Abstract
Caspase-6 (Casp6), a cysteinyl protease that induces axonal degeneration, is activated early in Alzheimer Disease (AD) brains. To determine whether Casp6 activation is responsible for early cognitive impairment, we investigated the abundance of Casp6 activity, paired helical filament–1 (PHF-1) phosphorylated Tau and amyloid beta peptide (Aβ) pathology by immunohistochemistry in the hippocampal formation of aged non–cognitively impaired (NCI) individuals. Casp6 activity was restricted to the entorhinal cortex (ERC) and CA1 regions of the hippocampus. Pathology scores were then correlated with cognitive scores obtained within 1 year of death. Regression analyses revealed that ERC and CA1 Casp6 activity were the main contributor to lower episodic memory performance, whereas ERC PHF-1 pathology predicted lower semantic and working memory performance. Aβ did not correlate with any of the cognitive tests. Because Casp6 activity and PHF-1 pathology are intimately associated with AD pathology and memory decline is an early event in AD, we conclude that Casp6 activity and PHF-1 immunoreactivity in ERC identifies aged individuals at risk for developing AD.
Keywords: Caspase-6, Alzheimer disease, Memory performance, Aged individuals and cognition, Episodic memory, Working memory, Semantic memory, Visuospatial abilities, Perceptual speed
1. Introduction
Caspase-6 (Casp6) activity is present in neurofibrillary tangles (NFT), neuropil threads (NPT), and neuritic plaques (NP) of sporadic Alzheimer’s disease (AD) brains and in familial AD caused by amyloid precursor protein (APP), presenilin I, or presenilin II mutations (Albrecht et al., 2009, Albrecht et al., 2007). Previously, we have shown that Casp6 activity occurs at all stages of AD and is equally abundant and distributed in mild, moderate, severe, and very severe forms of AD (Albrecht et al., 2007). Surprisingly, Casp6 activity was detected in some non–cognitively impaired (NCI) brains, and the levels of Casp6 activity correlated inversely with cognitive scores measured within a year of death. However, although Casp6 activity was quite abundant in the hippocampal formation and in the cortices of AD brains, high levels of Casp6 activity in NCI brains were mostly limited to the entorhinal cortex (ERC), the first area to be affected pathologically by AD according to Braak staging (Braak and Braak, 1995, Lace et al., 2009).
Casp6, is involved in inflammation and apoptosis, does not induce cell death when activated in AD neurons or in mammalian cell lines (Gray et al., 2010, Guo et al., 2004, Klaiman et al., 2009), but induces axonal degeneration in primary cortical human neurons and commissural and sympathetic mouse neurons (Klaiman et al., 2008, Nikolaev et al., 2009, Sivananthan et al., 2010). Casp6 cleaves several cytoskeletal (alpha-tubulin, Tau) or cytoskeletal-regulating proteins including post-synaptic actin regulating proteins, Drebrin, Spinophilin, actinin-1, and actinin-4 (Klaiman et al., 2008). In addition, Casp6 activation increases the production of the amyloid beta peptide (Aβ) (LeBlanc,1995); however, the effect is not through direct cleavage of APP as originally thought (Gervais et al., 1999, Pellegrini et al., 1999, Weidemann et al., 1999) but rather occurs by caspase-dependent cleavage of an inhibitor of the beta secretase (Tesco et al., 2007, Tesco et al., 2003). Furthermore, Casp6 cleaves valosin-containing protein and impairs its ability to target misfolded and ubiquitinated proteins to the proteasome for degradation (Halawani et al., 2010). Together, these studies indicate that Casp6 activity affects a number of parallel degenerative pathways that are likely to impair neuronal function.
To determine the impact of Casp6 activity on the cognitive performance of NCI individuals compared to accepted AD pathological markers, we analyzed the extent of active Casp6, amyloid beta peptide (Aβ), and PHF-1 immunopositive pathology in different areas of the hippocampal formation of 17 NCI individuals and correlated these pathological scores with cognitive scores obtained within a year of death.
2. Methods
2.1. Collection of brain tissues, fixation, and preparation of slides
Brain tissue was obtained from subjects who participated in the Religious Orders Study (ROS). Details of the clinical and pathologic evaluation have been previously reported (Bennett et al., 2012). The ROS includes more than 1150 older nuns, priests, and brothers who have agreed to yearly clinical evaluations and brain donation at time of death. The clinical evaluations include a medical history, and neurologic and cognitive assessments performed on a yearly basis. The cognitive evaluation includes 21 tests: the Mini Mental State Examination (MMSE), and 20 other tests, 19 of which can be summarized as follows: visuospatial ability (VSA); perceptual speed (PS); and episodic, semantic, and working memory by converting the individual tests within those domains to Z scores and averaging. All 19 Z-scores can be averaged to yield a global cognitive score. Participants were diagnostically classified by a clinician following the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) criteria. An AD diagnosis was assigned to persons with a history of cognitive decline and evidence of impairment in memory and other cognitive domains. Mild cognitive impairment (MCI) referred to individuals who displayed cognitive impairment upon neuropsychological evaluations but were not clinically diagnosed with dementia. Non–cognitive impairment (NCI) referred to persons without dementia or MCI. Standard neuropathologic assessment, including Braak Stage determination, was done at autopsy by a neuropathologist shielded from all clinical data.
2.2. Memory tasks and scores
The memory scores represent an average z scores based on the mean and standard deviation of 19 cognitive performance tests at the baseline clinical evaluation. Essentially, a score of 0 refers to the average score of the cohort at baseline, and =1 and −1 are 1 standard deviation above and below the mean. Details of the development and derivation of the scores can be found in prior papers (Wilson et al., 2002). The episodic memory scores were based on a composite score of the following seven instruments: logical memory, word list recall (immediate), word list recall (delayed), word list recognition from the Consortium to Establish a Registry for AD (CERAD), and immediate and delayed recall of story A from the logical memory subset of the Wechsler Memory Scale–revised, and immediate and delayed recall of the East Boston Story. Visuospatial abilities were composite scores of 2 instruments: the 15 items version of Judgment of Line orientation and a 16-item version of standard progressive matrices. Perceptual speed is a composite score of the oral version of the symbol digit modalities test and number comparison. Semantic memory scores are a composite score of 4 instruments: verbal fluency from CERAD, subsets of items from the Boston naming test, extended range vocabulary test, and the national adult reading test. Working memory scores are a composite score of 4 instruments: digit span subtests forward and backward of the Wechsler memory scale revised, digit ordering, and alpha span. The MMSE is the widely used 30-item standardized screening measure of dementia severity.
2.3. Immunostaining of tissue sections with anti-active Casp6, TauΔCasp6, PHF-1, and Aβ
Formalin-fixed, paraffin-embedded 4-µm thick hippocampal formation tissue sections were deparaffinized, rehydrated, and treated with antigen retrieval buffer (10 mmol/LTris Base, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.05% Tween 20, pH 9) for 20 minutes at 97 °C in the Pascal Dako Cytomation apparatus and immunostained using the Dako Autostainer Plus automated slide processor and the EnVision Flex system (Dako, ON). Tissue sections were treated with peroxidase for 5 minutes, blocked with Serum-Free Protein Block (Dako, Burlington, ON) for 30 minutes, and subjected to primary antibodies diluted in EnVision Flex Antibody Diluent for 30 minutes. Active Casp6 was detected with p20Casp6 10630 antiserum (1/5000) and TauΔCasp6 10635 antiserum (1/ 25,000) (Guo et al., 2004), Aβ was detected with F25276 antiserum (1/2000) (LeBlanc, 1995). The PHF-1 antibody (1/5000) was generously provided by Dr Peter Davies (Department of Neuroscience, Albert Einstein College of Medicine, New York, NY). Immunoreactivity was revealed with mouse/rabbit–horseradish peroxidase secondary antibodies for 30 minutes and diaminobenzidine (DAB; Dako, ON) for 10 minutes. Slides were counterstained with hematoxylin.
2.4. Assessment of immunohistochemical staining
The MIRAX SCAN was used to scan tissue sections and generate high-resolution digital images, which were analyzed using the MIRAX Viewer Program (Zeiss, Oberkochen, Germany). The Atlas of the Human Brain was used as a reference to identify the hippocampus proper (CA1–CA4 and subiculum) and the entorhinal cortex (ERC), trans-ERC, and temporal cortices in each tissue section (Mai et al., 2008). In some sections, not all of these brain areas were present (indicated as N.A. in Supplemental Tables 1–4). JR and SA did the scoring in a blinded manner. The densities of NFTs, NPs, and NPTs were scored semi-quantitatively as absent (0), rare (0.5), sparse (1), frequent (2), or abundant (3). Scoring diagrams developed by CERAD for assessing AD pathology in autopsy brains were used as guides (Mirra et al., 1993). Sparse consisted of 1 to 5/100 × field (grade 1); moderate, 6 to 19 NPs/100 × field (grade 2); and frequent, 20 or more NPs/100 × field (grade 3). When NFTs, NPs, and NPTs were unevenly distributed in a slide, the region with the highest density was rated. Rare (0.5) was used when the immunostaining was present but did not reach the sparse criteria.
2.5. Statistical evaluations
Separate Spearman rank (r) correlations for the ERC and CA1 brain regions were performed between the following: individual pathology scores; pathology scores and comprehensive cognitive scores; memory scores; and perceptual performance. The number of cases with identical scores is indicated next to the diamond in Figs. 3 to 7. Given the numerous correlation performed, we obtained a less conservative overall alpha level [ α′ = 1− (1 − overall α)1/k] (Cupples et al., 1984) and used a Bonferroni correction. Significance criteria are indicated in the figure captions. Stepwise linear regression analyses for the ERC and CA1 brain regions entering the 4 brain pathology markers to predict cognitive performance were done with SPSS17 software (IBM). The age and education level of NCI individuals was included as a potential variable in predicting cognitive outcomes.
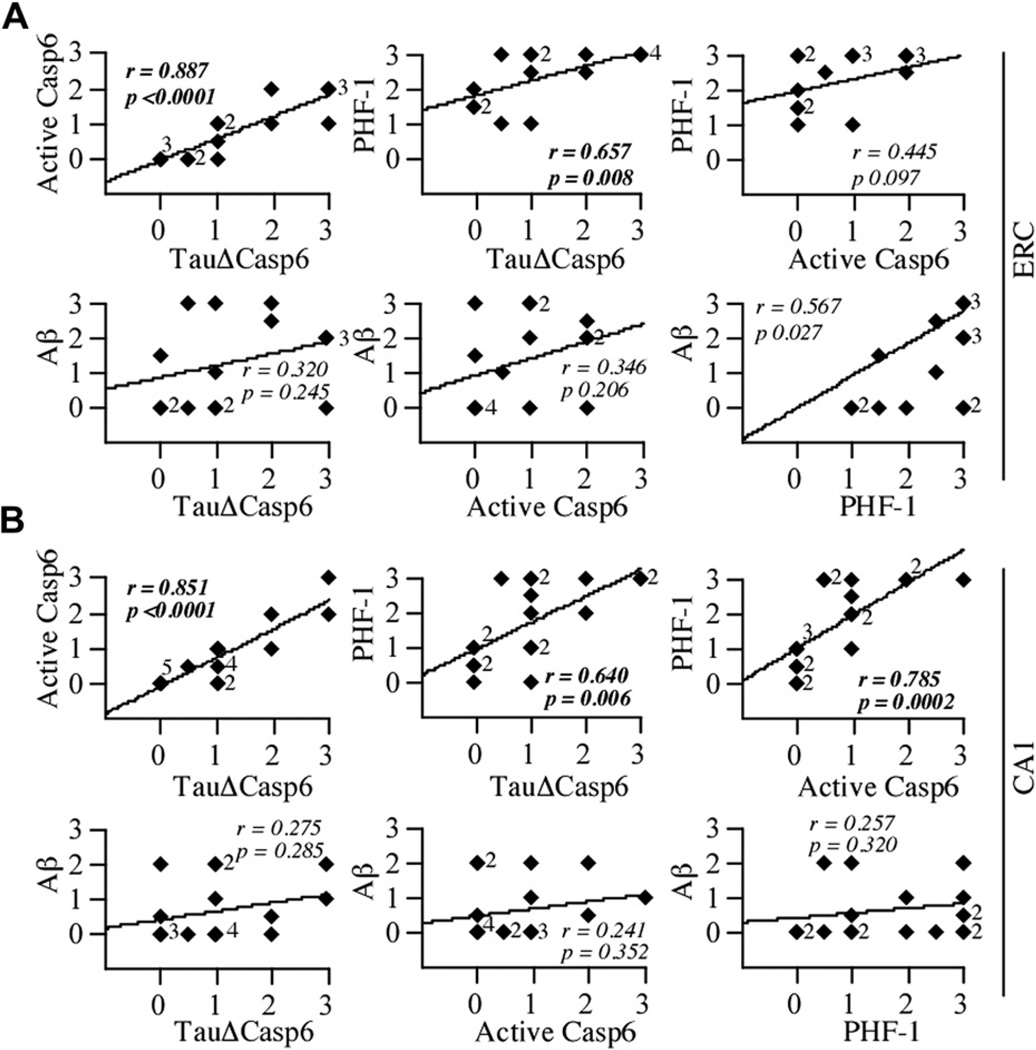
Fig. 3.
TauΔCasp6 staining correlate with active Casp6 and PHF-1 staining but not amyloid staining. Correlation between the active Casp6, TauΔCasp6, PHF-1, or Aβ immunostaining scores in the ERC (A; df = 15) and CA1 (B; df = 17). Because of memory and/or pathology score overlap, it appears as though some figures contain more cases than others; however, all cases are included within each comparison for this and consecutive figures. Diamonds represent individual or overlapping cases as indicated by a small number next to the diamond. A value of p < .02 defines a statistically significant difference.
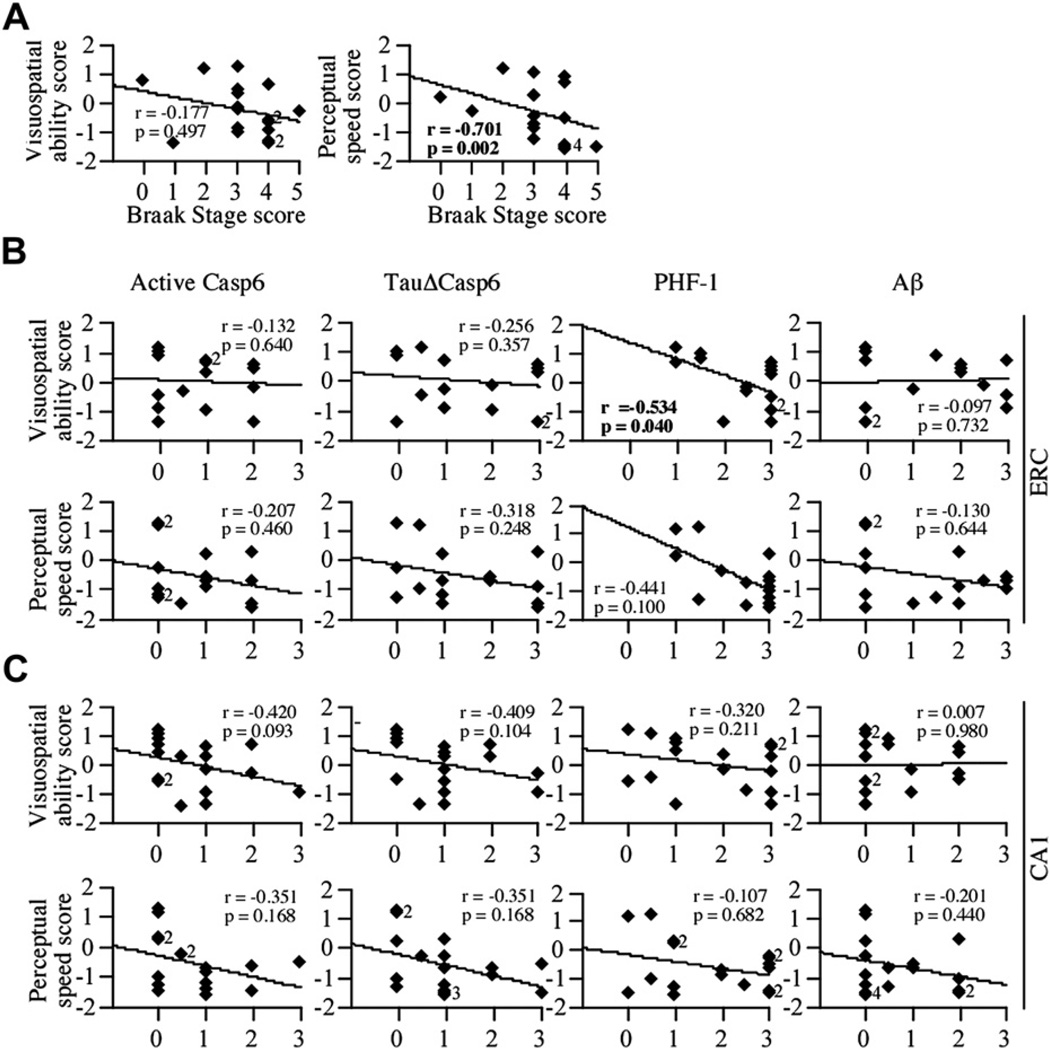
Fig. 7.
Casp6 activity staining does not correlate with either visuospatial ability or perceptual speed scores. (A) Correlation between the Braak stage scores and visuospatial ability or perceptual speed scores (df = 17). Correlation between the scores obtained by staining tissue sections with active Casp6, TauΔCasp6, PHF-1, or Aβ antibodies and visuospatial ability or perceptual speed scores in the ERC (B; df = 15) and CA1 (C; df = 17). Diamonds represent individual or overlapping cases as indicated by a small number next to the diamond. An r indicates Spearman rank correlation, with p values less than .02 considered significant.
3. Results
3.1. Cognitive characteristics, education levels, and Apo E genotypes of the 17 NCI cases
The 17 NCI individuals were 83.89 ± 6.12 years (range, 72.43–92.83) and scored 27.88 ± 2.18 on MMSE. Global cognitive, episodic memory, working memory, semantic memory, visuospatial abilities, and perceptual speed scores are provided in Table 1. These NCI individuals all achieved college-level education or beyond, ranging between 15 and 25 years of education (18.35 ± 2.74 years). Apo E genotypes were mostly Apo E3 with only 3 individuals carrying 1 Apo E4 allele. Only visuospatial ability (VSA) and perceptual speed (PS) scores correlated significantly with age (Table 1).
Table 1.
Demographics and clinical characteristics of the study group
| Case patient | Sex | Age (y) | Braak | GCS | MMSE | Memory |
VSA* | PS** | Education (y) | APOE allele | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Episodic | Working | Semantic | ||||||||||
| 1 | F | 79.12 | 3 | 1.22364 | 30 | 1.60395 | 0.95262 | 0.90475 | 1.0366 | 1.27859 | 18 | 3/3 |
| 2 | F | 84.75 | 3 | 0.71149 | 30 | 1.48841 | −0.15070 | 0.96480 | 0.29079 | −0.23121 | 16 | 3/3 |
| 3 | M | 72.43 | 2 | 0.69258 | 30 | 0.45572 | 0.40186 | 0.86009 | 1.18376 | 1.17663 | 23 | 3/3 |
| 4 | M | 74.29 | 0 | 0.59127 | 27 | 0.60954 | 0.74473 | 0.63723 | 0.73764 | 0.23726 | 19 | 3/3 |
| 5 | M | 83.73 | 4 | 0.52824 | 29 | 1.29833 | −0.28511 | 0.71240 | 0.89198 | −1.26619 | 16 | 3/3 |
| 6 | F | 85.48 | 4 | 0.40283 | 29 | 0.95316 | 0.31588 | −0.22709 | 0.71407 | −0.65848 | 20 | 3/3 |
| 7 | F | 89.41 | 3 | 0.33139 | 30 | 1.07033 | 0.09619 | 0.28166 | −0.46610 | −1.00030 | 19 | 3/4 |
| 8 | F | 77.48 | 3 | −0.09110 | 29 | −0.13994 | −0.50318 | −0.23723 | 0.44705 | 0.30807 | 18 | 3/3 |
| 9 | F | 92.67 | 1 | −0.14633 | 27 | 0.44284 | −1.19912 | 0.55572 | −1.37839 | −0.25670 | 18 | 3/3 |
| 10 | M | 92.83 | 3 | −0.18231 | 26 | −0.06912 | −0.66609 | 0.34252 | −0.14436 | −0.69320 | 20 | 3/3 |
| 11 | M | 90.29 | 5 | −0.23766 | 27 | −0.17313 | −0.18859 | 0.29502 | −0.26917 | −1.47179 | 16 | 3/3 |
| 12 | M | 78.54 | 4 | −0.40004 | 30 | −0.22092 | −0.68774 | 0.28922 | −0.58024 | −1.51319 | 25 | 3/4 |
| 13 | F | 91.05 | 4 | −0.61728 | 28 | −0.07310 | −1.25225 | 0.10650 | −1.35791 | −1.58537 | 20 | 3/3 |
| 14 | M | 82.97 | 3 | −0.62227 | 28 | −0.74148 | −1.12674 | −0.13982 | 0.30176 | −0.86870 | 16 | 3/3 |
| 15 | M | 84.16 | 4 | −0.68013 | 25 | −0.60590 | −0.83912 | −0.63451 | −0.92203 | −0.51771 | 15 | 3/3 |
| 16 | M | 83.54 | 4 | −1.13457 | 27 | −1.38893 | −1.36202 | −1.23970 | 0.60258 | −1.44238 | 18 | 3/4 |
| 17 | M | 83.40 | 3 | −1.20115 | 22 | −1.00056 | −1.31209 | −1.57093 | −0.89131 | −1.20052 | 15 | 2/3 |
| Average | 10 M | 83.89 | 3.12 | −0.04891 | 27.88 | 0.206121 | −0.41538 | 0.11180 | 0.011157 | −0.570895 | 18.35 | |
| SD | 7F | 6.12 | 1.20 | 0.69 | 2.18 | 0.89 | 0.74 | 0.73 | 0.88 | 0.89 | 2.74 | |
Key: APO E, apolipoprotein E; F, female; GCS, global cognitive score; M, male; MMSE, Mini-Mental State Examination; PS, perceptual speed; SD, standard deviation; VSA, visuospatial ability.
p = .0173.
p = .0062.
3.2. Pathological characteristics of the NCI brains
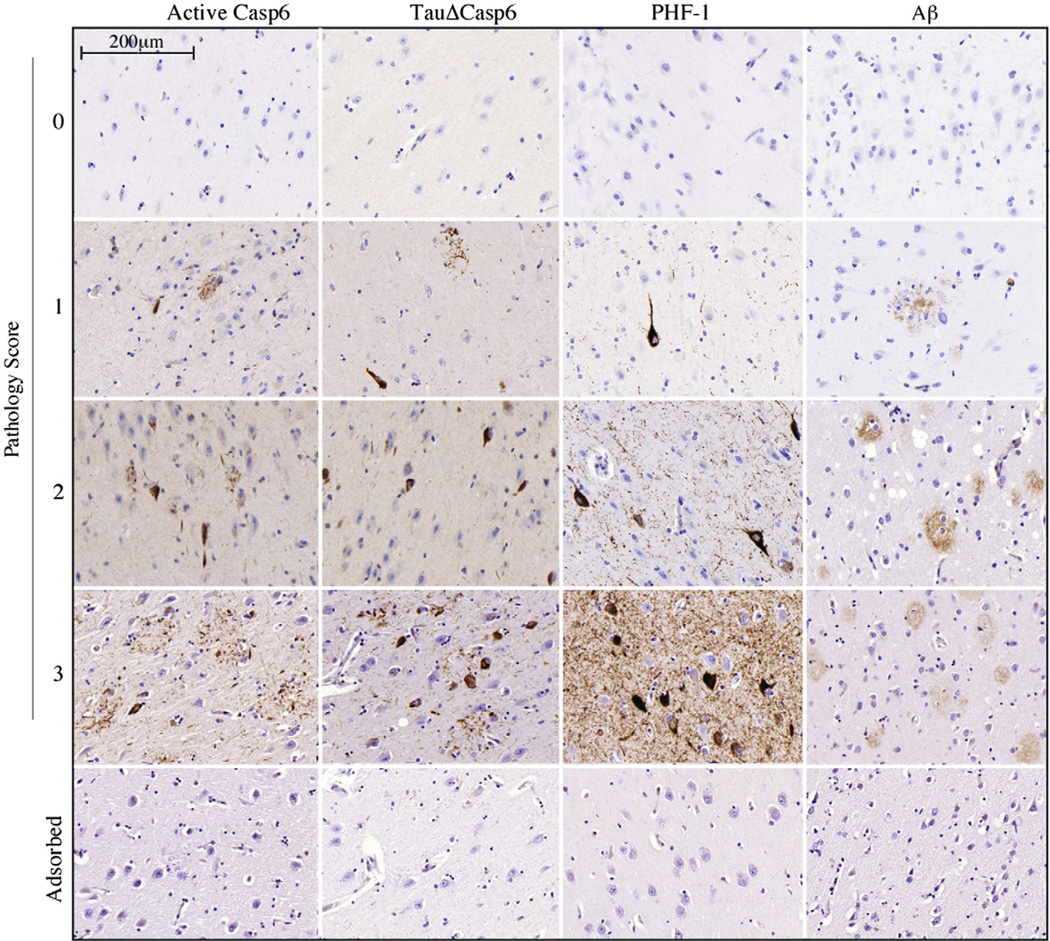
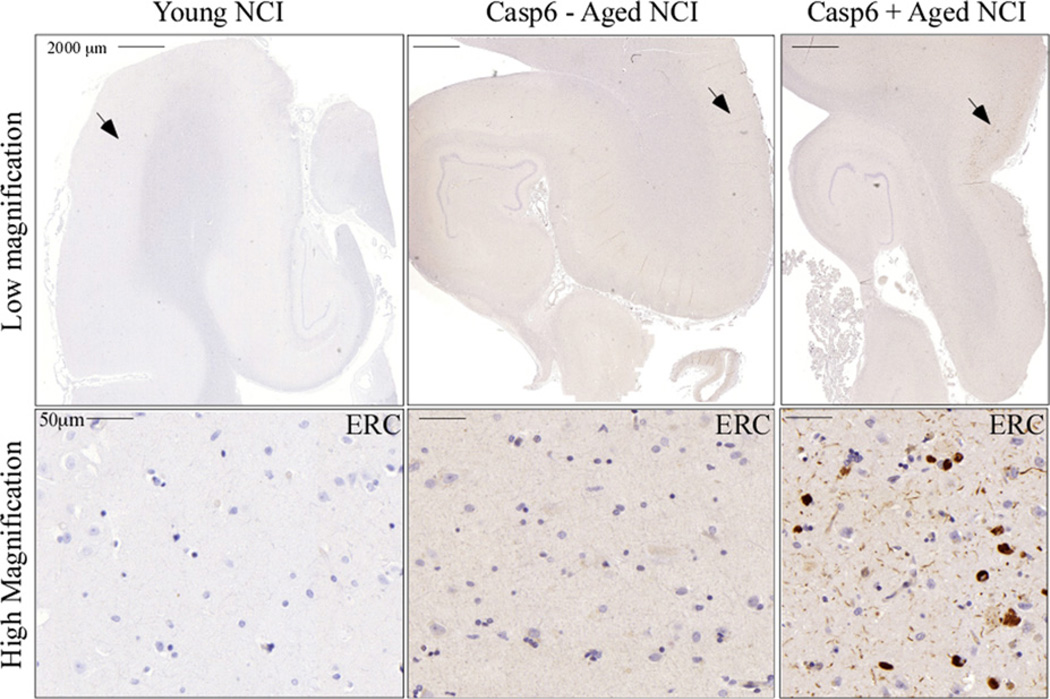
Braak stages (Table 1) were determined to be stage 3.12 ± 1.20 (0–5). An example of the pathological scores associated with immunopositivity is shown for all markers (Fig. 1). Specificity was confirmed by adsorptions (Fig. 1), the absence of immunoreactivity in younger normal brains (Fig. 2A) and in nonaffected areas of AD brains (Guo et al., 2004). Immunostaining of active Casp6 and Tau cleaved by Casp6 (TauΔCasp6) was always associated with NPT, NP, or NFT, sometimes pre-tangles, and was not detected in glial cells (Fig. 1). A range of immunopositivity from none (Fig. 2B) to abundant (Fig. 2C) was observed in the aged NCI cases. Individual pathology scores are presented in Supplemental Tables 1–4. Because only the ERC and CA1 were consistently present in the 17 cases and are well-known to be among the first areas involved in AD and responsible for memory function, subsequent analyses were limited to these 2 regions.
Fig. 1.
Semi-quantitative immunohistochemical scoring of non–cognitively impaired (NCI) hippocampal brain tissue sections. Hippocampal brain tissue sections from NCI (scores 0, 1, and 2) and from AD (score 3) were stained with active Casp6, TauΔCasp6, PHF-1, or Aβ antibodies. NFTs, NPs, and NPTs were scored as absent (0), sparse (1), frequent (2), or abundant (3). A score of 0.5 was used in situations in which there was immunopositive staining that did not reach the sparse criteria. Specificity for the immunostaining was confirmed by adsorptions (bottom panel).
Fig. 2.
Young non–cognitively impaired (NCI) brains lack TauΔCasp6 staining while aged NCI brains show a range of TauΔCasp6 staining. TauΔCasp6 immunostaining in the hippocampal formation of young, aged NCI negative for TauΔCasp6, and aged NCI positive for TauΔCasp6. Arrow indicates the area magnified in the lower panels.
There was a significant correlation (p < .0001) between the pathological scores of active Casp6 and TauΔCasp6 in the ERC (r = 0.887) and CA1 (r = 0.851), confirming that both antisera detect Casp6 activity similarly (Fig. 3A and B). The level of PHF-1 immunopositive pathology correlated significantly with TauΔCasp6 pathological scores in the ERC (r = 0.640; p < .008) and CA1 (r = 0.785; p < .006), indicating that both these epitopes are equally good detectors of Tau pathology. However, PHF-1 correlated significantly with active Casp6 levels only in the CA1 (p < .0002) and not in the ERC (p < .097). In contrast, Aβ did not correlate with either active Casp6, TauΔCasp6, or PHF-1 in the ERC or CA1. No association was observed between Casp6 activity and education levels or Apo E genotypes. Together, these results indicate the following: (1) active Casp6 and TauΔCasp6 antisera identify Casp6 activity in AD-like pathology in NCI brains; (2) PHF-1 and TauΔCasp6 immunopositive pathologies overlap considerably, but not completely, in the ERC and CA1; and (3) Casp6 activity does not associate with Aβ immunoreactivity in either the ERC or the CA1.
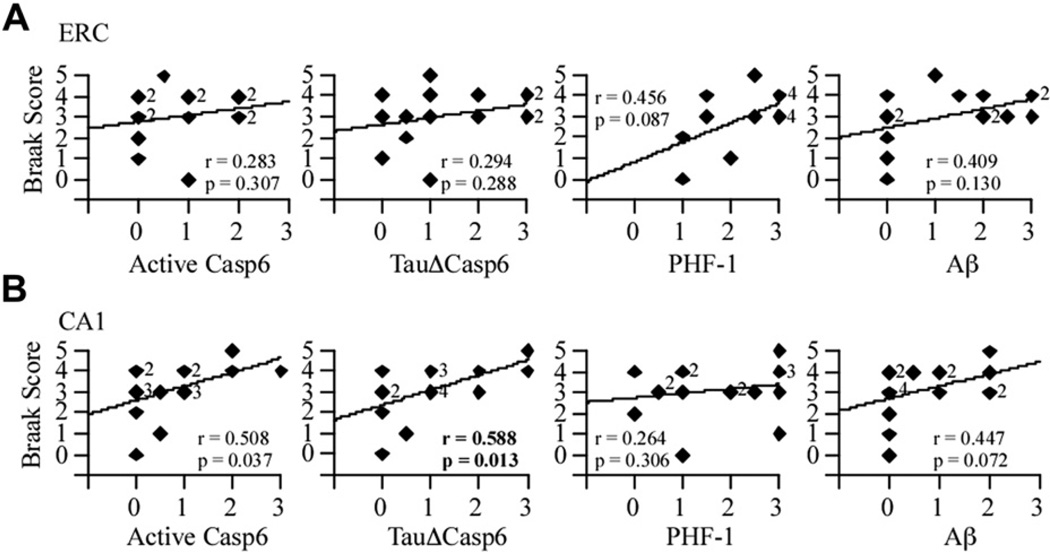
3.3. Casp6 activity in the CA1 of NCI brains correlates with the progression of Braak-defined AD pathology
To determine whether the level of active Casp6 was associated with defined stages of AD pathology, Spearman rank correlations were obtained between the levels of active Casp6, TauΔCasp6, PHF-1, or Aβ immunopositivity and the Braak stage (Fig. 4). None of the pathological markers in the ERC correlated with the Braak stage (Fig. 4A). However, a positive correlation was obtained between TauΔCasp6 in the CA1 and Braak stage (Fig. 4B). These results indicate that TauΔCasp6 in the CA1 of NCI individuals increase with the level and distribution of early Tau pathology defined by AT8 in the hippocampal formation (Bennett et al., 2006).
Fig. 4.
Casp6 activity staining correlates with Braak stage scores in the CA1 but not ERC. Correlation between the active Casp6, TauΔCasp6, PHF-1 or Aβ immunostaining scores and the Braak stage scores in the ERC (A; df = 15) and CA1 (B; df = 17). Diamonds represent individual or overlapping cases as indicated by a small number next to the diamond. An r indicates a Spearman rank correlation, with p values less than .02 considered significant.
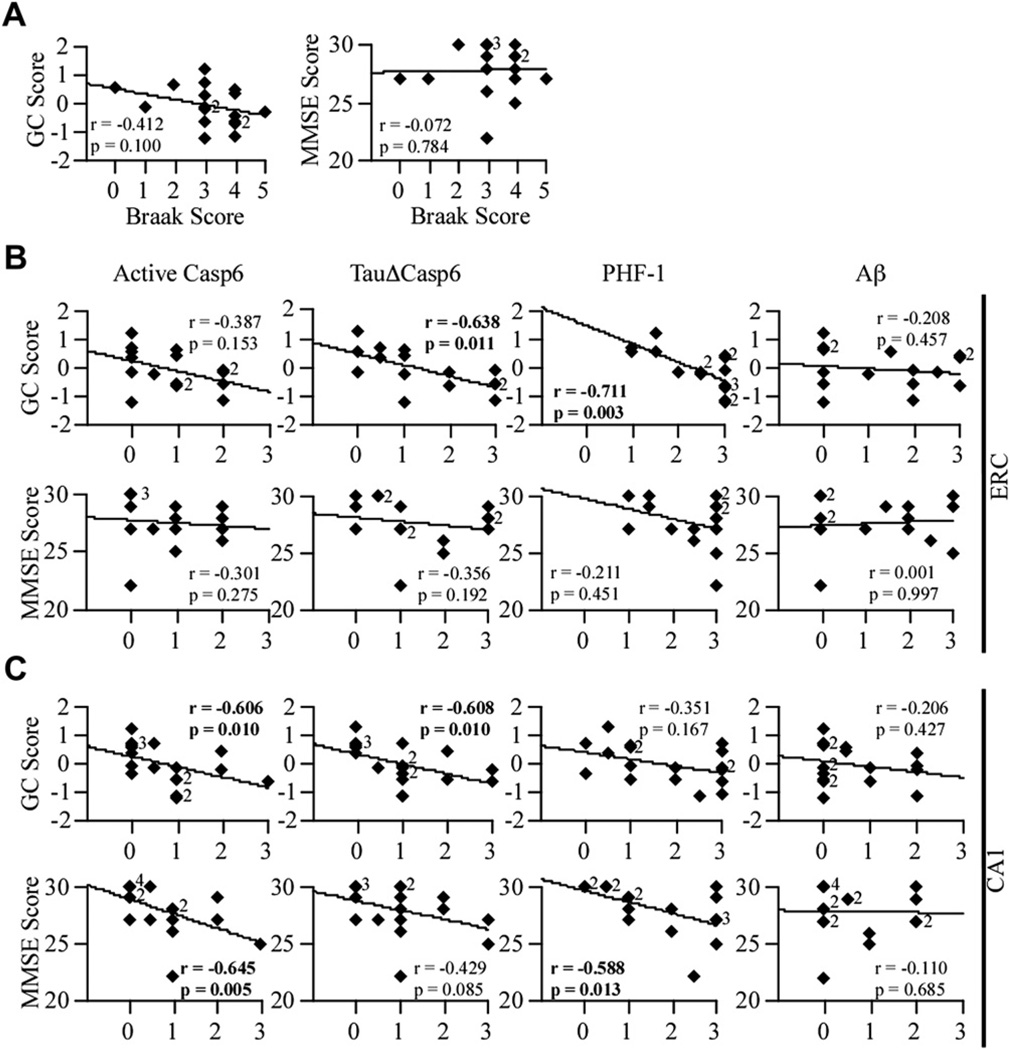
3.4. Casp6 activity in the CA1 of aged NCI brains correlates with lower global cognitive score
To assess whether Casp6 activity was associated with cognition, the immunostaining scores were correlated with GCS and MMSE scores (Fig. 5). Neither the GCS nor the MMSE scores decreased as Braak stage increased (Fig. 5A). The level of active Casp6 in ERC (Fig. 5B) did not correlate with GCS, but both TauΔCasp6 (p = .011) and PHF-1 (p = .003) pathology correlated negatively with GCS. The absence of significant correlation with the active Casp6 may reflect the transient nature of active Casp6, which is degraded rapidly by the proteasome (Tounekti et al., 2004), or a lower affinity of the anti-Casp6 antiserum for active Casp6 epitopes. Indeed, although TauΔCasp6 staining was often observed at a score of 3, active Casp6 did not reach a score of 3 in the ERC, and only 1 case was observed in the CA1. Neither Casp6 activity nor PHF1 immunopositivity in the ERC correlated with MMSE scores. In the CA1 region, both active Casp6 and TauΔCasp6 immunoreactivity correlated negatively (p = .01) with GCS (Fig. 5C). Active Casp6, but not TauΔCasp6, correlated with MMSE score (p = .005). PHF-1 levels did not correlate with GCS but did with MMSE scores (p = .013). No significant correlation was obtained with ERC or CA1 Aβ immunostaining (Fig. 5B and C). These results show that higher TauΔCasp6 in the ERC and CA1 region of the hippocampus is associated with lower GCS in aged NCI individuals, suggesting that Casp6-mediated damage in CA1 neurons may be responsible for the poorer cognitive performance of these individuals.
Fig. 5.
Casp6 activity staining correlates with global cognitive scores in the ERC and CA1. (A) Correlation between the Braak stage scores and GC or MMSE scores (df = 17). Correlation between the active Casp6, TauΔCasp6, PHF-1, or Aβ immunostaining scores in the ERC (B; df = 15) and CA1 (C; df = 17) and GC or MMSE scores. Diamonds represent individual or overlapping cases as indicated by a small number next to the diamond. An r indicates Spearman correlation, with p values less than .02 considered significant.
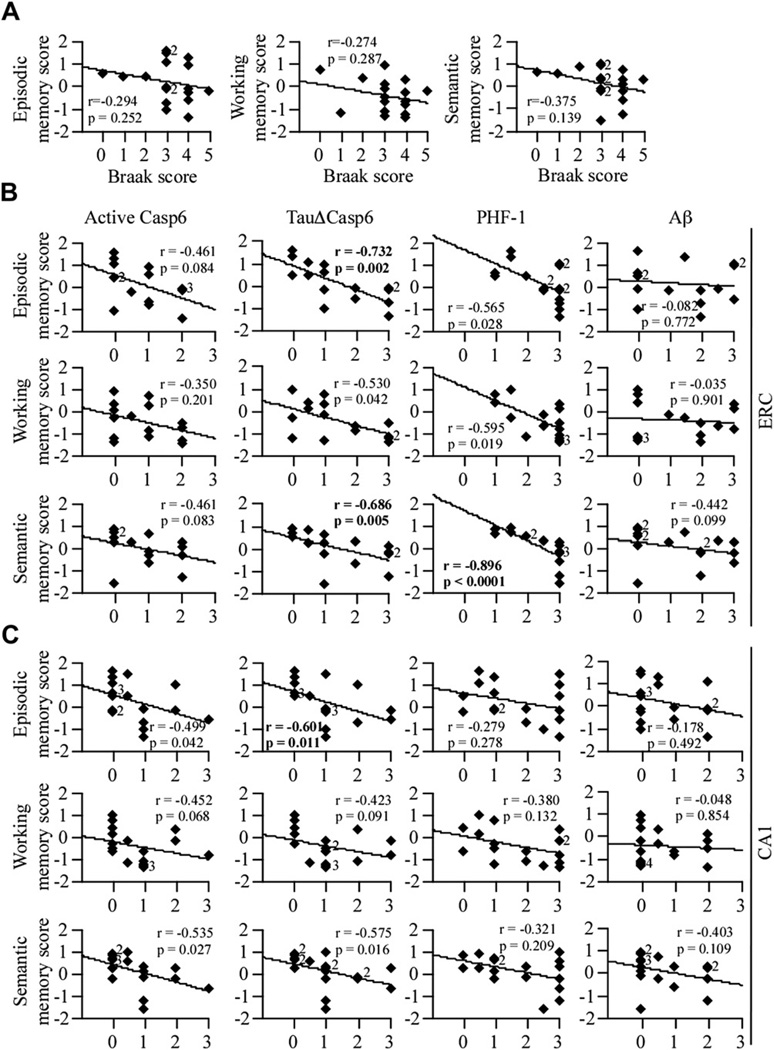
3.5. Casp6 activity is associated with lower episodic and semantic memory scores in aged NCI individuals
To determine whether Casp6 activity was associated with a specific memory function, the presence of Casp6 activity was correlated with episodic, working, and semantic memory scores (Fig. 6). None of the specific memory scores correlated with the Braak stage score (Fig. 6A). In the ERC, the levels of active Casp6 did not correlate with episodic, working, or semantic memory, whereas TauΔCasp6 (p = .05) correlated with episodic and semantic memory scores (Fig. 6B). In the ERC, PHF-1 correlated only with lower semantic memory performance (p = .001, Fig. 6B). In the CA1 (Fig. 6C), active Casp6 did not correlate with any of the 3 memory tasks, and TauΔCasp6 correlated negatively with episodic memory (p = .011). In contrast, PHF-1 and Aβlevels in the CA1 did not correlate with any of the specific memory tests. Together, these results suggest that Casp6 activity in AD-like pathologies of the ERC and CA1 is associated with lower cognitive performance in episodic and semantic memory in aged NCI individuals, whereas ERC PHF-1 is associated with semantic memory only.
Fig. 6.
Casp6 activity staining in the ERC and CA1 correlates with episodic and/or semantic memory scores, whereas PHF-1 staining in the ERC correlates with semantic memory. (A) Correlation between the Braak stage scores and episodic, working, or semantic memory scores (df = 17). Correlation between the active Casp6, TauΔCasp6, PHF-1, or Aβ immu-nostaining scores in the ERC (B; df = 15) and CA1 (C; df = 17) and episodic, working or semantic memory scores. Diamonds represent individual or overlapping cases as indicated by a small number next to the diamond. An r indicates Spearman rank correlation, with p values less than .01 considered significant.
3.6. Casp6 activity does not correlate with visuospatial or perceptual speed abilities
To assess whether late AD cognitive deficits might be associated with the presence of Casp6 activity, correlations were made between the level of Casp6 activity and PS or VSA scores. PS but not VSA scores correlate with Braak stage (p = .002) (Fig. 7A). In the ERC and the CA1, none of the pathologies correlated with either VSA or PS scores (Fig. 7B and C). These results indicate that Casp6 activity or classical AD pathologies in the ERC or CA1 of NCI individuals does not associate with lower VSA or PS performance.
3.7. Casp6 activity selectively predicts lower performance of episodic memory
A series of linear regression analyses was preformed to identify the most important pathological characteristics of NCI brain regions that influence cognitive scores. We performed 2 separate stepwise linear regression analyses for the ERC and CA1 brain regions entering the 4 brain pathology markers (Table 2). Although stepwise linear regression analyses identified a subset of independent variables that best predicted outcomes, all variance–covariance matrices were singular; thus, only 1 variable was always the best predictor in the following models. ERC and CA1 TauΔCasp6 best predicted episodic memory performance, whereas CA1 TauΔCasp6 also predicted GCS performance. In contrast, TauΔCasp6 did not predict semantic memory or working memory in either region. However, CA1-active Casp6 predicted lower semantic memory performance. ERC PHF-1 predicted lower GCS, semantic memory, and working memory performance. Neither Aβ nor age nor education variables were significant predictors of memory performance. None of the pathological markers predicted VSA and PS performance, which were nevertheless predicted best by age. These results show that ERC and CA1 Casp6 activity predict episodic memory, whereas ERC PHF-1 predicts semantic and working memory, thus suggesting that different pathways are impaired by Casp6 activity and PHF-1 pathology.
Table 2.
Stepwise linear regression analysis predicting cognitive performance from various brain pathologies, age, and education
| Predicting cognition | Brain region | Pathology variable | Estimate (SE) | p Value | Beta | Constant (SE) | Adjusted R2 |
|---|---|---|---|---|---|---|---|
| GCS | ERC | PHF-1 | −0.642 (0.172) | 0.003** | −0.719 | 1.466 (.434) | 0.497 |
| CA1 | TauCaspΔ6 | −0.349 (0.158) | 0.043* | −0.495 | 0.311 (.221) | 0.195 | |
| Episodic memory | ERC | TauCaspΔ6 | −0.543 (0.143) | 0.002** | −0.726 | 0.909 (.256) | 0.491 |
| CA1 | TauCaspΔ6 | −0.440 (0.205) | 0.048* | −0.485 | 0.659 (.287) | 0.184 | |
| Semantic memory | ERC | PHF-1 | −0.687 (0.178) | 0.002** | −0.731 | 1.692 (.447) | 0.499 |
| CA1 | Casp6 | −0.395 (0.185) | 0.050* | −0.483 | 0.414 (.213) | 0.182 | |
| Working memory | ERC | PHF-1 | −0.628 (0.214) | 0.012* | −0.631 | 1.093 (.539) | 0.352 |
| No significant predictor variables were entered. | NA | ||||||
| VSA | Age | −0.086 (0.027) | 0.006** | −0.635 | 7.254 (2.281) | 0.363 | |
| PS | Age | −0.083 (0.031) | 0.017* | −0.568 | 6.385 (2.607) | 0.278 | |
Key: ERC, entorhinal cortex; PHF-1, paired helical filament–1; PS, perceptual speed; SE, standard error; VSA, visuospatial ability.
p < 0.05.
p < 0.01.
4. Discussion
AD is thought to start several years before the clinical onset of symptoms. It has thus become very important to identify the underlying molecular mechanisms that lead to neuronal dysfunction and degeneration in aged individuals. In this manuscript, we show that Casp6 activity in the ERC and CA1 of aged NCI individuals predict lower performance in episodic memory, 1 of the first types of memory impaired in AD. Indeed, linear regression analyses showed that both the ERC and CA1 TauΔCasp6 predicted episodic memory performance in aged NCI individuals. Correlations between ERC or CA1 TauΔCasp6 with episodic memory scores were significant. However, correlations with active Casp6, while showing the same trend, did not reach statistical significance. Active Casp6 is normally more difficult to detect for the following reasons: (1) Casp6 being an enzyme, it is much less abundant in cells than Tau protein; (2) Casp6 active subunits are degraded rapidly by the proteasome (Tounekti et al., 2004); and (3) the active Casp6 epitope is less stable in human brain histological sections than the TauΔCasp6 epitope (personal observation). Indeed, although TauΔCasp6 staining was often observed at a score of 3, active Casp6 did not reach a score of 3 in the ERC, and only 1 case was observed in the CA1. Therefore, significant results obtained with TauΔCasp6, but not with active Casp6, are considered as evidence for Casp6 activity, whereas significant results with active Casp6, but not with TauΔCasp6, are not considered sufficient evidence for Casp6 activity. That Casp6 activity in the CA1 correlates with lower episodic memory scores is consistent with several studies highlighting the critical role of the CA1 in episodic memory. The human hippocampal CA1 region is involved in encoding spatial memory in human young adults (Suthana et al., 2009), and CA1 atrophy correlates with deficits of patients with transient global amnesia (Bartsch et al., 2011), with amnesic mild cognitive impairment (Fouquet et al., 2012), and with mild AD (Kerchner et al., 2010). Furthermore, synaptic and neuronal loss is observed in the CA1 of mild AD brains (Price et al., 2001; Scheff et al., 2007). Similarly, the ERC has been shown to be involved in episodic memory (Sauvage et al., 2010; Schultz et al., 2012; Suh et al., 2011). In healthy adults, shrinkage of the ERC in aging is associated with reduced episodic memory function (Rodrigue and Raz, 2004; Trivedi et al., 2011).
Our results suggest that the pathway between layer III of the ERC and the CA1 axis is involved in the propagation of Casp6 activation in the aged hippocampus. Although we observed Casp6 activity in both layers II and III of the ERC, considerable levels of active Casp6 were limited to the CA1 and were rare in the CA3. Layer II neurons project to the dentate gyrus, which then project to the CA3 and from there output to the CA1 (Andersen et al., 2007). There are also layer II neurons that projected directly to the CA3. Therefore, the absence of significant Casp6 activity in the CA3 excludes these pathways. In contrast, layer III neurons of the ERC project directly to the CA1, thus providing a direct link between ERC and CA1 Casp6 activity (Andersen et al., 2007).
The role of PHF-1 pathology is dissociated from that of Casp6 activity. PHF-1 pathology in ERC predicted lower performance in GCS and both semantic and working memory in linear regression analyses. Although active Casp6 and TauΔCasp6 almost identically identified AD-like pathology (r = 0.887), TauΔCasp6 pathology overlapped only partially with PHF-1 (r = 0.657). Furthermore, linear regression analysis indicated that TauΔCasp6 in the ERC significantly predicted only episodic performance. These results suggest 2 parallel pathologies that affect different pathways mediating specific types of memories.
That Casp6 activity and PHF-1 pathology predict lower performance of AD-associated memory types in individuals classified as NCI suggest that both may be very sensitive markers of the very early degenerative events that occur in the hippocampus and that lead to AD (Buckner et al., 2005). Pre-clinical memory deficits have been identified several years before the onset of symptoms in familial AD individuals, but the underlying molecular mechanism has not been elucidated (Fox et al., 1998). The presence of Casp6 activity in AD-associated pathological lesions (NFT, NPT, NP) in these NCI cases supports the hypothesis that Casp6 activity in the CA1 predicts eventual development of AD. Indeed, Casp6 activity induces axonal degeneration (Nikolaev et al., 2009; Sivananthan et al., 2010), possibly by Casp6 proteolytic processing of cytoskeletal and cytoskeletal-associated proteins that are essential for the proper maintenance and function of neurons (Guo et al., 2004; Harris and Weinberg, 2012; Klaiman et al., 2008).
Together, these results indicate that it might be worth developing imaging techniques or CSF biomarkers to survey Casp6 activity in the brain of living, aged individuals. The study findings also suggest that treatments designed to target both PHF-1 tangles and active Casp6 may be necessary to prevent early memory impairment in AD.
Supplementary Material
Acknowledgements
This work was supported by the Canadian Foundation of Innovation, CIHR IAP-102238, and 2011 MOP-243413-BCA-CGAG-45097 to ALB, P30AG10161 and R01AG15819 to DAB. The authors gratefully acknowledge the gift of PHF-1 antibody from Dr Peter Davies (Albert Einstein College of Medicine, NY).
Appendix. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2013.01.007
Footnotes
Disclosure statement
There are no actual or potential conflicts of interest between the authors and this work.
References
- Albrecht S, Bogdanovic N, Ghetti B, Winblad B, LeBlanc AC. Caspase-6 activation in familial Alzheimer disease brains carrying amyloid precursor protein or presenilin I or presenilin II mutations. J. Neuropathol. Exp. Neurol. 2009;68:1282–1293. doi: 10.1097/NEN.0b013e3181c1da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht S, Bourdeau M, Bennett D, Mufson EJ, Bhattacharjee M, LeBlanc AC. Activation of caspase-6 in aging and mild cognitive impairment. Am. J. Pathol. 2007;17:1200–1209. doi: 10.2353/ajpath.2007.060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Morris RG, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Bartsch T, Dohring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc. Natl. Acad. Sci. 2011;108:17562–17567. doi: 10.1073/pnas.1110266108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurol. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr. Alzheimer Res. 2012;9:628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples LA, Heeren T, Schatzkin A, Colton T. Multiple testing of hypotheses in comparing two groups. Ann. Intern. Med. 1984;100:122–129. doi: 10.7326/0003-4819-100-1-122. [DOI] [PubMed] [Google Scholar]
- Fouquet M, Desgranges B, La Joie R, Riviere D, Mangin JF, Landeau B, Mezenge F, Pelerin A, de La Sayette V, Viader F, Baron JC, Eustache F, Chetelat G. Role of hippocampal CA1 atrophy in memory encoding deficits in amnestic Mild Cognitive Impairment. NeuroImage. 2012;59:3309–3315. doi: 10.1016/j.neuroimage.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Seiffer AL, Agnew SK, Rossor MN. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer’s disease. A longitudinal prospective study. Brain. 1998;121:1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- Gervais F, Xu D, Robertson G, Vaillancourt J, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman M, Clarke E, Zheng H, Van Der Ploeg L, Ruffolo S, Thornberry N, Xanthoudakis S, Zamboni R, Roy S, Nicholson D. Involvement of caspases in proteolytic cleavage of Alzheimer’s β-amyloid precursor protein and amyloidogenic β-peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Gray DC, Mahrus S, Wells JA. Activation of specific apoptotic caspases with an engineered small-molecule-activated protease. Cell. 2010;142:637–646. doi: 10.1016/j.cell.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC. Active caspase-6 and caspase-6 cleaved tau in neuropil threads, neuritic plaques and neurofibrillary tangles of Alzheimer’s disease. Am. J. Pathol. 2004;165:523–531. doi: 10.1016/S0002-9440(10)63317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halawani D, Tessier S, Anzellotti D, Bennett DA, Latterich M, LeBlanc AC. Identification of caspase-6-mediated processing of the valosin containing protein (p97) in Alzheimer’s disease: a novel link to dysfunction in ubiquitin proteasome system-mediated protein degradation. J. Neurosci. 2010;30:6132–6142. doi: 10.1523/JNEUROSCI.5874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Weinberg RJ. Cold Spring Harbor Perspectives in Biology. Vol. 4. NY: Cold Spring Harbor; 2012. Ultrastructure of synapses in the mammalian brain; p. a005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Hess CP, Hammond-Rosenbluth KE, Xu D, Rabinovici GD, Kelley DA, Vigneron DB, Nelson SJ, Miller BL. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurol. 2010;75:1381–1387. doi: 10.1212/WNL.0b013e3181f736a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiman G, Champagne N, LeBlanc AC. Self-activation of caspase-6 in vitro and in vivo: caspase-6 activation does not induce cell death in HEK293T cells. Biochim. Biophys. Acta. 2009;1793:592–601. doi: 10.1016/j.bbamcr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Klaiman G, Petzke TL, Hammond J, LeBlanc AC. Targets of caspase-6 activity in human neurons and Alzheimer disease. Mol. Cell Proteom. 2008;7:1541–1555. doi: 10.1074/mcp.M800007-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace G, Savva GM, Forster G, de Silva R, Brayne C, Matthews FE, Barclay JJ, Dakin L, Ince PG, Wharton SB. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain. 2009;132:1324–1334. doi: 10.1093/brain/awp059. [DOI] [PubMed] [Google Scholar]
- LeBlanc A. Increased production of 4 kDa amyloid beta peptide in serum deprived human primary neuron cultures: possible involvement of apoptosis. J. Neurosci. 1995;15:7837–7846. doi: 10.1523/JNEUROSCI.15-12-07837.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. Third Edition. Netherlands: Elsevier, Amsterdam; 2008. [Google Scholar]
- Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch. Pathol. Lab. Med. 1993;117:132–144. [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pellegrini L, Passer BJ, Tabaton M, Ganjei JK, D’Adamio L. Alternative, non-secretase processing of Alzheimer’s beta-amyloid precursor protein during apoptosis by caspase-6 and −8. J. Biol. Chem. 1999;274:21011–21016. doi: 10.1074/jbc.274.30.21011. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J. Neurosci. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage MM, Beer Z, Ekovich M, Ho L, Eichenbaum H. The caudal medial entorhinal cortex: a selective role in recollection-based recognition memory. J. Neurosci. 2010;30:15695–15699. doi: 10.1523/JNEUROSCI.4301-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurol. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Schultz H, Sommer T, Peters J. Direct evidence for domain-sensitive functional subregions in human entorhinal cortex. J. Neurosci. 2012;32:4716–4723. doi: 10.1523/JNEUROSCI.5126-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivananthan SN, Lee AW, Goodyer CG, LeBlanc AC. Familial amyloid precursor protein mutants cause caspase-6-dependent but amyloid beta-peptideindependent neuronal degeneration in primary human neuron cultures. Cell Death Dis. 2010;1:e100. doi: 10.1038/cddis.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. J. Neurosci. 2009;29:10512–10519. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Tanzi RE. Caspase activation increases beta-amyloid generation independently of caspase cleavage of the beta-amyloid precursor protein (APP) J. Biol. Chem. 2003;278:46074–46080. doi: 10.1074/jbc.M307809200. [DOI] [PubMed] [Google Scholar]
- Tounekti O, Zhang Y, Klaiman G, Goodyer CG, LeBlanc A. Proteasomal degradation of caspase-6 in 17 beta-estradiol-treated neurons. J. Neurochem. 2004;89:561–568. doi: 10.1111/j.1471-4159.2004.02349.x. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Stoub TR, Murphy CM, George S, deToledo-Morrell L, Shah RC, Whitfield-Gabrieli S, Gabrieli JD, Stebbins GT. Entorhinal cortex volume is associated with episodic memory related brain activation in normal aging and amnesic mild cognitive impairment. Brain Imaging Behav. 2011;5:126–136. doi: 10.1007/s11682-011-9117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Paliga K, Durrwang U, Reinhard FB, Schuckert O, Evin G, Masters CL. Proteolytic processing of the Alzheimer’s disease amyloid precursor protein within its cytoplasmic domain by caspase-like proteases. J. Biol. Chem. 1999;274:5823–5829. doi: 10.1074/jbc.274.9.5823. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol. Aging. 2002;17:179–193. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.