Abstract
Purpose
To evaluate the functional outcomes of patients with polypoidal choroidal vasculopathy (PCV) who underwent intravitreal ranibizumab (IVR) treatment, compared with photodynamic therapy (PDT), after at least 2 years.
Methods
We retrospectively studied all the treatment-naïve patients with PCV who were scheduled to undergo IVR or PDT between August 2005 and June 2010. All the patients who had a 2-year or longer follow-up period were included in the study. The best-corrected visual acuity (BCVA) in the two groups was compared before treatment and at 3, 6, 12, 18 and 24 months after the initial treatment. The regression of the polyps was also assessed using indocyanine green angiography.
Results
A total of 77 patients were included in this study. Thirty-three eyes were treated with IVR, and 44 eyes were treated with PDT. Although no significant differences between the two groups were observed at baseline or at 3, 6, and 12 months after treatment, a significantly better BCVA was seen in the IVR group, compared with the PDT group, at 18 and 24 months after treatment (P=0.035 and P=0.021, respectively). No significant difference in the rate of polyp regression was observed between the two groups (P=0.092).
Conclusion
IVR was well tolerated and maintained or improved the vision of patients with PCV, compared with PDT, as evaluated at 2-year follow-up examinations. PDT for the treatment of PCV might result in unfavorable outcomes, with no superiority to achieving the involution of polyps.
Keywords: age-related macular degeneration, polypoidal choroidal vasculopathy, ranibizumab, intravitreal injection, vascular endothelial growth factor, photodynamic therapy
Introduction
Yannuzzi et al1, 2 was the first author to report polypoidal choroidal vasculopathy (PCV) as a variant of neovascular age-related macular degeneration (AMD). The branching vascular network and polypoidal structures derived from the choroid seem to be unique to PCV.
The natural course of PCV might differ from that of the typical exudative AMD, with favorable visual outcomes.3 However, some cases develop exudative changes and massive hemorrhagic complications, resulting in a severe visual loss.4 Regarding the treatment modalities for PCV, photodynamic therapy (PDT) has been widely used, resulting in a high frequency of the complete regression of polyps and an improvement in visual acuity.5, 6, 7, 8, 9, 10 However, the long-term outcomes of PDT for PCV indicate that frequent recurrences often result in a visual acuity loss.11, 12, 13, 14
On the other hand, ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA, USA) is a recombinant humanized anti-vascular endothelial growth factor (VEGF) antibody fragment targeting all isoforms of VEGF15 that has been used extensively for the treatment of AMD. The treatment of PCV using ranibizumab has also yielded promising results.16, 17 Therefore, whether PDT or ranibizumab should be selected for the treatment of PCV is an important matter. A recent study described that PDT with or without ranibizumab was superior to ranibizumab alone for the complete resolution of polyp lesions, and no significant differences in the improvement of best-corrected visual acuity (BCVA) were seen among the three groups.18 However, a longer follow-up period is needed to confirm the optimal treatment for PCV.
The purpose of this study was to evaluate the functional outcomes of patients with PCV who underwent intravitreal ranibizumab (IVR) treatment, compared with PDT, after a follow-up period of at least 2 years.
Patients and methods
We studied 77 eyes in 77 Japanese patients aged 50 years old or older who were diagnosed as having PCV. All the patients were initially treated at the Yokohama City University Medical Center between August 2005 and June 2010. The treatment modalities were selected according to the time period. PDT monotherapy was used between August 2005 and May 2009, and ranibizumab was administered between April 2009 and June 2010. The study was performed according to the principles of the Declaration of Helsinki. The study was conducted with the approval of the ethics committee of Yokohama City University Medical Center.
The inclusion criteria were the presence of PCV as diagnosed based on the presence of clinical, optical coherence tomography (OCT), fluorescein and confocal indocyanine green angiographic (ICGA) findings showing a branching vascular network and polypoidal structures; a treatment-naïve status; availability for follow-up at 24 months or longer after the first treatment; and a BCVA of 20/400 or better at baseline.
Patients who had previously received treatment for PCV (ie, laser photocoagulation, submacular surgery or the intravitreal injection of other anti-VEGF agents) were excluded from this study. Patients who received PDT combined with anti-VEGF agents or patients who underwent PDT that was subsequently switched to ranibizumab monotherapy within 2 years were also excluded. Furthermore, patients with eye diseases that could potentially influence visual acuity, such as glaucoma, macular hole, diabetic retinopathy or rhegmatogenous retinal detachment, were also excluded.
In the PDT group, verteporfin was administered for over 10 min, as per the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy protocol.19 Fifteen minutes after the start of the intravenous infusion, a 689-nm laser was applied for 83 s to deliver 50 J/cm2. The greatest linear dimension was determined so as to cover the polyp lesions and the surrounding abnormal vascular network. The need for additional retreatment was evaluated every 3 months using angiographic and OCT (Stratus or Cirrus high-definition OCT; Carl Zeiss, Dublin, CA, USA) findings.
In the IVR group, all the patients received three consecutive monthly intravitreal injections of 0.5 mg/0.05 ml of ranibizumab through the pars plana via a 30-gauge needle as an induction treatment. During the maintenance phase, IVR was repeated if any of the following changes were observed by the evaluating physician: 1) a visual acuity loss of 0.2 logarithm of the minimum angle of resolution (logMAR) vision or more, 2) new macular hemorrhage or 3) evidence of persistent or recurrent subretinal fluid (SRF) accumulation, intraretinal edema or the enlargement of an area of pigment epithelial detachment as diagnosed using SD-OCT at a 1-month follow-up examination.
The main outcome measure was the BCVA and the differences between the pre- and postoperative BCVA at 3, 6, 12, 18 and 24 months after the initial treatment. The BCVA was converted to the logMAR equivalent for the statistical analysis. Furthermore, the proportion of patients with changes in the BCVA of 0.3 logMAR vision or more was compared between the two groups. The secondary outcome was the regression of polyps assessed using ICGA at 24 months.
The postoperative visual acuity parameters were compared between the two groups using the Student's t-test. A Student's paired t-test was used to compare the preoperative and postoperative visual acuity in each group. A chi-square test was used to compare the proportion of improvement or deterioration in the visual acuity at 24 months and the rate of polyp regression between the two groups. Recurrence rate in either group was investigated by Fisher's exact test. Statistical analyses were performed using PASW Statistics (version 17.0; SPSS, Inc., Chicago, IL, USA). A P-value <0.05 was considered to denote statistical significance.
Results
The baseline characteristics and clinical data of the patients are shown in Table 1. All 77 patients were included in this study. There were 33 patients in the IVR group and 44 patients in the PDT group. Of the 77 patients, 49 were men and 28 were women, with the patients' ages ranging from 59 to 91 years (mean age±s.d., 73.2±7.5 years) in the IVR group and from 55 to 84 years (mean age, 71.0±7.8 years) in the PDT group. Both groups were comparable with regard to age, sex, preoperative logMAR visual acuity, greatest linear dimension, presence of retinal cysts, SRF, subretinal and/or subretinal pigment epithelial hemorrhage (SRH) and pigment epithelial detachment (Table 1).
Table 1. Patient characteristics of all study eyes with polypoidal choroidal vasculopathy.
| IVR group | PDT group | P-value* | |
|---|---|---|---|
| Number of eyes | 33 | 44 | |
| Number of patients | 33 | 44 | |
| Male/female (%) | 19/14 (58/42) | 30/14 (68/32) | 0.175 |
| Age, mean±s.d., years (range) | 73.2±7.5 (59–91) | 71.0±7.8 (55–84) | 0.104 |
| Preoperative logMAR visual acuity, mean±s.d. | 0.48±0.38 | 0.52±0.28 | 0.283 |
| Greatest linear dimention, mean±s.d. (μm) | 4171±2631 | 3640±2120 | 0.173 |
| Presence of retinal cysts, present/none (%) | 3/30 (9/91) | 8/36 (18/82) | 0.123 |
| Presence of subretinal fluid, present/none (%) | 25/8 (76/24) | 36/8 (82/18) | 0.265 |
| Presence of SRH, present/none (%) | 13/20 (39/61) | 16/28 (36/64) | 0.395 |
| Presence of PED, present/none (%) | 13/20 (39/61) | 16/28 (36/64) | 0.395 |
Abbreviations: logMAR, logarithm of the minimum angle of resolution; PED, pigment epithelial detachment; SRH, subretinal and/or subpigment epithelial hemorrhage.
P-value calculated using the Student's t-test.
The mean logMAR visual acuity at the baseline was 0.48±0.38 (median: 0.30) in the IVR group and 0.52±0.28 (median: 0.52) in the PDT group. The mean logMAR BCVA values at 3, 6, 12, 18 and 24 months after surgery were 0.38±0.42 (0.22), 0.37±0.42 (0.22), 0.38±0.42 (0.22), 0.37±0.44 (0.22) and 0.39±0.47 (0.22) in the IVR group and 0.50±0.35 (0.52), 0.47±0.37 (0.40), 0.54±0.41 (0.52), 0.54±0.41 (0.52) and 0.62±0.49 (0.52) in PDT group, respectively. In the IVR group, the postoperative BCVA improved significantly, compared with the preoperative visual acuity, throughout the 24-month period (P=0.006, P=0.002, P=0.007, P=0.009 and P=0.047 at 3, 6, 12, 18 and 24 months, respectively). In the PDT group, on the other hand, the mean logMAR BCVA was maintained throughout the 24-month period (P=0.305, P=0.087, P=0.383, P=0.336 and P=0.083 at 3, 6, 12, 18 and 24 months, respectively). Although the visual acuity at the baseline and at 3, 6 and 12 months was not significantly different between the IVR group and the PDT group, significant differences in the changes in the logMAR BCVA values were seen between the two groups at 18 and 24 months after the initial treatment (P=0.035 and P=0.021, respectively; Figure 1).
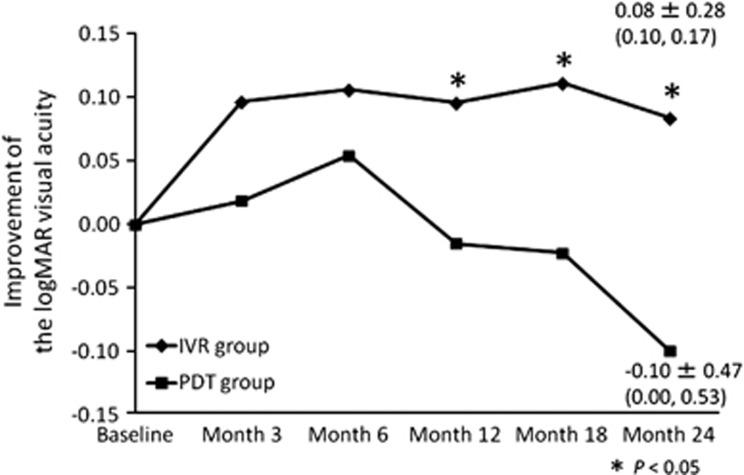
Figure 1.
Changes in the mean logMAR BCVA from the baseline to 24 months post treatment in the IVR and PDT groups. BCVA data at baseline and 24 months are shown as mean BCVA±s.d. (median, IQR). Although the visual acuity at the baseline and at 3, 6 and 12 months was not significantly different, significant differences in the change of the logMAR BCVA values were seen between the two groups at 18 and 24 months (P=0.035 and P=0.021, respectively).
The mean differences in the BCVA between the preoperative value and the postoperative values at 3, 6, 12, 18 and 24 months after surgery were 0.10±0.21 (median: 0.08), 0.11±0.20 (0.10), 0.10±0.21 (0.10), 0.11±0.26 (0.11) and 0.08±0.28 (0.10) in the IVR group and 0.02±0.24 (median: 0.06), 0.05±0.26 (0.10), −0.02±0.34 (0.00), −0.02±0.36 (0.00) and −0.10±0.47 (0.00) in the PDT group, respectively. The IVR group showed a greater degree of improvement in the visual acuity after the initial treatment at 12, 18 and 24 months (P=0.041, P=0.029, and P=0.018, respectively; Figure 2).
Figure 2.
Differences between the pre- and postoperative BCVA at 24 months after treatment (improvement in the visual acuity) in the IVR and PDT groups. Change in BCVA at 24 months are shown as mean change±s.d. (median, IQR). The IVR group showed a greater degree of improvement in visual acuity at the 12-, 18- and 24-month follow-up examinations (P=0.041, P=0.029 and P=0.018, respectively).
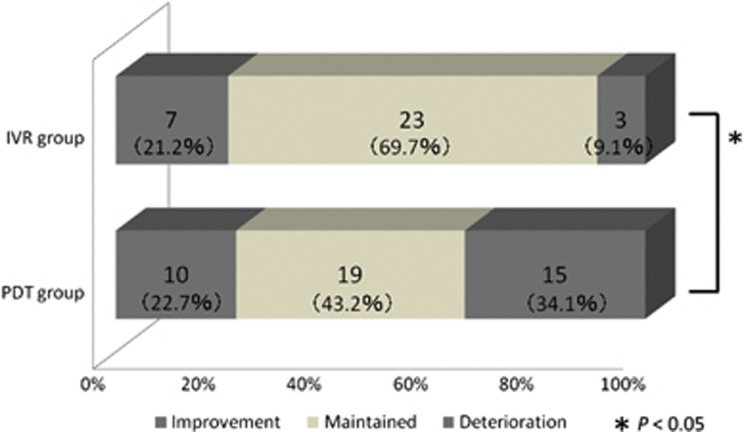
In the IVR group, 7 of the 33 eyes (21.2%) had an improvement in visual acuity of 0.3 logMAR or more at the 2-year follow-up. The visual acuity of 23 eyes (69.7%) remained unchanged, and the remaining 3 eyes (9.1%) exhibited a deterioration in visual acuity of 0.3 logMAR or more. In the PDT group, on the other hand, 10 eyes (22.7%) showed an improvement in visual acuity, 19 eyes (43.2%) showed no change and the remaining 15 eyes (34.1%) showed a deterioration. The deterioration in visual acuity following PDT was significantly higher than that following IVR (P=0.024; Figure 3).
Figure 3.
Graphs showing the proportion of patients with changes in their BCVA of 0.3 logMAR or more at the 2-year follow-up. A greater proportion of eyes in the PDT group showed a deterioration in the logMAR BCVA of ⩾0.3, compared with the IVR group (P=0.024).
During the 24-month study period, the mean number of ranibizumab injections that were administered was 7.1±5.2 in the IVR group. The ICGA findings showed the complete resolution of the polypoidal lesions in 20 eyes (60.6%), persistence in 11 eyes (33.3%) and growth in 2 eyes (6.1%), although the abnormal vascular networks remained the same in all the eyes. SRH occurred in two eyes in which the PCV lesions had grown during the follow-up period; however, these eyes were treated with an additional IVR treatment.
In the PDT group, on the other hand, the mean number of PDT treatments was 1.52±0.66 during the 2-year follow-up period. The ICGA findings showed the complete disappearance of the polypoidal lesions in 34 eyes (77.3%), persistence in 6 eyes (13.6%) and the development of a new PCV or choroidal neovascularization in 4 eyes (9.1%). SRH occurred in seven eyes, five of which developed after 1 year. SRH seriously damaged the visual acuity, with a score of 0.3 logMAR or more, in six eyes. No significant difference in the rate of polyp regression was observed between the two groups (P=0.092).
Recurrence rate in the PDT group was 43.2% (19/44), that is, 19 eyes in the PDT group needed more than two times of PDT during the 2 years. On the other hand, additional injection needed in the maintenance phase in the IVR group was 72.7% (24/33). IVR group showed significantly higher recurrence rate compared with PDT group in the maintenance phase (P=0.012).
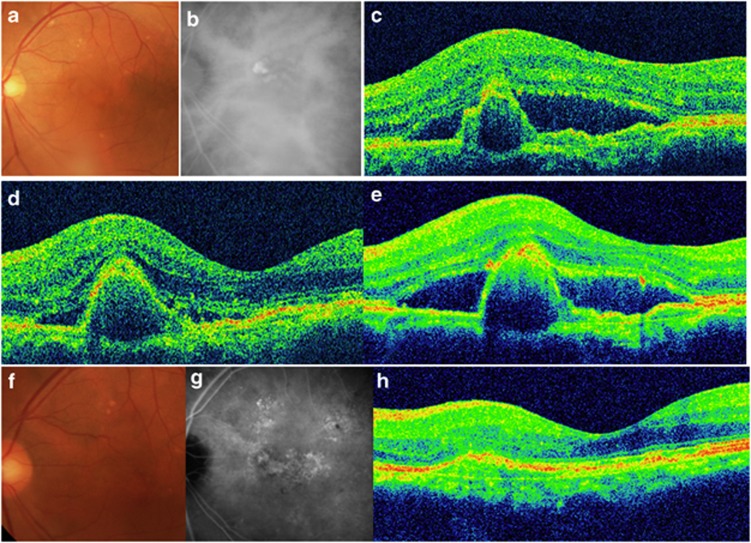
No cases of ocular adverse effects such as endophthalmitis, rhegmatogenous retinal detachment or any systemic complications were encountered in either group. Figure 4 shows the results for the eyes in the IVR.
Figure 4.
A 75-year-old man presented with reduced visual acuity in the left eye. (a) A color fundus photograph of the left eye shows a large area of SRF. (b) Indocyanine green angiographic (ICGA) image showed staining indicating PCV. (c) Spectral-domain optical coherence tomography (SD-OCT) at baseline revealed SRF with a polypoidal lesion. The visual acuity was 20/30 in the left eye, and the patient was diagnosed as having PCV. (d) At 3 months after the first injection, the SRF had resolved, although the polypoidal lesion persisted. The patient's visual acuity had improved to 20/25. (e) However, at 13 months after the initial treatment, the SRF had increased. Additional IVR treatments were administered. (f) At 2 years after the first treatment, the patient's visual acuity was maintained at 20/25. (g) An ICGA image shows the regression of the polyps, although the abnormal vascular network remains. (h) SD-OCT also shows the disappearance of PCV. In total, 11 IVR treatments were administered during the follow-up period.
Discussion
Our study demonstrated that IVR was effective for improving vision in patients with PCV, compared with PDT, as evaluated at 24 months after the treatment. A recent study comparing PDT with or without ranibizumab and ranibizumab monotherapy in Asian people reported that all three groups showed a gain in visual acuity over a 6-month period, although the PDT groups were able to achieve the complete regression of the polyps, compared with the IVR group.18 Although it was a prospective randomized clinical trial, the long-term outcomes were not clearly identified. The present report is the first to describe the functional outcome of IVR in Asian patients with PCV during the first 24 months after the initial treatment. Furthermore, the use of IVR and PDT for the treatment of PCV was compared.
In this study, the postoperative BCVA in the IVR group was significantly improved, compared with the baseline value, throughout the 24-month period. As previously described, IVR rapidly resolved the exudative findings, such as retinal edema and SRF, resulting in a good visual improvement.16, 17 We speculated that the good visual acuity continued during the 2-year period because of anatomic improvements resulting from the anti-VEGF therapy. Furthermore, although SRH occurred in two eyes in which the polyps were found to have grown based on the ICGA findings, additional IVR administrations prevented severe visual deterioration. A previous study reported that monthly IVR treatments reduced the risk of macular hemorrhage, compared with PDT in patients with AMD.20 Although IVR was repeated as needed during the maintenance phase in this study, prompt IVR after recurrences inhibited VEGF expression in the vascular endothelial cells of the PCV lesion, possibly decreasing the risk of massive SRH. However, careful monitoring of the size of polypoidal lesions may be needed in some cases.
On the other hand, in the PDT group, the mean logMAR BCVA tended to decrease over the 6-month period following the initial treatment. Furthermore, the proportion of eyes with a deterioration in visual acuity following PDT was significantly higher, compared with that following IVR. Similar to this study, the long-term outcomes of AMD after PDT have indicated several serious problems. For example, long-term influences of PDT, such as damage to the retinal pigment epithelium after PDT21 and choroidal hypoperfusion (which is correlated with chorioretinal atrophy),22 have been reported.
The continued decline in vision after PDT was found to be caused by progressive photoreceptor degeneration in the area over a scar resulting from CNV inactivation.23 We speculated that changes resulting from PDT treatment, such as photoreceptor degeneration, fibrosis and atrophic changes, might attenuate the visual acuity improvement even if the lesion is inactivated. Furthermore, choroidal ischemia caused by PDT, which further secretes VEGF, might introduce recurrent lesions and massive SRH with severe visual deterioration, as SRH after PDT could be a common complication in patients with PCV.24
Although previous reports have described that an estimated 33–43% of patients treated with IVR showed the complete regression of polyps, which seemed to be inferior to that in patients treated with PDT,16, 17, 18 in the present study, 60.6% of the patients in the IVR group showed no polyp lesions on the ICGA findings, and no significant difference between the two groups was observed. The rate of polyp regression over time is difficult to compare between the two groups because ICGA was performed only at baseline and at 24 months in the IVR group although the PDT group was evaluated every 3 months. However, these results showed that repeated IVR might be effective not only for suppressing exudative changes but also for the involution of polyp lesions during a long-term follow-up period (Figure 4).
Some concern about the data exists because the baseline visual acuity differed between the two groups, although a statistically significant difference was not observed. However, these results might explain the differences in the subsequent visual acuity outcome. Therefore, we also investigated the improvement in the visual acuity. As a result, the IVR group showed a greater degree of improvement in the visual acuity at 12, 18 and 24 months after the initial treatment. IVR might be superior to PDT with regard to the improvement in visual acuity during the long-term follow-up period.
In this study, the abnormal vascular networks had persisted in all the eyes when evaluated at a 2-year follow-up examination, consistent with the results of previous studies.16, 17 Although our study showed favorable results, the persistent abnormal vascular networks may indicate a high risk of recurrent polypoidal lesions.12 In fact, IVR group showed higher recurrence rate compared with PDT group during the follow-up period. Therefore, more investigations are needed to assess the further long-term efficacy of IVR in patients with PCV. The strict monitoring of patients beyond 2 years after the initial treatment should be considered.
Furthermore, although IVR was effective for improving vision compared with PDT, financial cost cumulates by the mean number of 7.1±5.2 ranibizumab injections. The total treatment cost is 1 292 449 yen (182 035 yen per one injection). On the other hand, the treatment cost of mean number of 1.52 PDT is 565 227 yen (371 860 yen per one). Bevacizumab might be an attractive alternative to ranibizumab from its lower cost to reduce the economic burden. However, bevacizumab has not received Food and Drug Administration approval for the treatment of AMD. Furthermore, we reported that bevacizumab injection was significantly higher to cause endophthalmitis compared with ranibizumab.25 To realize an appropriate cost-effectiveness treatment for AMD is needed in the future.
The main limitations of the present study were its small sample size and its retrospective nature. The retrospective nature of the study may have introduced some bias. However, our approach to PCV is highly homogenous, enabling patients to be compared retrospectively because they have all been treated in a similar way. Furthermore, we didn't compare the possibility of IVR or intravitreal bevacizumab combined with PDT or reduced fluence PDT. In 2010–2012, treatment of the PCV also focused on combined therapy.26, 27, 28, 29 However, in our institution, it was difficult to compare these patients because recurrent patients of combined therapy were changed into IVR monotherapy within 2 years. Although combined therapy could be also the main therapeutic choice in the present, Kim et al29 described that the benefit of combined therapy diminished in year 2 with no significant difference compared with baseline. Further investigations are needed to compare combined therapy and IVR monotherapy during the long follow-up periods.
In conclusion, IVR is well tolerated for maintaining or improving vision in patients with PCV, compared with PDT, as evaluated at the time of a long-term follow-up examination. PDT for the treatment of PCV might result in unfavorable outcomes with no superiority with regard to achieving the involution of polyps.
The authors declare no conflict of interest.
References
- Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10 (1:1–8. [PubMed] [Google Scholar]
- Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997;115 (4:478–485. doi: 10.1001/archopht.1997.01100150480005. [DOI] [PubMed] [Google Scholar]
- Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121 (10:1392–1396. doi: 10.1001/archopht.121.10.1392. [DOI] [PubMed] [Google Scholar]
- Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol. 2002;133 (5:639–648. doi: 10.1016/s0002-9394(02)01404-6. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, Slakter J, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002;22 (5:529–535. doi: 10.1097/00006982-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Lee SC, Seong YS, Kim SS, Koh HJ, Kwon OW. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004;218 (3:193–201. doi: 10.1159/000076844. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004;111 (8:1576–1584. doi: 10.1016/j.ophtha.2003.12.056. [DOI] [PubMed] [Google Scholar]
- Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K, Mori R. Role of photodynamic therapy in polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2007;51 (4:270–277. doi: 10.1007/s10384-007-0452-3. [DOI] [PubMed] [Google Scholar]
- Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115 (1:141–146. doi: 10.1016/j.ophtha.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Rouvas AA, Papakostas TD, Ntouraki A, Douvali M, Vergados I, Ladas ID. Photodynamic therapy, ranibizumab, and ranibizumab with photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Retina. 2011;31 (3:464–474. doi: 10.1097/IAE.0b013e3181f274ec. [DOI] [PubMed] [Google Scholar]
- Kurashige Y, Otani A, Sasahara M, Yodoi Y, Tamura H, Tsujikawa A, et al. Two-year results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2008;146 (4:513–519. doi: 10.1016/j.ajo.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Akaza E, Yuzawa M, Mori R. Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2011;55 (1:39–44. doi: 10.1007/s10384-010-0886-x. [DOI] [PubMed] [Google Scholar]
- Leal S, Silva R, Figueira J, Cachulo ML, Pires I, de Abreu JR, et al. Photodynamic therapy with verteporfin in polypoidal choroidal vasculopathy: results after 3 years of follow-up. Retina. 2010;30 (8:1197–1205. doi: 10.1097/IAE.0b013e3181d37486. [DOI] [PubMed] [Google Scholar]
- Lee WK, Kim KS, Kim W, Lee SB, Jeon S. Responses to photodynamic therapy in patients with polypoidal choroidal vasculopathy consisting of polyps resembling grape clusters. Am J Ophthalmol. 2012;154 (2:355–365.e1. doi: 10.1016/j.ajo.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94 (3:297–301. doi: 10.1136/bjo.2008.150029. [DOI] [PubMed] [Google Scholar]
- Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, et al. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol. 2012;154 (1:117–124.e1. doi: 10.1016/j.ajo.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, et al. EVEREST STUDY: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32 (8:1453–1464. doi: 10.1097/IAE.0b013e31824f91e8. [DOI] [PubMed] [Google Scholar]
- Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol. 1999;117 (10:1329–1345. [PubMed] [Google Scholar]
- Barbazetto I, Saroj N, Shapiro H, Wong P, Freund KB. Dosing regimen and the frequency of macular hemorrhages in neovascular age-related macular degeneration treated with ranibizumab. Retina. 2010;30 (9:1376–1385. doi: 10.1097/IAE.0b013e3181dcfb0b. [DOI] [PubMed] [Google Scholar]
- Schnurrbusch UE, Welt K, Horn LC, Wiedemann P, Wolf S. Histological findings of surgically excised choroidal neovascular membranes after photodynamic therapy. Br J Ophthalmol. 2001;85 (9:1086–1091. doi: 10.1136/bjo.85.9.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Schlotzer-Schrehard U, Cursiefen C, Michels S, Beckendorf A, Naumann GO. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44 (10:4473–4480. doi: 10.1167/iovs.02-1115. [DOI] [PubMed] [Google Scholar]
- Mataix J, Desco MC, Palacios E, Garcia-Pous M, Navea A. Photodynamic therapy for age-related macular degeneration treatment: epidemiological and clinical analysis of a long-term study. Ophthalmic Surg Lasers Imaging. 2009;40 (3:277–284. doi: 10.3928/15428877-20090430-09. [DOI] [PubMed] [Google Scholar]
- Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27 (3:335–341. doi: 10.1097/01.iae.0000233647.78726.46. [DOI] [PubMed] [Google Scholar]
- Inoue M, Kobayakawa S, Sotozono C, Komori H, Tanaka K, Suda Y, et al. Evaluation of the incidence of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor. Ophthalmologica. 2011;226 (3:145–150. doi: 10.1159/000329863. [DOI] [PubMed] [Google Scholar]
- Gomi F, Sawa M, Wakabayashi T, Sasamoto Y, Suzuki M, Tsujikawa M. Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010;150 (1:48–54. e1. doi: 10.1016/j.ajo.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Tomita K, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Otani A, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with intravitreal injections of ranibizumab. Am J Ophthalmol. 2012;153 (1:68–80. e1. doi: 10.1016/j.ajo.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Saito M, Iida T, Kano M. Combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2012;32 (7:1272–1279. doi: 10.1097/IAE.0b013e318236e624. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim K, Kim do G, Yu SY, Kwak HW. Two-year results of photodynamic therapy combined with intravitreal anti-vascular endothelial growth factor for polypoidal choroidal vasculopathy. Ophthalmologica. 2011;226 (4:205–213. doi: 10.1159/000330793. [DOI] [PubMed] [Google Scholar]