Abstract
Background
Liver transplantation offers life-saving therapy for patients with decompensated liver disease or T2 hepatocellular carcinomas. In the United States, deceased donor livers are primarily allocated by Model for End-Stage Liver Disease (MELD) score within each of the country's more than 50 donation service areas (DSAs). Variation in DSA size, population, and organ availability have engendered concern that unequal access to deceased donor livers across DSAs contributes to geographic variability in outcome.
Methods
To determine the extent to which DSA variability in organ availability correlated with combined waitlist and posttransplant mortality, we analyzed retrospectively national waitlist and posttransplant data for a 7-year period after implementation of the current MELD-based allocation system.
Results
Marked variation among DSAs was evident in death rate (3.3-fold), transplant rate (20-fold), and mean transplant MELD (>10 points). Death rate correlated with organ availability was assessed by transplant rate and transplant MELD. DSAs with low organ availability included the country's largest cities, had more new listings per capita, larger waitlists, more transplant centers per DSA, and a higher proportion of black and Asian patients. DSAs of organ shortage were also characterized by more frequent dual listing at another transplant center, more living donor liver transplants, and increased average length of the transplant admission.
Conclusions
Geographic differences in deceased donor organ availability contribute to variation in overall death rate of liver transplant patients, shape the clinical practice of transplant, and influence the resources consumed per transplant. Geographic variation in organ access results primarily from rates of listing rather than donation. Our findings highlight the need to restructure organ distribution areas to achieve equal access to deceased donor livers for transplantation in the United States.
Keywords: Liver transplantation, Allocation, Geographic variation, Organ demand, Listings per capita
To more objectively and accurately rank patients by illness severity, Model for End-Stage Liver Disease (MELD) (1, 2)-based allocation of deceased donor livers was adopted in 2002. Waitlist death rate has since decreased (3), but approximately 15%of listed patients still die or become too ill to be transplanted (United Network of Organ Sharing [UNOS] website). One limiting aspect of MELD-based allocation may be the fact that organs continue to be distributed primarily within donation service areas (DSAs), geographic entities that developed without regard to population need or organ availability. Consequently, there is marked disparity in the MELD score to which candidates progress before reaching the top of their DSA's waitlist, leading to patients in certain DSAs receiving deceased donor liver transplants before their sicker counterparts in other DSAs (4). Some authors have found that MELD score at the time of transplant correlates with posttransplant survival (5–7), especially in sicker patients (MELD score >24) (8), so it is possible that both pretransplant and posttransplant mortality rates are higher in areas with lower organ availability.
A previous analysis showed wide variability in transplant rates and waitlist death rates but found no correlation between the two, possibly because it adjusted for MELD at transplant, largely annulling the effect of transplant rate on waitlist mortality (9). Another found no relationship between transplant rates and 1-year posttransplant patient survival but broadly divided DSAs into large and small, rather than considering them individually (4). Neither examined pretransplant and posttransplant survival together, and both were limited to the 12 months after MELD implementation.
In the current analysis, we evaluated geographic variation in overall mortality for the 7 years after MELD implementation with an intent-to-transplant analysis encompassing both waitlist and posttransplant deaths, to reexamine the extent to which this was because of variations in organ access associated with arbitrary DSA boundaries. We then categorized DSAs into quartiles by the extent of relative organ shortage to allow examination of variation in clinical practice based on relative organ availability. This demonstrated novel, expected, and unexpected differences in quartile characteristics with respect to reliance on living donor liver transplant (LDLT), likelihood of patients listing at another center, use of high donor risk index (DRI) organs, and the cost of transplant. Our findings reveal that the impact of geographic variation in organ shortage extends beyond outcome and markedly influences the practice of transplant based on organ availability.
Results
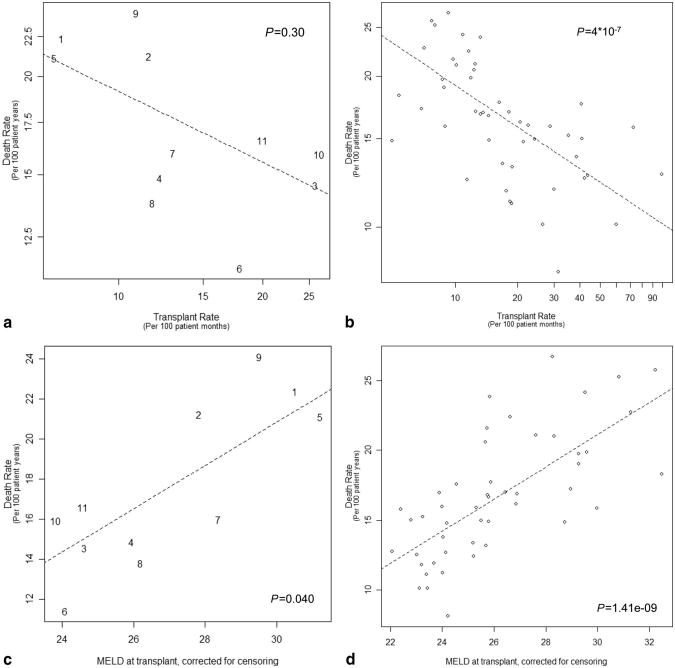
We examined the relationship between overall death rate and two measures of relative organ availability, transplant rate and corrected MELD at transplant. Both measures were significantly different among DSAs (P<0.0001), and both demonstrated a strong correlation with overall death rate by both UNOS region and DSA (Fig. 1a–d). At the regional level, death rate varied 2.1-fold and transplant rate varied 3.6-fold. There was a 7.4-point range (23.8–31.2) in average corrected MELD at transplant (Fig. 2a). DSA death rate varied 3.3-fold and transplant rate varied 20.1-fold. There was a 10.4-point range (22.1–32.5) among DSAs in average transplant MELD score. Thirty-six percent of the variation in death rate was attributable to corrected MELD at transplant. In O blood group patients who tend to wait the longest for transplant, the variation among DSAs was greater—3.7- and 36.6-fold for death and transplant rates, respectively.
Figure 1.
(a and b) Graphs are plotted on a log scale. Death rate and transplant rate are expressed in events per 100 patient years and per 100 patient months, respectively. Trend lines were determined by weighted linear regression, and P values were calculated using an F-test. (a) Correlation of death rate and transplant rate, by region. (b) Correlation of death rate and transplant rate, by donation service area (DSA). (c) Correlation of death rate with Model for End-Stage Liver Disease (MELD) at transplant, by region. (d) Correlation of death rate with MELD at transplant, by DSA.
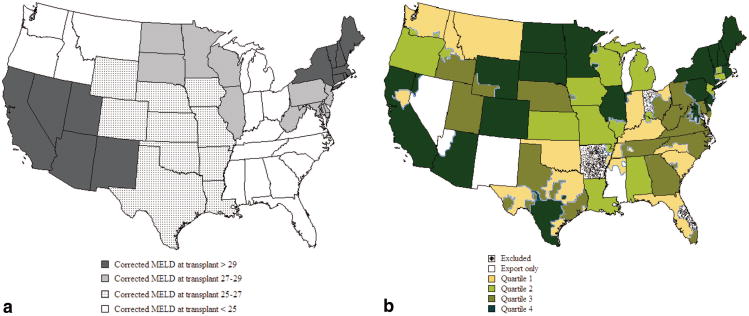
Figure 2.
Relative organ availability by United Network of Organ Sharing region (a) and donation service area (DSA) (b) stratified by corrected Model for End-Stage Liver Disease (MELD) at transplant/censoring and quartile of corrected MELD at transplant, increasing from quartile 1 to 4. DSAs not transplanting during the full study period are marked (stippled) as are export-only DSAs (white). (a) Relative organ availability, by region. (b) Relative organ availability, by DSA.
DSAs were ranked by MELD at transplant and grouped into quartiles with corrected MELD at transplant increasing from quartiles 1 to 4. Quartiles were similar in patient age and gender but revealed differences in blood type, race, and cause of end-stage liver-disease (Table 1, DSA characteristics). Number of centers, absolute list size, and new listings per capita increased with MELD at transplant, but neither eligible death nor donor rates correlated significantly with MELD at transplant (Table 1, Patient characteristics). Forty percent of states performing liver transplants contained DSAs or parts of DSAs in different quartiles (Fig. 2b). Eight of the 10 most populous cities in the United States were in quartile 4.
Table 1. DSA, patient, and transplant characteristics of DSA quartiled by MELD score at transplant/censoringa.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P | |
|---|---|---|---|---|---|
| DSA characteristics | |||||
| No. DSAs in quartile | 12 | 12 | 12 | 12 | |
| DSA size | 756.9±21 | 586.0±24 | 689.2±21 | 1849± 139 | <.001 |
| Transplant centers per DSA | 2.2±0.6 | 2.4±0.7 | 3.1±0.6 | 5.4±0.4 | <.001 |
| Eligible deaths per million | 75.6±5.8 | 89.9±4.7 | 91.2±4.6 | 75.4±3.6 | 0.973 |
| Eligible deaths recovered per million | 40.1±2.8 | 48.2±2.2 | 43.6±2.2 | 40.2±1.7 | 0.685 |
| Conversion rate | 55.2±3.0 | 54.0±2.5 | 48.5±2.4 | 53.7± 1.9 | 0.371 |
| Total deaths recovered per million | 43.7±3.6 | 51.7±2.9 | 46.0±2.8 | 44.6±2.2 | 0.822 |
| Liver donors per million | 35.0±3.0 | 42.8±2.4 | 38.1±2.4 | 35.4±1.9 | 0.757 |
| New listings per milliona | 16.9±3.3 | 21.6±2.7 | 23.2±2.6 | 27.9±2.1 | 0.008 |
| Patient characteristics | |||||
| Age (yr) | 52.8±0.3 | 52.4±0.2 | 52.3±0.2 | 53.2±0.3 | 0.513 |
| Male | 67.4±0.8 | 66.6±1.0 | 68.0±1.6 | 68.6±0.9 | 0.255 |
| Race (%) | |||||
| White | 80.2±2.6 | 80.2±2.3 | 77.6±3.4 | 62.3±4.2 | 0.005 |
| Black | 9.0±2.6 | 6.8± 1.6 | 8.4±3.0 | 21.5±4.0 | 0.023 |
| Hispanic | 7.3±0.9 | 10.3± 1.7 | 10.3± 1.3 | 8.0±1.3 | 0.669 |
| Asian | 2.6±0.8 | 2.1±0.4 | 2.7±0.7 | 6.9± 1.6 | 0.022 |
| Other | 1.0±0.4 | 0.7±0.2 | 0.9±0.4 | 1.2±0.3 | 0.42 |
| Blood type (%) | |||||
| O | 48.0±0.5 | 46.3± 1.1 | 46.5±0.8 | 46.2±0.8 | 0.134 |
| A | 38.5±0.6 | 38.3±0.8 | 39.1±0.9 | 36.7±0.8 | 0.197 |
| B | 10.9±0.4 | 11.7±0.7 | 11.5±0.6 | 12.9±0.5 | 0.021 |
| AB | 2.7±0.2 | 3.8±0.3 | 2.8±0.3 | 4.1±0.2 | 0.007 |
| Diagnosis (%) | |||||
| Noncholestatic cirrhosis | 73.1±2.0 | 70.0±2.5 | 72.1 ±2.1 | 71.9±3.0 | 0.905 |
| Malignant neoplasms | 7.1 ± 1.6 | 8.8±1.3 | 3.3 ±1.1 | 6.8± 1.3 | 0.293 |
| Cholestatic liver disease | 8.9±0.6 | 9.0±1.0 | 8.2±0.8 | 5.7±0.5 | 0.007 |
| Metabolic diseases | 2.0±0.2 | 2.6±0.4 | 2.6±0.4 | 1.5±0.2 | 0.122 |
| Other | 8.9± 1.4 | 9.6± 1.6 | 13.8±2.8 | 14.1 ±3.4 | 0.113 |
| Transplant characteristics | |||||
| Percent transplanted | 83.8±2.1 | 80.6±1.2 | 68.6±1.5 | 57.9± 1.5 | <.001 |
| Transplant rate (% per patient mo) | 4.8±0.4 | 3.1±0.5 | 1.6±0.4 | 1.0±0.3 | <.001 |
| Death rate (% per patient yr) | 0.13±0.01 | 0.14±0.01 | 0.20±0.01 | 0.21±0.01 | <.001 |
| MELD at transplant or censoring | 22.0±0.2 | 23.2±0.2 | 23.5±0.1 | 25.4±0.3 | <.001 |
| MELD at transplant | 22.2±0.2 | 23.4±0.2 | 24.4±0.2 | 28.0±0.5 | <.001 |
| MELD at listing (study population) | 21.1±0.2 | 21.8±0.2 | 21.8±0.1 | 22.9±0.3 | <.001 |
| Transplants at MELD <15 (%)a | 10.0±3.3 | 3.6±0.8 | 8.2±3.2 | 3.9± 1.2 | 0.197 |
| MELD when added to waitlista | 17.4±0.4 | 18.6±0.3 | 17.4±0.3 | 17.3±0.3 | 0.355 |
| Donation after cardiac death (%) | 4.9±1.7 | 4.6±0.9 | 4.1 ± 1.2 | 4.0±0.5 | 0.583 |
| Cold ischemia time | 7.3±0.1 | 6.9±0.3 | 7.9±0.4 | 7.3±0.4 | 0.374 |
| Cold ischemia time: imported organs | 7.8±0.3 | 8.0±0.6 | 9.1±0.6 | 8.9±0.4 | 0.103 |
| Cold ischemia time: organs within DSA | 7.0±0.1 | 6.7±0.3 | 7.5±0.4 | 6.9±0.3 | 0.554 |
| Relisted within 30 days of transplant | 3.0±0.3 | 3.1±0.3 | 4.0±0.4 | 3.9±0.6 | 0.122 |
| DRI | 1.5±0.05 | 1.5±0.02 | 1.5±0.03 | 1.6±0.06 | 0.393 |
| DRI: imported organs | 1.8±0.04 | 1.7±0.03 | 1.8±0.05 | 1.9±0.08 | 0.152 |
| DRI: organs from within DSA | 1.4±0.02 | 1.4±0.02 | 1.4±0.02 | 1.5±0.03 | 0.045 |
| Transplants from living donors (%)a | 0.4±0.2 | 0.8±0.5 | 3.9± 1.8 | 8.6± 1.3 | <.001 |
| Percent transplants with imported organs | 26.6±7.2 | 11.6±3.2 | 20.5±4.9 | 15.5±4.1 | 0.381 |
| Percent recovered organs exported | 23.5±5.2 | 21.8±5.7 | 27.6±6.6 | 7.8±1.4 | 0.018 |
| Dual listing | 0.27±0.11 | 0.35±0.06 | 1.10±0.43 | 0.98±0.12 | 0.003 |
| Life support at time of transplant (%) | 2.3±0.3 | 2.1±0.4 | 3.1±0.6 | 6.3±2.0 | 0.03 |
| Length of hospital stay | 14.3±0.5 | 15.3±0.6 | 18.5± 1.6 | 16.9± 1.2 | 0.03 |
DSAs were grouped into quartiles 1–4 by increasing corrected MELD at transplant. For certain analyses of transplant characteristics, patients outside the study population were considered included: MELD score < 15 and living donor transplants. Eligible deaths include patients younger than 70 yr who meet brain death criteria. Eligible deaths recovered are those eligible deaths from whom organs are recovered. Total recovered include donation after cardiac death and donors older than 70 yr. Conversion rate is the total eligible deaths leading to recovered organs divided by the total eligible deaths (data not shown). New listings/capita include all patients listed including those at MELD score < 15. Per capita donor data and listing data were analyzed for years 2002–2003 because of reliance on year 2000 census data and significant time-dependent changes in population. Patient characteristics, except age, are reported as percents ± SE. Age is expressed in yr.
Percent transplants before MELD score 15, MELD when added to waitlist, and transplants from living donors includes all patients in the 48 DSAs analyzed of any MELD. MELD at listing (study population) includes only those patients with two MELD scores > 15. A single DSA with more than 10% dual listing at its neighbor DSA, one of whose centers has transplant staff and patients in common with a center in the neighboring DSA, was excluded from the dual listing analysis as an outlier.
DSA, donation service area; MELD, Model for End-Stage Liver Disease; DRI, donor risk index; SE, standard error.
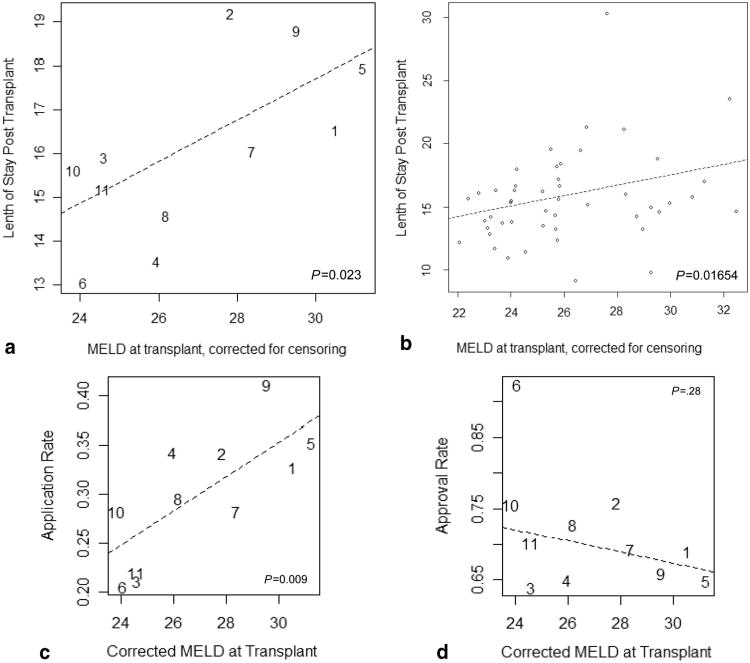
Listing MELD tended to follow corrected MELD at transplant, but this effect disappeared when patients with MELD score less than 15 were included (Table 1, Transplant characteristics). Donor after cardiac death (DCD) usage did not vary significantly. DRI (10) of local organs increased minimally with MELD at transplant. Quartile 4 DSAs exported the smallest percentage of organs recovered, but there was no clear trend in the percent of transplants performed using imported organs. Cold ischemia time (CIT) of imported organs increased, but this was not statistically significant. The average CIT of local and imported organs differed by 1.54 hr (P<0.001). Dual listing and LDLT were more common in the higher quartiles (Table 1, Transplant characteristics). Mean length of stay posttransplant, by region and DSA, increased significantly with corrected MELD at transplant (Table 1, Transplant characteristics and Fig. 3a and b), as did the proportion of patients who required life support at the time of transplant (Table 1, Transplant characteristics). Petitions to the regional review board (RRB) for MELD exception points increased with MELD at transplant, but the approval rate did not (Fig. 3c and d). There was a trend toward a higher percent of transplanted patients relisted within 30 days in DSAs with higher MELD at transplant, but this difference was not significant (Table 1, Transplant characteristics).
Figure 3.
Correlation of relative organ availability with posttransplant hospital length of stay and regional review board (RRB) petitions for additional priority points. Mean hospital length of stay by region (a) and donation service area (DSA) (b) is plotted versus corrected Model for End-Stage Liver Disease (MELD) at transplant as a measure of relative organ availability. Length of stay (a and b) is expressed in days posttransplant. It does not include pretransplant days in the hospital if the patient was in house at the time of transplant. The mean number of RRB applications (c) and percent approvals (d) for additional MELD priority points for special exception cases is shown by region versus correct MELD at transplant. Application rate was calculated from the number of applications to the RRB (excluding hepatocellular carcinoma meeting standard criteria) per number of patients listed. This included all patients on the waitlist in the 48 analyzed DSAs of any MELD Score. Approval rate was calculated as the number of approvals per application. (a) Hospital length of stay and MELD at transplant, by region. (b) Hospital length of stay and MELD at transplant, by DSA. (c) RRB petitions and MELD at transplant. (d) RRB approval and MELD at transplant.
There was greater representation of black and Asian patients in quartile 4 and overall mortality varied by race with the highest overall mortality in black patients. When analyzed by quartile, however, overall mortality did not differ significantly by race (Table 2, Mortality by race and quartile and Percent transplanted by race and quartile).
Table 2. Mortality and percent of patients transplanted by race and quartile.
| Quartile | White | Black | Hispanic | Asian | Other | P |
|---|---|---|---|---|---|---|
| Mortality by race and quartile | ||||||
| All | 31.1±1.2 | 35.2±1.6 | 32.8±1.8 | 24.7±1.6 | 35.3±2.6 | 0.0166 |
| 1 | 23.3± 1.0 | 25.4±2.9 | 21.8±1.8 | 20.8±3.9 | 25.8±2.9 | 0.5946 |
| 2 | 26.0±1.1 | 32.6±1.8 | 24.8±2.4 | 21.9±3.2 | 38.9±9.6 | 0.0806 |
| 3 | 33.2±1.6 | 35.7±3.0 | 33.1±4.6 | 21.5±2.9 | 33.9±5.9 | 0.1276 |
| 4 | 36.4±1.2 | 39.9± 1.9 | 35.5± 1.5 | 26.1±2.0 | 38.2±3.6 | 0.1847 |
| Percent transplanted by race and quartile | ||||||
| All | 69.5±2.2 | 71.0±2.2 | 61.2±2.8 | 70.6±2.9 | 67.4±3.8 | 0.1405 |
| 1 | 83.7±2.2 | 85.7±2.4 | 82.2±3.6 | 88.0±2.5 | 80.3±3.4 | 0.5239 |
| 2 | 80.3±1.3 | 81.3±1.6 | 81.7±1.0 | 86.8±3.7 | 77.8±9.8 | 0.5131 |
| 3 | 68.5±1.6 | 70.3±2.4 | 65.8± 1.3 | 72.3±3.5 | 72.9±8.5 | 0.6702 |
| 4 | 57.8± 1.8 | 61.3± 1.4 | 54.6±1.9 | 65.9±2.5 | 59.8±4.1 | 0.2707 |
Mortality is expressed as percent of patients who died during the study period, on the waitlist and posttransplant. Mortality rates were computed using least square means, adjusting for clustering by donation service area, and standard errors were transformed using the delta-method. P values were calculated using the generalized score test for generalized estimating equation models.
Discussion
Although MELD-based stratification of candidates for liver transplantation has been successful in targeting patients in greatest need of transplant within a given DSA, there remain marked differences in the mean MELD at which transplant occurs across regions and DSAs. In conjunction with the fact that a large fraction of deceased donor transplants are performed in recipients with a MELD score less than 15 who may not derive benefit from the procedure (11), these observations suggest that deceased donor livers are not reaching those in greatest need, the primary intent of MELD-based allocation. That geographic inequity in access to livers maybe responsible for a wide variation in death rates among DSAs has prompted a contentious discussion in transplant community about the relative merits of broader sharing of livers for transplant (12).
In the current analysis, we characterized the magnitude of regional and DSA variation in relative organ availability and demonstrated a strong correlation of overall mortality with relative organ availability as measured by the accepted surrogates, transplant rate, and MELD at transplant. DSAs exhibited a 20-fold range in transplant rate and 10 points in mean MELD at transplant. Categorizing DSA by quartiles based on the degree of relative organ shortage enabled us to characterize the transplant practice in high and low need areas and the nature of the organ imbalance from the perspective of supply and demand. Addressing the latter, we compared a panel of measures of organ supply per capita by DSA and found no significant correlation with the magnitude of the areas' relative organ shortage. Similarly, conversion rates, the percentage of candidate donors who actually donated, were not significantly different between quartiles. In contrast, a strong correlation between transplant demand measured by listing rates per capita, and the relative shortage of organs by quartile and DSA was evident, indicating that geographic variation in organ availability is largely demand driven. Further study will be required to assess whether variation in demand results from geographic or racial variation in disease incidence (13), greater access to care in urban areas (14, 15), or referral of patients toward major metropolitan areas where transplant programs are more numerous. Greater demand could also result from variation in transplant listing practices; however, we did not find significant differences in MELD score at listing among quartiles (Table 1, Transplant characteristics).
Perhaps related to the association of organ shortage with large urban areas, we observed that high need DSAs (and regions) were characterized by greater representation of African Americans and Asians and fewer white patients. Importantly, although race was associated with outcome in the overall cohort, as also noted by others (16), when stratified by quartile, race-related variation in mortality was no longer significant, suggesting that geographic differences in racial composition may account for race-associated variation in mortality (17).
A number of differences in the clinical practice of transplant apparently intended to mitigate organ unavailability were evident comparing relatively organ rich and organ poor DSAs. Most striking was a 20-fold greater frequency of LDLT in quartile 4 compared with quartile 1. Quartile 4 also had the greatest proportion of candidates listing at additional centers for transplant (dual listing), but overall, only approximately 1% of patients availed themselves of this opportunity to expedite transplant. The financial and logistic burden of distant travel or relocation may not be feasible for most patients, with the unfortunate effect of barring access to organs based on lack of economic resources. This may contribute to the general population's perception that organ allocation is discriminatory (18), discouraging organ donation (19). Candidates in areas of organ paucity were also more likely to petition their RRB for extra points with the belief that their calculated MELD score underestimated their mortality risk; however, despite the increase risk while waiting in areas of organ shortage, RRBs were no more likely to approve these requests.
Interestingly, although high-need DSAs were less likely to export donor livers to other areas, we did not find evidence for greater utilization of marginal organs in areas of greater organ shortage. Although DCD and DRI do not encompass all aspects of high risk, DCD liver utilization was comparable across quartiles, and the mean DRI, a measure of donor liver quality, was increased only marginally in quartile 4. We hypothesize that these practices result from the fact that high DRI organs have been found to function less well in high MELD recipients, exhibiting markedly increased rates of primary nonfunction (20, 21). The trend toward greater early relisting for another transplant in high need DSAs is compatible with this notion.
The higher frequency of transplant at low MELD scores (< 15) in quartile 1 paired with the higher frequency of patients reaching a MELD score more than 30 in quartile 4 (Table 1, Transplant characteristics) suggests that broader sharing of livers disregarding arbitrary DSA and regional boundaries may better target the sickest patients for transplant. Although redistribution of livers from low to high shortage areas will lead to corresponding increases in the MELD at transplant in the former, the non-linear relationship between MELD and mortality suggests that an overall reduction in mortality in liver transplantation should still be achieved.
The need for more equitable organ distribution targeting the sickest patients has been prominently recognized for some time (22). More than 10 years ago, the US Department of Health and Human Services declared that allocation of scarce organs should “be based on common medical criteria, not accidents of geography,” (10), and the Organ Procurement and Transplantation Network Final Rule stated that: “Policies for the equitable allocation of cadaveric organs shall not be based on the candidate's place of residence or place of listing, shall be designed to distribute organs over as broad a geographic area as feasible, reducing the intertransplant program variance to as small as can reasonably be achieved.” Because organ availability varies at both DSA and regional levels and because regional sharing was predicted by the Liver Simulation Allocation Model to decrease deaths by only 1.6% (23), it seems clear that regional sharing alone will not achieve these objectives.
The main objections to broader sharing have been fear of worse outcomes because of longer CIT, higher costs from shipping organs longer distances, and a decrease in donation rates because of the perception that donors prefer their organs to be used locally (24). We found average CIT for an imported organ to be only approximately 2 hr longer than that for a local organ. However, it is true that more expensive modes of transportation may be used to limit CIT when organs are shipped greater distances.
Although organ travel costs may increase, it is possible, if not likely, that broader sharing will yield a net cost savings by minimizing transplants at high MELD scores. Our finding that length of stay and need for life support increases with MELD at transplant is consistent with other reports that calculate a $4309 increase in cost of transplant alone for each additional MELD point (25). Because that analysis did not include the pretransplant care of patients sitting or dying on the waitlist, it likely significantly underestimated the added costs of delayed transplant in areas of low organ availability and the potential savings achievable by broader sharing.
It is difficult to assess the impact of broader sharing on donation rates in the absence of pilot studies addressing this issue specifically. The general public values equity most in making allocation decisions. Furthermore, in an open-ended survey asking what factors should be considered in organ allocation, respondents cited religion, citizenship, and living donor potential as important considerations, but none of them stated that geographic location should play a role in allocation (26). Fair distribution is likewise the primary concern of potential donors, especially racial and socioeconomic equality (19). In addition to obviating the need for dual listing, an option more easily taken advantage of by higher income patients, previously noted racial disparities in transplant rate and posttransplant outcome are likely to improve with broader sharing.
Maximal advantage from sharing will probably result from some trade-off between the increase in CIT/cost and the survival benefit gained from transporting the organ farther to reach a sicker patient, as suggested many years ago (27). This could be incorporated into the algorithm by requiring a graded differential in MELD score between a local candidate and one farther from the donor hospital in another DSA before triggering an offer to the more distant patient (e.g., 5 points for 500 miles, 10 points for 1000 miles, and 15 points for 1500 miles). Further analysis by Liver Simulation Allocation Model should help to define the optimal parameters of the MELD threshold required for sharing and the MELD differential that would prompt an increase in share distance. Of note, distribution areas for the sickest patients awaiting heart transplantation were recently redefined, as first a 500 miles, then a 1000-mile radius from the donor hospital (an area larger than the biggest UNOS region). One year later, waitlist death rates had decreased by 2- to 3-fold (28), establishing that organ distribution based on distance can be more effective than relying on the artificial boundaries of DSAs and regions.
In summary, the overall risk of death for a liver transplant candidate varies markedly based on geographic location and depends on that location's relative organ availability. In addition to impacting outcome, the practice of transplant is altered in areas of organ shortage as seen in greater reliance on LDLT, and there is a greater consumption of resources associated with transplanting sicker patients. We also report that the geography-associated imbalance in organ availability is largely demand driven, and high demand localizes around large metropolitan areas. Reorganizing organ distribution has the potential to decrease death rates nationally and simultaneously reduce cost by lowering the average MELD at transplant. Reducing perceived racial and socioeconomic bias in access to organs may help to ameliorate the overall organ shortage by encouraging donation.
Materials and Methods
We obtained a dataset from the Scientific Registry of Transplant Recipients, which included 172,663 listings. We included only those from February27, 2002 to February 27, 2009 (73,939) in DSAs with active waitlists over the entire 7-year period (73,707), removing three DSAs with an average of 2.9 years of activity. Two DSAs that allocated from a single list were combined. Pediatric end-stage liver disease score (PELD) listings (5222), retransplants (5170), status 1 (2502), and LDLTs (1406) were excluded (59,407 remaining). Patients were also excluded if delisted because they refused a transplant, were transferred to another center, listed in error, or improved and no longer needed a transplant (6695 excluded and 52,712 remaining). Patients with multiple listings were counted only once, at the center where they received their transplant if they received a transplant during the study period or at the first center of listing if they had not received a transplant (1120 listings excluded). Three hundred twenty patients were excluded for having no MELD score on record. As mean MELD at the time of initial listing may differ by DSA, we used the date of the second consecutive MELD score more than 15 as a uniform starting point to measure time spent on the waiting list and excluded 15,980 patients who had only one or no recorded MELD scores greater than or equal to 15. Hepatocellular carcinoma and other diagnoses that received exception points were analyzed using listing MELD rather than laboratory MELD. A total of 35,291 patients were included in the analysis.
The primary measure of relative organ availability was defined to be MELD at transplant, corrected for censored data. The correction was performed by calculating a Kaplan-Meier estimate of the mean MELD at transplant for each DSA, censoring patients if they had not received a transplant because of pretransplant death or waitlist removal, or if they remained listed at the date of last follow-up. DSAs were divided into quartiles by corrected MELD at transplant, quartile 1 containing the 12 DSAs with the lowest corrected MELD and quartile 4 containing the 12 with the highest.
To determine the percent of variation explained by the corrected MELD at transplant, a multiple linear regression weighted by DSA size was fit to the data aggregated by DSA, with death rate as the dependent variable and corrected MELD at transplant, MELD at listing, age, race, and blood type as the independent variables. The percent of variation explained by the corrected MELD at transplant was estimated as one minus the ratio of the mean square errors of models with and without this variable. Death rate and transplant rate were calculated as total number of deaths and transplants, respectively, divided by the total amount of time in the database, where time is the time from patients' start dates to pretransplant or posttransplant death, or last pretransplant or posttransplant follow-up for patients remaining alive.
DSA characteristics were analyzed using generalized estimating equations models on patient-level data, clustering on quartile, and DSA. The standard error (SE) was calculated using generalized estimating equation that accounted for clustering of patients within each DSA. Least-squares means ±SE are reported. Measurements that were only available on the DSA level, such as the difference between ischemia times of imported and exported organs, were modeled using weighted least squares with the DSA size as the weighting variable.
Per capita calculations were performed with UNOS and Scientific Registry of Transplant Recipients data from January 1, 2002 to December 31, 2003 and census data from 2000 (most recent available from UNOS) to minimize the effect of population fluctuations and expressed as events per million population per year. Per capita data were analyzed using Poisson regression; because Poisson regression is performed on the log scale, the delta-method was applied to transform the SEs. Conversion rates were calculated using logistic regression, with SEs again transformed using the delta-method.
Acknowledgments
The authors thank Drs. Frank Delmonico, A. Benedict Cosimi, and Daniel Pratt for their constructive review of the manuscript.
Footnotes
The data reported here have been supplied in part by the Scientific Registry of Transplant Recipients and in part by the United Network for Organ Sharing as contractors for the Organ Procurement and Transplant Network (OPTN). The interpretation of these data is the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US government.
References
- 1.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 2.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end stage liver disease. Hepatology. 2001;33:464. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 3.Freeman RB, Wiesner RH, Edwards E, et al. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 4.Trotter JF, Osgood MJ. MELD scores of liver transplant recipients according to size of waiting list: Impact of organ allocation and patient outcomes. JAMA. 2004;291:1871. doi: 10.1001/jama.291.15.1871. [DOI] [PubMed] [Google Scholar]
- 5.Saab S, Wang V, Ibrahim AB, et al. MELD score predicts 1-year patient survival post-orthotopic liver transplantation. Liver Transpl. 2003;9:473. doi: 10.1053/jlts.2003.50090. [DOI] [PubMed] [Google Scholar]
- 6.Habib S, Berk B, Chang CCH, et al. MELD and prediction of post-liver transplantation survival. Liver Transpl. 2006;12:440. doi: 10.1002/lt.20721. [DOI] [PubMed] [Google Scholar]
- 7.Jacob M, Copley LP, Lewsey JD, et al. Pretransplant Meld score and post liver transplantation survival in the UK and Ireland. Liver Transpl. 2004;10:903. doi: 10.1002/lt.20169. [DOI] [PubMed] [Google Scholar]
- 8.Desai NM, Mange KC, Crawford MD, et al. Predicting outcome after liver transplantation: Utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99. doi: 10.1097/01.TP.0000101009.91516.FC. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JP, Dykstra DM, Goodrich NP, et al. Geographic differences in event rates by model for end stage liver disease score. Am J Transplant. 2006;6:2470. doi: 10.1111/j.1600-6143.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: The concept of a donor risk index. Am J Transplant. 2006;6:783. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 11.Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Pondrom S. Location, location, location: A new national policy change broadens geographic access to donated livers. Am J Transplantation. 2009;9:2207. doi: 10.1111/j.1600-6143.2009.02835.x. [DOI] [PubMed] [Google Scholar]
- 13.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 14.Tuttle-Newhall JE, Rutledge R, Johnson M, et al. A statewide, population-based, time series analysis of access to liver transplantation. Transplantation. 1997;63:255. doi: 10.1097/00007890-199701270-00014. [DOI] [PubMed] [Google Scholar]
- 15.Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299:202. doi: 10.1001/jama.2007.50. [DOI] [PubMed] [Google Scholar]
- 16.Reid AE, Resnick M, Chang YC, et al. Disparity in use of orthotopic liver transplantation among blacks and whites. Liver Transpl. 2004;10:834. doi: 10.1002/lt.20174. [DOI] [PubMed] [Google Scholar]
- 17.Volk ML, Choi H, Warren GJW, et al. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9:2113. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 18.http://www.bloomberg.com/apps/news?pid=20601109&sid=amJlnzRxhdkM. Accessed 15 september 2009
- 19.Boulware LE, Troll MR, Wang NY, et al. Perceived transparency and fairness of the organ allocation system and willingness to donate organs: A national study. Am J Transplant. 2007;7:1778. doi: 10.1111/j.1600-6143.2007.01848.x. [DOI] [PubMed] [Google Scholar]
- 20.Amin MG, Wolf MP, TenBrook JA, Jr, et al. Expanded criteria donor grafts for deceased donor liver transplantation under the MELD system: A decision analysis. Liver Transpl. 2004;10:1468. doi: 10.1002/lt.20304. [DOI] [PubMed] [Google Scholar]
- 21.Schaubel DE, Sima CS, Goodrich NP, et al. Survival benefit of liver transplantation by MELD and donor risk index. Am J Transplant. 2006;6(suppl 2):95. [Google Scholar]
- 22.Bronsther O, Fung JJ, Tzakis A, et al. Prioritization and organ distribution for liver transplantation. JAMA. 1994;271:140. [PMC free article] [PubMed] [Google Scholar]
- 23.UNOS public policy proposal Pubcomment Prop Sub 239. http://optn.transplant.hrsa.gov/PublicComment/pubcommentPropSub_239.pdf.
- 24.OPTN/UNOS Liver and Intestinal Organ Transplantation Committee Report to the Board of Directors. 2009 Jun 22–23; http://optn.transplant.hrsagov/CommitteeReports/board_main_Liver&IntestinalOrganTransplantationCommittee_7_27_2009_14_27.pdf.
- 25.Axelrod DA, Koffron AJ, Baker T, et al. The economic impact of MELD on liver transplant centers. Am J Transplant. 2005;5:2297. doi: 10.1111/j.1600-6143.2005.01025.x. [DOI] [PubMed] [Google Scholar]
- 26.Ubel PA, Loewenstein G. Distributing scarce livers: The moral reasoning of the general public. Soc Sci Med. 1996;42:1049. doi: 10.1016/0277-9536(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 27.Starzl TE, Gordon RD, Tzakis A, et al. Equitable allocation of extrarenal organs: With special reference to the liver. Transplant Proc. 1988;20:131. [PMC free article] [PubMed] [Google Scholar]
- 28.UNOS News Bureau. [Accessed september 27 2009]; Available at http://www.unos.org/news/newsDetail.asp?id=103102/22/2008.