Abstract
Nontyphoidal Salmonellae are a major cause of life-threatening bacteremia among HIV-infected individuals. Although cell-mediated immunity controls intracellular infection, antibody protects against Salmonella bacteremia. We report that high titer antibodies specific for Salmonella lipopolysaccharide (LPS) associate with absent Salmonella-killing in HIV-infected African adults. Killing was restored by genetically shortening LPS from target Salmonella, or removing LPS-specific antibodies from serum. Complement-mediated killing of Salmonella by healthy serum is shown to be induced specifically by antibodies against outer membrane proteins. This killing is lost when excess antibody against Salmonella LPS is added. Thus our study indicates impaired immunity against nontyphoidal Salmonella bacteremia in HIV infection results from excess inhibitory antibodies against Salmonella LPS, whilst serum killing of Salmonella is induced by antibodies against outer membrane proteins.
The association between HIV infection and fatal disease with nontyphoidal strains of Salmonella (NTS) was first described at the outset of the AIDS pandemic 26 years ago (1, 2). This is a global problem affecting affluent countries (3, 4), but particularly Africa (5-8). The underlying mechanisms are not known. NTS, especially Salmonella enterica serovars Typhimurium and Enteritidis, are a major cause of invasive bacterial disease in Africa affecting young children (5, 9), as well as HIV-infected adults. Case-fatality and recrudescence rates are high (10), antibiotic resistance is an increasing problem (5) and currently no vaccine is available. Although Salmonellae are facultative intracellular pathogens (11) and cell-mediated immunity is important for controlling infection (12-14) we recently demonstrated an important protective role for antibody-induced complement-mediated killing of NTS in African children (15). Here we investigate antibodies to Salmonella in the context of HIV infection, because HIV causes extensive defects in the humoral immune system (16-18). Our studies reveal aberrant humoral immunity to NTS in HIV-infected African adults characterized by absent bactericidal activity resulting from dysregulated antibody production with excess IgG directed against S. Typhimurium lipopolysaccharide (LPS). We also show that antibodies against S. Typhimurium outer membrane proteins induce killing of NTS in HIV-uninfected African adults.
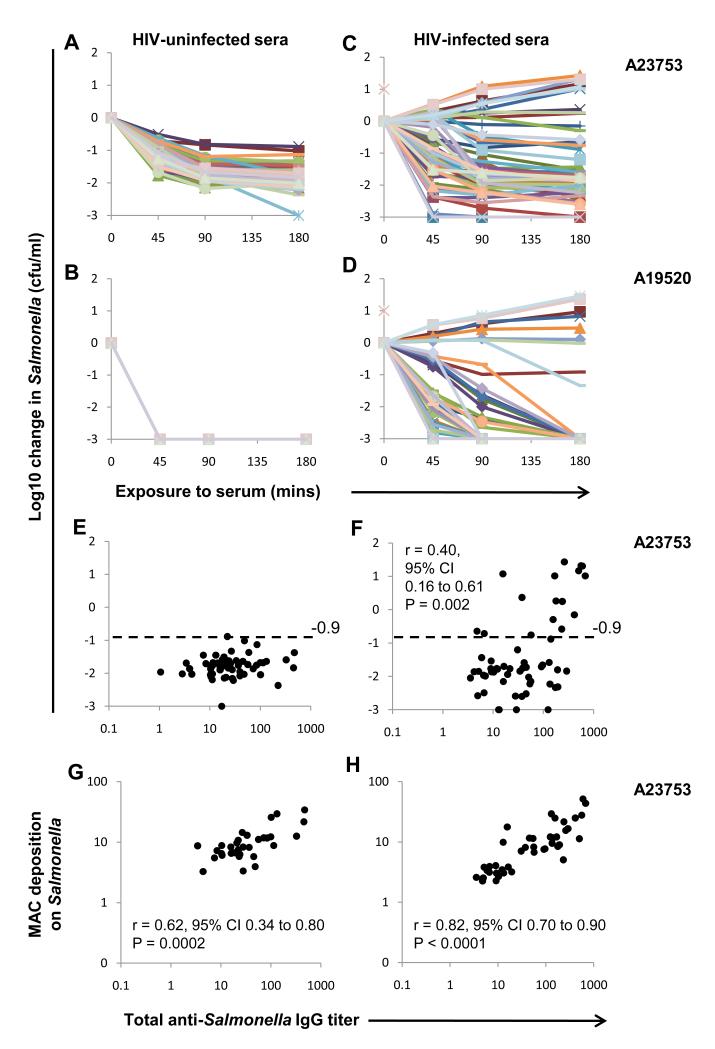
To determine whether HIV infection affects humoral immunity to NTS, we assessed in vitro killing of two invasive Malawian S. Typhimurium isolates by sera from Malawian adults (19). Isolate A23753 was killed by all sera from HIV-uninfected adults with a log10 kill at 180 minutes of ≥ 0.9 (designated ‘normal kill’) (Fig. 1A) and all effected a 3.0 log10 kill of A19520 by 45 minutes (Fig. 1B). In contrast, there was considerable variation in ability of sera from HIV-infected adults to kill both isolates. 28% of sera failed to effect a 0.9 log10 kill of A23753 by 180 minutes (Fig. 1C) and 59% failed to produce a 3.0 log10 kill of A19520 by 45 minutes (Fig. 1D). All sera had normal total and alternative pathway hemolytic complement activity (table S1), excluding complement degradation or impaired synthesis as reasons for impaired killing. HIV targets CD4+ T lymphocytes and lowered blood CD4+ lymphocyte numbers (CD4 counts) are associated with increased susceptibility to NTS bacteremia (20). CD4 counts of HIV-infected subjects with impaired serum killing of A23753 were lower than those with normal killing (P=0.05) (fig. S1).
Fig. 1.
Dysregulated humoral immunity to NTS in HIV infection. Killing of (A and C) S. Typhimurium isolate A23753, and (B and D) serum-sensitive S. Typhimurium isolate A19520 by sera at 45, 90 and 180 minutes. Negative values correspond with a decrease in viable Salmonellae compared with the initial concentration. (E and F) Serum titers of Salmonella A23753 IgG compared with killing of S. Typhimurium isolate A23753 at 180 minutes, and (G and H) C5b-9 MAC deposition on A23753. (A, B, E and G) sera from HIV-uninfected Africans (n=58). (C, D, F and H) sera from HIV-infected Africans (n=58). Each line or point represents data for serum from one individual. Note all lines are superimposed in (B). Horizontal dashed line indicates threshold for impaired killing of S. Typhimurium A23753 (−0.9 log10 change in Salmonellae cfu/ml).
Next, IgG binding to S. Typhimurium A23753 was measured in all sera to determine whether lack of antibody was the reason for impaired Salmonella killing. S. Typhimurium-specific IgG was present in all sera and, paradoxically, IgG titer positively correlated with impaired Salmonella-killing by HIV-infected sera (P=0.002) (Fig. 1, E and F). S. Enteritidis D24954-specific IgG was also present in all sera and positively correlated with S. Typhimurium IgG titer for HIV-uninfected and HIV-infected sera (fig. S2). Some impairment of killing of S. Enteritidis D24954 was observed with a subset of HIV-infected sera that could not kill S. Typhimurium D23580. S. Enteritidis IgG titer correlated impaired killing of S. Enteritidis (fig. S3).
In case Salmonella-specific antibody in HIV-infected sera could not activate complement, we measured deposition on A23753 of C5b-9 membrane attack complex (MAC), the final effector of complement-mediated bactericidal activity. MAC deposition was detected for all sera and strongly correlated with Salmonella-specific IgG titer for HIV-infected and HIV-uninfected sera (Fig. 1,G and H). We also detected IgG binding and C3 complement deposition for HIV-infected and -uninfected sera by confocal microscopy (fig. S4). This indicates failure to deposit complement is not responsible for absent Salmonella killing by HIV-infected serum.
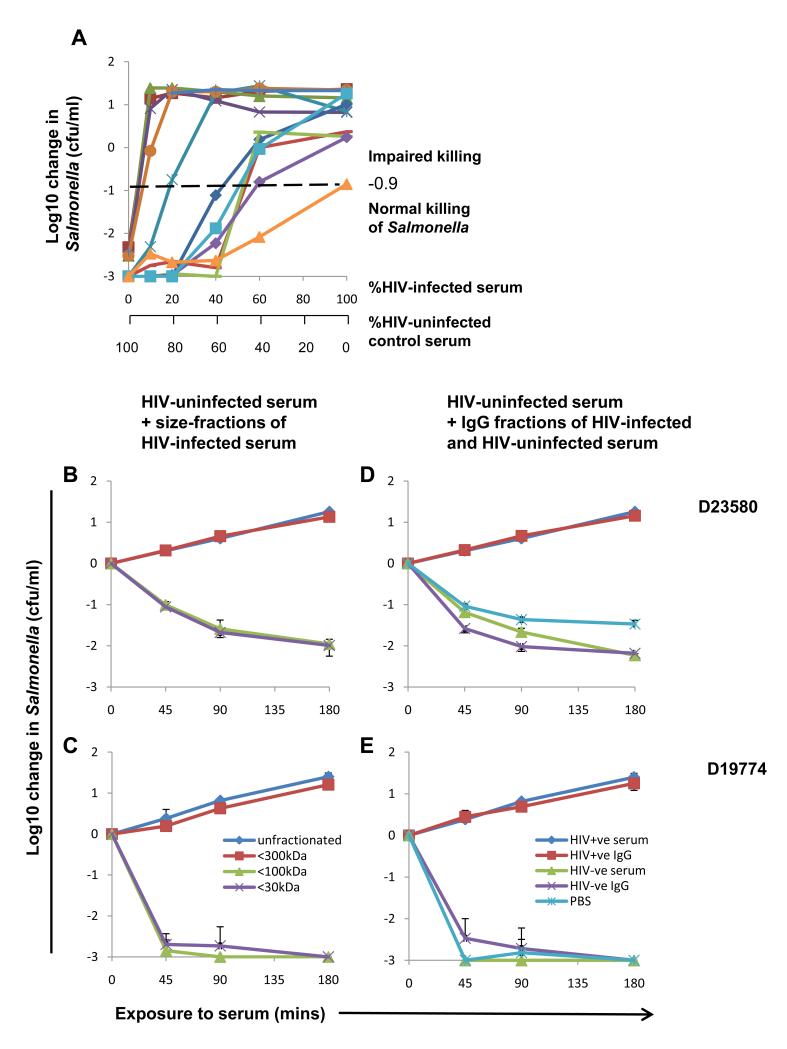
Killing of Salmonella A23753 was impaired when different proportions of HIV-infected sera that could not kill Salmonella were mixed with HIV-uninfected serum (Fig. 2A). For some HIV-infected sera, this impairment was observed with one part HIV-infected serum to nine parts control serum. Thus an inhibitor in HIV-infected serum blocks killing. The inhibitory factor was found to be between 100 and 300 kDa (Fig. 2, B and C). We tested whether this was an antibody, since IgG is approximately 160 kDa. Total IgG at 10 g/l extracted from inhibitory HIV-infected sera blocked killing of S. Typhimurium D23580 and D19774 by control sera (Fig. 2, D and E). Conversely, IgG from HIV-uninfected sera had no effect on killing.
Fig. 2.
Inhibition of HIV-uninfected control serum killing of NTS by HIV-infected sera with impaired Salmonella-killing ability. (A) Killing of S. Typhimurium isolate A23753 at 180 minutes by mixed sera consisting of different percentages of HIV-infected serum (n=12, serum from one HIV-infected subject per line) and control HIV-uninfected serum. Horizontal dashed line indicates threshold for impaired killing of S. Typhimurium A23753. (B-E) Inhibition of control serum killing of S. Typhimurium isolate D23580 (B and D), and serum-sensitive S. Typhimurium isolate D19774 (C and E) by size-fractionated (B and C) and IgG fraction (D and E) of HIV-infected serum. Data are means ± SD of 3 experiments. Inhibition of killing of both strains of Salmonella by HIV-uninfected sera with <300 kDa fraction of HIV-infected serum compared with <100 kDa fraction, and with IgG fraction of HIV-infected serum compared with IgG fraction of HIV-uninfected serum was significant by Student’s t test (P<0.0001).
We then tested whether inhibition results from excess total serum immunoglobulin because hypergammaglobulinemia is a well-recognized feature of HIV infection (16, 17). Although higher total IgG titers were present in HIV-infected compared with HIV-uninfected sera (P<0.0001), there was only a small, yet significant correlation between total serum IgG and IgA, but not IgM, and impaired killing of S. Typhimurium (fig. S5). This suggests inhibitory IgG binds specific targets on S. Typhimurium. We hypothesized that antibody targeting structures away from the bacterial membrane might prevent killing. NTS are surrounded by LPS with long polysaccharide side chains (O-antigen) extending from the outer membrane along with flagella (consisting of flagellin, H-antigen) (21). LPS and flagellin are highly immunogenic (22). We previously showed that O-antigen of invasive African S. Typhimurium protects against complement-mediated killing in the absence of antibody (15). Earlier studies found MAC deposited on LPS of S. Minnesota does not insert into the bacterial membrane (23) and rabbit LPS IgG can inhibit the bactericidal effect of bovine serum on S. Typhimurium (24). These considerations led us to test whether LPS or flagellin are targets of inhibitory IgG.
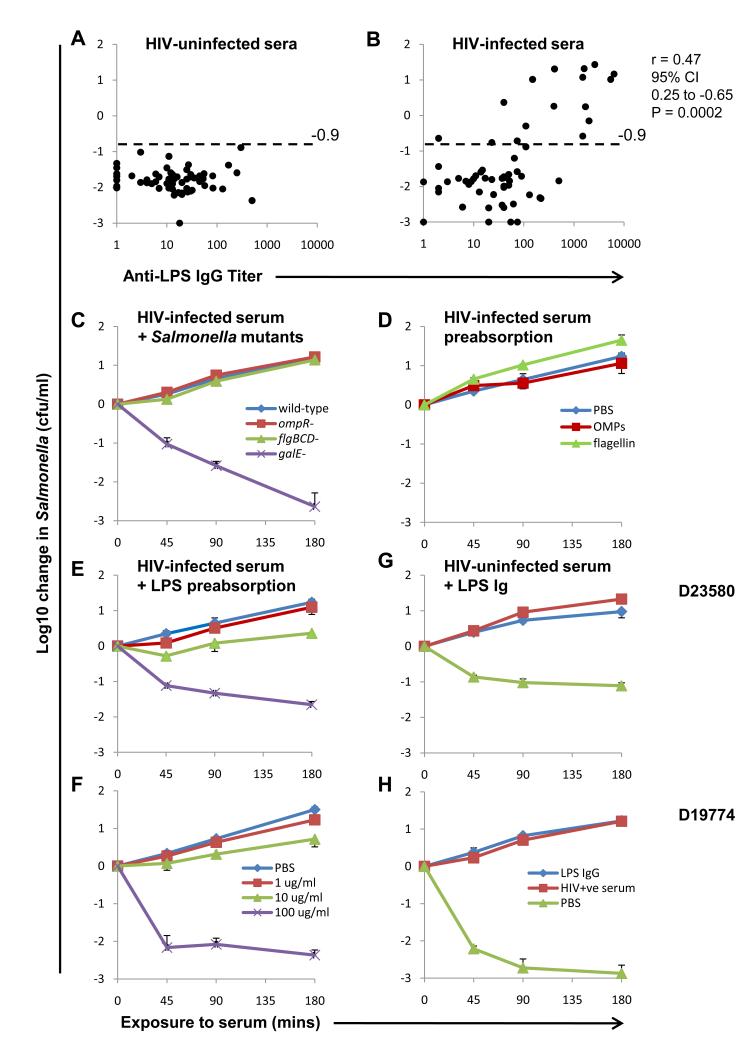
S. Typhimurium LPS IgG titers were selectively elevated in HIV-infected compared with HIV-uninfected sera (P<0.002) (Fig. 3, A and B), whereas flagellin-specific IgG titers were comparable (fig. S6). Impaired Salmonella-killing in HIV-infected sera correlated with LPS IgG titer (P=0.0002), but not flagellin IgG titer. We confirmed the correlation between LPS IgG and impairment of Salmonella-killing by measuring LPS IgG in a subset of HIV-infected and HIV-uninfected sera by fluorescent-bead-based immunoassay (fig. S7). These results are consistent with LPS IgG being the key inhibitor. Median IgM titers to LPS (as previously reported (25)) and flagellin were respectively higher or not significantly different in HIV-uninfected compared with HIV-infected sera (fig. S8), arguing against a role for IgM in inhibition of Salmonella killing.
Fig. 3.
LPS antibodies prevent killing of NTS by HIV-infected serum. (A and B) Killing of S. Typhimurium A23753 at 180 minutes by HIV-uninfected sera (A) and HIV-infected sera (B) compared with S. Typhimurium LPS IgG titer determined by ELISA. Horizontal dashed line indicates threshold for impaired killing of S. Typhimurium A23753. r values is Spearman correlation coefficient. Median IgG titer was higher in HIV-infected sera compared with HIV-uninfected sera (Mann-Whitney P<0.002, difference in medians 20 units, 95% CI 6.0 to 39). (C) Killing of indicated S. Typhimurium D23580 strains by HIV-infected serum. (D) Effect of preabsorbing HIV-infected serum with 100 μg/ml S. Typhimurium flagellin or outer membrane proteins on killing of S. Typhimurium D23580. (E and F) Effect of preabsorbing HIV-infected serum with 1, 10 or 100μg/ml LPS on serum killing of (E) D23580 and (F) D19774. (G and H) Effect of adding LPS antibodies at one tenth concentration in inhibitory HIV-infected source serum to HIV-uninfected serum on killing of (G) D23580 and (H) D19774. Data represent means ± SD of 3 experiments. Killing of both strains of Salmonella by HIV-infected sera preabsorbed with 100 μg/ml LPS compared with unabsorbed serum, and inhibition of killing of both strains of Salmonella by HIV-uninfected serum with exogenous LPS antibody added compared with PBS added was significant by Student’s t test P<0.0001.
To test further whether LPS IgG inhibits Salmonella-killing, ability of HIV-infected serum to kill without the LPS target antigen was examined using a galE mutant of S. Typhimurium D23580 lacking O-antigen polysaccharide (15). The mutant was fully susceptible to killing by inhibitory HIV-infected serum (Fig. 3C). Wild-type, flgBCD and ompR mutants of D23580, deficient in expression of flagellin and certain outer membrane proteins respectively, served as controls and could not be killed. These results indicate that inhibitory HIV-infected sera have inherent capacity to kill Salmonella and suggest inhibitory antibodies target O-antigen, further implicating LPS IgG as the inhibitor. We investigated the effect of absorbing LPS antibodies from HIV-infected serum. Preabsorption with S. Typhimurium flagellin and outer membrane proteins at 100 μg/ml did not affect bactericidal activity (Fig. 3D), but preabsorption with LPS fully restored killing of S. Typhimurium D23580 and D19774 (Fig. 3, E and F and fig. S9). For HIV-infected sera with partially-impaired Salmonella-killing ability, 1 μg/ml LPS restored normal killing (fig. S9).
Finally, LPS IgG extracted from inhibitory HIV-infected serum was added to HIV-uninfected serum. Inhibition of killing of S. Typhimurium D23580 and D19774 was induced at one tenth LPS IgG concentration present in source serum (Fig. 3, G and H), confirming LPS IgG as the inhibitor of Salmonella-killing. Killing of both strains by HIV-uninfected serum was also inhibited by LPS IgG from autologous HIV-uninfected serum at 10 times the original concentration in source serum (fig. S10) (relative concentration of LPS IgG in HIV-uninfected serum was 1/60 that in HIV-infected serum used). This indicates that LPS IgG titer rather than source of this antibody is critical for inhibition of Salmonella-killing.
Elevated IgG titers in HIV infection are characterized by antibodies to HIV viral proteins (26, 27) and self antigens (27, 28). This occurs in parallel with loss of antigen-specific antibodies, for example to tetanus toxoid and measles (29). The global reduction in T-dependent (30, 31) and T-independent (19, 31, 32) antibody responses after immunization in HIV-infected individuals contrasts with increased antibody to Salmonella-specific LPS. This indicates immune dysregulation, not immune deficiency, accounts for impaired humoral immunity to nontyphoidal Salmonella. The high proportion of HIV-infected subjects with elevated LPS IgG suggests that high titers are not the consequence of random expansion of antigen-specific B cell clones. The explanation may relate to elevated plasma LPS titers in HIV infection secondary to microbial translocation from the gastrointestinal tract (25). We found no correlation between serum LPS and S. Typhimurium LPS antibody titers (fig. S11) and no difference between serum LPS levels in HIV-infected and HIV-uninfected sera (P=0.33). However, LPS is likely to be cleared from the blood by the antibody it induces in immune complexes and become localized in secondary lymphoid tissue.
We hypothesized that LPS antibodies prevent killing of Salmonella by two possible mechanisms that are not mutually exclusive. One would act by diverting complement deposition away from the bacterial membrane, thereby preventing insertion of MAC into the membrane (fig. S12). The other would impede access of antibody and/or complement to the outer membrane by cross-linking O-antigen, the distal portion of the LPS molecule. To test these hypotheses, we investigated whether the inhibitory antibodies bind O-antigen, rather than proximal lipid A and core oligosaccharide moieties (21). Preabsorption of inhibitory HIV-infected sera with smooth S. Typhimurium LPS at 100 μg/ml enabled killing of S. Typhimurium D23580 (Fig. 3, E and F). However, preabsorption with 100 μg/ml lipid A or LPS from Rb, Rc, Rd and Re rough forms of Salmonella, where LPS is truncated in the core oligosaccharide (33), did not induce killing of Salmonella (fig. S13, A and B). These findings indicate that inhibitory antibodies target O-antigen. We also found that inhibitory antibodies could not be removed by preabsorbing with LPS from S. Enteritidis (Group D Salmonella) and S. Minnesota (Group L Salmonella) (fig. S13, C and D). This provides further evidence that O-antigen is targeted by inhibitory antibodies, since LPS from these three Salmonella serovars are distinguished by their non-cross-reactive O-antigens.
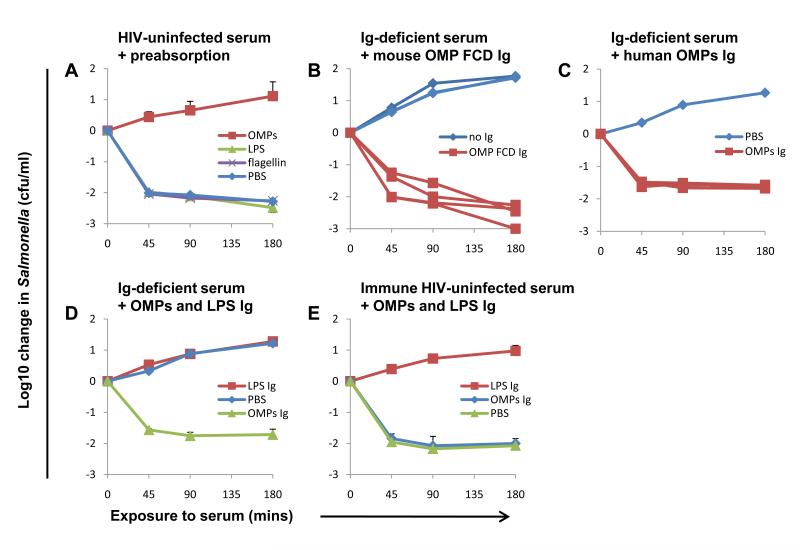
The concept that inhibitory antibodies act by binding O-antigen, a target distal to the Salmonella membrane, implies that protective bactericidal antibodies target molecules proximal to the membrane (fig S12), an idea we have previously suggested (15). This conclusion is consistent with recent reports that antibodies against S. Typhimurium outer membrane proteins, in particular porins OMP F, C and D, protect against Salmonella in the mouse (34). Consequently, we investigated whether such antibodies are responsible for S. Typhimurium killing by serum from Africans. First, we preabsorbed serum from HIV-uninfected Malawian adults with S. Typhimurium outer membrane proteins, LPS or flagellin. Although preabsorption with LPS and flagellin had no effect, killing was abrogated by preabsorption with outer membrane proteins (Fig. 4A). This indicates that antibodies against these proteins are bactericidal. Next, we immunized mice with OMP F, C and D porins, boosting at day 14 and used heat-inactivated sera from mice at day 21 as a source of OMP F, C and D-specific antibodies. Immunized sera, but not sera from unimmunized litter-mates, enabled antibody-deficient human serum to kill S. Typhimurium D23580 (Fig. 4B). This provides further evidence that antibodies against outer membrane proteins, in particular porins, cause Salmonella killing.
Fig. 4.
Antibodies targeted against outer membrane proteins mediate African serum killing of NTS. (A) Effect of preabsorbing HIV-uninfected serum with 200 μg/ml of S. Typhimurium outer membrane proteins, flagellin or LPS on killing of S. Typhimurium D23580. (B) Effect of adding OMP F, C and D antibodies from four mice immunized with S. Typhimurium OMP F, C and D to antibody-deficient HIV-uninfected human serum on killing of S. Typhimurium D23580 compared with adding antibody from four unimmunized mice. Each line represents log10 change of Salmonella induced by antibody from one mouse. (C) Effect of adding outer membrane protein antibodies from four HIV-uninfected sera (one per line) at one tenth concentration present in source serum, to antibody-deficient serum on killing of S. Typhimurium D23580. Note that lines are superimposed. (D and E) Effect of adding outer membrane protein antibodies and LPS antibodies extracted and purified from inhibitory HIV-infected serum to antibody-deficient serum (D) or immune HIV-uninfected serum (E) on killing of S. Typhimurium D23580. (A, E and F) Data represent means +/− SD of 3 experiments.
Finally, we purified antibodies to outer membrane proteins from HIV-uninfected and HIV-infected Malawian sera. These antibodies, when added to antibody-deficient serum at one-tenth the concentration in source serum, enabled killing of D23580 (Fig. 4C), even when extracted from HIV-infected inhibitory serum (Fig. 4D). The outer membrane protein antibodies had no effect when added to immune HIV-uninfected serum (Fig. 4E). This contrasts with absent killing of Salmonella observed after adding LPS antibody to antibody-deficient and immune serum (Fig. 4, D and E). The findings also indicate that individual sera contain antibodies that can kill Salmonella and block killing of Salmonella (fig. S14).
These results suggest killing of Salmonella by inhibitory HIV-infected sera could be restored by adding IgG from HIV-uninfected serum. We added human normal IgG immunoglobulin pooled from HIV-uninfected donors to inhibitory HIV-infected sera. This induced killing in a dose-dependent manner in three inhibitory sera, but not a fourth serum which had an LPS antibody titer over 10 times higher than the other sera (fig. S15). Finally, killing of Salmonella in antibody-deficient serum could be induced or prevented by adding combinations of IgG from HIV-uninfected and inhibitory HIV-infected sera depending on the proportion of IgG from each serum (fig. S16). This supports the concept of competition between blocking and killing anti-Salmonella antibodies.
Dysregulated humoral immunity in HIV-infected Africans could contribute to their susceptibility to invasive Salmonella by undermining protective antibody-mediated immunity that develops within the first two years of life (15). Together with impaired cellular immunity in HIV-infection, it is unsurprising that HIV-infected adults suffer from repeated episodes of Salmonella infection with associated high mortality (6, 10). A vaccine for nontyphoidal Salmonella is urgently required for Africa. The current study indicates that although an O-antigen polysaccharide-based vaccine might be ineffective and increase susceptibility to life-threatening extracellular Salmonella growth, an outer membrane protein-based vaccine could induce protective antibodies.
Supplementary Material
Acknowledgments
We thank the individuals who participated in this study and the staff at the Malawi-Liverpool-Wellcome Trust Clinical Research Programme and Queen Elizabeth Central Hospital, Blantyre, Malawi for their support. We are grateful to T. Plant, K. Stephens, A. Seeley and the Clinical Immunology Service, Birmingham, UK for technical assistance and F. Gotch and P. Kelleher for helpful discussions. This work was supported by a Clinical Research Fellowship from GlaxoSmithKline (to CAM), Tropical Research Fellowships from the Wellcome Trust (to CAM and MAG), a Programme Grant from the Wellcome Trust (to MEM), and a Scientific Projects Committee Research Grant from University of Birmingham (to CAM).
References
- 1.Clumeck N, Mascart-Lemone F, de Maubeuge J, Brenez D, Marcelis L. Lancet. 1983;1:642. doi: 10.1016/s0140-6736(83)91808-1. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs JL, Gold JW, Murray HW, Roberts RB, Armstrong D. Ann. Intern. Med. 1985;102:186. doi: 10.7326/0003-4819-102-2-186. [DOI] [PubMed] [Google Scholar]
- 3.Levine WC, Buehler JW, Bean NH, Tauxe RV. J. Infect. Dis. 1991;164:81. doi: 10.1093/infdis/164.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Gruenewald R, Blum S, Chan J. Clin. Infect. Dis. 1994;18:358. doi: 10.1093/clinids/18.3.358. [DOI] [PubMed] [Google Scholar]
- 5.Gordon MA, et al. Clin. Infect. Dis. 2008;46:963. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 6.Gilks CF, et al. Lancet. 1990;336:545. doi: 10.1016/0140-6736(90)92096-z. [DOI] [PubMed] [Google Scholar]
- 7.Vugia DJ, et al. J. Infect. Dis. 1993;168:564. doi: 10.1093/infdis/168.3.564. [DOI] [PubMed] [Google Scholar]
- 8.Kassa-Kelembho E, Mbolidi CD, Service YB, Morvan J, Minssart P. Acta. Trop. 2003;89:67. doi: 10.1016/j.actatropica.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Berkley JA, et al. N. Engl. J. Med. 2005;352:39. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MA, et al. AIDS. 2002;16:1633. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 11.Fields PI, Swanson RV, Haidaris CG, Heffron F. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5189. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanden RV, Mackaness GB, Collins FM. J. Exp. Med. 1966;124:585. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustamante J, et al. Curr. Opin. Immunol. 2008;20:39. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 14.MacLennan C, et al. J. Infect. Dis. 2004;190:1755. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan CA, et al. J. Clin. Invest. 2008;118:1553. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moir S, Fauci AS. Nat. Rev. Immunol. 2009;9:235. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane HC, et al. N. Engl. J. Med. 1983;309:453. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 18.Ammann AJ, et al. JAMA. 1984;251:1447. [PubMed] [Google Scholar]
- 19.Information on materials and methods is available in Science Online.
- 20.Gilks CF. Br. Med. Bull. 1998;54:383. doi: 10.1093/oxfordjournals.bmb.a011695. [DOI] [PubMed] [Google Scholar]
- 21.Chart H. In: Methods in Laboratory Bacteriology. Chart H, editor. CRC Press; London: 1994. pp. 11–20. [Google Scholar]
- 22.Fierer J, Guiney DG. J. Clin. Invest. 2001;107:775. doi: 10.1172/JCI12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joiner KA, Hammer CH, Brown EJ, Frank MM. J. Exp. Med. 1982;155:809. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Normann B, Stendahl O, Tagesson C, Edebo L. Acta. Pathol. Microbiol. Scand. [B] Microbiol. Immunol. 1972;80:891. doi: 10.1111/j.0365-5563.1973.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 25.Brenchley JM, et al. Nat. Med. 2006;12:1365. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 26.Allan JS, et al. Science. 1985;228:1091. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- 27.Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM. J. Clin. Invest. 1992;89:561. doi: 10.1172/JCI115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant MD, Weaver MS, Tsoukas C, Hoffmann GW. J. Immunol. 1990;144:1241. [PubMed] [Google Scholar]
- 29.De Milito A, et al. Blood. 2004;103:2180. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 30.Tejiokem MC, et al. PLoS ONE. 2007;2:e1260. doi: 10.1371/journal.pone.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opravil M, Fierz W, Matter L, Blaser J, Luthy R. Clin. Exp. Immunol. 1991;84:185. doi: 10.1111/j.1365-2249.1991.tb08146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.French N, et al. AIDS. 1998;12:1683. doi: 10.1097/00002030-199813000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Huber M, et al. Eur. J. Immunol. 2006;36:701. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 34.Gil-Cruz C, et al. Proc. Natl. Acad. Sci. USA. 2009;106:8903. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.