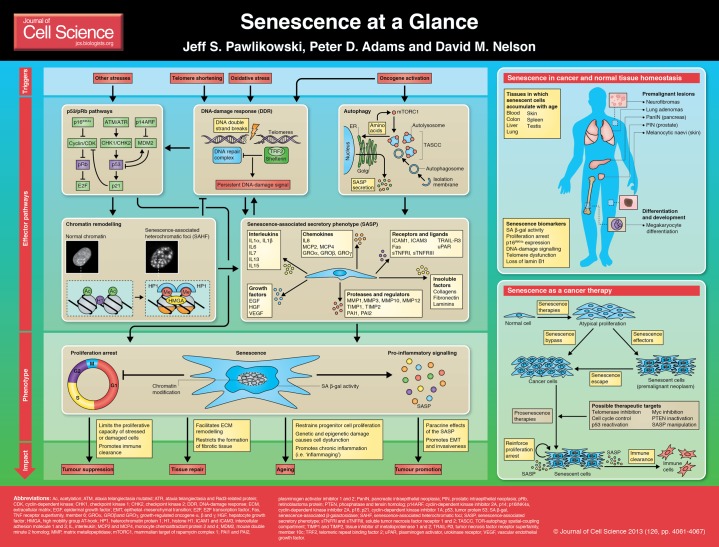
Summary
Cellular senescence is a stable proliferation arrest that is associated with extensive cellular remodelling and an altered secretory pathway. Through its numerous inducers that lead to altered gene expression, senescence is able to influence many contrasting functions and pathologies, namely tumour suppression, tumour promotion, wound healing and ageing. As senescence is able to control such important tissue functions, it is now being pinpointed as a possible route for novel therapies. This article and accompanying poster aim to provide a summary of the initiators, pathways and roles of senescence, as well as present examples of senescence and a possible use for senescence in therapy.
Introduction
Just over 50 years ago, Leonard Hayflick and Paul Moorhead reported their seminal observation that primary human cells possess a finite lifespan in culture, describing the phenomenon as a form of ‘senescence at the cellular level’ (Hayflick and Moorhead, 1961). Since then, significant progress has been made toward characterizing how damaged cells establish a stable proliferation arrest, a process that we now refer to as cellular senescence, and research has focused on identifying the molecular triggers, mechanisms, phenotypes and functional impact of engaging the senescence programme (Adams, 2009; Campisi and d'Adda di Fagagna, 2007; Kuilman et al., 2010). Here, and in the accompanying poster, we concisely summarise recent advances in our mechanistic and functional understanding of the senescence programme and discuss how it might be exploited for therapeutic purposes.
Cell senescence is a stable state of proliferation arrest that cells undergo in response to a variety of detrimental stimuli to limit the propagation of damaged and stressed cells. Considerable evidence supports a functional role for senescence in tumour suppression and wound healing, but also possibly in promoting tissue ageing. To date, a diverse array of cellular stressors have been identified as triggers of senescence.
One of the first reported molecular triggers of senescence was telomere attrition, the progressive shortening of the linear ends of chromosomes that occurs with repeated rounds of cell division (Harley et al., 1990). Consequently, senescence that occurs because of telomere shortening (and probably coupled with oxidative damage) is referred to as replicative senescence (RS). Senescence induction also occurs in response to the activation of oncogenes, termed oncogene-induced senescence (OIS) (Serrano et al., 1997). In addition, DNA-damaging agents and oxidative stress have been identified as potent initiators of senescence (d'Adda di Fagagna, 2008; Saretzki and Von Zglinicki, 2002). Each of these molecular triggers is fully capable of driving senescence by engaging an integrated network of effector pathways that collectively culminate in the establishment of a stable proliferation arrest and the expression of the senescence-associated secretory phenotype (SASP), an array of chemokines, cytokines, extracellular matrix remodelling enzymes and other soluble and insoluble factors secreted by senescent cells (see Box 1).
Box 1. Senescence-associated secretory phenotype.
In addition to the establishment of a stable proliferation arrest, the other hallmark of senescence is the senescence-associated secretory phenotype (SASP). Senescent cells remain metabolically active and express and secrete a broad spectrum of soluble and insoluble proteins, as well as other factors collectively termed the SASP. SASP factors can be classified into several defined categories including interleukins, chemokines and other inflammatory factors, proteases and regulators, growth factors and regulators, receptors and ligands, and extracellular matrix components (Coppé et al., 2010). The SASP is at least partially dependent upon a persistent DNA-damage signal, because depletion of the DDR mediators ATM, CHK2 or NBS1 in senescent cells attenuates secretion of the cytokine interleukin 6 (IL6) (Rodier et al., 2009). In addition, SASP factors including several chemokine receptor 2 (CXCR2) ligands (e.g. IL8) reinforce the senescence proliferation arrest by enhancing the DDR, underscoring the role of the SASP as not only a downstream phenotype of senescence, but also an integral effector mechanism (Acosta et al., 2008; Kuilman et al., 2008). Functionally, secretion of SASP factors into the extracellular milieu by senescent cells can elicit a variety of autocrine and paracrine responses. Non-cell-autonomous functions of the SASP include the attenuation of fibrosis in response to chemical-induced or physical injury, modulation of the immune response and transmission of senescence to normal neighbouring cells adjacent to senescent lesions (Acosta et al., 2013; Jun and Lau, 2010; Krizhanovsky et al., 2008; Lujambio et al., 2013; Nelson et al., 2012). Indeed, a number of reports ascribe a role for the SASP in the tumour suppressive function of senescence through the initiation of immune surveillance and clearance of senescent cells in vitro and in vivo (Kang et al., 2011; Krizhanovsky et al., 2008; Xue et al., 2007). By contrast, secretion of the SASP by senescent cells can also facilitate detrimental non-cell-autonomous effects, including enhancement of cell growth, induction of the epithelial-to-mesenchymal transition (EMT) and invasiveness and the promotion of tumorigenesis (Coppé et al., 2008; Krtolica et al., 2001; Yoshimoto et al., 2013). Consequently, the SASP reflects both a definitive and essential feature of senescence that can also potentiate tissue dysfunction and cancer in certain contexts.
Effectors of the senescence program
It is now well established that the p53 and p16INK4a-pRb tumour suppressor pathways are the master regulators of senescence and serve to initiate a state of stable proliferation arrest. Indeed, bypass of senescence in primary human cells requires inactivation of both the p53 and p16INK4a-pRb pathways (Bond et al., 1999; Hahn et al., 2002; Shay et al., 1991), as reviewed extensively elsewhere (Ben-Porath and Weinberg, 2005; Campisi, 2005). In culture, senescent cells frequently display an enlarged, flattened morphology that is accompanied by expression of p16INK4a and elevated lysosomal activity (senescence-associated β-galactosidase; SA β-gal) (Campisi and d'Adda di Fagagna, 2007). Recent studies have identified additional effector mechanisms involved in establishing senescence that are further discussed here, including the DNA-damage response, chromatin remodelling and autophagy (see Poster).
DNA-damage response
A common feature of many senescence triggers is the ability to produce DNA damage. Not surprisingly, the DNA-damage response (DDR) has emerged as a crucial senescence effector mechanism. Replicative senescent cells accumulate markers of DNA damage, including phosphorylated γ-H2AX, CHK1, CHK2, SMC1 and RAD17, and inactivation of the DDR enables replicative senescent cells to resume DNA replication (d'Adda di Fagagna et al., 2003; Takai et al., 2003). Similarly, oncogenic activation engages the DDR by driving hyper-replication, resulting in improperly terminated replication forks and DNA double-strand breaks (Bartkova et al., 2006; Di Micco et al., 2006). More recent findings suggest that when DNA damage occurs at telomeres, it cannot be effectively repaired, resulting in the presence of chronic DNA damage foci, a persistent DDR and senescence induction (Fumagalli et al., 2012; Hewitt et al., 2012). Intriguingly, oncogenic activation also induces telomeric lesions, stochastic telomere attrition and senescence (Hewitt et al., 2012; Suram et al., 2012). Inactivation of CHK2 not only abolishes oncogenic H-RAS-induced senescence, but also promotes cellular transformation, further illustrating the significance of an intact DDR for the establishment of OIS (Di Micco et al., 2006).
In addition, a persistent DDR is required for robust expression of the SASP (see Box 1). Indeed, inactivation of the DDR mediators ATM, CHK2 or NBS1 attenuates the SASP in radiation-induced senescent cells (Rodier et al., 2009). Thus, a sustained DDR is crucial for establishment of proliferation arrest and SASP expression, the two phenotypic hallmarks of senescence.
Chromatin remodelling
Upon senescence induction, cells undergo profound chromatin remodelling, which is also emerging as an important mediator of the senescence program, and its most striking manifestation is the formation of facultative heterochromatin structures termed senescence-associated heterochromatic foci (SAHF). Originally reported by Scott Lowe's laboratory, SAHF appear microscopically as large DNA foci when senescent cells are stained with 4′,6-diamidino-2-phenylindole (DAPI) (Narita et al., 2003). There are ∼30–50 foci per nucleus, and each focus arises from the compaction of an individual chromosome (Funayama et al., 2006; Zhang et al., 2007). Although SAHF probably reflect a heterochromatic state, pericentric and telomeric heterochromatin domains are largely excluded from SAHF (Chandra et al., 2012; Funayama et al., 2006; Narita et al., 2003; Zhang et al., 2007).
SAHF exhibit enrichment of histone H3 lysine 9 dimethylation and trimethylation (H3K9me2/3) and are devoid of the euchromatic histone H3 lysine 9 acetylation (H3K9ac) and histone H3 lysine 4 methylation (H3K4me) marks (Narita et al., 2006; Narita et al., 2003). Recent data indicate that SAHF result from the spatial reorganization of pre-existing domains of repressive histone modifications rather than through the acquisition of new repressive histone marks (Chandra et al., 2012). This study further showed that, whereas H3K9me2 is distributed across the entire SAHF focus, H3K9me3 is restricted to the SAHF core and is enveloped by a ring of H3K27me3. SAHF are also composed of additional heterochromatin components, including the histone H2A variant macroH2A and the heterochromatin protein 1 (HP1) and high-mobility group A (HMGA) proteins (Narita et al., 2006; Narita et al., 2003; Zhang et al., 2005).
Functionally, SAHF probably mediates senescence in two ways. First, SAHF is likely to restrict the expression of proliferation genes, such as cyclin A2, a gene required for cell cycle progression that is silenced and physically incorporated into SAHF (Narita et al., 2003; Zhang et al., 2007). Likewise, actively transcribed chromatin regions are largely excluded from SAHF (Funayama et al., 2006; Narita et al., 2003). Recent findings also support a role for SAHF in dampening the DDR and preserving cell viability following oncogene-induced replication stress (Di Micco et al., 2011). This study revealed that the DDR mediators γ-H2AX, RAD50, NBS1 and activated ATM are physically excluded from SAHF in OIS fibroblasts. Furthermore, inhibition of the H3K9me3 methyltransferase SUV39, or depletion of SUV39 or HP1 disrupts heterochromatin formation in oncogene-expressing cells, increases the DDR and leads to the accumulation of DDR markers at DAPI-rich regions, and ultimately results in cell death (Di Micco et al., 2011). Thus, chromatin remodelling reinforces senescence-mediated proliferation arrest and constrains the DDR.
Autophagy
More recently, evidence has emerged indicating that autophagy has an important role as a molecular mediator of cell senescence, including in the establishment of cell cycle exit and SASP expression. Autophagy is a program in which intracellular proteins, small organelles and other cytoplasmic constituents are degraded by lysosomes for their subsequent use by the cell as substrates in various metabolic and synthetic processes (Mizushima, 2007). Intriguingly, autophagic activity increases markedly during OIS in primary human fibroblasts (Young et al., 2009). Depletion of either ATG5 or ATG7, genes required for autophagy, attenuates SA β-galactosidase activity, a molecular marker of a senescence-specific expansion of the lysosomal compartment, and delays expression of SASP components, IL6 and IL8, in OIS fibroblasts. Remarkably, reduction of ATG5 or ATG7 protein levels also enables cells to bypass H-RAS-induced proliferation arrest, indicating that autophagy is an essential contributor to the establishment of OIS.
Additionally, senescent cells typically exhibit an enlarged morphology, contain more protein per cell than proliferating cells and maintain active protein synthesis (Young et al., 2009). To accommodate this significant energetic demand, OIS cells upregulate autophagy to increase nascent protein synthesis through the formation of a novel subcellular structure termed the TOR-autophagy spatial-coupling compartment (TASCC) (Narita et al., 2011). Findings from this study indicated that the recruitment of the mTOR complex to the TASCC is dependent on amino acids and Rag GTPase, and disruption of mTOR recruitment to the TASCC restricts IL6 and IL8 expression during OIS. As a component of the SASP (see Text Box 1), IL6 is involved in the activation of the senescence inflammatory transcriptome and is required for the proper establishment of OIS (Kuilman et al., 2008). Consequently, the spatial association of mTOR-regulated autophagy and protein synthesis in senescent cells appears to facilitate the synthesis of at least a subset of SASP proteins and thus represents a crucial effector mechanism in the establishment of OIS. Whether autophagy plays an essential role in all modes of senescence remains to be elucidated, because different studies have found that inhibition of mTOR, which is known to activate autophagy, alternately delays or potentiates aspects of senescence (Cao et al., 2011; Demidenko et al., 2009; Iglesias-Bartolome et al., 2012; Kennedy et al., 2011; Wall et al., 2013).
Interestingly, an autophagy and/or lysosomal pathway has been shown to process chromatin in senescent cells, leading to depletion in total histone content. Depletion of histones was shown to correlate with naevus maturation, an established histopathological parameter associated with proliferation arrest and clinical benignancy, therefore linking chromatin remodelling via an autophagic pathway with senescence and tumour suppression (Ivanov et al., 2013).
The diverse roles of senescence
Since the first description of senescence, it is becoming apparent that it has diverse functions and impinges on fundamental biological processes that can have opposing effects, such as tumour suppression, tissue repair, ageing and tumour promotion.
To form a tumour, cancer cells typically must acquire an unrestrained growth potential, a trait that is suppressed by senescence (Hanahan and Weinberg, 2000). Indeed, many of the same triggers that initiate cell transformation are also able to elicit a senescence response. Consequently, most, if not all, cancers have mutations in the p53 and p16INK4a-pRb pathways, allowing for a bypass or escape from senescence. In addition, many premalignant tissues contain senescent cells (discussed below).
Senescence has also been shown to regulate tissue repair using a mouse model of liver damage (Krizhanovsky et al., 2008). Upon the induction of liver damage by chemical treatment, hepatic stellate cells proliferate and secrete extracellular matrix (ECM), which results in the formation of a fibrotic scar. These cells soon senesce and display a SASP that results in the downregulation of ECM components and the increased expression of matrix metalloproteinases (MMPs), which can also degrade ECM proteins. Senescent hepatic cells also secrete chemokines to attract natural killer cells to resolve fibrosis once the healing has taken place to allow the restoration of normal tissue function. Furthermore, when senescence is inhibited in hepatic stellate cells severe fibrosis entails after acute liver injury, underscoring the importance of senescence in facilitating a portion of the wound-healing response (Krizhanovsky et al., 2008).
Cells with senescent properties have been shown to increase with age in a variety of mammalian tissues (Dimri et al., 1995; Herbig et al., 2006; Jeyapalan et al., 2007; Krishnamurthy et al., 2004; Sedelnikova et al., 2004; Wang et al., 2009), and, moreover, the accumulation of senescent cells is also related to age-associated tissue pathologies, such as osteoarthritis, atherosclerosis and liver cirrhosis (Minamino and Komuro, 2007; Price et al., 2002; Wiemann et al., 2002). Experimentally, inactivation of p16INK4a-pRb in a prematurely aged mouse model is able to prolong cell renewal and transgenic expression of telomerase, an enzyme that maintains telomere length and extends longevity in cancer-resistant mice (Baker et al., 2008; Janzen et al., 2006; Krishnamurthy et al., 2006; Molofsky et al., 2006; Tomás-Loba et al., 2008).
The contribution of senescence to ageing has been explained by the ‘theory of antagonistic pleiotropy’, which stipulates that a biological process can be both beneficial and deleterious, depending on the age of the organism (Williams, 1957). Specifically, a subset of genes responsible for negative effects are selected for if they can confer a reproductive advantage early in life, whereas they confer harmful effects in aged individuals. Antagonistic pleiotropy is based on the fact that most organisms evolve in environments with fatal extrinsic hazards: a so-called ‘survival of the fittest’. However, age-associated phenotypes are not under the control of natural selection and, therefore, processes that promote fitness in young individuals can be detrimental in aged organisms (Rodier and Campisi, 2011; Williams, 1957). According to this view, senescence is beneficial in young organisms through its ability to promote tumour suppression and wound healing, but it has detrimental effects on old organisms. This is in line with the finding that cellular senescence is associated with age-related phenotypes and the removal of senescent cells is able to prevent or delay tissue dysfunction and extend healthspan (Baker et al., 2011). Nonetheless, the notion of senescence as an example of antagonistic pleiotropy has also been challenged recently because there is little evidence that the positive effects of senescence on survival predominate at young ages and the negative effects predominate at late ages (Giaimo and d'Adda di Fagagna, 2012).
The theory of antagonistic pleiotropy can explain the tumour-promoting role of senescence. Although seemingly paradoxical, senescence might promote cancer owing to the fact that senescent cells are able to promote malignancy in the cells around them. Through the SASP (see Box 1), senescent cells secrete a large amount of cytokines and chemokines into their surrounding environment (Coppé et al., 2010). Because the number of senescent cells increases with age, the secretion of these factors can persist and stimulate the formation of a tumour. It is possible that a large, persistent amount of SASP signalling promotes the pro-tumorigenic effects, whereas their secretion in lower amounts during acute SASP promotes the tumour suppressive effects of senescence. Whatever the main contribution of SASP might be, either pro- or anti-tumorigenic, it appears to be clearly context dependent, as a result of many factors such as the interplay of tumour suppressors and the tissue microenvironment (Lujambio et al., 2013).
Senescence in cancer and normal tissue homeostasis
Senescence was initially considered an artefact of in vitro cell culture shock, but this view has now changed owing to many groups observing senescent cells in premalignant tissues (Sherr and DePinho, 2000). Analyses of human and murine tissues have shown the presence of senescent cells in lung adenomas, pancreatic intraductal neoplasia (PanIN lesions), prostatic intraepithelial neoplasia (PIN lesions) and melanocytic naevi, all of which are premalignant neoplasms (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005) (see Poster). It has also been shown that senescence is largely abolished in the corresponding malignant lesion – lung carcinomas, pancreatic ductal adenocarcinomas, prostate carcinomas and melanomas, respectively (Chen et al., 2005; Collado et al., 2005; Gray-Schopfer et al., 2006).
Mouse models have been used to alter tumour suppressor genes such as PTEN or oncogenes such as NRAS to induce senescence that is associated with the development of pre-malignant lesions without signs of apoptosis (Braig et al., 2005; Chen et al., 2005). Upon inactivation of senescence through deletion of senescence modulators such as p53 or chromatin modulators, fully developed malignancy then occurred, highlighting the role of senescence in tumour suppression. Senescence has also been observed in melanocytic naevi in mice with an activated mutant form of the oncogene Braf (BRAFV600E) expressed specifically in their melanocytes (Dhomen et al., 2009). Appropriately, the majority of benign human naevi have also been found to have a BRAFV600E mutation (Pollock et al., 2003). However, the efficiency of senescence in acting as a tumour suppression mechanism in murine naevi harbouring BRAFV600E-expressing melanocytes is reduced compared with human naevi because these mice typically develop melanomas within a year (Dhomen et al., 2009). Nonetheless, the rather long latency of tumours in these mice suggests that oncogenic BRAF alone is not sufficient for the induction of melanoma and that additional events are required to bypass senescence and for subsequent disease progression. Indeed, additional genetic alterations in oncogenes or tumour suppressors have been shown to accelerate progression into melanoma (Damsky et al., 2011; Dankort et al., 2009; Delmas et al., 2007; Vredeveld et al., 2012).
In addition to the role of senescence in tumour suppression and in ageing, there is also in vivo evidence for its role in normal cellular differentiation and development, such as the terminal differentiation of megakaryocytes (Besancenot et al., 2010). The proliferative arrest that is observed in mature megakaryocytes resembles a senescence arrest, because many markers of senescence are upregulated in this state. Interestingly, senescence is not observed in malignant megakaryocytes, which might be the underlying reason for their cancerous phenotype.
Senescence therapies
As discussed above, the bypass or escape of a senescence response in premalignant lesions is required for tumour progression (Bennecke et al., 2010; Braig et al., 2005; Chen et al., 2005; Collado and Serrano, 2010; Dankort et al., 2009; Dhomen et al., 2009). Therefore, if senescence can be either maintained or reactivated, progression towards a malignant state might be slowed or malignancy even averted, suggesting that senescence could be a therapy target. Supporting evidence from murine models shows that the reactivation of tumour suppressor genes such as p53 can induce senescence and tumour regression in a liver tumour model (Ventura et al., 2007; Xue et al., 2007). Similarly, tumour regression due to the inactivation of Myc can be associated with cell senescence, functionally supporting a therapeutic role for senescence (Wu et al., 2007).
The outcome of conventional cancer therapy is enhanced when a senescence response is present (Schmitt et al., 2002; te Poele et al., 2002). In addition, other strategies for pro-senescence therapy have been proposed, including inhibition of telomerase activity and alteration of CDK or CDK regulators (Campaner et al., 2010; Chen et al., 2005; Harley, 2008; Kennedy et al., 2011; Nardella et al., 2011; Puyol et al., 2010; Ventura et al., 2007; Wall et al., 2013; Xue et al., 2007) (see Poster).
Moreover, it has been shown that senescent cells can be cleared by the immune system owing to the SASP. For example, reactivation of p53 in a mouse model of hepatocellular carcinoma that is driven by RAS expression results in tumour regression that was associated with a strong SASP, which allows for tumour clearance (Kang et al., 2011; Xue et al., 2007). However, the clearance of senescent cells by the immune system is not universal because precancerous lesions such as naevi can persist for decades without any sign of malignant transformation (Michaloglou et al., 2005). The targeting of SASP might also provide a possible route for pro-senescence cancer therapy by enhancing cell clearance. However, it is important to remember that even though senescence acts primarily as a tumour suppression mechanism, it can also promote tumorigenesis (Krtolica et al., 2001). Therefore, pro-senescence therapy will have to be applied in a highly specific manner in the clinic and might require initial genotyping before use. Our expanding knowledge of the senescence mechanism will help to advance pro-senescence cancer therapy, so that it can reach the clinic as a cancer therapy in the near future.
Conclusions
In the 50 years since a role of senescence in tumour suppression and ageing was first proposed, the senescence phenotype has become much clearer. Once thought to be merely a phenomenon found in vitro, senescence is now being recognised as a relevant cellular mechanism in vivo. A major hurdle that the senescence field must overcome is labelling senescence with a strict definition. Because there are a multitude of distinctive initiators, pathways and markers of senescence, unambiguously defining a cell as senescent can be difficult. Senescence is increasingly referred to not as an irreversible growth arrest, but as a stable growth arrest.
Additional functions and signalling mechanisms of senescence have also recently been uncovered, including links to autophagy, the inflammatory response, chromatin structure, as well as its regulation by miRNAs, which have been shown to induce senescence by regulating key effectors of senescence pathways (Christoffersen et al., 2010; Feliciano et al., 2011). Powerful high-throughput analyses at the genomic and epigenomic level will continue to progress the field to a better understanding of senescence at the molecular level and further elucidate its potential in tumour suppression, ageing, wound healing and tumour promotion, as well as other roles that probably remain to be discovered. Further research into these mechanisms might address unanswered questions in the senescence field such as how to control the effects of senescence in such a way that it could be used in cancer therapy.
Footnotes
Funding
The work done in the Peter Adams lab is funded by Cancer Research UK [grant number C10652/A10250]; and the National Institutes of Health [grant number R01 CA129334-01]. Deposited in PMC for release after 12 months.
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.109728/-/DC1.
References
- Acosta J. C., O'Loghlen A., Banito A., Guijarro M. V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N. et al. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 10.1016/j.cell.2008.03.038 [DOI] [PubMed] [Google Scholar]
- Acosta J. C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J. P., Athineos D., Kang T-W., Lasitschka F., Andrulis M. et al. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol doi: 10.1038/ncb2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. D. (2009). Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol. Cell 36, 2–14 10.1016/j.molcel.2009.09.021 [DOI] [PubMed] [Google Scholar]
- Baker D. J., Perez-Terzic C., Jin F., Pitel K. S., Niederländer N. J., Jeganathan K., Yamada S., Reyes S., Rowe L., Hiddinga H. J. et al. (2008). Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 10, 825–836 10.1038/ncb1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. J., Wijshake T., Tchkonia T., LeBrasseur N. K., Childs B. G., van de Sluis B., Kirkland J. L., van Deursen J. M. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L-V. F., Kolettas E., Niforou K., Zoumpourlis V. C. et al. (2006). Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 10.1038/nature05268 [DOI] [PubMed] [Google Scholar]
- Ben-Porath I., Weinberg R. A. (2005). The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37, 961–976 10.1016/j.biocel.2004.10.013 [DOI] [PubMed] [Google Scholar]
- Bennecke M., Kriegl L., Bajbouj M., Retzlaff K., Robine S., Jung A., Arkan M. C., Kirchner T., Greten F. R. (2010). Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer Cell 18, 135–146 10.1016/j.ccr.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Besancenot R., Chaligné R., Tonetti C., Pasquier F., Marty C., Lécluse Y., Vainchenker W., Constantinescu S. N., Giraudier S. (2010). A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol. 8 10.1371/journal.pbio.1000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. A., Haughton M. F., Rowson J. M., Smith P. J., Gire V., Wynford-Thomas D., Wyllie F. S. (1999). Control of replicative life span in human cells: barriers to clonal expansion intermediate between M1 senescence and M2 crisis. Mol. Cell. Biol. 19, 3103–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig M., Lee S., Loddenkemper C., Rudolph C., Peters A. H. F. M., Schlegelberger B., Stein H., Dörken B., Jenuwein T., Schmitt C. A. (2005). Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 10.1038/nature03841 [DOI] [PubMed] [Google Scholar]
- Campaner S., Doni M., Hydbring P., Verrecchia A., Bianchi L., Sardella D., Schleker T., Perna D., Tronnersjö S., Murga M. et al. (2010). Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nat. Cell Biol. 12, 54–59 [DOI] [PubMed] [Google Scholar]
- Campisi J. (2005). Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 10.1016/j.cell.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Campisi J., d'Adda di Fagagna F. (2007). Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- Cao K., Graziotto J. J., Blair C. D., Mazzulli J. R., Erdos M. R., Krainc D., Collins F. S. (2011). Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in hutchinson-gilford progeria syndrome cells. Sci. Transl. Med. 3, 89ra58. [DOI] [PubMed] [Google Scholar]
- Chandra T., Kirschner K., Thuret J-Y., Pope B. D., Ryba T., Newman S., Ahmed K., Samarajiwa S. A., Salama R., Carroll T. et al. (2012). Independence of repressive histone marks and chromatin compaction during senescent heterochromatic layer formation. Mol. Cell 47, 203–214 10.1016/j.molcel.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Trotman L. C., Shaffer D., Lin H-K., Dotan Z. A., Niki M., Koutcher J. A., Scher H. I., Ludwig T., Gerald W. et al. (2005). Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 10.1038/nature03918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen N. R., Shalgi R., Frankel L. B., Leucci E., Lees M., Klausen M., Pilpel Y., Nielsen F. C., Oren M., Lund A. H. (2010). p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 17, 236–245 10.1038/cdd.2009.109 [DOI] [PubMed] [Google Scholar]
- Collado M., Serrano M. (2010). Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 10, 51–57 10.1038/nrc2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M., Gil J., Efeyan A., Guerra C., Schuhmacher A. J., Barradas M., Benguría A., Zaballos A., Flores J. M., Barbacid M. et al. (2005). Tumour biology: senescence in premalignant tumours. Nature 436, 642 10.1038/436642a [DOI] [PubMed] [Google Scholar]
- Coppé J-P., Patil C. K., Rodier F., Sun Y., Muñoz D. P., Goldstein J., Nelson P. S., Desprez P-Y., Campisi J. (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J-P., Desprez P-Y., Krtolica A., Campisi J. (2010). The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F. (2008). Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522 10.1038/nrc2440 [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F., Reaper P. M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N. P., Jackson S. P. (2003). A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 10.1038/nature02118 [DOI] [PubMed] [Google Scholar]
- Damsky W. E., Curley D. P., Santhanakrishnan M., Rosenbaum L. E., Platt J. T., Gould Rothberg B. E., Taketo M. M., Dankort D., Rimm D. L., McMahon M. et al. (2011). β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 20, 741–754 10.1016/j.ccr.2011.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D., Curley D. P., Cartlidge R. A., Nelson B., Karnezis A. N., Damsky W. E. J., Jr, You M. J., DePinho R. A., McMahon M., Bosenberg M. (2009). Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 41, 544–552 10.1038/ng.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas V., Beermann F., Martinozzi S., Carreira S., Ackermann J., Kumasaka M., Denat L., Goodall J., Luciani F., Viros A. et al. (2007). Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 21, 2923–2935 10.1101/gad.450107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko Z. N., Zubova S. G., Bukreeva E. I., Pospelov V. A., Pospelova T. V., Blagosklonny M. V. (2009). Rapamycin decelerates cellular senescence. Cell Cycle 8, 1888–1895 10.4161/cc.8.12.8606 [DOI] [PubMed] [Google Scholar]
- Dhomen N., Reis-Filho J. S., da Rocha Dias S., Hayward R., Savage K., Delmas V., Larue L., Pritchard C., Marais R. (2009). Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15, 294–303 10.1016/j.ccr.2009.02.022 [DOI] [PubMed] [Google Scholar]
- Di Micco R., Fumagalli M., Cicalese A., Piccinin S., Gasparini P., Luise C., Schurra C., Garre' M., Nuciforo P. G., Bensimon A. et al. (2006). Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 10.1038/nature05327 [DOI] [PubMed] [Google Scholar]
- Di Micco R., Sulli G., Dobreva M., Liontos M., Botrugno O. A., Gargiulo G., dal Zuffo R., Matti V., d'Ario G., Montani E. et al. (2011). Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat. Cell Biol. 13, 292–302 10.1038/ncb2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G. P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E. E., Linskens M., Rubelj I., Pereira-Smith O. et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano A., Sánchez-Sendra B., Kondoh H., Lleonart M. E. (2011). MicroRNAs regulate key effector pathways of senescence. J. Aging Res. 2011, 205378 10.4061/2011/205378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M., Rossiello F., Clerici M., Barozzi S., Cittaro D., Kaplunov J. M., Bucci G., Dobreva M., Matti V., Beauséjour C. M. et al. (2012). Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14, 355–365 10.1038/ncb2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama R., Saito M., Tanobe H., Ishikawa F. (2006). Loss of linker histone H1 in cellular senescence. J. Cell Biol. 175, 869–880 10.1083/jcb.200604005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaimo S., d'Adda di Fagagna F. (2012). Is cellular senescence an example of antagonistic pleiotropy? Aging Cell 11, 378–383 10.1111/j.1474-9726.2012.00807.x [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer V. C., Cheong S. C., Chong H., Chow J., Moss T., Abdel-Malek Z. A., Marais R., Wynford-Thomas D., Bennett D. C. (2006). Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br. J. Cancer 95, 496–505 10.1038/sj.bjc.6603283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn W. C., Dessain S. K., Brooks M. W., King J. E., Elenbaas B., Sabatini D. M., DeCaprio J. A., Weinberg R. A. (2002). Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22, 2111–2123 10.1128/MCB.22.7.2111-2123.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2000). The hallmarks of cancer. Cell 100, 57–70 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- Harley C. B. (2008). Telomerase and cancer therapeutics. Nat. Rev. Cancer 8, 167–179 10.1038/nrc2275 [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. (1990). Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 10.1038/345458a0 [DOI] [PubMed] [Google Scholar]
- Hayflick L., Moorhead P. S. (1961). The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- Herbig U., Ferreira M., Condel L., Carey D., Sedivy J. M. (2006). Cellular senescence in aging primates. Science 311, 1257 10.1126/science.1122446 [DOI] [PubMed] [Google Scholar]
- Hewitt G., Jurk D., Marques F. D. M., Correia-Melo C., Hardy T., Gackowska A., Anderson R., Taschuk M., Mann J., Passos J. F. (2012). Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 3, 708 10.1038/ncomms1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Bartolome R., Patel V., Cotrim A., Leelahavanichkul K., Molinolo A. A., Mitchell J. B., Gutkind J. S. (2012). mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 11, 401–414 10.1016/j.stem.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A., Pawlikowski J., Manoharan I., van Tuyn J., Nelson D. M., Rai T. S., Shah P. P., Hewitt G., Korolchuk V. I., Passos J. F. et al. (2013). Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 202, 129–143 10.1016/j.mad.2006.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V., Forkert R., Fleming H. E., Saito Y., Waring M. T., Dombkowski D. M., Cheng T., DePinho R. A., Sharpless N. E., Scadden D. T. (2006). Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443, 421–426 [DOI] [PubMed] [Google Scholar]
- Jeyapalan J. C., Ferreira M., Sedivy J. M., Herbig U. (2007). Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 128, 36–44 10.1016/j.mad.2006.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J-I., Lau L. F. (2010). The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 10.1038/ncb2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T-W., Yevsa T., Woller N., Hoenicke L., Wuestefeld T., Dauch D., Hohmeyer A., Gereke M., Rudalska R., Potapova A. et al. (2011). Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 10.1038/nature10599 [DOI] [PubMed] [Google Scholar]
- Kennedy A. L., Morton J. P., Manoharan I., Nelson D. M., Jamieson N. B., Pawlikowski J. S., McBryan T., Doyle B., McKay C., Oien K. A. et al. (2011). Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol. Cell 42, 36–49 10.1016/j.molcel.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J., Torrice C., Ramsey M. R., Kovalev G. I., Al-Regaiey K., Su L., Sharpless N. E. (2004). Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J., Ramsey M. R., Ligon K. L., Torrice C., Koh A., Bonner-Weir S., Sharpless N. E. (2006). p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 10.1038/nature05092 [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V., Yon M., Dickins R. A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S. W. (2008). Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A., Parrinello S., Lockett S., Desprez P. Y., Campisi J. (2001). Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. USA 98, 12072–12077 10.1073/pnas.211053698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T., Michaloglou C., Vredeveld L. C. W., Douma S., van Doorn R., Desmet C. J., Aarden L. A., Mooi W. J., Peeper D. S. (2008). Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 10.1016/j.cell.2008.03.039 [DOI] [PubMed] [Google Scholar]
- Kuilman T., Michaloglou C., Mooi W. J., Peeper D. S. (2010). The essence of senescence. Genes Dev. 24, 2463–2479 10.1101/gad.1971610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio A., Akkari L., Simon J., Grace D., Tschaharganeh D. F., Bolden J. E., Zhao Z., Thapar V., Joyce J. A., Krizhanovsky V. et al. (2013). Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 10.1016/j.cell.2013.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloglou C., Vredeveld L., Soengas M. S., Denoyelle C., Kuilman T., van der Horst C., Majoor D., Shay J., Mooi W., Peeper D. S. (2005). BRAF(E600)-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 10.1038/nature03890 [DOI] [PubMed] [Google Scholar]
- Minamino T., Komuro I. (2007). Vascular cell senescence: contribution to atherosclerosis. Circ. Res. 100, 15–26 10.1161/01.RES.0000256837.40544.4a [DOI] [PubMed] [Google Scholar]
- Mizushima N. (2007). Autophagy: process and function. Genes Dev. 21, 2861–2873 10.1101/gad.1599207 [DOI] [PubMed] [Google Scholar]
- Molofsky A. V., Slutsky S. G., Joseph N. M., He S., Pardal R., Krishnamurthy J., Sharpless N. E., Morrison S. J. (2006). Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452 10.1038/nature05091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella C., Clohessy J. G., Alimonti A., Pandolfi P. P. (2011). Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer 11, 503–511 10.1038/nrc3057 [DOI] [PubMed] [Google Scholar]
- Narita M., Nu˜nez S., Heard E., Narita M., Lin A. W., Hearn S. A., Spector D. L., Hannon G. J., Lowe S. W. (2003). Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 10.1016/S0092-8674(03)00401-X [DOI] [PubMed] [Google Scholar]
- Narita M., Narita M., Krizhanovsky V., Nuñez S., Chicas A., Hearn S. A., Myers M. P., Lowe S. W. (2006). A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell 126, 503–514 10.1016/j.cell.2006.05.052 [DOI] [PubMed] [Google Scholar]
- Narita M., Young A. R. J., Arakawa S., Samarajiwa S. A., Nakashima T., Yoshida S., Hong S., Berry L. S., Reichelt S., Ferreira M. et al. (2011). Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 10.1126/science.1205407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G., Wordsworth J., Wang C., Jurk D., Lawless C., Martin-Ruiz C., von Zglinicki T. (2012). A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349 10.1111/j.1474-9726.2012.00795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock P. M., Harper U. L., Hansen K. S., Yudt L. M., Stark M., Robbins C. M., Moses T. Y., Hostetter G., Wagner U., Kakareka J. et al. (2003). High frequency of BRAF mutations in nevi. Nat. Genet. 33, 19–20 10.1038/ng1054 [DOI] [PubMed] [Google Scholar]
- Price J. S., Waters J. G., Darrah C., Pennington C., Edwards D. R., Donell S. T., Clark I. M. (2002). The role of chondrocyte senescence in osteoarthritis. Aging Cell 1, 57–65 10.1046/j.1474-9728.2002.00008.x [DOI] [PubMed] [Google Scholar]
- Puyol M., Martín A., Dubus P., Mulero F., Pizcueta P., Khan G., Guerra C., Santamaría D., Barbacid M. (2010). A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18, 63–73 10.1016/j.ccr.2010.05.025 [DOI] [PubMed] [Google Scholar]
- Rodier F., Campisi J. (2011). Four faces of cellular senescence. J. Cell Biol. 192, 547–556 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F., Coppé J. P., Patil C. K., Hoeijmakers W. A. M., Muñoz D. P., Raza S. R., Freund A., Campeau E., Davalos A. R., Campisi J. (2009). Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 11, 973–979 10.1038/ncb1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saretzki G., Von Zglinicki T. (2002). Replicative aging, telomeres, and oxidative stress. Ann. N. Y. Acad. Sci. 959, 24–29 10.1111/j.1749-6632.2002.tb02079.x [DOI] [PubMed] [Google Scholar]
- Schmitt C. A., Fridman J. S., Yang M., Lee S., Baranov E., Hoffman R. M., Lowe S. W. (2002). A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 10.1016/S0092-8674(02)00734-1 [DOI] [PubMed] [Google Scholar]
- Sedelnikova O. A., Horikawa I., Zimonjic D. B., Popescu N. C., Bonner W. M., Barrett J. C. (2004). Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 6, 168–170 [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. (1997). Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 10.1016/S0092-8674(00)81902-9 [DOI] [PubMed] [Google Scholar]
- Shay J. W., Pereira-Smith O. M., Wright W. E. (1991). A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 196, 33–39 10.1016/0014-4827(91)90453-2 [DOI] [PubMed] [Google Scholar]
- Sherr C. J., DePinho R. A. (2000). Cellular senescence: mitotic clock or culture shock? Cell 102, 407–410 10.1016/S0092-8674(00)00046-5 [DOI] [PubMed] [Google Scholar]
- Suram A., Kaplunov J., Patel P. L., Ruan H., Cerutti A., Boccardi V., Fumagalli M., Di Micco R., Mirani N., Gurung R. L. et al. (2012). Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 31, 2839–2851 10.1038/emboj.2012.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H., Smogorzewska A., de Lange T. (2003). DNA damage foci at dysfunctional telomeres. Curr. Biol. 13, 1549–1556 [DOI] [PubMed] [Google Scholar]
- te Poele R. H., Okorokov A. L., Jardine L., Cummings J., Joel S. P. (2002). DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62, 1876–1883 [PubMed] [Google Scholar]
- Tomás-Loba A., Flores I., Fernández-Marcos P. J., Cayuela M. L., Maraver A., Tejera A., Borrás C., Matheu A., Klatt P., Flores J. M. et al. (2008). Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135, 609–622 10.1016/j.cell.2008.09.034 [DOI] [PubMed] [Google Scholar]
- Ventura A., Kirsch D. G., McLaughlin M. E., Tuveson D. A., Grimm J., Lintault L., Newman J., Reczek E. E., Weissleder R., Jacks T. (2007). Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 10.1038/nature05541 [DOI] [PubMed] [Google Scholar]
- Vredeveld L. C. W., Possik P. A., Smit M. A., Meissl K., Michaloglou C., Horlings H. M., Ajouaou A., Kortman P. C., Dankort D., McMahon M. et al. (2012). Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 26, 1055–1069 10.1101/gad.187252.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M., Poortinga G., Stanley K. L., Lindemann R. K., Bots M., Chan C. J., Bywater M. J., Kinross K. M., Astle M. V., Waldeck K. et al. (2013). The mTORC1 inhibitor everolimus prevents and treats Eμ-Myc lymphoma by restoring oncogene-induced senescence. Cancer Discov. 3, 82–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Jurk D., Maddick M., Nelson G., Martin-Ruiz C., von Zglinicki T. (2009). DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8, 311–323 10.1111/j.1474-9726.2009.00481.x [DOI] [PubMed] [Google Scholar]
- Wiemann S. U., Satyanarayana A., Tsahuridu M., Tillmann H. L., Zender L., Klempnauer J., Flemming P., Franco S., Blasco M. A., Manns M. P. et al. (2002). Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 16, 935–942 [DOI] [PubMed] [Google Scholar]
- Williams G. C. (1957). Pleiotrpy, Natural Selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 [DOI] [Google Scholar]
- Wu C-H., van Riggelen J., Yetil A., Fan A. C., Bachireddy P., Felsher D. W. (2007). Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc. Natl. Acad. Sci. USA 104, 13028–13033 10.1073/pnas.0701953104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W., Zender L., Miething C., Dickins R. A., Hernando E., Krizhanovsky V., Cordon-Cardo C., Lowe S. W. (2007). Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 10.1038/nature05529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S., Loo T. M., Atarashi K., Kanda H., Sato S., Oyadomari S., Iwakura Y., Oshima K., Morita H., Hattori M. et al. (2013). Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 10.1038/nature12347 [DOI] [PubMed] [Google Scholar]
- Young A. R. J., Narita M., Ferreira M., Kirschner K., Sadaie M., Darot J. F. J., Tavaré S., Arakawa S., Shimizu S., Watt F. M. et al. (2009). Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 10.1101/gad.519709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Poustovoitov M. V., Ye X., Santos H. A., Chen W., Daganzo S. M., Erzberger J. P., Serebriiskii I. G., Canutescu A. A., Dunbrack R. L. et al. (2005). Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 10.1016/j.devcel.2004.10.019 [DOI] [PubMed] [Google Scholar]
- Zhang R., Chen W., Adams P. D. (2007). Molecular dissection of formation of senescence-associated heterochromatin foci. Mol. Cell. Biol. 27, 2343–2358 10.1128/MCB.02019-06 [DOI] [PMC free article] [PubMed] [Google Scholar]