Abstract
Antibody arrays can be employed for the profiling glycan structures on proteins. Antibody arrays capture multiple, specific proteins directly from biological samples (such as serum), and lectin and glycan-binding antibodies probe the levels of specific glycans on the captured proteins. We use a practical method of partitioning microscope slides to enable the convenient processing of many detection reagents or samples. A critical first step in the procedure is the chemical derivatization of the glycans on the spotted capture antibodies, which prevents lectin binding to those glycans. We describe those methods along with the methods for preparing and treating serum samples, running the experiments, and designing and interpreting the experiments.
Keywords: antibody arrays, glycan profiling, lectin detection, serum biomarkers
1. Introduction
Antibody microarrays have proven to be very valuable for their ability to obtain high-sensitivity measurements of multiple proteins using low sample volumes (1). This capability has been applied to many different research topics, particularly in cancer research (2, 3). Recently we have further developed the antibody array method to enable the probing of carbohydrate modifications on proteins (4). The ability to accurately measure the variation in specific carbohydrate structures on specific proteins in biological samples has many important applications. For example, the more careful characterization of glycan alterations associated with cancer could be used to determine the prevalence of particular structural alterations or the correlations with clinical factors. Conventional technologies for studying carbohydrates, such as separations-based methods or mass spectrometry, do not have the quantitative precision necessary to make comparisons between samples, nor do they have the throughput to look at population statistics. The method described here addresses those limitations.
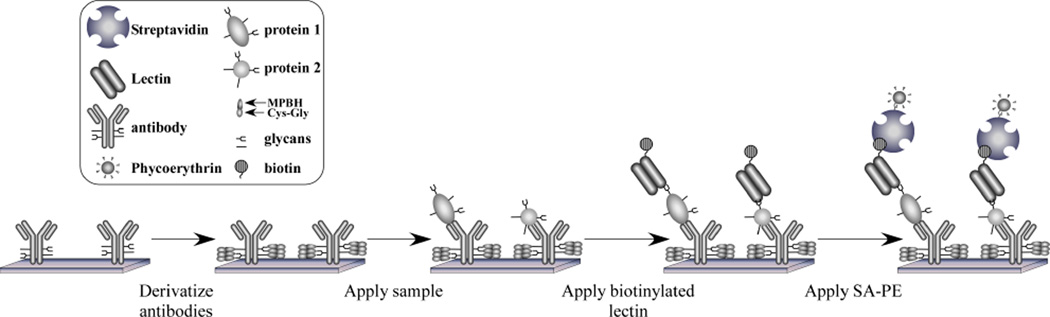
The basic principle of the method is presented in Figure 1. A biological sample, such as serum, is incubated on the surface of a microarray of immobilized antibodies. After proteins bind to the antibodies according to their specificities, the levels of specific glycan structures on the captured proteins are probed using lectins (proteins with glycan-binding activity) or antibodies targeting glycan epitopes. Different types of lectins and glycan-binding antibodies can be used to probe various glycan structures. An important first step in this procedure is a method to chemically derivatize the glycans on the immobilized antibodies. This step alters the glycans so that they are no longer recognized by the lectins or glycan-binding antibodies, ensuring that only the glycans on the captured proteins are probed. Each type of lectin recognizes its own, specific carbohydrate structure.
Figure 1.
The detection of glycans on proteins captured by antibody arrays. The drawing depicts antibodies immobilized on a planar surface. The glycans on the antibodies are derivatized to prevent lectin binding; a sample is incubated on the antibody array; proteins are captured by the antibodies; biotinylated lectins bind to the glycans on the captured proteins; and the level of bound lectin is determined by scanning for fluorescence from streptavidin-B-phycoerythrin.
A description of the validation and optimization of the method was presented earlier (4). The purpose of this chapter is to give detailed instructions on how to use the method in practice, along with the latest protocol enhancements. The description of this method will be presented in three sections: 1) chemical derivatization of the glycans on the capture antibodies; 2) sample preparation; and 3) processing the microarrays.
2. Materials
2.1 Reagents
NaIO4 (Pierce Biotechnology, Rockford, IL)
4-(4-N-Maleimidophenyl) butyric acid hydrazide hydrochloride (MPBH) (Pierce Biotechnology, Rockford, IL)
Cysteine-Glycine (CysGly) dipeptide (Sigma-Aldrich, St. Louis, MO)
Streptavidin-B-Phycoerythrin (Invitrogen, Carlsbad, CA)
Neuraminidase (New England Biolabs, Ipswich, MA)
Protease Inhibitors (1 tablet dissolved in 10 mL buffer) (Roche Applied Science, Indianapolis, IN).
Biotinylated lectins (Vector Labs, Burlingame, CA, and other suppliers)
Mouse, goat, sheep, and rabbit IgG antibodies, and chicken IgY antibodies (Jackson ImmunoResearch Labs, West Grove, PA)
Tween-20 (Sigma-Aldrich, St. Louis, MO)
Brij-35 (Sigma-Aldrich, St. Louis, MO)
2.2 Solutions
Coupling Buffer (0.04 M sodium acetate, pH 5.5)
Coupling Buffer + 0.1% Tween-20
Phosphate-buffered saline (PBS), pH 7.4 (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4)
Tris-buffered saline (TBS)
PBST0.1: PBS + 0.1% Tween-20
PBST0.5: PBS + 0.5% Tween-20
PBST0.1 + 1 mM CysGly (prepare immediately before use)
Coupling Buffer + 200 mM NaIO4 (prepare immediately before use)
Coupling Buffer + 1 mM MPBH + 1mM CysGly (prepare immediately before use)
PBST0.5 + 1% bovine serum albumin (BSA)
PBST0.5 + 0.1% (BSA) + 1 mg/mL Streptavidin-B-Phycoerythrin
10× Sample buffer (1% Tween-20 + 1% Brij-35 in 1× TBS)
4× IgG/Y cocktail: 400 mg/mL of goat, sheep, mouse, and chicken antibody, 800 mg/mL rabbit antibody, in TBS
20× protease inhibitor solution: dissolve one tablet of protease inhibitor into 0.5 mL of distilled water (prepare immediately before use).
2.3 Hardware and instruments
Microscope slide staining chambers with slide racks (Shandon Lipshaw, Pittsburgh, PA, cat. No. 121)
Microscope slide boxes (several versions available)
Wafer handling tweezers (Techni-Tool, Worcester, PA, cat. No. 758TW178, style 4WF)
Slide Imprinter, for printing wax partitions on slides (The Gel Company, San Francisco, CA)
Clinical centrifuge with flat swinging buckets for holding slide racks (Beckman Coulter, Fullerton, CA, among others)
Microarray scanner (several versions available)
3. Methods
This method described here assumes access to antibody microarrays. The selection and treatment of antibodies and the fabrication of antibody arrays are covered in previous publications (3, 5, 6). Antibody microarrays can be stored in a vacuum-sealed slide box with desiccant in a refrigerator for months without loss of activity (see Note 1).
3.1 Chemically derivatize the glycans on the capture antibodies
Because antibodies are glycoproteins, the lectins used as the detection probes could bind to the capture antibodies on the arrays, which would interfere with the specific detection of the glycans on the proteins captured by the antibodies. A reliable method to prevent that interference is to chemically modify the glycans on the capture antibodies after they have been immobilized. The cis-hydroxyl groups of the glycans on the spotted antibodies are gently oxidized to convert to aldehyde groups, which are reacted with a hydrazide-maleimide bi-functional crosslinking reagent, which then reacts with a cysteine-glycine (CysGly) dipeptide. The CysGly dipeptide adds bulk to the derivatized carbohydrates to hinder lectin binding.
The chemically blocking procedure should be finished within a working day without interruption. All chemical solutions, such as NaIO4, MPBH, and CysGly should be prepared right before the reactions.
Some lectins or antibodies target glycan structures which are rare in antibodies, such as the Lewis blood group structures or O-linked N-acetyl-Glucosamine (O-GlcNAc), so these reagents do not bind to the capture antibodies. Therefore the chemical derivatization step is not necessary in those cases. A preliminary experiment to look at the level of lectin and antibody binding to capture antibodies would help determine if glycan derivatization is necessary. A slight loss in affinity may be evident after chemical derivatization (4), so it may be important to make this determination. IgE antibodies seem to be particularly susceptible to affinity loss.
If the microarray slides are stored refrigerated in a vacuum-sealed pack, equilibrate the slides to room temperature for 30 minutes before opening vacuum seal. This equilibration will prevent condensation on the slides.
If using wax partitioning of the slides, print the desired pattern onto the slides using the SlideImprinter (see Figure 2 and section 3.3) (see Note 2).
Briefly rinse all the slides with PBST0.1 followed by Coupling Buffer + 0.1% Tween-20, and wash the slides in Coupling Buffer + 0.1% Tween for 10 minutes at room temperature with gentle shaking (see Note 3). The slide washing steps can be performed by placing the slides in slide racks in a slide staining chamber.
Prepare 200 mM NaIO4 in 1× Coupling Buffer (see Note 4).
Pour the NaIO4 solution into a flat-bottom glass or plastic container to a depth sufficient to easily cover a microscope slide lying flat (about 5 mm) (see Note 5). Place the microarray slides in the bottom of the container facing up, submerging them, and cover the cassette with aluminum foil to keep the slides dark. Incubate the slides for five hours with gentle shaking at 4 °C (see Note 6). This step could be performed in a cold-room.
Prepare the Coupling Buffer + 1 mM MPBH + 1 mM CysGly solution about 30 minutes before the end of the previous step (see Note 7).
Using the wafer-handling tweezers, remove the slides, briefly rinse them in Coupling Buffer + 0.1% Tween-20, and wash them for three minutes in the same buffer with gentle shaking at room temperature.
Pour the Coupling Buffer + 1 mM MPBH + 1 mM CysGly solution into a flat-bottom container as before, and place the slides in the bottom of the container facing up. Incubate at room temperature for two hours with gentle shaking.
Prepare the PBST0.1 + CysGly solution about 30 minutes before the previous step finishes.
Wash the slides in PBST0.1 for 3 minutes.
Pour the CysGly solution into a cassette, place the slides on the bottom of the cassette facing up, and incubate at 4 °C overnight with gentle shaking (see Note 8 and 9).
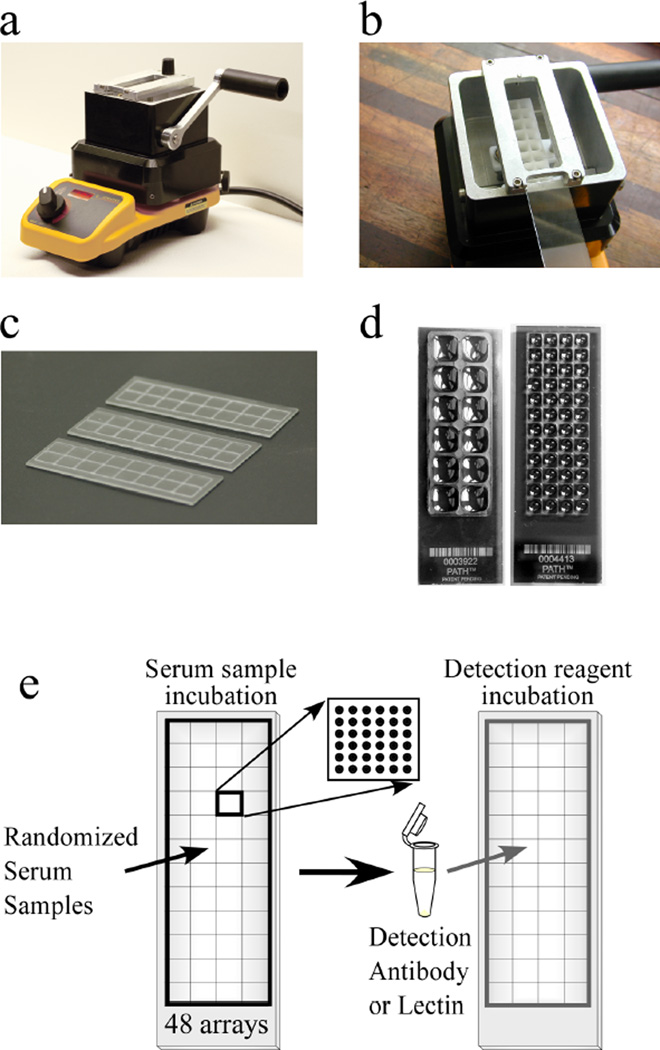
Figure 2.
High-throughput processing of antibody arrays. (a) A SlideImprinter device (The GelCompany, San Francisco, CA) is used to imprint wax patterns onto microscope slides. A bath of wax is melted on a hotplate, and (b) a slide is inserted upside-down into a holder above the bath. Pulling the level forward elevates a stamp out of the bath to contact the slide, (c) leaving a pattern of wax on the slide. (d) Various designs of stamps can be used, such as one that partitions 12 regions on a slide (left), or another that partitions 48 regions on a slide (right). The liquid loaded into each region remains segregated from the other regions. (e) Strategy for detecting a glycan structure on many samples. 48 different serum samples are randomized and incubated on 48 identical antibody arrays on a single microscope slide. Each array contains up to 144 spots. After the proteins bind to the antibodies, a glycan structure on the captured proteins are detected with a lectin or glycan-binding antibody.
3.2 Preparation of the serum samples
3.2.1 Serum sample dilution and mixture
Low concentrations of detergents, such as Tween-20 and Brij-35, should be added to the serum samples to reduce non-specific binding to the capture antibodies. We also recommend mixing in small amounts of non-specific antibodies to compete for possible serum protein binding to the capture antibodies. The types of antibodies that are represented on the microarrays should be added to the serum samples. For example, if mouse IgG antibodies are used on the arrays, then normal mouse IgG antibodies (from non-immunized mice) should be added to the sera. Tween-20 and Brij-35 are added to a final concentration of 0.1%, and the non-specific antibodies also are added to 100 mg/mL, using 1× PBS as a carrier buffer. In addition, some serum proteases may be activated if serum samples are highly diluted. We recommend using a protease inhibitor cocktail in the diluent buffers.
The serum should be diluted so that the antigens of interest are in the linear-response range of detection. Low-abundance antigens, such as cytokines, are best detected using low dilution factors, such as 1.5–2×, and high-abundance antigens, such as serum transferrin, may require dilution factors of several-thousand-fold. The optimal dilution factor can be determined experimentally. Serial dilutions of pooled sera should be analyzed on arrays (using the methods described below), and the signals should be plotted with respect to the dilution factors. The dilution factor for subsequent experiments should be set in the range that shows an approximately linear relationship between signal and dilution factor.
Another consideration in determining the appropriate dilution factor is that some detection lectins may show high backgrounds (high binding to the slide substrate) when using high concentrations (low dilution factors) of serum. The dilution factor may need to be increased in those cases to reduce backgrounds.
Here is an example of the preparation of 100 ml of a serum sample with a two-fold dilution factor:
50 ml serum
10 ml Sample buffer
10 ml 10× TBS.
25 ml of 4× IgG/Y cocktail
5 ml of 20× protease inhibitor solution
3.2.2 Enzymatic stepwise removal of terminal glycans on the serum proteins
This optional step can be used in addition to the standard serum preparation method to provide more information about the glycan structures on the captured proteins. Some sugar groups are not accessible to lectin detection because they are masked by terminal glycan groups. Through the sequential, enzymatic removal of terminal glycan structures, the inner glycan groups can be made accessible to lectin detection. Certain enzymes, such as neuraminidase and galactosidase, can function without denaturing the target proteins, so that the proteins are still recognized by the capture antibodies. The following procedure describes the removal of terminal sialic acid groups using the enzyme neuraminidase. Some optimization may be required for other enzymes.
Add 4 ml of 10× reaction buffer (from the neuraminidase reaction kit) and 2 ml of the 20× protease inhibitor solution into 34 ml of serum sample.
Add 1 ml of neuraminidase into the mixture, and incubate at 37 °C for 4 hours.
Continue the serum sample preparation as described in section 3.2.1 (see Note 10).
3.3 Detection of glycans on captured glycoproteins
This step describes the incubation of serum samples on the arrays and the probing of the captured proteins with glycan-binding reagents. We recommend partitioning the microscope slides so that multiple microarrays can be run on each slide, which saves on supply costs and makes it convenient to run many samples or detection reagents. A practical and versatile method to partition microscope slides is to imprint hydrophobic wax borders around each of the microarrays (Figure 2a-d). These borders prevent liquid from leaking from one array to another, and they are thin enough to allow scanning using conventional microarray scanners. The borders can be applied using a commercially-available instrument, and various patterns of borders can be applied to fit particular experimental needs. A design using 48 microarrays on a single slide is useful for probing many samples (Figure 2e).
The detection lectins or glycan-binding antibodies should be chosen to target the particular glycan structures specific to the needs of each project. The specificities of some widely-used lectins and antibodies for glycan detection are listed in Table 1, which contains information compiled from various sources (7–11). Some lectins have broad specificities or bind more than one sugar type, such as Lens Culinaris Agglutinin (LCA), which binds both a-Mannose and a-Fucose; other lectins recognize very specific sugar units, such as Phaseolus vulgaris Leucoagglutinin (L-PHA), which binds branched N-acetyl lactosamine (LacNAc). Anti-glycan antibodies can be a good alternate detection reagent, but antibodies against common sugars are not available due to difficulties in their generation. A caveat in using anti-glycan antibodies is that they may have higher cross-reactivity than other antibodies, as suggested by a specificity study using carbohydrate microarrays (12). Therefore, multiple lectins or antibodies may be used to obtain a reliable and a broader profile of the glycan structures present on the captured proteins. A good resource for searching glycan epitopes and their antibodies can be found in GlycoEpitope Database (http://www.glyco.is.ritsumei.ac.jp/epitope/) maintained by Kawasaki laboratory in Research Center for Glycobiotechnology, Ritsumeikan University.
If continuing from the chemical derivatization step (section 3.1), wash the microarray slides in three baths of PBST0.5 for three minutes each.
Incubate the slides in a blocking solution of PBST0.5 + 1% BSA for one hour with gentle shaking (see Note 11).
Wash the slides in three baths of PBST0.5 for three minutes each.
Spin the slides at 1000×g for one minute to dry the slides (see Note 12).
Place the slides in a slide chamber containing a moist paper towel at the bottom. The paper towel will humidify the chamber and prevent evaporation during the serum incubation.
Apply the serum solutions to their designated arrays and incubate at room temperature for one hour with gentle shaking. The volume applied to each array depends on the size of the array. Using the 48-array/slide design shown in Figure 2e, about 7 ml thoroughly covers the each array.
Prepare the glycan-binding detection reagents. Biotinylated lectins should be prepared in PBST0.1 with 0.1% BSA at a concentration of 10 mg/mL. The optimal concentration may be lower for certain high-affinity lectins; this value can be determined experimentally. Biotinylated antibodies (see Note 13) should be prepared at 1 mg/mL in the same buffer as the lectins.
Wash the slides in three baths of PBST0.1 for three minutes each.
Spin the slides at 1000×g for one minute to dry the slides.
Apply the biotinylated lectin or antibody solutions onto each array, and incubate the slides in a humidified slide box at room temperature for one hour with gentle shaking.
Prepare streptavidin-B-phycoerythrin in PBST0.1 + 0.1% BSA at a concentration of 1 mg/mL.
Wash the slides in three baths of PBST0.1 for three minutes each.
Spin the slides at 1000×g for one minute to dry the slides.
Apply streptavidin-B-phycoerythrin to each array, and incubate the slides in a humidified box at room temperature for one hour with gentle shaking.
Wash the slides in three baths of PBST0.1 for three minutes each.
Spin the slides at 1000×g for one minute to dry the slides.
Scan the slides using a microarray scanner with appropriate resolution (10 mm or better) and emission and excitation settings. If the slides will not be scanned immediately, store them vacuum-sealed with desiccant in the refrigerator.
Table 1.
| Glycans | GBPs | Lectin Name or antibody Clones | Lectin Specificities or Glycan Structures |
|---|---|---|---|
| Fucose (Fuc) | AAL | Aleuria Aurantia Lectin | Fuc (preferably (α1–6) linked Fuc) |
| LCA | Lens Culinaris Agglutinin | (α1–6) linked Fuc | |
| PSA | Pisum Sativum Agglutinin | (α1–6) linked Fuc | |
| UEA I | Ulex Europaeus Agglutinin I | Terminal or subterminal Fuc (α1–2) Gal | |
| LTL | Lotus Tetragonolobus Lectin | Fuc (α1–2) Gal (β1–4)[Fuc α1–3]Glc/GlcNAc), other difucosyl carbohydrates | |
| AAA | Anguilla Anguilla Agglutinin | Fuc (α1–3) GlcNAc | |
| α linked galactose (αGal) | antibody | 2.10G, Gal-13, M86 | Gal(α1–3)Gal(β 1–4)GlcNAc-R |
| GS IB4 | GSL I -isolectin B4 | Terminal aGal | |
| MOA | Marasmius oreades agglutinin | Gal (α1–3)Gal(β1–4) GlcNAc/Glc | |
| α linked N-acetyl galactosamine (αGalNAc) (including Tn antigen (GalNAc α1 – Ser/Thr)) | antibody | B1.1, 5F4, 83D4, BRIC 111, Ca3256, Ca3268, Ca3638, CU-1, ETn1.01, FBT3, HBTn1, IE3, MLS128, NCC-LU-35, NCC-LU-81, Tn5, | GalNAc α-Thr/Ser (Tn antigen) |
| HPA | Helix pomatia agglutinin | Terminal αGalNAc | |
| HAA | Helix aspersa agglutinin | α/βGalNAc | |
| VVL | Vicia Villosa Lectin | α/βGalNAc | |
| DBA | Dolichos Biflorus Agglutinin | GalNAc (α1–3) GalNAc | |
| LBL | Lima Bean Lectin (Phaseolus lunatus) | GalNAc (α1–3) Gal | |
| MPL | Maclura Pomifera Lectin | αGalNAc | |
| SBA | Soybean Agglutinin | αGalNAc | |
| WFA | Wisteria Floribunda Lectin | GalNAc (α/β1–6/3) Gal | |
| β linked galactose (βGal) | PNA | Peanut Agglutinin | Terminal βGal |
| RCA120 | Ricinus Communis Agglutinin I | Terminal βGal as in Lactose/LacNAc | |
| ABA | Agaricus bisporus agglutinin | βGal on T antigen | |
| ACA | Amaranthus Caudatus Lectin | βGal on T antigen | |
| β linked N-acetyl galactosamine (βGalNAc) | SJA | Sophora Japonica Agglutinin | βGal and βGalNAc |
| PTL II | Psophocarpus Tetragonolobus Lectin II | βGal and βGalNAc | |
| SBA | Soybean Agglutinin | βGalNAc and αGalNAc | |
| WFA | Wisteria Floribunda Lectin | GalNAc (α/β1–6) Gal and GalNAc (α/β1–3) Gal | |
| GalNAc(β1–3)Gal(β1–4)GlcNAc | antibody | TH2 | GalNAc(β1–3)Gal(β1–4)GlcNAc-R |
| GalNAc(β1–4)[Fuc(α1–3)]Glc(β1−) | antibody | AK97 | Gal(β1–4)[Fuc(α1–3)]Glc(β1−)-R |
| GalNAc(β1–4)[Neu5Ac(α2–3)]Gal(β1−) | antibody | 2A3D2, 2D11E2, CT1, CT2, DMAb-1, DMAb-2, DMAb-3, DMAb-4, DMAb-5, KM531, YHD-06 | GalNAc(β1–4)[Neu5Ac(α2–3)]Gal(β1−)-R |
| GalNAc(β1–4)Gal | antibody | 2D4 | GalNAc(β1–4)Gal-R |
| T antigen (Gal-β1–3 GalNAc α1-Ser/Thr) | antibody | 49H.24, 49H.8, 5A8, 8D8, A78-G, Ca3114, Ca3741, HB-T1, HH8, TF1, TF2, TF5 | Gal (β1–3) GalNAc a-Thr/Ser (T antigen) |
| BPL | Bauhinia Purpurea Lectin | Gal (β1–3) GalNAc or terminal αGalNAc | |
| PNA | Peanut Agglutinin | terminal Gal (β1–3) GalNAc a-Thr/Ser (T antigen), terminal βGal | |
| Jacalin | Jacalin | Gal (β1–3) GalNAc α-Thr/Ser,GalNAc α-Thr/Ser(Tn) | |
| VVL | Vicia Villosa Lectin | Terminal Gal β1–3 GalNAc α-Thr/Ser or terminal αGalNAc α-Thr/Ser | |
| Sialyl T antigen | antibody | Neu5Ac (α2–3)Gal(β1,3)GalNAc] α-Ser/Thr | |
| Disialyl T antigen | antibody | QSH2 | Neu5Ac(α2–3)Gal(β1–3)[Neu5Ac(α2–6)]GalNAc(α1−)Ser/Thr |
| Terminal Galactose 3-O-Sulfate | antibody | M14–376, Sulph 1 | HSO3(−3)Gal-R |
| Sialyl Tn antigen | antibody | B72.3, C1282, CC102, CC49, HB-STn1, 3C2, MLS132, | Neu5Ac (α2–3) GalNAc](α−)-Ser/Thr |
| Glucose (Glc) | VFA | Vicia faba agglutinin | α–linked Glc and α–linked Man |
| Con A | Concanavalin A | α–linked Glc and α–linked Man | |
| N-acetyl-Glucose (GlcNAc) | GSL II | Griffonia (βandeiraea) Simplicifolia Lectin II | Terminal βGlcNAc or αGlcNAc |
| PVL | Psathyrella velutina lectin | βGlcNAc | |
| O-linked GlcNAc (O-GlcNAc) | antibody | CTD110.6, HGAC39, HGAC85, RL2 | GlcNAc(β1−)Ser/Thr |
| Chitobiose (GlcNAc (β1–4)GlcNAc) | WGA | Wheat Germ Agglutinin | (GlcNAc (β1–4) GlcNAc)n repeats |
| DSL | Datura Stramonium Lectin | GlcNAc oligomer | |
| LEL | Lycopersicon Esculentum (Tomato) Lectin | GlcNAc oligomer | |
| STL | Solanum Tuberosum (Potato) Lectin | GlcNAc oligomer | |
| UDA | Urtica dioica agglutinin | GlcNAc oligomer | |
| LacNAc (Gal (β1–4) GlcNAc) | antibody | 1B2 (to Terminal LacNAc) | Terminal LacNAc |
| ECL | Erythrina Cristagalli Lectin (ECL, ECA) | Terminal LacNAc | |
| RCA120 | Ricinus Communis Agglutinin I | LacNAc (binding inhibited by terminal sialic acid) | |
| Branched (β1–6) LacNAc | PWM | Pokeweed mitogen lectin | Gal (β1–4) GlcNAc (β1–6) -R |
| L-PHA | Phaseolus vulgaris Leucoagglutinin | Gal (β1–4) GlcNAc (β1–6) -R | |
| 6-Sulfo LacNAc | antibody | AG107, DD1, DD2, M-DC8 | Gal(β1–4)[HSO3(−6)]GlcNAc -R |
| 6,6'-Sulfo LacNAc | antibody | DD1, M-DC8 | HSO3(−6)Gal(β1–4)[HSO3(−6)]GlcNAc(β1−)-R |
| 6,6,6'-Sulfo LacNAc | antibody | DD1 | HSO3(−6)Gal(β1–4)[HSO3(−6)]GlcNAc(β1–3)[HSO3(−6)]Gal(β1−)-R |
| LDN (LacdiNAc) | antibody | 100-2H5-A, 114-2H12-C, 259-2A1, 273-3F2, 99-2A5-B, SMLDN1.1, | GalNAc(β1–4)GlcNAc-R |
| LDN-F | antibody | 204-6A1, 294-2A1, SMLDNF1 | GalNAc(β1–4)[Fuc(α1–3)]GlcNAc-R |
| Acharan Sulfate | antibody | MW3G3 | -{[HSO3(−2)]IdoA(α1–4)GlcNAc(α1–4)}n− |
| Lewis a | antibody | 7LE, PR.5C5, PR 4D2, T174, B369, BC9-E5, CF4-C4 DG4-1, | Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1−)-R |
| Dimeric Lewis a | antibody | NCC-ST-421 | Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1–3)Gal(β1–3)[Fuc(α1–4)] GlcNAc(β1−)-R |
| Disialyl Lewis a | antibody | FH7 | Neu5Ac(α2–3)Gal(β1–3)[Neu5Ac(α2–6)][Fuc(α1–4)] GlcNAc(β1−)-R |
| 3'-Sulfo Lewis a | antibody | F2, 91.9H, | HSO3(−3)Gal(β1–3)[Fuc(α1–4)]GlcNAc-R |
| Sialyl Lewis a (CA19-9) | antibody | 121SLE, CA199.02, 2M1, SPM110, 241, 192 | Neu5Ac (α2–6))Gal(β1–3)[Fuc(α1–4)]GlcNAc-R |
| Lewis a- Lewis x Hybrid Antigen | antibody | 43-9F, NCC-ST-421 | Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1–3)Gal(β1–4)[Fuc(α1–3)] GlcNAc(β1−)-R |
| Lewis b | antibody | 2–25LE, T218, SPM194, 1116NS-10, YB-2, | Fuc(α1–2)Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1−)-R |
| Trifucosyl-Lewis b Antigen | antibody | IMH2 | Fuc(α1–2)Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1–3)Gal(β1–3)[Fuc(α1–4)] GlcNAc(β1−)-R |
| Lewis c | antibody | K21 | Fuc(α1–2)Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1–3)Gal(β1–3)[Fuc(α1–4)] GlcNAc(β1−)-R |
| Sialyl Lewis c | antibody | C-50, DUPAN-2 | Neu5Ac(α2–3)Gal(β1–3)GlcNAc(β1−)-R |
| Lewis x | antibody | 15C02, 28, ZC-18C, FR4A5, HI98, W6D3, P12, 1G10, 73-30, 80H5, FH2, LeuM1, MC-480, MMA, SH1, WGHS-29-1 | Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1−)-R |
| Sialyl Lewis x | antibody | CSLEX1 | Neu5Ac (α2–6)Gal(β1–4)[Fuc(α1–3)]GlcNAc–R |
| Dimeric Lewis x | antibody | FH4 | Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)[Fuc(α1–3)] GlcNAc(β1−)-R |
| 3'-Sulfo Lewis x | antibody | MIN/3/60, SU59 | HSO3(−3)Gal(β1–4)[Fuc(α1–3)]GlcNAc-R |
| 6-Sulfo Lewis x | antibody | AG107, AG273, AG97 | Gal(β1–4)[Fuc(α1–3)][HSO3(−6)]GlcNAc(β1−)-R |
| Sialyl 6-Sulfo Lewis x | antibody | 2F3, 2H5, G162, G72, HECA-452, | Neu5Ac(α2–3)Gal(β1–4)[Fuc(α1–3)][HSO3(−6)] GlcNAc(β1−)-R |
| 6'-Sulfo Sialyl Lewis x | antibody | 2F3, 2H5, CSLEX1 / CSLEX-1, HECA-452, SNH3 / SNH-3 | Neu5Ac(α2–3)[HSO3(−6)]Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1−)-R |
| 6,6'- Disulfo Sialyl Lewis x | antibody | 2F3, 2H5, G27011, G27037, G27039, G2706, HECA-452 | Neu5Ac(α2–3)[HSO3(−6)]Gal(β1–4)[Fuc(α1–3)][HSO3(−6)]GlcNAc(β1–3) Gal(β1−)-R |
| Sialyl Lewis i-x (VIM-2) | antibody | CF4, HE-10, VIM-2 | Neu5Ac(α2–3)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)[Fuc(α1–3)] GlcNAc(β1−)-R |
| Sialyl Lewis x-i | antibody | FH6 | Neu5Ac(α2–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4) GlcNAc(β1–3)Gal(β1−)-R |
| Cyclic Sialyl 6-Sulfo Lewis x | antibody | G159 | Cyclic Neu5Ac (α2–3) Gal(β1–4)[Fuc(α1–3)[HSO3(−6)]GlcNAc-R |
| Lewis y | antibody | BR55, F3, A70-C/C8, A63-D/B12, 3S193, AH6, BR96, H18A, YB-2, | Fuc(α1–2)Gal(β1–4)[Fuc(α1–3)]GlcNAc-(β1−)-R |
| Trifucosyl-Lewis y | antibody | KH1 | Fuc(α1–2)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)[Fuc(α1–3)] GlcNAc(β1−)-R |
| Mannose (Man) | Con A | Concanavalin A | Terminal α-Man, branched tri-Man |
| LCA | Lens Culinaris Agglutinin | Terminal α-Man | |
| GNL | Galanthus Nivalis Lectin | Terminal Man (α1–2)Man, Mana(α1–6) Man | |
| BanLec | Banana Lectin (Musa Acuminata) | a-Man, 3-O-a-Man, branched tri-Man | |
| O-Mannosyl Glycan (Mammalian) | antibody | IIH6 | Neu5Ac(α2–3)Gal(β1–4)GlcNAc(β1–2)Man(α1−)Ser/Thr |
| Sialic acid (Neu5Ac) | SNA | Sambucus Nigra Lectin | α2–6 linked Neu5Ac |
| MAL II | Maackia Amurensis Lectin II | Neu5Ac (α2–3) Gal | |
| SSA | S. sieboldiana agglutinin | NeuAc(α2–6)Gal/GalNAc | |
| TJA-I | Trichosanthes japonica agglutinin I | NeuA(α2–6)Gal | |
| PSL | Polyporus squamosus lectin | Neu5Ac (α2–6)Gal(β1–4)GlcNAc/Glc | |
| LPA | Limulus polyphemus agglutinin | Neu5Ac | |
| LFA | Limax flavus agglutinin | Neu5Ac | |
| 9-O-Acetyl Sialic Acid | antibody | 493D4, CH#-FcD, | Neu5Ac9Ac(α2−)-R |
| Poly-N-Acetylneuraminic Acid | antibody | 12E3, 1E6, 2-2B, 5A5, 735, H.46, OL28, S2–566 | (Neu5Ac) n |
| KDN-Gal (KDN: 3-deoxy-D-glycero-D-galacto-nonulosonic acid) | antibody | Kdn3G | KDN(α2–3)Gal(β1−)-R |
| Poly-KDN | antibody | Kdn8kdn | (KDN)n |
| Neu5Ac(α2–3)Gal (Monosialoganglioside) | antibody | 202 | Neu5Ac(α2–3)Gal-R |
| Neu5Ac(α2–3)Gal(β1) | antibody | FCM1, | Neu5Ac(α2–3)Gal(β1−)-R |
| Neu5Ac(α2–3)Gal(β1–4)Glc | antibody | M2590, MSG-1, | Neu5Ac(α2–3)Gal(β1–4)Glc-R |
| Neu5Ac(α2–3)Gal(β1–4)GlcNAc | antibody | M2590, NS24, SPS-20 | Neu5Ac(α2–3)Gal(β1–4)GlcNAc-R |
| Neu5Ac(α2–8)Neu5Ac(α2–3)Gal | antibody | A1–245, A1–267, HJM1, R24 | Neu5Ac(α2–8)Neu5Ac(α2–3)Gal-R |
| Neu5Ac(α2–8)Neu5Ac(α2–3)Gal(β1–4)Glc | antibody | DMAb-19, DMAb-7, DMAb-8, DSG-1, GMB7 | Neu5Ac(α2–8)Neu5Ac(α2–3)Gal(β1–4)Glc-R |
| Blood Group A Trisaccharide | antibody | 3–3a, AC1001, AC12, B2C114, CB, HB29–36, K7422, K7522, K7516, S10-11A6, | GalNAc(α1–3)[Fuc(α1–2)]Gal(β1−)-R |
| Blood Group A Type 1 | antibody | AH16, AH21, CB, CLH6, HB29–36, T36 | GalNAc(α1–3)[Fuc(α1–2)]Gal(β1–3)GlcNAc(β1−)-R |
| Blood Group A Type 2 | antibody | 11D4, 3A7, AH16, CB, HB29–36, T36 | GalNAc(α1–3)[Fuc(α1–2)]Gal(β1–4)GlcNAc(β1−)-R |
| Blood Group A Type 3 | antibody | CB, HB29–36, K45aF3, M2, MRG-1 | GalNAc(α1–3)[Fuc(α1–2)]Gal(β1–3)GalNAc(α1−)-R |
| Blood Group A Type 4 | antibody | CB, HB29–36, M2, | GalNAc(α1–3)[Fuc(α1–2)]Gal(β1–3)GalNAc(β1−)-R |
| Blood Group B Type 2 | antibody | 3A7, CLCP-19B, | Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)GlcNAc(β1−)-R |
| Blood Group H Disaccharide | antibody | 1E3, 3H1, B389 | Fuc(α1–2)Gal(β1−)-R |
| Blood Group H Type 1 | antibody | 0.BG.5, 17–206, 1E3, 1E5, 3H1, BE2, H1B4 | Fuc(α1–2)Gal(α1–3)GlcNAc(β1−)-R |
| Blood Group H Type 2 | antibody | 0.BG.5, 1E3, 1E5, 3A5, 3H1, 92 FR A2, B376, B389, B393, BE2, BRIC231, H1B4, YB02 | Fuc(α1–2)Gal(β1–4)GlcNAc(β1−)-R |
| Blood Group H Type 3 | antibody | 1E3, 3H1, MBr1 | Fuc(α1–2)Gal(β1–3)GalNAc(α1−)-R |
| Blood Group H Type 4 | antibody | 1E3, 3H1, MBr1 | Fuc(α1–2)Gal(β1–3)GalNAc(β1−)-R |
| SSEA-1 | antibody | SSEA-1 | Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)GlcNAc (β1–3)Gal(β1−)-R |
| SSEA-3 | antibody | MC631, SSEA-3 | Gal(β1–3)GalNAc(β1–3)Gal(α1−)-R |
| SSEA-4 | antibody | MC813-70 | Neu5Ac(α2–3)Gal(β1–3)GalNAc(β1–3)Gal(α1−)-R |
| Heparan Sulfate | antibody | 10E4, AO4B08, HepSS1, HK249, HS3A8, HS3B7, HS4C3, HS4E4, J403, JM13, MW3G3, NAH46, RB4EA12, | -[{[±(HSO3(-2))GlcA(β1–4)]or[±(HSO3(−2))IdoA(α1–4)]} (±(HSO3(−3)) ±[(HSO3 or CH3CO)(−2)]±(HSO3(−6))GlcN]n− |
| Delta-Heparan Sulfate | antibody | 3G10 | unsaturated HexA-R |
| Keratan Sulfate | antibody | 5D4 | -{HSO3(−6)GlcNAc(β1–3)±[HSO3(−6)]Gal(β1–4)}n− |
| P1 Antigen | antibody | P001, Pk002 | Gal(α1–4)Gal(β 1–4)GlcNAc-R |
| Pk Antigen | antibody | 38.13, P001, Pk002, PK67 | Gal(α1–4)Gal(β 1–4)Glc-R |
| HNK-1 | antibody | 334, 4F4, Elec39, HNK-1, L2, M6749, NC-1, NGR50, NSP-4, VC1.1, | HSO3(−3)GlcA(β1–3)Gal(β1–4)GlcNAc-R |
| I-Antigen | antibody | Anti-I Ma, anti-I Woj, C6 | Gal(β 1–4)GlcNAc(β 1–6)[Gal(β 1–4)GlcNAc(β 1–3)]Gal(β 1–4)GlcNAc(β 1−)-R |
3.4 Data analysis and interpretation
The lectin-binding levels on the arrays can be interpreted in light of the known binding specificities of the lectins. By using multiple lectins or glycan-binding antibodies, more information about the glycan structures can be gathered. When looking at changes in lectin-binding level between samples, it must be remembered that the underlying core protein concentrations might fluctuate along with the amount of a given glycan on the protein. We recommend running sandwich immunoassays to determine the core protein concentrations, which will allow a comparison between core protein changes and glycan changes. Sandwich immunoassays may be conveniently run using the same antibody microarrays as used for the assay described here, only detecting with antibodies against the core proteins rather than detecting with lectins or glycan-binding antibodies. Detailed descriptions of those types of experiments are found elsewhere (13–15).
The researcher should also be aware that lectin binding levels can be affected by other factors besides the glycan level or the protein level. These factors include the number of the glycan repeats, the location of the glycan epitope in the overall carbohydrate structure, and the presence of outer glycan groups that block binding. For example, Ricinus Communis Agglutinin I (RCA120) binding to LacNAc is inhibited by sialic acid groups at the terminus of glycan chains (4). Another consideration is that some lectins might bind more than one sugar group, so the relative contribution of the different sugar groups to the overall signal can be ambiguous. Therefore results must be interpreted with some caution. This method is ideal for high-throughput screening of the associations of glycan structures with disease or conditions, and complementary techniques can be used to further elucidate the glycan structures in selected samples.
Footnotes
Vacuum sealing helps to preserve the antibodies and proteins. Slide boxes can be vacuum sealed using a moderately-priced vacuum packaging device such as the FoodSaver. Punch a hole in the slide box to ensure the air in the slide box is removed. Further removal of moisture can be achieved by placing a small desiccant pack in the slide box.
The wax partition should stay on throughout all procedures. However, if some thinning or weakening of the lines in observed, the wax can be re-printed at any step. Simply wash and dry the slides and re-apply the wax. It may be most convenient to do that before the BSA blocking. Some surfaces, particularly hydrogel surfaces, may not hold wax well. To partition those surfaces, we recommend a gasket system available from several suppliers (The GelCompany, GraceBiolabs).
Two forms of the Coupling Buffer are used here: with and without Tween-20. We have observed that the addition of the detergent prevented the slide coating from peeling off during the long incubation in the oxidation step when using the PATH slide from GenTel Biosciences (Madison, WI); other slide coatings may behave differently. The Coupling Buffer without Tween-20 should be used for the other reactions.
The concentration of NaIO4 needed to oxidize the cis-hydroxyl groups varies somewhat between monosaccharides (4). Sialic acid is the easiest to oxidize, while mannose seems to be the most difficult. When profiling many types of glycans, we suggest using around 200 mM NaIO4, which will efficiently oxidize all types of glycans, but if the targets are only sialic acid or glucose, the NaIO4 concentration can be 50 mM or less. The optimal conditions for cis-hydroxyl oxidation are pH< 4 and 4 °C. Higher pH or temperature may decrease the reaction efficiency. At the concentration of 200 mM and 4 °C, sodium paraperiodate may precipitate onto the slides, but it can be washed off without negative effect. We recommend storing the NaIO4 in a vacuum-sealed package with desiccant at −20 °C and preparing the solution immediately before use.
The lid of a pipette-tip box works well for this step. About 50 mL of solution is sufficient for a typical lid, and five slides can lie flat in the lid.
Shaking is necessary to ensure even access of the solution to all areas of the slides. Shaking can also help to avoid precipitate formation. Push the slides to the bottom of the box so that they will not float on top of each other. To ensure the slides stay on the bottom of the container, a clip can be affixed to the side of the container to hold the edges of the slides down.
The optimum reaction pH for the maleimide groups on the MPBH is between 6.5 and 7.5. The purpose of the addition of CysGly at this step is to eliminate the possible reaction between the maleimide groups and the cysteine groups on antibodies, which could negatively affect the antibody activities.
The reaction between CysGly and the maleimide group of MPBH can be finished at room temperature in two hours. The overnight incubation at 4 °C is designed for a consecutive two-day protocol. If planning to store the slides after blocking, this step can be carried out at room temperature for two hours, followed by washing, drying, and storage.
Since the effect of long-term storage of the blocked slide has not been tested, we suggest finishing the lectin detection immediately after the chemically blocking. The whole procedure will take two consecutive days.
It is not necessary to remove or denature the enzymes in the reaction mixture. Heat denaturation of the enzyme may denature the serum proteins, and may not able to be captured by the capture antibodies. No negative result or increasing background was found when this reaction mixture was used in glycan detection assays.
Blocking reagents that contain glycans, such as milk, may result in high backgrounds, and should be avoided.
Higher drying speeds may cause the coatings of certain slides, such as the PATH slide, to peel slightly at this step. This possibility can be minimized by slower spinning or drying the slides in a flat, facing-up position.
Many of the lectins can be obtained biotinylated, but some lectins and most antibodies will need to be biotinylated by the user. This process can be achieved using commercially-available biotinylation reagents such as the NHS-biotin, Hydrazide-biotin, etc. from Pierce Biotech or other suppliers.
References
- 1.Kingsmore SF. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat Rev Drug Discov. 2006;5:310–320. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haab BB. Antibody arrays in cancer research. Mol Cell Proteomics. 2005;4:377–383. doi: 10.1074/mcp.M500010-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Haab BB. Applications of antibody array platforms. Curr Opin Biotechnol. 2006;17:415–421. doi: 10.1016/j.copbio.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Laroche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, et al. Multiplexed analysis of glycan variation on native proteins captured by antibody microarrays. Nat Methods. 2007;4:437–444. doi: 10.1038/nmeth1035. [DOI] [PubMed] [Google Scholar]
- 5.Haab BB, Zhou H. In: Protein Arrays. Fung E, editor. Totowa, NJ: Humana Press; 2004. pp. 33–45. [Google Scholar]
- 6.Orchekowski R, Hamelinck D, Li L, Gliwa E, vanBrocklin M, Marrero JA, et al. Antibody microarray profiling reveals individual and combined serum proteins associated with pancreatic cancer. Cancer Res. 2005;65:11193–11202. doi: 10.1158/0008-5472.CAN-05-1436. [DOI] [PubMed] [Google Scholar]
- 7.Kelly LS, Kozak M, Walker T, Pierce M, Puett D. Lectin immunoassays using antibody fragments to detect glycoforms of human chorionic gonadotropin secreted by choriocarcinoma cells. Anal Biochem. 2005;338:253–262. doi: 10.1016/j.ab.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, et al. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nat Methods. 2005;2:851–856. doi: 10.1038/nmeth803. [DOI] [PubMed] [Google Scholar]
- 9.Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray analysis of 24 lectins. Angew Chem Int Ed Engl. 2006;45:3607–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 10.Wearne KA, Winter HC, Goldstein IJ. Temporal changes in the carbohydrdates expressed on BF01 human embryonic stem cells during differentiation as embryoid bodies. Glycoconjugate Journal. 2007 doi: 10.1007/s10719-007-9064-x. In press. [DOI] [PubMed] [Google Scholar]
- 11.Wearne KA, Winter HC, O'Shea K, Goldstein IJ. Use of lectins for probing differentiated human embryonic stem cells for carbohydrates. Glycobiology. 2006;16:981–990. doi: 10.1093/glycob/cwl019. [DOI] [PubMed] [Google Scholar]
- 12.Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology. 2007;17:17C–23C. doi: 10.1093/glycob/cwm047. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Haab BB. In: Clinical Proteomics. Van Eyk J, Dunn M, editors. Weinheim, Germany: Wiley, VCH; 2007. [Google Scholar]
- 14.Forrester S, Kuick R, Hung KE, Kucherlapati R, Haab BB. Low-volume, high-throughput sandwich immunoassays for profiling plasma proteins in mice: identification of early-stage systemic inflammation in a mouse model of intestinal cancer. Molecular Oncology. 2007 doi: 10.1016/j.molonc.2007.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen UB, Geierstanger BH. Multiplexed sandwich assays in microarray format. J Immunol Methods. 2004;290:107–120. doi: 10.1016/j.jim.2004.04.012. [DOI] [PubMed] [Google Scholar]