Abstract
Peroxynitrite is the product of the diffusion-controlled reaction of nitric oxide and superoxide radicals. Peroxynitrite, a reactive short-lived peroxide with a pKa of 6.8, is a good oxidant and nucleophile. It also yields secondary free radical intermediates such as nitrogen dioxide and carbonate radicals. Much of nitric oxide- and superoxide-dependent cytotoxicity resides on peroxynitrite, which affects mitochondrial function and triggers cell death via oxidation and nitration reactions. Peroxynitrite is an endogenous toxicant but is also a cytotoxic effector against invading pathogens. The biological chemistry of peroxynitrite is modulated by endogenous antioxidant mechanisms and neutralized by synthetic compounds with peroxynitrite-scavenging capacity.
Keywords: Free Radicals, Nitric Oxide, Oxidative Stress, Superoxide Dismutase (SOD), Superoxide Ion, Peroxynitrite, Tyrosine Nitration
Peroxynitrite: A Product from a Radical-Radical Combination Reaction
Free radicals typically react fast with each other via radical-radical coupling reactions. Indeed, radical combination reactions usually occur at near diffusion-controlled rates (1). This unique type of reaction is, in many cases, kinetically and thermodynamically favored by the fact that it results in the formation of a new chemical bond at the expense of the unpaired electrons of the precursors. There are many possible radical-radical combination reactions that can happen biologically, but low steady-state levels of intermediates and competing reactions usually limit reaction yields and quantitative relevance. A prime example of a relevant radical species produced at high rates biologically is represented by the superoxide radical anion (O2⨪), the product of the univalent reduction of molecular oxygen (2). O2⨪ is ubiquitous and continuously formed during normal cellular metabolism, with its production rates increasing severalfold during the disruption of cellular redox homeostasis and with inflammatory stimuli. Although excess O2⨪ production has been associated with oxidative damage, more controlled fluxes can lead to redox signaling (3).
The discovery of nitric oxide (•NO) as an enzymatically generated free radical was paralleled by the recognition that it could readily react with O2⨪ (4, 5). •NO produced by a variety of nitric-oxide synthases (NOS)2 participates as a mediator in the regulation of vascular tone, neurotransmission, and immunity, among other metabolic and cell signaling effects (6, 7). Thus, the reaction of O2⨪ with •NO was first conceived as a mechanism of •NO “inactivation” (8). Notably, the combination reaction leads to peroxynitrite (9, 10), a peroxy acid originally studied in the chemical literature as a strong oxidizing and nitrating compound (11, 12) (Equation 1).
The reaction of •NO with O2⨪ occurs biologically even in the presence of superoxide dismutase (SOD), indicating that it is extremely fast to outcompete the enzyme-catalyzed dismutation (Equation 2).
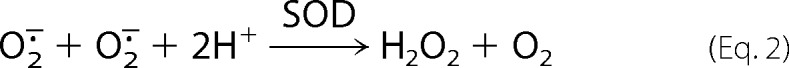 |
Indeed, the formation of peroxynitrite occurs with a k1 of ∼1010 m−1 s−1, ∼1 order of magnitude higher than that of enzymatic dismutation (∼1–2 × 109 m−1 s−1) (2, 13). Although SOD exists in cells in micromolar levels, •NO concentrations can, in some cases, reach close to micromolar values. Thus, under appropriate conditions, the formation of peroxynitrite is the only known reaction for O2⨪ in biology that can be similar or even substantially faster than the dismutation reaction (i.e. Equation 3).
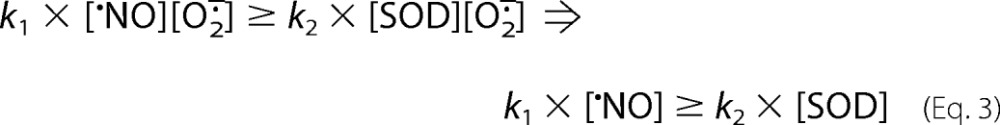 |
Although the proximal species formed from the •NO plus O2⨪ reaction is peroxynitrite anion, the pKa value of 6.8 and the rapid protonation imply that, under most biological conditions, ONOO− and ONOOH will both be present (13) (Equation 4).
For instance, at pH 7.4, ∼80% of peroxynitrite will be in the anionic form; conversely, at pH 6.2 (e.g. inside a macrophage phagocytic vacuole), up to 80% will be in the protonated form. The stability, reactivity, and capacity to permeate membranes of ONOO− and ONOOH are quite different (13, 14), and therefore, the biochemistry of peroxynitrite in biological systems is highly pH-dependent. This acid-base property of peroxynitrite contrasts with that of H2O2, which has a pKa of ∼11.6 and therefore is almost 100% protonated in the physiological pH range.
Early Evidence of the Oxidizing Capacity of Peroxynitrite in Biochemical Systems
As peroxide, the relatively labile O–O bond provides the possibility of homolysis to radicals (10, 12, 15, 16). Indeed, protonation weakens the O–O bond in ONOOH and leads to homolytic cleavage to hydroxyl radicals (•OH) and nitrogen dioxide (•NO2), two strongly oxidizing/hydroxylating and nitrating species, respectively (Equation 5).
The homolytic cleavage occurs with a k4 of 4.5 s−1 at 37 °C, resulting in a half-life of 0.8 s in phosphate buffer at pH 7.4 (i.e. kapp = 0.9 s−1) (13). The recognition that the homolysis of ONOOH could yield •OH led to the postulation of a new biologically relevant mechanism of oxygen radical-mediated molecular damage, without the requirement of transition metal-dependent reactions. Indeed, until the emergence of peroxynitrite, much of O2⨪-dependent oxidative damage was postulated to occur via the Haber-Weiss cycle and/or the Fenton reaction (2), where ultimately •OH was formed (Equation 6).
In fact, O2⨪ is not a strong oxidant, and actually, it can also act as a reductant (17). Therefore, its direct toxicity is usually limited (i.e. oxidation and disruption of the iron-sulfur cluster in [4Fe-4S]-containing dehydratases) (2). Moreover, despite high formation rates (18), the steady-state levels of O2⨪ are always quite low due to the abundance and thorough distribution of SOD that promote its preferential dismutation to H2O2 (unless •NO is present). Thus, considerable O2⨪-dependent toxicity resides in the formation of secondary reactive species; these include H2O2 (Equation 6), peroxynitrite (Equation 1; to be analyzed in this minireview), and, possibly, reactive hydroperoxides formed by the fast reactions of O2⨪ with biomolecule-derived radicals (19, 20).
Similarly, although •NO was recognized early as a cytotoxic effector during cellular immune responses mediated by macrophages and neutrophils (21), the biological effects of •NO did not correlate well with its chemical reactivity, i.e. a relatively stable radical with modest redox properties (22). Thus, •NO-mediated toxicity was also further rationalized considering the generation of •NO-derived oxidants (23) such as peroxynitrite.
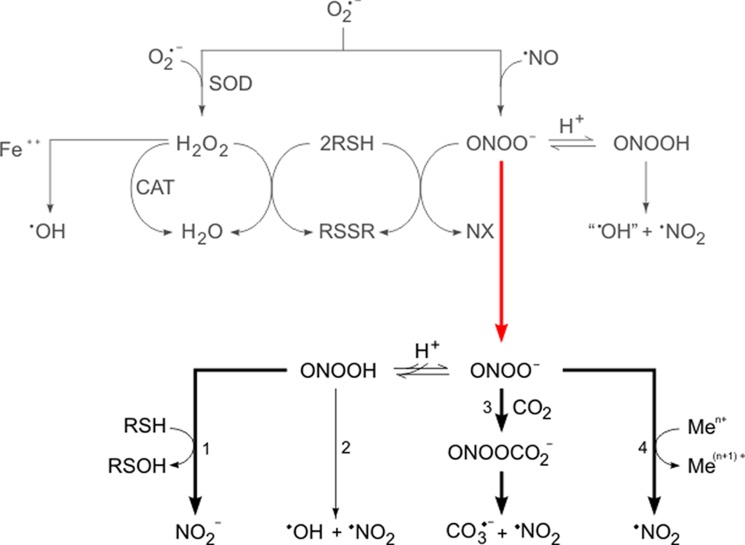
Soon after the proposal of the formation and homolysis of peroxynitrite in biological systems (10), it was reported that peroxynitrite could directly oxidize thiol groups at rates much faster than the homolytic cleavage (24). Overall, the initial observations (10, 24, 25) paved the way for a new paradigm of O2⨪- and •NO-mediated toxicity via peroxynitrite, which was schematized in JBC in 1991 (Fig. 1, upper) (24). The hypothesis led to predictions that could be tested experimentally, including how the inhibition of nitric oxide synthesis (e.g. using NOS inhibitors), the elimination of excess O2⨪ (e.g. overexpression of SOD), the catalytic decomposition of peroxynitrite, or the scavenging of peroxynitrite-derived radicals could influence oxidative processes and biological outcome. An updated version of the original proposal is shown in Fig. 1 (lower) and is analyzed below.
FIGURE 1.
Peroxynitrite as a mediator of superoxide radical and nitric oxide-dependent oxidative and cytotoxic processes. The figure represents the evolution of our understanding of the biochemically relevant reactions in which peroxynitrite participates. It integrates the initial proposal (24) with what is currently known. Upper, shown in gray is a reproduction of the original scheme in which the commonly accepted mechanism of O2⨪-mediated oxidative damage was challenged with an alternative mechanism that considered the participation of •NO. The proposal indicated that the reaction of O2⨪ with •NO yielded peroxynitrite anion, which could then start oxidation reactions directly or via secondary radicals. The original scheme showed two main reactions for peroxynitrite, namely thiol oxidation and homolysis. The lack of absolute certainty at the time regarding the products of the decomposition of peroxynitrous acid led us to write the hydroxyl radical as “•OH”. Similarly, NX indicated an uncharacterized nitrogen-containing product, later proved to be nitrite. The red arrow connects the early proposal with an updated scheme of the biological chemistry of peroxynitrite. Lower, shown in black are the main accepted reaction pathways of peroxynitrous acid and peroxynitrite anion, namely 1) two-electron oxidation of thiols, 2) homolysis, 3) nucleophilic addition to CO2 and evolution to radicals, and 4) reaction with transition metal centers. The width of the arrows symbolizes the preferential routes of peroxynitrite consumption in biological systems, underscoring the fact that homolysis is a quantitatively minor process.
Key Aspects of Peroxynitrite Biological Chemistry: Redox Reactions and Nucleophilic Addition
Peroxynitrite is both an oxidant and nucleophile, and these two chemical properties dictate much of its biochemical actions in vivo (13). First, as an oxidant, it can promote one- and two-electron oxidations by direct reactions with biomolecular targets. Indeed, the redox potentials of peroxynitrite at pH 7 (E′0) for the ONOO−/•NO2 and ONOO−/NO2− pairs have been estimated as 1.4 and 1.2 V, respectively (26), supporting its performance as a good biological oxidant from a thermodynamic viewpoint (17).
A prime example of two-electron oxidations corresponds to the reaction of peroxynitrite with thiols, which yields the sulfenic acid derivative (and nitrite) (24, 27) (Equation 7).
This reaction was originally described for cysteine and the single thiol group of albumin (Cys-34) and occurs with an apparent second-order rate constant of >103 m−1 s−1 at pH 7.4 and 37 °C (Table 1), ∼3 orders of magnitude faster than the same reaction with H2O2. These data underscored the high chemical reactivity of peroxynitrite in biological systems (24). Nonetheless, peroxynitrite is less reactive toward thiols than other biologically relevant two-electron oxidants such as hypochlorous acid (HOCl) and hypobromous acid (HOBr), which react with cysteine and glutathione on the order of 107 m−1 s−1 (28, 29). The reactivity of peroxynitrite with thiols is intermediary between that of H2O2 and that of hypohalous acids, resulting in a moderately strong and selective biological oxidant in a similar way to what has been proposed for thiol oxidation mediated by chloramines (k ∼ 100–200 m−1 s−1) (28). Importantly, peroxynitrite can oxidize at even more remarkable rates some “fast reacting thiols,” such as those present in mammalian and microbial peroxiredoxins (30). Indeed, peroxiredoxins react with peroxynitrite with constants on the order of 106–107 m−1 s−1 (Table 1) and represent a first line of enzymatic antioxidant defense against peroxynitrite.
TABLE 1.
Kinetic aspects of peroxynitrite-mediated oxidations: selected reactions of biochemical relevance
The majority of the information highlights the reactivity of peroxynitrite with biomolecules and provides considerations about its quantitative relevance in biological systems. The last three listed compounds are examples of synthetic molecules utilized to either decompose or detect peroxynitrite. Prx, peroxiredoxin.
| Reactant | ka | Process | Commentary |
|---|---|---|---|
| m−1s−1 | |||
| Tyrosine | 0 | Tyr oxidation and nitration | There is no direct reaction (40). Tyr oxidation and nitration is accomplished by peroxynitrite-derived radicals (23). |
| Tryptophan | 40 | Trp oxidation and nitration | The direct reaction is rather slow and can cause Trp nitration (96). |
| Methionine | 360 | Methionine sulfoxide formation | It can account for enzyme inactivation (97). It is sometimes used to scavenge peroxynitrite in biochemical systems. |
| Uric acid | 500 | A variety of oxidation products can be formed, including allantoin, alloxan, and triuret (83, 85). The intermediate formation of uric acid-derived radicals may promote secondary oxidation reactions and products such as urate hydroperoxide (83, 98). | It is good inhibitor of peroxynitrite-dependent processes in vitro and in vivo. The direct reaction is relatively slow, so protection is ascribed to reaction with peroxynitrite-derived radicals. Uric acid is also a physiological substrate of myeloperoxidase (98) and may therefore interfere in heme peroxidase-dependent nitration reactions as well. |
| Glutathioneb | 1400 | It evolves mainly to glutathione disulfide through the intermediacy of glutathione sulfenic acid (13). Glutathionyl radicals can be formed from peroxynitrite-derived radicals. | It is an endogenous compound that decomposes peroxynitrite. Considering a 5 mm intracellular concentration, the k[GSH]c product results in a value of 7 s−1, significantly faster that the rate constant of homolysis (0.9 s−1)d but much less than that of other direct reactions, so its direct reaction with peroxynitrite in biological systems is modest. |
| Cysteineb | 5900 | It evolves to cysteine disulfide (cystine) through the intermediacy of cysteine sulfenic acid (13, 24). | This was the first determination of a second-order rate constant of peroxynitrite reaction with a biomolecule. It provided the concept that direct reactions of peroxynitrite may be more relevant in biology than homolysis. |
| Human serum albumin | 9700 | About 40% of the direct reactivity is due to the reaction with the single thiol group (Cys-34) (40), leading to the sulfenic acid derivative. | A highly abundant plasma protein, it consumes a fraction of intravascular peroxynitrite but cannot outcompete the reaction with CO2. |
| Oxyhemoglobin | 2.3 × 104 | It isomerizes peroxynitrite to nitrate (99). | It is relevant for peroxynitrite detoxification in red blood cells. At a concentration of 5 mm, k[oxy-Hb] = 340 s−1, a remarkable velocity. However, peroxiredoxin-2 outcompetes oxyhemoglobin in peroxynitrite detoxification in the erythrocyte (13). |
| Mn-SOD | >104 | The reaction of peroxynitrite anion with the Mn2+ atom produces enzyme nitration at Tyr-34 (43). | The nitration of the critical Tyr residue leads to enzyme inactivation. This process is largely observed in vivo under inflammatory conditions. |
| CO2 | 5.8 × 104 | Nucleophilic addition of peroxynitrite anion to CO2 yields an unstable intermediate that undergoes homolysis (35, 36, 38). | This is a central reaction controlling peroxynitrite reactivity in biological system. A k[CO2] value of ∼60–100 s−1 has been established as a desirable starting range for a peroxynitrite scavenger to be competitive (13). |
| Aconitasee | 1.4 × 105 | Oxidation and disruption of the iron-sulfur cluster (57, 58) | A key reaction in mitochondria, aconitase inactivation slows down the Krebs cycle and causes iron release. |
| Peroxiredoxins | 106–107 | Fast reaction with the peroxidatic cysteine residue (30, 81) | Microbial and mammalian peroxiredoxins constitute a central catalytic mechanism for the detoxification peroxynitrite. The k[Prx] value ranges from >102 to 103 s−1 depending on cell types. |
| Ebselen | 4.6 × 106 | A synthetic seleno-containing compound that in the reduced state (selenol) undergoes two-electron oxidation, a reaction chemistry similar to that of thiols (100) | Ebselen readily decomposes peroxides and can create catalytic redox cycles at the expense of reducing compounds such as glutathione. It can be used pharmacologically to neutralize peroxynitrite (54). |
| MnP | >107 | Mn2+ reduces peroxynitrite to nitrite and is catalytically recycled by endogenous reductants and the electron transport chain (89). | These compounds are used pharmacologically and can achieve 5–10 μm concentrations in vivo. Thus, with k[MnP] > 100 s−1, they can effectively eliminate part of peroxynitrite (13). |
| Boronate-based compounds | >106 | Peroxynitrite anion reacts directly via two-electron oxidation with boronate-based compounds to yield their corresponding hydroxyl derivatives (95). In the case of aryl boronates, the corresponding phenols are the major final products. | These compounds are a novel class of probes that can be utilized for peroxynitrite detection. They react with peroxynitrite at rates ∼106 faster than hydrogen peroxide. The high rate constant and the lack of formation of probe-derived radical intermediates minimize secondary reactions and confounding results. |
a Stopped-flow spectrophotometry has been utilized to determine the rate constants of peroxynitrite reaction with most compounds, taking advantage of the distinctive optical absorption of ONOO− at 302 nm (ϵ = 1670 m−1 cm−1) as originally reported (24). Alternative approaches have been also used, with the application of competition kinetics with reference reactions of known rate constants (81).
b The actual reactants are peroxynitrous acid and the thiolate anion (Equation 7) (27); thus, the observed apparent reaction rate is strongly pH-dependent (24), with the thiol pKSH representing a relevant variable. The table shows kapp, which is on the order of 103 m−1 s−1; however, the actual (pH-independent) rate constant of the reaction is on the order of 105 m−1 s1 (27, 30).
c The product of the second-order rate constant times the concentration of the reactant provides a pseudo-first-order rate constant in s−1 that allows ready comparison of the kinetic biological relevance among different peroxynitrite targets.
d In fact, the homolytic yields of •NO2 and •OH are ∼30% of ONOOH due to “in cage” recombination of nascent radicals to nitrate (NO3−) before their diffusion to the bulk aqueous phase.
e Peroxynitrite also causes aconitase tyrosine nitration, but this is not related to the loss of activity, which is exclusively due to the oxidation of the [4Fe-4S] cluster (59).
Peroxynitrite also promotes one-electron oxidations directly (e.g. oxidation of cytochrome c2+ (31)) or secondarily through the homolysis of peroxynitrite. Indeed, •OH is the most potent (and less selective) known biological oxidant and reacts with biomolecules at rates approaching the diffusion limit to cause hydroxylation or one-electron oxidation; •NO2 is also a strong oxidant (32) and a key intermediate in nitration reactions (i.e. incorporation of a –NO2 group). Although peroxynitrite homolysis is an interesting chemical process, its actual quantitative relevance at the biochemical level is less likely (13). Indeed, a lesson obtained from kinetic data is that the first-order rate constant of homolysis can hardly compete with other bimolecular reactions of peroxynitrite (Table 1). At most, homolysis represents a small percentage of the peroxynitrite-consuming reactions in living systems; nonetheless, homolysis generates reactive secondary radicals that initiate radical chain reactions such as lipid peroxidation and amplify the oxidation processes in vitro (25, 33, 34) and presumably in vivo (34).
As a nucleophile, a central reaction of peroxynitrite in biology is the addition of the anion to carbon dioxide (CO2) to yield a nitrosoperoxocarboxylate adduct (ONOOCO2−) that undergoes a fast homolysis to •NO2 and carbonate radicals (CO3⨪) (16, 35, 36) (Equation 8).
This reaction is relevant because of the ubiquity of CO2 in biological systems (e.g. 25 mm HCO3− is in equilibrium with ∼1.3 mm CO2 at pH 7.4) and its relatively high rate constant (Table 1). The “CO2” reaction of peroxynitrite accounts for a substantial portion of its biological fate and chemistry (13). CO3⨪, rising from the reaction, is a good one-electron oxidant (32). Although the reaction of the HCO3−/CO2 pair with peroxynitrite was inferred during studies of peroxynitrite-induced luminol chemiluminescence (37), direct EPR studies during continuous flow of peroxynitrite to carbonated phosphate buffers unambiguously revealed the formation of CO3⨪ (38). Thus, the formation and subsequent reactions of CO3⨪ and •NO2 radicals are an integral part of the chemical biology of peroxynitrite. The nucleophilic addition of peroxynitrite anion to monocarbonyl- and dicarbonyl-containing compounds (e.g. glyoxal) (39) also occurs at significant rates; the adducts evolve to carbonyl-derived radical species and •NO2 (secondarily, singlet oxygen is produced), although the biological significance of these processes is unknown.
Another type of reaction in which peroxynitrite can evolve to secondary oxidants involves its interaction with transition metal centers. These can be part of metalloproteins (e.g. Mn-SOD and hemeproteins) or metal complexes (e.g. Mn-porphyrins (MnP)). Transition metals may be regarded as Lewis acids, which can react with ONOO− to yield a Lewis adduct (13). Usually, the metal-based Lewis adducts undergo homolysis to yield •NO2 and the corresponding oxyradical-metal complex, which rearranges to a strongly oxidizing oxo-metal complex (13, 23) (Equation 9).
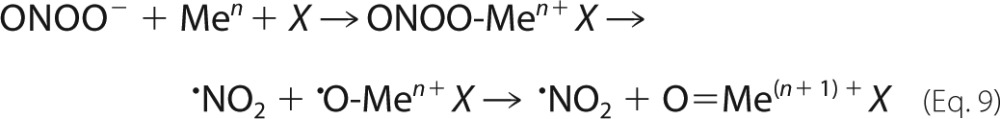 |
Peroxynitrite-mediated Protein Tyrosine Nitration
Peroxynitrite does not react directly with tyrosine (40). However, a recognized oxidative protein modification left by peroxynitrite in vitro and in vivo is the formation of 3-nitrotyrosine (23). Indeed, all of the secondary radicals arising from peroxynitrite (•OH, CO3⨪, oxo-metal complexes, lipid peroxyl radicals (33), and •NO2) promote protein tyrosine oxidation and nitration (41). The typical mechanism of tyrosine nitration in biological systems is a two-step radical process: a one-electron oxidant leading to the formation of a tyrosyl radical (Equation 10), which then combines at diffusion-controlled rates with •NO2 to yield 3-nitrotyrosine (Equation 11) (42).
The nitration process started by oxo-metal complexes is particularly relevant in the site-specific tyrosine nitration of metalloproteins (41); in this case, the metal promotes the initial one-electron oxidation and enhances nitration yields in vicinal tyrosine residues (e.g. in Mn-SOD; see below) (43).
A long-lasting debate on the actual yields of peroxynitrite-mediated tyrosine nitration generated by variable fluxes of O2⨪ and •NO (i.e. radical flux ratios ≠ 1) has been largely clarified by considering factors such the actual steady-state of nitrating species, secondary radical processes over tyrosyl radicals, the presence of SOD, and the diffusion of excess •NO across cellular compartments (44–46). Although originally described in biology to occur via peroxynitrite-dependent reactions (47, 48), protein tyrosine nitration can be also due to other •NO-mediated processes, most notably in heme peroxidase-catalyzed reactions (49–51). Nonetheless, peroxynitrite represents a substantial endogenous source of nitrating species (for a recent critical analysis, see Ref. 41 and references therein).
Nitric Oxide Interactions with Mitochondria and Peroxynitrite
At the cellular level, a prime locus related to the formation, reactions, and effects of peroxynitrite is represented by the mitochondrion (52). Indeed, mitochondria are central intracellular sources of O2⨪, and peroxynitrite formation is favored by the facile diffusion of •NO from the cytosol (53, 54). Peroxynitrite reactions with mitochondrial components irreversibly affect the activity of electron transport chain complexes (complexes I and II) and ATPase, altering mitochondrial bioenergetics and calcium homeostasis and further promoting O2⨪ formation (54–56). In this scenario, mitochondria become a key cellular “sink” of •NO and “source” of peroxynitrite.
Although the initial interactions of •NO with mitochondria lead to relative reversible processes regulating respiration, membrane potential, and calcium homeostasis, the effects of peroxynitrite are harsher and typically promote to toxic events. Indeed, much of the initial evidence reporting long-lasting and toxic actions of •NO on mitochondrial function was later demonstrated to be mainly peroxynitrite-dependent. For instance, its has been clarified that peroxynitrite is the main •NO-derived species responsible of the inactivation of mitochondrial aconitase (57–59), a [4Fe-4S] cluster-containing enzyme of the Krebs cycle (2). Disassembly of the cubane [4Fe-4S] cluster by peroxynitrite via oxidative attack leads to an inactive [3Fe-4S] enzyme (Equation 12).
 |
Iron released from aconitase can further propagate intramitochondrial oxidative damage by metal-mediated formation of oxidizing and nitrating species.
The relevance of the intramitochondrial formation of peroxynitrite is underscored by the established observation of mitochondrial protein nitration in pathological states and even under basal conditions (reviewed in Ref. 60). A key tyrosine-nitrated protein in mitochondria is Mn-SOD (61, 62), an essential antioxidant enzyme. Mn-SOD is found nitrated and inactivated in vitro and in vivo under conditions that facilitate intramitochondrial peroxynitrite formation (60). Not only is nitro-Mn-SOD a footprint of the process, but also its inactivation by nitration contributes to further amplify mitochondrial nitroxidative stress (54). Mn-SOD provides one of the few well established examples in which tyrosine nitration accounts for a physiologically relevant loss of function (i.e. the extent of nitration observed in vivo is sufficient to cause enough inactivation to alter metabolism) (23). Moreover, the nitration process leading to enzyme inactivation involves the site-specific manganese-catalyzed nitration of Tyr-34, the active site tyrosine lying only 5 Å from the manganese site) (43, 62, 63). Peroxynitrite anion enters the Mn-SOD active site through the same channel as O2⨪, reacts at relatively fast rates with manganese (approximately >104 m−1 s−1), and yields highly oxidant and nitrating species that cause nitration of Tyr-34. Once nitrated, the phenolic Tyr-34 hydroxyl group becomes partially deprotonated (i.e. its pKa drops from ∼10.3 to 7.3) (41), and therefore, the reaction of O2⨪ with manganese is hindered by both steric constraints and electrostatic repulsion (63, 64). The nitration of Mn-SOD Tyr-34 is strongly supportive of the intramitochondrial peroxynitrite formation (65, 66). Other oxidizing and nitrating species tend to react with tyrosines other than Tyr-34 and do not lead to enzyme inactivation.
Efforts have been also directed to understand how peroxynitrite-dependent nitration affects the redox properties of cytochrome c, an abundant mitochondrial protein and key partner of the electron transport chain (67). Recent evidence points to cytochrome c as an intramitochondrial heme peroxidase after a conformational change triggered by binding to cardiolipin and indicates that this process is related to apoptotic signaling (68). We have found that the preferential nitration of cytochrome by peroxynitrite-derived radicals in one of the solvent-exposed tyrosine residues (Tyr-74) leads to a conformational change that causes the displacement of the sixth ligand to the heme (Met-80) and a gain in peroxidase activity (67); the conformational change induced by nitration in cells also facilitates its translocation to the cytosol (even in non-apoptotic cells) (69). The biological significance of these events is currently under investigation.
Peroxynitrite-dependent Cytotoxicity
The reactions of peroxynitrite with biomolecules can led to cytotoxic events, which may result in apoptotic or necrotic cell death (54, 70, 71). The cytotoxic effects triggered by oxidation and nitration reactions usually involve the participation of a variety of effector molecules and processes. For example, peroxynitrite-mediated oxidation of mitochondrial membrane components facilitates the release of pro-apoptotic factors, whereas poly(ADP-ribose) polymerase activation secondary to DNA oxidative damage triggers necrosis (54). Although excess formation of peroxynitrite by mammalian tissues is deleterious in inflammatory and degenerative processes, it is also clear that our immune system can utilize peroxynitrite to repel microbial invasion (72). Indeed, whereas the shift in the signaling properties of •NO toward oxidative pathways after reacting with O2⨪ is associated with pathology in the vascular and nervous systems, the antimicrobial activity of •NO released by macrophages is largely dependent on the formation of peroxynitrite. Once formed, peroxynitrite can permeate cell membranes through either anion channels or passive diffusion of the anionic and protonated forms, respectively (14), and over a biological half-life of ∼5–20 ms, it promotes toxic effects locally at up to one- to two-cell diameters (∼10 μm) from its site of formation.
Endogenous Toxicant
The participation of peroxynitrite in pathophysiological conditions has been recognized in cellular and animal disease models and in human pathology (54, 70). For example, peroxynitrite contributes to apoptotic neuronal death in a variety of acute injuries and neurodegenerative conditions (54, 73). Notably, it has just been reported that nitration of Hsp90 plays a central role in motor neuron death through activation of the Fas pathway (71). In the vascular wall, conditions such as hyperglycemia, atherosclerosis, and hypertension lead to enhanced O2⨪ formation, shorten the biological half-life of •NO, and cause endothelial dysfunction; the inexorable formation of peroxynitrite results in oxidation and nitration of biomolecules that may further contribute to vascular degeneration (74). Peroxynitrite also participates in vascular aging and protein tyrosine nitration of the vessel wall, including the selective nitration and inactivation of prostacyclin synthase (75). It is important to note that, during vascular inflammation, neutrophil degranulation of myeloperoxidase also contributes to protein tyrosine nitration processes by peroxynitrite-independent mechanisms (51, 74).
Cytotoxic Effector against Invading Pathogens
The cytotoxic properties of peroxynitrite can be also utilized by immune system cells to combat infecting microorganisms. Indeed, early experiments demonstrated the cytotoxic capacity of peroxynitrite toward Escherichia coli (76) and Trypanosoma cruzi (77), which was later extended to several other pathogens. For instance, peroxynitrite formation by macrophages represents a cytotoxic effector mechanism, which requires immunostimulation with cytokines that induce iNOS expression. Then, upon macrophage interaction with the microbial pathogen and association with a phagocytic process, the plasma membrane NADPH oxidase is activated for O2⨪ production (i.e. the respiratory burst): the simultaneous formation of O2⨪ and •NO yields large levels of peroxynitrite in the phagosomal compartment over a 60–120-min period (72). For example, the intraphagosomal levels of peroxynitrite are sufficient for T. cruzi killing (i.e. the causative agent of Chagas disease). Nitrated and oxidized T. cruzi was evidenced inside the phagosome, confirming the diffusion of peroxynitrite from the macrophage to the pathogen (72). Instead, in other immune system cells such as neutrophils, alternative •NO-dependent mechanisms of cytotoxicity and pathogen protein tyrosine nitration operate, with the likely participation of myeloperoxidase-mediated reactions (51, 78, 79). The prolonged respiratory burst observed in macrophages (with respect to neutrophils) and the lack of significant myeloperoxidase activity (72) make the formation of peroxynitrite in immunostimulated macrophages a premier reaction pathway for the execution of •NO-derived cytotoxicity.
Modulation of the Redox Biochemistry of Peroxynitrite
The endogenous systems that cope with the toxic effects of peroxynitrite were established in initial work on microbial systems (80, 81). In fact, bacterial and parasitic peroxiredoxins were found to readily decompose peroxynitrite via a fast reaction with the “peroxidatic” cysteine residue of the enzyme active site (Equation 13),
where Prx is peroxiredoxin. This reaction was later shown in mammalian peroxiredoxin systems (13). The remarkable velocity for peroxiredoxin reactions with peroxynitrite (Table 1) extended earlier observations for H2O2 (30); the molecular determinants of such reactivity are under scrutiny but seem to depend of the stabilization of the enzyme-activated complex (30). The complete catalytic cycle to restore peroxiredoxin to the resting state is analyzed elsewhere (30). Due to the high concentration of peroxiredoxins, the fast rate constant, and its thorough distribution across various cellular compartments, it constitutes a prime endogenous antioxidant mechanism for the catalytic detoxification of peroxynitrite. In the case of microbial systems, peroxiredoxins have been recently revealed as virulence factors due to their capacity to detoxify peroxynitrite formed from macrophages both in vitro and in vivo (72, 82).
A large number of compounds have been used with pharmacological goals to cope with the toxic effects of peroxynitrite and peroxynitrite-derived radicals (54). A strong protector against peroxynitrite-mediated toxicity in vitro and in vivo is uric acid (54, 83–85), the end product of purine metabolism and an antioxidant compound in humans (86). The direct reaction of peroxynitrite with uric acid is rather slow to account for its protective effects (Table 1); thus, much of its effects may instead be due to the scavenging of peroxynitrite-derived radicals and the inhibition of tyrosine nitration reactions.
With respect to synthetic molecules that are effective against peroxynitrite in vitro and in vivo, MnP deserve special attention. This class of metal-based drugs, initially conceived as SOD mimics (87), readily reacts with peroxynitrite (13) and has been used to attenuate peroxynitrite-dependent cytotoxicity (54). Peroxynitrite can readily react with both the Mn2+ and Mn3+ states to yield nitrite or •NO2, respectively. Although the MnP are typically administered in the 3+ state, the most effective and likely antioxidant mechanism in biological systems involves the 2+ state in a catalytic cycle as follows (Equations 14–17).
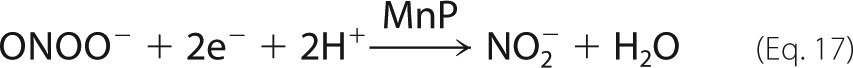 |
The reduction of the Mn3+P is carried out by a variety of flavoenzymes, glutathione, and electron transport chain complexes. Then, Mn2+ readily reduces peroxynitrite anion to nitrite (k ∼ 106–107 m−1 s−1); Mn4+=O is reduced back to the Mn3+ state by fast reactions with a variety of endogenous low molecular weight reductants, including ascorbate, glutathione, and uric acid (88, 89). Through the reactions shown in Equations 14–16, MnP can catalytically decompose peroxynitrite via a redox cycle of peroxynitrite reduction at the expense of endogenous reducing equivalents. Notably, cationic MnP can accumulate in mitochondria to micromolar levels that can exert antioxidant activity (89, 90), and we have found that complexes I and II of the mitochondrial electron transport chain can readily provide the electrons for MnP reduction to the Mn2+ state under physiologically relevant oxygen concentrations (5–30 μm O2) (91). Then, MnP protect mitochondria from peroxynitrite-mediated toxicity both in vitro and in vivo. Reduced MnP can also eliminate peroxynitrite-derived carbonate radicals (Table 1) (92). Even though MnP were initially conceived as SOD mimics, many of their protective antioxidant effects relate to peroxynitrite detoxification (93).
Another interesting strategy to connect the mechanisms of peroxynitrite-mediated toxicity with potential pharmacological applications is the use of cell-permeable tyrosine-containing peptides (73, 94). Tyrosine peptides do not react directly with peroxynitrite but interfere in the radical-dependent tyrosine nitration process and spare critical protein tyrosine residues. Although their precise mechanism of action in cells and in vivo remains to be established, intracellular delivery of tyrosine-containing peptides can protect cells from protein tyrosine nitration and death (73).
Concluding Remarks and Perspectives
Our current understanding of the biological chemistry of peroxynitrite provides a framework to understand the molecular mechanisms of oxidant-mediated cell and tissue injury in •NO-producing systems (Fig. 1). Although it is desirable to eliminate excess peroxynitrite to neutralize its toxicity in a variety of pathologies, peroxynitrite plays also a role as a strong antimicrobial agent, and therefore, tackling the peroxynitrite-detoxifying systems of microbes appears to be a good strategy for infection control. The elusive nature of peroxynitrite has made it difficult to determine its role as a key mediator in pathology, but the correct understanding of its redox biochemistry has greatly helped in the process (13). Moreover, the recent characterization of boronate-based probes that react quickly with peroxynitrite (95) (Table 1) provides possibilities for a more specific detection and even quantitation of peroxynitrite by bioanalytical and bioimaging techniques. Thus, although “stealthy” in nature, accumulated knowledge and new molecular tools are revealing peroxynitrite as a key redox mediator in pathological states and providing possible remedies for this furtive oxidant species.
Acknowledgments
I thank Drs. Larry Marnett, Silvina Bartesaghi, Madia Trujillo, Sebastián Carballal, and Gerardo Ferrer-Sueta for helpful suggestions and Dr. Valeria Valez for assistance with the artwork.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AI095173. This work was also supported by grants from the Universidad de la República, the Programa de Desarrollo de Ciencias Básicas (PEDECIBA), and the Howard Hughes Medical Institute. This is the first article in the Thematic Minireview Series on Redox-active Protein Modifications and Signaling.
- NOS
- nitric-oxide synthase(s)
- SOD
- superoxide dismutase(s)
- MnP
- Mn-porphyrin(s).
REFERENCES
- 1. Ross W. G. M., Helman W. P., Buxton G. V., Huie R. E., Neta P. (1995) NDRL/NIST Solution Kinetics Database, Version 3.0, National Institute of Standards and Technology, Gaithersburg, MD [Google Scholar]
- 2. Fridovich I. (1995) Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64, 97–112 [DOI] [PubMed] [Google Scholar]
- 3. Sies H., Jones D. P. (2007) Oxidative stress. in Encyclopedia of Stress (Fink G., ed) 2nd Ed., Vol. 3, pp. 45–48, Elsevier/Academic Press, London [Google Scholar]
- 4. Gryglewski R. J., Palmer R. M., Moncada S. (1986) Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320, 454–456 [DOI] [PubMed] [Google Scholar]
- 5. Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S., Chaudhuri G. (1988) Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J. Pharmacol. Exp. Ther. 244, 181–189 [PubMed] [Google Scholar]
- 6. Bredt D. S., Snyder S. H. (1994) Nitric oxide: a physiologic messenger molecule. Annu. Rev. Biochem. 63, 175–195 [DOI] [PubMed] [Google Scholar]
- 7. Hill B. G., Dranka B. P., Bailey S. M., Lancaster J. R., Jr., Darley-Usmar V. M. (2010) What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 285, 19699–19704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ignarro L. J. (1990) Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 30, 535–560 [DOI] [PubMed] [Google Scholar]
- 9. Blough N. V., Zafiriou. O. C. (1985) Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg. Chem. 24, 3502–3504 [Google Scholar]
- 10. Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 87, 1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halfpenny E., Robinson P. L. (1952) The nitration and hydroxylation of aromatic compounds by pernitrous acid. J. Chem. Soc. 939–946 [Google Scholar]
- 12. Mahoney L. R. (1970) Evidence for the formation of hydroxyl radicals in the isomerization of pernitrous acid to nitric acid in aqueous solution. J. Am. Chem. Soc. 92, 5262–5263 [Google Scholar]
- 13. Ferrer-Sueta G., Radi R. (2009) Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem. Biol. 4, 161–177 [DOI] [PubMed] [Google Scholar]
- 14. Denicola A., Souza J. M., Radi R. (1998) Diffusion of peroxynitrite across erythrocyte membranes. Proc. Natl. Acad. Sci. U.S.A. 95, 3566–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Augusto O., Gatti R. M., Radi R. (1994) Spin-trapping studies of peroxynitrite decomposition and of 3-morpholinosydnonimine N-ethylcarbamide autooxidation: direct evidence for metal-independent formation of free radical intermediates. Arch. Biochem. Biophys. 310, 118–125 [DOI] [PubMed] [Google Scholar]
- 16. Goldstein S., Merényi G. (2008) The chemistry of peroxynitrite: implications for biological activity. Methods Enzymol. 436, 49–61 [DOI] [PubMed] [Google Scholar]
- 17. Buettner G. R. (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 300, 535–543 [DOI] [PubMed] [Google Scholar]
- 18. Winterbourn C. C., Hampton M. B., Livesey J. H., Kettle A. J. (2006) Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome. Implications for microbial killing. J. Biol. Chem. 281, 39860–39869 [DOI] [PubMed] [Google Scholar]
- 19. Winterbourn C. C., Parsons-Mair H. N., Gebicki S., Gebicki J. M., Davies M. J. (2004) Requirements for superoxide-dependent tyrosine hydroperoxide formation in peptides. Biochem. J. 381, 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Möller M. N., Hatch D. M., Kim H. Y., Porter N. A. (2012) Superoxide reaction with tyrosyl radicals generates para-hydroperoxy and para-hydroxy derivatives of tyrosine. J. Am. Chem. Soc. 134, 16773–16780 [DOI] [PubMed] [Google Scholar]
- 21. Nathan C. F., Hibbs J. B., Jr. (1991) Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 3, 65–70 [DOI] [PubMed] [Google Scholar]
- 22. Radi R. (1996) Reactions of nitric oxide with metalloproteins. Chem. Res. Toxicol. 9, 828–835 [DOI] [PubMed] [Google Scholar]
- 23. Radi R. (2004) Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. U.S.A. 101, 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radi R., Beckman J. S., Bush K. M., Freeman B. A. (1991) Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266, 4244–4250 [PubMed] [Google Scholar]
- 25. Radi R., Beckman J. S., Bush K. M., Freeman B. A. (1991) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 288, 481–487 [DOI] [PubMed] [Google Scholar]
- 26. Koppenol W. H., Moreno J. J., Pryor W. A., Ischiropoulos H., Beckman J. S. (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 5, 834–842 [DOI] [PubMed] [Google Scholar]
- 27. Trujillo M., Radi R. (2002) Peroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: new insights into the reaction of peroxynitrite with thiols. Arch. Biochem. Biophys. 397, 91–98 [DOI] [PubMed] [Google Scholar]
- 28. Peskin A. V., Winterbourn C. C. (2001) Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic. Biol. Med. 30, 572–579 [DOI] [PubMed] [Google Scholar]
- 29. Pattison D. I., Davies M. J. (2004) Kinetic analysis of the reactions of hypobromous acid with protein components: implications for cellular damage and use of 3-bromotyrosine as a marker of oxidative stress. Biochemistry 43, 4799–4809 [DOI] [PubMed] [Google Scholar]
- 30. Ferrer-Sueta G., Manta B., Botti H., Radi R., Trujillo M., Denicola A. (2011) Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 24, 434–450 [DOI] [PubMed] [Google Scholar]
- 31. Thomson L., Trujillo M., Telleri R., Radi R. (1995) Kinetics of cytochrome c2+ oxidation by peroxynitrite: implications for superoxide measurements in nitric oxide-producing biological systems. Arch. Biochem. Biophys. 319, 491–497 [DOI] [PubMed] [Google Scholar]
- 32. Augusto O., Bonini M. G., Amanso A. M., Linares E., Santos C. C., De Menezes S. L. (2002) Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic. Biol. Med. 32, 841–859 [DOI] [PubMed] [Google Scholar]
- 33. Bartesaghi S., Wenzel J., Trujillo M., López M., Joseph J., Kalyanaraman B., Radi R. (2010) Lipid peroxyl radicals mediate tyrosine dimerization and nitration in membranes. Chem. Res. Toxicol. 23, 821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stadler K., Bonini M. G., Dallas S., Jiang J., Radi R., Mason R. P., Kadiiska M. B. (2008) Involvement of inducible nitric oxide synthase in hydroxyl radical-mediated lipid peroxidation in streptozotocin-induced diabetes. Free Radic. Biol. Med. 45, 866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lymar S. V., Hurst J. K. (1995) Rapid reaction between peroxynitrite ion and carbon dioxide: implications for biological activity. J. Am. Chem. Soc. 117, 8867–8868 [Google Scholar]
- 36. Denicola A., Freeman B. A., Trujillo M., Radi R. (1996) Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem. Biophys. 333, 49–58 [DOI] [PubMed] [Google Scholar]
- 37. Radi R., Cosgrove T. P., Beckman J. S., Freeman B. A. (1993) Peroxynitrite-induced luminol chemiluminescence. Biochem. J. 290, 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonini M. G., Radi R., Ferrer-Sueta G., Ferreira A. M., Augusto O. (1999) Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J. Biol. Chem. 274, 10802–10806 [DOI] [PubMed] [Google Scholar]
- 39. Massari J., Tokikawa R., Medinas D. B., Angeli J. P., Di Mascio P., Assunção N. A., Bechara E. J. (2011) Generation of singlet oxygen by the glyoxal-peroxynitrite system. J. Am. Chem. Soc. 133, 20761–20768 [DOI] [PubMed] [Google Scholar]
- 40. Alvarez B., Ferrer-Sueta G., Freeman B. A., Radi R. (1999) Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J. Biol. Chem. 274, 842–848 [DOI] [PubMed] [Google Scholar]
- 41. Radi R. (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 46, 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prütz W. A., Mönig H., Butler J., Land E. J. (1985) Reactions of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Arch. Biochem. Biophys. 243, 125–134 [DOI] [PubMed] [Google Scholar]
- 43. Quijano C., Hernandez-Saavedra D., Castro L., McCord J. M., Freeman B. A., Radi R. (2001) Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem. 276, 11631–11638 [DOI] [PubMed] [Google Scholar]
- 44. Goldstein S., Czapski G., Lind J., Merényi G. (2000) Tyrosine nitration by simultaneous generation of •NO and O2⨪ under physiological conditions. How the radicals do the job. J. Biol. Chem. 275, 3031–3036 [DOI] [PubMed] [Google Scholar]
- 45. Pfeiffer S., Schmidt K., Mayer B. (2000) Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite. Implications for tyrosine modification by nitric oxide/superoxide in vivo. J. Biol. Chem. 275, 6346–6352 [DOI] [PubMed] [Google Scholar]
- 46. Quijano C., Romero N., Radi R. (2005) Tyrosine nitration by superoxide and nitric oxide fluxes in biological systems: modeling the impact of superoxide dismutase and nitric oxide diffusion. Free Radic. Biol. Med. 39, 728–741 [DOI] [PubMed] [Google Scholar]
- 47. Ischiropoulos H., Zhu L., Beckman J. S. (1992) Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 298, 446–451 [DOI] [PubMed] [Google Scholar]
- 48. Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. (1994) Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler. 375, 81–88 [DOI] [PubMed] [Google Scholar]
- 49. Brennan M. L., Wu W., Fu X., Shen Z., Song W., Frost H., Vadseth C., Narine L., Lenkiewicz E., Borchers M. T., Lusis A. J., Lee J. J., Lee N. A., Abu-Soud H. M., Ischiropoulos H., Hazen S. L. (2002) A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 277, 17415–17427 [DOI] [PubMed] [Google Scholar]
- 50. Gaut J. P., Byun J., Tran H. D., Lauber W. M., Carroll J. A., Hotchkiss R. S., Belaaouaj A., Heinecke J. W. (2002) Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Invest. 109, 1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eiserich J. P., Baldus S., Brennan M. L., Ma W., Zhang C., Tousson A., Castro L., Lusis A. J., Nauseef W. M., White C. R., Freeman B. A. (2002) Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 296, 2391–2394 [DOI] [PubMed] [Google Scholar]
- 52. Radi R., Cassina A., Hodara R., Quijano C., Castro L. (2002) Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 33, 1451–1464 [DOI] [PubMed] [Google Scholar]
- 53. Poderoso J. J., Carreras M. C., Lisdero C., Riobó N., Schöpfer F., Boveris A. (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch. Biochem. Biophys. 328, 85–92 [DOI] [PubMed] [Google Scholar]
- 54. Szabó C., Ischiropoulos H., Radi R. (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6, 662–680 [DOI] [PubMed] [Google Scholar]
- 55. Radi R., Rodriguez M., Castro L., Telleri R. (1994) Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 308, 89–95 [DOI] [PubMed] [Google Scholar]
- 56. Bringold U., Ghafourifar P., Richter C. (2000) Peroxynitrite formed by mitochondrial NO synthase promotes mitochondrial Ca2+ release. Free Radic. Biol. Med. 29, 343–348 [DOI] [PubMed] [Google Scholar]
- 57. Hausladen A., Fridovich I. (1994) Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J. Biol. Chem. 269, 29405–29408 [PubMed] [Google Scholar]
- 58. Castro L., Rodriguez M., Radi R. (1994) Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J. Biol. Chem. 269, 29409–29415 [PubMed] [Google Scholar]
- 59. Tórtora V., Quijano C., Freeman B., Radi R., Castro L. (2007) Mitochondrial aconitase reaction with nitric oxide, S-nitrosoglutathione, and peroxynitrite: mechanisms and relative contributions to aconitase inactivation. Free Radic. Biol. Med. 42, 1075–1088 [DOI] [PubMed] [Google Scholar]
- 60. Castro L., Demicheli V., Tórtora V., Radi R. (2011) Mitochondrial protein tyrosine nitration. Free Radic. Res. 45, 37–52 [DOI] [PubMed] [Google Scholar]
- 61. MacMillan-Crow L. A., Crow J. P., Kerby J. D., Beckman J. S., Thompson J. A. (1996) Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. U.S.A. 93, 11853–11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamakura F., Taka H., Fujimura T., Murayama K. (1998) Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 273, 14085–14089 [DOI] [PubMed] [Google Scholar]
- 63. Moreno D. M., Martí M. A., De Biase P. M., Estrin D. A., Demicheli V., Radi R., Boechi L. (2011) Exploring the molecular basis of human manganese superoxide dismutase inactivation mediated by tyrosine 34 nitration. Arch. Biochem. Biophys. 507, 304–309 [DOI] [PubMed] [Google Scholar]
- 64. Quint P., Reutzel R., Mikulski R., McKenna R., Silverman D. N. (2006) Crystal structure of nitrated human manganese superoxide dismutase: mechanism of inactivation. Free Radic. Biol. Med. 40, 453–458 [DOI] [PubMed] [Google Scholar]
- 65. Xu S., Ying J., Jiang B., Guo W., Adachi T., Sharov V., Lazar H., Menzoian J., Knyushko T. V., Bigelow D., Schöneich C., Cohen R. A. (2006) Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 290, H2220–H2227 [DOI] [PubMed] [Google Scholar]
- 66. Redondo-Horcajo M., Romero N., Martínez-Acedo P., Martínez-Ruiz A., Quijano C., Lourenço C. F., Movilla N., Enríquez J. A., Rodríguez-Pascual F., Rial E., Radi R., Vázquez J., Lamas S. (2010) Cyclosporine A-induced nitration of tyrosine 34 MnSOD in endothelial cells: role of mitochondrial superoxide. Cardiovasc. Res. 87, 356–365 [DOI] [PubMed] [Google Scholar]
- 67. Abriata L. A., Cassina A., Tórtora V., Marín M., Souza J. M., Castro L., Vila A. J., Radi R. (2009) Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J. Biol. Chem. 284, 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kagan V. E., Tyurin V. A., Jiang J., Tyurina Y. Y., Ritov V. B., Amoscato A. A., Osipov A. N., Belikova N. A., Kapralov A. A., Kini V., Vlasova I. I., Zhao Q., Zou M., Di P., Svistunenko D. A., Kurnikov I. V., Borisenko G. G. (2005) Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 [DOI] [PubMed] [Google Scholar]
- 69. Godoy L. C., Muñoz-Pinedo C., Castro L., Cardaci S., Schonhoff C. M., King M., Tórtora V., Marín M., Miao Q., Jiang J. F., Kapralov A., Jemmerson R., Silkstone G. G., Patel J. N., Evans J. E., Wilson M. T., Green D. R., Kagan V. E., Radi R., Mannick J. B. (2009) Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proc. Natl. Acad. Sci. U.S.A. 106, 2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pacher P., Beckman J. S., Liaudet L. (2007) Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 87, 315–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Franco M. C., Ye Y., Refakis C. A., Feldman J. L., Stokes A. L., Basso M., Melero Fernández de Mera R. M., Sparrow N. A., Calingasan N. Y., Kiaei M., Rhoads T. W., Ma T. C., Grumet M., Barnes S., Beal M. F., Beckman J. S., Mehl R., Estévez A. G. (2013) Nitration of Hsp90 induces cell death. Proc. Natl. Acad. Sci. U.S.A. 110, E1102–E1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Alvarez M. N., Peluffo G., Piacenza L., Radi R. (2011) Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi. Consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J. Biol. Chem. 286, 6627–6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ye Y., Quijano C., Robinson K. M., Ricart K. C., Strayer A. L., Sahawneh M. A., Shacka J. J., Kirk M., Barnes S., Accavitti-Loper M. A., Radi R., Beckman J. S., Estévez A. G. (2007) Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J. Biol. Chem. 282, 6324–6337 [DOI] [PubMed] [Google Scholar]
- 74. Peluffo G., Radi R. (2007) Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc. Res. 75, 291–302 [DOI] [PubMed] [Google Scholar]
- 75. van der Loo B., Labugger R., Skepper J. N., Bachschmid M., Kilo J., Powell J. M., Palacios-Callender M., Erusalimsky J. D., Quaschning T., Malinski T., Gygi D., Ullrich V., Lüscher T. F. (2000) Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 192, 1731–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhu L., Gunn C., Beckman J. S. (1992) Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 298, 452–457 [DOI] [PubMed] [Google Scholar]
- 77. Denicola A., Rubbo H., Rodríguez D., Radi R. (1993) Peroxynitrite-mediated cytotoxicity to Trypanosoma cruzi. Arch. Biochem. Biophys. 304, 279–286 [DOI] [PubMed] [Google Scholar]
- 78. Evans T. J., Buttery L. D., Carpenter A., Springall D. R., Polak J. M., Cohen J. (1996) Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc. Natl. Acad. Sci. U.S.A. 93, 9553–9558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hurst J. K. (2012) What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 53, 508–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bryk R., Griffin P., Nathan C. (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 [DOI] [PubMed] [Google Scholar]
- 81. Trujillo M., Budde H., Piñeyro M. D., Stehr M., Robello C., Flohé L., Radi R. (2004) Trypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J. Biol. Chem. 279, 34175–34182 [DOI] [PubMed] [Google Scholar]
- 82. Piacenza L., Zago M. P., Peluffo G., Alvarez M. N., Basombrio M. A., Radi R. (2009) Enzymes of the antioxidant network as novel determiners of Trypanosoma cruzi virulence. Int. J. Parasitol. 39, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Santos C. X., Anjos E. I., Augusto O. (1999) Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch. Biochem. Biophys. 372, 285–294 [DOI] [PubMed] [Google Scholar]
- 84. Squadrito G. L., Cueto R., Splenser A. E., Valavanidis A., Zhang H., Uppu R. M., Pryor W. A. (2000) Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 376, 333–337 [DOI] [PubMed] [Google Scholar]
- 85. Robinson K. M., Morré J. T., Beckman J. S. (2004) Triuret: a novel product of peroxynitrite-mediated oxidation of urate. Arch. Biochem. Biophys. 423, 213–217 [DOI] [PubMed] [Google Scholar]
- 86. Ames B. N., Cathcart R., Schwiers E., Hochstein P. (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. U.S.A. 78, 6858–6862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Batinić-Haberle I., Rebouças J. S., Spasojević I. (2010) Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 13, 877–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ferrer-Sueta G., Batinić-Haberle I., Spasojević I., Fridovich I., Radi R. (1999) Catalytic scavenging of peroxynitrite by isomeric Mn(III) N-methylpyridylporphyrins in the presence of reductants. Chem. Res. Toxicol. 12, 442–449 [DOI] [PubMed] [Google Scholar]
- 89. Ferrer-Sueta G., Hannibal L., Batinić-Haberle I., Radi R. (2006) Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic. Biol. Med. 41, 503–512 [DOI] [PubMed] [Google Scholar]
- 90. Spasojević I., Chen Y., Noel T. J., Yu Y., Cole M. P., Zhang L., Zhao Y., St. Clair D. K., Batinić-Haberle I. (2007) Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radic. Biol. Med. 42, 1193–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Valez V., Cassina A., Batinić-Haberle I., Kalyanaraman B., Ferrer-Sueta G., Radi R. (2013) Peroxynitrite formation in nitric oxide-exposed submitochondrial particles: detection, oxidative damage and catalytic removal by Mn-porphyrins. Arch. Biochem. Biophys. 529, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ferrer-Sueta G., Vitturi D., Batinić-Haberle I., Fridovich I., Goldstein S., Czapski G., Radi R. (2003) Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J. Biol. Chem. 278, 27432–27438 [DOI] [PubMed] [Google Scholar]
- 93. Batinić-Haberle I., Cuzzocrea S., Rebouças J. S., Ferrer-Sueta G., Mazzon E., Di Paola R., Radi R., Spasojević I., Benov L., Salvemini D. (2009) Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 46, 192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Szeto H. H., Schiller P. W. (2011) Novel therapies targeting inner mitochondrial membrane–from discovery to clinical development. Pharm. Res. 28, 2669–2679 [DOI] [PubMed] [Google Scholar]
- 95. Zielonka J., Sikora A., Joseph J., Kalyanaraman B. (2010) Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide. Direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 285, 14210–14216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Alvarez B., Rubbo H., Kirk M., Barnes S., Freeman B. A., Radi R. (1996) Peroxynitrite-dependent tryptophan nitration. Chem. Res. Toxicol. 9, 390–396 [DOI] [PubMed] [Google Scholar]
- 97. Pryor W. A., Jin X., Squadrito G. L. (1994) One- and two-electron oxidations of methionine by peroxynitrite. Proc. Natl. Acad. Sci. U.S.A. 91, 11173–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Meotti F. C., Jameson G. N., Turner R., Harwood D. T., Stockwell S., Rees M. D., Thomas S. R., Kettle A. J. (2011) Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation. J. Biol. Chem. 286, 12901–12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Romero N., Radi R., Linares E., Augusto O., Detweiler C. D., Mason R. P., Denicola A. (2003) Reaction of human hemoglobin with peroxynitrite. Isomerization to nitrate and secondary formation of protein radicals. J. Biol. Chem. 278, 44049–44057 [DOI] [PubMed] [Google Scholar]
- 100. Masumoto H., Sies H. (1996) The reaction of ebselen with peroxynitrite. Chem. Res. Toxicol. 9, 262–267 [DOI] [PubMed] [Google Scholar]