Abstract
Purpose of review
We review recently published literature concerning early morbidity and mortality during antiretroviral therapy (ART) among patients in resource-limited settings. We focus on articles providing insights into this burden of disease and strategies to address it.
Recent findings
In sub-Saharan Africa mortality rates during the first year of ART are very high (8%-26%), with most deaths occurring in the first few months. This compares to 3%-13% in programmes in Latin America and the Caribbean and 11%-13% in South-East Asia. Risk factors generally reflect late presentation with advanced symptomatic disease. Key causes of morbidity and mortality include tuberculosis, acute sepsis, cryptococcal meningitis, malignancy, wasting syndrome/chronic diarrhoea. Current literature shows the fundamental need is for much earlier HIV diagnosis and initiation of ART. In addition, further studies provide data on the role of screening and prophylaxis against opportunistic diseases (particularly TB, bacterial sepsis and cryptococcal disease) and the management of specific opportunistic diseases and complications of ART. Effective and sustainable delivery of these interventions requires strengthening of programmes.
Summary
Strategies to address this disease burden should include earlier HIV diagnosis and ART initiation, screening and prophylaxis for opportunistic infections, optimised management of specific diseases and treatment complications, and programme strengthening.
Keywords: HIV, antiretroviral, mortality, death, morbidity, resource-limited, low-income
Introduction
In 2007 an estimated 33 million people worldwide were living with HIV and 2.0 million died [1]. Over 90% of infected people were in resource-limited settings, with sub-Saharan Africa accounting for 22.0 million of these and South & South-East Asia, Latin America and East & Central Europe accounting for a further 4.2 million, 1.7 million and 1.5 million people, respectively [1]. Following the commitment of United Nations member states to provide universal access to prevention, treatment, care and support by 2010, substantial progress has been made in scaling up antiretroviral treatment (ART) [2]. By the end of 2008, between 3.8 and 4.3 million people in resource-limited settings were estimated to have started ART, with sub-Saharan Africa accounting for approximately 3.0 million of these [3].
One of the key clinical and programmatic challenges to scale-up of ART in resource-limited settings is the very high rates of mortality and morbidity within the first year of treatment [4,5**,6**]. In this article, we review recent literature concerning this issue in adult patients. A large proportion of the literature arises from studies conducted in sub-Saharan Africa which has borne the brunt of the epidemic. However, we acknowledge that there is likely to be considerable geographical variation in the magnitude, causes and potential solutions to this problem.
Mortality
Considerable literature has now accumulated concerning the rates, risk factors and temporal distribution of mortality in ART programmes, but data on causes of death are comparatively scarce.
Mortality risk
A study published in 2006 by the Antiretroviral Therapy in Low Income Countries (ART-LINC) Collaboration was one of the first studies to highlight the disproportionately high mortality of patients treated in resource-limited settings compared to those treated in high-income settings [4]. Despite similar immunological and virological responses to ART, patients treated in low-income settings had a several-fold greater risk of death in the initial months of treatment, even after adjustment for baseline characteristics [4].
A subsequent review of data from sub-Saharan Africa included 18 ART cohort studies published between 2002 and 2008 [5**]. These cohorts had median baseline CD4 cell counts ranging between 43 and 147 cells/μL and represented almost 40,000 patients. Substantial mortality accrued in each of these cohorts; Kaplan-Meier estimates ranged between 8% and 26% during the first year of ART and only two cohorts reported estimates of <10%. These high rates may even be underestimates as a recent meta-analysis confirms earlier reports that in some cohorts in sub-Saharan Africa a substantial proportion of patients initially classified as ‘lost to follow-up’ have actually died [7].
Tuboi and colleagues reported on mortality in 7 ART sites in Latin America and the Caribbean [6**]. Median baseline CD4 cell counts in these cohorts ranged from 79 cells/μL to 163 cells/μL. Overall mortality at one year was 8.3% (95%CI, 7.6-9.1%), but ranged from 2.6% to 13.0% [6**]. In South-East Asia, the national ART programme in Thailand treated over 58,000 patients in 839 health care facilities between 2002 and 2007 and reported a one year mortality of 11% [8**]. This is similar to a rate of 13% in a single cohort in neighbouring Cambodia [9]. Thus, mortality rates in ART programmes in resource-limited settings are higher than in industrialised countries, but are heterogeneouswith generally much higher rates in sub-Saharan Africa compared to other regions [5**].
Temporal distribution of mortality
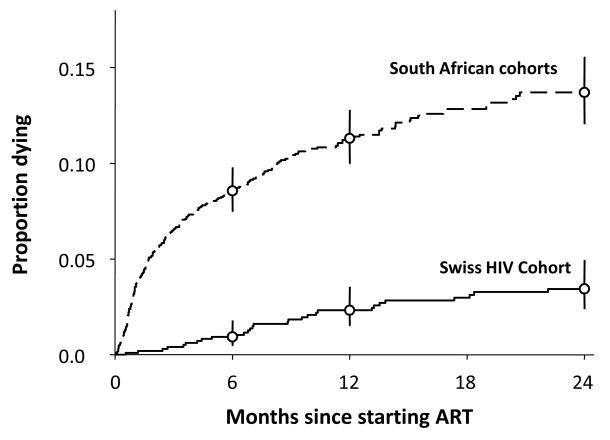
In most cohorts mortality risk is substantially higher in the first few months of ART and especially in those with the greatest overall cumulative mortality [5**, 6**, 8**]. This is illustrated by comparison of mortality risk in cohorts in South Africa and Switzerland (Figure 1) [10]. Compared to the Swiss cohort, those treated in South Africa had more advanced immunodeficiency at baseline and yet similar virological and immunological responses to ART. It is clearly seen that the great disparity in mortality risk was particularly marked within the first 3-6 months of treatment (Figure 1).
Figure 1.
Kaplan Meier plots comparing the cumulative mortality during ART in South African (Gugulethu and Khayelitsha) cohorts and the Swiss HIV Cohort Study. Baseline CD4 cell counts were 80 cells/μL and 204 cells/ μL, respectively. Graph reproduced from [10].
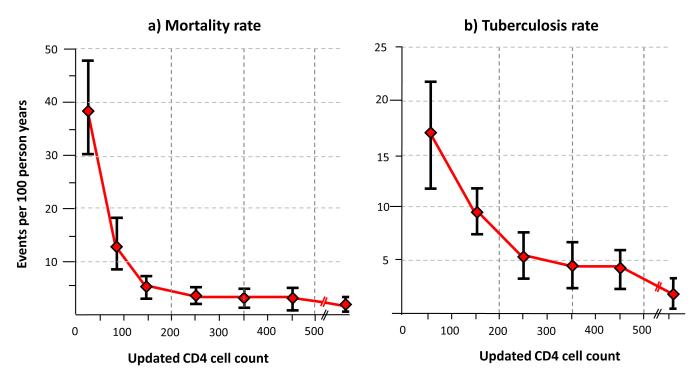
The immunological response to ART is the key modifiable variable that is likely to explain temporal changes in mortality risk [11**]. In a study from South Africa, changes in CD4 cell counts measured every 4 months during ART were directly related to changes in mortality risk (Figure 2a). Mortality rates decreased rapidly as CD4 cell counts increased during early ART. However, once CD4 cell counts exceeded a threshold of 200 cells/μL, no further significant reductions in mortality were observed during ongoing CD4 count recovery [11**]. Those with poor CD4 cell recovery, however, remained at high mortality risk.
Figure 2.
Graphs showing (a) mortality rates and (b) tuberculosis (TB) incidence rates (95% confidence intervals, deaths per 100 person-years) plotted against CD4 counts measured at baseline and updated every 4-months during ART (updated CD4 counts). As CD4 cell counts increased, the mortality rate is seen to fall very steeply. Above a CD4 count threshold of 200 cells/μL, however, no further significant reductions occurred with further CD4 cell count recovery. TB rates similarly decreased with increasing CD4 cell counts, but substantial rates persisted at CD4 counts of 200-500 cells/ μL but significantly decreased above a threshold of 500 cells/μL. Data adapted from [11**] and [12**].
Mortality risk factors
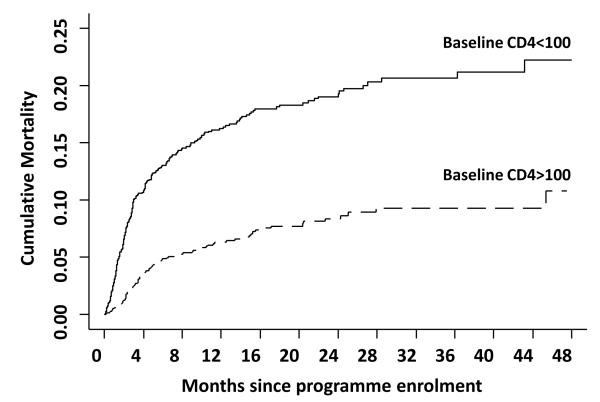
Baseline risk factors for death are generally indicative of advanced immunodeficiency – low blood CD4 cell counts, advanced WHO clinical stage of disease, low body mass index and anaemia [4,5**,6**, 8**, 13]. Patients enrolling in a South African ART programme with CD4 cell counts <100 cells/μL had approximately twice the cumulative mortality risk of patients enrolling with CD4 counts >100 cells/ μL (Figure 3). A key overall conclusion of these studies is the need to provide ART to patients earlier in the disease course. Confirming the findings of previous publications [5**], recent studies have also specifically highlighted men as having higher risk of early mortality compared to women, which is only in part explained by late presentation [8**, 14, 15].
Figure 3.
Kaplan Meier plot showing the cumulative mortality in the Gugulethu cohort in groups of patients with baseline CD4 cell counts <100 cells/μL or >100 cells/μL from the time of enrolment into the programme (includes mortality accruing in one month pre-ART period plus mortality on ART). Data adapted from [11**].
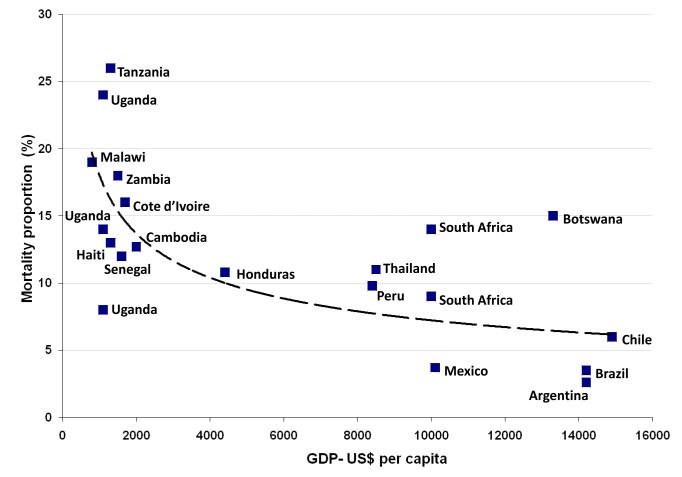
The need to pay for treatment is another strong predictor of poor treatment outcomes and it is clear that provision of free treatment is essential [4, 16, 17]. Macro-economic factors may also be important, with lower national gross domestic product (GDP) per capita being broadly associated with poorer treatment outcomes (Figure 4). During the initial months of ART in patients with advanced immunodeficiency, medical management can be complex and resource-intensive. Thus, mortality rates may in part be higher in resource-constrained environments as a result of limitations in healthcare provision.
Figure 4.
Graph showing mortality after one year of ART for 21 cohorts plotted against the Gross Domestic Product (GDP) per capita (in US dollars) for the countries represented. Cohorts included are from countries in sub-Saharan Africa, Latin America and the Caribbean and South East Asia from references [5**, 6**, 8**, 18]. The countries from which the data are derived are indicated and some are represented by more than one cohort. Mortality risk is very heterogeneous, but cohorts in countries with lower GDP tend to be associated with higher mortality risk.
Causes of death
Very few data are available concerning the key causes of death during early ART and these data are largely based on clinical assessments rather than post-mortem examinations. Comprehensive studies of the causes of death in different geographical regions are needed. In cohort studies from sub-Saharan Africa, the most commonly identified causes of death were tuberculosis (TB), cryptococcal meningitis, acute sepsis, Kaposi’s sarcoma (KS) and wasting syndrome / chronic diarrheoa [5**,19*]. In Haiti wasting syndrome was by far the commonest cause [20, 21]. In India, most deaths were reported as being due to TB, Pneumocystis jirovecii pneumonia, cerebral toxoplasmosis and cerebrovascular disease [22].
Patients with prevalent TB at ART initiation have high mortality risk [23-26]. However, a study from South Africa suggested that prevalent TB was not an independent risk factor for death but might simply reflect an association with advanced immunodeficiency in that setting [27*]. While this is consistent with data from other African cohorts [28, 29], these analyses may fail to account for deaths occurring very early during TB treatment prior to ART initiation and also for the high burden of TB in this patient group that remains unascertained [30].
Consistent with previous data from Africa [23, 24], a study from Haiti found that patients who develop TB during the first 3 months of ART have high (27%) mortality risk during the first year of ART [25]. This study also reported that the mortality risk of such patients greatly exceeded that of a comparator group consisting of pooled data from patients with either prevalent TB at baseline or incident TB presenting after 3 months of ART. Selection and survival biases, however, may in part account for this apparent difference [31].
It has been speculated that immune reconstitution disease might be associated with increased mortality risk although it should be borne in mind that this complication develops in those with the most advanced immunodeficiency who have high pre-existing risk. Overall mortality associated with TB immune reconstitution disease, however, appears to be low [19*,32]. Even among patients with involvement of the central nervous system, just 3 of 23 (13%) of patients died during 6 months follow-up in a recent study from South Africa, although some were lost to follow-up [33]. In Uganda where KS-associated immune reconstitution disease is relatively frequent, it is rarely life-threatening [34]. Cryptococcal meningitis and cryptococcal immune reconstitution disease have been highlighted as important causes of mortality [19*,35-38]. Cryptococcal antigenaemia present at baseline has been found to be an independent risk factor for mortality in a study from South Africa [39*], potentially providing a means to direct pre-emptive treatment.
Morbidity
Key causes of serious morbidity are likely to largely reflect the causes of death described above and are likely to vary substantially between geographical regions. Detailed studies of morbidity during ART, however, are relatively few with the exception of TB.
Bacterial sepsis
In a West African study cohort with a median baseline CD4 cell count of 252 cells/μL, the two key causes of severe morbidity were identified as TB and invasive bacterial infections [40]. Bacterial diseases were predominantly episodes of pneumonia, bacteraemia, enteritis and pyelonephritis and the most commonly isolated pathogens were Streptococcus pneumoniae, non-typhoidal Salmonella and Escherichia coli [40]. Gastrointestinal mucosal dysfunction, which has emerged as an important component of HIV pathogenesis [41], might serve as an important factor underlying systemic gram-negative sepsis as well as wasting syndrome.
Tuberculosis
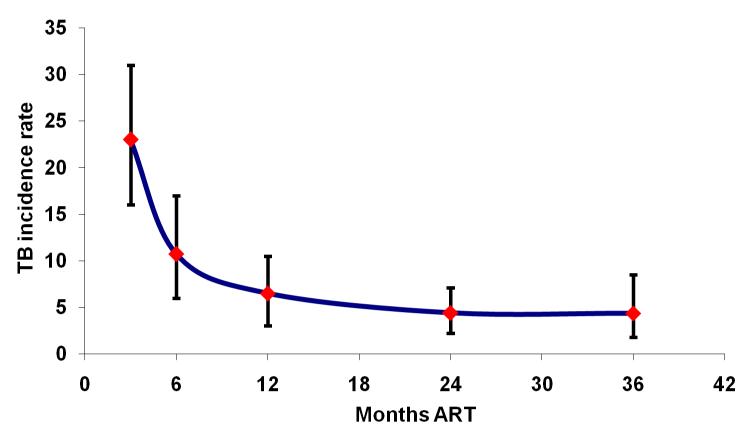
TB incidence rates are very high during the initial months of ART and decrease with increasing duration of treatment [23, 24, 42-44] (Figure 5). Although overall rates are much higher in resource-limited settings, the proportionate reduction in rates during ART is similar in high- and low-income settings [44]. In a South African study, TB risk was strongly associated with changes in CD4 cell counts measured serially during ART [12**]. The risk was almost 10-fold higher when patients had CD4 counts <100 cells/μL compared to when counts exceeded >500 cells/μL (Figure 2b) [12**]. In contrast to mortality (Figure 2a), substantial rates of TB persisted at CD4 counts of 200-500 cells/μL (Fig 2b).
Figure 5.
Graph showing changing tuberculosis (TB) incidence rate (95% confidence intervals, cases per 100 person-years) with increasing duration of antiretroviral therapy (ART) in a South African cohort. The incidence rate is extremely high in the first 3 months but rapidly decreases thereafter. Data adapted from [23, 12].
This South African study also found that during long-term ART, there was a substantial excess TB incidence rate (1.7-fold) during the first 4 months of ART that was not explained by CD4 cell counts and other covariates. From these data, the authors reasoned that approximately 40% of the TB cases presenting in the first 4 months of ART were likely to be attributable to immune-mediated ‘unmasking’ of sub-clinical TB that was not recognised at baseline [12**, 45]. This is supported by subsequent findings in the same cohort that systematic culture-based screening detected a very high prevalence (25%) of sputum culture-positive disease at baseline [46*]. Much of this disease was sub-clinical or pauci-symptomatic.
Immune reconstitution disease
In addition to TB, a range of other opportunistic infections is associated with development of immune reconstitution disease. In a prospective South African cohort with a median baseline CD4 cell count of 115 cells/μL, 10% (n=44) of patients developed immune reconstitution disease during the first 6 months of ART [47*]. Almost half were associated with TB and a majority of the remainder were associated with cutaneous abscesses and folliculitis, varicella zoster, herpes simplex, cryptococcal meningitis, molluscum contagiosum and Kaposi’s sarcoma. Although most cases were mild, one quarter of cases required hospital admission and two (4.5%) resulted in death (one case of TB and one case of cryptococcal disease) [47*]. The overlap between paradoxical immune reconstitution disease in patients with MDR-TB has been highlighted in South Africa [48]; this complicates both diagnosis and management.
Higher rates of morbidity and mortality in low-resource settings
Much higher rates of early morbidity and mortality among patients receiving ART in resource-limited settings compared to high-income settings are only partially explained by the presentation of patients with more advanced immunodeficiency [4-6, 8]. This may reflect much higher event frequencies during the natural history of HIV in resource-limited settings [49]. For example, in a South African natural history cohort, patients with CD4 cell counts in the range 50-200 cells/μL who either have WHO stage 1&2, stage 3 or stage 4 disease had very high 6-month mortality risks of 3-5%, 6-12% and 14-22%, respectively [49]. This has important implications for the appropriate CD4 count thresholds at which to start ART as discussed below.
Strategies to reduce early morbidity and mortality
A number of strategies are likely to be needed to reduce early morbidity and mortality during ART:
1. Earlier HIV diagnosis
A critical stumbling block is that most infected people do not know their HIV status and most HIV diagnoses are only made once patients present to the health services with WHO stage 3 or stage 4 (ie advanced symptomatic) disease. Such patients by definition have a high burden of morbidity and high mortality risk [49].
Revised WHO guidelines now recommend that all individuals accessing health care services in high HIV prevalence settings (antenatal prevalence >1%) should be tested for HIV unless they specifically opt out [50]. In a community-based study in South Africa in which testing increased from 4% to 20% between 2001 and 2006, however, median CD4 counts and WHO stage of disease among newly diagnosed patients remained stable rather than improving [51]. Rates of HIV testing may need to be even more radically improved to promote earlier diagnosis and should also be provided within communities outside the context of health care facilities.
2. Availability of CD4 count testing
CD4 count testing needs to be scaled up at the district and health centre level to assess ART eligibility. With the current impetus to decentralise HIV care and treatment services to more peripheral sites, this will require much simpler technology such as a point-of-care CD4 count tests, which are currently under development and evaluation.
3. Pre-ART care
The majority of health services in sub-Saharan Africa fail the HIV-infected people who are not yet eligible for ART and the concept of “pre-ART” care needs to be championed. A package of regular care and support that includes clinical assessment, CD4 count measurement and co-trimoxazole and isoniazid preventive therapy would go a long way to decreasing subsequent late presentation and high early mortality on ART. The mortality risk of patients just prior to starting ART is extremely high [37, 52] and delays within the health system need to be minimised.
4. Guidelines recommending earlier ART
Current international [53] and national guidelines in resource-limited settings recommend starting treatment at CD4 cell counts of either up to 200 cells/μL or up to 200-350 cells/μL. Observational data from South Africa, however, show that the 12-month mortality for untreated patients with CD4 counts of 200-350 cells/μL is 22% [54] compared to 4% in industrialised settings [55]. These data strongly suggest the need for earlier ART initiation as has been clearly demonstrated in the CIPRA HT001 trial from Haiti. This relatively small randomised controlled study (n=816) compared initiation of ART at CD4 counts between 200-350 cells/μL versus deferral of ART until the CD4 count had decreased to <200 cells/μL [56**]. The mortality rate was 4-fold higher and the TB rate two-fold higher in the deferred treatment arm, providing the first data from a randomised study to provide compelling evidence to support earlier ART initiation in resource-limited settings.
5. Co-trimoxazole prophylaxis
WHO/UNAIDS published provisional and updated recommendations in 2000 and 2006 for prophylaxis with trimethoprim-sulphamethoxazole (co-trimoxazle) in all patients with symptomatic HIV infection in resource-limited settings [57]. However, many countries have not widely implemented this important intervention for a variety of reasons, including concerns over efficacy in the context of high rates of resistant bacteria. Thus, a further randomized controlled trial was completed in one such country (Zambia) in 2008 and this confirmed that this is a safe intervention that reduces mortality among HIV-infected patients with TB [58]. In an ART programme in Cambodia, co-trimoxazole use was associated with an 85% reduction in adjusted hazards of death with benefit observed among those with baseline CD4 cell counts <200 cells/μL [18].
6. TB screening and prophylaxis
In 2008, the WHO launched the 3I’s policy - a new initiative to reduce the burden of TB in HIV-infected people [59]. This comprises the use of intensified case finding, isoniazid prophylaxis and infection control to be scaled up in tandem with ART.
The tools available for intensified case finding in patients with advanced HIV infection are blunt, however [60]. Recent studies from South Africa found a prevalence of sputum culture-positive TB of 20%-25% in patients just prior to ART initiation [46*, 61] but culture-based diagnosis was slow, the sensitivity of fluorescence smear microscopy was < 20% and chest radiology performed poorly [62]. These data suggest the potential need for routine culture-based screening in this setting and the need for new rapid and point of care diagnostics.
Following the fatal outbreak of multidrug- and extensively drug-resistant TB (MDR-TB and XDR-TB) among ART patients attending a facility in Kwazulu Natal, South Africa, in 2005-2006 [63], the WHO has recommended the implementation of molecular line-probe assays in resource-limited settings [64]. This should greatly expedite the diagnosis of MDR-TB.
Isoniazid prophylaxis substantially reduces TB risk and shows a trend towards a reduction in mortality in patients not receiving ART and who have a positive tuberculin skin test [65]. Using observational data from Brazil and South Africa, it has been suggested that there may also be an additive effect of isoniazid preventive therapy when administered prior to or during ART [66, 67]. A number of considerations, however, suggest that initiation of IPT at the same time as ART initiation might be inadvisable in patients with low CD4 cell counts since there may be a high prevalence of active undiagnosed TB [46, 61], the negative predictive value of TB screening algorithms is limited [60] and combined use of isoniazid during the initial weeks of stavudine-containing ART is associated with a high incidence of peripheral neuropathy [68]. Initiation of isoniazid after completion of the initial months of ART has therefore been suggested as an alternative strategy [12**].
7. Screening and prophylaxis for cryptococcal disease
Two retrospective studies from Africa have found that detectable cryptococcal antigenaemia in patients starting ART is an independent predictor of mortality [37*, 69]. In the more recent study, the prevalence of antigenaemia was 13% among patients with CD4 cell counts <100 cells/μL and an antigen titre of ≥1:8 was 100% sensitive and 96% specific for predicting development of cryptococcal meningitis during the first year of ART [39*]. Use of this as a screening test pre-ART might permit implementation of a targeted pre-emptive treatment strategy.
Importantly, a study from Cambodia found that use of fluconazole prophylaxis during ART was associated with a 50% reduction in adjusted hazards of death, with benefit being observed among those with baseline CD4 cell counts of <100 cells/μL [18]. No comment was made on whether this was due to prevention of cryptococcosis, oesophageal candidiasis or other locally prevalent fungal pathogens such as Penicillium marneffei.
8. Optimised management of opportunistic diseases
Management strategies need to be optimised to address serious and life-threatening morbidity. Expertise in the treatment TB and HIV concurrently is gradually evolving through observational studies [70*]. Use of efavirenz rather than nevirapine-containing ART appears to be preferable when combined with rifampicin-containing TB treatment, both with regard to ART efficacy and toxicity [71]. Results of randomised controlled trials informing the optimum time to initiate ART during TB treatment are awaited, but interim results from the South African SAPIT trial found a very high mortality among patients deferring ART initiation to the end of TB the 6-month treatment phase compared to initiation during or at the end of the 2-month intensive phase [72]. MDR-TB and HIV co-infection carries a poor prognosis and much remains to be learned about MDR-TB management during ART.
A randomised placebo-controlled trial of corticosteroids has been evaluated in the management of moderate (non-life-threatening) TB immune reconstitution disease in South Africa [73*]. Although no mortality benefit was observed, there was a significant reduction in the need for hospitalisation and interventions in the treatment arm.
Optimum regimens are needed for treatment of cryptococcal meningitis. Although high dose intravenous amphotericin and flucytosine evaluated in South Africa may be the ideal [74], this is neither logistically feasible nor affordable in many low-income settings. Data from Uganda suggest dose oral fluconazole at a high dose (1200 mg per day) may be more rapidly fungicidal than currently used lower doses [75*].
9. Improving treatment access and retention in care
Recognition of and pragmatic solutions to address the factors that hinder treatment access and retention in care are essential. These include the need for free treatment [4, 16, 17], the critical issue of high transport costs to and from clinics [76], the importance of uninterrupted drug supplies in health facility pharmacies and decentralisation of services so that they are nearer to patients’ homes. Every attempt must be made by ART clinics to link their services with the community and particularly with associations of people living with HIV/AIDS. Care packages such as home treatment of opportunistic infections, support to family carers, referral of patients with possible adverse drug reactions, continuing adherence counselling and defaulter tracing are, not surprisingly, associated with better ART treatment outcomes [77]. The community is an unrecognised and largely unexploited resource that could play an important contributory role in improving ART retention provided there are well defined structured links to the health services.
Conclusions
Rates of early morbidity and mortality are much higher among patients treated in resource-limited settings compared to those in high-income settings. However, these are very heterogeneous across resource-limited settings and a range of biomedical, economic and social factors may underlie this. The high burden of disease needs to be addressed by strategies that broadly enhance earlier HIV diagnosis and access to ART, screening and prophylaxis for opportunistic infections, optimised opportunistic disease management and strengthening of ART services. Managing patients with advanced immunodeficiency and high rates of morbidity and mortality is a major challenge using the simplified public health approach to ART delivery. Nevertheless, this has been very successful indeed in enabling rapid expansion to treatment to permit millions of people to gain the benefits of ART.
Acknowledgements
SDL is funded by the Wellcome Trust, London, UK. RW is funded in part by the National Institutes of Health (NIH) through a CIPRA grant 1U19AI53217-01 and RO1 grant (A1058736-01A1).
Abbreviations
- AIDS
acquired immune deficiency syndrome
- ART
antiretroviral treatment
- HIV
human immunodeficiency virus
- KS
Kaposi’s sarcoma
- MDR-TB
multi-drug resistant tuberculosis
- TB
tuberculosis
- WHO
World Health Organization
- XDR-TB
extensively resistant tuberculosis
Footnotes
Conflicts of Interest The authors have no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- (1).UNAIDS . 2008 Report on the global AIDS epidemic. UNAIDS; Geneva: 2008. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. [Google Scholar]
- (2).World Health Organization . Progress report 2008. World Health Organization; Geneva: 2008. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. Accessible at: http://www.who.int/entity/hiv/pub/towards_universal_access_report_2008.pdf. [Google Scholar]
- (3).Souteyrand Y, Akwara P, Warner Smith M, et al. Scaling up access to antiretroviral therapy (ART) in low- and middle-income countries: global and regional progress in 2008. Abstracts of the 5th International AIDS Society conference on HIV pathogenesis, treatment and prevention; Cape Town, South Africa. International AIDS Society; Jul, 2009. Abstract WELBD105. [Google Scholar]
- (4).Braitstein P, Brinkhof MW, F Dabis, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- (5)**.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. A comprehensive of the mortality rates (8%-26% in the first year) risk factors, causes and potential interventions to address this in ART programmes in sub-Saharan Africa.
- (6)**.Tuboi SH, Schechter M, McGowan CC, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009;51:615–23. doi: 10.1097/QAI.0b013e3181a44f0a. Data from ART programmes in Latin America are scarce. This paper reports on mortality rates in 6 programmes in the region and one from Haiti. The overall mortality rate was 8.3% in the first year of ART.
- (7).Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS ONE. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8)**.Chasombat S, McConnell MS, Siangphoe U, et al. National expansion of antiretroviral treatment in Thailand, 2000-2007: program scale-up and patient outcomes. J Acquir Immune Defic Syndr. 2009;50:506–12. doi: 10.1097/QAI.0b013e3181967602. A comprehensive report of the national ART scale-up programme in Thailand, which includes outcomes for over 58,000 patients and shows a one year mortality of 11%.
- (9).Ferradini L, Laureillard D, Prak N, et al. Positive outcomes of HAART at 24 months in HIV-infected patients in Cambodia. AIDS. 2007;21:2293–301. doi: 10.1097/QAD.0b013e32828cc8b7. [DOI] [PubMed] [Google Scholar]
- (10).Keiser O, Orrell C, Egger M, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5:e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11)**.Lawn SD, Little F, Bekker LG, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–42. doi: 10.1097/QAD.0b013e328321823f. This paper is the first to report how mortality risk changes in relationship to the CD4 count measured at 4-monthly intervals during ART. CD4 counts are the dominant determinant of mortality risk and the rapid temporal changes in mortality rates.
- (12)**.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009 doi: 10.1097/QAD.0b013e32832d3b6d. epub ahead of print. This paper is the first to report how risk of incident TB during ART changes in relationship to the CD4 count measured at 4-monthly intervals. CD4 counts were the dominant determinant of TB risk and the temporal changes in rates. Data also suggest that approximately 40% of TB presenting in the first 4 months of ART is due to immune-mediated ‘unmasking’ of TB that was not recognised at ART initiation.
- (13).Srasuebkul P, Lim PL, Lee MP, et al. Short-term clinical disease progression in HIV-infected patients receiving combination antiretroviral therapy: results from the TREAT Asia HIV observational database. Clin Infect Dis. 2009;48:940–50. doi: 10.1086/597354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Chen SC, Yu JK, Harries AD, et al. Increased mortality of male adults with AIDS related to poor compliance to antiretroviral therapy in Malawi. Trop Med Int Health. 2008;13:513–9. doi: 10.1111/j.1365-3156.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- (15).Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14:722–31. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Boyer S, Marcellin F, Ongolo-Zogo P, et al. Financial barriers to HIV treatment in Yaounde, Cameroon: first results of a national cross-sectional survey. Bull World Health Organ. 2009;87:279–87. doi: 10.2471/BLT.07.049643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–9. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- (19)*.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965–72. doi: 10.1086/605500. Consistent with other studies from Africa, the most common causes of mortality during early ART in this Ugandan cohort were tuberculosis and cryptococcal disease and the overall contribution of immune reconstitution inflammatory syndrome was limited.
- (20).Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- (21).Dillingham RA, Pinkerton R, Leger P, et al. High early mortality in patients with chronic acquired immunodeficiency syndrome diarrhea initiating antiretroviral therapy in Haiti: a case-control study. Am J Trop Med Hyg. 2009;80:1060–4. [PMC free article] [PubMed] [Google Scholar]
- (22).Kumarasamy N, Venkatesh KK, Devaleenol B, et al. Factors associated with mortality among HIV-infected patients in the era of highly active antiretroviral therapy in southern India. Int J Infect Dis. 2009 doi: 10.1016/j.ijid.2009.03.034. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- (23).Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- (24).Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- (25).Koenig SP, Riviere C, Leger P, et al. High mortality among patients with AIDS who received a diagnosis of tuberculosis in the first 3 months of antiretroviral therapy. Clin Infect Dis. 2009;48:829–31. doi: 10.1086/597098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Yu JK, Bong CN, Chen SC, et al. Outcomes in HIV-infected patients who develop tuberculosis after starting antiretroviral treatment in Malawi. Int J Tuberc Lung Dis. 2008;12:692–4. [PubMed] [Google Scholar]
- (27)*.Westreich D, MacPhail P, Van RA, et al. Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS. 2009;23:707–15. doi: 10.1097/QAD.0b013e328325d115. These data from South Africa suggest that although TB patients within ART programmes have high mortality risk, this simply reflects an association with more advanced immunodeficiency.
- (28).Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–60. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- (29).Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- (30).Lucas SB, Hounnou A, Peacock C, et al. The mortality and pathology of HIV infection in a west African city. AIDS. 1993;7:1569–79. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- (31).Lawn SD, Edwards DJ, Wood R. Reducing the burden of tuberculosis during the initial months of antiretroviral therapy in resource-limited settings. Clin Infect Dis. 2009 doi: 10.1086/649007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- (33).Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48:e96–107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]
- (34).Martin J, Laker M, Clutter D, et al. Kaposi’s sarcoma-associated IRIS in Africa: initial findings from a prospective evaluation. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. Feb, 2009. Abstract 31. [Google Scholar]
- (35).Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- (36).Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- (37).Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- (38).Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39)*.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–62. doi: 10.1086/597262. Detection of cryptococcal antigenaemia just prior to starting ART is a strong independent predictor of mortality and of incident cryptococcal disease during ART. It’s detection therefore may permit pre-emptive targeted treatment.
- (40).Moh R, Danel C, Messou E, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–91. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- (41).Paiardini M, Frank I, Pandrea I, Apetrei C, Silvestri G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev. 2008;10:36–46. [PubMed] [Google Scholar]
- (42).Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- (43).Bonnet MM, Pinoges LL, Varaine FF, et al. Tuberculosis after HAART initiation in HIV-positive patients from five countries with a high tuberculosis burden. AIDS. 2006;20:1275–9. doi: 10.1097/01.aids.0000232235.26630.ee. [DOI] [PubMed] [Google Scholar]
- (44).Brinkhof MW, Egger M, Boulle A, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007;45:1518–21. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–5. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46)*.Lawn SD, Edwards SD, Kranzer K, Vogt M, Bekker L-G, Wood R. Urine lipoarabinomannan assay for tuberculosis screening prior to ART: diagnostic yield and association with immune reconstitution disease. AIDS. 2009 doi: 10.1097/qad.0b013e32832e05c8. epub ahead of print. This study from South Africa found a prevalence of sputum culture-positive TB among patients just prior to ART of 25% and highlights the need for culture-based screening of all patients in this setting.
- (47)**.Murdoch DM, Venter WD, Feldman C, Van RA. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–10. doi: 10.1097/QAD.0b013e3282f4a607. This is the first prospective cohort study from sub-Saharan Africa documenting the spectrum of immune reconstitution disease. TB and cutaneous infections were the most commonly associated opportunistic diseases.
- (48).Meintjes G, Rangaka MX, Maartens G, et al. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis. 2009;48:667–76. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–9. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- (50).WHO/UNAIDS . Guidance on provider-initiated HIV testing and counselling in health facilities. World Health Organization, Joint United Nations Programme on HIV/AIDS; Geneva., Switzerland: 2007. Available at: http://www.who.int/hiv/pub/guidelines/9789241595568_en.pdf. 2009. [Google Scholar]
- (51).April MD, Walensky RP, Chang Y, et al. HIV testing rates and outcomes in a South African community, 2001-2006: implications for expanded screening policies. J Acquir Immune Defic Syndr. 2009;51:310–6. doi: 10.1097/qai.0b013e3181a248e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–9. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).World Health Organisation [Accessed 14 Aug 09];Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2006 revision. Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- (54).Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, Wood R. Initiating highly active antiretroviral therapy in sub-Saharan Africa: an assessment of the revised World Health Organization scaling-up guidelines. AIDS. 2004;18:1159–68. doi: 10.1097/00002030-200405210-00009. [DOI] [PubMed] [Google Scholar]
- (55).Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197(8):1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- (56)**.Fitzgerald D. Early versus delayed ART: results from Haiti. Abstracts of the 5th International AIDS Society conference on HIV pathogenesis, treatment and prevention; Cape Town, South Africa. International AIDS Society; Jul, 2009. 2009. Abstract WESY201. This randomised controlled trial from Haiti compared initiation of ART among patients with CD4 counts of 200-350 cells/μL or deferring until counts had decreased to <200 cells/μL. Deferred treatment was associated with 4-fold high mortality risk and two-fold high TB risk, providing very strong evidence for the need for earlier ART initiation.
- (57).World Health Organisation [Accessed 14th Aug 2009];Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults. Recommendations for a public health approach. 2006 Accessible at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf.
- (58).Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;337:a257. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).World Health Organization . WHO three I’s meeting. Report of a joint WHO HIV/AIDS and TB Department Meeting, 2008. WHO; Geneva: 2008. Accessible at: http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf. [Google Scholar]
- (60).Reid MJ, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9:173–84. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- (61).Bassett I, Chetty S, Wang B, et al. Intensive TB screening for HIV-infected patients ready to start ART in Durban, South Africa: limitations of WHO guidelines. Program and Abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. Feb, 2009. Abstract #779. [Google Scholar]
- (62).Dawson R, Masuka P, Edwards DJ, et al. Chest radiograph reading and reporting system (CRRS): evaluation for TB screening in advanced HIV patients. Int J Tuberc Lung Dis. 2009 in press. [PMC free article] [PubMed] [Google Scholar]
- (63).Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- (64).World Health Organization Molecular line probe assays for rapid screning of patients at risk of multidrug-resistant tuberculosis (MDR-TB) Policy statement. 2008 Jun; www.who.int/entity/tb/dots/laboratory/lpa_policy.pdf. 2008.
- (65).Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2004;(1):CD000171. doi: 10.1002/14651858.CD000171.pub2. [DOI] [PubMed] [Google Scholar]
- (66).Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–6. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–8. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Westreich DJ, Sanne I, Maskew M, et al. Tuberculosis treatment and risk of stavudine substitution in first-line antiretroviral therapy. Clin Infect Dis. 2009;48:1617–23. doi: 10.1086/598977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- (70)*.Harries AD, Zachariah R, Lawn SD. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa. Int J Tuberc Lung Dis. 2009;13:6–16. A state of the art review of the management of TB/HIV-coinfected patients in sub-Saharan Africa.
- (71).Boulle A, Van CG, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–9. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- (72).Abdool Karim S, Naidoo K, Grobler A, et al. Iniitating ART during TB treatment significantly increases survival: results of a randomized controlled trial in TB/HIV-co-infected patients in South Africa. Programme and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections (CROI); Montreal, Canada. Feb, 2009. Abstract no. 36a. [Google Scholar]
- (73)*.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomised placebo-controlled trial of predisone for TB IRIS. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. Feb, 2009. Abstract no. 34. This, the only randomised placebo-controlled study of the use of corticosteroids in the management of mild and moderate TB immune reconstitution disease, found steroid use was associated with significantly reduced hospital stay and need for interventions.
- (74).Bicanic T, Wood R, Meintjes G, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis. 2008;47:123–30. doi: 10.1086/588792. [DOI] [PubMed] [Google Scholar]
- (75)*.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–61. doi: 10.1086/593194. Fluconazole is a sub-optimal fungistatic treatment for cryptococcal meningitis, but this study examining rate of clearance of the organism from cerebrospinal fluid suggests that high dose fluconazole may improve outcomes.
- (76).Zachariah R, Harries AD, Manzi M, et al. Acceptance of anti-retroviral therapy among patients infected with HIV and tuberculosis in rural Malawi is low and associated with cost of transport. PLoS ONE. 2006;1:e121. doi: 10.1371/journal.pone.0000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Zachariah R, Teck R, Buhendwa L, et al. Community support is associated with better antiretroviral treatment outcomes in a resource-limited rural district in Malawi. Trans R Soc Trop Med Hyg. 2007;101:79–84. doi: 10.1016/j.trstmh.2006.05.010. [DOI] [PubMed] [Google Scholar]