Abstract
Mirror self-recognition is often considered as an index of self-awareness. Neuroimaging studies have identified a neural circuit specialised for the recognition of one’s own current facial appearance. However, faces change considerably over a lifespan, highlighting the necessity for representations of one’s face to continually be updated. We used fMRI to investigate the different neural circuits involved in the recognition of the childhood and current, adult, faces of one’s self. Participants viewed images of either their own face as it currently looks morphed with the face of a familiar other or their childhood face morphed with the childhood face of the familiar other. Activity in areas which have a generalised selectivity for faces, including the inferior occipital gyrus, the superior parietal lobule and the inferior temporal gyrus, varied with the amount of current self in an image. Activity in areas involved in memory encoding and retrieval, including the hippocampus and the posterior cingulate gyrus, and areas involved in creating a sense of body ownership, including the temporo-parietal junction and the inferior parietal lobule, varied with the amount of childhood self in an image. We suggest that the recognition of one’s own past or present face is underpinned by different cognitive processes in distinct neural circuits. Current self-recognition engages areas involved in perceptual face processing, whereas childhood self-recognition recruits networks involved in body ownership and memory processing.
Keywords: Self, Face, Recognition, fMRI, Ownership, Plasticity
1.0 Introduction
The face is the most distinctive feature of our appearance, the one by which we come to be known to ourselves and to others as an individual (Tsakiris, 2008). The importance of faces to the self-concept and for social cognition leads to their physical features being processed in a specialised neural circuit (Downing et al., 2001; Kanwisher, 2000). In turn, there is some evidence to suggest that representations of one’s own facial appearance may be stored in a similarly specialised network of areas, which is engaged when a face is recognised as one’s own (Devue and Bredart, 2011; Kircher et al., 2000; Platek et al., 2008; Uddin et al., 2007).
Although there has been considerable neuroimaging research investigating self-face recognition (Devue and Bredart, 2011; Platek et al., 2008), one important question that has not previously been asked relates to the issue of continuity and plasticity of self-face representations as one’s facial appearance changes over time. How are different visual representations of one’s own face maintained in the brain and how are they integrated to form a continuous sense of self over time? To deal with changes in our appearance, areas in the brain must have plastic properties to update representations of one’s current facial appearance. In addition, humans are also able to recognise themselves in photos from their past. There must therefore be areas of the brain that store representations of one’s physical appearance from the past.
To date, only one previous functional imaging study has examined the issue of self-faces are processed in the context of changes with age (Arzy et al., 2009). Participants viewed current, younger or older versions of their own face, or that of George Clooney. The stimuli were created by artificially ageing photos of their current facial appearance, creating stimuli that appeared to be the participant at several different ages. They were asked to perform a subjective mental time judgement task, indicating whether an imagined event occured before or after their face was at the age they were viewing. They reported activity in the Temporo-Parietal Junction (TPJ), the Inferior Parietal Lobe (IPL), the Insula and the Inferior Frontal Gyrus (IFG) when the age of a participant’s face and the time-stamp of the imagined event were incongruent. This raises the possibility that these regions may process representations of one’s past facial appearances. However, as the participants were not performing a task that required them to judge the stimuli as themselves from the past, it is unclear whether these regions are explicitly involved in past self-recognition. In addition, the artificial nature of the stimuli used make it unclear whether participants were processing the face as their own, or simply as a face that is similar to their own that has been synthetically aged. As such, no previous study has directly examined how images of one’s face from the past are processed. In this study, we aimed to address these limitations. Participants performed a self-recognition task, judging whether a stimulus was their own face or the face of a personally familiar other, on real photos from their childhood and also photos of their current face. This enabled us to examine the areas of the brain engaged when processing images of one’s self from the past.
Interestingly, the regions identified by Arzy et al. (2009) during the mental subjective time judgement task, partially overlap with regions that are typically considered as important for self-recognition processing (Devue and Bredart, 2011; Devue et al., 2007; Feinberg and Keenan, 2005; Gillihan and Farah, 2005; Kaplan et al., 2008; Morita et al., 2008; Platek and Kemp, 2009; Platek et al., 2006; Platek et al., 2008; Turk et al., 2002; Uddin et al., 2005). A study by Uddin et al. (2005) reported that similar portions of the IPL and the IFG in the right hemisphere responded parametrically to the amount that a facial stimulus looked like the participant’s current facial appearance. In their task, participants were presented with pictures of faces. The faces were morphs that contained varying degrees of the participant’s face and the face of another person with whom the participant was personally familiar (e.g. 0%, 20%, 40%, 60%, 80% or 100% of the participant’s own face).The IPL and IFG showed a parametric response, increasing with the amount of current self in the stimuli. Given that similar portions of the IPL and the IFG are activated when processing one’s current facial appearance and also when making decisions whilst viewing one’s past facial appearance, this highlights these regions as candidates for integrating one’s past and current facial appearances and processing representations of both. In this study we examined whether such areas are engaged by both one’s current and past facial appearances.
In addition to the regions reported by Uddin et al. (2005) that overlapped with those reported by Arzy et al. (2009), Uddin et al. (2005) also reported activity in the inferior occipital gyrus (IOG), a portion of which is often referred to as the occipital face area (OFA) due to the presence of face-selective neurons. The activity in the IOG showed an increasing response the greater the percentage of the participant’s own face present in the morphed stimuli. Uddin et al. (2005) also reported activity in the ventromedial portions of the frontal lobe, the precuneus, the superior frontal gyrus, the Superior Partietal Lobe (SPL) and medial portions of a region covering the right inferior temporal gyrus (ITG) and the right middle temporal gyrus (MTG), that showed an increasingly negative response the more that the stimuli contained of the self-face. A recent review of the literature highlights that these regions are those that are typically reported as activated in studies that investigate the neural basis of current self-face recognition (Devue and Bredart, 2011). This therefore suggests that a network consisting of the regions reported by Uddin et al. (2005) may have plastic properties, updating and maintaining a representation of one’s current facial appearance. However, previous studies examining brain activity during self-other judgements, typically do not have a comparable control condition, in which “self” judgements are made on stimuli that are the participant’s own face, but not their current facial appearance. Thus, it is not clear which areas within this network are engaged exclusively by one’s current facial appearance. In our study, we address this confound by examining areas that respond to one’s current facial appearance, excluding areas that respond to a childhood self image. This design allows areas that maintain and update a representation of one’s current facial appearance to be identified.
The aim of this study was to investigate the areas of the brain involved in recognising one’s current and past facial appearances. Participants performed a self-face recognition task on morphed stimuli that were similar to those used by Uddin et al. (2005). The stimuli were created by morphing the current facial appearance of the participant into the current face of a familiar other, or alternatively, the childhood facial appearance of the participant into the childhood face of the same familiar other. We examined activity time-locked to the presentation of each morphed image. This design enabled us to test the predictions that (i) ventromedial portions of the frontal lobe, the precuneus, the SFG, SPL and medial portions of the ITG/MTG respond parametrically to the percentage of current self in the stimuli and activity in these regions will not vary parametrically with the percentage of childhood self (ii) the TPJ and Insula respond parametrically and exclusively to the amount of childhood self in the stimuli and (iii) the IPL and the IFG respond parametrically to the percentage of childhood and current self in the stimuli.
2.0 Materials and Methods
2.1 Participants
16 right-handed participants (10 female) between the ages of 19 and 33 years (M = 24.31, S.D. = 4.3) were recruited in pairs of eight acquaintances. The mean length of acquaintance between the participants and the paired partner was 30.43 months. Handedness was determined using the Edinburgh Handedness Inventory. All participants gave written informed consent; the study was approved by the local Ethics Committee, and the study conformed to regulations set out in the CUBIC MRI Rules of Operations (http://www.pc.rhul.ac.uk/sites/cubic/).
2.2 Apparatus
Photographs were taken on a Nikon 5 megapixel colour digital camera. Stimuli were created using Abrosoft Fantamorph 4 and Adobe Photoshop. Participants lay supine in an MRI scanner with the fingers of the right hand positioned on a four-button MRI-compatible response box. Stimuli were projected onto a screen behind the participant and viewed via a mirror positioned above the participants face. Presentation software (NeurobehavioralSystems, Inc., USA) was used to deliver stimuli and record responses. Brain images were acquired with a 3 Tesla Siemens Trio Magnetic Resonance Imaging scanner (Royal Holloway, University of London). Behavioural and fMRI Data were analysed in Matlab 2006a, SPSS 19 and SPM8.
Stimuli and Experimental design
Stimuli were idiosyncratically tailored for each scanned participant. Each participant saw images of faces that were constructed from a photo of themselves and a gender-matched personally familiar other. We used photos of individuals with whom the participant was personally familiar to control for the confounding effects of differences in the familiarity of the participant’s face and that of the other person that are present when using famous or unknown faces as the “other” stimulus. We note that participants were not personally familiar with the “other” individual at the time of their childhood. Whilst this acts as a limitation, as the childhood self and other faces were not equally personally familiar, it was necessary to use such stimuli as alternative stimuli (e.g. siblings or family members, celebrities) would have been confounded by the differences in frequency at which the faces were currently viewed (i.e., family members are seen at a much lower frequency during adulthood compared to childhood). Thus, alternative facial stimuli would have been currently, visually less familiar than the participant’s own face. The stimuli used in this study therefore were as closely matched in terms of visual and personal familiarity as possible.
Stimuli were created from current photos of themselves and the familiar other, and also from photos of each of their childhoods. Images of the participants’ current faces were taken from frontal position, pulling a neutral facial expression, under uniform lighting conditions. Participants also provided a photo of themselves from their childhood, with them aged between 10-14 years (M = 12.43, S.D. = 1.5) at the time the photograph was taken. Images of the participants’ current and childhood faces were flipped in the horizontal plane such that the actual image was reversed. This was to control for the fact that people typically see their own face in a mirror. Pictures were matched in size (height = 1000 pixels) and edited into greyscale.
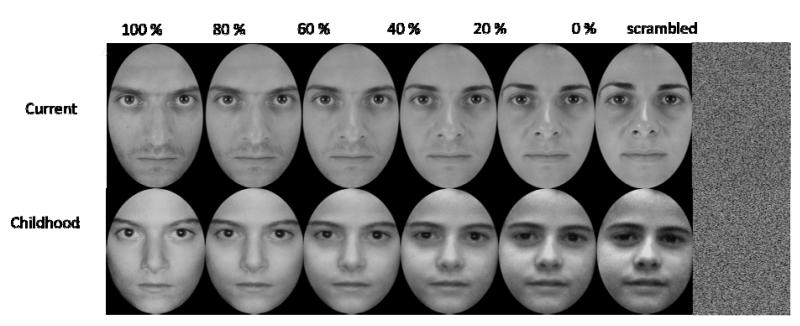
The main aim of this experiment was to examine whether activity in any area of the brain varied parametrically with the amount of one’s own face that was present in an image. We therefore used stimuli in which the amount of “self” was varied (see figure 1). To create stimuli that varied with the amount of “self” present, morphs were created between the current image of the participant and the current image of the familiar other. Morphs were also created between the images of the participant as a child and the images of the familiar other as a child. Morphs were created by varying the amount of “self” in the stimuli in 6 increments of 20% (i.e., 0%, 20%, 40%, 60%, 80% and 100% self in the image). It is notable that we did not morph the current self into the childhood self, given that this could be an alternative design that could potentially allow us to examine the neural basis of processing one’s past and current faces. We used stimuli in which the self was morphed with the familiar other, in order to perform a parametric analysis (see below). This design enabled us to analyse whether activity in the brain varied parametrically with the amount of current self, the amount of childhood self and with both the amount of current and childhood self. A design in which current self was morphed into childhood self would not have enabled us to examine activity that varied with each of these parameters, as some of the morphs would contain high percentages of both of the different self faces, making current and childhood self parameters collinear. In addition to the face stimuli, two scrambled images were created by randomly rearranging the pixels in a current stimulus and also in a childhood stimulus. Thus, there were 14 stimuli in total (see figure 1). Participants were presented with one of these images on each trial and were required to perform a self-recognition task. On each trial they were instructed to press one button on the keypad with their index finger, if they thought the image they were seeing was more themselves and another button with their middle finger if the image they were seeing was more the familiar other or the scrambled image. Stimuli were presented in four blocks, each of which lasted approximately nine minutes. Each stimulus was presented five times in a block, such that there were 70 stimuli presented in each block and 280 trials in total (20 repetitions of each stimulus). Stimuli were presented for 2s. Stimuli were randomly arranged in a different order within each block and for each participant. Participants were required to make their response during or after the stimuli were presented on the keypad. Inter-trial intervals (ITI) ranged from 2s-10s (M = 5.5s) and were varied according to a Poisson distribution.
Figure 1.
Stimuli. Each stimulus was a morph that contained different percentages of the participants’ own face. There were two sets of morphs: one set were morphs between the participants’ current facial appearance and the appearance of a personally familiar other (a). The second set of morphs were between the participants’ childhood face and the childhood face of the same personally familiar other (b). Participants were presented with one morphed image on each trial, or a scrambled image. The morphed images in this figure are of two of the experimenters, used for illustrative purposes only.
2.3 Image Acquisition
For each participant, T2* weighted echoplanar (EPI) images were acquired. The field of view covered most of the brain (36 axial slices; field of view = 192mm × 192mm; voxel size = 3mm × 3mm × 3mm; image matrix = 64mm × 64mm; TR = 2.5s; TE = 32s; flip angle = 90°). Prior to the functional scans, high resolution T1-weighted structural images were acquired at a resolution of 1×1×1mm using an MPRAGE sequence (TR = 1830; TE = 5.56ms; flip angle = 11°).
2.4 Image Analysis
All preprocessing and statistical analyses were conducted using SPM8 (www.fil.ion.ucl.ac.uk/spm). The EPI images were first realigned, and coregistered to the subject’s own anatomical image. The structural image was processed using a unified segmentation procedure combining segmentation, bias correction, and spatial normalization to the MNI template (Ashburner and Friston, 2005); the same normalization parameters were then used to normalize the EPI images. Lastly, a Gaussian kernel of 8mm FWHM was applied to spatially smooth the images in order to conform to the assumptions of the GLM implemented in SPM8.
2.5 Statistical Analysis
To analyse the functional data we used a parametric approach. For each participant, regressors were created in a design matrix by convolving the event onset delta functions with the canonical haemodynamic response function (HRF) for 3 different events in each block (12 event-related regressors in total in the four blocks): one regressor for the current faces (both self and familiar other), one regressor for the childhood faces (both self and familiar other) and a third for the scrambled images (concatenated across both current and childhood scrambled). Six head motion parameters (3 translations and 3 rotations), estimated during Realignment, were incorporated as confounding regressors for each functional run, i.e., for each session (24 in total). To examine whether activity in any voxel covaried parametrically with the amount of one’s own face that was present in an image, parametric modulators were created for both the current and childhood face event-related regressors. These parametric modulators scaled the amplitude of the HRF to correspond with the percentage of one’s own face that was present in each stimulus, i.e., the parameter predicted a maximal response when the image was 100% self, with incrementally decreasing responses for images when there was less of the participant’s face in an image. Separate parameters were created for the current images and the childhood images in each block. SPM{t} images were then created for each event-related regressor in each session.
Second-Level
The second-level analysis strategy was similar to that used in a previous paper (Apps et al., 2012). SPM{t} contrast images from the first-level were input into a second-level full factorial (one factor was ‘event’ and the second factor was ‘block’) random effects ANOVA with pooled variance. F-contrasts were applied at the second level to look for areas in which activity varied statistically with a linear combination of the betas corresponding to the parametric modulators across the blocks. To look for areas that varied parametrically with the amount of self in the current images, voxels in which activity varied parametrically with the amount of self in the childhood images were excluded (P<0.05unc) and vice versa for examining activity varying with the amount of self in the childhood images. To create peristimulus time histogram (PSTH) plots of the response in ‘activated’ voxels a secondary analysis was conducted that used a 2×2 factorial design. One factor was ‘agent’ (self or other) and the second factor was ‘time’ (childhood or current). In this analysis the images that contained 80% or 100% self were categorized as “self” in the agent factor and the images that contained 0% or 20% self images were categorized as “other”. Recent studies have also examined whether there are differences in activity between the response “self” and the response “other” for morphed images containing the amount of the self-face (Ma and Han, in press). Unfortunately, given the large number of stimuli used in this study and therefore the limited number of repetitions, there was not enough statistical power to compare activity between different responses within morphs containing the same percentage of self. The analysis we report here therefore reflects the best possible manner of examining the hypotheses of this study.
False-discovery rate (FDR; p < 0.05) was used for whole-brain correction for multiple comparisons. Small volume corrections (SVC) were also applied around the coordinates of Uddin et al. (2005). The coordinates from Uddin et al. (2005) were chosen as the stimuli and task used in this study were similar to those that they employed. An alternative approach could have been to use another study examining self-face recognition or the coordinates from a meta-analysis. However, very few studies have used stimuli where the personally familiar face of another is morphed into one’s own facial appearance and examined the parametric nature of activity during a self-face recognition task, in the same manner as Uddin et al. (2005) did. In addition, the available meta-analyses include studies that do not examine differences between self and the faces of familiar others, and studies that do not perform self-recognition tasks. As a result, many of the available meta-analyses are likely to exhibit anatomical variability in terms of the location of their activity, meaning that correcting around their coordinates may result in false negatives. As such, we felt that the similarity of the design of Uddin et al. (2005) and the design in the current study made the coordinates reported by Uddin et al. (2005) the most appropriate to use for correction. We would like to note that there is a limitation in using the coordinates of Uddin et al., (2005) for correcting for multiple for comparisons for the childhood parameter. In their study, the stimuli were morphs between current adult faces and not childhood faces. Ideally a correction would be applied around the coordinates of a study that examined self-recognition using childhood faces. However, no previous study using such a design has been conducted. We therefore used the coordinates of a study that was matched in terms of the task performed by participants and that used morphed facial stimuli. The corrections were applied around each of the coordinates reported for both the Self>Other and the Other>Self contrasts listed in their results. We applied these corrections to each of the three contrasts that were conducted. We only report areas as activated if their activation is significant following whole brain correction or if it survived small volume correction around one of Uddin et al.’s (2005) coordinates. Anatomical localization was performed using the brain atlas of Duvernoy (1988).
3.0 Results
3.1 Behavioural Results
Participants performed a self-other recognition task on stimuli that were morphs between their own childhood face and the childhood face of a personally familiar other and also on stimuli that were morphs between their own current face and the face of the same personally familiar other. On each trial they were presented with one facial stimulus, or a scrambled image that contained a percentage (0%, 20%, 40%, 60%, 80% or 100%) of their own face. They were required to indicate whether the face looked more like them, or more like the other person or the scrambled image.
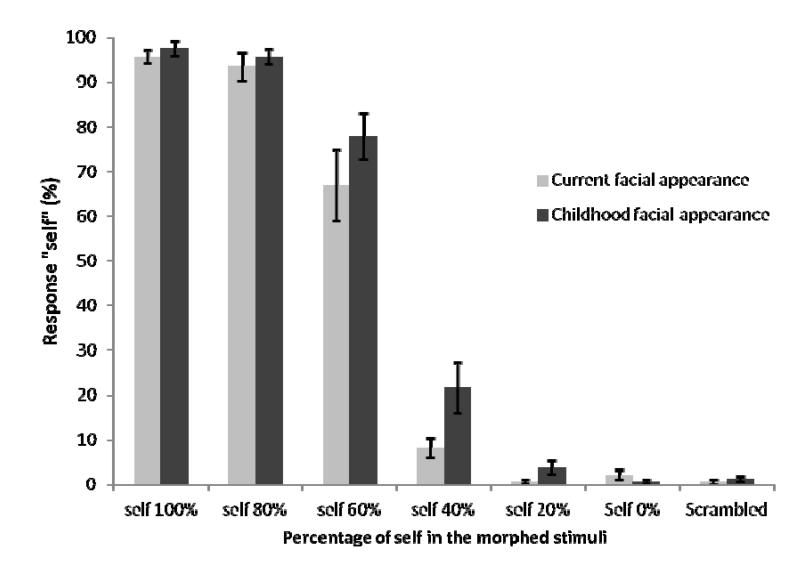
To examine whether participants performed self-other judgements differently for the childhood and current facial stimuli, we performed a repeated measures ANOVA on the percentage of “self” responses. We used a 2×6 factorial design in which the first factor was the Age of the individuals in the facial stimuli (Current, Childhood) and the second was the percentage of Self in the stimuli (0%, 20%, 40%, 60%, 80% and 100%). The results showed a main effect of the percentage of Self in the stimuli (F(1.8, 35.8 (greenhouse-geisser corrected)) = 332.1; p < 0.001), which also showed a significant linear trend (F(1,15) = 2007.1, p < 0.001). Examination of Figure 2 shows that this effect is being driven, unsurprisingly, by increased numbers of “self” responses being made as the percentage of self increases in the stimuli. There was also a main effect of the Age of the individuals in the stimuli (F(1, 35.8) = 6.7, p < 0.05), with overall a greater percentage of responses as “self” for the current stimuli than for the childhood stimuli. There was no significant linear interaction between Age and percentage of Self (F(2.4, 35.8) = 1.9, p > 0.05). However there was a significant quadratic trend to the interaction (F(976.3, 3027.2 = 4.8 p < 0.05). Examination of the histogram in Figure 2 shows that this quadratic interaction appears to be driven by a greater number of “self” responses for the current 20% and 40% stimuli, compared to the childhood 20% and 40% stimuli. In summary, our results show that participants were more likely to judge morphs containing their current face as “self” than those containing their childhood face. Such effects were particularly prevalent when the percentage of “self” in the morph was less than 80%. Importantly, overall our results show that participants were able to distinguish between self and other, indicating that they understood the task sufficiently.
Figure 2.
Behavioural results. Percentage of “self” responses for each of the current and childhood stimuli. Error bars depict the between-subject standard error of the mean.
3.2 fMRI Results
This study examined whether activity in any area of the brain varied parametrically with the amount of a participant’s own current or childhood face that was morphed into the current or childhood face of a personally familiar other. We used a parametric approach to examine activity that statistically varied with (i) the amount of one’s own current face in an image, (ii) the amount of one’s own childhood face in an image and (iii) with both the amount of one’s own childhood and current face in the images. To avoid false negative results in the whole brain analysis, we also applied small volume corrections around the coordinates of Uddin et al. (2005). Previously they identified a set of brain areas that show differential responses to morphs that contain a high percentage of a participant’s current face, compared to morphs that contain a high percentage of the current face of a familiar other. We applied these corrections to each of the three analyses outlined above, to examine whether these areas were sensitive to the current, childhood or both faces of the participants.
3.2.1 Current Faces
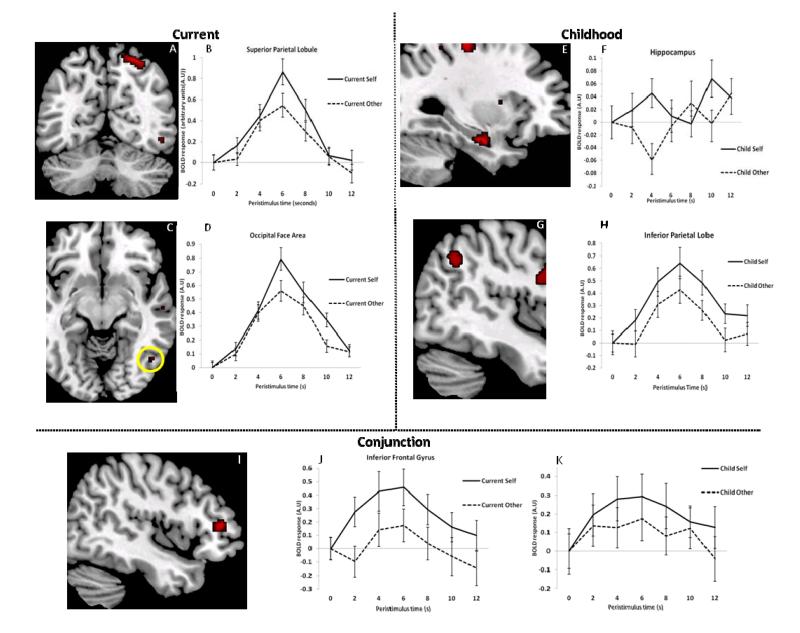
To examine whether activity in any area of the brain was scaled parametrically with the amount of one’s own face, an F-contrast was performed on the parametric modulators of the current face events. To ensure that any voxels that varied parametrically with this regressor did not also vary parametrically with the amount of one’s childhood face in the stimuli, voxels in which the response varied parametrically with the amount of childhood face in an image were excluded (F-contrast; p < 0.05 uncorrected). Whole brain analysis did not find any voxels that varied parametrically with the amount of participants’ current self in the image. However, small volume corrections (spheres with 8mm diameter) around the peak coordinates of Uddin et al. (2005) revealed activation (see figure 3) in the right ITG (BA 20) (MNI coordinates: 62, −12, −16, Z = 3.04, p < 0.05svc), the right inferior occipital gyrus (IOG; BA18/19), putatively in the OFA (48, −62, −8, Z = 3.21, p < 0.05svc) and the right SPL (BA 7/BA19) (28, −62, −8, Z = 3.71, p < 0.005svc) that varied with the amount of the participant’s current face in the stimuli. No other regions reported by Uddin et al. (2005) survived small volume correction. Examination of the PSTH plots reveals that activity in the SPL and the IOG was not exclusive to the current face stimuli. However, both areas showed an increased response to one’s own current face in comparison to the current face of the familiar other.
Figure 3.
fMRI results. Current self: Activity shown in (a) the Superior Parietal Lobule (SPL) and (c) in the Inferior Occipital Gyrus (IOG) – putatively in the Occipital Face Area (OFA) - that varied with the amount of current self present in the morphed stimuli; activity in these regions did not vary with the amount of childhood self in the stimuli. Peristimulus time histogram (PSTH) plots of activity from the peak voxel in the SPL (b) and the IOG (d) time-locked to the stimuli containing current facial appearances. Childhood self: Activity shown in (e) the Hippocampus and (g) the Inferior Parietal Lobule (IPL) that varied with the amount of childhood self present in the morphed stimuli.; activity in these regions did not vary with the amount of current self in morphed stimuli. PSTH plots of activity from the peak voxel in the Hippocampus (f) and the IPL (h) time-locked to the stimuli containing childhood facial appearances. Conjunction: Activity shown in the Inferior Frontal Gyrus (IFG) (i) that varied with both the amount of childhood and current self in the morphed stimuli. PSTH plots of activity from the peak IFG voxel, time-locked to (j) the current stimuli and (k) the childhood stimuli. The current and childhood self data in the PSTHs are plots of activity evoked by the stimuli containing 100% and 80% “self”, and the current and childhood other data are plots of the activity evoked by the stimuli containing 0% and 20% “self”.
3.2.2 Childhood Faces
To examine whether activity in any area of the brain was scaled parametrically with the amount of one’s own childhood face in a stimulus, an F-contrast was performed on the parametric modulator of the childhood face events. To ensure that activity in any voxels that varied parametrically with the amount of self in the childhood images did not also vary parametrically with the amount of one’s current face in the image, voxels in which the response varied parametrically (F-contrast; p < 0.05) with the amount of current face in an image were excluded (p < 0.05 uncorrected). Whole brain correction for multiple comparisons revealed activity in several areas (see figure 3) that varied parametrically with the amount of one’s own childhood face in the stimuli (see table 1). Small volume corrections around the coordinates of Uddin et al. (2005) did not find any additional regions activated to those identified in the whole brain analysis.
Table 1.
Full list of fMRI results for activity varying with the amount of childhood self in the stimuli.
| Anatomical region | Hemisphere | Brodmann Area (BA) |
MNI Coordinate (x, y, z) |
Z-value1 |
|---|---|---|---|---|
| Parietal | ||||
| Inferior Parietal Lobule - Angular Gyrus |
R | BA 7 | 30 −62 36 | 4.53 |
| Intraparietal Sulcus | L | BA 7 | −18 −42 60 | 3.61 |
| Precuneus | L | BA 7 | −32 −34 −6 | 3.34 |
| Frontal | ||||
| Precentral Gyrus | L | BA 6 | −12 −4 62 | 5.47 |
| Middle Frontal Gyrus | L | BA 9/46 | −54 18 34 | 5.33 |
| Medial Superior Frontal Gyrus | L | BA 8 & BA 32 | −4 38 54 | 4.97 |
| Orbital Gyrus | L | BA 13 | −28 26 −16 | 4.44 |
| Middle Frontal Gyrus | R | BA 9/46 | 50 16 28 | 4.3 |
| Orbital Gyrus | R | BA 13 | 24 30 −16 | 3.90 |
| Precentral Gyrus | R | BA 6 | 18 0 60 | 3.77 |
| Superior Frontal Gyrus | R | BA 8 | 22 32 56 | 3.62 |
| Cingulate | ||||
| Posterior Cingulate Gyrus | L | BA 23 | −2 −14 32 | 3.31 |
| Isthmus of the Posterior Cingulate Gyrus |
L | BA 26 or29 | −4 −36 30 | 3.11 |
| Temporal | ||||
| Hippocampus | L | −34 −10 −20 | 4.4 | |
| Inferior Temporal Gyrus | R | BA 37 | 54 −48 −16 | 3.55 |
| Inferior Temporal Gyrus | L | BA 37 | −44 −46 −14 | 3.42 |
| Temporal Parietal Junction | L | BA 39or 40 | −54 −38 14 | 3.2 |
| Temporal Parietal Junction | R | BA 39 or40 | 60 −26 10 | 3.13 |
| Cerebellum | ||||
| Lobule VI | R | 16, −46, 16 | 4.06 |
All results are whole-brain FDR corrected for multiple comparisons. Activity in these areas did not vary with the amount of current self in the stimuli.
3.2.3 Conjunction between Childhood and Current Faces
To examine whether activity in any area of the brain was scaled parametrically with the amount of one’s own face in the images, regardless of whether it was a childhood or current face, a conjunction was performed between the two F-contrasts outlined above. Whole-brain analysis did not reveal any effects that survived correction for multiple comparisons. Small volume correction around the peak coordinates from Uddin et al. (2005) revealed activity (see figure 3) in the inferior frontal gyrus (IFG; BA 46; see figure 5) that covaried with the amount of one’s own face in an image (48, 42, 6, Z = 3.41, p < 0.05svc). No other regions reported by Uddin et al. (2005) survived small volume correction. Examination of the PSTH plots reveals that the IFG showed increasing responses the more self present in the stimuli, regardless of whether it was a current or childhood face.
4.0 Discussion
This study recorded neural activity while participants made self-recognition judgements when looking at their own current or childhood faces morphed, respectively, with the current or childhood faces of a personally familiar other. Our first aim was to identify areas in the brain that have plastic properties and process one’s own current facial appearance and not one’s past appearance. Second, we aimed to identify areas that store representations of one’s past appearance, processing only one’s childhood facial appearance and not one’s current appearance. Thirdly, we aimed to identify areas that process one’s appearance independently of the age of the face. The behavioural results indicate that participants could easily distinguish self from other for both the current and childhood faces. However, participants showed a bias for identifying a current image that contained only low percentages (e.g., 20% and 40%) of their own face as “self”, as compared to the childhood stimuli that contained the same percentage of their face. These results suggest that the greater salience of one’s own present facial appearance, as compared to one’s past facial appearance, leads to a greater sensitivity to features of the current self face. This sensitivity biases participants more towards a response of “self” even when a stimulus contains only a small percentage of their own current appearance.
For the fMRI results, we examined activity that varied with the three parameters using two statistical thresholds, a whole brain analysis (p < 0.05 FDR corrected) and small volume corrections (p < 0.05 FWE corrected) around the coordinates of a previous paper (Uddin et al., 2005). Whole brain analysis revealed activity in several areas that covaried with the amount of the participant’s childhood face that was morphed into the childhood face of the familiar other, with no response to the current facial stimuli. The small volume correction analysis revealed no regions that responded to the amount of childhood self in the stimuli, reflecting the fact that childhood and current self-recognition may recruit separate neural circuits. The small volume correction analysis did reveal activity in the ITG, the SPL and the IOG (putatively in the OFA) that varied with the percentage of the participants’ current facial appearance that was morphed into the face of the familiar other, with no response to any childhood stimuli. In addition, the small volume correction analysis revealed activity in only one area, a portion of the IFG, that was sensitive to both the percentage of the participant’s current and childhood face present in the image. Overall, although the results for the childhood parameter survived a more stringent statistical threshold than the results for the current self or conjunction analyses, they support the notion that activity in distinct networks is modulated by the amount of one’s past and current appearance that is present in a stimulus during self-face recognition. This is indicative of distinct cognitive processes being engaged when recognising a face as one’s own from the past or from the present.
4.1 Recognition of the current self-face
This study is not the first to investigate activity in the brain when participants are performing a self-other recognition task on their own face and that of a familiar other (Devue and Bredart, 2011; Platek et al., 2008). However, a unique feature of the design of our study enables us to shed further light on the neural processes that underpin self-face recognition. Specifically, in this study we used a parametric design, with stimuli created by morphing photos of the participant with a personally familiar other. By using this approach we did not make direct comparisons between brain activity evoked by current and childhood stimuli, as one typically would for a factorial design. The advantage of this approach is that our results cannot be explained by the potentially confounding effects of differences in difficulty for recognising past and present images as “self” or “other”. Previously, Uddin et al. (2005) used a very similar design and stimuli to investigate the neural antecedents of recognising one’s current facial appearance. However, whilst their design was parametric in nature, their analysis involved only the subtraction of activity between all “self” (60%, 80% and 100% self) and “other” (0%, 20% and 40%) conditions, as such they did not test statistically whether activity in these areas was scaled parametrically with the percentage of “self” in the images. Our results support our hypotheses and also the results of Uddin et al., (2005) by showing that activity in the SPL, the ITG and the OFA varies statistically with the percentage of current self in a facial stimulus, but not in the other areas reported by Uddin et al. (2005). Although we note that these areas did not survive whole-brain correction in this study and were only significant when corrections were applied around the coordinates of Uddin et al. (2005). However, despite this caveat the results of our study still support the claim made by Uddin et al. (2005) that activity in these three areas varies with the extent to which a face is recognised as one’s own, but not the wider network that has previously been reported by Uddin and others (Devue and Bredart, 2011; Platek et al., 2008).
Whilst our results support previous claims that activity in the SPL, the ITG and the OFA (Kircher et al., 2000; Uddin et al., 2008; Uddin et al., 2005) varies with the amount of current self in an image, it is interesting to note that activity in these areas was not exclusive to the processing of one’s own current face. Rather, these areas were activated when participants viewed the stimuli that contained a large percentage of the familiar other, but exhibited a greater response to the stimuli which contained high percentages of the current self. This would support the notion that these areas are face sensitive but do not process one’s own face selectively. Indeed, each of these three areas has been found to contain patches which are face selective in both humans and monkeys. The ITG contains several face-selective regions and in addition, the portion of the IOG activated in the study also contains a face selective region (Barraclough and Perrett, 2011; Freiwald and Tsao, 2010; Perrett et al., 1992; Perrett et al., 1982; Pitcher et al., 2011; Rajimehra et al., 2009). It has been reported that some neurons in the ITG become selective for the processing of one face, with the highest spike rate evoked by that specific face (Barraclough and Perrett, 2011). In addition, a subset of these neurons have been shown to increase their spiking rate each time that the same face is presented (Li et al., 1993). A functional imaging study has also reported differential responses in the ITG and the IOG to familiar and unfamiliar faces (Rotshtein et al., 2005), suggesting that these areas have plastic properties, increasing their response as a particular face becomes gradually more familiar. Thus, in our study, whilst activity in these areas is increased when viewing one’s own current image, the faces of oneself and others were still processed in both of these areas. One’s current facial appearance evoked a quantitatively different response at the population level in each of these regions. This differential response may be a result of the increased familiarity and regular, recent exposure to one’s own current facial appearance compared to another’s face.
We also found activity in the SPL, in a region in close proximity to a portion of the intraparietal sulcus that purportedly contains a face-selective region in humans and non-human primates. This area has been found to process somatosensory and visual information about the spatial location of stimuli in reference to one’s own face (Avillac et al., 2005; Duhamel et al., 1998; Sereno and Huang, 2006). In our study, this region responded the more a stimulus contained the participant’s own current facial appearance than the current appearance of another or any childhood face. This would suggest that this region also has plastic properties, updating information about the physical properties of one’s current facial appearance, in order to process sensorimotor information about the face. This therefore suggests that recognising one’s own current face, may involve multisensory processes.
The results of this study therefore argue against the notion that there is a large network of areas which are specialised for coding a representation of one’s own current facial appearance. Rather, current self-recognition may result from quantitative differences in the spiking activity of neurons in areas of the brain which have a more generalised specialisation for recognising faces. In addition, this information about the visual properties of one’s face may be integrated with somatosensory information about one’s body in areas that process multisensory information about one’s own face.
4.2 Recognition of the past self-face
This study is the first to examine the neural mechanisms that underpin the process of recognising a past facial appearance as one’s own, and it is therefore the first to support the hypothesis that activity in the TPJ is modulated by the percentage of childhood self present in facial stimuli. Furthermore, we found a similar profile of activity in the IPL. The activated regions reported in this study did not overlap with the portions of the IPL and the TPJ that were reported by Uddin et al. (2005) and were not activated following small-volume correction around their coordinates, only in a whole brain analysis. This therefore suggests that the portions of the TPJ and the IPL activated are not those that have previously been found to be engaged during self-face recognition. In contrast, these regions are well known for their role in integrating information from different sensory systems creating a sense of ownership over one’s body (Farrer and Frith, 2002; Tsakiris, 2010). TMS to the right TPJ has been shown to disrupt one’s ability to maintain a sense of ownership of a rubber hand, during the rubber hand illusion (Tsakiris et al., 2008). In this illusion, tactile stimulation of one’s own hand and the viewing of synchronous stimulation on a rubber hand causes a sense of ownership over the rubber hand. TMS to this region has also been shown to cause out-of-body experiences (Blanke et al., 2005; Blanke et al., 2002), where participants become detached from their body, often having the sense that they are observing their body from a remote viewing position. Thus, the integrity of activity in the TPJ may be important for creating and maintaining a sense of ownership over body parts. In addition to these findings, TMS to the right TPJ has been shown to disrupt behaviour on a self-face recognition morphing task (Heinisch et al., 2011). The TPJ may therefore play an important role in maintaining a sense of ownership of the “material me”, i.e., the recognition and ownership of one’s past facial appearance, as shown here.
The adjacent portion of the IPL has also been implicated in creating a sense of ownership, although it may have a more important role in the ownership of actions, or “agency”. Previous fMRI studies have found that this region is activated when participants have a sense that they were the agent that caused an action (Farrer et al., 2003; Farrer and Frith, 2002; Ruby and Decety, 2001). Specifically, activity in the IPL is greater when viewing an action performed by oneself, compared to when viewing an action performed by another (Buccino et al., 2004; Iacoboni et al., 2005; Van Overwalle and Baetens, 2009). There is also previous evidence that highlights this region as important for recognising a face as one’s own. rTMS to this region has also been shown to alter behaviour on self-face recognition tasks (Uddin et al., 2006). Activity in the IPL is therefore central to normal self-face recognition and perception. Thus, whilst the IPL and the TPJ may have distinct functional properties, the two areas share the common property of creating a sense of ownership over one’s own body. We argue that the TPJ and the IPL play an important role in recognising one’s childhood face, by creating a sense of ownership over images of one’s past facial appearances.
In addition to activity in areas involved in body-ownership, activity in the hippocampus and the isthmus of the posterior cingulate gyrus was also found to vary with the amount of childhood self in the stimuli. The portion of the posterior cingulate gyrus activated in this study and the hippocampal formation are typically known for their role in memory encoding and retrieval (Fink et al., 1996; Maguire and Mummery, 1999; Poppenk et al., 2010; Vann et al., 2009). Previously, the hippocampus has also been found to be activated during self-face recognition tasks, where participants are required to distinguish their own face from that of unfamiliar others (Kircher et al., 2000). However, studies which compare activity when participants view their own face with either famous or personally familiar faces do not report differential responses to self and other stimuli in the hippocampus (see Devue and Bredart, 2011). This suggests that these areas are activated when faces which have previously been perceived are processed, regardless of whether it is one’s own face or that of another person. It has been argued that these areas are engaged when autobiographical memories are processed following the presentation of a familiar face (Ramasubbu et al., 2011). However, we note that the portions of the PCC and Hippocampus we report as activated for the childhood self parameter are distinct from those reported in Ramasubbu et al. (2011). Given the extent of functional heterogeneity in both the PCC (Cauda et al., 2010) and the Hippocampus (Carr et al., 2010) the discrepancy in locations would suggest that the portions activated in our study may be unrelated to autobiographical processing. We therefore suggest that self-face recognition may require the recollection of stored representations of faces when participants are familiar with the faces they are viewing.
Our study supports this notion, as activity was evident in the hippocampus for images of the participant’s own appearance from childhood, but a decreased response was found for the stimuli which contained a high proportion of the childhood familiar other. As participants were not personally familiar with the other individual at the time that the childhood photo was taken and therefore with the physical features of the childhood other in the stimuli, it is unlikely that a representation of their features had previously been encoded by the participant. Thus, the decreased response to the childhood “other” face, which was the face that the participants were the least familiar with, but sustained response for faces which the participant was familiar with, suggests that these areas are sensitive to the familiarity of faces. Thus, circuits involved in memory processing may play an important role in processing faces, by storing representations of facial features. These representations are then retrieved when viewing a familiar face.
Activity was also found to vary with the amount of childhood self in large clusters extending over the dorsal medial superior frontal gyrus and adjacent portions of the middle frontal gyrus. Many have argued that these areas, in particular the portions of the superior frontal gyrus on the medial wall, are important for self-referential processing and for making decisions about one’s own personal characteristics (Gusnard et al., 2001; Kelley et al., 2002; Northoff and Bermpohl, 2004; Northoff et al., 2006). However, these areas are known to have strong connections to the motor system, suggesting that they may play an important role in guiding action selection, rather than self-referential processing (Petrides and Pandya, 2006). Neuroimaging studies have shown that activity in these areas occurs during the preparation of actions when stimulus-response mappings are abstract and must be recalled from memory (Lau et al., 2007; Lau et al., 2004). Tentatively, we suggest that judging an image of one’s own face from childhood as “self”, may reflect the processing of the abstract association between one’s childhood image and the finger movement required to indicate the response of “self” on the keypad. Such an abstract stimulus-response mapping may be required for childhood self images, as the image is not their current facial appearance and thus there is a more abstract link between the childhood image and the response “self” than there is for the current image and the response “self”. Similarly, there is a less abstract mapping between the response “other” and the images of the other person, as these images have always been considered “other” by the participant. We argue that the portions of the superior frontal gyrus that were activated in this experiment may be related to the stimulus-response mappings required to perform the task, rather than these areas being engaged by self-referential processes.
4.3 Recognition of the self-face across time
Our results supported the hypothesis that activity in the IFG would vary with both the amount of current and childhood self in the stimuli. Indeed this was the only region to show such a profile. Previous fMRI studies have found activity in this area when processing self-face images compared to the faces of familiar or unfamiliar others (Devue et al., 2007; Platek et al., 2006; Sugiura et al., 2008; Uddin et al., 2005). It has been suggested that this area is engaged when making evaluative judgements about one’s own face (Morita et al., 2008; Platek et al., 2006; Platek et al., 2008; Sugiura et al., 2008; Uddin et al., 2005). Whilst the results of this study also support this notion, there is a limitation to the tasks used in each of the studies that report self-face evoked activity in the IFG, including the task which was used in this study (Devue et al., 2007; Kaplan et al., 2008; Platek and Kemp, 2009; Platek et al., 2009; Platek et al., 2006; Platek et al., 2008; Sugiura et al., 2008; Sugiura et al., 2006; Uddin et al., 2008; Uddin et al., 2005). Specifically, participants were required to indicate their self-other judgement in a task where there was a one-to-one mapping between a judgement (“self” or “other”) and the effector which must perform an action to implement the rule, i.e., one button was always “self” and one was always “other”. However this portion of the IFG is best known for its role in preparing abstract rule-related actions (Petrides and Pandya, 1999; Wallis et al., 2001). Specifically, this portion of the prefrontal cortex is engaged by symbolic cues which instruct the performance of an action by a particular effector in order to implement a rule (Balsters and Ramnani, 2008; Bunge et al., 2003). Thus, in our study and in others which investigate self-face recognition, activity in the IFG may be driven by the one-to-one mapping between the “self” and “other” stimuli and the specific effector, which must be used to perform the action required to indicate the self-other judgement. Future studies should aim to disentangle such action-rule mappings to examine whether the IFG plays an important role in self-recognition or is engaged in processing task-related preparations of motor responses.
4.4 Limitations
One limitation of this study is that there are potentially differences in cognitive demand and difficulty when making self-other judgements on the childhood faces compared to the current faces, due to the differences in the salience of the faces. This confound is unfortunately unavoidable, as it was not possible to control for differences in salience between one’s current and one’s past facial appearance. Such a confound limits the interpretation we can make about the behavioural data and may potentially explain why there were differences between the behaviour on the self-recognition task on the childhood faces compared to the current faces. This may also explain why the results for the current facial stimuli did not reach the same statistical threshold as those for the childhood stimuli. However, such effects do not alter our interpretation of the fMRI results, as we did not make direct comparisons between childhood and current stimuli and we examined whether activity increased linearly with the amount of current and childhood self.
4.5 Conclusions
In conclusion, the results of this study suggest that recognising one’s past and current facial appearances relies on processing in distinct neural circuits. Recognition of one’s physical appearance from the past engages areas involved in body-ownership and recognition memory. This suggests that identifying a past facial appearance as one’s own requires the physical features of the face to be retrieved from memory and coded for as a part of one’s body. In contrast, a representation of one’s current self-image is maintained and updated through plastic processes in areas of the brain that are specialised for processing faces, but not specifically one’s own face. The absence of overlap between the systems engaged in self-recognition of one’s past and current physical appearance, suggests that recognising oneself from the past recruits different cognitive processes from those recruited when recognising one’s current appearance in a mirror. These findings pave the way for understanding the neural mechanisms underlying the plasticity of self-representations as a result of changes in appearance due to normal ageing as well as due to traumatic events or reconstructive surgery.
Highlights.
Separate neural circuits process one’s current and childhood face.
Current self-recognition engages areas within the face perception network
Childhood self-recognition recruits body-ownership and memory retrieval networks
Acknowledgements
The authors would like to thank Ari Lingeswaran, Dr. Matt Wall, Dr. Velia Cardin and Dr. Marcello Contstantini for help with the design and with data collection.
Funding: ESRC First Grant (RES-061-25-0233) and a European Research Council (ERC-2010-StG-262853) grant to MT, Bial Foundation Bursary for Scientific Research 2010/2011 to AT-J and MT.
References
- Apps MAJ, Balsters JH, Ramnani N. The anterior cingulate cortex: Monitoring the outcomes of others’ decisions. Social neuroscience. 2012;7:424–435. doi: 10.1080/17470919.2011.638799. [DOI] [PubMed] [Google Scholar]
- Arzy S, Collette S, Ionta S, Fornari E, Blanke O. Subjective mental time: the functional architecture of projecting the self to past and future. European Journal of Neuroscience. 2009;30:2009–2017. doi: 10.1111/j.1460-9568.2009.06974.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avillac M, Deneve S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nature Neuroscience. 2005;8:941–949. doi: 10.1038/nn1480. [DOI] [PubMed] [Google Scholar]
- Balsters JH, Ramnani N. Symbolic representations of action in the human cerebellum. Neuroimage. 2008;43:388–398. doi: 10.1016/j.neuroimage.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Barraclough NE, Perrett DI. From single cells to social perception. Philosophical Transactions of the Royal Society B-Biological Sciences. 2011;366:1739–1752. doi: 10.1098/rstb.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. Journal of Neuroscience. 2005;25:550–557. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Neuropsychology: Stimulating illusory own-body perceptions - The part of the brain that can induce out-of-body experiences has been located. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: An event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Carr VA, Rissman J, Wagner AD. Imaging the Human Medial Temporal Lobe with High-Resolution fMRI. Neuron. 2010;65:298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE. Functional Connectivity of the Posteromedial Cortex. Plos One. 2010;5 doi: 10.1371/journal.pone.0013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devue C, Bredart S. The neural correlates of visual self-recognition. Consciousness and Cognition. 2011;20:40–51. doi: 10.1016/j.concog.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Devue C, Collette F, Balteau E, Dequeldre C, Luxen A, Maquet P, Bredart S. Here I am: The cortical correlates of visual self-recognition. Brain Research. 2007;1143:169–182. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang YH, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: Congruent visual and somatic response properties. Journal of Neurophysiology. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod A. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18:324–333. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Keenan JP. Where in the brain is the self? Consciousness and Cognition. 2005;14:661–678. doi: 10.1016/j.concog.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiwald WA, Tsao DY. Functional Compartmentalization and Viewpoint Generalization Within the Macaque Face-Processing System. Science. 2010;330:845–851. doi: 10.1126/science.1194908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch C, Dinse HR, Tegenthoff M, Juckel G, Bruene M. An rTMS study into self-face recognition using video-morphing technique. Social Cognitive and Affective Neuroscience. 2011;6:442–449. doi: 10.1093/scan/nsq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. Plos Biology. 2005;3:529–535. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nature Neuroscience. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kaplan JT, Aziz-Zadeh L, Uddin LQ, Iacoboni M. The self across the senses: an fMRI study of self-face and self-voice recognition. Social Cognitive and Affective Neuroscience. 2008;3:218–223. doi: 10.1093/scan/nsn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SC, Bartels M, David AS. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain research. Cognitive brain research. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Passingham RE. Manipulating the experienced onset of intention after action execution. Journal of Cognitive Neuroscience. 2007;19:81–90. doi: 10.1162/jocn.2007.19.1.81. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus-familiarity in anterior inferior temporal cortex. Journal of Neurophysiology. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Ma Y, Han S. Functional dissociation of the left and right fusiform gyrus in self-face recognition. Human Brain Mapping. doi: 10.1002/hbm.21356. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Morita T, Itakura S, Saito DN, Nakashita S, Harada T, Kochiyama T, Sadato N. The role of the right prefrontal cortex in self-evaluation of the face: A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:342–355. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bennpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain - A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Experimental brain Research. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. Journal of Comparative Neurology. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Experimental Brain Research. 2011;209:481–493. doi: 10.1007/s00221-011-2579-1. [DOI] [PubMed] [Google Scholar]
- Platek SM, Kemp SM. Is family special to the brain? An event-related fMRI study of familiar, familial, and self-face recognition. Neuropsychologia. 2009;47:849–858. doi: 10.1016/j.neuropsychologia.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Platek SM, Krill AL, Wilson B. Implicit trustworthiness ratings of self-resembling faces activate brain centers involved in reward. Neuropsychologia. 2009;47:289–293. doi: 10.1016/j.neuropsychologia.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, Panyavin IS, Langleben DD. Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping. 2006;27:91–98. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Wathne K, Tierney NG, Thomson JW. Neural correlates of self-face recognition: An effect-location meta-analysis. Brain Research. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Poppenk J, McIntosh AR, Craik FIM, Moscovitch M. Past Experience Modulates the Neural Mechanisms of Episodic Memory Formation. Journal of Neuroscience. 2010;30:4707–4716. doi: 10.1523/JNEUROSCI.5466-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajimehra R, Young JC, Tootell RBH. An anterior temporal face patch in human cortex, predicted by macaque maps. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Masalovich S, Gaxiola I, Peltier S, Holtzheimer PE, Heim C, Goodyear B, MacQueen G, Mayberg HS. Differential neural activity and connectivity for processing one’s own face: A preliminary report. Psychiatry Research-Neuroimaging. 2011;194:130–140. doi: 10.1016/j.pscychresns.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nature Neuroscience. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nature Neuroscience. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Jeong H, Horie K, Sato S, Kawashima R. Face-specific and domain-general characteristics of cortical responses during self-recognition. Neuroimage. 2008;42:414–422. doi: 10.1016/j.neuroimage.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, jeong HJ, Miura N, Akitsuki Y, Horie K, Sato S, Kawashima R. Multiple brain networks for visual self-recognition with different sensitivity for motion and body part. Neuroimage. 2006;32:1905–1917. doi: 10.1016/j.neuroimage.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. Looking for Myself: Current Multisensory Input Alters Self-Face Recognition. Plos One. 2008;3 doi: 10.1371/journal.pone.0004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Costantini M, Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia. 2008;46:3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Heatherton TF, Kelley WM, Funnell MG, Gazzaniga MS, Macrae CN. Mike or me? Self-recognition in a split-brain patient. Nature Neuroscience. 2002;5:841–842. doi: 10.1038/nn907. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Davies MS, Scott AA, Zaidel E, Bookheimer SY, Iacoboni M, Dapretto M. Neural Basis of Self and Other Representation in Autism: An fMRI Study of Self-Face Recognition. Plos One. 2008;3 doi: 10.1371/journal.pone.0003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. Neuroimage. 2005;25:926–935. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Social Cognitive and Affective Neuroscience. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009;10:792–U750. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Verosky SC, Todorov A. Differential neural responses to faces physically similar to the self as a function of their valence. Neuroimage. 2010;49:1690–1698. doi: 10.1016/j.neuroimage.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]