Background: Yeast mitochondrial cytochrome oxidase has been proposed to assemble from three modules.
Results: The Cox1 module assembles independently of the two other modules and contains mitochondrial- encoded Cox1p and three nuclear encoded subunits.
Conclusion: The composition of the Cox1p module reflects the subunit interactions in the holoenzyme.
Significance: Cytochrome oxidase is assembled from several rather than a single linear pathway.
Keywords: Cytochrome Oxidase, Membrane Biogenesis, Mitochondria, Respiratory Chain, Yeast Genetics, Biogenesis, Cox1p Intermediates, Saccharomyces cerevisiae
Abstract
Mitochondrial-encoded Cox1p, one of the three core subunits of yeast cytochrome oxidase (COX), was previously shown to associate with regulatory proteins and nuclear-encoded subunits into five high molecular weight complexes that were proposed to constitute the pathway for biogenesis of the Cox1p assembly module. One of the intermediates (D5) was inferred, but not directly shown to exist. In the present study mitochondria of strains expressing C-terminal-tagged subunits of COX that had not been looked at previously were pulse-labeled and analyzed for the presence of newly translated Cox1p in the immunoprecipitates. These studies revealed that of the eight nuclear-encoded COX subunits, only Cox5ap, Cox6p, and Cox8p are present in the Cox1p module. Both Cox5ap and Cox8p share interfaces with Cox1p in the holoenzyme, whereas Cox6p interacts indirectly through Cox5ap. These results suggest that the subunit contacts in the holoenzyme are probably established during biogenesis of the Cox1p module. To confirm the existence of the largest Cox1p intermediates (D5), which was only inferred previously, radiolabeled Cox1p with a C-terminal tag was expressed in COX-deficient pet111 and pet494 mutants. Pulldown assays confirmed the presence of newly translated Cox1p in D5, which in wild type cannot be demonstrated directly because of its co-migration with COX in the native electrophoresis system used to separate the intermediates. Jointly, the results of these analyses substantiate our previous proposal that COX is assembled from separate assembly modules, each containing one of the mitochondrial-translated core subunits in association with a unique set of nuclear-encoded subunits.
Introduction
Cytochrome oxidase (COX) is the terminal complex of mitochondrial and bacterial respiratory chains. The catalytic core of COX consists of Cox1p, Cox2p, and Cox3p, which are products of mitochondrial DNA (mtDNA) in fungi and animal mitochondria (1). In addition to the core subunits, the mitochondrial enzyme contains eight other subunits that are absent in bacteria. This group of proteins is encoded in nuclear DNA, translated on cytoplasmic ribosomes, and routed to the appropriate mitochondrial compartments. Although the functions of the latter proteins are not known, studies in yeast indicate that with a few exceptions, they are required for assembly and stability of an optimally active enzyme (2, 3).
Biogenesis of yeast COX is a regulated process requiring a balanced output of the mitochondrial and nuclear gene products. In Saccharomyces cerevisiae, the rate of Cox1p translation is adjusted to its utilization during assembly of the holoenzyme (4–7). The regulation depends on several accessory proteins. Foremost is Mss51p, a Cox1p-specific translational activator that interacts not only with the COX1 mRNA to initiate translation but also with the protein product itself (8). The Cox14p-Coa3p complex and Coa1p also play important roles in translational regulation by stabilizing the Cox1p-Mss51p interaction (4, 9, 10) and preventing aggregation of unassembled Cox1p with other hydrophobic proteins (11). Sequestration of Mss51p in this complex prevents it from exercising its function as a translational activator of the mRNA until it is released during recruitment of Cox1p for COX assembly.
Five assembly intermediates (D1-D5) have been detected by immunoprecipitation of tagged Cox1p from digitonin extracts of yeast mitochondria pulse-labeled in vitro with radioactive amino acids (12). The complexes differ both in size and abundance and in pulse-chase experiments display a precursor-product relationship with COX. Several approaches have been used to study the compositions of Cox1p intermediates (6, 8, 12). An early intermediate was shown to consist of Cox1p complexed to Ssc1p (6). Other Cox1p intermediates contain Mss51p, Cox14p, Coa3p, Coa1p, and subunits 5a and 6 of COX (4, 8, 9, 12, 13). Based on pulse-chase analysis of the intermediates in isolated mitochondria, we proposed that COX is assembled from three different modules, each containing one of the core proteins with its own unique complement of nuclear-encoded subunits and/or regulatory/assembly factors (12).
The present studies were undertaken to answer several questions related to the Cox1p assembly module. The first was to determine if in addition to Cox5ap and Cox6p, this module contained other nuclear-encoded subunits for the purpose of gaining a better understanding of the relationship between the composition of the Cox1p intermediate(s) and the known subunit contacts Cox1p makes in the holoenzyme. Second, we examined if the mutation in these subunits affected the steady-state distribution of Cox1p in the different Cox1p intermediates. Last, we sought to obtain additional evidence for the existence of the largest Cox1p intermediate (D5), which was inferred to exist but was not demonstrated directly in the prior study (12).
MATERIALS AND METHODS
Strains and Growth Media
The genotypes and sources of the strains of S. cerevisiae are listed in Table 1. The compositions of solid and liquid YPD (yeast extract/peptone/dextrose), YPGal (yeast extract/peptone/galactose), and YEPG (yeast extract/peptone/glycerol/ethanol) used to grow wild type and mutant yeast have been described (15).
TABLE 1.
Genotypes and sources of S. cerevisiae strains
| Strain | Genotype | mtDNA | Source |
|---|---|---|---|
| W303-1A | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 | ρ + | –a |
| W303-1B | MATα ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 | ρ + | –a |
| DFK160/COX1-HAC | MAT α kar1-1 ade-10 arg8::URA3 lys2 leu2Δura3–52 | COX1-HAC, ρ + | (12) |
| MRSIo/COX1-HAC | MATα ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1arg8::HIS3 | COX1-HAC | (12) |
| aW303ΔCOX7 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox7::URA3 | ρ+ | (4) |
| aW303/COX7-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox7::URA3 trp1::pG199/ST10 | ρ+ | This study |
| aW303ΔCOX7/COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox7::URA3 | COX1-HAC | This study |
| aW303ΔCOX8 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 | ρ+ | This study |
| W303ΔCOX8 | MATα ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 | ρ+ | This study |
| aW303ΔCOX8/COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 | COX1-HAC allele | This study |
| W303/COX8-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 ura3::pCOX8/ST9 | ρ+ | This study |
| aW303ΔCOX12 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox12::HIS3 | ρ+ | This study |
| aW303ΔCOX12/COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox12::HIS3 | COX1-HAC allele | This study |
| W303/COX12-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 ura3::pCOX12/ST4 | ρ+ | This study |
| aW303ΔCOX13 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox13::HIS3 | ρ + | This study |
| aW303ΔCOX13/COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox13::HIS3 | COX1-HAC allele | This study |
| W303/COX13-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 ura3::pCOX13/ST4 | ρ+ | This study |
| aW303ΔRCF1 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 rcf1::HIS3 | ρ+ | This study |
| aW303ΔRCF1/COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 rcf1::HIS3 | COX1-HAC allele | This study |
| W303/RCF1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 ura3::pRCF1/ST4 | ρ+ | This study |
| aW303ΔRCF2 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 rcf2::HIS3 | ρ+ | This study |
| aW303ΔRCF2/COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 rcf2::HIS3 | COX1-HAC allele | This study |
| W303/RCF2-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox8::HIS3 ura3::pRCF2/ST4 | ρ+ | This study |
| aW303ΔCYC1 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cyc1::HIS3 | ρ + | (14) |
| aW303ΔCYC7 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cyc1::TRP1 | ρ+ | (14) |
| aW303ΔCYC1,CYC7 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cyc1::URA3 cyc7::TRP1 | ρ + | (14) |
| aW303ΔCYC1,CYC7 ΔCOX14 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cyc1::URA3 cyc7::TRP1 cox14::HIS3 | ρ + | This study |
| aW303ΔCYC1,CYC7/COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cyc1::URA3 cyc7::TRP1 | COX1-HAC allele | This study |
| aMRSIoΔPET111 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1arg8::HIS3 pet111::HIS3 | ρ | (4) |
| aMRSΔPET111/ COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1arg8::HIS3 pet111::HIS3 | COX1-HAC allele | This study |
| aMRSΔPET494 | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1arg8::HIS3 pet494::HIS3 | ρ + | This study |
| aMRSΔPET494/ COX1-HAC | MATa ade-1 his3-1,15 leu2-3,112 trp1-1 ura3-1arg8::HIS3 pet494::HIS3 | COX1-HAC allele | This study |
a R. Rothstein, Dept. of Human Genetics and Development, Columbia University.
Construction of a COX8 Null Allele
COX8 with ∼500 bp of 5′ and 3′ sequences was amplified from W303-1A nuclear DNA with primers Cox8-1, 5′-ggcgagtctgcaagcatcaagagcaataa, and Cox8-2, 5′-ggcgagctcactcattgcaacaaagtaaac. The 1.3-kb PCR fragment was digested with SacI and cloned into pUC19. The resultant plasmid (pCOX8/ST1) was used as a template for removal of the COX8 coding sequence with the bidirectional primers Cox8-3, 5′-ggcggatcctgcgtaatggaaatggataacttttctgtt, and Cox8-4, 5′-ggcggatccacccccataagtttgaaggatagatgtgt. The amplified plasmid plus COX8 flanking sequences was digested with BamH1 and ligated to a 1-kb BamH1 fragment containing the yeast HIS3 gene to yield pCOX8/ST5. The SacI fragment with the cox8::HIS3 null allele plasmid obtained from pCOX8/ST5 was used to introduce the null allele into W303-1A and W303-1B by homologous recombination (16). Histidine-independent clones obtained from the transformation were verified to have the null allele by PCR amplification of nuclear DNA with primers Cox8-1 and Cox8-2. Similar protocols were used to obtain null alleles of COX12, COX13, RCF1, and RCF2 (the sequences of the primers used for these constructs are described in Table 2).
TABLE 2.
Primers for construction of null alleles and hybrid genes expressing tagged proteins
| Primer name | Primer sequence |
|---|---|
| Primers used to amplify gene plus 5′- and 3′-flanking sequences | |
| cox7-1 | 5′-GGCAGATCTCAGTTATTTACCTTCCTTTCTC |
| cox1-1 | 5′-GGCGAGCTCGGAAAGCAAAGCTCTAAATGA |
| cox12-2 | 5′-CGGCTGCAGTTCTCAACATGTTCAGCGTTT |
| cox13-1 | 5′-GGCGAGCTCTTAAACACTACTAATGTAATT |
| cox13-2 | 5′-CGGCTGCAGTAGATTTGCCCCTCATTGAAA |
| rcf1-1 | 5′-GGCGAGCTCACAGCCCAAGATAGAGTACAA |
| rcf1-2 | 5′-CGGCTGCAGTCGCTTGACCATATAGTAAAT |
| rcf-1 | 5′-GGCGAGCTCCATGTATGTGTAGATATGTAC |
| rcf2-2 | 5′-CGGCTGCAGCCTCGTCGTCCACTGTTATAG |
| Bidirectional primers used to delete coding sequence | |
| cox12-3 | 5′-GGCGGATCCGGATCTTAGTTTCGTTTTTAG |
| cox12-4 | 5′-GGCGGATCCGTCTATTATACTGTATTTATG |
| cox13-3 | 5′-GGCGGATCCTTAATTGATTAAGTGAGAATA |
| cox13-4 | 5′-GGCGGATCCTTTTCTATTGTTTGTTATTTG |
| rcf1-3 | 5′-GGCGGATCCTTTGGCTCTTCGATTTGCTGT |
| rcf1-4 | 5′-GGCGGATCCCTTGAACGTATAACTGTGATG |
| Rcf2-3 | 5′-GGCGGATCCACCTCGTCGGTTTATCTGCCT |
| rcf2-4 | 5′-GGCGGATCCTGTGCGATGTTGGTGAGTATC |
| Primers used to add HA and protein C epitope tags | |
| cox7-HA | 5′-GCCTGCAGTCAAGCGTAGTCTGGGACGTCGTATGGGTATGCCTTCTTGGCTTTGATACC |
| cox7-C | 5′-GGCGGTACCTCATTTACCATCGATTAATCTTGGATCTACTTGATCTTCTCCTCCAGCGTAGTCTGGGACGTCGTATGG |
| cox12-HA | 5′-GGCCTGCAGTCAAGCGTAGTCTGGGACGTCGTATGGGTAGTCTGAGTTGATATCACCTGC |
| cox12-C | 5′-GGCCTGCAGTCATTTACCATCGATTAATCTTGGATCTACTTGATCTTCTCCTCCAGCGTAGTCTGGGACGTCGTATGG |
| cox13-HA | 5′-GGCCTGCAGTCAAAGCGTAGTCTGGGACGTCGTATGGGTAATCGTCGTGCTCGATGTGCCT' |
| cox13-C | 5′-GGCCTGCAGTCATTTACCATCGATTAATCTTGGATCTACTTGATCTTCTCCTCCAGCGTAGTCTGGGACGTCGTATGG |
| rcf1-HA | 5′-GCCTGCAGTCAAGCGTAGTCTGGGACGTCGTATGGGTACTTCTTTCCAAGCTTATTTTC |
| rcf1-C | 5′-GGCCTGCAGTCATTTACCATCGATTAATCTTGGATCTACTTGATCTTCTCCTCCAGCGTAGTCTGGGACGTCGTATGG-3′ |
| rcf2-HA | 5′-GCCTGCAGTCAAGCGTAGTCTGGGACGTCGTATGGGTACGACTTGAAGATCTTGGAGTT |
| rcf2-C | 5′-GGCCTGCAGTCATTTACCATCGATTAATCTTGGATCTACTTGATCTTCTCCTCCAGCGTAGTCTGGGACGTCGTATGG-3′ |
Construction of COX8-HAC
To construct the COX8-HAC allele expressing Cox8p with a C-terminal tag consisting of HA followed by the protein C epitope, the 5′-UTR plus coding sequence was first amplified from W303-1A nuclear DNA with primers Cox8-1 and Cox8-5, 5′-tgactgcagtcaagcgtaatctgggacgtcgtatgggtaaaaagcacctgactttttcaa. The resultant fragment was used as a template for the addition of a sequence coding for the protein C tag with primers Cox8-1 and Cox6-C, 5′-ggcctgcagtcatttaccatcgattaatcttggatctacttgatcttctcctccagcgtagtctgggacgtcgtatgg. The PCR product was digested with SacI plus PstI and cloned in YIp352 (17) to yield pCOX8/ST9. The latter construct was linearized at the unique NcoI site in URA3 and integrated at the ura3 locus of the cox8 null mutants W303ΔCOX8 and aW303ΔCOX8.
Preparation of Mitochondria and Labeling of Mitochondrial Gene Products
Mitochondria were prepared by the method of Herrmann et al. (18) from yeast grown in rich galactose medium (YPGal) to early stationary phase. Mitochondria were labeled with a mixture of [35S]methionine and [35S]cysteine (3000 Ci/mmol, MP Biochemicals, Solon, OH) (19), and tagged proteins were extracted and purified on protein C antibody beads (Roche Applied Sciences) as described previously (12). The details of individual experiments are included in the legends to the figures.
Miscellaneous Procedures
Standard procedures were used to prepare and ligate DNA and to transform Escherichia coli (20). Mitochondrial gene products were labeled in whole cells as described previously (21). The lithium acetate method of Schiestl and Gietz (22) was used to transform yeast. Proteins were separated by SDS-PAGE on different concentrations of polyacrylamide gels in the buffer system described by Laemmli (23). Mitochondrial protein complexes were separated under non-denaturing conditions by BN-PAGE2 on 4–13% polyacrylamide gels (24). The second dimension in SDS-PAGE was run on 12% polyacrylamide gels. Western blots were treated with antibodies against the appropriate proteins followed by a second reaction with anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase (Sigma). Proteins were detected with the SuperSignal chemiluminescent substrate kit (Pierce). Protein concentrations were estimated by the method of Lowry et al. (25).
RESULTS
Association of Cox8p (subunit 8) with the Cox1p Assembly Module
Yeast expressing a subset of COX subunits and assembly factors tagged at their C termini with HA followed by the protein C epitope were previously used to examine the compositions of different Cox1p intermediates (12). The intermediates were analyzed by native gel electrophoresis of the tagged proteins immuno-adsorbed from digitonin extracts of isolated mitochondria labeled in organello with radioactive amino acids. The same approach was used in this study to analyze Cox1p intermediates for the presence of Cox8p, the only nuclear-encoded subunit other than Cox5ap in direct contact with Cox1p in the holoenzyme (26).
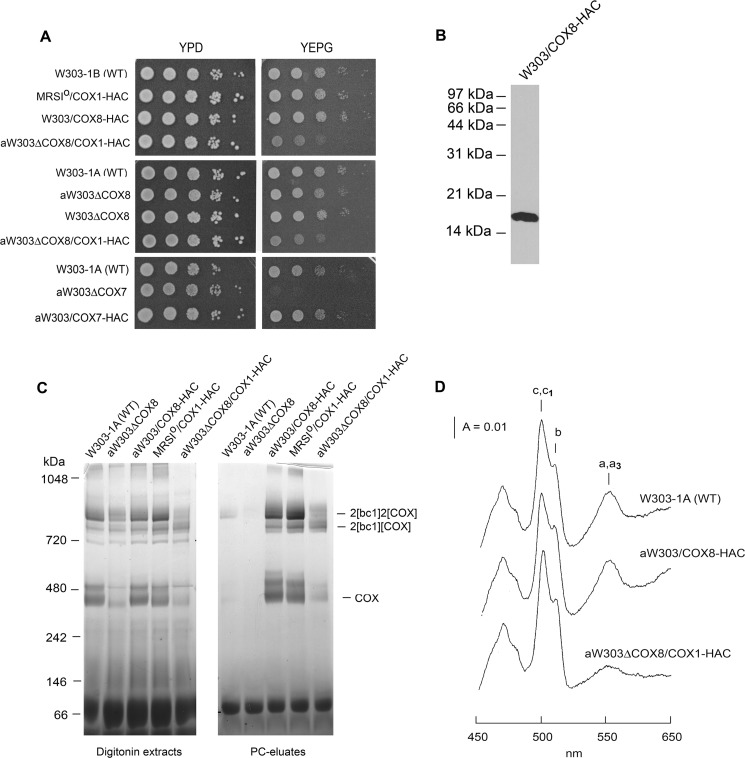
W303/COX8-HAC, a cox8 null mutant harboring a chromosomally integrated copy of COX8-HAC, was verified to express Cox8p with a C-terminal HAC tag and, based on its growth on rich glycerol/ethanol, in-gel enzyme activity, and spectra of mitochondrial cytochromes, to contain normal amounts of COX (Fig. 1, A–D). Mitochondria of this strain were labeled with [35S]methionine plus cysteine. After extraction with digitonin, Cox8p-HAC was purified on protein C antibody beads. The same protocol was used with mitochondria expressing Cox1p-HAC in the respiratory-competent haploid strain MRSIo/COX1-HAC and in the cox8 mutant aW303ΔCOX8/COX1-HAC. Labeled mitochondrial gene products associated with Cox8p-HAC and Cox1p-HAC in the fractions eluted from the antibody beads were analyzed by SDS-PAGE and by BN-PAGE. The eluates from mitochondria with either tagged Cox8p or Cox1p were enriched for newly translated Cox1p, Cox2p, and Cox3p (Fig. 2A). The one-dimensional BN-PAGE showed the expected radiolabeled intermediates D1-D5 in the eluates from the two strains with double-tagged Cox1p-HAC (Fig. 2B). The two-dimensional gels of the eluates from these strains were very similar except that there was less newly translated Cox3p in COX and the supercomplex bands of the cox8 null mutant, indicating reduced COX assembly (Fig. 2C). This is consistent with the growth property, the in-gel COX assay, and the cytochrome spectrum of mitochondria of this strain (Fig. 1, A, C, and D). The decrease of COX in the cox8 null mutant suggests that C-terminal modification of Cox1p renders it less assembly-competent in the absence of Cox8p or reduces the stability of the holoenzyme.
FIGURE 1.
Properties of yeast-expressing Cox8p double-tagged with HA and a protein C epitope. A, the wild type W303-1B, the respiratory competent haploid strain MRSIo/COX1-HAC expressing Cox1p-HAC, the cox8 null mutant, and transformant (W303/COX8-HAC) expressing Cox8p-HAC were serially diluted and spotted on rich glucose (YPD) and rich glycerol/ethanol (YEPG) plates. The plates were incubated at 30 °C for 2 days. B, W303/COX8-HAC expressing Cox8p-HAC was grown in rich 2% galactose medium (YPGal) to early stationary phase. Mitochondria were prepared, and the equivalent of 20 μg of protein was separated by SDS-PAGE on a 15% polyacrylamide gel. Proteins were transferred to nitrocellulose and probed with a monoclonal antibody against the HA tag. C, mitochondria from the indicated strains were suspended at 10 mg of protein/ml of extraction buffer and mixed with 1.2 volumes of 4% digitonin in extraction buffer as described under “Materials and Methods.” The suspension was centrifuged at 100,000 × gav, and samples of the supernatants representing 80 μg of starting mitochondrial protein were separated by BN-PAGE on a 4–13% polyacrylamide gel (left panel). Samples of the digitonin extracts were also purified on protein C antibody beads before BN-PAGE (right panel). The gel was stained for COX activity in a solution containing 0.5 mg/ml DAB and 1 mg/ml horse heart cytochrome c for several h. D, cytochrome spectra were recorded at room temperature after extraction of mitochondria at a protein concentration of 5 mg/ml with potassium deoxycholate (27). The positions of the α absorption bands are indicated.
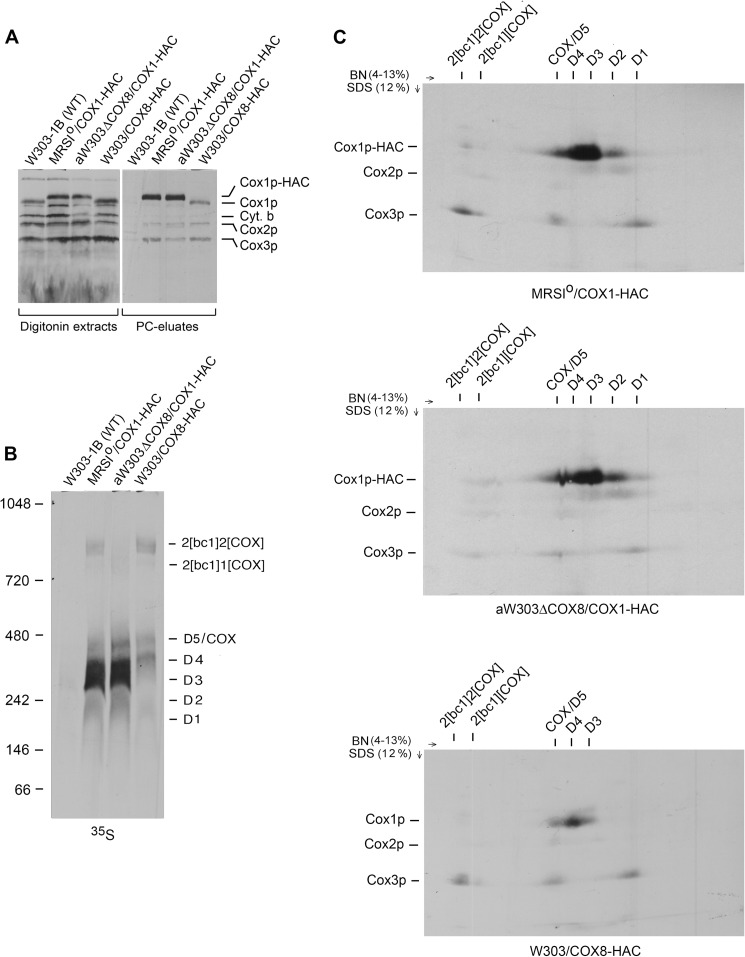
FIGURE 2.
Cox8p is a component of the Cox1p module. A, mitochondria were prepared from the wild type W303-1B and the respiratory competent haploid strains MRSIo/COX1-HAC and W303/COX8-HAC expressing, respectively, Cox1p and Cox8p with C-terminal HAC tags and from the cox8 null mutant aW303ΔCOX8/COX1-HAC harboring mtDNA with COX1-HAC. Mitochondria were labeled for 20 min as described under “Materials and Methods,” and samples of the digitonin extracts and the fraction eluted from the protein C antibody beads (PC eluate) were separated by SDS-PAGE on a 17% polyacrylamide gel, transferred to nitrocellulose, and exposed to x-ray film. The radiolabeled mitochondrial gene products are identified in the margins. B, the protein C eluates obtained in A were separated by BN-PAGE on a 4–13% polyacrylamide gel, transferred to a PVDF membrane, and exposed to x-ray film. The bands corresponding to the supercomplexes, COX, and the D1-D5 Cox1p intermediates identified previously (12) are indicated in the margin. C, the protein C antibody eluates obtained in A were separated in the first dimension as in B. A slice of the gel containing the separated proteins was equilibrated with stacking gel buffer (23), layered on a 12% polyacrylamide gel, and separated by SDS-PAGE in the second dimension. Proteins were transferred to a PVDF membrane and exposed to x-ray film. The radiolabeled bands separated under native conditions are identified above the gels and in the denaturing gel on the left margins. As discussed previously, the non-stoichiometric incorporation of newly radiolabeled Cox1p into the supercomplexes is probably a consequence of dilution by the large steady-state concentrations of unlabeled Cox1p intermediates present in mitochondria (12).
The antibody eluate obtained from the strain expressing Cox8-HAC indicated co-immunoprecipitation of the D4 intermediate mainly, although some radiolabel was also associated with D3 and D5/COX (Fig. 2, B and C, lower panel). The radiolabel in D4 and D3 was contributed entirely by Cox1p, whereas D5/COX contained both Cox1p and Cox3p. The co-purification of the D3-D5 intermediates with Cox8p-HAC confirms this subunit to be a constituent of the Cox1 module.
Cox7p, Cox12p, Cox13p, Rcf1p, and Rcf2p Are Absent in the Cox1p Assembly Intermediates
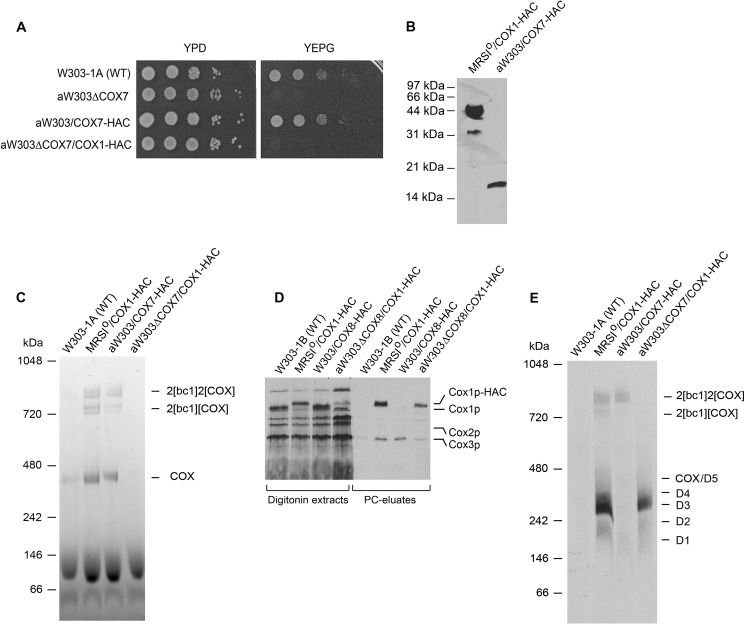
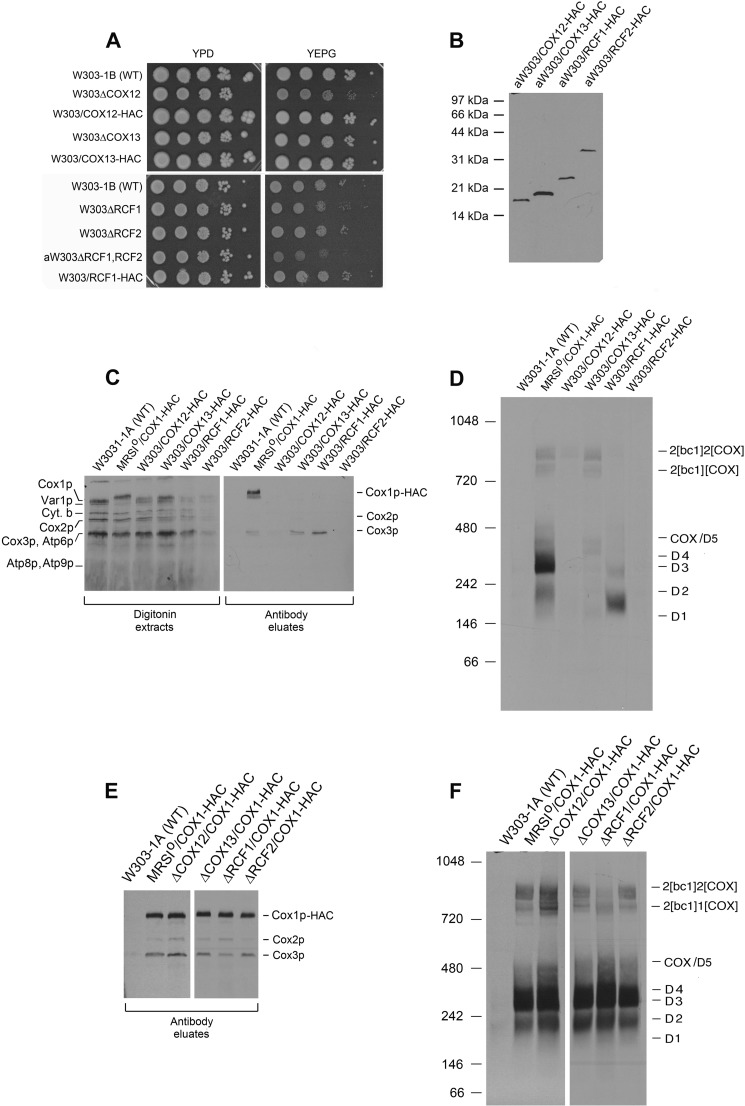
Cox1p intermediates were also analyzed for the presence of other previously untested COX subunits and assembly factors. These included subunits Cox7p, Cox12p, and Cox13p and assembly factors Rcf1p and Rcf2p. Of the six genes, only the deletion of COX7 elicits a clear respiratory deficiency (Fig. 3, A and C), whereas the deletion of COX12 and RCF1 only partially retarded growth on glycerol/ethanol (Fig. 4A). The gene for each protein, modified to express its product with the HAC double tag, was cloned in an integrative plasmid and introduced into the corresponding null mutant by homologous recombination (16). Transformants, verified to express the tagged proteins (Figs. 3B and 4B) grew as well as wild type on non-fermentable carbon sources and displayed wild type COX activity, indicating that the modified proteins were fair substitutes for the native subunits or factors (Figs. 3, A and C, and 4A).
FIGURE 3.
Properties of yeast expressing HAC tagged Cox7p and analysis of Cox1p intermediates in a cox7 mutant. A, the indicated mutants and transformants were serially diluted and spotted on rich glucose and rich glycerol/ethanol media. B, total mitochondrial proteins (20 μg of protein) W303/COX7-HAC, a respiratory-competent haploid strain with an integrated copy of COX7-HAC and from MRSIo/COX1-HAC expressing Cox1p-HAC, were separated by SDS-PAGE, and proteins were blotted and probed with a monoclonal antibody against the HA tag as in Fig. 1B. C, digitonin extracts of mitochondria from the indicated strains were purified on protein C antibody beads, and the eluates equivalent to 80 μg of starting mitochondria, were separated by BN-PAGE on a 4–13% polyacrylamide gel. The gel was stained for COX activity as in Fig. 1C. D, mitochondria were labeled. and digitonin extracts were purified on protein C antibody beads as in Fig. 2A. The digitonin extracts and eluates from the beads (PC eluates) were separated by SDS-PAGE on a 17% polyacrylamide gel and exposed to x-ray film after transfer to nitrocellulose. E, the antibody eluates were separated by BN-PAGE on a 4–13% polyacrylamide gel and exposed to x-ray film after transfer to a PVDF membrane.
FIGURE 4.
Properties and analysis of Cox1p intermediates in respiratory competent cox12, cox13, rcf1, and rcf2 mutants. A, wild type, mutants, and transformants expressing the HAC-tagged protein were serially diluted, spotted on rich glucose (YPD) and rich glycerol/ethanol (YEPG) media, and incubated at 30 °C either for 4 days (upper panel) or 2 days (lower panel). B, mitochondria (25 μg of protein) from the indicated strains expressing Cox12p, Cox13p, Rcf1p, and Rcf2p with the C-terminal HAC tag were separated by SDS-PAGE on a 15% polyacrylamide gel, transferred to nitrocellulose, and probed with an antibody against the protein C tag. C, mitochondria prepared from wild type and mutant strains grown to early stationary phase in YPGal were labeled and fractionated as in Fig. 2A. Samples of the digitonin extracts and eluates from the protein C antibody beads (PC eluates) were separated on a 17% polyacrylamide gel by SDS-PAGE, transferred to nitrocellulose, and exposed to x-ray film. The radiolabeled mitochondrial gene products of COX are identified in the margin. D, the antibody eluates from C were separated by BN-PAGE on a 4–13% polyacrylamide gel, transferred to PVDF membrane, and exposed to x-ray film. E, mitochondria of wild type and mutant strains in the W303 nuclear background containing the COX1-HAC hybrid gene in their mtDNA were labeled and fractionated on protein C antibody beads as in Fig. 2A. The Cox1p-HAC-enriched fractions eluted from the beads were separated by SDS-PAGE on a 17% polyacrylamide gel, transferred nitrocellulose, and exposed to x-ray film. The radiolabeled COX subunits are identified in the margin. F, the antibody eluates obtained in E were separated by BN-PAGE on a 4–13% polyacrylamide gel, transferred to a PVDF membrane, and exposed to x-ray. The radiolabeled supercomplexes COX and Cox1p intermediates are identified in the margin.
The association of the different subunits and accessory factors with Cox1p intermediates and the effect of each mutation on the steady-state levels of the intermediates was assessed by the pulldown assay described in the previous section for the analysis of Cox8p. None of the antibody eluates obtained from the strains expressing the tagged subunits and assembly factors were enriched for Cox1p or Cox1p intermediates, indicating that these subunits and accessory proteins are not components of the Cox1p module (Figs. 3, D and E, and 4, C and D). The antibody eluates of strains with tagged Cox7p, Cox13p, and Rcf1p, however, were enriched for newly translated Cox3p. The interaction of Rcf1p with Cox3p before its assembly into COX has been reported (28). On a native gel the labeled Cox3p co-immunoprecipitated with Rcf1p-HAC separates into two distinct complexes, the more abundant of which migrates distinctly from the D1-D5 Cox1p intermediates (Fig. 4D). Co-purification of Cox7p and Cox13p with Cox3p suggests that these subunits may be components of another assembly module containing Cox3p.
Each mutant was also made ρ0 with ethidium bromide and repopulated with mitochondrial DNA containing Cox1p-HAC by cytoduction with the kar1 donor strain DFK160/COX1-HAC. Mutants in the four nuclear COX genes and two assembly factors, each harboring a COX1-HAC in mtDNA, were appraised for the effect of each mutation on the steady-state levels of Cox1p intermediates. The results of these assays revealed that the distribution of radiolabeled Cox1p among the intermediates was the same in each mutant (Figs. 3, D and E, and 4, E and F).
These together with previous results (12) indicate that Cox5ap, Cox6p, and Cox8p are the only COX subunits associated with the Cox1p subassembly and that only the steady-state level but not the relative abundance of the different Cox1p intermediates is influenced by the absence of subunits that are essential for assembly of the holoenzyme. The subset of nuclear gene products in the Cox1p module is consistent with the known subunit contacts in the holoenzyme (Fig. 5).
FIGURE 5.
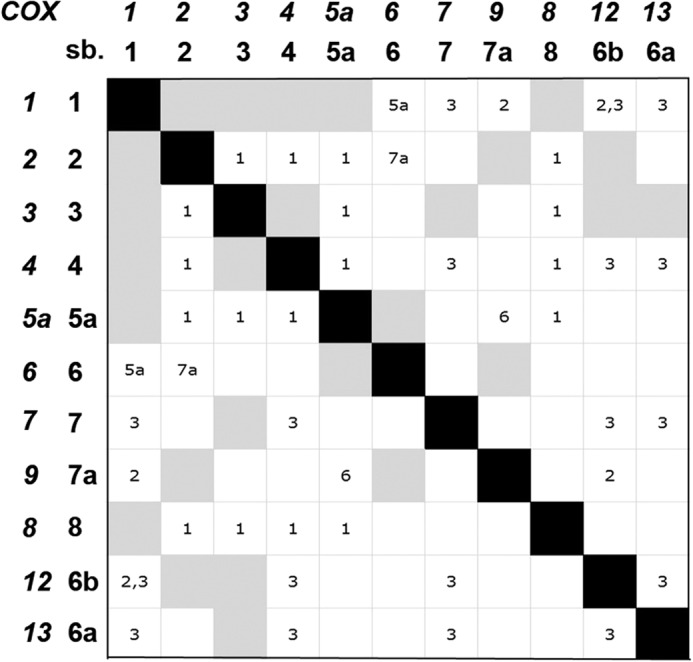
Subunit contacts in COX. The COX gene numbers and the corresponding subunit numbers are shown on the abscissa and ordinate. Direct contact between subunits (sb.) is depicted by the gray squares. Interactions through intermediary subunits such as Cox1p with Cox6 through Cox5a are indicated in the white squares by the number of the intermediary subunit. The contacts shown in the matrix are based on the structure of the bovine enzyme (26).
Cytochrome c Is Necessary for Optimal Translation of Cox1p but Not for Formation of Cox1p Intermediates
Translation of Cox1p is modulated by the efficiency with which it is assembled with other subunits into functional COX (4–7). Mutants lacking COX as a result of mutations in catalytic or structural subunits essential for assembly as well as in accessory factors involved in synthesis or maturation of the three catalytic centers display a severe reduction in Cox1p translation (4). Translation of Cox1p in such mutants, however, is restored by mutations in either COX14 or COA3, presumably due to the instability of the Cox1p-Mss51p interaction in the absence of these proteins (4, 9, 10).
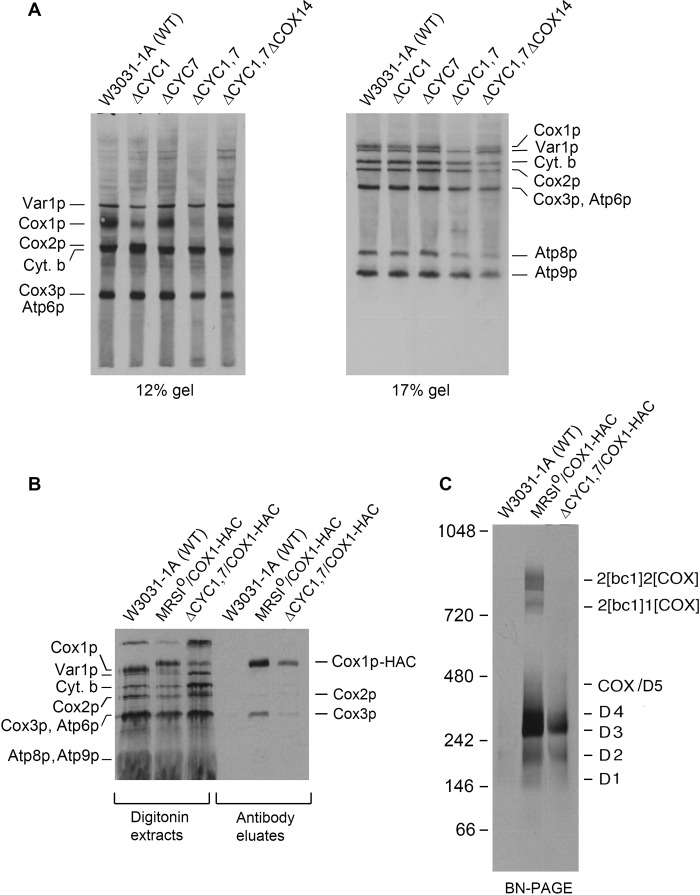
Even though cytochrome c is not an integral subunit of COX, the absence of this protein also leads to a deficiency of the enzyme (29). This is evident in mutants lacking both isoforms of cytochrome c or the lyase that catalyzes covalent attachment of protoheme to apocytochrome c (30). Furthermore, the requirement of cytochrome c is structural in nature and independent of the ability of the protein to function in electron transport (14). The odd requirement of cytochrome c for COX assembly prompted us to measure Cox1p translation in a double cyc1,cyc7 mutant with and without an added cox14 mutation. Strains with single and multiple mutations were labeled in the presence of cycloheximide, and total cellular proteins were separated by SDS-PAGE. The in vivo assays disclosed a sharply reduced rate of Cox1p translation in the cyc1,cyc7 double mutant that was restored by a cox14 mutation, similar to what was previously observed with other assembly-arrested COX mutants (Fig. 6A). The effect of cytochrome c deficiency on Cox1p intermediates was examined in a cyc1,cyc7 double mutant expressing Cox1p-HAC. As expected, the steady-state concentration of the intermediates was lower in the mutant, but the relative abundance of the different intermediates was not significantly different from the wild type control (Fig. 6, B and C). Attempts to determine if cytochrome c co-immunoprecipitates with Cox1p intermediates were hampered by the high background of other radiolabeled mitochondrial products in the precipitate due to the low ionic strength of the wash buffers used to prevent dissociation of cytochrome c from other proteins.
FIGURE 6.
A mutant lacking both isoforms of cytochrome c (Cyt. b) has the hallmarks of a COX assembly-arrested mutant. A, the wild type and the single cyc1,cyc7, double cyc1,cyc7, and triple cyc1,cyc7,cox14 mutants were labeled in vivo with [35S]methionine and cysteine in the presence of cycloheximide for 20 min (21). Total cellular proteins were separated by SDS-PAGE on a 12 and 17% polyacrylamide gel, transferred to nitrocellulose, and exposed to x-ray film. The radiolabeled mitochondrial gene products are identified in the margins. B, mitochondria from the respiratory competent and double cyc1 and cyc7 mutant expressing Cox1p-HAC were labeled for 20 min with [35S]methionine plus cysteine as described under “Materials and Methods.” The mitochondria were extracted with digitonin and further purified on protein C antibody beads. Samples of the extracts and eluates from the beads were separated by SDS-PAGE on a 17% polyacrylamide gel, transferred to nitrocellulose, and exposed to x-ray film. C, the eluates from B were separated by BN-PAGE on a 4–13% polyacrylamide gel, transferred to a PVDF membrane, and exposed to x-ray film.
These results show that like the integral subunits, a deficiency of cytochrome c affects assembly of COX with a consequent decrease in translation of Cox1p. Restoration of Cox1p translation in the cox14 mutant indicated that the release of Mss51p from the Cox1p module depends on the presence of cytochrome c.
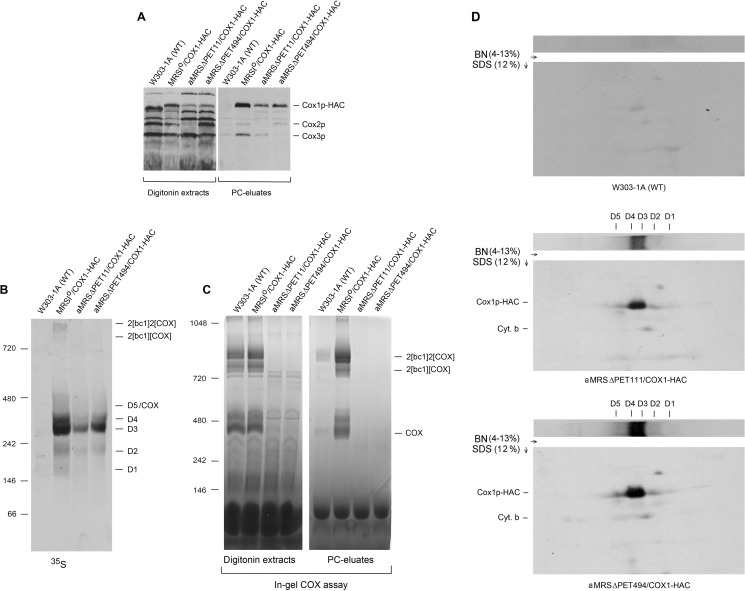
Evidence for the Presence of a Cox1p Intermediate That Co-migrates with COX on Native Gels
The co-migration of D5 with COX made it difficult to demonstrate this intermediate by direct means in respiratory competent yeast (12). Direct evidence for the existence of this intermediate was obtained in the present study by analyzing Cox1p intermediates in pet111 and pet494 mutants expressing Cox1p-HAC. Both pet111 and pet494 mutants are COX-deficient due to their failure to translate Cox2p and Cox3p, respectively (31, 32). Mitochondrial gene products in each stain were pulse-labeled in organello, and proteins associated with Cox1p-HAC were purified from digitonin extracts with protein C antibody beads. The SDS gels of the Cox1-HAC-enriched fractions eluted from the antibody beads contained Cox1-HAC but lacked Cox2p and Cox3p in the pet111 and pet494 mutants, respectively (Fig. 7A). The native gels of this fraction indicated the presence in both mutants of all the Cox1p intermediates, although their concentrations, due to down-regulation of Cox1p translation, were less than in the wild type control (Fig. 7, A and B). The COX defect was corroborated by the absence in both strains of the supercomplexes and COX activity (Fig. 6, B and C). The presence of D5 in the antibody eluates of the two strains was confirmed by two-dimensional gel electrophoresis, which achieves a better resolution of the D5 region (Fig. 6D). Radiolabeled Cox1p-HAC separated into a distinct band separate from D4 in both mutants.
FIGURE 7.
Detection of the D5 intermediate in COX-deficient pet111 and pet494 mutants. A, mitochondria of the wild type W303-1B and of the pet111 and pet494 mutants, each expressing Cox1p-HAC, were labeled with [35S]methionine and cysteine for 20 min as described under “Materials and Methods.” Mitochondria were extracted with digitonin, and tagged proteins were purified on protein C (PC) antibody beads. Samples of the extracts and the fraction eluted from the beads were separated by SDS-PAGE on a 17% polyacrylamide gel and transferred to nitrocellulose. B, eluates from the antibody beads were separated by BN-PAGE on a 4–13% polyacrylamide gel, transferred to a PVDF membrane, and exposed to x-ray film. C, in-gel COX activity of the digitonin extracts and antibody-purified proteins were separated by BN-PAGE on a 4–13% polyacrylamide gel. D, the antibody-purified fractions of the W303-1A control and of the pet111 and pet494 mutants expressing Cox1p-HAC were separated by BN-PAGE on a 4–13% gel in the first dimension and on a 12% polyacrylamide gel by SDS-PAGE in the second dimension. All the blots were exposed to x-ray film.
DISCUSSION
Several factors have contributed to our still partial understanding of the process by which mitochondria assemble COX. The most serious of these has been the extensive turnover of COX constituents in all assembly-arrested mutants. This is glaringly evident by the almost complete absence in such mutants of the hydrophobic core subunits, a circumstance that has hampered the use of COX mutants to study and reconstruct the assembly pathway from stalled intermediates under steady-state conditions. To circumvent this experimental drawback, Cox1p complexes have been analyzed after labeling isolated mitochondria in pulse-chase experiments. This approach led to the identification of five different Cox1p complexes that were proposed to be intermediates of a Cox1p assembly module (12). We further conjectured that COX is assembled from three different modules, each consisting of one of the core subunits partnered with a select set of nuclear gene products reflective of the subunit interactions observed in the fully assembled enzyme.
A substantial amount of information has been gained about the compositions of some Cox1p intermediates (4–7, 12, 13, 33). However, their analysis remained partial as only a subset of COX proteins had been studied. To remedy this shortcoming we constructed strains that expressed each of the previously unstudied subunits with tags suitable for their detection and purification. These studies have led us to conclude that the Cox1p assembly module utilized in the final stages of the pathway contains only three of the eight nuclear encoded subunits. Two of the proteins, Cox5ap and Cox8p, interact directly with Cox1p in the holoenzyme (Ref. 26 and Fig. 5). The third protein, Cox6p, does not have a common interface with Cox1p but is a constituent of the Cox1p module through its interaction with Cox5ap (Fig. 5). A still open question is whether Cox5ap and Cox6p associate with Cox1p sequentially or as a preformed unit. The instability of Cox6p in Cox5ap mutants (34) suggests that the two proteins may form a complex before their incorporation into the Cox1p module.
Cox1p intermediates were also characterized in strains lacking a COX subunit or accessory factor. The absence of a protein essential for COX assembly (Cox7p, Cox2p, and Cox3p) decreased Cox1p translation but did not affect the relative distribution of the newly translated protein in the intermediates. This was also true of a double cyc1 and cyc7 mutant missing both isoforms of cytochrome c. Predictably, cox12, cox13, rcf1, and rcf2 mutants with wild type levels or only partial reductions of COX displayed normal translation of Cox1p and steady-state concentrations of the intermediates. The two sets of results indicate that assembly of the Cox1p module is a separate and independent pathway that is not influenced by other COX components.
The previously postulated existence of a D5 intermediate that co-migrates with COX in blue native gels was confirmed to be present in pet111 and pet494 mutants that are deficient in COX due to their failure to translate Cox2p and Cox3p. Both mutants contained radiolabeled Cox1p that migrated at the position of COX. The size and composition of this intermediate suggest that it is likely to be a late and perhaps immediate precursor of COX. At present the proteins known to be present in D5 include the regulatory factors Mss51p, Cox14p, Coa3p, Coa1, the heme a chaperone Shy1p, and the three structural subunits Cox5ap, Cox6p, and Cox8.
This work was supported, in whole or in part, by National Institutes of Health Grant GM 50187.
- BN-PAGE
- blue native PAGE.
REFERENCES
- 1. Barrientos A., Barros M. H., Valnot I., Rötig A., Rustin P., Tzagoloff A. (2002) Cytochrome oxidase in health and disease. Gene 286, 53–63 [DOI] [PubMed] [Google Scholar]
- 2. McEwen J. E., Ko C., Kloeckner-Gruissem B., Poyton R. O. (1986) Nuclear functions required for cytochrome c oxidase biogenesis in Saccharomyces cerevisiae. Characterization of mutants in 34 complementation groups. J. Biol. Chem. 261, 11872–11879 [PubMed] [Google Scholar]
- 3. Tzagoloff A., Dieckmann C. L. (1990) PET genes of Saccharomyces cerevisiae. Microbiol. Rev. 54, 211–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrientos A., Zambrano A., Tzagoloff A. (2004) Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 23, 3472–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez-Martinez X., Butler C. A., Shingu-Vazquez M., Fox T. D. (2009) Dual functions of Mss51 couple synthesis of Cox1 to assembly of cytochrome c oxidase in Saccharomyces cerevisiae mitochondria. Mol. Biol. Cell 20, 4371–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fontanesi F., Soto I. C., Horn D., Barrientos A. (2010) Mss51 and Ssc1 facilitate translational regulation of cytochrome c oxidase biogenesis. Mol. Cell. Biol. 30, 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mick D. U., Fox T. D., Rehling P. (2011) Inventory control. Cytochrome c oxidase assembly regulates mitochondrial translation. Nat. Rev. Mol. Cell Biol. 12, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez-Martinez X., Broadley S. A., Fox T. D. (2003) Mss51p promotes mitochondrial Cox1p synthesis and interacts with newly synthesized Cox1p. EMBO J. 22, 5951–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mick D. U., Vukotic M., Piechura H., Meyer H. E., Warscheid B., Deckers M., Rehling P. (2010) Coa3 and Cox14 are essential for negative feedback regulation of COX1 translation in mitochondria. J. Cell Biol. 191, 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fontanesi F., Clemente P., Barrientos A. (2011) Cox25 teams up with Mss51, Ssc1, and Cox14 to regulate mitochondrial cytochrome c oxidase subunit 1 expression and assembly in Saccharomyces cerevisiae. J. Biol. Chem. 286, 555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McStay G. P., Su C. H., Tzagoloff A. (2013) Stabilization of Cox1p intermediates by the Cox14p-Coa3p complex. FEBS Lett. 587, 943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McStay G. P., Su C. H., Tzagoloff A. (2013) Modular assembly of yeast cytochrome oxidase. Mol. Biol. Cell 24, 440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pierrel F., Bestwick M. L., Cobine P. A., Khalimonchuk O., Cricco J. A., Winge D. R. (2007) Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 26, 4335–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barrientos A., Pierre D., Lee J., Tzagoloff A. (2003) Cytochrome oxidase assembly does not require catalytically active cytochrome c. J. Biol. Chem. 278, 8881–8887 [DOI] [PubMed] [Google Scholar]
- 15. Myers A. M., Pape L. K., Tzagoloff A. (1985) Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 4, 2087–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rothstein R. J. (1983) One-step gene disruption in yeast. Methods Enzymol. 101, 202–211 [DOI] [PubMed] [Google Scholar]
- 17. Hill J. E., Myers A.M., Koerner T. J., Tzagoloff A. (1986) Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2, 163–167 [DOI] [PubMed] [Google Scholar]
- 18. Herrmann J.M., Foelsch H., Neupert W., Stuart R. A. (1994) Cell Biology: A Laboratory Handbook (Celis J. E., ed) Vol. I, pp. 538–544, Academic Press, Inc., San Diego, CA [Google Scholar]
- 19. Rak M., Gokova S., Tzagoloff A. (2011) Modular assembly of yeast mitochondrial ATP synthase. EMBO J. 30, 920–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 21. Rak M., Tzagoloff A. (2009) F1-dependent translation of mitochondrially encoded Atp6p and Atp8p subunits of yeast ATP synthase. Proc. Natl. Acad. Sci. U.S.A. 106, 18509–18514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schiestl R. H., Gietz R. D. (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16, 339–346 [DOI] [PubMed] [Google Scholar]
- 23. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 24. Wittig I., Braun H. P., Schägger H. (2006) Blue native PAGE. Nat. Protoc. 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 25. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 26. Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 27. Tzagoloff A., Akai A., Needleman R. B., Zulch G. (1975) Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J. Biol. Chem. 250, 8236–8242 [PubMed] [Google Scholar]
- 28. Strogolova V., Furness A., Robb-McGrath M., Garlich J., Stuart R. A. (2012) Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Mol. Cell. Biol. 32, 1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Downie J. A., Stewart J. W., Brockman N., Schweingruber A. M., Sherman F. (1977) Structural gene for yeast iso-2-cytochrome c. J. Mol. Biol. 113, 369–384 [DOI] [PubMed] [Google Scholar]
- 30. Dumont M. E., Ernst J. F., Hampsey D. M., Sherman F. (1987) Identification and sequence of the gene encoding cytochrome c heme lyase in the yeast Saccharomyces cerevisiae. EMBO J. 6, 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poutre C. G., Fox T. D. (1987) PET111, a Saccharomyces cerevisiae nuclear gene required for translation of the mitochondrial mRNA encoding cytochrome c oxidase subunit II. Genetics 115, 637–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costanzo M. C., Fox T. D. (1986) Product of Saccharomyces cerevisiae nuclear gene PET494 activates translation of a specific mitochondrial mRNA. Mol. Cell. Biol. 6, 3694–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mick D. U., Wagner K., van der Laan M., Frazier A. E., Perschil I., Pawlas M., Meyer H. E., Warscheid B., Rehling P. (2007) Shy1 couples Cox1 translational regulation to cytochrome c oxidase assembly. EMBO J. 26, 4347–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glerum D. M., Tzagoloff A. (1997) Submitochondrial distributions and stabilities of subunits 4, 5, and 6 of yeast cytochrome oxidase in assembly defective mutants. FEBS Lett. 412, 410–414 [DOI] [PubMed] [Google Scholar]