Background: The mechanism of substrate recognition for IPK1 (inositol 1,3,4,5,6-pentakisphosphate 2-kinase) is unresolved.
Results: Binding and activity data reveal specific roles for each phosphate of IP5.
Conclusion: The phosphate profile of IP5 is mechanistically critical to IPK1 activation.
Significance: Identifying determinants of substrate specificity will aid in the design of selective inhibitors for IPK1.
Keywords: Enzyme Mechanisms, Inositol Phosphates, Isothermal Titration Calorimetry, Ligand-binding Protein, Phosphorylation, IP6, IPK1, Kinase Activation, Substrate Recognition
Abstract
Inositol phosphate kinases (IPKs) sequentially phosphorylate inositol phosphates (IPs) to yield a group of small signaling molecules involved in diverse cellular processes. IPK1 (inositol 1,3,4,5,6-pentakisphosphate 2-kinase) phosphorylates inositol 1,3,4,5,6-pentakisphosphate to inositol 1,2,3,4,5,6-hexakisphosphate; however, the mechanism of IP recognition employed by IPK1 is currently unresolved. We demonstrated previously that IPK1 possesses an unstable N-terminal lobe in the absence of IP, which led us to propose that the phosphate profile of the IP was linked to stabilization of IPK1. Here, we describe a systematic study to determine the roles of the 1-, 3-, 5-, and 6-phosphate groups of inositol 1,3,4,5,6-pentakisphosphate in IP binding and IPK1 activation. The 5- and 6-phosphate groups were the most important for IP binding to IPK1, and the 1- and 3-phosphate groups were more important for IPK1 activation than the others. Moreover, we demonstrate that there are three critical residues (Arg-130, Lys-170, and Lys-411) necessary for IPK1 activity. Arg-130 is the only substrate-binding N-terminal lobe residue that can render IPK1 inactive; its 1-phosphate is critical for full IPK1 activity and for stabilization of the active conformation of IPK1. Taken together, our results support the model for recognition of the IP substrate by IPK1 in which (i) the 4-, 5-, and 6-phosphates are initially recognized by the C-terminal lobe, and subsequently, (ii) the interaction between the 1-phosphate and Arg-130 stabilizes the N-terminal lobe and activates IPK1. This model of IP recognition, believed to be unique among IPKs, could be exploited for selective inhibition of IPK1 in future studies that investigate the role of higher IPs.
Introduction
Inositol phosphates (IPs)2 are a group of small molecules that play critical roles in cellular signaling (1). IP signaling regulates DNA editing and repair (2), vesicle transport (3), and ion channel regulation (4) and has been implicated in diseases such as cancer and diabetes (5). IPs are produced by sequential phosphorylation of inositol 1,4,5-trisphosphate by a family of enzymes known as IP kinases (IPKs) (1). Similarity between IPs, which sometimes differ by only one phosphate group on the inositol ring, demands that IPKs use mechanisms to recognize and phosphorylate specific positions of their IP substrates while excluding highly similar molecules. Crystal structures from each of the IPK subfamilies have revealed that the structural determinants for IP discrimination vary between IPKs. IP3K (Inositol 1,4,5-trisphosphate 3-kinase) employs shape complementarity to recognize precisely positioned phosphate and hydroxyl groups of inositol 1,4,5-trisphosphate (6). In contrast, ITPK1 (inositol 1,3,4-trisphosphate 5/6-kinase/inositol 3,4,5,6-tetrakisphosphate 1-kinase) discriminates among IPs using phosphate affinity and stereochemical features to establish contacts with phosphates that are sufficient for substrate recognition (7). Crystal structures of IPK1 (inositol 1,3,4,5,6-pentakisphosphate 2-kinase) in its IP substrate- and product-bound forms reveal extensive contacts with all phosphate groups of the bound IPs (8). These structures reveal how inositol 1,3,4,5,6-pentakisphosphate (IP5) is phosphorylated on its axial 2′-hydroxyl, yielding inositol 1,2,3,4,5,6-hexakisphosphate, but they do not suggest a mechanism through which IPK1 selectively recognizes IP5 as its substrate while excluding other highly phosphorylated IPs with free axial 2′-hydroxyl groups. We recently determined the crystal structure of wild-type IPK1 in an IP-free state, which exhibited disorder within its N-terminal lobe (N-lobe) of the kinase, centered at Arg-130 (9). This IP-free structure suggests that binding of IP substrate plays a role in stabilization of the N- and C-lobes of the kinase, which is an important step in the activation of protein kinases (10–12).
Our current objective was to define the contributions of the individual phosphate groups of the IP to binding and to recognition of bound IP as a substrate. The results demonstrate that each phosphate group of the IP plays a different role in binding and activation of IPK1 and that there are three critical contacts formed between IPK1 and the IP that mediate IPK1 activation.
EXPERIMENTAL PROCEDURES
Generation of Alanine Mutants
Residues that interact with IP5, either directly or through solvent molecules, were identified using previous crystal structures (9). Mutation of these residues to alanine was performed by site-directed mutagenesis using the QuikChange method (Stratagene). A pET28a vector containing wild-type Arabidopsis thaliana IPK1 and a hexahistidine tag was used as a template (a kind gift from Dr. C. A. Brearley, University of East Anglia). All mutations were verified by DNA sequencing.
Protein Expression and Purification
Wild-type IPK1 and alanine mutants were expressed in BL21-AI cells (Invitrogen) that were grown in Terrific Broth to A600 = 1.5 and induced with 0.5 mm isopropyl β-d-thiogalactopyranoside and 0.1% l-arabinose at 18 °C for 20 h. Cells were lysed in 10 mm Tris-HCl (pH 8.0), 250 mm NaCl, and 50% glycerol using a sonicator. The supernatant was separated from the lysate by centrifugation at 45,000 × g. The supernatant was then diluted 5-fold using 20 mm Tris-HCl (pH 8.0) and 500 mm NaCl, and 25 mm imidazole was added. IPK1 was purified using nickel-nitrilotriacetic acid beads (Thermo Scientific) in a gravity column using 4 ml of dry beads/250 ml of culture. The beads were washed with 20 column volumes of 50 mm KPO4 (pH 8.0), 800 mm NaCl, 1% Triton X-100, and 1.7 mm β-mercaptoethanol. Protein was eluted using 250 mm imidazole in 20 mm Tris-HCl (pH 8.0) and 300 mm NaCl, and then 2 mm DTT was added to the eluate. The protein concentration was determined by Bradford assay (Thermo Scientific) using BSA as a standard. Protein was stored at 4 °C and used within 72 h.
Isothermal Titration Calorimetry (ITC)
Experiments were performed on a MicroCal iTC200 titration calorimeter (GE Healthcare). Wild-type IPK1 was purified and dialyzed into ITC buffer containing 50 mm HEPES (pH 7.5), 6 mm MgCl2, 150 mm NaCl, and 1 mm tris(2-carboxyethyl)phosphine (pH 7.0). After protein dialysis was complete, dialysis buffer was used to dissolve the ligands, IP, and AMP-PNP (Jena Bioscience). Titration experiments were performed at 25 °C with 100 μm IPK1 and 1 mm AMP-PNP in the cell and 1–2 mm IP in the syringe to ensure a final IP:IPK1 molar ratio of at least 2:1. Titration experiments were performed at least twice for each IP, and one set was chosen to represent data. Calorimetric data were analyzed using Origin 7.0 (MicroCal). Data were fitted with a one-site model using Equation 1,
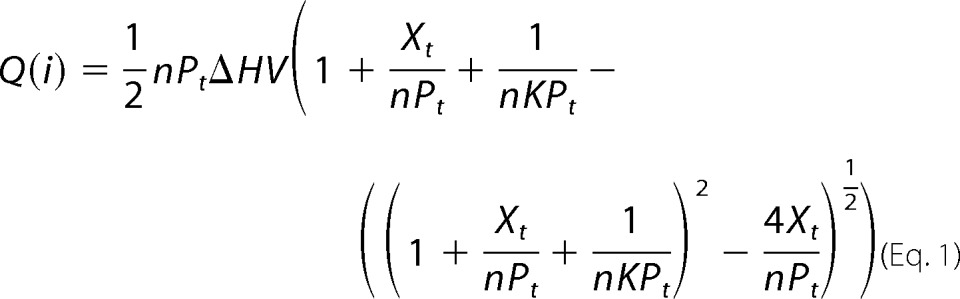 |
where n is the number of IP-binding sites on IPK1, Pt is the total concentration of IPK1, Xt is the total concentration of IP, V is the cell volume, K is the binding constant, and ΔH corresponds to the enthalpy change due to IP-IPK1 binding. The heat corresponding to the ith injection only, ΔQ(i), is equal to the difference between Q(i) and Q(i − 1) and is given by Equation 2,
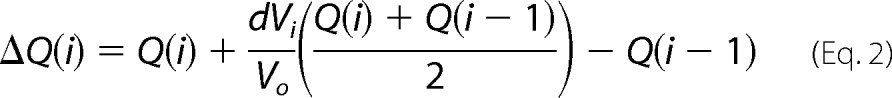 |
which is corrected by the injection volume (dVi) for the displaced volume.
IPK1 Activity Assay
IPs (IP5, 1,3,4,5-inositol tetrakisphosphate (IP4), 1,4,5,6-IP4, 1,3,4,6-IP4, and 3,4,5,6-IP4) were purchased from Cayman Chemical Company. A source for 1,3,5,6-IP4 was not located. IPK1 kinase activity was assessed using the Kinase-Glo Max luminescent kinase assay (Promega) following the manufacturer's instructions. Kinase reactions were performed in 25-μl volumes on black 96-well plates at 25 °C. The reaction mixture contained 50 mm HEPES (pH 7.5), 6 mm MgCl2, 50 mm NaCl, and 300 μm ATP. 25 μl of Kinase-Glo reagent was added to stop the reaction, and luminescence was measured after 20 min on a Berthold Orion II microplate luminometer. Initially, 80 μm IP was used, and the amount of enzyme was varied to determine conditions in which product formation was linear over 30 min. Subsequently, an array of reactions with varying concentrations of IP (20, 40, 60, 80, 100, 120, and 140 μm) stopped at various time points (2, 5, 10, 20, and 30 min) were performed in triplicate. The rate of product formation versus IP concentration was plotted and fitted to the Michaelis-Menten equation using nonlinear regression to determine Km and Vmax (GraphPad Software). The kcat values were calculated using the equation kcat = Vmax/[E], where [E] is the micromolar enzyme concentration.
IPK1 Alanine Mutant Activity Assay
Initially, 150 ng of each mutant was tested for kinase activity with 80 μm IP5 after 30 min. Mutants that exhibited little or no activity were retested using 750 ng of enzyme. Active mutants were further characterized for their kinetic parameters with IP5 as a substrate using the abovementioned approach.
RESULTS
5- and 6-Phosphates Are Important for Binding
To determine the role of phosphates at each position of the inositol ring, we measured the effect on binding affinity when using IPs lacking a phosphate group at the 1-, 3-, 5-, or 6-position. Using ITC, we obtained KD values for each IP4 and IP5 (Table 1). As expected, IP5, the native substrate for IPK1, displayed the highest binding affinity, with KD = 0.60 μm. IP4s exhibited a range of binding affinities. The KD values of 1,4,5,6-IP4 and 3,4,5,6-IP4 were 13-fold higher than that of IP5, whereas the KD values of 1,3,4,6-IP4 and 1,3,4,5-IP4 were at least 30-fold higher. These results indicate that different phosphate groups have varying contributions to the binding affinity of IP5 for IPK1. Comparison of the IP4 KD:IP5 KD ratios revealed that the 5- and 6-phosphates contributed the most to the binding affinity of the IP, as the absence of either phosphate group dramatically increased the KD (Table 1).
TABLE 1.
Binding data of IPK1 for IP5 and IP4s
| IP | N (sites) | K | KD | ΔH | ΔS |
|---|---|---|---|---|---|
| m−1 | μm | cal/mol | cal/mol/degrees | ||
| IP5 | 0.895 | 1.68 × 106 | 0.60 | −11,900 | −11.4 |
| 1,3,4,6-IP4 | 0.895 | 3.72 × 104 | 26.88 | −8720 | −8.34 |
| 1,4,5,6-IP4 | 1.14 | 1.22 × 105 | 8.20 | −7551 | −2.05 |
| 3,4,5,6-IP4 | 0.656 | 1.33 × 105 | 7.52 | −18,300 | −37.9 |
| 1,3,4,5-IP4 | 1.19 | 5.49 × 104 | 18.21 | −10,140 | −12.3 |
1- and 3-Phosphates Are Important for Substrate Recognition
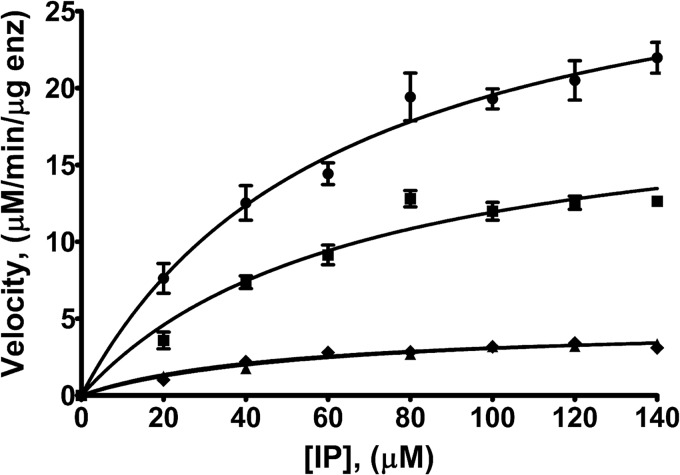
We tested the kinase activity of IPK1 for IP5 and IP4s using a luminescence-based assay to determine which phosphates identify an IP as a substrate for IPK1 (Fig. 1 and Table 2). The kinase activity of IPK1 was maximal in the presence of IP5, with kcat = 44.02 ± 3.19 nmol/min. With 1,3,4,6-IP4, an IP lacking the 5-phosphate, there was a modest decrease in kinase activity, with kcat = 27.48 ± 4.00 nmol/min. In contrast, IPs lacking 1- and 3-phosphate groups exhibited a substantial 85% decrease in activity compared with IP5. No activity was detected when 1,3,4,5-IP4 was used as a substrate, suggesting that the 6-phosphate may also be important for activation. These results indicate that the 1- and 3-phosphates are important for the IP to be recognized as a substrate by IPK1. We also observed that the KD values for IPs lacking phosphates varied considerably, whereas the Km for each IP remained nearly constant (Table 2). This disconnect suggests that the kinetic parameters of ligand binding or catalysis change along with the binding affinity, but we cannot define with the current set of assays how they change.
FIGURE 1.
Kinetic analysis of the kinase activity of IPK1 for IP5 and IP4s. IPK1 kinase activity was assessed using a luminescence-based assay. The rate of product formation versus IP concentration was plotted and fitted to the Michaelis-Menten equation. Each point represents the mean ± S.D. of triplicate experiments. ●, IP5; ■, 1,3,4,6-IP4; ♦, 3,4,5,6-IP4; ▴, 1,4,5,6-IP4. enz, enzyme.
TABLE 2.
Kinetic parameters of IPK1 for IP5 and IP4s
Data represent the mean ± S.D. of triplicate experiments. ND, no activity detected.
| IP | Km | kcat |
|---|---|---|
| μm | min−1 | |
| IP5 | 63.05 ± 10.77 | 44.02 ± 3.19 |
| 1,3,4,6-IP4 | 66.81 ± 22.37 | 27.48 ± 4.00 |
| 1,4,5,6-IP4 | 55.99 ± 13.95 | 6.60 ± 0.66 |
| 3,4,5,6-IP4 | 47.54 ± 13.94 | 6.30 ± 0.68 |
| 1,3,4,5-IP4 | ND | ND |
Alanine Mutants Identify Active Site Residues Critical for IPK1 Activity
To identify the contacts between IPK1 and IP that are essential for activity, we mutated to alanine those IPK1 residues that interact with the IP and tested these mutants for activity for IP5 (Table 3). To compare the kinase activity between mutants, we determined kcat values for each mutant. For wild-type IPK1, kcat = 44.02 ± 3.19 nmol/min. Mutation of Arg-130, the only residue that interacts with the 1-phosphate of the IP, resulted in no detectable activity. Mutations of Lys-168 and Asp-368, which mediate phosphoryl transfer from ATP to the 2′-hydroxyl, also exhibited no activity, as expected. The R45A mutant, which abolished the contact with the 3-phosphate, displayed a 40% decrease in kinase activity (kcat = 27.16 ± 1.79 nmol/min), whereas the R415A and Y419A mutants, which abolished contacts with the 4-phosphate, both displayed activity equivalent to wild-type IPK1. In contrast, the K411A mutant, which abolished contacts with both the 3- and 4-phosphates, showed no activity. The R192A mutant displayed a modest decrease in kinase activity (kcat = 22.01 ± 2.05 nmol/min), and the H196A mutant had no effect on kinase activity (kcat = 34.12 ± 4.33 nmol/min), demonstrating that interactions with the 5-position have modest impact on catalytic activity. The K170A mutant, which abolished interactions with both the 5- and 6-phosphates, showed no activity. Finally, the K200A, N238A, and N239A mutants, which eliminated contacts with the 6-phosphate, displayed reduced activity compared with wild-type IPK1. Fig. 2 summarizes the effect of alanine mutations in the inositide-binding site on IPK1 activity. Here, we observed that the residues that interact with more than one phosphate play important roles in substrate recognition, whereas most residues that interact with a single phosphate play lesser roles in this process.
TABLE 3.
Kinetic parameters of IPK1 alanine mutants for IP5
Data represent the mean ± S.D. of triplicate experiments. The last column indicates the IP phosphate that interacts with the mutant side chain, either directly or indirectly, through ordered water molecules. ND, no activity detected.
| Mutant | Km | kcat | PO4 interaction |
|---|---|---|---|
| μm | min−1 | ||
| R45A | 54.28 ± 9.07 | 27.16 ± 1.79 | 3 |
| R130A | ND | ND | 1 |
| K168A | ND | ND | 2 |
| K170A | ND | ND | 5, 6 |
| R192A | 59.35 ± 13.41 | 22.01 ± 2.05 | 5 |
| H196A | 43.48 ± 15.62 | 34.12 ± 4.33 | 5 |
| K200A | 39.79 ± 19.52 | 16.74 ± 2.77 | 6 |
| N238A | 33.72 ± 7.918 | 14.74 ± 1.07 | 6 |
| N239A | 83.30 ± 9.53 | 15.89 ± 0.87 | 6 |
| D368A | ND | ND | 2 |
| K411A | ND | ND | 3, 4 |
| R415A | 62.27 ± 21.89 | 38.71 ± 5.73 | 3, 4 |
| Y419A | 76.86 ± 16.94 | 41.62 ± 4.25 | 4 |
FIGURE 2.
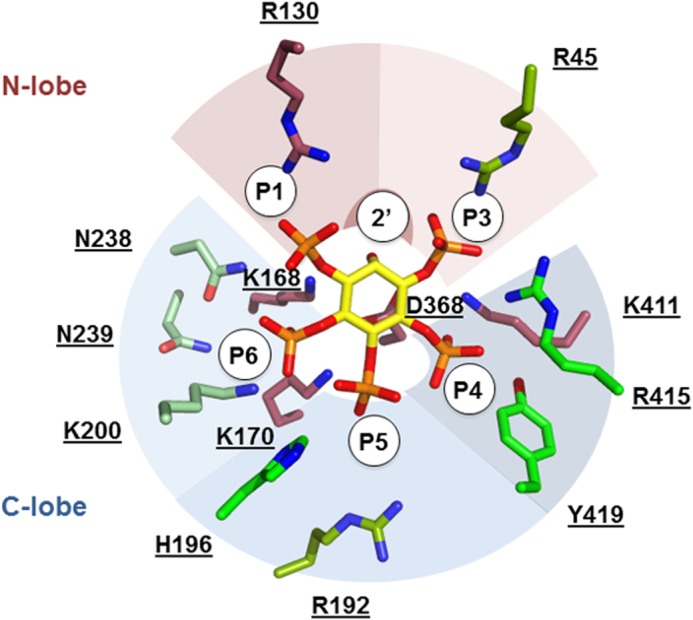
Structural representation of kinetic parameters of alanine mutants. IP5 is shown in yellow stick form. The side chains of IP-binding residues are shown in stick form and colored according to alanine mutant kcat. Maroon indicates no activity. Darker green shades indicate reduced activity compared with wild-type IPK1. Green indicates equivalent activity to wild-type IPK1. Side chains are grouped according to bound phosphate, overlaid with colored arcs (red, N-lobe; blue, C-lobe).
DISCUSSION
Roles of Different Phosphate Groups: Binding versus Activation
Here, we performed a systematic study to identify the relative contributions of the 1-, 3-, 5-, and 6-phosphates to IP5 binding affinity and recognition as an IPK1 substrate. Initially, we used ITC to determine the binding affinity of IPK1 for IP5 and for a set of IP4s, each lacking a single phosphate group. We observed a spectrum of affinities for these differently phosphorylated IP4 molecules, indicating that phosphates contribute differently to binding. IPs lacking the 5- or 6-phosphate displayed the lowest binding affinity for IPK1, whereas IPs lacking the 1- or 3-phosphate displayed only moderately decreased binding affinity (Table 1). The structure of nucleotide-bound IPK1 (Protein Data Bank code 3UDS) revealed the N-lobe to be unstable compared with the IP-bound state, and in a recent structure of IPK1 engineered to crystallize in the absence of IP (Protein Data Bank code 4AXC), the N-lobe was too far away from the C-lobe to form a complete inositide-binding pocket (9, 13). In both wild-type and mutant structures, the N-lobe fails to assemble into the active conformation. Our ITC binding data, which indicate that the C-lobe-binding 5- and 6-phosphates contribute substantially more to binding, are consistent with the C-lobe playing a dominant role in substrate recruitment. This collection of structures and the binding data support the model that substrate recruitment likely occurs though the stable C-lobe, which comprises half of the IP-binding site. To complete assembly of the IP-binding site, IP must bind to both the N- and C-lobes, thereby coupling binding of the substrate to stabilization of the kinase.
We further investigated the contribution of each phosphate group of the IP to its recognition as a substrate and to activation of IPK1. We determined the Km and kcat of IPK1 in the presence of IP5 and our series of IP4s. These data indicate that the kcat for IPK1 is substantially decreased for IPs lacking the 1- or 3-phosphate compared with IP5 and 1,3,4,6-IP4 (Table 2). Preliminary studies of IPK1 substrate specificity also revealed the use of 1,3,4,6-IP4 as a substrate, but not 3,4,5,6-IP4; however a kinetic analysis of IPK1 with each IP4 was not performed (14). Our observations are consistent with the 1- and 3-phosphates stabilizing the bilobed structure of IPK1, thereby promoting its activation though recruitment of the N-lobe using a mechanism similar to that reported for protein kinases (12).
IPK1 was unable to use 1,3,4,5-IP4 as a substrate, which suggests that the 6-position may play a dual role in both IP binding and activation; abolishing both functions decreased its use as a substrate to levels below the detection limit of our assay. The KD values for 1,3,4,5-IP4 and 1,3,4,6-IP4 were similar; however, 1,3,4,6-IP4 could be used as a substrate, whereas 1,3,4,5-IP4 could not. This indicates that the decreased binding affinity of 1,3,4,5-IP4 for IPK1 was not by itself the underlying factor for its inability to be used as a substrate (Table 1). The 6-phosphate-binding site plays a key role in IPK1 activation by preventing clasp formation (a critical step in the IPK1 catalytic cycle) in the absence of IP substrate. Binding of the 6-phosphate to Lys-200 disrupts the interaction between Lys-200 and Glu-255, and this newly freed Glu-255 binds to Trp-129, thereby promoting clasp formation between the L3 loop and α6 helix (13). However, the K200A mutant, as well as other mutants that disrupted interaction with the 6-phosphate (K170A, N238A, and N239A), did not show any activity for 1,3,4,5-IP4, so intramolecular changes in the 6-phosphate-binding site are not essential for IPK1 activation (data not shown). It is possible that 1,3,4,5-IP4 adopts alternative binding orientations in which the 2′-hydroxyl is not accessible for phosphorylation (7, 15). Further experimentation will be required to ascertain why 1,3,4,5-IP4 displays no activity.
Critical Roles of Arg-130 and 1-Phosphate in IPK1 Activation
On the basis of previous crystal structures, we mutated each substrate-binding residue to alanine to determine the critical contacts between IPK1 and the IP. Each mutation abolished an interaction with one or two phosphates (Table 3), and we determined the Km and kcat for each mutant in the presence of IP5. The impacts of the mutations and the activities of the IP4s were largely symmetric; abolishing single contacts with a phosphate group through mutation had a similar impact as removing the phosphate from the substrate. A notable difference between the IPK1 mutant and IP4 data was the asymmetric impact of abolishing the interaction with the 1-phosphate. Given that Arg-130, located on the N-lobe, is the only residue that interacts with the 1-phosphate, we expected that an IP lacking the 1-phosphate would correspond with the activity of this mutant. However, the R130A mutant displayed no detectable activity, whereas 3,4,5,6-IP4 could be used by the wild-type enzyme, albeit with low activity (Tables 2 and 3). Structures of IPK1 in complex with IP show that Arg-130 forms a bond with Gly-254, establishing an interaction between the L3 loop and α6 helix and promoting clasp formation (Fig. 3) (9). This interaction, and therefore clasp formation, cannot occur in the R130A mutant, rendering IPK1 inactive. Transient clasp formation can occur in wild-type IPK1, even in the absence of a 1-phosphate, which allows the use of 3,4,5,6-IP4 as a substrate with low activity. The 1-phosphate interaction with Arg-130 is conducive to clasp formation, thereby rendering IPK1 fully active (Fig. 3). Thus, the 1-phosphate stabilizes the active conformation of IPK1.
FIGURE 3.
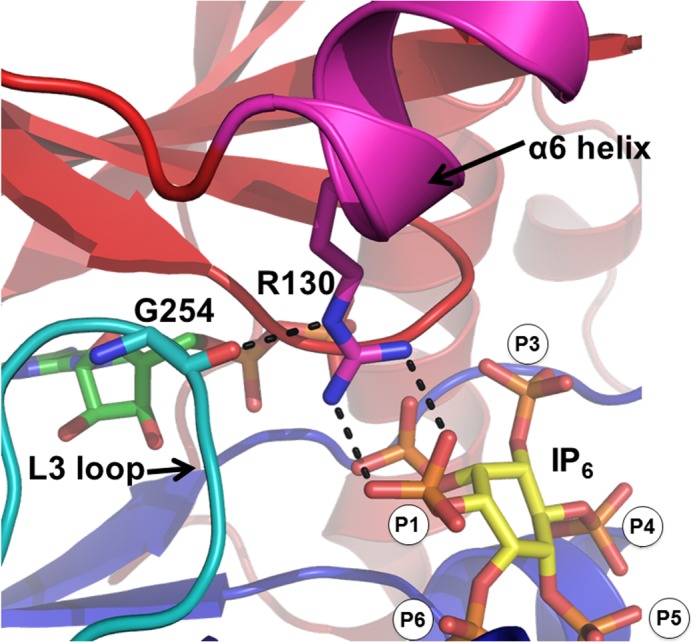
Clasp formation between the α6 helix and L3 loop. Interactions between Arg-130 (magenta stick form) and Gly-254 (cyan stick form) mediate clasp formation between the L3 loop (cyan) and α6 helix (magenta) of the C-lobe (blue) and of the N-lobe (red), respectively. Arg-130 interacts with the 1-phosphate of the IP, which is conducive to clasp formation. This image was created using PyMOL and a product-bound structure of IPK1 (Protein Data Bank code 3UDZ). ADP (green) and inositol 1,2,3,4,5,6-hexakisphosphate (IP6; yellow) are shown in stick form. Dashed lines indicate hydrogen bonds.
One Critical Residue at Each Phosphate-binding Site
There are 13 residues that interact with the IP in the IPK1 active site, either directly or indirectly through ordered water molecules, and each of these residues results in a bond to a phosphate group. For each phosphate of the IP, there is a contact residue that plays a critical role, and mutation of that residue abolishes activity. As discussed above, Arg-130 interacts with the 1-phosphate of the substrate and is a primary contact between the IP and the kinase N-lobe. Arg-130 is essential due to its structural role in stabilizing the active state and not exclusively for its role in binding the 1-phosphate, as 3,4,5,6-IP4 can bind and be recognized as a substrate. An IP lacking the 3-phosphate also displayed low activity; however, the R45A mutation, which disrupted the second contact between the IP and N-lobe, moderately impaired activity (Table 3). Thus, it appears that N-lobe interaction with IP is mediated primarily through Arg-130 and the 1-phosphate. Mutation of Lys-411, which interacts with both the 3- and 4-phosphates, was critical for activity, yet mutations of Arg-415 and Tyr-419, which mediate other interactions with the 4-phosphate, did not markedly affect IPK1 activity, nor did R45A, which is the second contact with the 3-phosphate, as described above (Table 3). Both the 5- and 6-phosphates bind to Lys-170, which is critical for activity; however, other 5-phosphate interactions with Arg-192 and His-196 and other 6-phosphate interactions with Lys-200, Asn-238, and Asn-239 are dispensable for activity. Wild-type IPK1 displayed activity with IPs lacking the 3- or 5-phosphate, yet mutations of Lys-170 and Lys-411 rendered IPK1 inactive, likely due to the fact that these residues bind more than one phosphate (Tables 2 and 3). Sequence alignments of IPK1 reveal that Arg-45, Arg-415, and Tyr-419 are the only inositide-binding residues that are not conserved among plant, human, rat, and yeast (8). Accordingly, R45A, R415A, and Y419A mutations retained at least 60% of wild-type activity. The conservation of Arg-130, but not Arg-45, suggests that N-lobe contact with the IP is mediated primarily through the 1-phosphate. In summary, our data indicate that each phosphate has a critical residue in the active site without which the enzyme cannot function, notably Arg-130, Lys-170, and Lys-411 (Table 3). Whether Lys-170 and Lys-411 impact binding affinity to the extent that substrate recognition cannot occur or if these residues play structural roles in shaping the binding site into an active conformation, as Arg-130 does, remains to be determined.
Model of IP-induced Stabilization as a Mechanism of Selectivity of IPs
Our previous crystal structures revealed that IPK1 possesses a stable C-lobe and a destabilized N-lobe in the absence of IP, such that the inositide-binding pocket remains partially formed (9). We proposed a model wherein IPK1 links its interactions with IP substrate phosphate groups to stabilization of the N- and C-lobes and kinase activation. The stability of the C-lobe in the absence of IPs suggested that the roles of 4-, 5-, and 6-phosphates were to mediate the initial contact of the IP with IPK1. Our present study reveals that the 5- and 6-phosphates impact binding affinity more than the 1- and 3-phosphates. Our model also proposed that the 1- and 3-phosphates were required for N-lobe stabilization, as they act to bridge the N-lobe with the C-lobe. In our present study, IPK1 displayed substantially lower kcat values with 3,4,5,6-IP4 and 1,4,5,6-IP4 than with IP5 or an IP4 lacking the 5-phosphate. IP-free crystal structures of IPK1 reveal that the destabilization of the N-lobe is centered at Arg-130, which directly binds to the 1-phosphate of the IP substrate (9). The mutation of R130A impaired IPK1 activity, suggesting that the N-lobe is required to be stabilized for IPK1 activation, a key feature of kinase activation (12). In short, our study reveals specific roles for each of the IP phosphates, linking IPK1 substrate specificity to IPK1 stability.
Conclusions
In this work, we have demonstrated that the phosphate profile of IP5 is mechanistically linked to IPK1 activation. We have identified phosphates at the 1-, 3-, and 6-positions as the most important for activation of IPK1, whereas phosphates at the 5- and 6-positions are more important for binding than those at the 1- and 3-positions. Identification of the roles of the phosphates of the IP supports our proposed model of IPK1 substrate specificity and may provide a basis for selective targeting of IPK1, as similarity among IP substrates continues to hinder development of specific inhibitors for IPKs. Inhibition of IPK1 would prove valuable in the investigation of the roles of higher IPs whose production is dependent on inositol 1,2,3,4,5,6-hexakisphosphate, the product of IPK1, as well as functional roles of IPK1 in mammals.
Acknowledgments
We thank Dr. C. A. Brearley for the gift of the pET28-AtIPK1 vector, Dr. Guennadi Kozlov (McGill University) for technical assistance with ITC, Dr. Dan Bernard (McGill University) for access to the luminometer used in enzyme assays, Dr. Hatem Dokainish (McGill University) for helpful discussions, and Anne W. Coventry for thoughtful reading of the manuscript.
This work was supported by Canadian Institutes of Health Research (CIHR) Operating Grant MOP-93687 (to G. J. M.) and a CIHR Strategic Training Initiative in Chemical Biology grant (to V. G.).
- IP
- inositol phosphate
- IPK
- IP kinase
- IP5
- inositol 1,3,4,5,6-pentakisphosphate
- N-lobe
- N-terminal lobe
- C-lobe
- C-terminal lobe
- ITC
- isothermal titration calorimetry
- AMP-PNP
- adenosine 5′-(β,γ-imido)triphosphate
- IP4
- inositol tetrakisphosphate.
REFERENCES
- 1. Irvine R. F., Schell M. J. (2001) Back in the water: the return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2, 327–338 [DOI] [PubMed] [Google Scholar]
- 2. Hanakahi L. A., Bartlet-Jones M., Chappell C., Pappin D., West S. C. (2000) Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell 102, 721–729 [DOI] [PubMed] [Google Scholar]
- 3. Hilton J. M., Plomann M., Ritter B., Modregger J., Freeman H. N., Falck J. R., Krishna U. M., Tobin A. B. (2001) Phosphorylation of a synaptic vesicle-associated protein by an inositol hexakisphosphate-regulated protein kinase. J. Biol. Chem. 276, 16341–16347 [DOI] [PubMed] [Google Scholar]
- 4. Vajanaphanich M., Schultz C., Rudolf M. T., Wasserman M., Enyedi P., Craxton A., Shears S. B., Tsien R. Y., Barrett K. E., Traynor-Kaplan A. (1994) Long-term uncoupling of chloride secretion from intracellular calcium levels by Ins(3,4,5,6)P4. Nature 371, 711–714 [DOI] [PubMed] [Google Scholar]
- 5. Shi Y., Azab A. N., Thompson M. N., Greenberg M. L. (2006) Inositol phosphates and phosphoinositides in health and disease. Subcell. Biochem. 39, 265–292 [DOI] [PubMed] [Google Scholar]
- 6. González B., Schell M. J., Letcher A. J., Veprintsev D. B., Irvine R. F., Williams R. L. (2004) Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase. Mol. Cell 15, 689–701 [DOI] [PubMed] [Google Scholar]
- 7. Miller G. J., Wilson M. P., Majerus P. W., Hurley J. H. (2005) Specificity determinants in inositol polyphosphate synthesis: crystal structure of inositol 1,3,4-trisphosphate 5/6-kinase. Mol. Cell 18, 201–212 [DOI] [PubMed] [Google Scholar]
- 8. González B., Baños-Sanz J. I., Villate M., Brearley C. A., Sanz-Aparicio J. (2010) Inositol 1,3,4,5,6-pentakisphosphate 2-kinase is a distant IPK member with a singular inositide binding site for axial 2-OH recognition. Proc. Natl. Acad. Sci. U.S.A. 107, 9608–9613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gosein V., Leung T. F., Krajden O., Miller G. J. (2012) Inositol phosphate-induced stabilization of inositol 1,3,4,5,6-pentakisphosphate 2-kinase and its role in substrate specificity. Protein Sci. 21, 737–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. (1991) Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 414–420 [DOI] [PubMed] [Google Scholar]
- 11. Ozkirimli E., Post C. B. (2006) Src kinase activation: a switched electrostatic network. Protein Sci. 15, 1051–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sicheri F., Moarefi I., Kuriyan J. (1997) Crystal structure of the Src family tyrosine kinase Hck. Nature 385, 602–609 [DOI] [PubMed] [Google Scholar]
- 13. Baños-Sanz J. I., Sanz-Aparicio J., Whitfield H., Hamilton C., Brearley C. A., González B. (2012) Conformational changes in inositol 1,3,4,5,6-pentakisphosphate 2-kinase upon substrate binding. Role of N-terminal lobe and enantiomeric substrate preference. J. Biol. Chem. 287, 29237–29249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sweetman D., Johnson S., Caddick S. E., Hanke D. E., Brearley C. A. (2006) Characterization of an Arabidopsis inositol 1,3,4,5,6-pentakisphosphate 2-kinase (AtIPK1). Biochem. J. 394, 95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chamberlain P. P., Qian X., Stiles A. R., Cho J., Jones D. H., Lesley S. A., Grabau E. A., Shears S. B., Spraggon G. (2007) Integration of inositol phosphate signaling pathways via human ITPK1. J. Biol. Chem. 282, 28117–28125 [DOI] [PMC free article] [PubMed] [Google Scholar]