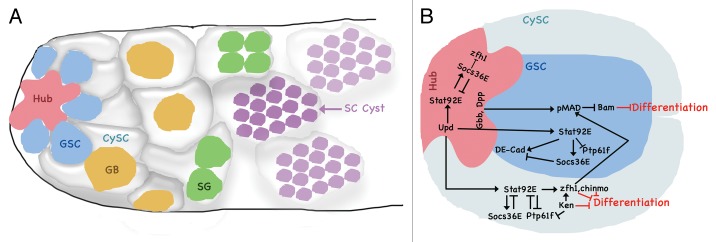
Figure 1. (A) The anterior tip of the testis hosts the stem cell niche consisting of the hub and CySCs, which are in physical contact with the GSCs. Asymmetric division produces GBs, and transient amplification continues to form spermatogonia (SG) and spermatocytes (SC). (B) JAK-STAT signaling from the hub and CySCs promotes GSC self-renewal and prevents differentiation. GSC intrinsic JAK-STAT activity is required for cell adhesion to the hub. See text for details.
The hub therefore establishes a microenvironment required to maintain two populations of stem cells—GSCs and CySCs. However, the hub shares the task of GSC maintenance with the CySCs (discussed below). Interestingly, CySCs and hub cells are derived from common progenitors, and CySCs retain the plasticity to differentiate into hub cells if required.13 Hub formation first becomes apparent in late embryogenesis, and is highlighted by high expression levels of the homophilic adhesion molecules Fasciclin III (FasIII), DE-, and DN-cadherins. Whereas FasIII is predominantly present in between hub cells, the cadherins are also prominent at the hub-stem cell interface,14 essential for anchoring stem cells to the niche. Physical attachment to the niche was assumed a prerequisite for stem cell identity and maintenance, since they are lost when DE-cadherin is removed.13 However, more recent findings suggest that altered signaling conditions allow viable GSCs detached from the hub,15 which will be discussed below. Another main function of the hub is the secretion of signaling molecules for the stem cell populations. One of the earliest sexual dimorphisms during hub formation is the production of Upd, which is specific for male stem cell niche formation,14 and the JAK-STAT pathway remains the prominent feature of stem cell:niche interaction throughout development. The fact that Upd secretion from the hub activates JAK-STAT signaling in GSCs and CySCs, and is essential for their maintenance, has been known for some time,16,17 but detailed cell-specific analyses have unraveled a more complicated picture than previously suggested.
In contrast to earlier assumptions, a more recent shift in paradigm suggests that JAK-STAT signaling does not act alone, but in conjunction with bone morphogenic protein (BMP) signaling to ensure GSC renewal. The two BMP ligands glass bottom boat (Gbb) and decapentaplegic (Dpp) are expressed in hub cells and CySCs, from where they signal to GSCs. BMP activation in GSCs suppresses the differentiation factor Bag of marbles (Bam), thereby preventing the GSC to GB transition. Thus, BMP signaling is essential for GSC maintenance.18,19
Bringing these lines of research together, Leatherman et al. recently provided evidence for a model of stem cell maintenance which integrated the BMP and JAK-STAT pathways with cell adhesion.20 The authors propose that CySCs contribute to the niche, as they are required for GSC maintenance and self-renewal. JAK-STAT signaling in the CySCs, via its targets zfh1 and chinmo, activates BMP effectors in GSCs. Interestingly, JAK-STAT signaling in GSCs themselves is not needed for self-renewal, but for adhesion to the niche. Reduction of STAT92E in GSCs leads to mislocalization of DE-cadherin and detachment from the hub (Fig. 1B). This model, however, has recently been challenged by findings from Lim and Fuller,15 who specifically ablated CySCs without affecting GSCs, and discovered that GSCs are maintained and accumulate irrespective of physical attachment to the hub. Those GSCs in contact with the hub still receive Upd and have active JAK-STAT signaling, as well as correctly oriented centrosomes—a prerequisite for asymmetric division. GSCs displaced from the hub are STAT92E negative, but express BMP targets, and divide with randomly oriented centrosomes. The fact that GSCs can proliferate without CySCs, but never express Bam, led the authors to propose that CySCs and their progenitors are actually needed for differentiation of GSCs. It will be very interesting to further investigate the details of the relationship between the somatic and germline stem cells, both attached to and displaced from the hub. It is also currently unclear how BMP signaling is activated in GSCs if CySCs are not the ligand source. The putative matricellular protein Magu (also called Pentagone) could provide a clue though. It is normally expressed from the hub and is required for short range BMP signaling in GSCs.21 However, Magu is also part of a regulatory feedback loop controlling BMP signaling to enhance long range, low BMP signals in other tissues.22 It might be possible that non-physiological accumulation of GSCs detached from the hub activate the Magu-mediated BMP amplification loop.
Another interesting aspect of the role of JAK-STAT in testis stem cells is their target gene profile. Given that the pathway is active in the niche and both somatic and germline cells, but serves different roles in these cell types, it is not surprising that differentially regulated targets and control mechanisms are necessary to distinguish between the tasks at hand. One example is zfh1, which is expressed in CySCs, but not in GSCs.23 A potential mechanism for this selective expression comes from the JAK-STAT target gene inhibitor ken and barbie (ken),24 which in this case regulates expression of zfh1 in a STAT92E-independent manner25 (Fig. 1B). Like zfh1 and chinmo, ken is necessary for CySC renewal, and notably, all three of them are transcriptional repressors.
ptp61F is another STAT92E target as well as a phosphatase important for pathway regulation.6,26 Whereas its expression is normally positively regulated by STAT92E, ptp61F expression is downregulated in response to JAK-STAT in testis, providing evidence for an uncommon role of STAT92E as a repressor in this tissue. Ptp61f is also negatively regulated by Ken, which could suggest a cooperative repression by Ken and STAT92E.25
Socs6E is an established JAK-STAT target gene and feedback inhibitor. In testis, Socs6E is highly regulated, not only by STAT92E, but also by the demethylase dUtx, which positively regulates Socs36E mRNA expression by removing histone modifications in its regulatory region.27Socs36E is expressed in the hub and CySC to control JAK-STAT signaling. In hub cells, it prevents ectopic zfh1 expression, and thus inhibits the adoption of CySC fate. In GSCs, Socs36E expression fine-tunes JAK-STAT signaling to control cell attachment by regulating correct levels of DE-cadherin. Similarly, SOCS36E might limit integrin-mediated adhesion of CySCs to the hub.28 Whereas loss of adhesion leads to depletion of stem cells from the niche, overattachment can lead to hub invasion in the case of GSCs, or, in the case of CySCs, to GSC loss from the niche due to increased CySC attachment interfaces, which competitively replace GSCs from the niche.28,29
Other signaling pathways involved in stem cell regulation in the Drosophila testis are the epidermal growth factor receptor (EGFR) and the Hedgehog (Hh) pathway. EGFR signaling seems to have two distinct roles during spermatogenesis. In GSCs, it regulates the frequency of cell division, as attenuation of the EGFR signal leads to increased speed in cell cycle completion.30 Independently, EGFR signaling from germline cells to their associated cyst cells is necessary for normal cyst encapsulation and differentiation.31,32
Hh signaling has only recently joined the group of stem cell supporting pathways in the testis. The eponymous ligand Hh is produced by the hub,33 from where it signals to CySCs and GSCs via the transmembrane proteins Patched (Ptc) and Smoothened (Smo) to activate the transcription factor Cubitus interruptus (Ci) (reviewed in ref. 34). Recent independent findings show that Hh signaling is required for CySC maintenance and prevents premature CySC differentiation.35,36 It is not required for cell-autonomous GSC maintenance, but might be necessary for proper BMP activity in GSCs and thus contributes to the inhibition of differentiation. Over activation of Hh signaling leads to over proliferation of CySCs, which can outcompete GSCs for a place at the hub and thus indirectly contribute to GSC loss. Interestingly, Hh and JAK-STAT signaling act in parallel and independently, and Hh in CySC does not contribute to their niche function. Therefore, JAK-STAT signaling remains the main pathway responsible for GSC maintenance via the hub and CySC niche (Fig. 1B).
In summary, the apical tip of Drosophila testis provides a region of clearly defined cellular organization, hosting a stem cell niche of apparent simplicity, with germline and somatic stem cells clustering around a central pool of somatic support cells. However, looks are deceiving, as stem cell maintenance is ensured by a complex three way communication between the hub and the two stem cell populations. JAK-STAT and BMP signaling are so far the main authorities responsible for the balance between self-renewal and differentiation, which is essential for tissue homeostasis. Upd emitted from the hub activates JAK-STAT signaling in both CySCs and GSCs, where it regulates a cell-specific set of target genes. In CySCs these seem to have two roles, which are currently under debate. Conflicting results show that JAK-STAT activity in CySCs might or might not be required for GSC maintenance. Different sets of experiments have shown that GSCs are lost when STAT92E is depleted from CySCs.20 However, more recent evidence finds that GSCs can still be found in complete absence of CySCs, and suggests the main task of CySCs is to enable GSC differentiation.15 However, agreement prevails on the fact that loss of JAK-STAT signaling in CySCs, or indeed loss of CySCs, leads to an accumulation of tumorous GSCs, which can proliferate independently of the hub, and they can still activate BMP signaling. JAK-STAT signaling within GSCs is not required for GSC maintenance as such, but is necessary for attachment to the hub. Consistent with this, GSCs accumulating away from the hub do not express STAT92E anymore. Strictly speaking, their identity is subject to debate as they have lost some stem cell attributes—JAK-STAT activity and physical contact with hub cells, but have maintained others—their morphology and hallmark spherical fusome.16 It would be interesting to investigate whether these displaced GSCs still retain the ability to fully differentiate into sperm.
As JAK-STAT plays distinct roles in all three niche cell types, numerous cell specific regulatory mechanisms must be in place. One of these is the feedback inhibitor SOCS36E. It represses JAK-STAT signaling in hub cells, which secrete the ligand Upd, but do not express STAT92E unless SOCS36E is lost.29 In CySCs, SOCS36E keeps JAK-STAT activity low, to ensure correct integrin expression levels.28,29 SOCS36E is also present in GSCs, but does not seem to be essential for their survival, whereas it is required non-cell autonomously for the CySCs. Different levels of JAK-STAT signal therefore activate different downstream events, via specific target genes. Of these, only zfh1 and chinmo are known, and despite their proven relevance for stem cell maintenance, their mechanisms of action are still unknown.
Given the exciting progress and available tools, the testis stem cells will no doubt remain a favorite playground of JAK-STAT enthusiasts for a while. However, further investigations of the involvements of other signaling pathways, as well as their interactions with each other, should be imminent. The exciting appearance of the Hedgehog pathway on the testis stage is a first indication, but niche and stem cell-specific expression data strongly suggests there will be more to come.37 Furthermore, epigenetic regulation adds another layer of complexity to stem cell regulation in testis. An example is provided by the nucleosome remodeling factor (NURF) complex, which is expressed throughout the apex of the testis and antagonizes GSC and CySC differentiation by positively regulating STAT92E.38
Ovarian Stem Cell Niches
The Drosophila ovaries are dominant structures residing in the abdomen of the adult female, resembling two artichokes in shape. Each ovary contains 16 to 21 tubular ovarioles, which contain 6 to 8 egg chambers, each of which holds a series of maturing eggs at different stages. The anterior tip of each ovariole converges into the germarium, which houses the niche for GSCs and escort stem cells (ESC), one of the two types of somatic stem cells. The niche itself is mainly composed of cap cells (CC) and terminal filament (TF) cells (Fig. 2A). However, ESC-derived escort cells (EC, also called inner germarial sheath cells) have been found to also contribute to the GSC niche.39 At the onset of oogenesis, each GSC divides asymmetrically, producing a new GSC and a cystoblast. As the cystoblasts leave the stem cell niche, they “crowd-surf” their way from one escort cell to another, always in extensive physical contact through membrane protrusions.40 The cystoblast then undergoes four rounds of mitotic division with incomplete cytokinesis to form an interconnected 16-cell cyst. At this stage, the associated ECs undergo apoptosis and the cyst gets enveloped by a single layer of follicle cells, which accompany them throughout development, continuously proliferating to adjust to the growing egg.41 Only one of the 16 cystocytes, marked by the presence of the microtubule organizing center, will go through meiosis to become the oocyte, whereas the remaining cells develop into oocyte-supporting nurse cells. The second type of somatic stem cells, the epithelial follicle stem cells (FSC), are located a few cells away from the TF and CCs, and have their own niche (discussed below). FSCs proliferate and differentiate to give rise to polar cells, stalk cells, and follicle cells surrounding the developing cyst cells, thus forming the follicle per se.
Figure 2. (A) The germarium located in the anterior tip of each ovariole contains the GSC niche, consisting of TF, CC, and ESC. The FSCs are positioned more posterior and attach to the surrounding basement membrane. (B) JAK-STAT signaling partly controls BMP signaling, which is required for GSC self-renewal and inhibition of differentiation. (C) Antagonistic Hh and Upd gradients determine the position of FSCs within the germarium. JAK-STAT activity within FSCs regulates Integrin-mediated adhesion to the basement membrane (BM). See text for details.
Given the extensive involvement of JAK-STAT signaling in GSCs in the testis (see above) and intestinal stem cells (see below), its role in the ovarian germline is surprisingly limited. However, JAK-STAT activity is present in the TF, CCs, and ESCs, and signaling has a clear regulatory role for GSCs. Ectopic pathway activation results in a tumorous GSC phenotype, whereas loss of STAT92E leads to the disappearance of GSCs from the niche.41 More detailed analyses have elucidated that JAK-STAT signaling specifically regulates dpp transcription in CCs and ESCs, thus controlling levels of BMP signaling (discussed below and Fig. 2B).
Interestingly, JAK-STAT signaling does not regulate gbb, implying that JAK-STAT activity manages the ovarian stem cell niche and limits GSC differentiation by a very precise specification of BMP levels.42,43 Similar to its role in testis GSCs, JAK-STAT signaling has not been found to be required cell-autonomously within the germline.
The major regulatory system for GSC renewal and differentiation however is the BMP signaling network, which represses the differentiation-promoting transcription factor Bam in GSCs, thus ensuring GSC maintenance.44-46 Dpp and Gbb are expressed by the TF and CCs and signal through BMP type I (Thickveins [Tkv] and Saxophone [Sax]) and type II (Punt [Put]) receptor complexes. In the GSCs, the activated BMP effector Mothers against Dpp (Mad) binds its co-factor Medea to regulate transcription of target genes, including and Daughters against Dpp (Dad) (Fig. 2B). In order to allow cystoblast differentiation, BMP signaling has to be switched off in the GSC daughter cell, the cystoblast, demanding a very steep change in response to the BMP gradient from the TF to the GSCs, but not their daughters. Recent findings demonstrate that this is achieved by rapid degradation of the BMP type I receptor Thickveins (Tkv) by the Fused/Smurf complex during the GSC–cystoblast transition.47,48 Other mechanisms include the translation regulator Brain tumor (Brat), which represses Mad,49 and the microRNA mir-184, which regulates the BMP receptor Sax.50 These findings not only show that BMP activity is necessary for GSC maintenance, but also that it is sufficient to determine cell fate after stem cell division. An additional extrinsic mechanism of limiting the range of BMP signaling might act via the extracellular matrix proteoglycan Dally, which restricts ligand diffusion.51 BMP also acts on the translation regulator pelota, which inhibits differentiation in a Bam-independent manner.52 Combined evidence suggests that BMP signaling is indeed the guardian of GSC maintenance, with more than one rabbit up its hat to prevent precocious differentiation.
Other factors important for GSC maintenance include female sterile (1)Yb (Yb), piwi, and hh, all of which are expressed in the TF and CCs. Although the precise mechanisms of their actions are not entirely clear, it is intriguing that all three are interconnected. Yb is believed to regulate piwi and hh expression in TF and CCs, which in turn controls GSC proliferation independently of Dpp.53 Within the GSCs, Piwi represses expression of the differentiation factor Bam, as releasing Bam would de-repress Pumilio and Nanos, two differentiation repressors, thus securing GSC maintenance.54 Notch (N) signaling is a key factor for initial niche (TF and CC) formation during development, and thus plays a—possibly indirect—role for GSC maintenance, as loss of N leads to a defective niche and loss of GSCs.55
The importance of JAK-STAT signaling within the ovary is by no means limited to the GSC niche, as it also controls FSC maintenance, as well as specification and /or migration of the specialized FSC descendants stalk cells, polar cells, and border cells. FSCs are not located in the stem cell niche at the anterior tip of the germarium, but reside more posterior, and provide a source of epithelial follicle cells needed to surround the germline cells and then the entire maturing egg chamber. However, some of the follicle cells differentiate into specialized cells early on, to form polar cells at either end of the developing egg chambers, or stalk cells, which act as spacers between the egg chambers. FSCs differ from GSCs in several aspects; although they do depend on a niche, it is neither as well defined nor located adjacent to the FSCs like the GSC niche. Instead, FSCs partly share the GSC niche and still respond to long-range signaling from the TF and CCs. Recently, Vied et al.56 investigated the interplay of the different pathways involved in FSC maintenance and discovered that fine-tuning of signal intensity is of great importance, since FSCs will only respond appropriately to exactly the right amount of ligand. The two main pathways responsible for FSC maintenance are Hh and JAK-STAT (Fig. 2C). RNAi experiments revealed that FSC are exactly positioned to receive a relatively weak long-range Hh signal from the CCs and TF, regulated by the Hh sequestering protein Brother of iHog (Boi),57 and a strong JAK-STAT signal from specified polar cells located just posterior to the FSCs (Fig. 2C). Wingless (Wg) signaling emanating from the GSC niche also contributes to the optimal FSC positioning within the germarium, although it only supplements Hh and JAK-STAT signals, and is insufficient to rescue loss of either of these.58 However, considering Wg forms a gradient along the anterior-posterior axis, and gradient levels detected even slightly more anterior than the FSCs have been found to be detrimental for FSC establishment, it is possible that Wg signaling is needed to prevent FSC formation too close to the CC/TF niche. BMP signaling is also essential for FSC maintenance by preventing differentiation,59 but it is as yet unclear how it interacts with the other pathways.
Adhesion molecules, such as cadherins, are structurally important components of every stem cell niche, and the FSC niche is no exception. DE-cadherin and Armadillo/β-catenin anchor FSC to the neighboring inner germarial sheath cells60 and are essential for FSC maintenance. Indeed, a recent genetic screen for modulators of the FSC niche suggests that adhesion molecules play a primary role during FSC maintenance, possibly of greater importance than proliferation.61
Surprisingly, hyperactivation of either Hh or JAK-STAT signaling can compensate for DE-cadherin-mediated FSC loss, suggesting that these pathways can strongly influence adhesive properties to support FSC maintenance.58 Evidence suggests that JAK-STAT signaling promotes Integrin-mediated FSC:basement membrane interactions, indicating a potential mechanism.
All of these data establish JAK-STAT signaling as the main control element of FSC maintenance, niche regulation and early cell fate determination, partly in conjunction with Hh and other signaling pathways. FSC-intrinsic JAK-STAT activity in response to nearby Upd secretion is sufficient for FSC retention, and can even compensate for loss of adhesive properties (Fig. 2C).
An important claim to fame for the JAK-STAT pathway during oogenesis has been its role during border cell specification and migration (reviewed in refs. 62 and 63). A very elegant mechanism, in which the two antagonistic pathway targets apontic (Apt) and slow border cells (Slbo) respond to different levels of JAK-STAT activity, which determines border cell fate.64 Subsequent migration of border cells is also controlled by JAK-STAT, which is required to activate the hormone ecdysone.65
In summary, the Drosophila ovary has established itself as an easily accessible and compact system to study a wide range of different processes. It is in the unique situation to host germline stem cells as well as two different types of somatic stem cells. Even though both of them essentially accompany and support the GSCs at different stages throughout their development, they have very different tasks. ESCs mainly give rise to somatic ECs, and were only recently identified as a distinct subpopulation of the inner germarial sheath cells.41 Similar to CyCs in the Drosophila testis, ESCs divide once, and either self-renew or differentiate, and then escort the early GSC daughters on the first leg of their journey. In contrast to CySCs though, which remain associated with the maturing sperm, ECs undergo cell death once the procession reaches the follicle cells. It will be interesting to investigate the mechanisms triggering and controlling EC apoptosis, and to identify the spatial and/or temporal cues. Given the recent finding that JAK-STAT signaling is responsible for the apoptosis of super-numerous polar cells during cell fate specification,58 it would be exciting to investigate whether JAK-STAT’s regulation of ECs also extends to their programmed cell death.
The responsibilities of JAK-STAT signaling toward FSCs and the follicle cell lineage are surprisingly versatile in mechanism. By communicating with other signaling pathways like Hh or Wg, it establishes a morphogen pattern that positions FSCs in the correct location. In addition, JAK-STAT signaling strongly supports FSC adhesion to the basement membrane, which might be the reason why high levels of pathway activity can compensate for loss of DE-cadherin. However, the mechanism for this still remains to be elucidated. FSCs are continuously providing new follicle cells for the maturing egg chamber, which in turn support nurse cells and the oocyte. In contrast to ESCs, FSC daughters can adopt different fates—stalk cells, polar cells, epithelial follicle cells, or, later on, border cells, depending on their positions relative to the ligand source and on their interpretation and translation of JAK-STAT activity.
GSCs ultimately all have the same objective—to provide the germ cell for fertilization. It is interesting to note that female GSCs in Drosophila still give rise to two different cell types—nurse cells and the oocyte, and are thus multipotent, in contrast to their male counterparts.
JAK-STAT signaling is without doubt one of the key players during oogenesis, even though it is not active within the GSCs itself. It is however essential for the stem cell niche and thus acts as an extrinsic factor for GSC maintenance. By controlling ESC and EC morphology and proliferation, JAK-STAT signaling organizes niche structure, and thus indirectly regulates GSC maintenance.
Intestinal Stem Cell Niche in the Drosophila Midgut
The relatively recent discovery of multi-potent intestinal stem cells (ISC) in the posterior midgut of adult Drosophila,66,67 a system that is remarkably similar to vertebrate digestive systems,68 has led to a rapidly expanding new research field. The architecture of the midgut consists of a basement membrane (BM), which provides a structural barrier between the underlying visceral muscle (VM) and the epithelium lining the midgut lumen (Fig. 3). Like the vertebrate intestine, the Drosophila midgut epithelium undergoes a rapid turnover, with complete renewal occurring within one week. In addition, dietary stress, injury, or infection triggers apoptosis within the epithelial layer, activating ISCs that are essential for replenishing lost cells.
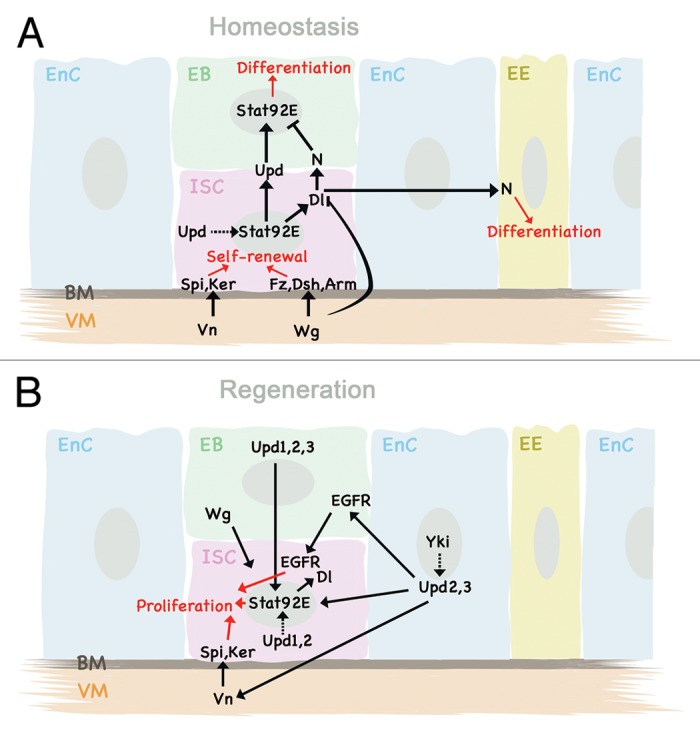
Figure 3. (A) During normal tissue homeostasis in the Drosophila midgut, JAK-STAT signaling, together with EGFR and Wg signaling, is necessary for ISC maintenance, and promotes differentiation in EB. (B) In response to dietary stress, injury, or infection, JAK-STAT signaling is strongly upregulated, and signals back from EnC and EB to ISC to promote ISC proliferation. See text for details.
The majority of the epithelial monolayer is made up of large absorptive enterocytes (commonly referred to as EC, but termed EnC for the purpose of this review), with interspersed smaller, hormone-secreting enteroendocrine cells (EE) (Fig. 3). Both of these cell types derive from enteroblasts (EB), which are the non-dividing daughter cells of the ISCs. ISCs therefore give rise to three different cell types. However, the final cell fate is already decided in the ISCs, rather than the EBs, and depends on levels of the N ligand, Delta (Dl). ISCs with low expression of Dl become EEs, whereas high Dl levels ultimately lead to EnC specification.69,70 The overall ratio of EEs to EnCs has been reported as 1:9,67 although this might depend on the region within the highly compartmentalized midgut.69,71,72
Unlike adult stem cells in other Drosophila tissues, ISCs do not seem to reside in a morphologically defined niche of stromal cells, but are scattered throughout the epithelium and attached to the BM and the underlying VM. However, these structures still function like a niche. The BM provides the necessary structural support, as the ISCs are anchored to it,73,74 while VM secretes pathway ligands to regulate ISC proliferation and differentiation.75
While base levels of ISC proliferation and regeneration ensure tissue homeostasis, rapid regeneration of the midgut can be triggered by various means. These include dietary changes, epithelial injury, bacterial infection, and DNA damage induced by compounds such as bleomycin. ISC division in response to epithelial damage can be elicited by a caspase-independent mechanism, involving c-Jun N-terminal kinase (JNK), or by a caspase-dependent process.76 However, the common essential denominator for epithelial repair during regeneration seems to be the JAK-STAT pathway, acting independently of JNK activation.76 Interestingly, the precise mechanism of the response is still subject to controversy and conflicting data.
Upon either bacterial infection of the midgut or JNK activation, EnCs induce expression of all three Upd ligands, with Upd3 responding most strongly.75-77 Secreted JAK-STAT ligands then trigger pathway activation non-cell autonomously in different cell types, which leads to ISC proliferation, differentiation, and regeneration of the epithelium. Although all three Upd molecules are involved in stress response, they act in different, only partly redundant, ways. Osman et al.77 dissected their different functions in meticulous detail, and found that upon challenge, Upd3 is upregulated in and secreted by the EnC, Upd2 in progenitor cells (ISCs and EBs) as well as in EnCs, whereas Upd expression levels are only slightly elevated in ISCs and EBs after infection. However, loss of Upd inhibits epithelial regeneration, indicating that even low levels of cytokine are essential for ISC proliferation, as are the downstream components Dome and STAT92E.76 Upd2 and 3 both contribute to ISC mitosis, and both of them trigger synthesis of the antimicrobial peptide drosomycin-3.77 Upd-mediated paracrine signaling is sufficient to activate JAK-STAT pathway activity in ISCs, which controls expression of Dl69,76 and activation of EGFR signaling,77,78 resulting in ISC differentiation and proliferation, respectively.
A recent report by Zhou et al.75 showed in a very similar set of experiments that Upd3 is synthesized by EnCs and EBs after infection, and that it signals specifically to the VM and EB, triggering expression of the EGFR ligands Vein (in VM) and Spitz (in EB). These then activate EGFR signaling in the ISCs to commence proliferation. The authors thus postulate that epithelial regeneration requires JAK-STAT signaling in the VM as well as in EBs, but not directly in the ISCs (Fig. 3B).
Another area of debate is the role of JAK-STAT signaling during normal stem cell homeostasis in the midgut in the absence of external stress factors. By generating clones of cells missing components of the JAK-STAT pathway, and following these over time, it should be possible to establish whether JAK-STAT activity is essential for ISC maintenance (in which case clones would be lost over time), or not. However, the jury is still out to answer this question, with some groups reporting a requirement of JAK-STAT activity for basal ISC proliferation or maintenance, and thus loss of clones over time,79,80 whereas others observe JAK-STAT deficient clones long after induction.69,76 The devil might be in the detail however, as Osman et al. find Upd, but not Upd2 or 3, are essential for ISC homeostasis, although the latter two contribute increasingly during aging.77 Additionally, loss of JAK-STAT signaling correlates with loss of stem cell adhesion, which might complicate loss of function clonal analysis.69 A wider analysis including interactions between different pathways suggests that Wingless (Wg), EGFR, and JAK-STAT signaling all contribute to basal ISC maintenance and proliferation in a somewhat redundant manner, and that only loss of all three pathways completely and rapidly abolishes ISC preservation.80
Either way, JAK-STAT activity, marked by STAT-GFP reporter expression or nuclear accumulation of STAT92E protein is evident in ISCs and EBs, but not in EnCs and EEs under unchallenged conditions, which implies a role for JAK-STAT signaling in the ISCs. The observations that (a) JAK-STAT activity correlates with expression of Dl,76 and that (b) STAT92E is indeed required for expression of Dl69 strongly suggest that JAK-STAT signaling drives ISC differentiation. Indeed, clones lacking STAT92E never develop into EEs or EnCs. The fact that nuclear STAT92E translocation can also be suppressed by N activity,80 but that JAK-STAT signaling is required for EB differentiation independently of N,69 paint a very intricate picture in which JAK-STAT could act both upstream and downstream of N signaling to allow for tightly controlled differentiation events (Fig. 3A).
Evidently, JAK-STAT signaling does not act on its own but is part of a greater regulatory network. The roles of additional pathways involved in midgut tissue homeostasis and regeneration must therefore not be omitted from this discussion. As mentioned above, the EGFR ligand Vein is secreted by the VM, and, together with autocrine EGFR regulation by the ligands Spitz and Keren ensures ISC maintenance and proliferation, both during steady-state levels and in response to stress or injury.81,82 Secretion of the Wnt pathway ligand Wg by the VM also signals to the ISCs to ensure self-renewal and proliferation via regulation of frizzled, dishevelled, and armadillo. Wg also antagonizes N signaling within the ISC, thus co-determining terminal differentiation of the EB into either EnC or EE.83 During regeneration after injury however, Wg secreted from the epithelial EB to the ISC is required for ISC proliferation84 (Fig. 3A and B).
The Hippo (Hpo) signaling pathway has also been implicated in ISC control in the midgut. Its precise role during normal ISC homeostasis is still controversial; conflicting reports suggest that it might, or might not be required to limit ISC proliferation.85,86 However, these results and other findings87,88 agree on the fact that Hpo signaling via its co-transcription factor Yorkie (Yki) is essential for ISC proliferation during midgut regeneration. Yki acts in the EnC to activate non-cell autonomous EGFR and JAK-STAT signaling in the ISC.85
In summary, the adult Drosophila midgut is an important model for stem cell behavior and niche communication, as well as for investigating cellular events during stress response and disease onset. Interestingly, inhibition of the Drosophila ortholog of the human adenomatous polyposis coli (APC) gene, apc1, mimics the early events of colorectal cancer (CRC). Evidence that the Wg inhibitor, Apc1 activates JAK-STAT and EGFR pathways, via the proto-oncogene myc, to drive intestinal hyperplasia, suggests Drosophila may represent an influential system to study the mechanisms underlying the disease, but also to identify potential novel therapeutic approaches for CRC.78,89
Although some details regarding the role of JAK-STAT signaling in the midgut epithelium maintenance remain contentious, important points have become clear over the last years. JAK-STAT signaling is strongly activated in response to bacterial infection and other challenges, as well as by JNK activation, and is essential for epithelial regeneration and for host survival. Under challenged conditions, paracrine JAK-STAT signaling promotes ISC proliferation and is necessary for ISC differentiation. In line with this, activation of JAK-STAT signaling by overexpressing pathway components results in ISC proliferation, differentiation, and hyperplasia, emphasizing that the JAK-STAT-mediated stress response has to be tightly controlled to prevent overgrowth. Whether JAK-STAT activity alone is essential for basal ISC turnover is debatable, but it is definitely required for tissue homeostasis in conjunction with EGFR and Wg activity. Discrepancies between results reported by different research groups could have several underlying reasons. First, whereas the posterior midgut is often treated as a homogenous tissue, regional differences in JAK-STAT activity and expression patterns in ISC have been observed,69 which could affect proliferation rates and cell fate decisions. Second, the midgut epithelium is constantly exposed to external factors ingested with the diet, and might differ between research groups. Indeed, low levels of stress response and apoptosis have been reported in presumably unchallenged midguts.67,90 Third, tissue homeostasis and regeneration are age dependent processes; whereas the former increases with age, the latter slows down. It is therefore essential to compare age-matched individuals.77
The Drosophila intestine has become a well-established model system for adult stem cell investigation with great potential, and has proven itself as a valid invertebrate model for human disease. Both aspects will hopefully be thoroughly exploited to extend our knowledge of adult tissue regeneration, but also to better understand the influences of external factors taken up in the diet; how they trigger cellular responses, and how these responses can sometimes lead to inflammation and disease.
Conclusions
The three Drosophila adult stem cell niches discussed in this review have contributed enormously to the advancement of stem cell research, especially with regards to the important stem cell–niche relationship. In addition, they have furthered our understanding of signaling pathways, their regulations, and the networking between them.
Concluding from the results discussed here, JAK-STAT signaling plays multiple roles in the stem cell niche, depending on the cell type and context of interaction (Table 1). The applications of pathway activity are accordingly diverse; a signal gradient determining the correct positioning of FSCs in the ovary, paracrine activation of Notch and EGFR pathways during injury response in the midgut epithelium, and localization of adhesion molecules in GSCs in the testis are just a few examples for the wide variety of potential downstream effects of JAK-STAT signaling. In addition, JAK-STAT signaling has also adopted tissue specific roles for each set of stem cells, such as the response to epithelial damage in the Drosophila intestine. However, a few common themes have emerged that are consistent throughout the systems, unless the findings are controversial.
Table 1. Requirement of JAK-STAT activity.
| |
Testis |
Ovary |
Midgut |
|
|---|---|---|---|---|
| Homeostasis | Regeneration | |||
| GSC/ISC |
yes |
yes |
? |
yes |
| CySC/ESC |
? |
(yes) |
n.a. |
|
| FSC | n.a. | yes | n.a. | |
Overview of the requirement of JAK-STAT signaling for the different cell lineages in the three Drosophila stem cell systems discussed in this review. n.a., not applicable. Brackets indicate implicated requirement. Question marks indicate controversial or missing evidence. See text for details.
1) JAK-STAT signaling is essential for stem cell maintenance:
Loss of JAK-STAT activity generally leads to loss of the stem cell population, usually by differentiation. Loss of STAT92E in the stem cell niche of the Drosophila ovary leads to depletion of both GSC and ESC populations, and of the GSC pool in testis. This supports the idea that JAK-STAT is required for stem cell self-renewal and/or prevention of differentiation. However, this mechanism does not have to be intrinsic to the stem cell, as JAK-STAT activity can be provided in a non-cell autonomous manner. As an example, JAK-STAT signaling in testis CySCs is essential for GSC survival, whereas it is not directly required within GSCs.
2) JAK-STAT signaling is pro-proliferative and can cause tumorous phenotypes:
Overactivation of JAK-STAT activity leads to aberrant proliferation and accumulation of stem cells. Interestingly, this phenomenon is associated with a loss of stem cell dependency on the niche. Instead, stem cells can survive and proliferate detached and even at considerable distance from their normal microenvironment, while still maintaining at least partial stem cell identity. This is consistent with the findings that perturbations in JAK-STAT activity play a fundamental role in many human malignancies and cancers.91,92
3) JAK-STAT signaling is required for stem cell adhesion:
Physical contact with its supporting niche a characteristic feature of stem cells, and JAK-STAT signaling is emerging as a key control factor for stem cell adhesion to the niche. In the testis, both somatic and germline stem cells rely on JAK-STAT pathway activity to anchor them to the niche, via regulation of adhesion molecules. Similar JAK-STAT-mediated mechanisms are in place to promote FSC attachment to the basement membrane in the ovary. ISCs in the Drosophila midgut are in intimate contact with the BM. The nature and regulation of this attachment still remains to be explored, but loss of JAK-STAT activity is associated with ISC dispersal.69 Conversely, reports from mammalian systems show that DE-cadherin regulates JAK-STAT signaling.93 Further research should establish whether these two mechanisms are conserved and whether they are independent or contribute to a feedback loop. It should also be considered that integrins and cadherins are able to participate in intracellular signaling,94 and a similar requirement has also been observed in the Drosophila adult stem cells.73,74,95 It will be interesting to investigate the details and interdependence of JAK-STAT activity and adhesion in the Drosophila stem cell systems.
Considering all three characteristics of JAK-STAT activity and the consequences of its misregulation, it is worth speculating how the findings from Drosophila could translate into an improved understanding of human malignancies. JAK-STAT over activation is associated with aberrant stem cell proliferation and mislocalization (2), providing a scenario for initial tumor formation and cell dispersal. The association of JAK-STAT activity with stem cell identity (1) and cell adhesion (3) would then provide a model for metastasis and the formation of secondary tumor sites. Together with the increasing understanding of the interactions of JAK-STAT with the network of other major signaling pathways, derived from the model systems described in this review, could provide important explanations of the multiple links of misregulated JAK-STAT components with various cancers and leukemias.
The well-established Drosophila stem cell systems described in this review have proven invaluable for demonstrating that JAK-STAT signaling is an important pathway in stem cell regulation and maintenance. Since this trait is conserved from flies to humans,96 shedding light on the precise mechanisms of this involvement will have direct implications for human stem cell and cancer research.
Acknowledgments
I am grateful to K Fisher, W Stec, S Brown and MP Zeidler for critical comments and helpful discussions. I apologize to authors whose work could not be discussed here due to space constraints. NB is supported by a Cancer Research-UK senior fellowship held by Martin Zeidler.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/25686
References
- 1.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–3. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persano L, Rampazzo E, Basso G, Viola G. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol. 2013;85:612–22. doi: 10.1016/j.bcp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Comen EA. Tracking the seed and tending the soil: evolving concepts in metastatic breast cancer. Discov Med. 2012;14:97–104. [PubMed] [Google Scholar]
- 4.Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–50. doi: 10.1016/S1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 5.Hombría JC, Brown S, Häder S, Zeidler MP. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol. 2005;288:420–33. doi: 10.1016/j.ydbio.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 2005;19:1861–70. doi: 10.1101/gad.1320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene. 2002;21:4812–21. doi: 10.1038/sj.onc.1205618. [DOI] [PubMed] [Google Scholar]
- 8.Karsten P, Häder S, Zeidler MP. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mech Dev. 2002;117:343–6. doi: 10.1016/S0925-4773(02)00216-2. [DOI] [PubMed] [Google Scholar]
- 9.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Canadian Critical Care Trials Group Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217:169–80. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 12.Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–76. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–6. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol. 2006;294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Lim JG, Fuller MT. Somatic cell lineage is required for differentiation and not maintenance of germline stem cells in Drosophila testes. Proc Natl Acad Sci U S A. 2012;109:18477–81. doi: 10.1073/pnas.1215516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–5. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 17.Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–9. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 18.Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–75. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- 19.Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr Biol. 2003;13:2065–72. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 20.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–11. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Q, Wang Y, Vargas E, DiNardo S. magu is required for germline stem cell self-renewal through BMP signaling in the Drosophila testis. Dev Biol. 2011;357:202–10. doi: 10.1016/j.ydbio.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuilleumier R, Springhorn A, Patterson L, Koidl S, Hammerschmidt M, Affolter M, et al. Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol. 2010;12:611–7. doi: 10.1038/ncb2064. [DOI] [PubMed] [Google Scholar]
- 23.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbouzova NI, Bach EA, Zeidler MP. Ken & barbie selectively regulates the expression of a subset of Jak/STAT pathway target genes. Curr Biol. 2006;16:80–8. doi: 10.1016/j.cub.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Issigonis M, Matunis E. The Drosophila BCL6 homolog Ken and Barbie promotes somatic stem cell self-renewal in the testis niche. Dev Biol. 2012;368:181–92. doi: 10.1016/j.ydbio.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, Boutros M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature. 2005;436:871–5. doi: 10.1038/nature03869. [DOI] [PubMed] [Google Scholar]
- 27.Tarayrah L, Herz HM, Shilatifard A, Chen X. Histone demethylase dUTX antagonizes JAK-STAT signaling to maintain proper gene expression and architecture of the Drosophila testis niche. Development. 2013;140:1014–23. doi: 10.1242/dev.089433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–6. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SR, Zheng Z, Wang H, Oh SW, Chen X, Hou SX. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J Cell Physiol. 2010;223:500–10. doi: 10.1002/jcp.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parrott BB, Hudson A, Brady R, Schulz C. Control of germline stem cell division frequency--a novel, developmentally regulated role for epidermal growth factor signaling. PLoS One. 2012;7:e36460. doi: 10.1371/journal.pone.0036460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar A, Parikh N, Hearn SA, Fuller MT, Tazuke SI, Schulz C. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol. 2007;17:1253–8. doi: 10.1016/j.cub.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 32.Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development. 2002;129:4523–34. doi: 10.1242/dev.129.19.4523. [DOI] [PubMed] [Google Scholar]
- 33.Michel M, Kupinski AP, Raabe I, Bökel C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development. 2012;139:2663–9. doi: 10.1242/dev.075242. [DOI] [PubMed] [Google Scholar]
- 34.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amoyel M, Sanny J, Burel M, Bach EA. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development. 2013;140:56–65. doi: 10.1242/dev.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Lv X, Jiang J, Zhang L, Zhao Y. Dual roles of Hh signaling in the regulation of somatic stem cell self-renewal and germline stem cell maintenance in Drosophila testis. Cell Res. 2013;23:573–6. doi: 10.1038/cr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry NA, Tulina N, Matunis E, DiNardo S. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev Biol. 2006;294:246–57. doi: 10.1016/j.ydbio.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 38.Cherry CM, Matunis EL. Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell. 2010;6:557–67. doi: 10.1016/j.stem.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirilly D, Wang S, Xie T. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 2011;138:5087–97. doi: 10.1242/dev.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138:2207–15. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–10. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 42.López-Onieva L, Fernández-Miñán A, González-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–40. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–8. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–91. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–64. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 46.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–60. doi: 10.1016/S0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 47.Xia L, Jia S, Huang S, Wang H, Zhu Y, Mu Y, et al. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 2010;143:978–90. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Xia L, Zheng X, Zheng W, Zhang G, Wang H, Tao Y, et al. The niche-dependent feedback loop generates a BMP activity gradient to determine the germline stem cell fate. Curr Biol. 2012;22:515–21. doi: 10.1016/j.cub.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 49.Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell. 2011;20:72–83. doi: 10.1016/j.devcel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iovino N, Pane A, Gaul U. miR-184 has multiple roles in Drosophila female germline development. Dev Cell. 2009;17:123–33. doi: 10.1016/j.devcel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Guo Z, Wang Z. The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range BMP signaling in the Drosophila ovary. Development. 2009;136:3627–35. doi: 10.1242/dev.036939. [DOI] [PubMed] [Google Scholar]
- 52.Xi R, Doan C, Liu D, Xie T. Pelota controls self-renewal of germline stem cells by repressing a Bam-independent differentiation pathway. Development. 2005;132:5365–74. doi: 10.1242/dev.02151. [DOI] [PubMed] [Google Scholar]
- 53.King FJ, Szakmary A, Cox DN, Lin H. Yb modulates the divisions of both germline and somatic stem cells through piwi- and hh-mediated mechanisms in the Drosophila ovary. Mol Cell. 2001;7:497–508. doi: 10.1016/S1097-2765(01)00197-6. [DOI] [PubMed] [Google Scholar]
- 54.Szakmary A, Cox DN, Wang Z, Lin H. Regulatory relationship among piwi, pumilio, and bag-of-marbles in Drosophila germline stem cell self-renewal and differentiation. Curr Biol. 2005;15:171–8. doi: 10.1016/j.cub.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–80. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- 56.Vied C, Reilein A, Field NS, Kalderon D. Regulation of stem cells by intersecting gradients of long-range niche signals. Dev Cell. 2012;23:836–48. doi: 10.1016/j.devcel.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartman TR, Zinshteyn D, Schofield HK, Nicolas E, Okada A, O’Reilly AM. Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. J Cell Biol. 2010;191:943–52. doi: 10.1083/jcb.201007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borensztejn A, Boissoneau E, Fernandez G, Agnès F, Pret AM. JAK/STAT autocontrol of ligand-producing cell number through apoptosis. Development. 2013;140:195–204. doi: 10.1242/dev.079046. [DOI] [PubMed] [Google Scholar]
- 59.Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell. 2005;9:651–62. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci U S A. 2002;99:14813–8. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang ZA, Huang J, Kalderon D. Drosophila follicle stem cells are regulated by proliferation and niche adhesion as well as mitochondria and ROS. Nat Commun. 2012;3:769. doi: 10.1038/ncomms1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Godt D, Tepass U. Breaking a temporal barrier: signalling crosstalk regulates the initiation of border cell migration. Nat Cell Biol. 2009;11:536–8. doi: 10.1038/ncb0509-536. [DOI] [PubMed] [Google Scholar]
- 63.Silver DL, Geisbrecht ER, Montell DJ. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development. 2005;132:3483–92. doi: 10.1242/dev.01910. [DOI] [PubMed] [Google Scholar]
- 64.Starz-Gaiano M, Melani M, Wang X, Meinhardt H, Montell DJ. Feedback inhibition of Jak/STAT signaling by apontic is required to limit an invasive cell population. Dev Cell. 2008;14:726–38. doi: 10.1016/j.devcel.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Jang AC, Chang YC, Bai J, Montell D. Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt. Nat Cell Biol. 2009;11:569–79. doi: 10.1038/ncb1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 67.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 68.Casali A, Batlle E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 2009;4:124–7. doi: 10.1016/j.stem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 70.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–92. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 71.Buchon N, Osman D, David FP, Yu Fang H, Boquete JP, Deplancke B, et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013;3:1725–38. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Strand M, Micchelli CA. Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc Natl Acad Sci U S A. 2011;108:17696–701. doi: 10.1073/pnas.1109794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goulas S, Conder R, Knoblich JA. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell. 2012;11:529–40. doi: 10.1016/j.stem.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin G, Zhang X, Ren J, Pang Z, Wang C, Xu N, et al. Integrin signaling is required for maintenance and proliferation of intestinal stem cells in Drosophila. Dev Biol. 2013;377:177–87. doi: 10.1016/j.ydbio.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 75.Zhou F, Rasmussen A, Lee S, Agaisse H. The UPD3 cytokine couples environmental challenge and intestinal stem cell division through modulation of JAK/STAT signaling in the stem cell microenvironment. Dev Biol. 2013;373:383–93. doi: 10.1016/j.ydbio.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osman D, Buchon N, Chakrabarti S, Huang YT, Su WC, Poidevin M, et al. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. J Cell Sci. 2012;125:5944–9. doi: 10.1242/jcs.113100. [DOI] [PubMed] [Google Scholar]
- 78.Cordero JB, Stefanatos RK, Myant K, Vidal M, Sansom OJ. Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development. 2012;139:4524–35. doi: 10.1242/dev.078261. [DOI] [PubMed] [Google Scholar]
- 79.Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109:992–9. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354:31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 81.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–93. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–23. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 84.Cordero JB, Stefanatos RK, Scopelliti A, Vidal M, Sansom OJ. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. EMBO J. 2012;31:3901–17. doi: 10.1038/emboj.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010;107:21064–9. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–7. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–45. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–58. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–64. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 90.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–55. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Constantinescu SN, Girardot M, Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. 2008;33:122–31. doi: 10.1016/j.tibs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hombría JC, Sotillos S. Disclosing JAK/STAT links to cell adhesion and cell polarity. Semin Cell Dev Biol. 2008;19:370–8. doi: 10.1016/j.semcdb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–65. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells. 2008;13:1219–27. doi: 10.1111/j.1365-2443.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 96.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]