Background: TCRγδ and NKG2D are two important receptors for γδT cell cytotoxicity.
Results: γδT cell cytotoxicity is TCRγδ-dependent and requires the activation of Vav1-PLC-γ1 pathway.
Conclusion: γδT cell cytotoxicity requires a strong signal to overcome the inhibitory threshold set by Cbl-b.
Significance: Our finding provides new insights into the molecular mechanisms underlying the activation of γδT cell cytotoxicity.
Keywords: Cancer Therapy, Cell Signaling, Innate Immunity, Phospholipase C, T Cell
Abstract
T cell antigen receptor γδ (TCRγδ) and natural killer group 2, member D (NKG2D) are two crucial receptors for γδT cell cytotoxicity. Compelling evidences suggest that γδT cell cytotoxicity is TCRγδ-dependent and can be co-stimulated by NKG2D. However, the molecular mechanism of underlying TCRγδ-dependent activation of γδT cells remains unclear. In this study we demonstrated that TCRγδ but not NKG2D engagement induced lytic granule polarization and promoted γδT cell cytotoxicity. TCRγδ activation alone was sufficient to trigger Vav1-dependent phospholipase C-γ1 signaling, resulting in lytic granule polarization and effective killing, whereas NKG2D engagement alone failed to trigger cytotoxicity-related signaling to overcome the inhibitory effect of Cbl-b; therefore, NKG2D engagement alone could not induce effective killing. However, NKG2D ligation augmented the activation of γδT cell cytotoxicity through the Vav1-phospholipase C-γ1 pathway. Vav1 overexpression or Cbl-b knockdown not only enhanced TCRγδ activation-initiated killing but also enabled NKG2D activation alone to induce γδT cell cytotoxicity. Taken together, our results suggest that the activation of γδT cell cytotoxicity requires a strong activation signal to overcome the inhibitory effect of Cbl-b. Our finding provides new insights into the molecular mechanisms underlying the initiation of γδT cell cytotoxicity and likely implications for optimizing γδT cell-based cancer immunotherapy.
Introduction
On the basis of different expression of rearranged TCR3 chains, T cells are usually divided into two major subsets: γδT cells and αβT cells (1). The majority of CD3+ cells in blood and secondary lymphoid organs are αβT cells, whereas γδT cells only represent a small subset (1–10%) of CD3+ cells in the peripheral blood (2). With striking diversity of TCRαβ chains originating from a large number of Vα and Vβ genes as well as extensive junctional diversity, αβT cells recognize a large array of antigenic peptides in complex with polymorphic presenting MHC class I or class II molecules (3). Although there are limited numbers of Vγ and Vδ genes available and preferential usage of a few V segments in Vγ/Vδ combinations, extensive junctional diversity in particular in CDR3δ makes TCRγδ chains substantially diverse in theory. However, only a few TCRγδ ligands have been identified so far, and most of them are endogenous antigens/ligands, such as MHC class I-related chains A and B (MICA/B) (4), UL16-binding proteins (ULBPs) (5, 6), F1-ATPase-apolipoprotein A-I complex (7), human MutS homologue 2 (hMSH2) (8), and phosphoantigens (9).
γδT cells contribute to all aspects of innate and adaptive immune responses to viruses, bacteria, parasites, allergy, and autoimmunity (3, 10). γδT cells have also recently become a promising effector candidate in targeting a broad spectrum of tumors for its unique properties, including MHC-unrestricted recognition, abundant IFN-γ secretion, and potent cytotoxicity (10). In our previous studies we focused on the identification of TCRγδ ligands including ULBP4 (6) and hMSH2 (8), both of which can also be recognized by NKG2D. Interestingly, although γδT cell cytotoxicity may be enhanced through multiple non-TCR receptors including NKG2D (6, 8), γδT cells appear to recognize antigens in a TCR-dependent manner (11, 12). This unique activation may result in a faster initiation of cell cytotoxicity, making γδT cells the crucial first line of defense against tumors and infections. However, detailed signal pathways related to TCRγδ-dependent activation of γδT cell cytotoxicity remain elusive. In αβT cells, upon TCRαβ engagement, guanine nucleotide exchange factor Vav1 is rapidly phosphorylated and recruited to a TCRαβ proximal signaling complex and subsequently elicits the activation of phospholipase C-γ1 (PLC-γ1), intracellular calcium flux, and PI3K (13). However, TCRαβ stimulation activates only a minimal level of Vav due to selective suppression by Cbl-b, so that the activation of αβT cell cytotoxicity requires the synergistic engagement of TCRαβ and co-stimulatory molecule CD28 (14). In NK cells, the synergistic engagement of NKG2D and 2B4 are required to overcome inhibition by c-Cbl ubiquitin ligase for the activation of NK cell cytotoxicity (15). Therefore, TCRγδ-dependent activation of γδT cell cytotoxicity may be involved in a specific signal pathway.
In this study we found that TCRγδ activation alone could be sufficient to induce lytic granule polarization and promote γδT cell cytotoxicity by triggering Vav1-dependent PLC-γ1 signaling to overcome Cbl-b-mediated inhibition. In the presence of TCRγδ activation, NKG2D ligation augmented the activation of γδT cell cytotoxicity. To our knowledge it is the first time to illustrate the molecular mechanism underlying TCRγδ-dependent γδT cell cytotoxicity. This finding may provide new insights into the biology of γδT cells and will have clinical significance by optimizing the current γδT-based biotherapy regimens.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Purified anti-pan-TCRγδ mAb (IMMU510) and FITC-conjugated anti-human TCRγδ (IMMU510) was from Beckman Coulter Immunotech; mouse IgG1 (11711), human NKG2D allophycocyanin mAb (149810), CD3ϵ (UCHT1), and NKG2D (149810) were from R&D Systems; phosphatidylethanolamine (PE) anti-human CD107a (LAMP-1) (H4A3), PE anti-human CD3 (HIT3a), Alexa Fluor® 488 anti-human perforin (dG9), FITC anti-human Fas (CD95) (DX2), LEAFTM purified anti-human CD178 (FasL) (NOK-1), and phospho-Akt (Ser-473) (Poly6490) were from Biolegend; Isopentenyl pyrophosphate (IPP) triammonium salt solution, concanamycin A (CMA), rottlerin, U73122,and U73343 were from Sigma; latrunculin A was from Calbiochem; cyclosporin A was from Novartis; phospho-PLC-γ1 (Tyr-783) (2821), PLC-γ1 (2822), phospho-Erk1/2 (Thr-202/Tyr-204) (4370), Erk1/2 (4695), Akt (4691), wortmannin (9951), Vav1 (2153), and Vav3 (2398) were from Cell Signaling; phospho-Vav1 (ab4763) and Vav2 (EP1067Y) were (ab52640) from Abcam; c-Cbl (7G10) was from Upstate; Cbl-b (G-1) and anti-β-actin mAb (C4) were from Santa Cruz; GFP-Vav1 expression plasmid and ProLong Gold Antifade Reagent were from Invitrogen; GFP-Cbl-b expression plasmid was from Origene, and normal donkey serum was from Jackson ImmunoResearch Laboratories. ULBP5 and hMSH2 proteins were expressed by our laboratory.
Cells
γδT cells were expanded from peripheral blood mononuclear cells (PBMCs) freshly isolated from healthy donors. Briefly, PBMCs isolated from healthy donors by density-gradient centrifugation on Ficoll-Hypaque (GE Healthcare) were cultured in RPMI 1640 medium (Invitrogen) with 10% fetal calf serum (FCS) (HyClone) and 200 IU/ml interleukin 2 (IL-2) (Read United Cross Pharmaceutical Co. Ltd.) in 24-well culture plates coated with 1 μg/ml anti-pan-TCRγδ mAb (Immunotech). After 2 weeks of culture, the purity of Vγ9Vδ2 T cells was >90%. γδT cells were rested in RPMI 1640 medium with 5% FCS (without IL-2) for 24 h before the phenotypic and functional analyses. Alternatively, fresh γδT cells (>90% Vγ9Vδ2T cells) were purified by negative selection using a human TCRγ/δ+ T Cell Isolation kit (MiltenyiBiotec) and directly used for functional assays. All tumor cells and P815 cells were obtained from the Cell Culture Center, Institute of Basic Medicine, Chinese Academy of Medical Sciences. Daudi (human Burkitt's lymphoma), NCI-H446 (human small-cell lung carcinoma), HR8348 (human rectal carcinoma), and MGC-803 (human gastric cancer) cells were cultured in complete RPMI 1640 medium with 10% FCS. G401 cells derived from human rhabdoid tumor were cultured in McCoy's 5a medium (Invitrogen) with 10% FCS. P815 (murine mastocytoma) cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) with 10% FCS.
Flow Cytometry
Cells were collected and sustained with appropriate surface antibodies. Cytometric data were acquired by using a BD Accuri C6 flow cytometer (BD Biosciences). The data were analyzed with FlowJo Software (Tree Star Inc.). Either the percentage of positive staining or mean fluorescence intensity is presented.
IFN-γ Secretion Assay
γδT cells (1 × 105/well) together with targets cells (1 × 104/well) were cultured for 6 h in 96-well plates. Cell-free supernatants were collected to detect IFN-γ levels using the human IFN-γ immunoassay kit (R&D Systems) following the manufacturer's instructions.
Cytotoxicity Assay
To determine specific cytotoxicity, we used the CytoTox 96® Non-radioactive Cytotoxicity Assay (Promega) based on the colorimetric detection of the released enzyme lactate dehydrogenase. In P815-mediated T cell activation assays, P815 cells were incubated with different antibodies for 1 h at 37 °C before co-culture with γδT cells at an E:T ratio of 10:1. The effects of pharmacological inhibitors and neutralization antibody on γδT cell cytotoxicity were measured after pretreating the cells at the indicated doses for 1 h at 37 °C. After 6 h, culture supernatant was used to detect lactate dehydrogenase activity according to the manufacturer's instructions.
Polarization Assay
Polarization assays were performed as described previously (16). In brief, P815 cells were preincubated with different antibodies for 1 h at 37 °C before co-culture with γδT cells at an E:T ratio of 1:1. Cells were pelleted at 20 × g for 3 min and then incubated for 20 min at 37 °C and 5% CO2. Cells were plated on poly-d-lysine-coated 2-well culture slides (BD Biosciences) for 1 h at room temperature followed by fixation with 4% paraformaldehyde and permeabilization in PBS containing 10% normal donkey serum and 0.5% Triton X-100. Anti-perforin antibody was used to stain intracellular perforin-containing granules for 1 h at room temperature. After washing, the samples were sealed on slides with coverslips using ProLong Gold Antifade Reagent as the mounting medium. Images were taken with a Leica DMIRE2 inverted microscope fitted with a Leica TCS SP2 SE confocal imager. Perforin-containing granules were considered polarized when most of the fluorescence was concentrated in the lower quadrant of the γδT cell (i.e. the quadrant that was closest to the target cell).
Receptor Cross-linking Experiments
For antibody-mediated cross-linking of γδT receptors, γδT cells were preincubated with 10 μg/ml isotype control mAb or mAbs specific for γδT receptors for 20 min on ice. After washing, γδT cells were stimulated by cross-linking with 30 μg/ml goat anti-mouse F(ab′)2Ab at 37 °C for the indicated time periods. Cells were moved to ice and then lysed for further analysis.
Ca2+ Flux Analysis
Measurement of the intracellular Ca2+ levels were performed in γδT cells loaded with 2 μm Fluo-4 AM (Invitrogen) for 45 min at room temperature in Hanks' balanced salt solution. γδT cells were washed and resuspended in Hanks' balanced salt solution with 1% FCS. Cells were prewarmed at 37 °C (for antibodies stimulation assay, cells were preincubated with different antibodies on ice for 20 min) and seeded on Lab-Tek glass chamber slides (Nunc). Measurements of intracellular Ca2+ responses were performed at 37 °C with an UltraVIEWVoX3D Live Cell Imaging System (PerkinElmer Life Sciences). After 1 min, 30 μg/ml goat F(ab′)2 anti-mouse IgG was added to cross-link the receptors. Alternatively, IPP (6 μg/ml), ULBP5 (40 μg/ml), or hMSH2 (40 μg/ml) were added to mimic physiological receptor-ligand interactions. Changes in fluorescence are shown as a function of time.
RNA Interference and Plasmid DNA Transfection
For RNA interference, γδT cells were transfected with 300 pmol of siRNAs using an AmaxaNucleofector system. A total of 2 × 107 cells were resuspended in 100 μl of Amaxa Kit solution V, mixed with siRNA, and immediately transfected using program I-24. After transfection γδT cell survival rates were >90%. Cells were incubated for 36 h at 37 °C and 5% CO2, with the last 24 h for resting before the assays were performed as indicated. Three siRNA sequences were used, as described previously (15): Vav1, CGUCGAGGUCAAGCACAUU; c-Cbl, CCUCUCUUCCAAGCACUGA; Cbl-b, GGACAGACGAAAUCUCACA. Pre-validated Vav2- and Vav3-specific siRNAs were purchased from Qiagen. The negative siRNA control was obtained from Invitrogen.
For plasmid DNA transfection, γδT cells were transfected with 8 μg of plasmid DNA using the AmaxaNucleofector kit V, program T-23. Transfected cells were assayed 24 h post-transfection after a rest period. Dead cells were removed by Dead Cell Removal kit (MiltenyiBiotec).
Western Blot
A total of 1 × 107 γδT cells were harvested and lysed in 100 μl CytoBusterTM Protein Extraction Reagent (71009, Novagen) in the presence of Halt Protease and Phosphatase Inhibitor Single-Use Mixture, EDTA-Free (Thermo). Equal amounts of proteins were separated by 8–12% SDS-PAGE, transferred onto nitrocellulose membranes, and blotted with appropriate antibodies. Data were analyzed with ImageJ software (National Institutes of Health).
Statistical Analysis
The results are expressed as the mean ± S.D. The data were analyzed by one-way analysis of variance (SPSS Version 16.0 software) followed by Tukey-Kramer multiple comparisons. In both analyses, the minimum acceptable level of significance was p < 0.05.
RESULTS
NKG2D Ligation Augments γδT Cell Cytotoxicity Mediated by TCRγδ Engagement
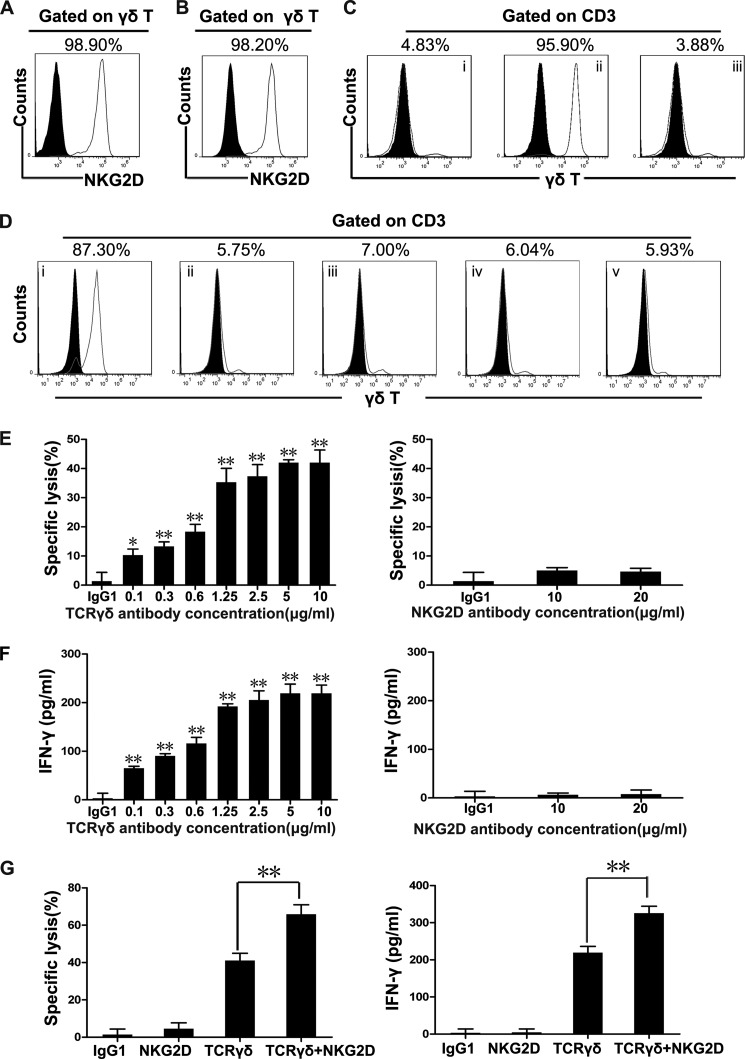
Most of the freshly isolated peripheral blood γδT cells and in vitro expanded peripheral blood γδT cells constitutively expressed NKG2D and TCRγδ receptors (Fig. 1, A and B). NKG2D is a well characterized activating receptor on NK cells and invariant NKT (iNKT) cells (15, 17). In vitro cell culture showed that γδT cells could be expanded by immobilized anti-TCRγδ activating antibodies but not NKG2D activating antibodies or the IgG1 isotype control (Fig. 1, C and D), indicating a TCRγδ-dependent activation exists. Therefore, to verify whether γδT cell cytotoxicity was also TCRγδ-dependent and can be co-stimulated by NKG2D as previous studies reported, redirected lysis assays were performed using antibody-coated FcR+P815 cells (15, 18–21). Our results showed that anti-TCRγδ but not anti-NKG2D activating antibodies initiated γδT cell-specific killing of P815 target cells (Fig. 1E), accompanied by the significant release of IFN-γ (Fig. 1F). However, TNF-α production remained low after the stimulation of TCRγδ or NKG2D (data not shown). Interestingly, NKG2D ligation augmented TCRγδ activation-mediated cytotoxicity and IFN-γ production (Fig. 1G). To test whether this response pattern was unique to TCRγδ antibody or an inherent feature of TCRγδ activation, we further compared the TCRγδ and CD3 activating antibodies in their ability to mediate the killing of redirected P815 cells. We found that anti-CD3 antibody also redirected γδT cell cytotoxicity toward P815 cells (data not shown). Furthermore, freshly isolated γδT cells purified by negative selection showed a similar response to those expanded γδT cells in the killing of TCRγδ or TCRγδ+NKG2D antibody-coated P815 target cells (data not shown).
FIGURE 1.
NKG2D plays a co-stimulatory effect on γδT cell cytotoxicity. A, γδT cells freshly isolated from human peripheral blood were stained with isotype Ab (black solid histogram) and Ab against NKG2D (gray line histogram). Data are representative of at least three independent experiments. B, flow cytometry analysis of NKG2D expression on γδT cells expanded with anti-pan-TCRγδ mAb. γδT cells were expanded in vitro for 14 days and then stained with isotype Ab (black solid histogram) and Ab against NKG2D (gray line histogram). Flow cytometry showed the expression of NKG2D with a gate set on γδT cells. Data are representative of at least three independent experiments. C, PMBCs were stimulated with immobilized IgG1 (i), anti-pan-TCRγδ mAb (ii), or anti-NKG2D mAb (iii). γδT cells were expanded in vitro for 14 days before staining with isotype Ab (black solid histogram) and Ab against TCRγδ (gray line histogram). Data are representative of at least three independent experiments. D, the percentages of γδT cells were analyzed by flow cytometry on day 14 after stimulation with immobilized 1 μg/ml anti-pan-TCRγδ mAb (i) or 0.25 μg/ml (ii), 0.5 μg/ml (iii), 1 μg/ml (iv) or 2 μg/ml (v) anti-NKG2D mAb. Data are representative of at least three independent experiments. E, redirected lysis of P815 cells by resting γδT cells at an E:T ratio of 10:1. P815 cells were preincubated with isotype control antibody (10 μg/ml) or mAb specific for TCRγδ or NKG2D at the indicated antibody concentrations, and γδT cell-mediated cytotoxicity against P815 cells was determined by lactate dehydrogenase assay. Error bars represent the S.D. *, p < 0.05, **, p < 0.01. F, 1 × 105 resting γδT cells and 1 × 104 preincubated P815 cells per well were co-cultured in 96-well plates for 6 h. IFN-γ in the supernatant was measured by ELISA. Values represent the mean ± S.D. *, p < 0.05, **, p < 0.01. G, γδT cell-mediated cytotoxicity against P815 cells (at an E:T ratio of 10:1) preincubated with isotype IgG control (10 μg/ml) or anti-pan-TCRγδ antibody (10 μg/ml) and/or anti-NKG2D antibody (10 μg/ml) was determined by lactate dehydrogenase assay. IFN-γ in the supernatant was measured by ELISA. Error bars represent the S.D. **, p < 0.01.
TCRγδ-induced Lytic Granule Polarization Is Critical for γδT Cell Cytotoxicity
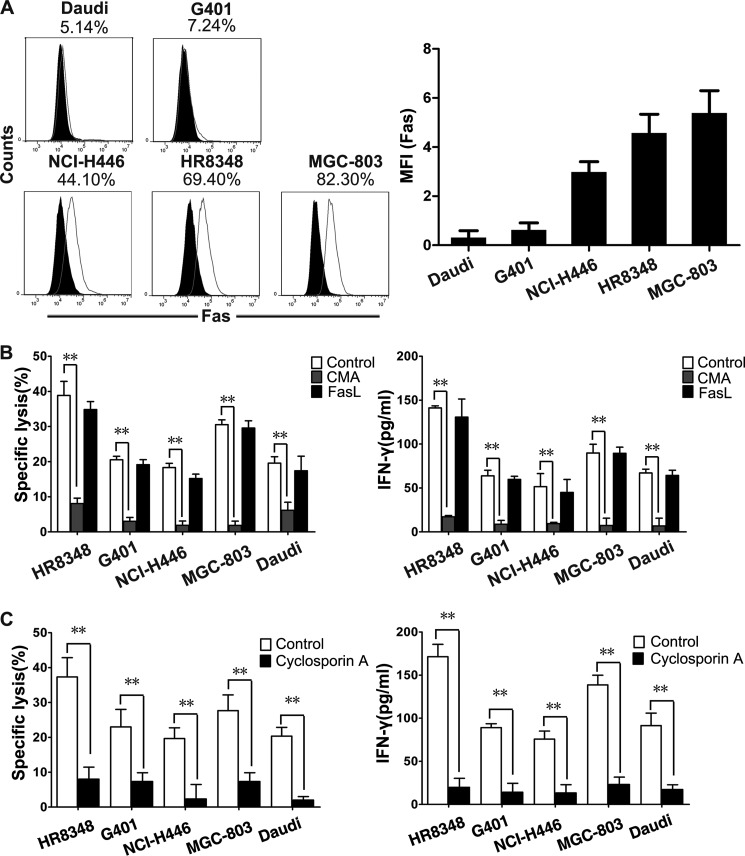
γδT cell cytotoxicity was achieved mainly by two pathways: perforin-granzyme pathway and Fas-FasL pathway (22–24). The contributions of these two pathways were compared in the killing of tumor cell lines by γδT cells. Five tumor cell lines with different levels of Fas expression were selected as the target cells (Fig. 2A). The blockage of Fas-FasL signaling with supplemented soluble anti-FasL antibody did not impair the specific lysis of tumor target cells or the release of IFN-γ by γδT cells. In contrast, CMA, a perforin inhibitor that accelerates perforin degradation within lytic granules (25), blocked 80–95% of γδT cell cytotoxicity (Fig. 2B). Hence, we can conclude that the perforin-granzyme pathway mainly contributes to γδT cell cytotoxicity. A previous study demonstrated that the perforin-granzyme pathway was achieved by lytic granule polarization and degranulation, which were controlled primarily by the TCRαβ in cytotoxic T cells (26). Therefore, TCRγδ dominant killing was further confirmed using tumor cells as targets. Cyclosporin A, a TCR blocking immunosuppressive drug (6), caused a large reduction of γδT cell-mediated tumor killing as well as IFN-γ release (Fig. 2C).
FIGURE 2.
TCRγδ-dependent perforin-granzyme pathway plays a critical role in γδT cell cytotoxicity toward tumor cells. A, flow cytometry analysis of Fas ligand expression on the surface of different tumor cells. Five tumor cell lines (Daudi, NCI-H446, MGC-803, HR8348, and G401) were stained with isotype control Ab (black solid histogram) and with anti-Fas Ab (gray line histogram). Data are representative of at least three independent experiments. MFI, mean fluorescence intensity. B, γδT cell-mediated cytotoxicity and IFN-γ production were abolished by the inhibition of the perforin-granzyme pathway with 100 nm CMA but not by the blockage of the Fas-FasL pathway with 10 μg/ml soluble anti-FasL antibody. Values represent the mean ± S.D. **, p < 0.01. C, γδT cell-mediated cytotoxicity and IFN-γ production could be largely decreased by TCR inhibitor cyclosporin A (CsA) (100 ng/ml). Data represent mean ± S.D. **, p < 0.01.
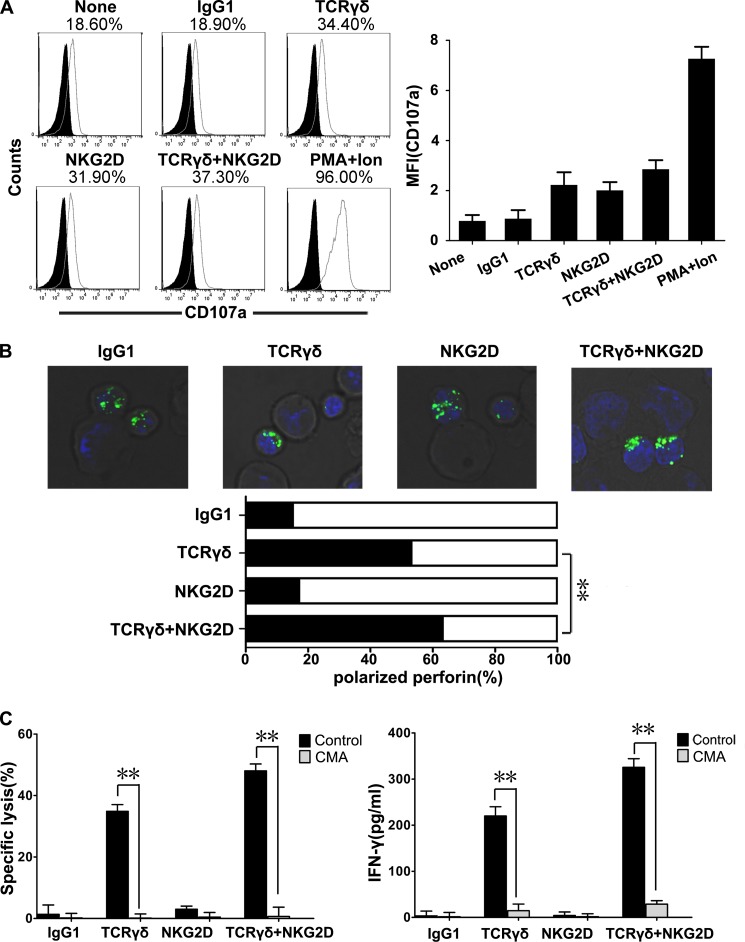
Perforin-granzyme pathway involves two processes, lytic granule polarization and degranulation, which can be coupled or uncoupled process (27, 28). We first measured cellular degranulation based on cell surface expression of CD107a (LAMP-1). No significant difference was observed in CD107a expression of γδT cells after TCRγδ activation versus NKG2D activation (Fig. 3A), indicating that degranulation alone does not guarantee the occurrence of specific killing. Intracellular staining of the perforin-containing granules showed that TCRγδ but not NKG2D engagement induced lytic granule polarization, and NKG2D in combination with TCRγδ activation merely enhanced this effect (Fig. 3B). In contrast to cytotoxic T cells, the lytic granule polarization of γδT cells was co-stimulated by NKG2D but not by LFA-1 (data not shown) (26). Noteworthily, pretreatment of γδT cells with CMA almost completely blocked the killing function and IFN-γ release of γδT cells (Fig. 3C). These results taken together suggest that TCRγδ-induced T cell cytotoxicity mainly depends on lytic granule polarization.
FIGURE 3.
TCRγδ-dependent lytic granule polarization is critical for γδT cell cytotoxicity. A, flow-cytometric analysis of CD107a (degranulation marker) expression in γδT cells co-cultured with antibody-preincubated P815 cells. FITC-labeled anti-TCRγδ mAb and phosphatidylethanolamine-labeled anti-CD107a mAb were used for γδT cell staining. By gating on γδT, the percentage of CD107a+ cells and its mean fluorescence intensity (MFI) was calculated. Data are representative of at least three independent experiments. B, representative confocal images of γδT lytic granule polarization toward preincubated P815 cells. Intracellular perforin (upper panel) staining was positive in γδT cells (the smaller cells) but not in P815 cells (the larger cells). Polarization of perforin-containing granules toward target cells was quantified (lower panel) as polarized (black) and nonpolarized (white). The number of synapses studied was 100 in each case. Data are representative of three independent experiments. **, p < 0.01. C. γδT cell-mediated lysis of target cells (left panel) and the IFN-γ release (right panel) could be blocked by 100 nm perforin inhibitor CMA. Values represent the mean ± S.D. **, p < 0.01.
The PLC-γ1 Pathway Plays a Critical Role in γδT Cell Cytotoxicity
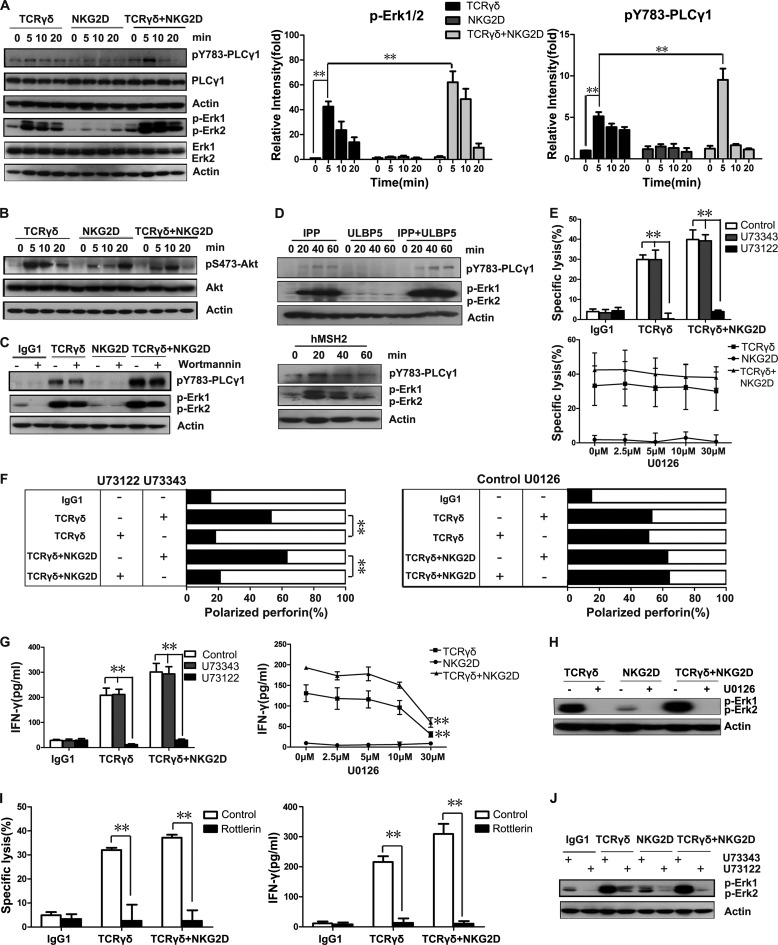
Next we questioned which molecules participate in TCRγδ-dependent lytic granule polarization and the molecular mechanism of the functional difference between TCRγδ and NKG2D. Three important signaling pathways are associated with αβT cell and NK cell cytotoxicity: PLC-γ, MAPK/Erk, and PI3K (15, 29). We tested whether γδT cells use similar signaling pathways. Stimulation of TCRγδ but not NKG2D induced strong phosphorylation of PLC-γ1 and Erk, which were enhanced when both TCRγδ and NKG2D were engaged (Fig. 4A). Phosphorylation of Erk induced by TCRγδ or TCR and NKG2D co-engagement reached a maximum at 5 min after stimulation and then decreased gradually. TCRγδ-induced phosphorylation of PLC-γ1 lasted longer than Erk phosphorylation. When both TCRγδ and NKG2D were engaged, the phosphorylation of PLC-γ1 reached a maximum at 5 min and then quickly disappeared within 20 min after stimulation (Fig. 4A). In contrast, phosphorylation of Akt, a downstream target of PI3K activation, showed no enhancement after the co-engagement of TCRγδ and NKG2D compared with either TCRγδ or NKG2D stimulation alone (Fig. 4B). Blocking PI3K signaling by Wortmannin did not significantly impair the phosphorylation of PLC-γ1 or Erk (Fig. 4C), suggesting that unlike the PLC-γ1 and Erk pathways, the PI3K-Akt pathway does not play a major role in TCRγδ- and NKG2D-mediated cytotoxicity.
FIGURE 4.
Activation of the PLC-γ1 pathway is required for γδT cell cytotoxicity. A, Western blot analysis of the activation of the PLC-γ and Erk pathways during γδT cell-mediated cytotoxicity. Resting γδT cells were preincubated with mAbs specific for TCRγδ and/or NKG2D on ice for 20 min and stimulated by cross-linking with secondary goat F(ab′)2 anti-mouse IgG at 37 °C for the indicated times. The levels of p-PLC-γ1, PLC-γ1, p-Erk1 and p-Erk2, and Erk1 and Erk2 were assessed by immunoblots with individual specific antibodies. Actin expression was also detected as the loading control. Normalized relative intensities of phosphorylated PLC-γ1 and Erk1/2 were quantified using ImageJ software. Values represent the mean ± S.D. **, p < 0.01. B, Western blot analysis of the activation of the PI3K-Akt pathway in γδT cell cytotoxicity. Resting γδT cells were preincubated with mAbs specific for TCRγδ and/or NKG2D on ice for 20 min and stimulated by cross-linking with secondary goat F(ab′)2 anti-mouse IgG at 37 °C for the indicated times. The levels of p-Akt and Akt were assessed by immunoblots with individual specific antibodies. Actin expression was also detected as the loading control. Data are representative of three independent experiments. C, resting γδT cells were preincubated for 1 h at 37 °C with 1 μm PI3K inhibitor wortmannin or an equal amount of DMSO before stimulation with isotype control mAb or mAbs specific for TCRγδ and/or NKG2D. The levels of p-PLC-γ1, p-Erk1, and p-Erk2 were assessed by immunoblots with individual specific antibodies. Data are representative of three independent experiments. D, Western blot analysis of the activation of the PLC-γ1 and Erk in response to physiological receptor-ligand interactions in γδT cells. Resting γδT cells were stimulated by IPP, ULBP5, IPP+ULBP5, and hMSH2 at 37 °C for the indicated time. Cell lysate was blotted for p-PLC-γ1, p-Erk1, and p-Erk2. Actin expression was also detected as the loading control. Data are representative of three independent experiments. E, resting γδT cells were pretreated for 1 h at 37 °C with 5 μm U73122, with its inactive analog U73343, or with U0126 at the indicated concentrations. P815 cells were preincubated with isotype control antibody (10 μg/ml) or mAb specific for TCRγδ (10 μg/ml), NKG2D (10 μg/ml), or both. After washing, inhibitor-pretreated γδT cells and coated P815 cells were mixed for cytotoxicity assays at a 10:1 E:T ratio. Values represent the mean ± S.D. **, p < 0.01. F, resting γδT cells were pretreated for 1 h at 37 °C with 5 μm U73122, with its inactive analog U73343, or with U0126 at 30 μm before quantification of polarization of perforin-containing granules toward target cells. **, p < 0.01. G, resting γδT cells were pretreated for 1 h at 37 °C with 5 μm U73122, with its inactive analog U73343, or with U0126 at the indicated concentrations. IFN-γ production was detected in the supernatant from 1 × 105 inhibitor-pretreated γδT cells and 1 × 104 preincubated P815 cells co-culture. Values represent the mean ± S.D. **, p < 0.01. H, resting γδT cells were incubated for 1 h at 37 °C with 30 μm U0126 before stimulation with mAbs specific for TCRγδ and/or NKG2D. The levels of p-Erk1 and p-Erk2 were assessed by Western blot. I, resting γδT cells were treated with 100 μm rottlerin at 37 °C for 1 h before the lactate dehydrogenase assay. Cytotoxicity was measured at an E:T ratio of 10:1. Supernatant IFN-γ was measured by ELISA. The values represent the mean ± S.D. **, p < 0.01. J, resting γδT cells were pretreated for 1 h at 37 °C with 5 μm U73122, with its inactive analog U73343 before Western blot analysis for p-Erk1 and p-Erk2. The blot was stripped and reprobed for actin. Data are representative of three independent experiments.
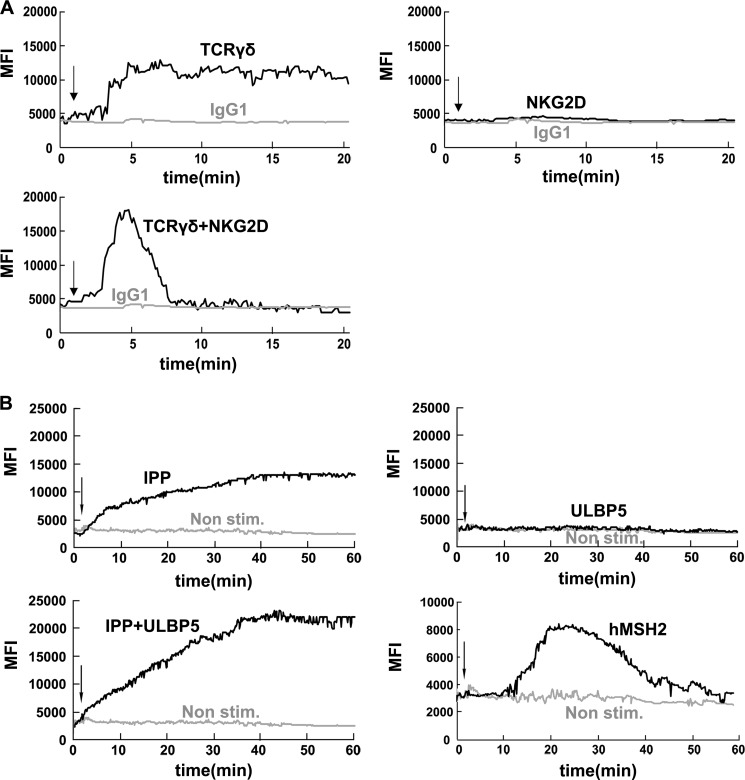
To further test the receptor-ligand interactions in a physiological relevant situation, we analyzed the phosphorylation of PLC-γ1 and Erk1/2 in the presence of isopentenyl pyrophosphate (a natural ligand for TCRγδ), ULBP5 (a natural ligand for NKG2D), either alone or in combination, and hMSH2 (a natural ligand for γδT cells that can be dually recognized by TCRγδ and NKG2D). As shown in Fig. 4D, the tyrosine phosphorylation of PLC-γ1 and Erk1/2 was induced by IPP but not ULBP5. Combination of IPP and ULBP5 resulted in enhanced phosphorylation of PLC-γ1 and Erk1/2. Unlike anti-TCRγδ+anti-NKG2D stimulation, IPP+ULBP5-induced phosphorylation of PLC-γ1 did not quickly disappear after reaching the peak. In addition, the phosphorylation of Erk1/2 was more sustained in comparison with anti-TCRγδ+anti-NKG2D stimulation (data not shown). When γδT cells were stimulated with hMSH2, the phosphorylation of Erk1/2 reached a maximum at 20 min and then gradually decreased within 60 min, whereas the phosphorylation of PLC-γ1 reached a peak at 20 min and then quickly disappeared within 40 min (Fig. 4D).
Next, we applied the inhibitors of PLC-γ and Erk pathways to clarify their roles in γδT cell cytotoxicity. The PLC-γ inhibitor U73122, but not its inactive analog U73343, fully abrogated γδT cell cytotoxicity. MEK inhibitor U0126, an upstream activator of Erk, did not abrogate γδT cell cytotoxicity even under high concentrations (Fig. 4E). As a consequence, U73122 but not U0126 blocked lytic granule polarization (Fig. 4F). Intriguingly, IFN-γ production was inhibited by both U73122 and U0126 (30 μm) (Fig. 4G). U0126 at the concentration of 30 μm could completely abrogate the phosphorylation of Erk (Fig. 4H). These results suggest that, unlike IFN-γ production, γδT cell cytotoxicity is an independent event downstream of PLC-γ1 but not Erk. Because the activation of PLC-γ1 pathway can induce protein kinase C-θ (PKC-θ) activation through diacylglycerol (30, 31), we tested the roles of PKC-θ in γδT cell cytotoxicity. As shown in Fig. 4I, PKC-θ inhibitor could completely abrogate γδT cell cytotoxicity and IFN-γ production, confirming that PLC-γ pathway plays a critical role in γδT cell cytotoxicity induced by TCRγδ or TCRγδ+NKG2D. In addition, Erk phosphorylation was inhibited by U73122 (Fig. 4J), indicating that Erk might be a downstream molecule of the PLC-γ pathway.
Engagement of TCRγδ and NKG2D Induces Ca2+ Mobilization
Calcium (Ca2+) mobilization typically depends on PLC-γ activation (32, 33). Elevation of intracellular free Ca2+ concentration is a key triggering signal for αβT cell activation (34, 35). Therefore, we analyzed the intensity of Ca2+ responses in the presence of TCRγδ and/or NKG2D stimulation. Stimulation of TCRγδ but not NKG2D induced strong Ca2+ mobilization, and this Ca2+ response was sustained within 30 min. With NKG2D co-stimulation, Ca2+ mobilization was enhanced, but this Ca2+ response was dramatically quickened and shortened as compared with TCRγδ engagement (Fig. 5A). These data fall in the same pattern as in the phosphorylation of PLC-γ1 (Fig. 4A). Ca2+ mobilization was also evaluated after stimulation with IPP, either alone or in combination with ULBP5, and hMSH2 (Fig. 5B). These Ca2+ mobilization results were consistent with the above PLC-γ1 phosphorylation results (Fig. 4D). Taken together, these results suggest that the phosphorylation of PLC-γ1 and Ca2+ mobilization are essential for γδT cell cytotoxicity in response to the natural ligands such as IPP, ULBP5, and hMSH2.
FIGURE 5.
Activation of PLC-γ1 associated Ca2+ mobilization is required for γδT cells cytotoxicity. A, Ca2+ mobilization in responses to stimuli with TCRγδ, NKG2D, or both. Resting γδT cells were incubated with Fluo-4 at room temperature for 45 min. After washing, the cells were incubated with mAbs for TCRγδ and/or NKG2D in Hanks' balanced salt solution with 1% FCS on ice for 20 min. Then the cells were prewarmed at 37 °C before analysis with the UltraVIEW VoX 3D Live Cell Imaging System. After 1min goat F(ab′)2 anti-mouse IgG was added to cross-link the receptors. Changes in fluorescence were shown as a function of time. Data are representative of three independent experiments. MFI, mean fluorescence intensity. B, analysis of Ca2+ mobilization during physiological receptor-ligand interactions in γδT cells. Resting γδT cells were incubated with Fluo-4 at room temperature for 45 min. After wash, the cells were prewarmed at 37 °C before analysis with UltraVIEW VoX 3D Live Cell Imaging System. After 1min, IPP, ULBP5, IPP+ULBP5, and hMSH2 were applied to mimic physiological receptor-ligand interactions. Changes in fluorescence are shown as a function of time. Data are representative of three independent experiments.
Vav1 Is Required for γδT Cell Cytotoxicity
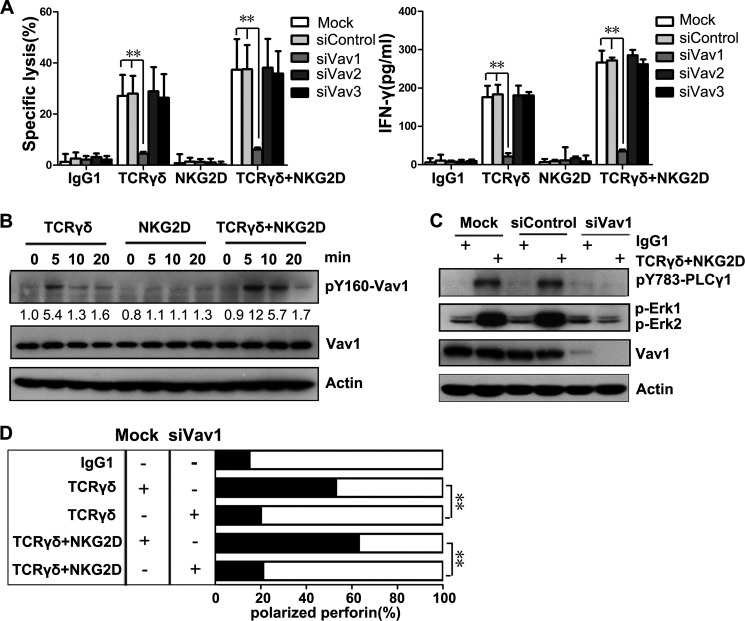
Vav proteins, which include Vav1, Vav2, and Vav3, play central roles in the regulation of lymphocyte development, activation, and proliferation (13). γδT cell cytotoxicity toward MHC class I-deficient Daudi cells is blocked by the forced expression of MHC class I molecule (36), and Vav1 is a preferred substrate of MHC class I-specific inhibitory receptor (37, 38), suggesting that Vav1 may be crucial for γδT cell cytotoxicity. Furthermore, NKG2D signals mainly through either PI3K or Grb2-Vav (39, 40). The above finding that NKG2D co-stimulatory effect is independent of PI3K signaling suggests that NKG2D might signal through Vav1 to co-stimulate TCRγδ. Therefore, we detected whether Vav proteins were involved in γδT cell cytotoxicity-related signaling and tested if they were located at the upstream of PLC-γ1 as shown in NK cells and αβT cells by knocking down Vav1, Vav2, and Vav3 using specific siRNAs (15, 32, 41, 42). γδT cell-mediated killing and IFN-γ release were markedly diminished by the knockdown of Vav1, but not Vav2 or Vav3 (Fig. 6A), by specific small interfering RNAs (siRNAs). This result is consistent with previous findings in Vav1−/−mouse γδT cells (43).
FIGURE 6.
γδT cell cytotoxicity is Vav1-dependent. A, lysis of P815 cells and IFN-γ production by rested γδT cells transfected with the indicated siRNAs before stimulation with mAb to TCRγδ, NKG2D, or both. Values represent the mean ± S.D. **, p < 0.01. B, Western blot analysis of phosphorylation of Vav1 (pY160-Vav1) during the activation of γδT cells. The normalized intensities of the phosphorylated Vav1 relative to actin were quantified using ImageJ software. Data are representative of three independent experiments. C, Western blot analysis of p-PLC-γ1, p-Erk1, p-Erk2, and Vav1 in γδT cells transfected with Vav1-specific siRNAs or control siRNAs. The cells were stimulated with isotype control mAb or mAb specific for TCRγδ and NKG2D. D, rested γδT cells were mock-transfected or transfected with Vav1-specific siRNAs. Polarization of perforin-containing granules toward target cells was quantified. Data are representative of three independent experiments. **, p < 0.01.
Activation of Vav1 is accompanied by Vav1 tyrosine phosphorylation, including tyrosine 160 (44). Vav1 phosphorylation was observed after TCRγδ stimulation and was more prominent under the combinational activation of TCRγδ and NKG2D (Fig. 6B). We then investigated whether Vav1 controlled PLC-γ1 and Erk signaling. Knockdown of Vav1 completely abrogated the phosphorylation of PLC-γ1 and Erk (Fig. 6C), indicating that both PLC-γ1 and Erk might be downstream molecules of Vav1 signaling in γδT cells. It was further supported by the fact that knockdown of Vav1 fully disrupted lytic granule polarization in γδT cells (Fig. 6D).
Cbl-b Inhibits Vav1-dependent γδT Cell Activation Signals
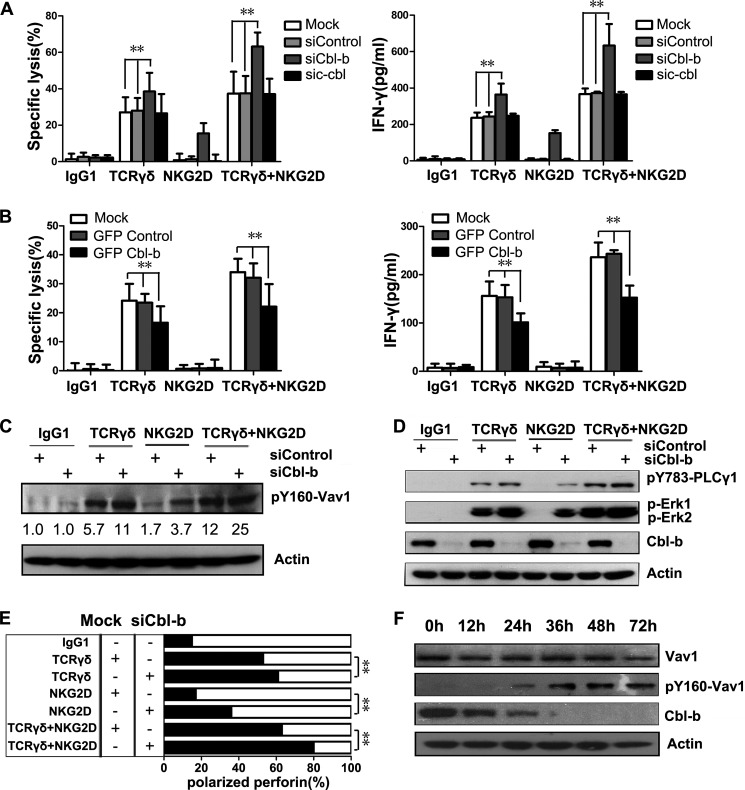
TCR signaling is negatively regulated by the Cbl family proteins (45). Mammalian cells express three Cbl genes, c-Cbl (Cbl), Cbl-b, and Cbl-3 (46). Cbl-3 is primarily expressed in epithelial cells (47). c-Cbl inhibits NK cell cytotoxicity, and Cbl-b negatively regulates peripheral T lymphocyte functions (14). Thus, we investigated the potential role of Cbl in γδT cell cytotoxicity by knocking down c-Cbl and Cbl-b using specific siRNAs. Knockdown of Cbl-b enhanced γδT cell cytotoxicity toward redirected P815 cells after TCRγδ and/or NKG2D engagement, whereas knockdown of c-Cbl had no effect (Fig. 7A). Interestingly, knockdown of Cbl-b enabled NKG2D to induce cytotoxicity. Cbl-b knockdown also promoted IFN-γ secretion in response to TCRγδ and/or NKG2D engagement (Fig. 7A).
FIGURE 7.
Cbl-b sets a threshold for γδT cell cytotoxicity and imposes the requirement of TCRγδ-dependent activation. A, lysis of P815 cells and IFN-γ production by rested γδT cells that were transfected with the indicated siRNAs. Values represent the mean ± S.D. **, p < 0.01. B, lysis of P815 cells and IFN-γ production by rested γδT cells transfected with the indicated plasmids. Values represent the mean ± S.D. **, p < 0.01. C, Western blot analysis of the phosphorylation of Vav1 (pY160-Vav1) in γδT cells transfected with control siRNAs or Cbl-b-specific siRNAs before stimulation with isotype control mAb or mAb against TCRγδ, NKG2D, or both. Normalized relative intensities of phosphorylated Vav1 to actin are presented. Data are representative of three independent experiments. D, rested γδT cells were transfected with control siRNAs or Cbl-b-specific siRNAs, and lysates were immunoblotted for p-PLC-γ1, p-Erk1, and p-Erk2, or Cbl-b. Blots were reprobed for actin as loading controls. Data are representative of three independent experiments. E, rested γδT cells were mock-transfected or transfected with Cbl-b-specific siRNAs. Polarization of perforin-containing granules toward target cells was quantified. Data are representative of three independent experiments. **, p < 0.01. F, rested γδT cells were transfected with Cbl-b-specific siRNAs for the indicated time periods before incubation with mAb specific to NKG2D. Lysates were immunoblotted for Vav1, p-Vav1 and Cbl-b. Actin was used as the sample loading control. Data are representative of three independent experiments.
Of note, knockdown of Cbl-b rendered γδT cells to acquire cytotoxicity by NKG2D engagement. When TCRγδ was engaged, γδT cell cytotoxicity toward redirected P815 cells was largely augmented by NKG2D ligation. This finding raised the question of whether NKG2D signaling might be more sensitive to Cbl-b-mediated inhibition. We hypothesized that Cbl-b overexpression would negatively regulate the co-stimulatory effect of NKG2D. To test this hypothesis, γδT cells were transiently transfected with GFP-tagged Cbl-b. Cbl-b overexpression resulted in a decreased cytotoxicity and IFN-γ production toward anti-TCRγδ-redirected P815 cells. However, no stronger inhibition was observed in the cytotoxicity or IFN-γ production toward anti-TCRγδ and anti-NKG2D-redirected P815 cells (Fig. 7, A and B). Therefore, Cbl-b might be the gatekeeper for NKG2D, and once the Cbl-b inhibition signal is eliminated, NKG2D may exhibit a strong co-stimulatory effect on TCR-ligated γδT cells.
After Cbl-b knockdown, phosphorylated Vav1, PLC-γ1, and Erk were all up-regulated after TCRγδ or NKG2D activation (Fig. 7, C and D). NKG2D engagement induced the phosphorylation of Vav1 and PLC-γ1 after Cbl-b knockdown. Taken together, these results suggest that Vav1 and PLC-γ1 are critical signals for γδT cell cytotoxicity. When the phosphorylation of Vav1 and PLC-γ1 reached a certain level, γδT cells became cytotoxic. In line with this finding, lytic granule polarization toward target cells was also increased after Cbl-b knockdown (Fig. 7E).
Cbl-b is an E3 ubiquitin ligase that ubiquitylates cytosolic signaling molecules and leads to proteasome-mediated degradation of target proteins. Because Cbl-b knockdown increased Vav1 phosphorylation, we hypothesized that Cbl-b regulates Vav1 ubiquitylation and degradation. In a time-course study, we found that knockdown of Cbl-b resulted in the up-regulation of phosphorylated Vav1, but the total amount of Vav1 remained constant (Fig. 7F). Thus, Cbl-b might regulate γδT cell cytotoxicity by targeting phosphorylated Vav1.
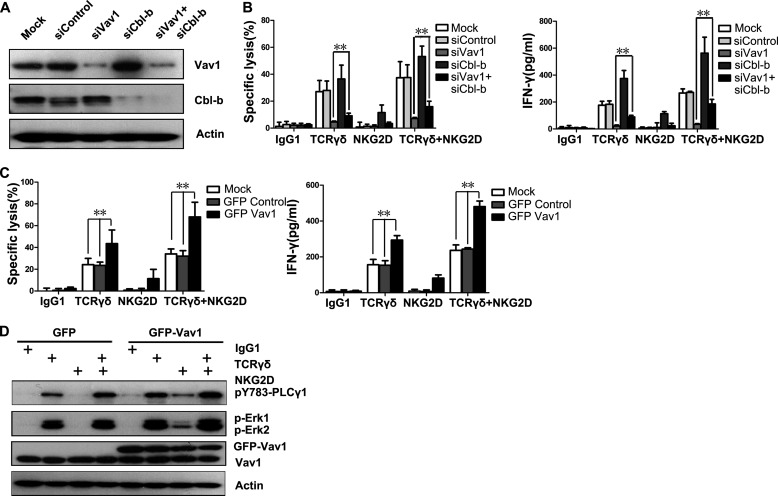
Increased Vav1 Expression Overcomes Cbl-b-mediated Inhibition
A previous finding that Vav1 is unessential for Cbl-mediated regulation of conventional αβT cell development, shown in Vav1−/−Cbl−/− mice (48), indicates that a Vav1-independent bypass activation pathway might exist. To test whether a similar mechanism is present in γδT cells, double knockdown of Vav1 and Cbl-b was performed (Fig. 8A). The deficiency of γδT cell cytotoxicity and IFN-γ production due to Vav1 knockdown was partially rescued by the Cbl-b knockdown (Fig. 8B). Although γδT cell responses were rescued to some extent by double knockdown of Vav1 and Cbl-b, cytotoxicity and IFN-γ production were far from normal (Fig. 8B). Thus, the absence of Cbl-b might just release a bypass activation pathway such as Vav2 or Vav3 signaling that functions more weakly than Vav1. This result raised the possibility that Vav1 overexpression could override Cbl-b-mediated inhibition and bypass the dependence on TCRγδ activation. Meanwhile, if NKG2D signals through a Cbl-b-sensitive pathway apart from the Vav1-PLC-γ1 axis, Vav1 overexpression would not override the Cbl-b-mediated inhibition of NKG2D signaling and would not increase the co-stimulatory effect of NKG2D on TCRγδ. To test this hypothesis, γδT cells were transiently transfected with GFP-tagged Vav1. Overexpression of GFP-tagged Vav1 resulted in increased γδT cell cytotoxicity and IFN-γ production even after NKG2D engagement (Fig. 8C). Vav1 overexpression bypassed the Cbl-b-mediated inhibition by stimulating the phosphorylation of PLC-γ1 and Erk after NKG2D engagement (Fig. 8D). These data suggest NKG2D co-stimulation with TCRγδ through the Vav1-PLC-γ1 axis. Vav1 overexpression can circumvent the necessity for TCRγδ-dependent activation and enable NKG2D alone to become sufficient for activation. Taken together, our data suggest that Cbl-b imposes a requirement of TCRγδ-dependent activation, and the activation of γδT cell cytotoxicity requires a strong signal to overcome the activation threshold set by the inhibitory effect of Cbl-b.
FIGURE 8.
Increased Vav1 expression overcomes Cbl-b-mediated inhibition. A, Western blot analysis of the knockdown efficiency of siRNAs specific for Vav1 or Cbl-b in γδT cells. B, redirected lysis of P815 cells and the release of IFN-γ by rested γδT cells transfected with the indicated siRNAs at an E:T ratio of 10:1. γδT cells were stimulated with mAb to TCRγδ, NKG2D, or both. Values represent the mean ± S.D. **, p < 0.01. C, lysis of P815 cells and IFN-γ production by rested γδT cells that were mock-transfected or transfected with GFP control or GFP-Vav1 plasmid before stimulation with mAb to TCRγδ, NKG2D, or both. Values represent the mean ± S.D. **, p < 0.01. D, Western blot analysis of the p-PLC-γ1, p-Erk1, and p-Erk2 in γδT cells transfected with the indicated plasmids before stimulation with mAb to TCRγδ, NKG2D, or both. Data are representative of three independent experiments.
DISCUSSION
The major finding of this study is that TCRγδ-dependent lytic granule polarization makes the major contribution to γδT cell cytotoxicity through a Vav1-dependent PLC-γ1 signaling pathway in which NKG2D plays an augmentative role. TCRγδ and NKG2D are two important receptors on γδT cells responsible for tumor lysis. Our results show that TCRγδ ligation alone could initiate downstream Vav1-dependent PLC-γ1 signaling in γδT cells, resulting in lytic granule polarization and active killing, whereas NKG2D engagement alone could not trigger effective cytotoxicity-related signaling and, therefore, could not overcome the inhibitory effect of Cbl-b. However, NKG2D augmented the existing TCRγδ-mediated signaling. This finding provides new insights into the molecular mechanisms behind the cytotoxicity of γδT cells against tumor cells.
The fact that γδT cell cytotoxicity is TCRγδ-dependent and can be co-stimulated by NKG2D has been partially characterized. One question addressed here is why TCRγδ alone, but not NKG2D, can activate γδT cell cytotoxicity. Using TCRγδ and/or NKG2D-activating antibody-redirected P815 cells as a target cell model, we demonstrated that γδT cell cytotoxicity was truly TCRγδ-dependent, whereas NKG2D only played a supporting role in the presence of a TCRγδ signal. This is not consistent with the Rincon-Orozco et al. (49) study that γδT cells might also be directly activated by NKG2D. We also discovered that γδT cell cytotoxicity largely depended on the polarized release of cytotoxic granules toward target cells induced by TCRγδ ligation but not the degranulation triggered by TCRγδ or NKG2D ligation. The ability to induce lytic granule polarization was the functional difference between TCRγδ and NKG2D. It is also true in NK cells that natural cytotoxicity is achieved by polarized release of perforin and granzymes at the NK cell-target cell immunological synapse, whereas degranulation without lytic granule polarization does not lead to the effective lysis of target cells (16). In NK cells, only the synergistic engagement of NKG2D (CD314) and 2B4 (CD244) or 2B4 and DNAM-1 (CD226) can activate NK cells (50). However, in iNKT cells, NKG2D plays not only a direct, TCR-independent, NK-like cytolysis role, but also plays a co-stimulatory role in the CD1d-induced activation of iNKT cells (17). These findings suggest that NKG2D might play different roles in different cells.
Then why is the TCRγδ signal alone able to induce the polarized release of cytotoxic granules and does how the co-stimulatory effect mediated by NKG2D takes place? We discovered that TCRγδ activation through antibody or IPP can induce phosphorylation of PLC-γ1 and Ca2+ responses, which were enhanced when additional NKG2D engaged through antibody or ULBP5. Particularly, co-stimulation of TCRγδ with NKG2D antibody led to a transient up-regulation of PLC-γ1 phosphorylation and Ca2+ responses, peaked at 5 min, and then quickly disappeared; this was also seen in hMSH2-stimulated γδT cells. Our results are consistent with the previous finding that TCR/CD3 stimulation of γδT cells induced sustained intracellular calcium mobilization, and NKG2D co-engagement led to the strongest calcium mobilization at 5 min and subsequently quick disappearance (21). Different from TCR and NKG2D activation through anti-TCRγδ+anti-NKG2D activating antibodies or hMSH2 protein, IPP+ULBP5 caused a more sustained PLC-γ1 phosphorylation and Ca2+ mobilization. These variances in γδT cell downstream signaling may come from different levels of TCR and/or NKG2D cross-linking caused by the above ligands. Our results suggested that TCRγδ and NKG2D dually recognized ligands can induce stronger activation of PLC-γ1 pathway, leading to more potent γδT cell cytotoxicity. Therefore, γδT cells may perform strong immunosurveillance to TCRγδ and NKG2D dually recognized ligands expressing tumor or infected cells.
The Vav family proteins (Vav1, Vav2, and Vav3) are guanine nucleotide exchange factors (GEFs) for Rho-family GTPases. In conventional αβT cells, Vav1 regulates the phosphorylation of PLC-γ1 and plays a central role in the regulation of lymphocyte development, activation, and proliferation (13). Vav1 regulates PLC-γ1 phosphorylation via PI3K-dependent and -independent pathways (41, 42, 51, 52). In the present study we found that the phosphorylation of PLC-γ1 was unaffected by the PI3K inhibitor, indicating that Vav1 might regulate the phosphorylation of PLC-γ1 through the PI3K-independent pathway in γδT cells.
The mammalian Cbl family proteins include three homologues known as c-Cbl, Cbl-b, and Cbl-3. Although all three share the highly conserved TKB (tyrosine kinase-binding), linker, and RING domains, which enable them to function as E3 ubiquitin ligases, they are involved in the degradation of different phosphorylated proteins through the ubiquitin proteasome system (41). In this study we found that Cbl-b inhibited γδT cell cytotoxicity by decreasing the level of phosphorylated Vav1, indicating that Cbl-b may be largely involved in functional modifications of signaling molecules in the earlier phase of γδT cell cytotoxicity activation. Vav1 overexpression and Cbl-b knockdown resulted in permissive γδT cell activation in the presence of NKG2D engagement, suggesting that the NKG2D co-stimulatory effect requires the Vav1-PLC-γ1 axis. Cbl-b could enforce a TCRγδ dependence of γδT cell cytotoxicity.
In contrast to an earlier study showing that NKG2D fulfilled its co-stimulatory function through the PKC-θ pathway and PKC-θ inhibitor could not abrogate anti-CD3 antibody-induced γδT cell cytotoxicity (21), we found that PKC-θ pathway also played an important role in TCRγδ-dependent cytotoxicity, as PKC-θ inhibitor could completely abrogate γδT cell cytotoxicity. In this respect, γδT cells were similar to the conventional αβT cells, as PKC-θ is crucial for the activation of mature T cells and cytokine production (53). PKC-θ is a novel PKC isoform whose activation requires diacylglycerol but not Ca2+ (30). The phosphorylation of PLC-γ1 triggers the generation of diacylglycerol and 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate and results in the induction of Ca2+influx (31). TCRγδ engagement can induce strong activation of PLC-γ1 pathway resulting in the generation of diacylglycerol, which can in turn activate PKC-θ. PKC-θ signaling leads to the degradation of Cbl-b (54), which induces polarized release of cytotoxic granules and activation of γδT cell cytotoxicity. In the presence of TCRγδ stimulation, NKG2D ligation, dispensed with Cbl-b inhibition, can turn on downstream cytotoxicity-related signaling.
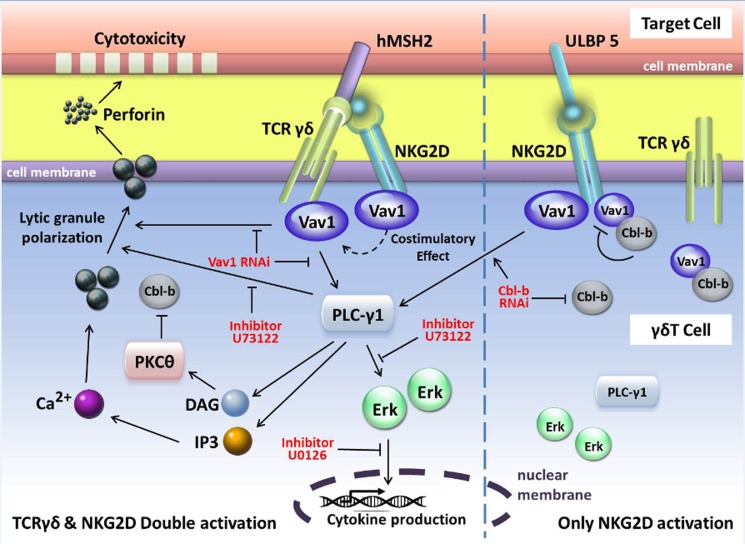
Based on the findings above, we inferred a model of the TCRγδ and NKG2D signaling network in γδT cells (Fig. 9), indicating that both TCRγδ signaling and NKG2D signaling rely on the Vav1-PLC-γ1 pathway for cytolytic function. TCRγδ works independently, whereas NKG2D functions as a co-stimulatory receptor in the presence of TCRγδ stimulation. Vav1 is a critical upstream activator of PLC-γ1 and Erk signaling in γδT cells, whereas PLC- γ1 is upstream of Erk. Vav1 is negatively regulated by Cbl-b. Inhibition of both Vav1 and PLC-γ1 abrogates γδT cell cytotoxicity and cytokine release, whereas inhibition of Erk only blocks cytokine release but not lytic granule polarization. This model implies the existence of separate regulatory mechanisms of cytokine release versus cytotoxicity in γδT cells. Cbl-b serves as a gatekeeper that negatively regulates the activation of γδT cells by targeting phosphorylated Vav1. In summary, Vav1-PLC-γ1-dependent TCRγδ activation of γδT cell can overcome Cbl-b-mediated inhibition of cytotoxicity. Our findings provide a new understanding of the molecular mechanisms behind the cytotoxic function of γδT cells, which may have implications for γδT-based cancer immunotherapy by optimizing γδT cell effector function, most likely by employing NKG2D ligands as adjuvant molecules or by modulating signaling pathways in γδT cells.
FIGURE 9.
Schematic diagram of the signaling pathways associated with γδT cell cytotoxicity and cytokine production. The phosphorylation of Vav1 and PLC-γ1 is essential to lytic granule polarization and cytokine release during γδT cell-mediated cytotoxicity, whereas the phosphorylation of Erk is only associated with cytokine release. Cbl-b has an inhibitory effect on Vav1-dependent γδT cell activation signals, and PKC-θ plays a critical role in the activation of γδT cell cytotoxicity by regulating the balance of phosphorylation of Vav1 and Cbl-b. DAG, diacylglycerol; IP3, 1,4,5-trisphosphate.
Acknowledgments
We thank Dr. Wei Xu (Baylor Institute for Immunology Research) and Dr. Mingxiao He (Duke University for Immunology Research) for critical reading of this manuscript. We thank Professor Eric O. Long (Laboratory of Immunogenetics, NIAID, National Institute of Health) for useful ideas.
This work was supported by Beijing Natural Science Foundation Grant 5122031, National Natural Science Foundation of China Grant 30972776, and National High Technology Research and Development Program (863 Program) Grants 2006AA02Z480 and 2007AA021109.
- TCR
- T cell receptor
- Ab
- antibody
- CMA
- concanamycin A
- hMSH2
- human MutS homologue 2
- IPP
- isopentenyl pyrophosphate
- NKG2D
- natural killer group 2 member D
- PLC-γ1
- phospholipase C-γ1
- TNF-α
- tumor necrosis factor-α
- ULBP
- UL-16 binding proteins
- E:T
- effector cells to target cells
- iNKT
- invariant NKT.
REFERENCES
- 1. Haas W., Pereira P., Tonegawa S. (1993) γ/δ cells. Annu. Rev. Immunol. 11, 637–685 [DOI] [PubMed] [Google Scholar]
- 2. Kabelitz D., Wesch D., He W. (2007) Perspectives of γδ T cells in tumor immunology. Cancer Res. 67, 5–8 [DOI] [PubMed] [Google Scholar]
- 3. Champagne E. (2011) γδ T cell receptor ligands and modes of antigen recognition. Arch. Immunol. Ther. Exp. 59, 117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Groh V., Steinle A., Bauer S., Spies T. (1998) Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279, 1737–1740 [DOI] [PubMed] [Google Scholar]
- 5. Lança T., Correia D. V., Moita C. F., Raquel H., Neves-Costa A., Ferreira C., Ramalho J. S., Barata J. T., Moita L. F., Gomes A. Q., Silva-Santos B. (2010) The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to γδ T-cell cytotoxicity. Blood 115, 2407–2411 [DOI] [PubMed] [Google Scholar]
- 6. Kong Y., Cao W., Xi X., Ma C., Cui L., He W. (2009) The NKG2D ligand ULBP4 binds to TCRγ9/δ2 and induces cytotoxicity to tumor cells through both TCRγδ and NKG2D. Blood 114, 310–317 [DOI] [PubMed] [Google Scholar]
- 7. Scotet E., Martinez L. O., Grant E., Barbaras R., Jenö P., Guiraud M., Monsarrat B., Saulquin X., Maillet S., Estève J. P., Lopez F., Perret B., Collet X., Bonneville M., Champagne E. (2005) Tumor recognition following Vγ9Vδ2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22, 71–80 [DOI] [PubMed] [Google Scholar]
- 8. Dai Y., Chen H., Mo C., Cui L., He W. (2012) Ectopically-expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human γδ T cells to induce innate anti-tumor/virus immunity. J. Biol. Chem. 287, 16812–16819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morita C. T., Lee H. K., Leslie D. S., Tanaka Y., Bukowski J. F., Marker-Hermann E. (1999) Recognition of nonpeptide prenyl pyrophosphate antigens by human γδ T cells. Microbes Infect. 1, 175–186 [PubMed] [Google Scholar]
- 10. Bonneville M., O'Brien R. L., Born W. K. (2010) γδ T cell effector functions. A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 [DOI] [PubMed] [Google Scholar]
- 11. Bukowski J. F., Morita C. T., Tanaka Y., Bloom B. R., Brenner M. B., Band H. (1995) Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J. Immunol. 154, 998–1006 [PubMed] [Google Scholar]
- 12. Peng G., Wang H. Y., Peng W., Kiniwa Y., Seo K. H., Wang R. F. (2007) Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 27, 334–348 [DOI] [PubMed] [Google Scholar]
- 13. Tybulewicz V. L. (2005) Vav-family proteins in T-cell signalling. Curr. Opin. Immunol. 17, 267–274 [DOI] [PubMed] [Google Scholar]
- 14. Huang F., Gu H. (2008) Negative regulation of lymphocyte development and function by the Cbl family of proteins. Immunol. Rev. 224, 229–238 [DOI] [PubMed] [Google Scholar]
- 15. Kim H. S., Das A., Gross C. C., Bryceson Y. T., Long E. O. (2010) Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity 32, 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das A., Long E. O. (2010) Lytic granule polarization, rather than degranulation, is the preferred target of inhibitory receptors in NK cells. J. Immunol. 185, 4698–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuylenstierna C., Björkström N. K., Andersson S. K., Sahlström P., Bosnjak L., Paquin-Proulx D., Malmberg K. J., Ljunggren H. G., Moll M., Sandberg J. K. (2011) NKG2D performs two functions in invariant NKT cells. Direct TCR-independent activation of NK-like cytolysis and co-stimulation of activation by CD1d. Eur. J. Immunol. 41, 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Estefanía E., Flores R., Gómez-Lozano N., Aguilar H., López-Botet M., Vilches C. (2007) Human KIR2DL5 is an inhibitory receptor expressed on the surface of NK and T lymphocyte subsets. J. Immunol. 178, 4402–4410 [DOI] [PubMed] [Google Scholar]
- 19. Cantoni C., Bottino C., Vitale M., Pessino A., Augugliaro R., Malaspina A., Parolini S., Moretta L., Moretta A., Biassoni R. (1999) NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J. Exp. Med. 189, 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parsons M. S., Zipperlen K., Gallant M., Grant M. (2010) Killer cell immunoglobulin-like receptor 3DL1 licenses CD16-mediated effector functions of natural killer cells. J. Leukoc. Biol. 88, 905–912 [DOI] [PubMed] [Google Scholar]
- 21. Nedellec S., Sabourin C., Bonneville M., Scotet E. (2010) NKG2D co-stimulates human Vγ9Vδ2 T cell antitumor cytotoxicity through protein kinase Cθ-dependent modulation of early TCR-induced calcium and transduction signals. J. Immunol. 185, 55–63 [DOI] [PubMed] [Google Scholar]
- 22. Li Z., Xu Q., Peng H., Cheng R., Sun Z., Ye Z. (2011) IFN-γ enhances HOS and U2OS cell lines susceptibility to γδ T cell-mediated killing through the Fas/Fas ligand pathway. Int. Immunopharmacol. 11, 496–503 [DOI] [PubMed] [Google Scholar]
- 23. Mami-Chouaib F., Flament C., Asselin-Paturel C., Gaudin C., Chouaib S. (1996) TCR α/β and TCR γ/δ CD4/CD8-HLA-DR alloreactive CTL clones do not use Fas/Fas ligand pathway to lyse their specific target cells. Hum. Immunol. 51, 13–22 [DOI] [PubMed] [Google Scholar]
- 24. Dalton J. E., Howell G., Pearson J., Scott P., Carding S. R. (2004) Fas-Fas ligand interactions are essential for the binding to and killing of activated macrophages by γδ T cells. J. Immunol. 173, 3660–3667 [DOI] [PubMed] [Google Scholar]
- 25. Kataoka T., Shinohara N., Takayama H., Takaku K., Kondo S., Yonehara S., Nagai K. (1996) Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156, 3678–3686 [PubMed] [Google Scholar]
- 26. Gross C. C., Brzostowski J. A., Liu D., Long E. O. (2010) Tethering of intercellular adhesion molecule on target cells is required for LFA-1-dependent NK cell adhesion and granule polarization. J. Immunol. 185, 2918–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bryceson Y. T., March M. E., Barber D. F., Ljunggren H. G., Long E. O. (2005) Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 202, 1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anikeeva N., Somersalo K., Sims T. N., Thomas V. K., Dustin M. L., Sykulev Y. (2005) Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 102, 6437–6442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith-Garvin J. E., Koretzky G. A., Jordan M. S. (2009) T cell activation. Annu. Rev. Immunol. 27, 591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quann E. J., Liu X., Altan-Bonnet G., Huse M. (2011) A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat. Immunol. 12, 647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y., Wange R. L. (2004) T cell receptor signaling. Beyond complex complexes. J. Biol. Chem. 279, 28827–28830 [DOI] [PubMed] [Google Scholar]
- 32. Knyazhitsky M., Moas E., Shaginov E., Luria A., Braiman A. (2012) Vav1 oncogenic mutation inhibits T cell receptor-induced calcium mobilization through inhibition of phospholipase C-γ1 activation. J. Biol. Chem. 287, 19725–19735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sieger N., Fleischer S. J., Mei H. E., Reiter K., Shock A., Burmester G. R., Daridon C., Dörner T. (2013) CD22 ligation inhibits downstream B-cell receptor signaling and Ca2+ flux upon activation. Arthritis Rheum. 65, 770–779 [DOI] [PubMed] [Google Scholar]
- 34. Lewis R. S. (2001) Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 [DOI] [PubMed] [Google Scholar]
- 35. Feske S. (2007) Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702 [DOI] [PubMed] [Google Scholar]
- 36. Rothenfusser S., Buchwald A., Kock S., Ferrone S., Fisch P. (2002) Missing HLA class I expression on Daudi cells unveils cytotoxic and proliferative responses of human γδ T lymphocytes. Cell Immunol. 215, 32–44 [DOI] [PubMed] [Google Scholar]
- 37. Peterson M. E., Long E. O. (2008) Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity 29, 578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stebbins C. C., Watzl C., Billadeau D. D., Leibson P. J., Burshtyn D. N., Long E. O. (2003) Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol. Cell. Biol. 23, 6291–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Graham D. B., Cella M., Giurisato E., Fujikawa K., Miletic A. V., Kloeppel T., Brim K., Takai T., Shaw A. S., Colonna M., Swat W. (2006) Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J. Immunol. 177, 2349–2355 [DOI] [PubMed] [Google Scholar]
- 40. Upshaw J. L., Arneson L. N., Schoon R. A., Dick C. J., Billadeau D. D., Leibson P. J. (2006) NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat. Immunol. 7, 524–532 [DOI] [PubMed] [Google Scholar]
- 41. Reynolds L. F., Smyth L. A., Norton T., Freshney N., Downward J., Kioussis D., Tybulewicz V. L. (2002) Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 195, 1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reynolds L. F., de Bettignies C., Norton T., Beeser A., Chernoff J., Tybulewicz V. L. (2004) Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J. Biol. Chem. 279, 18239–18246 [DOI] [PubMed] [Google Scholar]
- 43. Swat W., Xavier R., Mizoguchi A., Mizoguchi E., Fredericks J., Fujikawa K., Bhan A. K., Alt F. W. (2003) Essential role for Vav1 in activation, but not development, of γδ T cells. Int. Immunol. 15, 215–221 [DOI] [PubMed] [Google Scholar]
- 44. Bustelo X. R. (2001) Vav proteins, adaptors, and cell signaling. Oncogene 20, 6372–6381 [DOI] [PubMed] [Google Scholar]
- 45. Thien C. B., Langdon W. Y. (2005) c-Cbl and Cbl-b ubiquitin ligases. Substrate diversity and the negative regulation of signalling responses. Biochem. J. 391, 153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nau M. M., Lipkowitz S. (2003) Comparative genomic organization of the cbl genes. Gene 308, 103–113 [DOI] [PubMed] [Google Scholar]
- 47. Griffiths E. K., Sanchez O., Mill P., Krawczyk C., Hojilla C. V., Rubin E., Nau M. M., Khokha R., Lipkowitz S., Hui C. C., Penninger J. M. (2003) Cbl-3-deficient mice exhibit normal epithelial development. Mol. Cell. Biol. 23, 7708–7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chiang J., Hodes R. J. (2011) Cbl enforces Vav1 dependence and a restricted pathway of T cell development. PLoS ONE 6, e18542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rincon-Orozco B., Kunzmann V., Wrobel P., Kabelitz D., Steinle A., Herrmann T. (2005) Activation of Vγ9Vδ2 T cells by NKG2D. J. Immunol. 175, 2144–2151 [DOI] [PubMed] [Google Scholar]
- 50. Bryceson Y. T., March M. E., Ljunggren H. G., Long E. O. (2006) Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lewis C. M., Broussard C., Czar M. J., Schwartzberg P. L. (2001) Tec kinases. Modulators of lymphocyte signaling and development. Curr. Opin. Immunol. 13, 317–325 [DOI] [PubMed] [Google Scholar]
- 52. Cruz-Orcutt N., Houtman J. C. (2009) PI3 kinase function is vital for the function but not formation of LAT-mediated signaling complexes. Mol. Immunol. 46, 2274–2283 [DOI] [PubMed] [Google Scholar]
- 53. Sun Z., Arendt C. W., Ellmeier W., Schaeffer E. M., Sunshine M. J., Gandhi L., Annes J., Petrzilka D., Kupfer A., Schwartzberg P. L., Littman D. R. (2000) PKC-θ is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404, 402–407 [DOI] [PubMed] [Google Scholar]
- 54. Schmitz M. L. (2009) Activation of T cells. Releasing the brakes by proteolytic elimination of Cbl-b. Sci. Signal. 2, pe38. [DOI] [PubMed] [Google Scholar]