Background: Before constriction ZipA anchors FtsZ to the E. coli inner membrane as part of the cell division proto-ring.
Results: Dynamic FtsZ polymers shrink ZipA-containing vesicles whereas excess of ZipA invaginates the E. coli membrane destroying the permeability barrier.
Conclusion: Constriction forces can be evidenced both in bacteria and in vesicles.
Significance: Defined bacterial elements reproduce division functions when assembled in vitro.
Keywords: Cell Division, Escherichia coli, Membrane Function, Protein-Protein Interactions, Synthetic Biology, FtsZ, ZipA, Bacterial Membrane, Giant Vesicles
Abstract
Permeable vesicles containing the proto-ring anchoring ZipA protein shrink when FtsZ, the main cell division protein, polymerizes in the presence of GTP. Shrinkage, resembling the constriction of the cytoplasmic membrane, occurs at ZipA densities higher than those found in the cell and is modulated by the dynamics of the FtsZ polymer. In vivo, an excess of ZipA generates multilayered membrane inclusions within the cytoplasm and causes the loss of the membrane function as a permeability barrier. Overproduction of ZipA at levels that block septation is accompanied by the displacement of FtsZ and two additional division proteins, FtsA and FtsN, from potential septation sites to clusters that colocalize with ZipA near the membrane. The results show that elementary constriction events mediated by defined elements involved in cell division can be evidenced both in bacteria and in vesicles.
Introduction
Cell division in rod-shaped bacteria is a highly dynamic process in which a septum is positioned at mid-cell. As a result the diameter of the cell gradually decreases until two daughters separate (1). It has been postulated that some of the proteins involved in septum formation have mechanical properties that are responsible for the constriction of the bacterial envelope, including the invagination of the cytoplasmic membrane. Among them, the FtsZ, FtsA, and ZipA proteins have been described in Escherichia coli as forming the proto-ring (2), an initial complex that directs the assembly of a division ring in which at least 10 essential proteins participate (3). It is accepted that ZipA and FtsA, the two proto-ring elements that can associate with the cytoplasmic membrane, act as anchoring points for the cytoplasmic FtsZ protein (4). FtsZ is attributed to supply force to drive constriction (5).
FtsZ, widely conserved among prokaryotic organisms, is a GTPase that can form polymers and shares a common ancestor with eukaryotic tubulin (6). The assembly and disassembly reactions of FtsZ mediated by GTP are essential for the constriction of the division ring (7). ZipA contains a short N-terminal region that is integrated in the membrane and connected to the C-terminal FtsZ-interacting domain by a flexible, unstructured, linker region (8). In addition, this linker allows ZipA to undergo a coil-to-brush conformational transition upon lateral compression of the monolayers in which it has been incorporated (9). FtsA, a member of the actin family using a short amphipathic helix for membrane attachment, may also contribute to anchoring FtsZ (10).
An FtsZ chimera, which can bind to the membrane through an engineered amphipathic segment in the absence of ZipA or FtsA, has been described to become internalized and accumulate at constricted regions of tubular liposomes (11). The incorporation of polymers formed by the chimeric FtsZ protein onto the external face of the liposomes produced their deformation, and lipid tubules were observed (12, 13). The native forms of FtsZ and FtsA have been incorporated inside electro-formed giant vesicles obtained from the inner bacterial membrane in which ZipA is naturally present (14, 15). In this case the assembly of FtsZ alters the spatial distribution of the soluble proto-ring elements resulting in the dislodgement of FtsA from the membrane into the vesicle lumen where it colocalizes with FtsZ polymers. Following the release of caged GTP in E. coli vesicles in which ZipA has been inserted at both sides of the membrane, polymerization of FtsZ seems to modulate membrane plasticity (16).
In this work we have used phosphatidylcholine giant unilamellar vesicles containing FtsZ in their lumen and ZipA inserted into the inner surface of the lipid phase. These vesicles have been made permeable by integration of the pore-forming protein α-hemolysin allowing us to control the polymerization of FtsZ from outside the vesicle upon the addition of GTP and magnesium. The procedure used for obtaining the vesicles, droplet transfer, attains a high yield of vesicle production and protein encapsulation. The mechanical properties of the complexes formed upon interaction of FtsZ polymers with the ZipA-containing vesicles have been compared with the morphological and biological changes observed in living cells in which ZipA has been overproduced.
EXPERIMENTAL PROCEDURES
Reagents
Reagents, salts, mineral oil (M5904), and buffer components were from Sigma and Thermo Scientific. Fluorescent dyes, Alexa Fluor 488, Alexa Fluor 647, and DiIC184 were from Molecular Probes. l-α-Phosphatidylcholine from egg yolk and DOGS-NTA-Ni were purchased from Avanti Polar Lipids.
Escherichia coli Strains and Growth Conditions
Bacterial strains used in this study were MC1061 (17, 18) for in vivo studies of His-ZipA overproduction, BL21(DE3) for gene overexpression and protein purification, and VIP205 to deplete the amount of FtsZ by removing 30 μm isopropyl 1-thio-β-d-galactopyranoside from the culture, or restore it by its readdition (19). Strain VIP983, MC1061 transformed with pASV003 (see below), was inoculated in Luria-Bertani (LB) broth (20) supplemented with 100 mg ml−1 ampicillin and 0.2% glucose to repress gene expression. Cells were grown overnight at 37 °C in a shaking water bath with aeration and then diluted 1:100 into fresh and prewarmed medium supplemented as before. Absorbance at 600 nm (A600) was measured periodically with a CO8000 Cell Density Meter (WPA biowave) and always kept below 0.3 by serial dilutions with prewarmed medium to attain exponential balanced growth. After at least six mass doublings cells were filtered and transferred to arabinose-containing medium for 150 min to overproduce His-ZipA. Cells under these conditions were also maintained at exponential growth phase (as measured by absorbance increase), and aliquots were removed at 30-min intervals.
Cell Parameter Measurements
Cell samples were obtained at the times indicated and processed for checking cell number and cell volume after inducing his-zipA expression with 0.2% arabinose. Fixed cells (0.75% formaldehyde) were counted with a multichannel analyzer Beckman Coulter Multisizer 3 equipped with a 30-μm-diameter orifice. Cell length was measured using the image analysis software Object-Image version 2.21 (21). At least 100 cells were analyzed for each sample.
Plasmid Construction and DNA Manipulation
The his-zipA gene in pET-15Zip (22) was excised with NcoI and XhoI and ligated to pBAD22 vector (23) digested with NcoI and SalI to obtain pASV003 (his-zipA).
Proteins and Peptides
E. coli FtsZ was purified as described previously (24). sZipA, a soluble variant of ZipA lacking its hydrophobic N-terminal domain (amino acids 1–25), was overexpressed in E. coli strain BL21/DE3 and purified as described in Ref. 25. The peptide CTZ-MUT, with sequence (KRNDWTNIMAFLKKQAD) (26), was synthesized as described in Ref. 27. The pore-forming protein α-hemolysin from Staphylococcus aureus was obtained from Sigma-Aldrich (H9395).
Protein Labeling
FtsZ and ZipA were labeled with Alexa Fluor probes as described (28, 29). The degree of labeling was 0.9–0.2 mol of fluorophore/mol of protein in both cases.
Production of Permeable Giant Unilamellar Vesicles (GUVs)
FtsZ-containing GUVs were prepared according to a water-in-oil emulsion method (30) to optimize the procedure described in Ref. 31. This method generated rather high encapsulation efficiency and protein entrapment control without loss of activity compared with other vesicle preparation methods (32). Lipid films, dried from chloroform solutions under a gentle stream of nitrogen gas, were dissolved in mineral oil at a total concentration of 0.5 mg/ml. When it was required, 10 mol % of DOGS-NTA-Ni was previously mixed with egg yolk phosphatidylcholine. A volume of 5 ml of oil-lipid mixture was then sonicated for 30 min. The oil-lipid mixture was heated to 50 °C for 3 h, cooled down to room temperature, and stored at 4 °C for up to a week.
FtsZ (12–40 μm) was equilibrated in 50 mm Hepes, pH 7.5, buffer containing 100 mm KCl, 100 mm sucrose, 0.1 mm GDP, and 1 mm EDTA (I-buffer) with 50 g/liter Ficoll. When present, ZipA (from 2 to 10 μm) was added to the FtsZ-containing solutions. Alexa Fluor 488-labeled and unlabeled FtsZ were used at 9:1 molar ratio. When present, CTZ-MUT peptide was used at a final concentration of 24 μm. Protein solutions (10–25 μl) were added to 500 μl of phospholipid-containing oil and dispersed in droplets by gentle back-and-forth pumping for ∼1 min with a 1-ml pipette until the emulsion looked homogeneously cloudy. In the meantime, 500 μl of the oil-lipid mixture was placed in a tube on top of the same volume of 50 mm Hepes, pH 7.5, buffer containing 100 mm KCl and 100 mm glucose (O-buffer) to allow the assembly of a lipid monolayer at the interface. A volume of 500 μl of the emulsion was gently poured on top of the oil-lipid mixture, thus resulting in a three-phase sample with O-buffer at the bottom, the oil-lipid mixture in the middle, and the emulsion on top. The three-phase preparation was centrifuged twice for 10 min at 300 × g allowing for the emulsion droplets to pass through the lipid monolayer. After the second pelleting liposomes were rinsed with O-buffer to remove oil contaminations and nonencapsulated solutes from the vesicles. The osmolarity of all solutions was adjusted with a cryoscopic osmometer (Osmomat 030). Vesicles were made permeable by the addition of the pore-forming protein α-hemolysin (7 μm; (33)) to the O-buffer (33).
Confocal Microscopy Assays
Precipitated GUVs (500 μl) were placed in an 8-well visualization chamber from LabTech (155411) and were visualized by confocal microscopy. Images were collected with a Leica TCS SP5 AOBS inverted confocal microscope with a 63× (N.A. = 1,4–0,6/Oil HCX PL APO, Lbd.Bl.) immersion objective (Leica, Mannheim, Germany). 514-, 488-, and 633-nm laser lines excited DiIC18, Alexa Fluor 488, and Alexa Fluor 647, respectively.
Flow Cytometry Analysis
Staining of bacteria was done using the propidium iodide solution contained in the LIVE/DEAD BacLight Bacterial Viability Kit (L-7012; Invitrogen). One milliliter of cells at the indicated time points was mixed 1:1 with 0.85% NaCl. After an incubation period of 5 min on ice, cells were briefly spun down and resuspended in the same starting volume of propidium iodide diluted 1:1000 in 0.85% NaCl. Red fluorescence of 100,000 cells at each time point was recorded with a Gallios Flow Cytometer (Beckman Coulter). Controls of cell populations completely permeable to propidium iodide (data not shown) were obtained by treating the cell samples, at each time point, with 1 ml of isopropyl alcohol before staining. These controls also allowed for the setup and adjustment of the different cytometer parameters. Results are the mean of four independent experiments.
Immunofluorescence Microscopy
Anti-polyhistidine monoclonal antibody clone His-1 (peroxidase conjugate; Sigma-Aldrich A7058) was used specifically to detect the overproduced His-ZipA protein, whereas both endogenous and overproduced proteins were detected simultaneously using the anti-ZipA MVC1 polyclonal antibody (34). Alexa Fluor 488 anti-mouse (Invitrogen A11029) and Alexa Fluor 594 anti-rabbit (Invitrogen A11037) were used, respectively, as the secondary antibodies. FtsZ, FtsA, or FtsN was, respectively, detected using the MCV2, MVC3P, or MVG1 polyclonal antibody (35). Permeabilized cells were first incubated with the purified anti-ZipA MVC1 polyclonal antibody overnight at 4 °C and, after 10 washing steps with PBS 1×, cells were incubated with the anti-His monoclonal antibody for another 120 min at room temperature. Unbound primary antibodies were removed by extensively washing before incubation with a mixture (1:1) of secondary Alexa Fluor 594-conjugated anti-rabbit serum and Alexa Fluor 488-conjugated anti-mouse serum diluted to 1:500 with blocking solution. Finally, chromosomes were stained with DAPI (25 μg ml−1) in VECTASHIELD mounting medium (Vector Laboratories). Cells were observed and photographed using the BX61 Olympus fluorescence microscope with a 100× immersion oil lens coupled to a charge-coupled device camera DP70. Image processing was performed using Adobe Photoshop CS3.
Cryo-transmission Electron Microscopy
Cryo-transmission EM of frozen-hydrated sections of E. coli cells overproducing His-ZipA was used to examine cell organization. Cells were imaged with a Jeol JEM-1011 microscope operating at 100 kV coupled to an Erlangshen ES 1000 W camera device. Cells from uninduced or induced cultures were fixed for 30 min at 4 °C using a mixture of 4% paraformaldehyde, 0.1% glutaraldehyde in 1× PBS, washed in buffered glycerol, and quickly immersed in liquid ethane to achieve vitrification. Cells were freeze-substituted with 0.5% uranyl acetate in methanol at −90 °C for 50 h and then embedded in Lowicryl HM20 resin at −40 °C for 48 h and at 20 °C for 24 h. Thin sections (60–70 nm) of cells were obtained with a Leica EM UC6 ultramicrotome and counterstained with uranyl acetate and lead citrate. For ZipA immunolabeling, sections not counterstained with heavy metal stains were first blocked with TBST (30 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% BSA, and 0.02% Tween) and then incubated 1 h at room temperature with the MVC1 anti-ZipA antibody (34) diluted 1:100 in the same buffer. Incubation with the immunogold conjugate EM goat (Fab′)2 anti-rabbit IgG (10 nm; BBI International) secondary antibody was done following the manufacturer's instructions.
Thin Section Transmission EM
Cells from uninduced or induced cultures were fixed with 2% glutaraldehyde, 1% tannin in 0.4 m Hepes, pH 7.2, for 1 h at room temperature. Samples were treated with 1% OsO4 for 1 h at 4 °C and with 2% uranyl acetate for 30 min, dehydrated with acetone and infiltrated with the EPON 812 resin. This was finally polymerized for 50 h at 60 °C.
RESULTS
Encapsulated FtsZ Polymerizes Inside Permeable GUVs upon Addition of GTP and Magnesium
Giant vesicles made from egg yolk phosphatidylcholine were produced at high yield by an optimized droplet transfer protocol (30) under physiological potassium concentrations (100 mm) and in the presence of inert macromolecules at high concentration (50 mg/ml Ficoll 70) to mimic natural crowding conditions and to help to visualize FtsZ polymers inside the vesicle (28). The vesicles were made permeable upon the incorporation of the pore-forming protein α-hemolysin which allows us to trigger FtsZ polymerization by the addition of GTP and magnesium, as has been reported for actin assembly (36, 37).
FtsZ in its GDP form (nonassembled state), incorporated inside the vesicles during the process of GUV formation, remained soluble and homogeneously distributed inside the vesicles (Fig. 1Aa). The efficiency of FtsZ entrapment was high as the majority of vesicles contained FtsZ inside. The vesicles were unilamellar, ranging in size from 10 to 20 μm. A tracer amount of Alexa Fluor 488-labeled FtsZ was used to help visualize the protein by confocal microscopy whereas the vesicle boundary was evidenced by incorporating DiIC18 as a lipid membrane probe (see “Experimental Procedures”).
FIGURE 1.
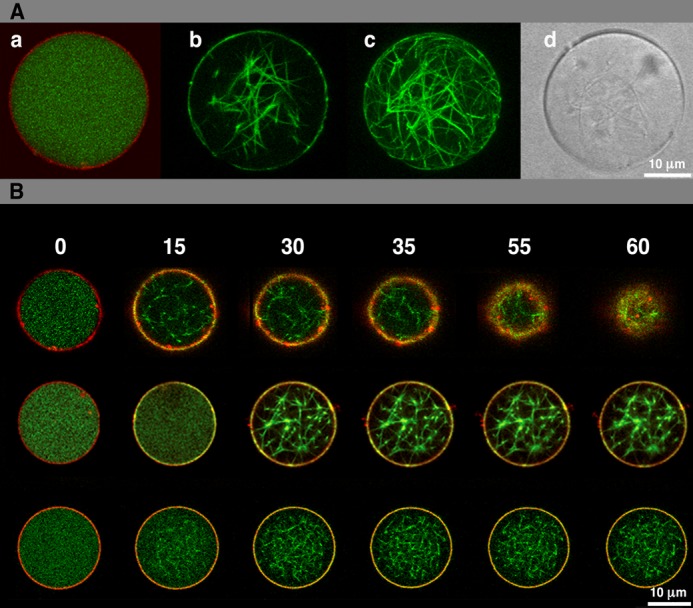
Encapsulation and polymerization of FtsZ inside permeable vesicles (A) and vesicle shrinkage and collapse induced by interaction of membrane bound ZipA with FtsZ polymers (B). A, panel a shows an equatorial confocal image of permeable vesicles containing 12 μm FtsZ-Alexa Fluor 488 in the presence of GDP; the membrane layer is stained with the lipid dye DiIC18. Panel b shows a similar image of FtsZ polymers after addition of α-hemolysin, GTP, and magnesium, whereas panel c is a full reconstruction of all confocal sections. The differential interference contrast image of the vesicle containing FtsZ polymers is shown in panel d. B, equatorial cross-sectional merged images of permeable vesicles containing 5 μm sZipA-Alexa Fluor 647 attached to the membrane through DOGS-NTA lipids and 12 μm Alexa Fluor 488-FtsZ in the absence (top row) or presence of the CTZ-MUT peptide inhibitor of FtsZ-ZipA interaction (middle row). Vesicles similar to those shown in top row containing 2 μm sZipA-Alexa Fluor 647 are shown in the bottom row. Frames were taken at the times (seconds) indicated after external addition of GTP and Mg2+. Further details are under “Results.” Movies reconstructed from the whole collection of images can be found in supplemental Movies S1–S4).
The α-hemolysin pores allowed the immediate permeation from the medium into the vesicle. The addition of millimolar concentrations of GTP/Mg2+ to the medium was quickly followed by the formation of FtsZ polymers inside the vesicles (Fig. 1A, b–d). FtsZ polymers were not distributed homogeneously but mostly localized as condensed filaments stochastically distributed (as an entangled network) inside the vesicle lumen. A faint signal of FtsZ was detected at the inner vesicle surface, indicating that a small fraction of FtsZ may be associated with the membrane. No morphological changes in the vesicles were detected after triggering FtsZ polymerization. Control experiments revealed that no visible changes in FtsZ distribution ever happened if the vesicles did not contain α-hemolysin, or if Mg2+ or GTP was not supplied.
GUVs with ZipA Inserted in the Membrane Shrink upon Polymerization of FtsZ
The assembly of the proto-ring in E. coli proceeds through the interaction of the cytoplasmic FtsZ protein with FtsA or ZipA. These two proteins provide a physical anchoring to the membrane in which ZipA is embedded in the lipid bilayer (2). We then tested the effect of FtsZ polymerization on vesicles in which ZipA had been inserted.
A soluble variant of ZipA, sZipA, lacking the transmembrane region, was integrated at the inner face of the vesicle. This was achieved by means of the interaction between the N-terminal His tag of the protein and tracer amounts of a nickel-chelating lipid (DOGS-NTA) incorporated to the lipid mixture during vesicle formation (abbreviated as NCL-sZipA). The proper localization of NCL-sZipA at the inner face of the vesicle was verified by confocal microscopy using a tracer amount of NCL-sZipA labeled with Alexa Fluor 647 (supplemental Fig. S1, top). The incorporation of the full-length ZipA protein into the vesicles, although technically feasible (38), was not considered because it requires the presence of detergents during the vesicle production that alter the properties of FtsZ. The sZipA variant has the same FtsZ binding properties as the full-length protein incorporated in phospholipid bilayer nanodiscs (25, 27).
To confirm that once inside the vesicle FtsZ and NCL-sZipA can interact, as observed in solution (25), FtsZ was incorporated inside vesicles produced in the absence of DOGS-NTA lipids in which sZipA could not be associated to the membrane. In the presence of GDP, most of the FtsZ and sZipA were homogeneously distributed throughout the vesicle lumen (supplemental Fig. S1, middle). Once FtsZ polymerization was triggered by addition of GTP, sZipA was localized, together with FtsZ polymers, in the vesicle lumen (supplemental Fig. S1, bottom). No alterations in the morphology of these vesicles were observed.
We then studied the effect of the polymerization of a fixed concentration of FtsZ (12 μm) on vesicles in which different concentrations of sZipA protein (2, 5, and 10 μm) were anchored to the membrane using DOGS-NTA lipids (see “Experimental Procedures”). These protein concentrations are close to the estimated physiological concentrations of FtsZ (2–10 μm (34)) and ZipA (0.5–1.0 μm (34, 39)) in E. coli. Moreover, they are in the range of the FtsZ-ZipA binding affinities obtained for either the soluble ZipA variant (25) or the full-length version of the protein incorporated in nanodiscs (27). In all cases addition of external GTP and magnesium was used to allow polymerization of FtsZ inside the vesicles. Although FtsZ was found as polymeric structures localized inside the vesicle and, together with NCL-sZipA, at the inner face of the membrane, no changes in the size and shape of the vesicles were detected during the time frame of the observations (5 min) at 2 μm sZipA concentration (Fig. 1B, bottom row, and supplemental Movie S1).
The polymerization of FtsZ inside vesicles in which 5 μm NCL-sZipA was attached to their inner surface had a striking effect on their morphology and behavior (Fig. 1B, top row). Upon inducing FtsZ assembly, the initial distribution of the two proteins was similar to the case of vesicles containing 2 μm NCL-sZipA. However, after 30 s the vesicles gradually shrank and evolved to finally collapse (Fig. 1B, top row, and supplemental Movies S2 and S3). Prior to their collapse, no obvious invagination associated to individual points of contact between FtsZ proto-fibril termini and the membrane surface was observed (supplemental Movies S2 and S3). Control experiments done in the absence of NCL-sZipA (Fig. 1A), or using sZipA not anchored to the membrane (vesicles without DOGS-NTA lipids; supplemental Fig. S1, bottom) showed that these vesicles did not change in size or shape over time. Vesicles containing 10 μm ZipA collapsed immediately after addition of GTP and magnesium precluding further analysis.
To test the relationship between the concentration of FtsZ inside the vesicles and the morphological effects observed at high NCL-sZipA, similar experiments were done using ZipA vesicles, containing 2 or 5 μm NCL-sZipA, in which FtsZ was included at 5, 12, or 40 μm. Shrinkage was only observed in the vesicles containing 5 μm NCL-sZipA, but not at 2 μm, independently of the FtsZ concentration used. In the absence of Ficoll, the thin FtsZ proto-filaments were not visible in the vesicle lumen; however, shrinkage of vesicles containing 5 μm sZipA occurred after the addition of GTP and magnesium following the accumulation of the FtsZ fluorescence signal in the vicinity of the membrane.
Vesicle Shrinkage Is Prevented by an Inhibitor of the Binding of FtsZ to ZipA and Can Be Modulated by FtsZ Polymer Dynamics
A C-terminal FtsZ peptide (CTZMUT) has been used to test whether the observed morphological effects on the vesicles are due to the interaction between FtsZ polymers and membrane-associated ZipA. This peptide has been previously shown to inhibit the binding of FtsZ polymers to ZipA incorporated in nanodiscs (27). The presence of 24 μm CTZMUT prevented the collapse of vesicles containing 5 μm ZipA and 12 μm FtsZ, without altering FtsZ polymerization inside the vesicles once GTP and magnesium were added (Fig. 1B, middle, and supplemental Movie S4). To demonstrate further that CTZMUT is interfering with the FtsZ-NCL-sZipA interaction but it does not affect FtsZ polymerization, FtsZ was induced to polymerize inside the vesicles in the presence of the inhibitor and sZipA not anchored to the membrane (vesicles without DOGS-NTA). In these vesicles sZipA did not colocalize with FtsZ polymers (supplemental Fig. S2) as it did when the experiment was done in the absence of the inhibitor (supplemental Fig. S1, bottom).
We reasoned that if vesicle collapse is due to the interaction between ZipA and FtsZ polymers the process could be altered when modifying the dynamics of the polymer. To test this prediction we performed experiments in which a nonhydrolyzable (GMP-PCP) or a slowly hydrolyzable form of the nucleotide (GMP-CPP) was added to collapsible vesicles containing 12 μm FtsZ and 10 μm ZipA. These GTP variants induced a slow polymerization of FtsZ in the presence of Ficoll. Whereas GTP caused a quick collapse of the vesicles as described above, the nonhydrolyzable GMP-PCP did not modify the physical appearance of the vesicles even after prolonged observation (24 h). On the other hand, the slowly hydrolyzable GMP-CPP produced a gradual contraction in vesicle diameter leading to a delayed collapse after 15–20 min (Fig. 2 and supplemental Movies S5 and S6). These slowly collapsing vesicles allow an inspection of their morphological evolution. A ruffling of the vesicle surface was followed by the progressive thickening of the region containing the fluorescence FtsZ or ZipA signals.
FIGURE 2.
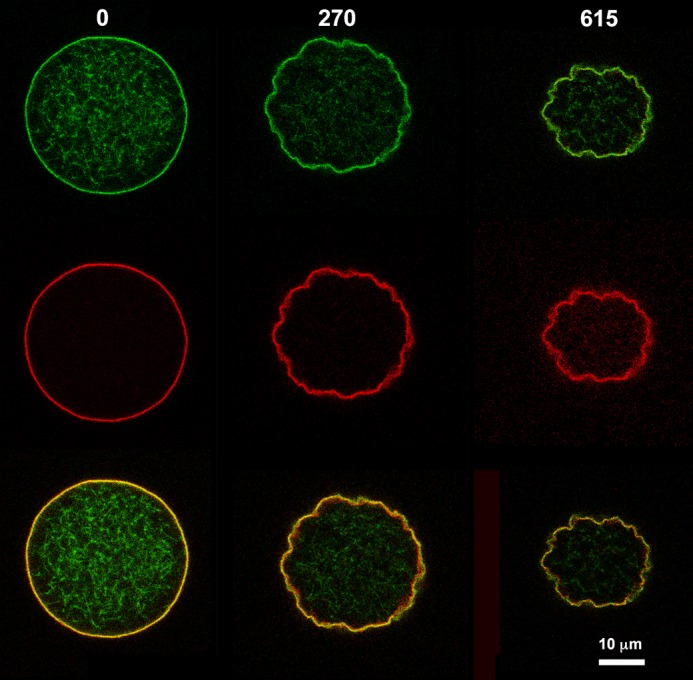
Membrane invagination of vesicle during shrinkage induced by interaction of membrane bound ZipA with FtsZ polymers in the presence of GMP-CPP. Equatorial cross-sectional images show a permeable vesicle containing 10 μm sZipA-Alexa Fluor 647 (middle row) attached to the membrane through DOGS-NTA lipids and 12 μm FtsZ-Alexa Fluor 488 (upper row) in the presence of 1 mm GMP-CPP. Columns show the frames that were taken at the times (seconds) indicated after GMP-CPP and Mg2+ addition. Merged images are shown in bottom row. Supplemental Movies S5 and 6 show vesicle collapse mediated by the interaction of GMPCPP-FtsZ polymers and ZipA bound to the inner face of the membrane. The two movies correspond to the time course of the collapse progression as detected by observing FtsZ (supplemental Movie S5) or ZipA (supplemental Movie S6).
Localization of Overproduced ZipA Protein in Vivo
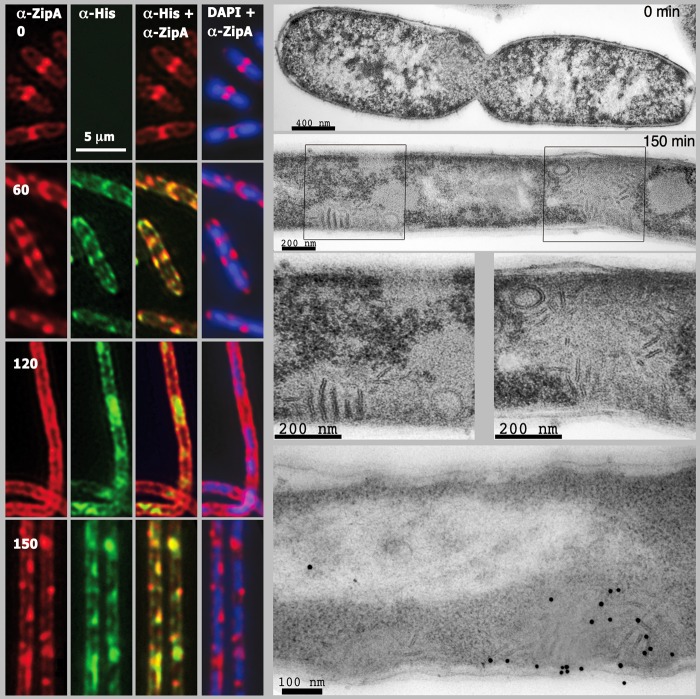
The results obtained with GUVs suggested that the local amount of membrane-attached ZipA is important for the contraction of the membrane when FtsZ polymerizes. We next investigated whether these findings correlate with cellular events that can be observed in vivo by studying the effect of ZipA overproduction in living cells. For this purpose we have induced the production of a His-ZipA fusion in E. coli. Although mild overproduction of GFP-tagged ZipA is known not to interfere with cell division (40), overproduction of ZipA in higher amounts causes cells to stop dividing while their length increases (supplemental Fig. S3 (41)). We then measured the amounts and cellular localization of the overproduced His-ZipA using specific antibodies against the histidine tail and the amounts of the background protein using antibodies against ZipA. Following induction, His-ZipA levels increased very rapidly and remained elevated throughout the whole experiment (supplemental Fig. S4). The immunolocalization pattern of the protein varied along time (Fig. 3, left). At time 0 the endogenous protein was distributed mainly as closed rings at mid-cell and along the membrane. At 60 min after induction the overproduced protein was localized equally at the membrane and in open rings, at 120 min and later times the protein decorated the membrane and accumulated at the nucleoid-free regions in the cytoplasm without forming any distinct structures (Fig. 3, left).
FIGURE 3.
Effects of elevated ZipA levels on ultracellular structure. The four left side columns show immunofluorescence images obtained from cells in which expression of an extrachromosomal copy of zipA had been induced during 0 (top row), 60, 120, 150 min (bottom row) before fixation. The images have been developed using antibodies against ZipA and Alexa Fluor 594 anti-rabbit conjugated to detect the total amount of ZipA (first column), against His tail and Alexa Fluor 488-anti-mouse conjugated to specifically detect the overproduced protein (second column). The third and fourth columns are overlays showing the overproduced protein over the total protein (third column) and the DAPI-stained nucleoid image over the total amount of ZipA (fourth column). The panels on the right show transmission electron micrographs of thin sections of cells overproducing ZipA. The top row is a longitudinal section of a cell before zipA induction. The second row is a longitudinal section of a segment of a filamentous cell after 150-min induction of zipA. The two framed sections reproduced at higher magnification are shown in the third row. The bottom row shows immunogold staining of ZipA in a filamentous cell 150 min after induction of zipA. See “Results” for growth and induction conditions.
ZipA Overproduction Induces Membrane Deformations in Vivo
We obtained electron microscopy images of sections that were stained using two different procedures to reveal subcellular structures. Distinct regions were differentiated within the filaments, the unstained nucleoid surrounded by a dense electronegative region likely formed by ribosomes and clear zones in which multilayered membrane structures were prominent (Fig. 3, right). Whereas the nucleoid and ribosomes were evident in uninduced controls, the multilayered membrane structures were absent. Immunogold staining was used to examine by electron microscopy the localization of ZipA in thin sections of filamented cells in which ZipA was overproduced. The resulting images indicated that the stained material was located both in the cytoplasmic membrane and in the membrane structures found inside the cytoplasm (Fig. 3, right).
ZipA Overproduction Interferes with FtsZ Ring Formation in Vivo
Within the long nonseptated filaments obtained after His-ZipA overexpression, nucleoids were regularly spaced and properly segregated (Fig. 3, left). Immunostaining with anti-FtsZ (MVC2 (34)) showed that no Z-rings were assembled at potential septation sites within these ZipA-induced filaments (Fig. 4A). The signal for the FtsZ protein was found near the membrane, colocalizing with the overproduced His-ZipA fusion. FtsA, the third component of the proto-ring, and FtsN, the last divisome assembling protein, were similarly displaced from potential septation sites by the excess of ZipA (Fig. 4, B and C). These results suggest that the effect of His-ZipA overproduction is specifically affecting the divisome elements, and it might explain the absence of cell division.
FIGURE 4.
Effects of elevated ZipA levels on localization of other cell division proteins. Immunofluorescence images were obtained from cells in which expression of an extrachromosomal copy of zipA had been induced during 0 (top row) or 150 min (bottom row) before fixation. The images have been developed using antibodies against FtsZ, FtsA, or FtsN and Alexa Fluor 594 anti-rabbit conjugated to detect FtsZ (first column), against His tail and Alexa Fluor 488-anti-mouse conjugated to specifically detect the overproduced His-ZipA protein (second column). The third and fourth columns are overlays showing the overproduced His-ZipA protein (third column) and the DAPI-stained nucleoid image (fourth column) over FtsZ, FtsA, or FtsN.
High Amounts of ZipA Affect the Biological Properties of the Bacterial Membrane
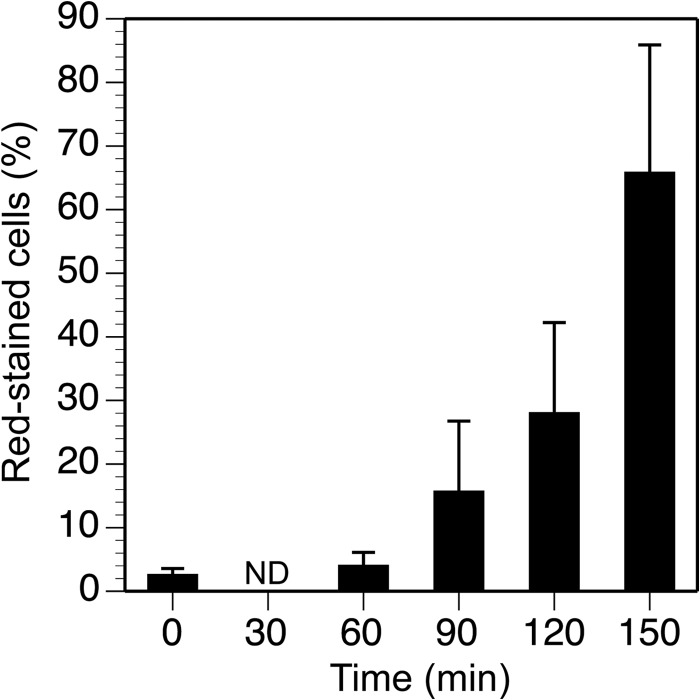
As the proper localization of several division proteins within the cytoplasmic membrane depends on the maintenance of a correct transmembrane potential (42), we have analyzed whether the cells overproducing His-ZipA are proficient at maintaining it. We have examined the proportion of cells that stain with propidium iodide, as cells with an intact membrane remain unstained. This occurs as a consequence of the fluorophore being actively pumped out from the cytoplasm when the membrane, including the transmembrane potential, functions correctly. Cells with an altered membrane stain red as they fail to expel propidium iodide. Results from flow cytometry (Fig. 5) show that the percentage of cells that stained red in cultures in which His-ZipA was overexpressed increased with time after induction. We reasoned that the increase in the amount of ZipA affected their ability to expel the fluorophore as a likely consequence of the impairment of the membrane.
FIGURE 5.
Modification of membrane permeability during ZipA overproduction. Shown is the percentage of propidium iodide-stained cells present at different times in a population in which ZipA is overproduced. Error bars indicate the S.D.
DISCUSSION
We have constructed permeable vesicles encasing varying amounts of two of the most important elements, FtsZ and ZipA, which drive constriction in E. coli. FtsZ likely produces the initial force to constrict and ZipA anchors it to the cytoplasmic membrane. The natural anchoring provided by ZipA was used to reconstruct the effects of FtsZ polymerization on the vesicle membrane. During the resting stage, in the absence of GTP, ZipA associates with the inner surface of the vesicle, and FtsZ largely remains soluble inside the lumen. Addition of GTP triggers the polymerization of FtsZ resulting in the shrinkage of the vesicles. Initially the remodeling of the membrane produces substantial ruffling of the surface and is later followed by a shriveling and shrinking of the vesicle, leading to its eventual collapse (Fig. 2).
Vesicle collapse requires the interaction between the two proteins as it is prevented by the addition of a peptide that blocks the FtsZ-ZipA interaction; this also proves that the vesicles are osmotically stable. Vesicle shrinkage occurs after ZipA attains a high surface density threshold. This suggests that it may involve a cooperative network effect linked to multipoint contacts between ZipA and the membrane. Under these conditions, the vesicle surface is almost saturated with ZipA, and therefore membrane deformation cannot be caused by the differential accumulation of the DOGS-NTA lipid in specific areas of the vesicle. Furthermore, polymerization of FtsZ is unlikely to actively concentrate ZipA as low amounts of ZipA do not shrink the vesicles. Poorly hydrolyzable GTP analogs can slow down or altogether abolish vesicle collapse, indicating that the dynamics of polymerization modulate the mechanical forces exerted by FtsZ polymers on the membrane. It is accepted that in the dividing cell constriction is initially due to the force generated by FtsZ polymers producing an unstable deformation on the cytoplasmic membrane that is stabilized by the subsequent synthesis of rigid peptidoglycan. Similarly, the deformations produced in the vesicles after GTP-induced FtsZ polymerization could result from a reorganization of the membrane responding to the local interaction of the membrane-anchored ZipA with FtsZ polymers and to the forces generated by GTP hydrolysis.
After squeezing, multilamellar liposomes may be converted into tubular structures in which an externally added FtsZ fused to the MinD membrane targeting sequence occasionally internalizes (11). In these conditions the chimeric protein seems to concentrate at discrete regions and to produce a limited increase in membrane curvature. Constriction in genetically induced spherical E. coli cells, as those formed by rodA strains, requires the emergence of distinct polar and equatorial positions (43). In these spherical cells, constriction is initiated by the assembly of an incomplete ring (44–46). Shrinking and collapse, rather than equatorial constriction, could then be expected to occur in a vesicle in which no molecular markers are available to delineate a discrete position where pulling forces concentrate. However, contrary to the squeezed tubular liposomes used in previous reports (11, 12), we have obtained vesicles using procedures that allow an accurate control of the amounts and biochemical environment of the proteins involved and even the exact timing of the reactions.
The polymerization of tubulin or actin inside vesicles with a composition similar to those we have used, produce protrusions and discoid-like structures large enough and sufficiently rigid to push the membrane (45, 46). These two proteins play important roles either as molecular motors in the motive behavior of eukaryotic cells or as structures in the formation of pedestals induced by enteropathogenic E. coli in the gut epithelial cells (47, 48). As we have observed, the flexible and shorter FtsZ polymers, when linked to ZipA, exert pulling rather than pushing forces, a behavior compatible with a role in membrane constriction. Interestingly, the directionality of the forces involved in these processes, pushing in the case of actin fibers and pulling in the case of FtsZ polymers, is consistent with the membrane deformations observed when these proteins polymerize at the outer face of the vesicles. Whereas actin polymerization produces inward protrusions (49) the polymerization of FtsZ when placed outside the vesicles results in outward radiating spikes (12, 50).5
In parallel, to correlate the observations obtained from the in vitro analysis with its role in the cell, we have investigated the effect of increased ZipA amounts on the membrane properties and on the localization of several divisome proteins, FtsZ among them, in the living cell. The accumulation of ZipA inside living cells resulted in the production of cytoplasmic membrane invaginations that were internalized forming several ZipA-containing layers inside the cytoplasm. Coinciding with the increased amounts of membranous layers, the FtsZ protein together with FtsA and FtsN were displaced from potential septation sites to abnormal positions where ZipA was more concentrated. FtsA is, together with FtsZ and ZipA, the third element of the E. coli proto-ring, and FtsN is the last protein to be recruited into the divisome and is required for the stability of the completed proto-ring (35). We conclude that the alterations produced by an excess of ZipA affect a number of essential cell division proteins, and therefore they are not unspecific.
Contrary to the extensive shrinkage observed upon FtsZ polymerization in the ZipA-containing vesicles, a more limited membrane invagination is observed in dividing cells. In this case the peptidoglycan and outer membrane layers that are not affected by the increase of ZipA may offer additional structural support. From our data the FtsZ polymerization-induced shrinkage of vesicles only occurs at ZipA densities of 10,000 molecules/μm2 or higher, but not at lower densities. Unperturbed E. coli cells naturally contain nearly 1500 ZipA molecules/cell (34) and consequently, assuming that they were distributed uniformly, from 300 to 500 molecules/μm2. The induction level attained under our overproduction procedure in E. coli cells produces protein amounts that moderately exceed the ZipA density needed for vesicle shrinkage. We conclude then that the behavior observed in vesicles approximates the one found in living cells at similar densities of the protein.
To investigate whether the formation of intracellular membrane structures has any physiological consequence, we measured the fraction of cells that, upon ZipA overproduction, had lost their permeability barrier to find that indeed, as the induction time progressed, their numbers increased drastically. Our results suggest that the force to constrict the cell envelope may induce modifications in the architecture of the cytoplasmic membrane resulting in the production of membrane structures that are prone to split and reseal. This membrane plasticity, also observed as producing dilation of E. coli lipid vesicles in which ZipA is simultaneously incorporated at both sides of the membrane (16), may serve to facilitate constriction, but on the other hand it may also increase the fragility of the envelope and destroy the permeability barrier. Together with the misplacement of the divisome proteins, the loss of the permeability barrier when ZipA is at high levels may then be deleterious and render the cell unable to drive a normal constriction process resulting in filamentation.
Internalization of the cytoplasmic membrane has been described to occur in cells depleted from the gene products encoded by the mre and mrd operons (51). In these conditions the synthesis of peptidoglycan is severely perturbed, producing a largely defective cell wall. The morphology of the cell is greatly modified as a response to the internal turgor pressure and yields cells with an aberrant quasispherical shape. Under our experimental design, we have limited the changes introduced in vivo to one single variable, the overproduction of ZipA. As a consequence, septation is blocked, and the cells elongate forming long filaments that maintain the basic cylindrical aspect of rod-shaped cells. We can conclude therefore that a layer of peptidoglycan of sufficient rigidity to prevent the loss of a rod shape is maintained during the experiment and that this rigid layer prevents the collapse of the living cells.
An excess of cell membrane has been reported to occur in Bacillus subtilis cells in which AccDA, encoding the carboxyltransferase subunit of acetylcoenzyme A (CoA) carboxylase, has been overproduced. It has been claimed that division of the wall-less L forms of this organism results from this excess of membrane (52). However, in lipid vesicles, where no peptidoglycan is available, and coinciding with the observation of Bendezú and de Boer (51), the force exerted by FtsZ polymers through their interaction with the membrane-anchored ZipA results in the remodeling of the membrane leading to vesicle collapse, which is also reminiscent of the division observed in L forms (52). Overproduction of FtsA, a protein that contains a short amphipathic domain able to establish a weak contact with the cytoplasmic membrane, has been suggested to induce a limited membrane distortion in E. coli cells (53), but not a collapse. This is in agreement with the behavior of FtsA and FtsZ contained inside vesicles formed from natural E. coli membranes. These membranes contain ZipA at relatively low physiological levels, and their attached FtsA can be dislodged and captured into the vesicle lumen by FtsZ polymers (14, 15). ZipA is anchored to the membrane through a transmembrane domain. This anchoring may sustain a stronger force that can be transferred to the membrane when the ZipA levels increase, causing an extensive disruption of the cell membrane and a significant shrinkage of vesicles.
Membrane constriction, besides being a crucial stage in the division of present day cells, represents the ancestral mechanism in the evolution of division that may even have predated the appearance of cells in a world where peptidoglycan was still to come. Our in vitro system neatly shows that the reconstruction of a minimal division machinery assembly, formed by just two proteins: ZipA acting as a membrane anchor to transmit the motor force generated by FtsZ polymerization, and a permeable vesicle, can reproduce inside the vesicle basic events mimicking those that drive constriction in the bacterial cell.
Acknowledgments
We thank Allen Minton for useful comments and for critically reading the manuscript and the Electron Microscopy and the Flow Cytometry services at the Centro Nacional de Biotecnología, and the Confocal Microscopy service at the Centro de Investigaciones Biológicas both at Consejo Superior de Investigaciones Científicas for technical support.
This work was supported by Human Frontier Science Program Grant RGP0050/2010, European Commission Contract HEALTH-F3-2009-223431, and Spanish Government Grant BIO2011-28941-C03 (all to M. V. and G. R.).

This article contains supplemental Figs. S1–S4 and Movies S1–S6.
A. Martos, I. López-Montero, A. Raso, L. H. Moleiro, G. Rivas, F. Monroy, and M. Jiménez, unpublished information.
- DiIC18
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
- DOGS-NTA-Ni
- 1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxypentyl)iminodiacetic acid]succinyl} nickel salt
- GMP-CPP
- guanosine-5'-[(α,β)-methyleno]triphosphate
- GMP-PCP
- guanosine 5′-(β,γ-methylenetriphosphate)
- GUV
- giant unilamellar vesicle
- NCL-sZipA
- interaction between the N-terminal His tag of the protein and tracer amounts of a nickel-chelating lipid (DOGS-NTA) incorporated to the lipid mixture during vesicle formation.
REFERENCES
- 1. Adams D. W., Errington J. (2009) Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat. Rev. Microbiol. 7, 642–653 [DOI] [PubMed] [Google Scholar]
- 2. Vicente M., Rico A. I. (2006) The order of the ring: assembly of Escherichia coli cell division components. Mol. Microbiol. 61, 5–8 [DOI] [PubMed] [Google Scholar]
- 3. Vicente M., Rico A. I., Martínez-Arteaga R., Mingorance J. (2006) Septum enlightenment: assembly of bacterial division proteins. J. Bacteriol. 188, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haney S. A., Glasfeld E., Hale C., Keeney D., He Z., de Boer P. (2001) Genetic analysis of the Escherichia coli FtsZ-ZipA interaction in the yeast two-hybrid system: characterization of FtsZ residues essential for the interactions with ZipA and with FtsA. J. Biol. Chem. 276, 11980–11987 [DOI] [PubMed] [Google Scholar]
- 5. Sun S. X., Jiang H. (2011) Physics of bacterial morphogenesis. Microbiol. Mol. Biol. Rev. 75, 543–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mingorance J., Rivas G., Vélez M., Gómez-Puertas P., Vicente M. (2010) Strong FtsZ is with the force: mechanisms to constrict bacteria. Trends Microbiol. 18, 348–356 [DOI] [PubMed] [Google Scholar]
- 7. Erickson H. P., Anderson D. E., Osawa M. (2010) FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 74, 504–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohashi T., Hale C. A., de Boer P. A., Erickson H. P. (2002) Structural evidence that the P/Q domain of ZipA is an unstructured, flexible tether between the membrane and the C-terminal FtsZ-binding domain. J. Bacteriol. 184, 4313–4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. López-Montero I., López-Navajas P., Mingorance J., Rivas G., Vélez M., Vicente M., Monroy F. (2013) Intrinsic disorder of the bacterial cell division protein ZipA: coil-to-brush conformational transition. FASEB J. 27, 3363–3375 [DOI] [PubMed] [Google Scholar]
- 10. Pichoff S., Lutkenhaus J. (2005) Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol. Microbiol. 55, 1722–1734 [DOI] [PubMed] [Google Scholar]
- 11. Osawa M., Anderson D. E., Erickson H. P. (2008) Reconstitution of contractile FtsZ rings in liposomes. Science 320, 792–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osawa M., Anderson D. E., Erickson H. P. (2009) Curved FtsZ protofilaments generate bending forces on liposome membranes. EMBO J. 28, 3476–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osawa M., Erickson H. P. (2011) Inside-out Z rings: constriction with and without GTP hydrolysis. Mol. Microbiol. 81, 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiménez M., Martos A., Vicente M., Rivas G. (2011) Reconstitution and organization of Escherichia coli proto-ring elements (FtsZ and FtsA) inside giant unilamellar vesicles obtained from bacterial inner membranes. J. Biol. Chem. 286, 11236–11241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martos A., Monterroso B., Zorrilla S., Reija B., Alfonso C., Mingorance J., Rivas G., Jiménez M. (2012) Isolation, characterization and lipid-binding properties of the recalcitrant FtsA division protein from Escherichia coli. PLoS One 7, e39829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. López-Montero I., López-Navajas P., Mingorance J., Vélez M., Vicente M., Monroy F. (2013) Membrane reconstitution of FtsZ-ZipA complex inside giant spherical vesicles made of E. coli lipids: large membrane dilation and analysis of membrane plasticity. Biochim. Biophys. Acta 1828, 687–698 [DOI] [PubMed] [Google Scholar]
- 17. Casadaban M. J., Cohen S. N. (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138, 179–207 [DOI] [PubMed] [Google Scholar]
- 18. Durfee T., Nelson R., Baldwin S., Plunkett G., 3rd, Burland V., Mau B., Petrosino J. F., Qin X., Muzny D. M., Ayele M., Gibbs R. A., Csörgo B., Pósfai G., Weinstock G. M., Blattner F. R. (2008) The complete genome sequence of Escherichia coli DH10B: insights into the biology of a laboratory workhorse. J. Bacteriol. 190, 2597–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garrido T., Sánchez M., Palacios P., Aldea M., Vicente M. (1993) Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 12, 3957–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., A.1, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 21. Vischer N. O. E., Huls P. G., Woldringh C. L. (1994) Object-Image: an interactive image analysis program using structured point collection. BINARY 6 [Google Scholar]
- 22. RayChaudhuri D. (1999) ZipA is a MAP-Tau homolog and is essential for structural integrity of the cytokinetic FtsZ ring during bacterial cell division. EMBO J. 18, 2372–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rivas G., López A., Mingorance J., Ferrándiz M. J., Zorrilla S., Minton A. P., Vicente M., Andreu J. M. (2000) Magnesium-induced linear self-association of the FtsZ bacterial cell division protein monomer. J. Biol. Chem. 275, 11740–11749 [DOI] [PubMed] [Google Scholar]
- 25. Martos A., Alfonso C., López-Navajas P., Ahijado-Guzmán R., Mingorance J., Minton A. P., Rivas G. (2010) Characterization of self-association and heteroassociation of bacterial cell division proteins FtsZ and ZipA in solution by composition gradient-static light scattering. Biochemistry 49, 10780–10787 [DOI] [PubMed] [Google Scholar]
- 26. Kenny C. H., Ding W., Kelleher K., Benard S., Dushin E. G., Sutherland A. G., Mosyak L., Kriz R., Ellestad G. (2003) Development of a fluorescence polarization assay to screen for inhibitors of the FtsZ/ZipA interaction. Anal. Biochem. 323, 224–233 [DOI] [PubMed] [Google Scholar]
- 27. Hernández-Rocamora V. M., Reija B., García C., Natale P., Alfonso C., Minton A. P., Zorrilla S., Rivas G., Vicente M. (2012) Dynamic interaction of the Escherichia coli cell division ZipA and FtsZ proteins evidenced in nanodiscs. J. Biol. Chem. 287, 30097–30104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. González J. M., Jiménez M., Vélez M., Mingorance J., Andreu J. M., Vicente M., Rivas G. (2003) Essential cell division protein FtsZ assembles into one monomer-thick ribbons under conditions resembling the crowded intracellular environment. J. Biol. Chem. 278, 37664–37671 [DOI] [PubMed] [Google Scholar]
- 29. Reija B., Monterroso B., Jiménez M., Vicente M., Rivas G., Zorrilla S. (2011) Development of a homogeneous fluorescence anisotropy assay to monitor and measure FtsZ assembly in solution. Anal. Biochem. 418, 89–96 [DOI] [PubMed] [Google Scholar]
- 30. Carrara P., Stano P., Luisi P. L. (2012) Giant vesicles “colonies”: a model for primitive cell communities. ChemBiochem 13, 1497–1502 [DOI] [PubMed] [Google Scholar]
- 31. Pautot S., Frisken B. J., Weitz D. A. (2003) Production of unilamellar vesicles using an inverted emulsion. Langmuir 19, 2870–2879 [Google Scholar]
- 32. Walde P., Cosentino K., Engel H., Stano P. (2010) Giant vesicles: preparations and applications. ChemBiochem 11, 848–865 [DOI] [PubMed] [Google Scholar]
- 33. Song L., Hobaugh M. R., Shustak C., Cheley S., Bayley H., Gouaux J. E. (1996) Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 274, 1859–1866 [DOI] [PubMed] [Google Scholar]
- 34. Rueda S., Vicente M., Mingorance J. (2003) Concentration and assembly of the division ring proteins FtsZ, FtsA, and ZipA during the Escherichia coli cell cycle. J. Bacteriol. 185, 3344–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rico A. I., García-Ovalle M., Palacios P., Casanova M., Vicente M. (2010) Role of Escherichia coli FtsN protein in the assembly and stability of the cell division ring. Mol. Microbiol. 76, 760–771 [DOI] [PubMed] [Google Scholar]
- 36. Limozin L., Bärmann M., Sackmann E. (2003) On the organization of self-assembled actin networks in giant vesicles. Eur. Phys. J. E Soft Matter 10, 319–330 [DOI] [PubMed] [Google Scholar]
- 37. Takiguchi K., Negishi M., Tanaka-Takiguchi Y., Homma M., Yoshikawa K. (2011) Transformation of ActoHMM assembly confined in cell-sized liposome. Langmuir 27, 11528–11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yanagisawa M., Iwamoto M., Kato A., Yoshikawa K., Oiki S. (2011) Oriented reconstitution of a membrane protein in a giant unilamellar vesicle: experimental verification with the potassium channel KcsA. J. Am. Chem. Soc. 133, 11774–11779 [DOI] [PubMed] [Google Scholar]
- 39. Hale C. A., de Boer P. A. (1999) Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J. Bacteriol. 181, 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hale C. A., de Boer P. A. (2002) ZipA is required for recruitment of FtsK, FtsQ, FtsL, and FtsN to the septal ring in Escherichia coli. J. Bacteriol. 184, 2552–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hale C. A., de Boer P. A. (1997) Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 88, 175–185 [DOI] [PubMed] [Google Scholar]
- 42. Strahl H., Hamoen L. W. (2010) Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. U.S.A. 107, 12281–12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corbin B. D., Yu X. C., Margolin W. (2002) Exploring intracellular space: function of the Min system in round-shaped Escherichia coli. EMBO J. 21, 1998–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Addinall S. G., Lutkenhaus J. (1996) FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol. 22, 231–237 [DOI] [PubMed] [Google Scholar]
- 45. Hotani H., Nomura F., Suzuki Y. (1999) Giant liposomes: from membrane dynamics to cell morphogenesis. Curr. Opin. Colloid Interface Sci. 4, 358–368 [Google Scholar]
- 46. Takiguchi K., Yamada A., Negishi M., Honda M., Tanaka-Takiguchi Y., Yoshikawa K. (2009) Construction of cell-sized liposomes encapsulating actin and actin-cross-linking proteins. Methods Enzymol. 464, 31–53 [DOI] [PubMed] [Google Scholar]
- 47. Bugyi B., Carlier M. F. (2010) Control of actin filament treadmilling in cell motility. Annu. Rev. Biophys. 39, 449–470 [DOI] [PubMed] [Google Scholar]
- 48. Croxen M. A., Finlay B. B. (2010) Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8, 26–38 [DOI] [PubMed] [Google Scholar]
- 49. Liu A. P., Richmond D. L., Maibaum L., Pronk S., Geissler P. L., Fletcher D. A. (2008) Membrane-induced bundling of actin filaments. Nat. Phys. 4, 789–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martos A., Jiménez M., Rivas G., Schwille P. (2012) Towards a bottom-up reconstitution of bacterial cell division. Trends Cell Biol. 22, 634–643 [DOI] [PubMed] [Google Scholar]
- 51. Bendezú F. O., de Boer P. A. (2008) Conditional lethality, division defects, membrane involution, and endocytosis in mre and mrd shape mutants of Escherichia coli. J. Bacteriol. 190, 1792–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mercier R., Kawai Y., Errington J. (2013) Excess membrane synthesis drives a primitive mode of cell proliferation. Cell 152, 997–1007 [DOI] [PubMed] [Google Scholar]
- 53. Szwedziak P., Wang Q., Freund S. M., Löwe J. (2012) FtsA forms actin-like protofilaments. EMBO J. 31, 2249–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]