Abstract
Objective
To determine whether pretreatment evaluation with contrast-enhanced ultrasonography (CEUS) is effective for percutaneous radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC) with poor conspicuity on conventional ultrasonography (US).
Materials and Methods
This retrospective study was approved by the institutional review board and informed consent was waived. From June 2008 to July 2011, 82 patients having HCCs (1.2 ± 0.4 cm) with poor conspicuity on planning US for RFA were evaluated with CEUS prior to percutaneous RFA. We analyzed our database, radiologic reports, and US images in order to determine whether the location of HCC candidates on planning US coincide with that on CEUS. To avoid incomplete ablation, percutaneous RFA was performed only when HCC nodules were identified on CEUS. The rate of technical success was assessed. The cumulative rate of local tumor progression was estimated with the use of the Kaplan-Meier method (mean follow-up: 24.0 ± 13.0 months).
Results
Among 82 patients, 73 (89%) HCCs were identified on CEUS, whereas 9 (11%) were not. Of 73 identifiable HCCs on CEUS, the location of HCC on planning US corresponded with that on CEUS in 64 (87.7%), whereas the location did not correspond in 9 (12.3%) HCCs. Technical success was achieved for all 73 identifiable HCCs on CEUS in a single (n = 72) or two (n = 1) RFA sessions. Cumulative rates of local tumor progression were estimated as 1.9% and 15.4% at 1 and 3 years, respectively.
Conclusion
Pretreatment evaluation with CEUS is effective for percutaneous RFA of HCCs with poor conspicuity on conventional US.
Keywords: Hepatocellular carcinoma, Ultrasonography, Radiofrequency ablation, Contrast-enhanced ultrasonography, SonoVue
INTRODUCTION
Radiofrequency ablation (RFA) has been accepted as a promising technique for treating small hepatocellular carcinomas (HCCs) (1-3). Among the various guiding modalities for percutaneous RFA, ultrasonography (US) is widely used for advantages, such as real-time control, easy accessibility, relatively low cost and no radiation hazard, to the patient. However, not all HCCs are suitable for percutaneous US-guided RFA. For example, very small-sized HCCs or HCCs with subphrenic location are sometimes difficult to detect on conventional US (4). In addition, local tumor progression after transcatheter arterial chemoembolization or thermal ablation is also hard to detect on US due to the difficulty of differentiating viable HCCs from the previously-treated area of tumors (5-7). A recent study reported that mistargeting, which indicates ablation of a pseudolesion that is not a true lesion, was found in approximately 2% of percutaneous US-guided RFA mainly due to confusion with cirrhotic nodules and poor conspicuity of HCCs (8). Therefore, accurate localization of the true index tumor is essential for successful local ablation therapy of HCCs.
Contrast-enhanced US (CEUS) allows better visualization of focal hepatic lesions that cannot be clearly visualized on conventional US for RFA (9-11). In these studies, either SH U 508A (Levovist; Schering, Berlin, Germany) or perfluorocarbon microbubbles (Sonazoid, Daiichi-Sankyo, Tokyo, Japan) were used for localizing the index tumors with poor sonographic conspicuity during percutaneous US-guided RFA of HCCs. However, to our knowledge, there are few data regarding the results of RFA using sulfur hexafluoride microbubbles (SonoVue, Bracco, Milan, Italy), which is another second generation US contrast agent. In this study, we evaluate whether pretreatment evaluation with CEUS using SonoVue is effective for percutaneous US-guided RFA of HCCs, which has poor conspicuity on conventional US.
MATERIALS AND METHODS
Patients and Inclusion Criteria
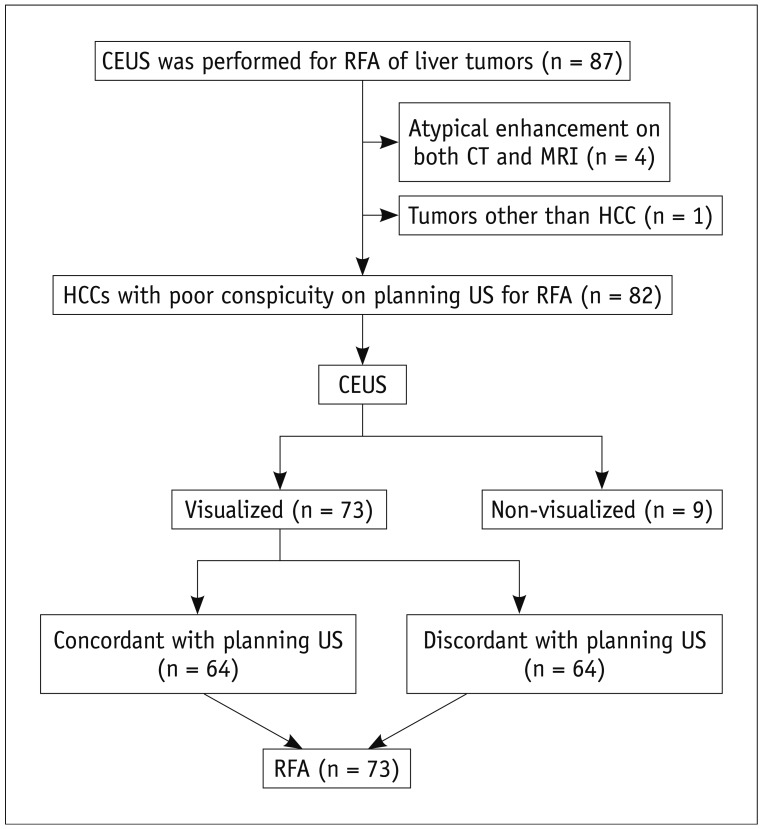
This retrospective study was approved by the institutional review board of our institution and the informed consent was waived. When SonoVue was first introduced to our hospital (Samsung Medical Center) in June 2008, the treatment guideline for HCCs in our hospital was modified. CEUS was added to the guideline in order to ablate HCCs with poor conspicuity, which widened the indication of RFA after the introduction of SonoVue. Therefore, patients having HCCs with poor sonographic conspicuity for RFA were evaluated with CEUS prior to percutaneous RFA. We retrospectively analyzed our electronic database (Microsoft Office Access and Excel; Microsoft, Redmond, WA, USA), which was recorded immediately after the RFA procedure, along with radiologic reports and US images of percutaneous RFA in which CEUS was used prior to RFA for localizing the index tumor. During the period from June 2008 to July 2011, a total of 87 consecutive patients with liver tumors, who underwent CEUS for RFA due to poor sonographic conspicuity, were found in this study (Fig. 1). Among them, 5 patients were excluded for the following reasons: 1) tumors showing atypical enhancement pattern of HCCs on both CT and MRI (n = 4) and 2) metastasis from colon cancer (n = 1). Consequently, a total of 82 consecutive patients were included in this study and their baseline characteristics are summarized in Table 1. Although all HCCs were ablated in the same RFA session for patients with multiple HCCs, only one index tumor with poor sonographic conspicuity was included and evaluated in this study because tumors with good sonographic conspicuity were out of our interest. Therefore, 82 HCCs (mean size ± SD, 1.2 ± 0.4 cm; range, 0.5-2.2 cm) in 82 patients were included (Table 2).
Fig. 1.
Flowchart of this study.
Table 1.
Demographics of 82 Patients
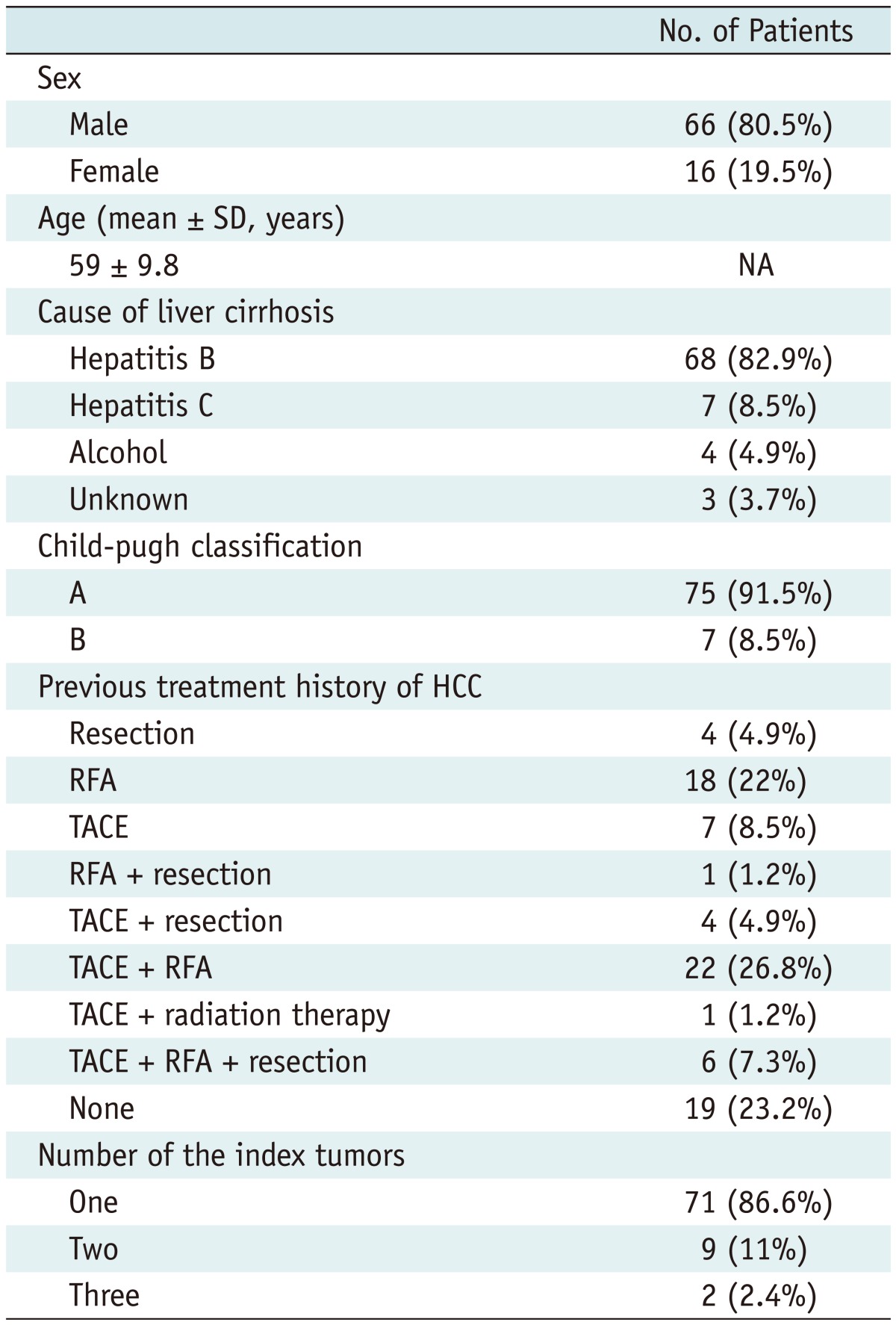
Note.- HCC = hepatocellular carcinoma, RFA = radiofrequency ablation, TACE = transcatheter arterial chemoembolization, NA = not applicable
Table 2.
Tumor Characteristics of 82 Patients
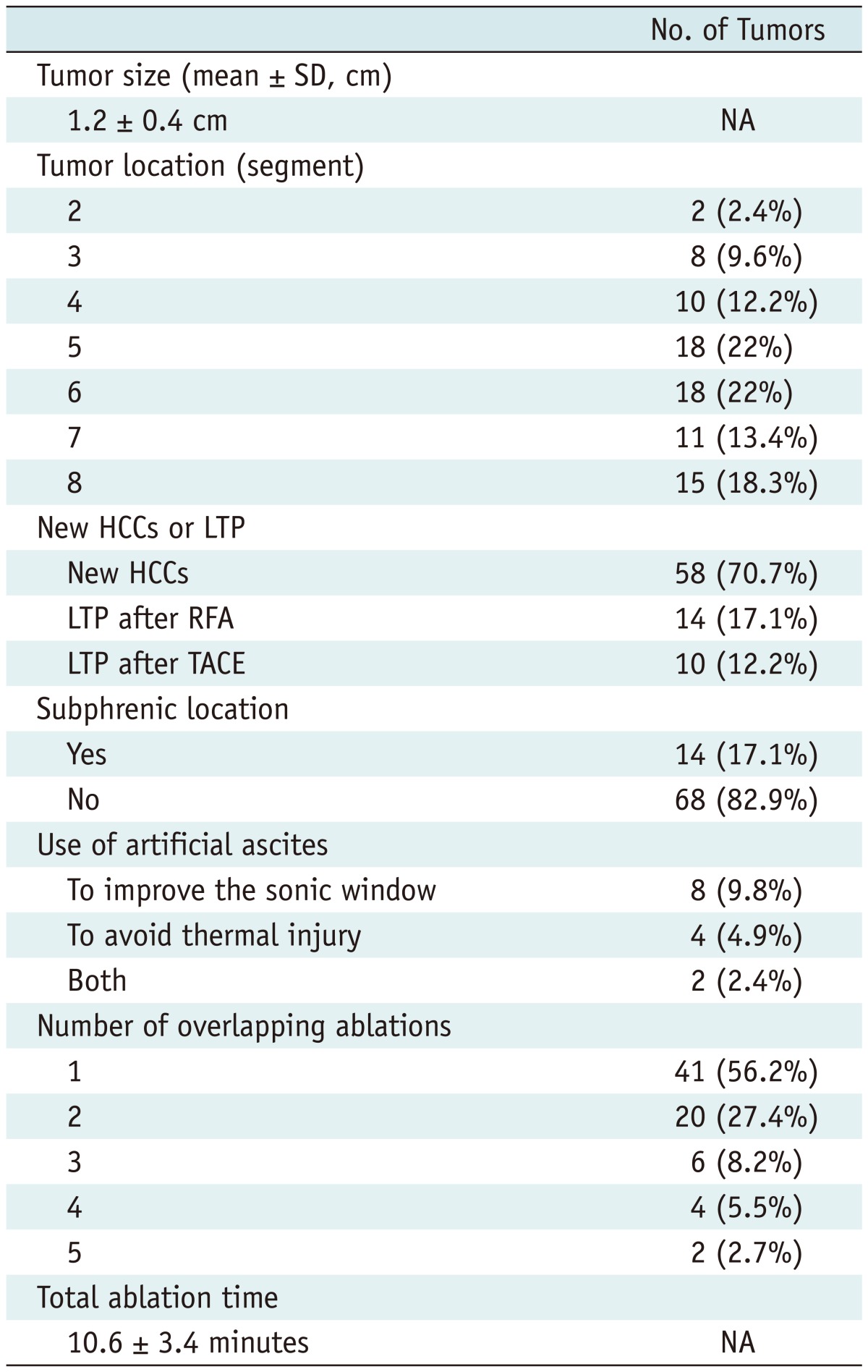
Note.- HCC = hepatocellular carcinoma, LTP = local tumor progression, RFA = radiofrequency ablation, TACE = transcatheter arterial chemoembolization, NA = not applicable
The inclusion criteria for percutaneous RFA in our institution were as follows: a single tumor ≤ 5 cm in the longest diameter, multinodular tumors (≤ 3) with each tumor ≤ 3 cm in the longest diameter, Child-Pugh class A or B, no portal vein thrombosis or extrahepatic metastasis, a prothrombin time ratio of > 50%, and a platelet count > 50000 cells/mm3 (50 cells × 109/L).
The diagnosis of HCC was based on the typical imaging features (arterial enhancement followed by portal or delayed washout) of the dynamic contrast-enhanced CT and/or MRI (12). A nodule < 1 cm was also considered to be an HCC if it showed the typical imaging features on the CT and/or MRI (13, 14).
CT and MR Imaging
Contrast-enhanced three-phase liver CT or gadoxetic acid disodium-enhanced MR images were used for image fusion. A 64-detector CT scanner (LightSpeed VCT; GE Healthcare) and a 3.0-T whole-body MR system (Intera Achieva 3.0-T; Philips Healthcare, Best, the Netherlands) with a 16-channel phased-array coil were also used.
Planning US
Prior to the RFA procedure, planning US for RFA of the HCCs was performed by one of four radiologists in order to determine the feasibility of percutaneous RFA. The radiologists were certificated, experienced abdominal radiologists who each had more than 4 years of experience and more than 200 cases of RFA procedures at the starting point of this study. All planning US were performed using an Acuson Sequoia 512 system with a multi-frequency 4C1 convex array probe (Siemens Medical Solutions, Mountain View, CA, USA). At the time of planning US, the radiologist was aware of the patients' clinical information and liver CT and MR images. After a careful evaluation of the liver CT and MR images, the radiologist carefully searched for the HCC nodule. The radiologists graded the conspicuity score using a 4-point scale: 1, definitely identifiable, highly confident for identifying the index tumor; 2, identifiable, confident for identifying the index tumor; 3, probably identifiable, but less confident due to poor lesion conspicuity; and 4, definitely unidentifiable (15). Tumors with a conspicuity score 3 were considered to have the risk of incomplete ablation due to the difficulty of differentiating the index tumor from the surrounding cirrhosis-related pseudolesions; thus, patients with such tumors were scheduled for CEUS before initiating the RFA procedures.
Pretreatment Contrast-Enhanced Ultrasonography
All CEUS were performed using the Acuson Sequoia 512 system with a multi-frequency 4C1 convex array probe immediately prior to RFA. We used a second-generation contrast agent named SonoVue. A vial of the contrast agent was divided into two doses of 2.4 mL each. The first dose was injected intravenously for the pretreatment CEUS and was immediately followed by a 10 mL normal saline flush. Imaging was performed in a split-screen mode, which displayed the CEUS image on the left side and the background B-mode US image on the right side on a single monitor. The mechanical index (MI) was 0.21.
Immediately after the injection, the radiologist searched for a lesion with arterial enhancement and/or portal or delayed washout at the hepatic region, where a HCC candidate was identified on planning US. If the index tumor was not identified within 5 min, the injected microbubbles were disrupted by imaging the liver in the high mechanical index mode for several minutes; then, the pretreatment CEUS was repeated with the second dose in order to detect the index tumor. If the second CEUS also did not reveal any index tumor, RFA was not performed. Such cases were counted as technical failures. However, when the index tumor was localized on CEUS, the radiologist recorded the enhancement pattern (arterial enhancement and/or portal or delayed washout) of the index tumor and determined whether the location of HCC candidates on planning US coincide with that on CEUS. When the location of HCC candidate was concordant between conventional US and CEUS, it was defined as concordant HCC, whereas if not, it was defined as discordant HCC.
RFA Procedure
Immediately after CEUS, all percutaneous RFA procedures were performed for tumors that were localized by CEUS by one of the four radiologists. They each had more than 20 cases of experience with CEUS for focal hepatic lesions at the starting point of this study.
For local anesthesia, 2% lidocaine hydrochloride (Huons Lidocaine HCI INJ; Hwaseong, Korea) was injected at the puncture site. In addition, for pain control, 50 mg of pethidine hydrochloride (Pethidine, Samsung Pharmaceutical, Seoul, Korea) mixed with 50 mL of 5% dextrose in water was dripped continuously by intravenous infusion. If needed, 50-100 µg of fentanyl citrate (Fentanyl Citrate Gu Ju INJ; GUJU Pharma, Seoul, Korea) mixed with 20 mL of normal saline was injected intravenously.
All RFA procedures were performed using a free-hand technique with an internally cooled electrode (Adjustable Type [Proteus RF Electrode; STARmed, Goyang, Korea] or Non-Adjustable Type [Well-point RF Electrode; STARmed, Gyeonggi-do, Korea]) with a 200 W generator (VIVA RF System; STARmed, Gyeonggi-do, Korea). The radiologists inserted an electrode under B-mode US guidance after the injected microbubbles were disrupted. CEUS was performed for pretreatment localization of the index tumor and was not performed for real-time guidance of an electrode placement. The algorithm for energy deposition followed the manufacturer's instructions for each device. Multiple overlapping ablations were applied where it was needed in order to achieve an adequate ablative margin of at least 0.5 cm. Artificial ascites was introduced prior to the RFA procedure whenever necessary in order to improve the sonic window or to decrease the level of thermal injury to the adjacent diaphragm or the colon (16, 17). At the end of the RFA procedure, the electrode path was cauterized to prevent bleeding or tumor seeding during retraction of the electrode.
Analysis on Therapeutic Efficacy
Immediate post-RFA evaluation was performed not by CEUS, but by contrast-enhanced CT scan. All patients were transferred to CT rooms immediately after the RFA procedure. All liver CT protocols were the same as for imaging prior to RFA. The radiologist who performed the RFA procedure also evaluated the CT images in order to determine the therapeutic response and occurrence of any immediate complications. When an unablated residual tumor was identified at the immediate follow-up CT examinations, additional RFA was performed the day after the procedure. The patients were then followed up with contrast-enhanced CT one month later and then every three months after treatment. The overall follow-up time was defined as the interval between the first RFA and either local tumor progression or the last follow-up visit by March 31, 2012. Patients who underwent liver transplantation during the follow-up period were censored.
Therapeutic efficacy of RFA was assessed according to the proposal for standardization of terms and the reporting criteria of image-guided tumor ablation (18). Technical success was assessed based on the immediate post-RFA CT images. Technique effectiveness was documented based on the one-month follow-up CT scan. A follow-up CT scan showing an enhanced area or enlargements at the margins of the treated tumors was determined to be local tumor progression. The rate of local tumor progression was compared between new HCCs and HCCs with local tumor progressions. It was also compared between concordant HCCs and discordant HCCs. The cumulative rate of local tumor progression was estimated with the use of the Kaplan-Meier method.
RESULTS
Pretreatment CEUS
The time interval between the last CT/MR and CEUS was 21.3 ± 12.4 days (range, 0-50 days). In 10 patients, pretreatment CEUS was repeated with the second dose in order to search for the index tumor, because the index tumors were not identifiable with the first dose injection. Among them, in one patient, the index tumor was found to be localized at the same site of planning US and was successfully ablated. However, tumor localization failed in the remaining nine HCCs (1.1 ± 0.2 cm; range, 0.8-1.5 cm) even after the second dose injection. Therefore, of the 82 HCCs with poor conspicuity on conventional US, 73 (89.0%) HCCs were localized on CEUS, whereas 9 (11.0%) were not, resulting in 11.0% technical failure of CEUS. Of 73 identifiable HCCs on CEUS, the location of HCC on planning US corresponded with that on CEUS in 64 (87.7%) HCCs and did not in 9 (12.3%) HCCs (Figs. 2, 3). Of 73 HCCs identifiable on CEUS, 69 (94.5%) HCCs showed arterial enhancement after contrast injection, whereas 4 (5.5%) lesions did not. These 4 lesions were also considered as index tumors because portal or delayed washout was found in these lesions, and their locations corresponded with that on planning US. Thus, RFA was performed not only for 69 HCCs which showed arterial enhancement, but also for these 4 tumors which demonstrated atypical enhancement patterns.
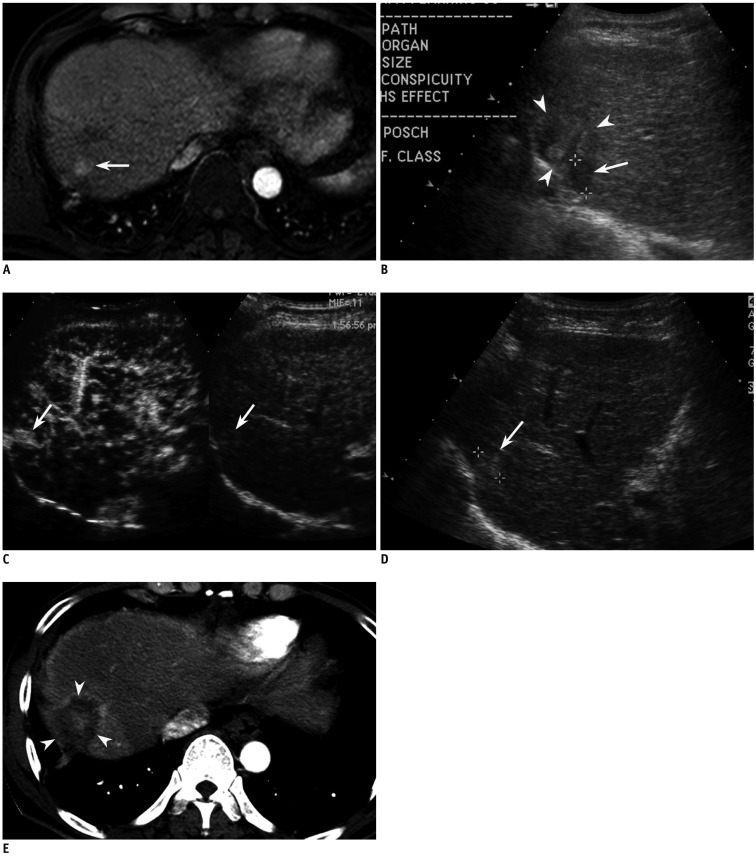
Fig. 2.
Fifty eight-year-old man with 1.6-cm-sized hepatocellular carcinoma (HCC) in segment VII of liver. This patient has history of hepatic resection and chemoembolization due to HCCs.
A. Arterial phase MR image (TR/TE, 3.1/1.5; flip angle, 10°; matrix size, 228 × 211; bandwidth, 724.1 Hz/pixel) shows 1.6-cm-sized enhancing lesion (arrow) in segment VII of liver. B. Small HCC candidate is seen as low echoic nodule (arrow) in segment VII of liver on planning ultrasonography (US). However, surrounding liver (arrowheads) has heterogeneous echo texture. Therefore, it is unclear whether lesion (arrow) is true index tumor. C. On CEUS, which was conducted to preclude mistargeting, small enhancing nodule (arrows) was identified in different location from that on planning US. Therefore, lesion identified on planning US was considered to be pseudolesion. D. Re-evaluation with conventional B-mode US reveals small echogenic nodule (arrow) at same site as (C). E. Arterial phase CT image obtained immediately after RFA shows technical success with sufficient ablative margin (arrowheads).
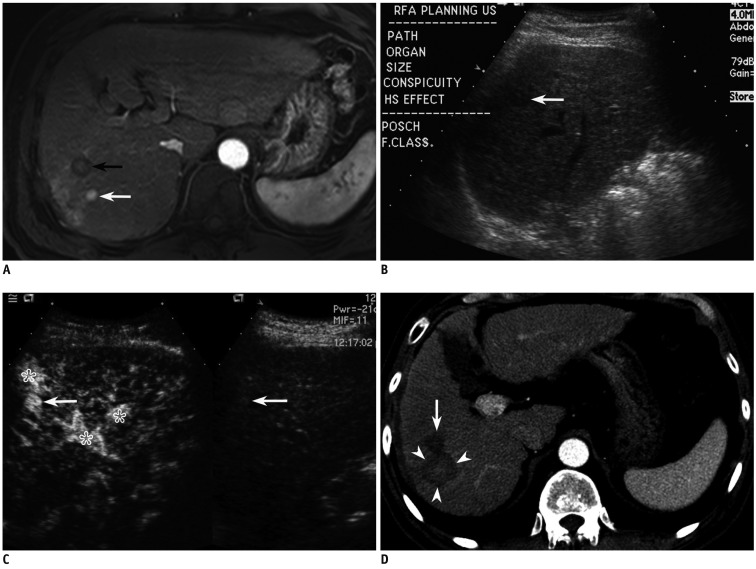
Fig. 3.
Fifty nine-year-old man with 1 cm-sized hepatocellular carcinoma (HCC) in segment VII of liver.
A. Arterial phase MR image (TR/TE, 3.1/1.5; flip angle, 10°; matrix size, 228 × 211; bandwidth, 724.1 Hz/pixel) shows 1.1 cm-sized enhancing HCC (white arrow) in segment VII of liver. Black arrow indicates previous radiofrequency ablation (RFA) zone. B. Small HCC candidate is seen as subtle low echoic nodule (arrow) with poor conspicuity in segment VII of liver on planning ultrasonography. C. On CEUS, which was performed to be certain, small enhancing nodule (arrow) was identified at same site as (B), suggestive of HCC. Asterisks indicate arterioportal shunts around index tumor. D. Arterial phase CT image obtained immediately after RFA shows large ablation zone (arrowheads) with sufficient ablative margin. Arrow indicates previous RFA zone.
Therapeutic Outcome
Of 73 HCCs ablated after localization of CEUS, residual viable tumor was identified in one patient on immediate post-RFA CT examination; thus, a second RFA session was performed the day after the first procedure. Technical success was achieved for all 73 identifiable HCCs in a single (n = 72) or two (n = 1) RFA sessions. For 9 (12.3%) of 73 HCCs in which the location of HCC candidate on planning US was discordant with that on CEUS, the technical success was also achieved in a single RFA session. Technique effectiveness was achieved in 72 of 72 patients (100%), except for one patient who was lost to follow-up. There were no major RFA-related complications or mortality.
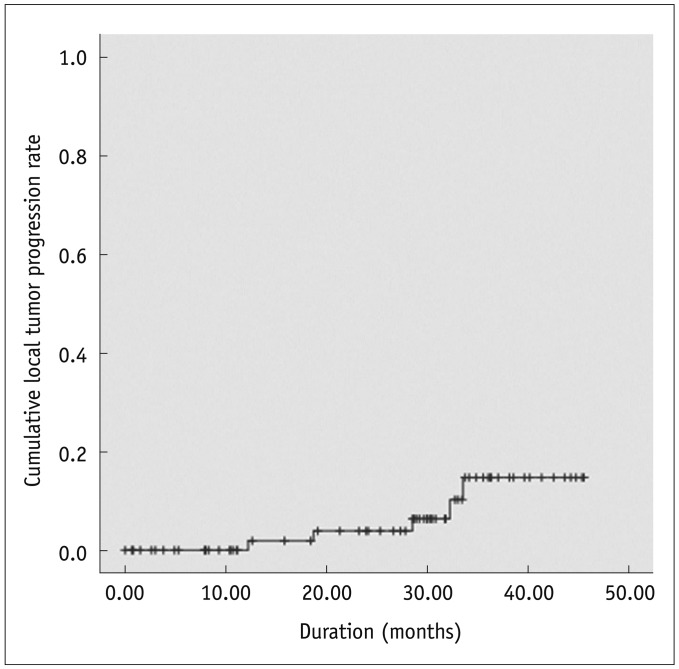
Local tumor progression was found in 5 (6.8%) of 73 HCCs during the follow-up period (mean ± SD, 24.1 ± 13.4 months; median, 28.5 months; and range, 0.0-45.5 months). The size of these 5 HCCs ranged from 1.5 cm to 2.0 cm (mean ± SD, 1.7 ± 0.2 cm). The rate of local tumor progression was not significantly different between new HCCs (5.2%, 3/58) and local tumor progressions (8.3%, 2/24) (p = 0.63). It was also not significantly different between concordant HCCs (6.3%, 4/64) and discordant HCCs (11.1%, 1/9) (p = 0.49). Cumulative rates of local tumor progression were estimated as 1.9% and 15.4% at 1 and 3 years, respectively (Fig. 4).
Fig. 4.
Local tumor progression rate of 73 hepatocellular carcinomas after percutaneous radiofrequency ablation.
Cumulative rates of local tumor progression were estimated as 1.9% and 15.4% at 1 and 3 years, respectively. '+' marks indicate censored data.
DISCUSSION
Ultrasonography-guided RFA of small HCCs with poor sonographic conspicuity can be challenging due to the risk of mistargeting or incomplete ablation (8). Therefore, in this study, we used CEUS prior to percutaneous RFA in order to achieve technical success for HCCs with poor sonographic conspicuity (conspicuity score 3). This study demonstrated that CEUS improved the therapeutic efficacy of percutaneous RFA for HCCs that were not clearly identified on conventional US, because CEUS allowed the operators to accurately localize and target the tumor with high confidence. In this study, 89% (73/82) HCCs with poor sonographic conspicuity were successfully ablated with percutaneous US-guided RFA with the aid of pretreatment CEUS. Moreover, 12.3% (9/73) of HCC candidates detected on planning US were found to be pseudolesions based on the findings of CEUS. Therefore, mistargeting and/or incomplete ablation were avoided in these 9 patients.
However, localization failure of CEUS was noted in 9 (11.0%) of 82 HCCs, even though the second dose was injected. This result seems to be unsatisfactory compared to a recent study by Liu et al. (19), which used CEUS using SonoVue for percutaneous microwave ablation of HCCs; as a result, localization failure was noted in 2 (1.9%) of 107 HCCs. This dissimilarity can be explained by the difference of the tumor size (this study: 1.2 ± 0.4 cm; range, 0.5-2.2 cm vs. the earlier study: 1.95 ± 0.85 cm; range, 0.8-4.4 cm). Given that smaller HCCs have a tendency of poor sonographic conspicuity (4), localization failure of CEUS for 9 small HCCs (1.1 ± 0.2 cm; range, 0.8-1.5 cm) in the current study may be within the acceptable range.
In terms of targeting HCC under CEUS guidance, realtime targeting has been used to facilitate accurate needle positioning (20). However, in this study, instead of real-time targeting, we used CEUS only for pretreatment localization of the index tumor for the following reasons: First, CEUS only provides a short temporal window of the arterial phase, making it difficult for us to insert an electrode to small tumors. In general, a cirrhotic liver usually has portal hypertension, thus decreasing the portal inflow but increasing the arterial inflow. Consequently, during the arterial phase, small arterial enhancing HCCs are likely to be obscured soon because the surrounding liver is also enhanced earlier by the dominant arterial inflow in patients with liver cirrhosis. This phenomenon was clearly described in a recent study in which the arrival time parametric imaging using CEUS was well correlated with the degree of hepatic fibrosis (21). Second, poor conspicuity of an electrode under CEUS guidance using a low MI technique makes real-time targeting technically difficult. Based on our experience, the location and direction of an electrode using low MI technique is not as clear as to that of conventional B mode US. Third, even though a guiding device attached to an US probe is used, the real electrode path may differ from the expected path during the targeting process due to the stiffness of the liver and breathing motions of patients. Therefore, all interventional radiologists in our institution have preferred a free-hand technique rather than a guiding device. Hence, they inserted an electrode into the tumor using the free hand technique under B-mode US guidance after the injected microbubbles were disrupted. Meanwhile, we did not perform pre-treatment CEUS for HCCs which were totally invisible on B-mode US. Since these tumors have poor sonographic conspicuity on B mode US, it would still be difficult for us to insert the electrode into the tumors on B-mode US even though the index tumors were successfully localized on CEUS. In this situation, real-time targeting using the guiding device during the arterial phase of CEUS may be better than targeting using the free hand technique on B-mode US after the disruption of the injected microbubbles.
In terms of the treatment outcome of CEUS-guided RFA of HCC, unlike Sonazoid (Daiichi-Sankyo, Tokyo, Japan), there are few data regarding the results of SonoVue-enhanced US-guided RFA. Compared to Liu et al.'s (19) study in which SonoVue was used as a contrast agent, the rate of local tumor progression was not significantly different between the present study (6.8%, 5/73) and the earlier study (1.9%, 2/105) (p = 0.1247, Fisher's exact test). Although a direct comparison of therapeutic outcome between the present study and the earlier study is difficult due to ablation modality (radiofrequency vs. microwave), guidance method (B-mode guidance after pretreatment CEUS vs. CEUS guidance), and immediate post-RFA assessment (CT vs. CEUS), the mean number of treatment sessions, follow-up period, and patient population are different between each other. B-mode US guidance after pretreatment evaluation using CEUS is likely to be comparable to the real-time targeting under CEUS guidance.
Recently, CEUS guidance using Sonazoid has been widely used for local ablation therapy of liver tumors and has also shown promising results (10, 22). Unlike other contrast agents, Sonazoid offers both vascular and post-vascular phases. Post-vascular phase, which is also called Kupffer phase, is typically presented 10 min after the injection of Sonazoid. Hepatic lesions, such as HCCs that contain few Kupffer cells, are clearly delineated as contrast defects surrounded by enhanced normal hepatic parenchyma on the post-vascular phase (23, 24). Since the post-vascular phase provides a long temporal window, targeting can be easily performed at the post-vascular phase (10, 22). Therefore, Sonazoid is likely to be more useful for the guidance of RFA than other US contrast agents. However, until now, a comparison study between Sonazoid- and SonoVue-guided RFA is not available.
This study had some limitations. First, this study may suffer from patient selection bias. Determining the lesion conspicuity classification of a HCC candidate on planning US was not done from an observers' agreement but by a decision from a single radiologist, which might have been subjective and biased. Second, the lack of biopsy proof is a limitation, particularly for subcentimeter sized HCCs. Although lesions < 1 cm can be diagnosed as HCC according to a practice guideline (13), imaging-based diagnosis of subcentimeter-sized HCC is not yet widely accepted in most practice guidelines (1-3). Third, this was a single arm study with no control group. We did not compare our results of RFA using CEUS with those of conventional RFA in the historical control. However, even if a control group is determined after matching the tumor size, it would be biased because the lesion conspicuity of the historical control group would be better than that of the case group. This was because we began to ablate small HCCs with poor sonographic conspicuity after the introduction of SonoVue, which would otherwise not have been ablated. Therefore, it was very difficult to define an appropriate control group. Nevertheless, the high technical success rate mostly after a single RFA session with an acceptable rate of local tumor progression in this study reflects that pretreatment CEUS using SonoVue is useful for RFA of HCCs with poor sonographic conspicuity. Fourth, using CEUS as a reference standard for HCC localization on US may be another limitation. In this study, however, the number of overlapping ablations was 1.7 ± 1.0, and only 6 (8.2%) out of 73 lesions were ablated using more than 3 overlapping treatments. Therefore, the probability of the index tumor incidentally being covered within the ablation zone would not be high. Fifth, although not analyzed, artificial ascites might have improved the sonographic conspicuity of the 10 HCCs, in which artificial ascites were introduced in order to enhance the sonic window. In these 10 patients, artificial ascites might have induced a synergistic effect along with CEUS for localizing the index tumor.
In conclusion, pretreatment evaluation with CEUS is effective for percutaneous RFA of HCCs with poor conspicuity on conventional US. This allows us to identify true HCCs by differentiating the index tumor from the surrounding cirrhosis-related pseudolesions. Therefore, it can increase the operator's confidence and the accuracy of the procedures, and ultimately, result in an increased rate of technical success.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010;194:W396–W400. doi: 10.2214/AJR.09.3171. [DOI] [PubMed] [Google Scholar]
- 5.Meloni MF, Goldberg SN, Livraghi T, Calliada F, Ricci P, Rossi M, et al. Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrastenhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. AJR Am J Roentgenol. 2001;177:375–380. doi: 10.2214/ajr.177.2.1770375. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 7.Min JH, Lee MW, Rhim H, Choi D, Kim YS, Kim YJ, et al. Recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: planning sonography for radio frequency ablation. J Ultrasound Med. 2011;30:617–624. doi: 10.7863/jum.2011.30.5.617. [DOI] [PubMed] [Google Scholar]
- 8.Lee MW, Lim HK, Kim YJ, Choi D, Kim YS, Lee WJ, et al. Percutaneous sonographically guided radio frequency ablation of hepatocellular carcinoma: causes of mistargeting and factors affecting the feasibility of a second ablation session. J Ultrasound Med. 2011;30:607–615. doi: 10.7863/jum.2011.30.5.607. [DOI] [PubMed] [Google Scholar]
- 9.Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Shiozaki H. Treatment of hepatocellular carcinoma with percutaneous radiofrequency ablation: usefulness of contrast harmonic sonography for lesions poorly defined with B-mode sonography. AJR Am J Roentgenol. 2004;183:153–156. doi: 10.2214/ajr.183.1.1830153. [DOI] [PubMed] [Google Scholar]
- 10.Masuzaki R, Shiina S, Tateishi R, Yoshida H, Goto E, Sugioka Y, et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:759–764. doi: 10.1111/j.1440-1746.2010.06559.x. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010;78(Suppl 1):40–45. doi: 10.1159/000315229. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 13.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 15.Rhim H, Choi D, Kim YS, Lim HK, Choe BK. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol. 2010;75:253–258. doi: 10.1016/j.ejrad.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 17.Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging. 2009;34:371–380. doi: 10.1007/s00261-008-9408-4. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S377–S390. doi: 10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Yu X, Liang P, Cheng Z, Han Z, Dong B. Contrastenhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperthermia. 2011;27:555–562. doi: 10.3109/02656736.2011.564262. [DOI] [PubMed] [Google Scholar]
- 20.Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51(Suppl):S19–S23. doi: 10.1016/j.ejrad.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Wakui N, Takayama R, Kanekawa T, Ichimori M, Otsuka T, Shinohara M, et al. Usefulness of arrival time parametric imaging in evaluating the degree of liver disease progression in chronic hepatitis C infection. J Ultrasound Med. 2012;31:373–382. doi: 10.7863/jum.2012.31.3.373. [DOI] [PubMed] [Google Scholar]
- 22.Minami Y, Kudo M, Hatanaka K, Kitai S, Inoue T, Hagiwara S, et al. Radiofrequency ablation guided by contrast harmonic sonography using perfluorocarbon microbubbles (Sonazoid) for hepatic malignancies: an initial experience. Liver Int. 2010;30:759–764. doi: 10.1111/j.1478-3231.2010.02226.x. [DOI] [PubMed] [Google Scholar]
- 23.Moriyasu F, Itoh K. Efficacy of perflubutane microbubbleenhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2009;193:86–95. doi: 10.2214/AJR.08.1618. [DOI] [PubMed] [Google Scholar]
- 24.Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, et al. Differential diagnosis of hepatic tumors: value of contrastenhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology. 2008;51(Suppl 1):61–69. doi: 10.1159/000122600. [DOI] [PubMed] [Google Scholar]