Abstract
Genetic alterations affecting the JAK-STAT signaling pathway are linked to several malignancies and hematological disorders in humans. Despite being one of the most extensively studied pathways, there remain many gaps to fill. JAK-STAT components are widely conserved during evolution. Here, we review the known roles of the JAK-STAT pathway in Drosophila immunity: controlling the different steps of hematopoiesis, both under physiological conditions and in response to immune challenge, and contributing to antiviral responses. We then summarize what is currently known about JAK-STAT signaling in renewal of the adult intestine, under physiological conditions or in response to ingestion of pathogenic bacteria.
Keywords: Drosophila, lymph gland, wasp infection, hematopoiesis, gut
Introduction
The Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway was discovered from studies on the role of interferon in the control of immune responses in mammals.1 This pathway, which is highly conserved throughout evolution, transduces the activity of a variety of cytokines and growth factors in many important biological processes, such as embryonic development, hematopoiesis and immunity, and stem cell maintenance.2 Inappropriate JAK-STAT activation is linked to the development of several malignancies in humans, especially those derived from hematopoietic lineages, and to immunological disorders such as inflammatory disease, autoimmune disease, and allergy.3 Innate immunity, which is defined by its activation following pathogen recognition by germ-line encoded receptors, is the most ancient form of immune defense shared by all metazoans. Insects rely entirely on innate immunity for protection against external threats.4 The first, external protection against pathogens is the cuticle that constitutes a physical barrier, preventing microbe entry into the hemolymph (circulating body fluid). Overtaking this barrier generates two types of immune response, humoral and cellular. The humoral response, also called systemic response, leads to the synthesis of dedicated antimicrobial peptides (AMPs) and contributes to hemolymph coagulation and melanization. The cocktail of AMPs synthetized in the fat body (equivalent to the mammalian liver), epithelia, and hemocytes (the insect blood cells) kills the pathogens. The Toll and IMD signaling pathways and their downstream effectors, the NFκB like transcription factors Dif and Relish, respectively, control this process. The Toll and IMD pathways are homologous to the mammalian Toll-like receptor and tumor necrosis factor pathways, respectively. The initial discovery, in Drosophila, of the major role of Toll receptors/signaling in immunity, established this insect as a major model for the study of innate immune mechanisms. The cellular immune response is achieved by hemocytes responsible for phagocytosis of microbes, melanization, and encapsulation of large pathogens (for reviews see refs 4 and 5). We review here the roles of JAK-STAT signaling in Drosophila immunity.
The JAK-STAT Signaling Pathway
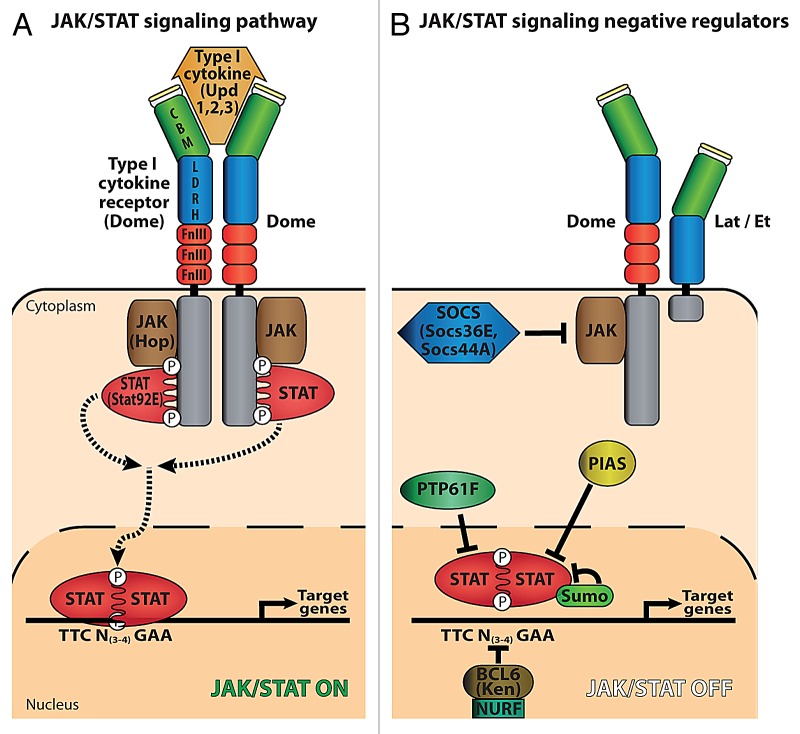
JAKs and STATs mediate intracellular signaling in response to secreted type I cytokines. JAK tyrosine kinases are associated with the intracellular part of single pass transmembrane proteins that form homo- or heteromeric receptors. Ligand binding induces a conformational change that triggers pathway activation, via trans-phosphorylation of JAK molecules associated with the intracellular part of the receptor. Phosphorylated (activated) JAKs then phosphorylate the receptor, creating docking sites for members of the STAT family of transcription factors, which in turn become phosphorylated. Phosphorylated (activated) STATs homo- or heterodimerize prior to nuclear translocation and transcriptional activation of target genes (Fig. 1). Four JAKs, seven STATs, and more than 30 different cytokines and growth factors have been identified in mammals.6 In contrast, in Drosophila there is only one active type I cytokine receptor (Domeless, Dome), one JAK (Hopscotch/Hop), one STAT (Stat92E/Marelle), and three cytokines called Unpaired (Upd, Upd2, and Upd3).2 Negative regulators of the pathway have been identified, including three suppressors of cytokine signaling (SOCS), one PIAS (dPIAS/ Su[var]2–10), the nucleosome remodeling factor NURF,7 one BCL-6 homolog Ken and Barbie, the nuclear STAT phosphatase PTP61, and the sumoylation of STAT 92E8. One short form of the cytokine receptor encoded by CG14225, called Eye Transformer (Et) or Latran (Lat), was recently shown to act as a tissue-specific dominant negative receptor (Fig. 1 and refs. 9 and 10). Beside the canonical JAK-STAT pathway, recent reports suggest that the association of STAT92E/HP1 complexes to heterochromatin, in the absence of JAK signaling, represents an alternative mechanism by which STAT could regulate transcription in Drosophila.11,12 Finally, recent data obtained for vertebrate STAT proteins indicate non canonical functions as they can be involved in chromatin organization, mitochondrial respiration and the regulation of tubulin dynamics.13-16
Figure 1. The JAK-STAT signaling pathway in Drosophila melanogaster. (A) Activation of JAK-STAT signaling. Binding of either cytokine, Unpaired (Upd1, 2, and 3), to the type I cytokine receptor Domeless activates trans-phosphorylation of the JAK kinase Hopscotch (Hop) and Dome phosphorylation, creating a docking site for STAT (Stat92E). Hop-phosphorylated STAT forms dimers which translocate into the nucleus and activate target genes via binding to TTCN(3–4)GAA sites. The green box in Dome corresponds to the cytokine binding domain (CBM), the blue box to a conserved region between Lat/Et and Dome (LDHR), the fibronectin III (Fn III) motifs are in red, the signal peptide in yellow and the intra-cytoplasmic region in gray. (B) Negative regulation of JAK-STAT signaling. Lat/Et acts as a dominant negative Dome co-receptor. Suppressor of cytokine signaling (SOCS) (Socs36E, Socs44A) prevents Stat92E recruitment onto the receptor. Protein inhibitors of activated STAT (PIAS) and PTP61F inhibit Stat92E function. Sumoylation of Stat92E has a repressive role in the regulation of the JAK-STAT pathway in Drosophila. BCL6 (Ken and Barbie, marked Ken) and NURF compete with Stat92E for binding to DNA.
Drosophila Hematopoiesis
Circulating hemocytes are the cellular component of the fly immune system.5,17,18 Three types of blood cells have been described in Drosophila: plasmatocytes, crystal cells, and lamellocytes. Plasmatocytes display phagocytic activity and represent functional equivalents of mammalian monocytes/macrophages; crystal cells are platelet-like cells involved in melanization, an insect-specific reaction that contributes to wound healing; lamellocytes, corresponding to a cryptic, stress-induced cell fate, encapsulate foreign bodies too large to be phagocytosed, such as eggs laid in Drosophila larvae by parasitoid wasps. Drosophila hematopoiesis occurs in two waves during development. First, a population of precursor cells for plasmatocytes and crystal cells is specified from the embryonic head mesoderm. Some of these cells differentiate before dispersing in the embryo, while others divide and differentiate later in circulation at the larval stage. A fraction of the embryonic hemocytes attach to the inner surface of the larval cuticle.19 Whether these “sessile” hemocytes, which can be mobilized upon immune challenge, perform specific functions remains unclear.19,20 A second population of plasmatocytes and crystal cells is released at the onset of metamorphosis, from a specific larval hematopoietic organ called the lymph gland (LG).21,22 Lamellocytes rarely differentiate under normal conditions but massively differentiate in the LG in response to wasp parasitism. The circulating hemocyte population in pupae and adults thus consists of a mixture of hemocytes of both embryonic and larval origins.23 No hematopoietic organ has, so far, been identified in adult flies.
JAK-STAT Signaling and STAT in the Larval Hematopoietic Organ, the Lymph Gland
The larval lymph gland (LG) is specified from the embryonic cardiogenic mesoderm.24,25,26 At the end of embryogenesis the LG is composed of two lobes located on either side of the anterior portion of the heart, the aorta. Growth of these primary/anterior lobes during larval development is accompanied by the formation of more posterior, secondary lobes, although the cells/mechanisms at the origin of these secondary lobes remain unclear. In third instar larvae, while the posterior lobes contain only progenitor cells (called pro-hemocytes), three zones can be distinguished in each primary lobe27: the medullary zone (MZ) composed of tightly packed pro-hemocytes, which are most akin to the common myeloid progenitor in mammals, the cortical zone (CZ) containing differentiating hemocytes and intermediate progenitors,27,28 and a group of about 20–30 cells at the posterior end of each lobe, forming the so-called posterior signaling center (PSC) (Fig. 2).
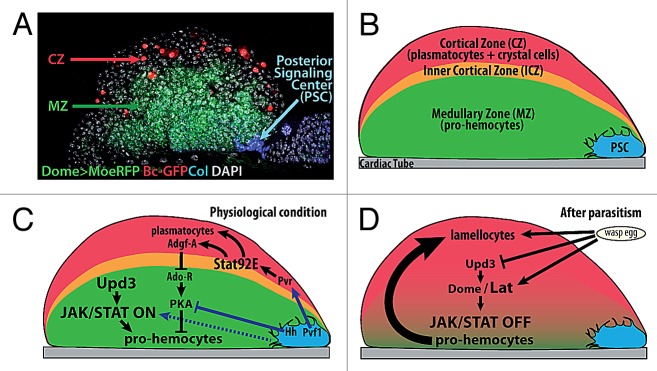
Figure 2. JAK-STAT signaling and STAT functions in the Drosophila larval hematopoietic organ, the lymph gland. (A) Confocal view of one primary lobe of the 3rd instar Drosophila lymph gland and (B) diagrammatic representation. Three zones can be distinguished, based on morphology and expression of specific markers: a small group of cells extending filopodia, the posterior signaling center (PSC, blue), the medullary zone (MZ, green) containing pro-hemocytes, and the cortical zone (CZ, red) containing differentiated hemocytes (plasmatocytes and crystal cells) and the intermediate progenitors (inner cortical zone, ICZ, orange). The cardiac tube (CT) is in gray. (C) Under normal conditions, Upd3 activates the JAK-STAT pathway in the MZ, and this is required to maintain a pool of pro-hemocytes. Binding of Pvf1 produced by the PSC to its receptor Pvr, in CZ cells, activates Stat92E expression. Stat92E is required non-cell autonomously in ICZ cells, and autonomously in CZ cells, for plasmatocyte differentiation. Stat92E also activates Adgf-A expression, leading to downregulation of Ado-R, which upregulates of PKA activity in the MZ. PKA activity, which contributes to the pro-hemocyte to hemocyte transition, is negatively regulated by Hh signaling from the PSC. (D) In response to wasp parasitism, there is a simultaneous decrease of Upd3 and Dome and increase of lat expression, leading to complete switching off of the JAK-STAT pathway in the MZ, a prerequisite to the massive differentiation of lamellocytes.
PSC cells were first identified in third instar larvae by their expression of the Notch ligand Serrate.29 They are specified in the embryo by the expression of the transcription factors Collier/Knot (Col), the Drosophila ortholog of mammalian early B-cell factor (EBF), and Antennapedia, a Hox protein.25,30 Two independent reports showed that the PSC plays a key role in third instar larvae, by maintaining the balance between multipotent pro-hemocytes in the MZ and hemocyte differentiation. This role is similar to that of the vertebrate hematopoietic niche in bone marrow, a cellular micro-environment, which controls self-renewal and differentiation of hematopoietic stem cells (HSCs).30,31 More specifically, the PSC cells were shown to express Hedgehog (Hh), which acts in a non-cell-autonomous manner to maintain the MZ. Since this founding work, it was shown that PSC cells are the source of several diffusible signals such as Wingless (Wg, a Wnt member) and Pvf1, one ligand of the platelet-derived growth factor (PDGF) signaling pathway,32 which, together with Hh, are necessary to maintain hemocyte homeostasis in the LG under normal conditions. The number of PSC cells is controlled by Dpp, a member of the TGF-β family of cytokines, together with Wg signaling, thus re-enforcing the parallels observed between the PSC and the vertebrate HSC niche (reviews in refs. 33–35). Finally, Spitz, one EGF-R ligand is released from the PSC in response to wasp parasitism and required for induction of lamellocyte differentiation.36 Important questions remain however to be addressed, such as the mechanism through which pro-hemocytes integrate the different PSC signals, or the role of the filopodial extensions emitted by PSC cells and which can contact MZ cells over several cell diameters (Fig. 2).31
JAK-STAT Signaling in Drosophila Hematopoiesis
Beside their morphological aspect, hematopoietic progenitors in the MZ can be distinguished by their expression of GFP under the control of a Gal4 driver inserted in dome (Dome > GFP;27,37). In the embryonic mesoderm, JAK-STAT activity controls the expression of its own receptor, Dome, through binding of Stat92E to an enhancer called Dome-MESO.38,39 The Dome-MESO enhancer was cloned upstream of the LacZ reporter gene to generate transgenic lines, called dome-MESO-LacZ, with LacZ expression being used as a read out for JAK-STAT signaling. Overlap between high expression levels of Dome > GFP and dome-MESO-lacZ in MZ cells indicated that the JAK-STAT pathway is active in these cells. Loss of tep4 expression, a MZ marker, in Stat92E hypomorphic mutants, further showed that the JAK-STAT pathway is required in the LG for the maintenance of prohemocytes.31 Wasp parasitism induces disruption of JAK-STAT signaling in the MZ and massive differentiation of pro-hemocytes into lamellocytes. lat/et, the gene next to dome on the X chromosome, encodes a Dome-related receptor with a truncated intracellular domain.40 Unlike dome, lat is neither expressed in embryos, nor required for fly viability and fertility. lat is expressed, however, in larval MZ cells. While the absence of LG morphological defects in the lat mutant indicates that the gene is not required for LG ontogeny or hemocyte homeostasis under physiological conditions, mutant larvae are unable to massively produce lamellocytes in response to wasp parasitism. Detailed analysis established that lat is specifically required for switching off JAK-STAT signaling in the MZ following parasitism, thereby licensing pro-hemocytes to differentiate. In vivo and cell culture assays showed that Lat and Dome form inactive heteromers and that Lat antagonizes Dome activity in a dose-dependent manner. In response to wasp parasitism, there is an increase of the Lat/Dome ratio and a strong decrease in the upd3 mRNA level, leading to a complete inhibition of JAK-STAT signaling in the MZ that allows massive differentiation of lamellocytes (Fig. 2).9 Altogether, these data revealed the key role of JAK-STAT signaling regulation in mediating a dedicated cellular immune response in Drosophila. The type I cytokine receptor family has considerably expanded in vertebrates,41 resulting both from an increased number of receptor genes and the generation of various protein isoforms, including truncated receptors that can act as co-receptors. Studies on IL13Ra2 or GP130/GP130-like receptors in cell culture indeed suggested that short membrane-anchored receptors can behave as dominant-negative receptors.42,43 That Lat acts as a dominant-negative receptor rather than a co-receptor in Drosophila is an in vivo example of the observations made in mammalian cell culture. Whether and when regulated expression of long and short receptor isoforms is employed in controlling specific aspects of vertebrate immunity, as it does in Drosophila, remains to be investigated.
The col, lat, and Stat92E mutant phenotypes indicate that JAK-STAT signaling is essential to preserving the pro-hemocyte status of cells in the MZ. This was rather unexpected, since constitutive activation of JAK-STAT signaling resulting from a dominant gain-of-function JAK mutation, hopTum-l, a mutation first described 20 years ago, induces an overproliferation of circulating plasmatocytes and differentiation of lamellocytes in the absence of immune challenge, leading to the formation of melanotic masses in larvae and adult flies.44-46 A similar phenotype is observed upon ubiquitous expression of a constitutively active form of Stat92E, Stat92EΔNΔC,47 consistent with high JAK-STAT activity being able to induce hemocyte differentiation. Accordingly, hemocytes located in the outer CZ and lacking Stat92E fail to undergo final differentiation into plasmatocytes32,48 (Fig. 2). Inhibition of STAT92E in the inner CZ, which is enriched in intermediate progenitors,28 revealed an additional, non-cell autonomous role of Stat92E in preventing differentiation of surrounding cells into plasmatocytes. STAT92E expression in CZ cells also contributes non-cell autonomously to the maintenance of the MZ. STAT92E expression in these cells is dependent upon platelet-derived growth factor/vascular endothelial growth factor-like (PDGF/PVR) signaling. PDGF/PVR signaling is activated upon binding of Pvf1 (platelet-derived growth factor/vascular endothelial growth factor-like factor) that is produced by PSC cells and transported to differentiating hemocytes in the CZ. Thus, Pvf1/PVR signaling has been proposed to link Stat92E’s CZ role in maintaining LG homeostasis to the PSC function.32
One downstream target of both PDGF/PVR signaling and Stat92E in the CZ is Adenosine deaminase growth factor A (Adgf-A), whose function is to reduce the amount of extracellular adenosine. In the absence of Stat92E activity, adenosine is free to bind its receptor Ado-R, a seven pass trans-membrane domain receptor, is expressed in the MZ and signals through G proteins to activate adenylate cyclase and protein kinase A (PKA). On the contrary, Hedgehog (Hh) signaling inhibits PKA activity. Hh signaling is activated in MZ cells upon reception of Hh secreted from the PSC, and it is required to maintain a pool of progenitors. PKA activity in the MZ is thus regulated positively by adenosine originating from the CZ32 and negatively by Hh signaling from the PSC30 (Fig. 2). The cross-talk between the PSC and the CZ that occurs at the level of PKA activity in the MZ is therefore responsible for maintaining the equilibrium between hemocyte differentiation and pro-hemocyte maintenance. In summary, JAK-STAT signaling plays several roles in the LG: it is required in the MZ for maintaining the multi-lineage capacity of pro-hemocytes; STAT, independent of JAK signaling, is required cell-autonomously for plasmatocyte differentiation; STAT in CZ cells contributes in a non-cell autonomous manner to hemocyte homeostasis. Many questions however remain open. First, the fact that the loss of JAK-STAT signaling in MZ cells leads to the loss of pro-hemocyte markers, but is not sufficient to induce their differentiation into mature hemocytes, suggests that JAK-STAT signaling is only one of several pathways contributing to maintain the progenitor state. Second, the mechanisms linking the loss of JAK-STAT signaling in pro-hemocytes and their exit from the MZ remain unknown. Third, the mechanisms by which high levels of JAK-STAT signaling enforce lamellocyte differentiation remain to be deciphered. While several screens for modifiers of JAK-STAT signaling have been performed, either in vivo, or in cultured cells, identification of JAK-STAT targets in hemocytes are not known.49-53 Finally, how STAT92E acts in CZ cells, independent of JAK-STAT signaling remains to be establihed.
The JAK-STAT Pathway in Circulating Hemocytes
A role for JAK-STAT signaling in cellular immunity was first suggested by studies of the hopTum-l mutant. It was observed early on that this mutation leads to an increased number of plasmatocytes and the massive differentiation of lamellocytes eager to encapsulate “self” tissue, leading to the formation of black masses/melanotic pseudo-tumors.45,46,54 A more recent study explored the role of JAK-STAT signaling in the interaction between hemocytes and tumors, generated in imaginal tissue by hyperactivation of Ras signaling.55 It showed that activation of Jun N-terminal kinase (JNK) signaling in tumors as well as in aseptic wounds, causes expression of JAK-STAT-activating cytokines from the injured tissue. Cytokine (mainly Upd3) production is amplified into a systemic response, through the induction of additional cytokine production by the plasmatocytes that adhere to injured tissue (upon their detection of basement membrane disruption), eventually resulting in hemocyte proliferation. Activation of JAK-STAT signaling in hemocytes is thus required for their increased proliferation in response to both tumors56-58 and wounds.55 Basement membrane components are remarkably conserved throughout the animal kingdom, providing a unique structure for the immune system to sense tissue integrity. A similar innate reaction may thus underlie the response to tumors and tissue damage in vertebrates and humans.55 Secretion of JAK-STAT activating cytokines by hemocytes also regulates the humoral systemic response following a septic injury.44 The TurandotA (TotA) gene is upregulated in both hopTum-l mutant flies and in response to bacterial infection. Its induction by septic injury is abolished in a hop loss of function mutant, showing that it requires JAK-STAT signaling. TotA protein subsequently found in the hemolymph, is mainly produced by the larval fat body. This indicates that stimulation of Upd3 expression in hemocytes in response to septic injury, activates the JAK-STAT pathway in fat body cells. Thus a global picture emerges with the circulating hemocytes as a central component in the regulation of Drosophila humoral and anti-tumoral responses. Signal(s) and the signaling pathway(s) involved in Upd3 upregulation in hemocytes remain to be identified.
JAK-STAT in the Immune Response to Viral Infection
Virus transmission and spread by insects is of major economical and public health importance. Viruses evolved diverse strategies to circumvent their host’s antiviral defenses. Drosophila is a host to numerous viruses and a good model to study the mechanisms of antiviral defense.59 Current data point to the existence of two general mechanisms: RNAi inhibition of viral RNAs, such as the piwi-interacting (pi) pathway60,61 and an induced response calling on the expression of specific antiviral proteins.62 As in mammals, the induced response involves the activity of several signaling pathways, among which JAK-STAT signaling.63 Drosophila C virus (DCV), a picorna-like virus, which infects natural populations of D. melanogaster, is a classical model to study antiviral responses. Genome-wide profiling identified some 140 genes that are upregulated upon DCV infection. Several induced genes, including virus-induced RNA 1 (vir-1) contain STAT-binding sites in their promoter.64 Genetic analyses confirmed that hop and Dome activity is required for the induction of vir-1 in response to DCV infection. Correlatively, hop mutant flies express low levels of vir-1, have high viral titers and succumb rapidly to DCV infection. Altogether, these data suggest a model in which DCV infected cells produce a cytokine that activates the JAK-STAT pathway and the immune defense in non-infected cells. An essential role of the JAK-STAT pathway in the antiviral response is also supported by the increased titers of SINV viruses after their inoculation into heterozygous STAT mutant flies.65 Of note, hop activity is required but not sufficient for the activation of some DCV-induced genes, indicating that additional regulation are needed. Moreover, microarray analyses of the Drosophila immune response to infection by different viruses (DCV, DXV, SIGMAV, FHV, and SINV), indicate that the response is virus-specific. Drosophila thus represents an excellent model to study the complexity of antiviral immune defenses.
The JAK-STAT Pathway and Gut Regeneration
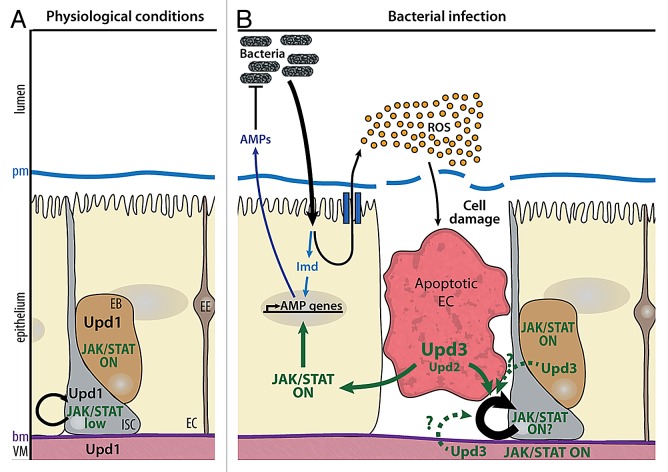
The gut lumen of mammals and insects contains an abundant flora of resident, commensal bacteria. Two complementary effector mechanisms are essential to control infection by enteric bacteria in the Drosophila gut: generation of microbicidal reactive oxygen species (ROS) by intestinal cells leading to elimination of the ingested pathogenic bacteria, and local production of AMPs66. Exposure to enteric pathogens can cause the loss of gut cells, as collateral effects of bacterial killing by ROS. Loss of gut cells, in turn, promotes intestinal stem cell (ISC) division and ISC-mediated epithelial repair, thereby maintaining gut homeostasis. Intestine epithelial renewal is an important aspect of the gut host defense. Infection of the Drosophila gut thus provides a powerful model to study the mechanistic links between the immune and repair pathways. The adult midgut, equivalent to the mammalian intestine, is composed of a single layer of specialized epithelial cells surrounded by a layer of visceral muscles. ISCs are scattered along the midgut and located basally, immediately adjacent to the basement membrane close to the visceral muscle (Fig. 3).67-69 Under normal physiological conditions, homeostasis of the intestinal epithelium is maintained by the production of new cells by stem cell division. ISCs undergo asymmetric divisions throughout adult life, to giving rise to one cell that retains ISC properties and one transient progenitor, called an enteroblast (EB).67,70 EBs ultimately differentiate into either secretory entero-endocrine cells (EEs) or absorptive enterocytes (ECs) depending on Notch signaling. Under physiological conditions, the JAK-STAT pathway is required for ISC proliferation and the differentiation of ECs.71,72 Low levels of JAK-STAT signaling are detected in ISCs and required to ensure their basal proliferation rate.72-74 The detection of Upd in visceral muscle cells indicated that Upd secreted by muscle cells could activate signaling in ISCs, but recent results suggest that Upd also controls ISC maintenance in an autocrine manner75 (Fig. 3). The situation in response to stress conditions due to bacterial infection becomes more complex. Feeding flies with toxic compounds or invasive bacteria such as Erwinia carotovora carotovora or Pseudomonas entomophila, induces the rapid accumulation of ROS which, in turn, cause gut damage. Renewal of the damaged epithelium occurs through an increase of the ISC division rate.72,76-80 One recent model proposed that ROS-challenged enterocytes produce Upd3, and to a lesser extent Upd2 cytokines which activate the mitogenic activity of JAK-STAT signaling in ISCs, thereby increasing their division rate. Independent data confirmed Upd3 expression in ECs, but also in EBs, and suggested that ISC division in response to bacterial infection requires activation of JAK-STAT signaling in EBs and visceral muscles (VMs), but not in ISCs.81 In this scenario, it is the JAK-STAT stimulation of epidermal growth factor (EGF) production by EBs and VMS, which controls the rate of EGF-R-dependent ISC divisions. Finally, a recent study proposed that high Wg signaling in ISCs, resulting from mutations in adenomatous polyposis coli (APC), leads to non-cell-autonomous upregulation of Upd3 in ECs, and subsequent activation of JAK-STAT signaling in ISCS, leading to ISC hyper proliferation.82 These results reveal novel parallels with the vertebrate intestine, since inactivating APC mutations are detected in a large fraction of colorectal cancers.83 Interestingly, suppressing either JAK-STAT signaling, or EGF-R signaling in ECs suppressed APC-dependent ISC hyper proliferation.82 Despite variations in interpretation between these different groups, the current data clearly point to a key role of JAK-STAT signaling in the control of ISC proliferation and intestinal epithelium repair following injury (Fig. 3 and for review, see ref. 84). They also underline the requirement of additional studies in order to define the molecular details of the signaling cascades that lead gut cells exposed to pathogenic bacteria to trigger immune and repair responses. A recent study on the pathogenicy of P. entomophila identified a new layer of regulation: translational inhibition by the bacteria can block both immune and regenerative epithelial responses of the host.85 As a consequence, Upd3 is not translated in P. entomophila infected guts, despite the strong induction of upd3 transcription in stressed ECs.
Figure 3. Roles of JAK-STAT signaling in gut homeostasis. (A) Under physiological conditions, Upd is expressed by visceral muscles (VM), enteroblasts (EB), and intestinal stem cells (ISC). Basal activation of the JAK-STAT pathway in ISCs and EBs allows ISC renewal and differentiation of EBs into enteroendocrine cells (EE) or enterocytes (EC). (B) Upon ingestion of pathogenic bacteria, the Imd pathway is activated in ECs, leading to the expression of antimicrobial peptides (AMPs). Bacterial infection also results in the production of high levels of reactive oxygen species (ROS) in the gut lumen, which induces cell damage and EC apoptosis. Upd3, and to a lower extent, Upd2 produced in apoptotic ECs induce both ISC proliferation and EB differentiation, in order to regenerate gut epithelium. The cells in which JAK-STAT signaling is required to stimulate ISC proliferation and the role of EGF-R signaling in this process remain to be firmly established. The position of the peritrophic and basal membranes (pm and bm respectively) is indicated on the left.
General Conclusion
The JAK-STAT pathway provides a simple membrane-to-nucleus mechanism to rapidly induce specific gene transcription and plays key roles in vertebrate immunity. Yet, despite 20 years of extensive studies, we are still surprisingly ignorant about this pathway’s tissue-specific functions and target genes. While the complexity of vertebrate immune responses is an obstacle to rapid progress, Drosophila has become a powerful genetic model to study the humoral and cellular aspects of innate immunity. Drosophila JAK and STAT are central players in both the genesis of immune cells and the fight against pathogens as diverse as viruses, bacteria and parasitic insects. Genetics, and genome-wide reverse-genetics and molecular screens, continue to reveal new components of the JAK-STAT pathway. Together with detailed studies of the cellular components of Drosophila immunity, they highlight the value of studying JAK-STAT signaling in Drosophila and its relevance to the understanding of JAK-STAT related human diseases.
Acknowledgments
We thank E Agius, M Meister, and C Monod-Wissler for critical reading of the manuscript. Work in authors’ laboratory is supported by CNRS, University Toulouse III, Ministère de la Recherche (ANR-11 BSV2 00801), ARC (Association pour la Recherche sur le Cancer), FRM (Fondation pour la Recherche Médicale: DEQ 20090515429), and a MRT fellowship for IM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/25700
References
- 1.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 2.Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–16. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- 3.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–50. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 5.Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–26. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 6.Levy DE, Darnell JE., Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 7.Kwon SY, Xiao H, Glover BP, Tjian R, Wu C, Badenhorst P. The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev Biol. 2008;316:538–47. doi: 10.1016/j.ydbio.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Grönholm J, Ungureanu D, Vanhatupa S, Rämet M, Silvennoinen O. Sumoylation of Drosophila transcription factor STAT92E. J Innate Immun. 2010;2:618–24. doi: 10.1159/000318676. [DOI] [PubMed] [Google Scholar]
- 9.Makki R, Meister M, Pennetier D, Ubeda JM, Braun A, Daburon V, et al. A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol. 2010;8:e1000441. doi: 10.1371/journal.pbio.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallio J, Myllymäki H, Grönholm J, Armstrong M, Vanha-aho LM, Mäkinen L, et al. Eye transformer is a negative regulator of Drosophila JAK/STAT signaling. FASEB J. 2010;24:4467–79. doi: 10.1096/fj.10-162784. [DOI] [PubMed] [Google Scholar]
- 11.Yan SJ, Lim SJ, Shi S, Dutta P, Li WX. Unphosphorylated STAT and heterochromatin protect genome stability. FASEB J. 2011;25:232–41. doi: 10.1096/fj.10-169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi S, Calhoun HC, Xia F, Li J, Le L, Li WX. JAK signaling globally counteracts heterochromatic gene silencing. Nat Genet. 2006;38:1071–6. doi: 10.1038/ng1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18:545–51. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark GR, Darnell JE., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolas CS, Peineau S, Amici M, Csaba Z, Fafouri A, Javalet C, et al. The Jak/STAT pathway is involved in synaptic plasticity. Neuron. 2012;73:374–90. doi: 10.1016/j.neuron.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gough DJ. sehgal P, Levy D E. Nongenomic functions of STAT3. In Jak-Stat signaling: from Basics to Disease, TDecker and M Müller, eds 2011. [Google Scholar]
- 17.Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–90. doi: 10.1016/S1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 18.Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5:573–80. doi: 10.1046/j.1462-5822.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 19.Márkus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, Sipos B, et al. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2009;106:4805–9. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–91. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grigorian M, Mandal L, Hartenstein V. Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev Genes Evol. 2011;221:121–31. doi: 10.1007/s00427-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–57. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 23.Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–62. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- 24.Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–23. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- 25.Crozatier M, Ubeda JM, Vincent A, Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2004;2:E196. doi: 10.1371/journal.pbio.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krzemien J, Crozatier M, Vincent A. Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int J Dev Biol. 2010;54:1117–25. doi: 10.1387/ijdb.093053jk. [DOI] [PubMed] [Google Scholar]
- 27.Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–33. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 28.Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 2010;346:310–9. doi: 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–53. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee UA. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–4. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krzemień J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–8. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- 32.Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, et al. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev Cell. 2009;16:756–63. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pennetier D, Oyallon J, Morin-Poulard I, Dejean S, Vincent A, Crozatier M. Size control of the Drosophila hematopoietic niche by bone morphogenetic protein signaling reveals parallels with mammals. Proc Natl Acad Sci U S A. 2012;109:3389–94. doi: 10.1073/pnas.1109407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crozatier M, Vincent A. Drosophila: a model for studying genetic and molecular aspects of haematopoiesis and associated leukaemias. Dis Model Mech. 2011;4:439–45. doi: 10.1242/dmm.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinenko SA, Shim J, Banerjee U. Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 2012;13:83–9. doi: 10.1038/embor.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourbon HM, Gonzy-Treboul G, Peronnet F, Alin MF, Ardourel C, Benassayag C, et al. A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech Dev. 2002;110:71–83. doi: 10.1016/S0925-4773(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 38.Rivas ML, Cobreros L, Zeidler MP, Hombría JC. Plasticity of Drosophila Stat DNA binding shows an evolutionary basis for Stat transcription factor preferences. EMBO Rep. 2008;9:1114–20. doi: 10.1038/embor.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hombría JC, Brown S, Häder S, Zeidler MP. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol. 2005;288:420–33. doi: 10.1016/j.ydbio.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 40.Hombría JC, Brown S. The fertile field of Drosophila Jak/STAT signalling. Curr Biol. 2002;12:R569–75. doi: 10.1016/S0960-9822(02)01057-6. [DOI] [PubMed] [Google Scholar]
- 41.Boulay JL, O’Shea JJ, Paul WE. Molecular phylogeny within type I cytokines and their cognate receptors. Immunity. 2003;19:159–63. doi: 10.1016/S1074-7613(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 42.Rahaman SO, Sharma P, Harbor PC, Aman MJ, Vogelbaum MA, Haque SJ. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002;62:1103–9. [PubMed] [Google Scholar]
- 43.Diveu C, Venereau E, Froger J, Ravon E, Grimaud L, Rousseau F, et al. Molecular and functional characterization of a soluble form of oncostatin M/interleukin-31 shared receptor. J Biol Chem. 2006;281:36673–82. doi: 10.1074/jbc.M607005200. [DOI] [PubMed] [Google Scholar]
- 44.Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–50. doi: 10.1016/S1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 45.Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14:2857–65. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo H, Hanratty WP, Dearolf CR. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 1995;14:1412–20. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ekas LA, Cardozo TJ, Flaherty MS, McMillan EA, Gonsalves FC, Bach EA. Characterization of a dominant-active STAT that promotes tumorigenesis in Drosophila. Dev Biol. 2010;344:621–36. doi: 10.1016/j.ydbio.2010.05.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minakhina S, Tan W, Steward R. JAK/STAT and the GATA factor Pannier control hemocyte maturation and differentiation in Drosophila. Dev Biol. 2011;352:308–16. doi: 10.1016/j.ydbio.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flaherty MS, Zavadil J, Ekas LA, Bach EA. Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of notch signaling by the JAK/STAT pathway. Dev Dyn. 2009;238:2235–53. doi: 10.1002/dvdy.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–68. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mukherjee T, Schaefer U, Zeidler MP. Identification of Drosophila genes modulating Janus Kinase/signal transducer and activator of transcription signal transduction. Genetics. 2006;172:1683–97. doi: 10.1534/genetics.105.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, Boutros M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature. 2005;436:871–5. doi: 10.1038/nature03869. [DOI] [PubMed] [Google Scholar]
- 53.Baeg GH, Zhou R, Perrimon N. Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 2005;19:1861–70. doi: 10.1101/gad.1320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanratty WP, Dearolf CR. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet. 1993;238:33–7. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- 55.Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1:144–54, discussion 153. doi: 10.1242/dmm.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163:203–15. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, et al. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101:14192–7. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorrentino RP, Melk JP, Govind S. Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics. 2004;166:1343–56. doi: 10.1534/genetics.166.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huszar T, Imler JL. Drosophila viruses and the study of antiviral host-defense. Adv Virus Res. 2008;72:227–65. doi: 10.1016/S0065-3527(08)00406-5. [DOI] [PubMed] [Google Scholar]
- 60.van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, et al. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–95. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Q, Luo Y, Lu R, Lau N, Lai EC, Li WX, et al. Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc Natl Acad Sci U S A. 2010;107:1606–11. doi: 10.1073/pnas.0911353107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beutler B, Eidenschenk C, Crozat K, Imler JL, Takeuchi O, Hoffmann JA, et al. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7:753–66. doi: 10.1038/nri2174. [DOI] [PubMed] [Google Scholar]
- 63.Wang JH, Valanne S, Rämet M. Drosophila as a model for antiviral immunity. World J Biol Chem. 2010;1:151–9. doi: 10.4331/wjbc.v1.i5.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6:946–53. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 65.Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Royet J. Epithelial homeostasis and the underlying molecular mechanisms in the gut of the insect model Drosophila melanogaster. Cell Mol Life Sci. 2011;68:3651–60. doi: 10.1007/s00018-011-0828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 68.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 69.Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–5. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- 70.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–92. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 71.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 72.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–44. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 75.Osman D, Buchon N, Chakrabarti S, Huang YT, Su WC, Poidevin M, et al. Autocrine and paracrine unpaired signaling regulate intestinal stem cell maintenance and division. J Cell Sci. 2012;125:5944–9. doi: 10.1242/jcs.113100. [DOI] [PubMed] [Google Scholar]
- 76.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–55. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009;220:664–71. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9:402–11. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou F, Rasmussen A, Lee S, Agaisse H. The UPD3 cytokine couples environmental challenge and intestinal stem cell division through modulation of JAK/STAT signaling in the stem cell microenvironment. Dev Biol. 2013;373:383–93. doi: 10.1016/j.ydbio.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cordero JB, Stefanatos RK, Myant K, Vidal M, Sansom OJ. Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development. 2012;139:4524–35. doi: 10.1242/dev.078261. [DOI] [PubMed] [Google Scholar]
- 83.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 84.Ferrandon D. The complementary facets of epithelial host defenses in the genetic model organism Drosophila melanogaster: from resistance to resilience. Curr Opin Immunol. 2013;25:59–70. doi: 10.1016/j.coi.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Chakrabarti S, Liehl P, Buchon N, Lemaitre B. Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe. 2012;12:60–70. doi: 10.1016/j.chom.2012.06.001. [DOI] [PubMed] [Google Scholar]