Summary
The reduction in breast cancer risk attributed to early-age pregnancy is mediated in part by changes in the mammary epithelium. Here, we address the role of the mammary stroma in this protection. Utilizing tumor cells capable of transitioning from indolent to proliferative or invasive states, we demonstrate that mammary extracellular matrix (ECM) from parous rats (parous matrix) decreases tumor growth and impedes cellular phenotypes associated with tumor cell invasion compared with that observed using nulliparous matrix. Proteomic analysis identifies an increased abundance of collagen I in parous matrix, an observation extended to breast tissue of parous women. Given the pro-tumorigenic attributes of fibrillar collagen, these results were unexpected. Second-harmonic generation imaging and atomic force microscopy revealed that the abundant collagen observed in the mammary glands of parous rats is less linearized and associated with a decrease in stromal stiffness, implicating collagen organization and stiffness in parity-induced protection. Using 3D cell culture models, we demonstrate that linearized (fibrillar) collagen I induces cellular phenotypes consistent with an invasive behavior in mammary tumor cells and alters the subcellular distribution of β1 integrin. Conversely, high-density non-fibrillar collagen I induces tumor-suppressive attributes, including increases in junctional E-cadherin in tumor cells, upregulation of genes encoding components of cell–cell junctions, and downregulation of mesenchymal-specific and metalloproteinase-encoding genes. These data show that collagen organization, rather than density alone, is a key contributor to the invasive phenotype. Furthermore, our data show that parity alters the composition and organization of mammary ECM, particularly fibrillar collagen, in a manner consistent with tumor suppression.
Key words: E-cadherin, Collagen organization, Integrin signaling, Tumor-suppressive extracellular matrix, ECM
Introduction
A striking epidemiological characteristic of human breast cancer is the inverse relationship between early-age first full-term pregnancy and a woman's lifetime risk of developing breast cancer (MacMahon et al., 1970; Reinier et al., 2007; Schonfeld et al., 2011). The reduction in risk associated with early-age pregnancy has been termed the ‘protective effect of pregnancy’. In support of these epidemiologic observations, tumor incidence is reduced in rodents when carcinogens are administrated following pregnancy or after exposure to pregnancy-levels of estrogen (Medina and Smith, 1999; Russo et al., 2005; Sinha et al., 1988). However, mammary epithelial morphology of nulliparous rodents is very similar to parous rodents (Sinha et al., 1988). One explanation for how full-term pregnancy confers protection is that pregnancy gives rise to a distinct population of differentiated progenitor epithelial cells that have reduced susceptibility to transformation (Russo et al., 2005; Wagner et al., 2002). Another explanation for this protection is a systemic effect caused by the decrease in circulating growth hormone in parous rodents compared with nulliparous rodents, which may result in suppression of tumor-initiating cells (Dearth et al., 2010). In addition, the protein composition of mammary extracellular matrix (ECM) changes with pregnancy, thus, ECM has been suggested as a potential mediator of pregnancy-induced protection (Schedin et al., 2004).
ECM contributes to mammary tumorigenesis and has pleiotropic effects on cellular programs including adhesion, polarity, proliferation, survival, invasion and differentiation (Lu et al., 2012; Schedin and Keely, 2011). A prominent component of mammary ECM is fibrillar collagen (Maller et al., 2010). High fibrillar collagen density correlates with increased mammographic density in women, which augments breast cancer risk by ∼4 fold (Li et al., 2005; Reinier et al., 2007). In addition to collagen abundance, fibrillar collagen organization and stiffness are emerging as key mediators of mammary tumor cell growth and invasion (Levental et al., 2009; Lyons et al., 2011; Provenzano et al., 2008). Furthermore, relevance to breast cancer patients has been demonstrated by a recent study where radial collagen fiber orientation at the tumor border independently predicted poor outcomes (Conklin et al., 2011).
To evaluate whether mammary ECM contributes to parity-induced tumor suppression, we investigated function, composition and spatial organization of mammary ECM in nulliparous and parous rats. Functionally, ECM of parous rodents decreases tumor growth in vivo and supports adherens junction formation and a rounded and compact cell morphology in vitro, which are phenotypes consistent with tumor suppression. Our proteomic studies show fibrillar collagen content is increased in mammary glands of parous rats, which is paradoxical given the documented tumor-promoting roles of fibrillar collagen (Paszek et al., 2005; Provenzano et al., 2009; Provenzano et al., 2008). However, our data suggest that organization of fibrillar collagen, and not density, is a primary determinant of whether mammary ECM suppresses or activates cellular programs associated with tumor cell invasion. Understanding how parity influences mammary ECM composition and collagen organization might uncover novel mechanisms of breast cancer latency as well as new approaches for prevention.
Results
Parous host and mammary ECM of parous rats have tumor suppressive attributes
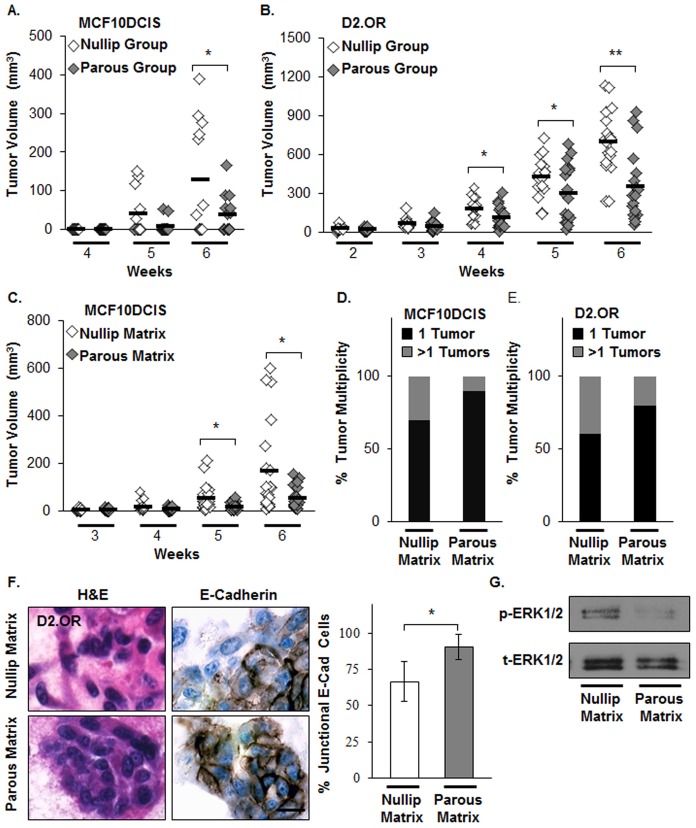
Earlier experimental work has revealed that mammary glands from parous mice and rats were less susceptible to chemically induced tumor formation compared with those from nulliparous rodents (Medina and Smith, 1999; Russo and Russo, 1980; Sinha et al., 1988). To address whether the parous host presents tumor cells with a suppressive environment, human breast cancer MCF10DCIS cells or murine mammary tumor D2.OR cells were injected into the mammary fat pads of nulliparous and parous murine hosts, and tumor growth monitored. Both cell lines were selected because their invasiveness and metastatic capabilities can be influenced by tissue microenvironments (Barkan et al., 2010; Lyons et al., 2011). For both cell lines, tumor volumes were reduced in mammary glands of parous hosts (Fig. 1A,B). To assess the role of the ECM in this suppression, mammary ECM was isolated from age-matched nulliparous (nulliparous matrix) and parous (parous matrix) rats and utilized as a tumor cell substratum for in vitro and in vivo assays. To ensure matrix performance, we confirmed that both nulliparous and parous matrix supported cell viability and branching organization of non-transformed human mammary epithelial MCF12A cells when overlaid onto these respective matrices (supplementary material Fig. S1A,B). We next addressed the influence of these matrices on tumor cells. MCF10DCIS or D2.OR cells were mixed with 20 µl of nulliparous or parous matrix and injected into the mammary fat pads of nulliparous mice. Mice co-injected with MCF10DCIS cells and parous matrix had a significant reduction in tumor growth and multiplicity relative to mice in the nulliparous matrix group (Fig. 1C,D). Mice co-injected with D2.OR cells and parous matrix also had reduced tumor multiplicity compared with those in the nulliparous matrix group (Fig. 1E); however, differences in D2.OR tumor growth between groups were not observed. Previously, we have shown that decreased mammary tumor multiplicity in an orthotropic xenograft model correlates with decreased tumor cell dispersion, suggesting inhibition of invasion (Lyons et al., 2011). Thus, these in vivo data suggest that parous matrix suppresses tumor cell growth and/or the invasive phenotype.
Fig. 1.
Mammary microenvironment from parous rodents reduces tumor growth and phenotypes associated with tumor cell invasion. (A,B) Human breast cancer MCF10DCIS cells or murine mammary tumor D2.OR cells injected into the mammary fat pads of parous SCID mice display reduced tumor growth compared with nulliparous hosts. n = 6–7 per group for MCF10DCIS and n = 9–10 per group for D2.OR. *P<0.025, **P<0.0005, unpaired Student's t-test. (C,D) MCF10DCIS cells and (E) D2.OR cells co-injected with nulliparous or parous matrix into mammary fat pads of nulliparous SCID mice; (C) tumor growth, and (D,E) tumor multiplicity. n = 10 per group for each cell line, *P<0.01, unpaired Student's t-test. (F) H&E and E-cadherin staining images of D2.OR cells grown in nulliparous or parous matrix in 3D cell culture. Scale bar: 20 µm. The graph (right panel) displays the ratio of cells with junctional E-cadherin to total number of cells. n = 3 wells per condition, *P<0.002, unpaired Student's t-test. (G) The amount of phosphorylated ERK1/2 (p-ERK1/2) levels (top) and total ERK1/2 (t-ERK1/2) (bottom) were evaluated in cells from F by immunoblotting (IB). Model 1 was used to perform the 3D cell culture experiments described in F,G.
Mammary ECM from parous hosts reduces tumorigenic behaviors in vitro
Next, we utilized D2.OR cells in three-dimensional (3D) cell culture to examine whether parous matrix can suppress in vitro cellular phenotypes associated with invasion. We focused these analyses on the D2.OR cells because these cells are quiescent on Matrigel (Barkan et al., 2008; Shibue and Weinberg, 2009), yet become proliferative on fibrillar collagen I (Barkan et al., 2010), potentially providing a robust readout for functional differences between nulliparous and parous matrices. D2.OR cells cultured within parous matrix formed more-compact and less-stellate multicellular structures compared with those cultured within nulliparous matrix (Fig. 1F, left panel). Consistent with this compact morphology, the level of junctional E-cadherin was increased (Fig. 1F, left and right panels) and extracellular-signal-regulated kinase 1/2 (ERK1/2) phosphorylation decreased in comparison with cells cultured in nulliparous matrix (Fig. 1G). These data show that parous matrix can normalize tumor cell junctional complexes, reduce elongated cell morphology, and suppress ERK1/2 signaling, a proliferation-related pathway (Onder et al., 2008). One prediction that arises from these in vitro data is that mammary tumors that develop within the parous host (Fig. 1A,B) or in the presence of parous matrix (Fig. 1C) would display increased junctional E-cadherin staining. However, at the study end, junctional E-cadherin staining was absent in all tumor groups, suggesting that with progression, all tumor groups lose E-cadherin at cell junctions (data not shown).
Collagen I is a potential mediator of suppression induced by parity
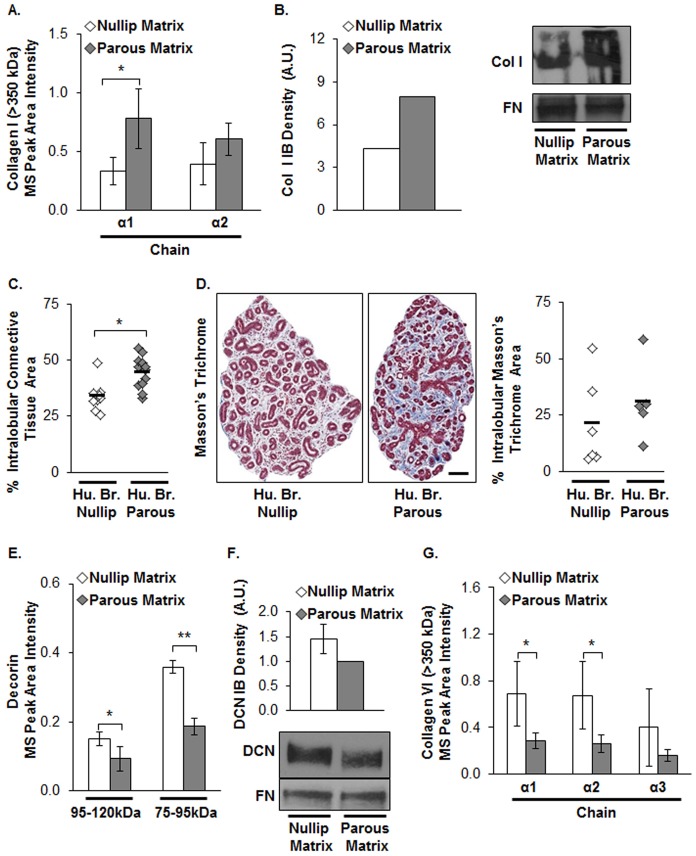
To identify compositional changes between nulliparous and parous matrix that could account for the observed functional differences, liquid chromatography tandem mass spectrometry (LC-MS/MS)-based proteomics and label-free quantitative analyses were performed. Initially, relative abundances of ECM proteins were measured from MS spectra via two different label-free quantitative approaches; average total ion current (TIC) and spectral counting. Using these quantitative approaches, we found that nulliparous and parous matrices had very similar proteomes (supplementary material Tables S1,S2). To increase the sensitivity of the proteomic analyses, in-gel tryptic digestion was performed to extract LC-MS/MS data from gel bands with specific molecular masses. Next, a label-free quantitative approach was utilized whereby peptide peak area intensities are measured, as this method more accurately assesses protein ratios between groups (Old et al., 2005). We assessed the peptide peak intensity measurements at specific molecular masses to examine relative abundances of ECM proteins and found high-molecular-mass collagen I α1 and α2 chains were increased ∼2 fold in parous relative to nulliparous matrix (Fig. 2A). These results were validated by immunoblot (IB) analysis (Fig. 2B), with collagen data normalized to fibronectin (FN) levels, because FN has previously been demonstrated to not differ between nulliparous and parous matrix (Schedin et al., 2004). Increased high-molecular-mass collagen I in the parous matrix was unanticipated owing to the established tumor-promoting roles of fibrillar collagens (Levental et al., 2009; Provenzano et al., 2008) and the documented tumor-suppressive role of parity. We also observed increased intralobular connective tissue (Fig. 2C) and fibrillar collagen (Fig. 2D) in breast tissues from premenopausal parous compared with that in nulliparous women. These data demonstrate that parity increases fibrillar collagen density in the mammary stroma of two different species and raise the possibility that parity results in a fibrillar collagen that is tumor suppressive rather than promoting.
Fig. 2.
Evidence for parity-induced changes in fibrillar collagen in rat mammary glands and human breast tissues. (A) Collagen I (Col I) α1 and α2 chain abundances measured in mammary matrices from nulliparous or parous rats by MS-based proteomics and label-free quantitative analyses. n = 3–4 analyses per group, *P<0.05, unpaired Student's t-test, and for across collagen I chains; P<0.05 across groups by ANOVA. (B) Collagen I and FN (as loading control) were evaluated by immunoblotting (IB). The graph is the densitometric analysis of the collagen I immunoblot (right panel). (C) Quantification of intralobular connective tissue using H&E images and (D) fibrillar collagen stain using Masson's trichrome in type 3 lobules of human breast tissues (Hu. Br.) from nulliparous or parous women, normalized for lobular area. n = 9–12 breast tissues for H&E and five or six for trichrome stain. *P<0.001, unpaired Student's t-test. Representative images are shown. Scale bar: 100 µm. (E) Decorin (DCN) abundance and mean values evaluated as described in A. *P<0.05, **P<0.0001, unpaired Student's t-test; P<0.05 across groups by ANOVA. (F) DCN (75–100 kDa) and FN (250 kDa) assessed by IB. Graph shows densitometric analysis of the decorin IB. Parous matrix values were normalized to 1 and paired with nulliparous matrix. n = 4. (G) Collagen VI α1, α2, and α3 chain abundances and mean values evaluated as described in A. For statistical relationships in individual chains, *P<0.05, unpaired Student's t-test; P<0.05 for across collagen VI chains, ANOVA.
Collagen organization is altered in mammary stroma between nulliparous and parous rats
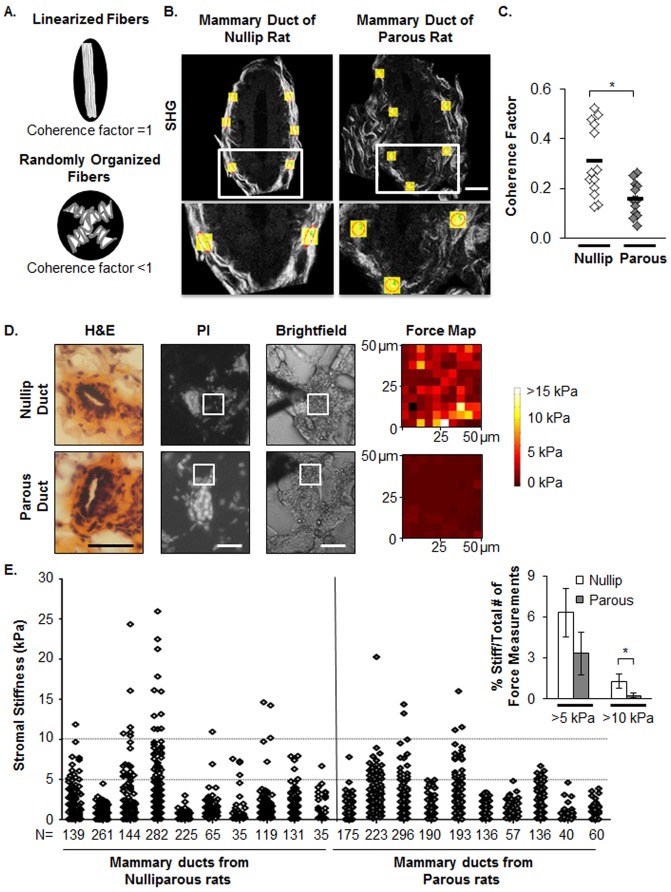
To assess for potential changes in collagen organization with parity status, we examined the ECM proteomes for differences in proteins associated with the formation of high order collagen structure. Interestingly, ECM proteins that participate in collagen fiber assembly, specifically decorin, collagen XIV α1 chain, and dermatopontin (Ansorge et al., 2009; Danielson et al., 1997; Takeda et al., 2002) were decreased in parous relative to nulliparous matrix (Fig. 2E and supplementary material Fig. S2A,B, respectively). The decrease in decorin protein in parous matrix was confirmed by IB (Fig. 2F). Furthermore, trimeric collagen VI, an ECM protein that is deposited in lung fibrosis (Specks et al., 1995) and promotes mammary tumor progression in a PyMT breast cancer model (Iyengar et al., 2005), was significantly reduced in parous compared with nulliparous matrix (Fig. 2G). Overall, these proteomic data raise the possibility that in addition to changes in fibrillar collagen abundance, there are differences in collagen organization between mammary glands from nulliparous and parous rats. To directly evaluate mammary collagen organization in vivo, fiber linearization was measured using second-harmonic generation (SHG) imaging. As shown in the diagram, non-linearized collagen has low fiber directionality (Fig. 3A) (Provenzano et al., 2008). In size-matched terminal mammary ducts, we found that glands from parous rats display higher abundance of randomly aligned collagen fibers and lower coherence factor values relative to ducts from the nulliparous group (Fig. 3B,C). Moreover, atomic force microscopy (AFM) indicates a decrease in mammary stromal stiffness in the parous group, as the number of highly stiff areas as detected by Young's modulus values of >5 kPa and >10 kPa (Fig. 3D,E) was significantly reduced in the parous group. Others have demonstrated that reduced tissue stiffness and decreased fibrillar collagen linearization are associated with tumor suppression in mouse models (Levental et al., 2009; Provenzano et al., 2008), providing a plausible explanation for how the high collagen density observed in mammary glands from parous hosts could be associated with a tumor-suppressive microenvironment.
Fig. 3.
Parity status associated with alterations in mammary fibrillar collagen organization and stromal stiffness. (A) Diagram describing the relationship between collagen fibers and coherence. (B) Second-harmonic generation (SHG) imaging was used to visualize fibrillar collagens adjacent to size-matched mammary ducts. Coherence factors were measured in selected areas and are shown as yellow boxes. Red circles depict isotropic and red ovals depict anisotropic fiber directionality. Scale bar: 15 µm. (C) Coherence factors were quantified via ImageJ with the OrientationJ plug in. n = 13 size-matched mammary terminal ducts from seven nulliparous and seven parous female rats. *P<0.002 unpaired Student's t-test. (D) Left panels are H&E staining, left middle panels are propidium iodide (PI) fluorescence images, right middle panels are brightfield images, and right panels are AFM force maps (50 µm×50 µm areas). White squares delineate the selected areas for force measurements. Scale bars: 50 µm. (E) The graph represents all force measurements (stromal stiffness) that were taken for each group. Each column represents a mammary gland and N indicates the number of force measurements per gland. Stromal areas were selected adjacent to terminal ducts (1–3 ducts per gland) from a total of ten rat mammary glands (1 gland per rat) per group. These data were collected from two independent rat studies. The inset on the top right represents the mean±s.e.m. percentage of stiff (above 5 kPa or 10 kPa) to total force measurements per gland. *P<0.05 unpaired Student's t-test.
Functions of collagen I density uncoupled from collagen I organization
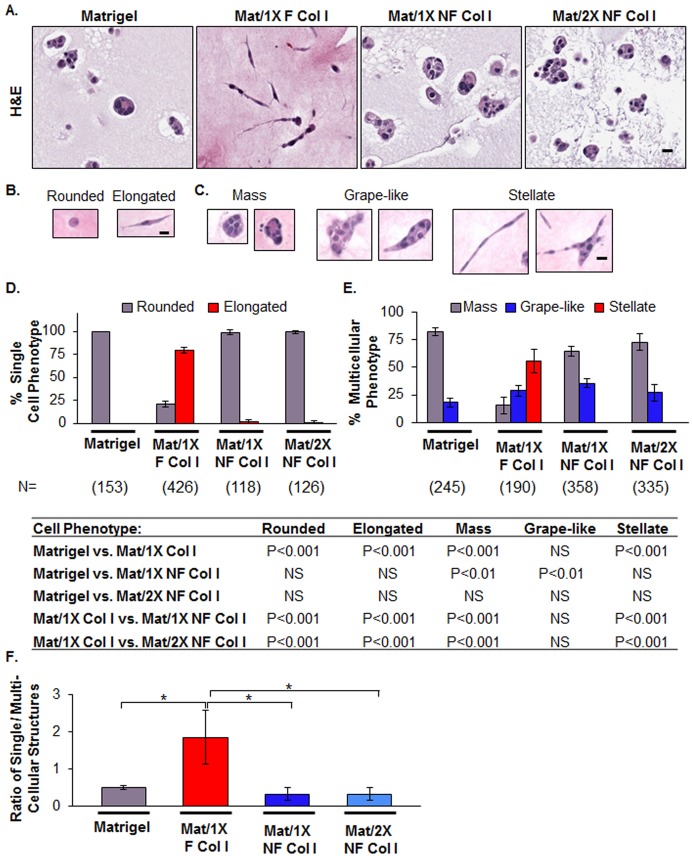
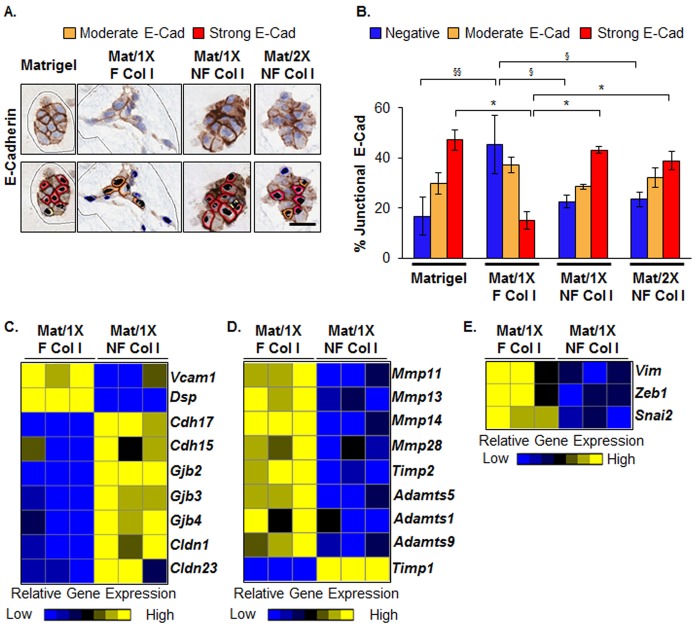
In order to distinguish the effects of fibrillar collagen organization from density, we disrupted collagen I organization and evaluated the functional consequences in 3D cell culture. To this end, we identified moderate sonication conditions that shifted the 250 kDa collagen I band to ∼140 kDa (supplementary material Fig. S3A) and which failed to generate an SHG signal, indicative of fiber disruption (supplementary material Fig. S3B). We refer to this moderately sonicated collagen I as non-fibrillar (NF). D2.OR cells were embedded in 4 mg/ml Matrigel alone or a mixture of 4 mg/ml Matrigel and 4 mg/ml collagen I and labeled (1X) for a final concentration of 1.6 mg/ml fibrillar (F) collagen I (Mat/1X F Col I) or NF collagen I (Mat/1X NF Col I), and (2X) for 3.2 mg/ml NF collagen I (Mat/2X NF Col I). Mammary tumor D2.OR cells cultured within fibrillar collagen I formed elongated structures with extensive protrusions, whereas cells within the Matrigel and Mat/1X NF Col I conditions did not (Fig. 4A) after 6 days in culture. A correlation between cell protrusions and the speed of cell migration in 3D cell culture has been demonstrated previously (Fraley et al., 2010). Hence, the cellular structures formed in fibrillar collagen I are consistent with a migratory or invasive phenotype (Fraley et al., 2010; Park et al., 2006). To quantify the morphological changes on different matrices, single round cells without protrusions were defined as having rounded morphology, and single cells with filopodia-like protrusions as having elongated cell morphology (Fig. 4B). Furthermore, the morphology of multicellular structures was grouped into three categories: compact spheroids (mass), mildly elongated spheroids without cellular protrusions (grape-like), and highly elongated spheroids with extensive filopodia-like protrusions (stellate) (Fig. 4C), as previously described (Kenny et al., 2007). The dominant morphology observed in Mat/1X F Col I was consistent with an elongated-stellate morphology (Fig. 4D,E), whereas D2.OR cells in Matrigel as well as in Mat/1X NF Col I overwhelmingly formed rounded-mass structures (Fig. 4D,E). Importantly, Mat/2X NF Col I did not support elongated-stellate morphology (Fig. 4D,E). We confirmed that the morphological changes in the Mat/1X NF Col I condition are due to disruption of collagen fiber organization, rather than lack of NF collagen I incorporation into Matrigel, by identifying specific gene signatures for Matrigel and Mat/1X NF Col I conditions (supplementary material Fig. S4 and Table S3). These data demonstrate that increasing collagen I density does not compensate for the disruption of its organization. In addition, a threefold decrease in the ratio of single cells to multicellular structures was observed in the Mat/1X NF Col I compared with that in the Mat/1X F Col I condition, providing additional evidence that NF collagen supports formation of rounded and compact multicellular structures (Fig. 4F).
Fig. 4.
Disruption of fibrillar collagen I organization reverts tumor cell morphology. (A) H&E images of D2.OR cells cultured in 3D Matrigel, Matrigel and fibrillar collagen I (Mat/1X F Col I), Matrigel and non-fibrillar collagen I (1X or 2X NF Col I). Representative images are shown. Scale bar: 20 µm. (B,C) Morphological classifications were determined on the basis of H&E images. Scale bars: 20 µm. (D,E) Quantitative analyses of cell morphology were performed on four wells per condition. The table shows the statistical relationships using ANOVA with Bonferroni multiple comparisons post test. NS, not significant. (F) Evaluation of the ratio of single cell to multicellular structures for each condition described in A. *P<0.01, **P<0.001, ANOVA with Bonferroni multiple comparisons post test. Model 2 was used to perform the 3D cell culture experiments in this figure.
Consistent with the decrease in elongated-stellate morphology observed in D2.OR cells cultured in Mat/1X NF Col I, the cells also had a quantifiable increase in junctional E-cadherin staining (Fig. 5A,B). Transcriptome microarray analysis revealed that several gene categories known to be involved in cell–cell junctions were upregulated in D2.OR cells grown in Mat/1X NF Col I relative to those in Mat/1X F Col I, including genes associated with gap, tight and adherens junctions (Fig. 5C). Further, gene expression of the pro-metastatic vascular cell adhesion molecule 1 (Vcam1) (Lu et al., 2011) and two distinct groups of metalloproteinase genes were downregulated in D2.OR cells isolated from Mat/1X NF Col I (Fig. 5C,D). Moreover, tissue inhibitor of metalloproteinases 1 (Timp1) gene expression was upregulated in D2.OR cells grown in Mat/1X NF Col I (Fig. 5D). Also downregulated in Mat/1X NF Col I relative to Mat/1X F Col I were expression levels of the mesenchymal genes vimentin (Vim), Zinc finger E-box-binding homeobox 1 (Zeb1) and snail homolog 2 (Snai2) (Fig. 5E). Transcriptome microarray data were validated for selected genes with RT-PCR (supplementary material Fig. S5). These data further demonstrate that cellular programs associated with invasive properties of tumor cells are suppressed at the transcriptional levels in Mat/1X NF Col I conditions relative to the Mat/1X F Col I conditions.
Fig. 5.
Collagen I organization mediates tumor cell–cell junctions, metalloproteinase and mesenchymal gene expression. D2.OR cells were cultured in Matrigel, Mat/1X F Col I or Mat/1X NF or Mat/2X NF Col I. (A) Representative immunohistochemistry images of E-cadherin staining. The bottom panel illustrates Aperio-software-generated intensities of membranous E-cadherin staining; orange (moderate intensity) to red (high intensity). Scale bar: 25 µm (B) Quantitative analysis of junctional E-cadherin intensity. n = 4 per condition. §P<0.01, §§,*P<0.001, ANOVA with Bonferroni multiple comparisons post test. (C–E) Heat maps of genes associated with cell–cell junctions, metalloproteinases and mesenchymal markers, respectively, from D2.OR cells isolated from Mat/1X F Col I or Mat/1X NF Col I. Yellow indicates upregulation and blue downregulation of gene expression. n = 3 per condition. Model 2 was used to perform the 3D cell culture experiments described in this figure.
It is possible that sonication of collagen causes the release of a soluble ‘anti-invasion factor’. To address this possibility, D2.OR cells were mixed with 200 µg/ml fibrillar or NF collagen I prior to plating onto a Mat/1X F Col I matrix pad (supplementary material Fig. S3C). Under both of these conditions, cells acquired an elongated-stellate morphology (supplementary material Fig. S3C). In addition, culturing D2.OR cells in Mat/1X denatured Col I (gelatin) did not result in stellate structure formation (supplementary material Fig. S6). Collectively, these data identify collagen I organization as a main contributor of tumor cell morphology in vitro.
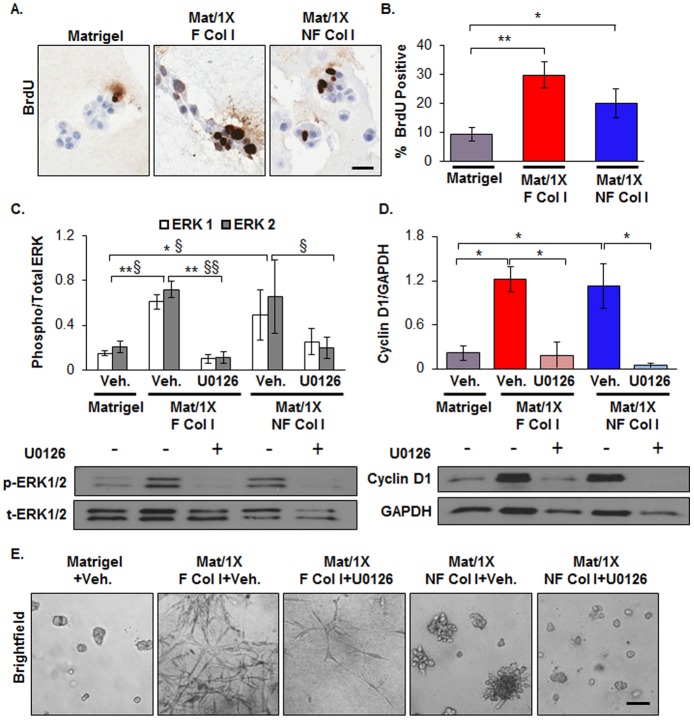
Collagen I density induces ERK1/2-mediated proliferation
Although collagen I organization had profound effects on cell morphology, our data indicate that disrupting its organization does not significantly affect tumor cell proliferation. Specifically, D2.OR cell proliferation, as measured by a BrdU incorporation assay, was increased 3- and 2-fold under Mat/1X F Col I and Mat/1X NF Col I conditions, respectively, compared with cells in Matrigel (Fig. 6A,B). Consistent with collagen I promoting proliferation, when the ERK1/2-mediated growth signaling pathway was examined, ERK1/2 phosphorylation was significantly increased with both Mat/1X F Col I and Mat/1X NF Col I compared with cells in Matrigel (Fig. 6C). Next, we blocked mitogen-activated protein kinase (MAPK) kinase (MEK1/2), which is upstream of ERK1/2, using the U0126 inhibitor, to decrease collagen-I-induced ERK1/2 phosphorylation (Fig. 6C). This blockade diminished protein expression of the proliferation marker cyclin D1 (Fig. 6D) and reduced cell growth (Fig. 6E; supplementary material Fig. S7). However, elongated cell morphology was not visibly influenced by blocking ERK1/2, suggesting that the elongated-stellate morphology induced by fibrillar collagen I is independent of ERK1/2 activity (Fig. 6E, compare second and third panels).
Fig. 6.
Collagen I promotes ERK1/2-mediated proliferation independent of its organization. (A) Representative immunohistochemistry images of BrdU staining as a readout of cell proliferation. (B) Quantitative analysis of BrdU staining in A. n = 4 wells per condition, *P<0.05, **P<0.001, ANOVA with Bonferroni multiple comparisons post test. (C–E) D2.OR cells were treated with vehicle (Veh.) or MEK1/2 inhibitor (U0126) and cultured in Matrigel, Mat/1X F Col I or Mat/1X NF Col I for 6 days. (C) Phosphorylated ERK1/2 levels (p-ERK1/2) and total ERK1/2 (t-ERK1/2) were evaluated by immunoblotting (lower panel). The graph in the upper panel shows the ratio of p-ERK1/2 to t-ERK1. n = 3 wells per condition, statistical values for ERK1, *P<0.05, **P<0.01, for ERK2 §P<0.05, §§P<0.01. (D) Cyclin D1 levels determined in conditions described in above by immunoblotting (lower panel) and plotted after normalization to the level of GAPDH (upper panel). n = 3 wells per condition. *P<0.001, ANOVA with Bonferroni multiple comparisons post test. (E) Brightfield images of cells cultured as described above. Model 2 was used to perform the 3D cell culture experiments in this figure. Scale bars: 25 µm.
Cell morphology and proliferation are mediated via β1 integrin
β1 integrin is a partner in several integrin receptors that bind fibrillar collagens, and mammary tumor cells can depend on ECM–β1-integrin interactions to induce cell proliferation and invasion (Paszek et al., 2005; Provenzano et al., 2009). We tested whether β1 integrin mediates the distinct cellular phenotypes observed in Mat/1X F Col I and Mat/1X NF Col I conditions by performing blocking experiments with antibody against β1 integrin. Blocking β1 integrin resulted in decreased cyclin D1 and ERK1/2 phosphorylation in Mat/1X Col I conditions (supplementary material Fig. S8A,B,and data not shown, respectively), and support a previous report demonstrating that ERK1/2-dependent proliferation is downstream of β1 integrin when D2.OR cells are grown in collagen I (Barkan et al., 2010). In addition, blocking β1 integrin also impeded the elongated-stellate morphology induced by Mat/1X F Col I (supplementary material Fig. S8A,C). We also confirmed that blocking β1 integrin caused reversion of elongated-stellate morphology in human breast cancer MCF10DCIS cells (supplementary material Fig. S9A,B). These results indicate that cellular elongation induced by fibrillar collagen I is dependent on β1 integrin, but not ERK1/2, and are consistent with β1 integrin influencing tumor cell morphology and proliferation through distinct signaling pathways.
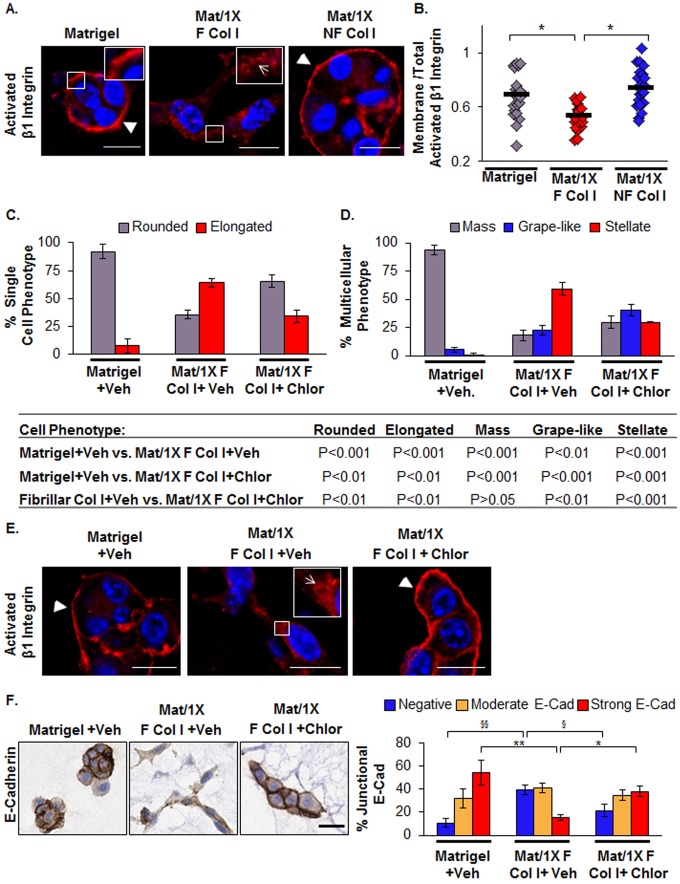
Collagen I organization mediates β1 integrin subcellular distribution
We next examined whether differences in β1 integrin downregulation account for the compact-non-stellate cell morphology observed under NF Col I conditions. However, no significant differences were found in total β1 integrin levels in D2.OR cells cultured in the two distinct collagen conditions (supplementary material Fig. S10A). We then explored whether collagen I organization could alter the distribution of activated β1 integrin, as others have shown that β1 subcellular distribution influences lung and ovarian cancer cell migration in vitro (Caswell et al., 2008; Muller et al., 2009). In 3D cell culture, we assessed changes in the subcellular distribution of β1 integrin by determining the ratio between activated β1 integrin at the plasma membrane to the total cellular signal. We observed a 28% decrease in activated β1 integrin at the plasma membrane in Mat/1X F Col I condition compared with the Mat/1X NF Col I condition (Fig. 7A,B). We also evaluated activated β1 integrin staining in MCF10DCIS cells cultured in Mat/1X F Col I and observed a decrease in staining at the cell membrane concomitant with the appearance of elongated-stellate morphology (supplementary material Fig. S9). We further evaluated the relationship between β1 integrin subcellular distribution and cell morphology in D2.OR cells cultured in Mat/1X F Col I by using chlorpromazine, a clathrin-dependent endocytotic inhibitor (Wang et al., 1993). Chlorpromazine treatment of D2.OR cells cultured in Mat/1X F Col I was associated with phenotypic reversion from highly elongated-stellate to rounded-mass-like structures (Fig. 7C,D; supplementary material Fig. S11). This reversion in morphology correlated with re-localization of β1 integrin to the plasma membrane (Fig. 7E), although similar total β1 integrin levels were maintained across vehicle and treatment groups (supplementary material Fig. S10B). Strikingly, increased localization of activated β1 integrin to the plasma membrane was associated with re-establishment of E-cadherin at the cell–cell junction (Fig. 7F). Collectively, these data show causal relations between changes in β1 integrin subcellular distribution, loss of E-cadherin at cell–cell junctions and acquisition of elongated-stellate morphology.
Fig. 7.
Fibrillar collagen I promotes alterations in the β1 integrin subcellular distribution. (A) Representative immunofluorescence images of activated β1 integrin in D2.OR cells cultured in Matrigel, Mat/1X F Col I or Mat/1X NF Col I. Arrowheads and the arrow point to activated β1 integrin at the plasma membrane or cytoplasm, respectively. Activated β1 integrin is stained red, nuclei are stained blue (Hoechst stain). (B) Quantification of the ratio of activated β1 integrin at plasma membrane to total activated β1 integrin in the cells from immunofluorescence images. n = 21–22 images (total of 37–45 structures) per group taken from three wells per condition. *P<0.01, ANOVA with Bonferroni multiple comparisons post test. (C,D) Morphological quantitative analyses of single cell and multicellular structures for D2.OR cells treated with vehicle or chlorpromazine and cultured in Matrigel or Mat/1X F Col I. Morphological classifications are as shown in Fig. 4B,C. Each group includes three wells per condition. Table shows the statistical relationship using ANOVA with Bonferroni multiple comparisons post test. (E) Immunofluorescence images of activated β1 integrin in D2.OR cells treated with vehicle (Veh) or chlorpromazine (Chlor), a clathrin-dependent endocytotic inhibitor. (F) Left panel: immunohistochemistry images of E-cadherin staining in D2.OR cells treated with vehicle (Veh) or chlorpromazine (Chlor). Colors are described in C. Right panel: quantification of junctional E-cadherin intensity. n = 3 wells per condition, *P<0.05, **P<0.001, §P<0.01, §§P<0.001 by ANOVA with Bonferroni multiple comparisons post test. Model 2 was used to perform the 3D cell culture experiments described in this figure. Scale bars: 25 µm.
Discussion
Mammary epithelial cells in parous rodents are more resistant to tumorigenesis than those in the nulliparous rodents (Medina and Smith, 1999; Sinha et al., 1988). Epithelial-specific changes probably contribute directly to this reduced tumor susceptibility, as decreased mammary epithelial cell proliferation in the parous rodent has been demonstrated, and elevated expression of epithelial-specific differentiation markers have been reported in breast tissues from parous women (Russo et al., 2008; Russo et al., 2005). However, parous hosts demonstrate decreased tumor incidence in transplantation experiments, implicating non-epithelial changes as well (Abrams et al., 1998). Considering ECM and epithelial cells form the functional unit of the mammary gland (Bissell and Barcellos-Hoff, 1987), parity-induced changes in mammary stroma are anticipated. We previously demonstrated decreased branching formation when normal mammary epithelial cells are cultured on parous matrix in vitro (Schedin et al., 2004). Here, we demonstrate for the first time that parous matrix suppresses tumor cell growth and cellular phenotypes associated with tumor cell invasion, and we suggest a mechanistic role for fibrillar collagen organization in this suppression.
Proteomic analysis of mammary ECM isolated from parous rats revealed increased fibrillar collagen I, which is consistent with our previous picro-sirus staining results (O'Brien et al., 2010b). Our current work establishes relevance in human tissues, as we observed increased intralobular collagen deposition in breast tissues of premenopausal parous relative to that in nulliparous women. Collectively, our data indicate that increased collagen density is a primary stromal alteration associated with parity. This observation was unexpected, as collagen density is associated with breast cancer promotion (Paszek et al., 2005; Provenzano et al., 2009; Provenzano et al., 2008). For example, fibrillar collagen accumulation increased tumor formation and metastasis in a transgenic mammary tumor model (Provenzano et al., 2008), which is consistent with collagen density contributing to increased breast cancer risk in women with dense breasts (Li et al., 2005). In addition, increasing collagen I density in vitro supports tumor cell proliferation and formation of non-polarized disorganized multicellular structures through a β1-integrin–ERK1/2 signaling axis (Paszek et al., 2005; Provenzano et al., 2009). However, in a previous study, we observed elevated fibrillar collagen in mammary glands of rats treated with the chemopreventive agent tamoxifen. Furthermore, mammary ECM isolated from tamoxifen-treated rats reduced breast cancer cell growth in vivo and motility in vitro (Hattar et al., 2009). Together, our previous and current studies demonstrate that, contrary to existing premise, dense fibrillar collagen can associate with tumor-suppressive mammary microenvironments.
We obtained insight into how dense fibrillar collagen from mammary glands of parous rats could impart tumor suppression by evaluating collagen fiber organization and stromal stiffness via SHG and AFM analyses. In mammary tissue from parous rats, collagen fiber linearization and stromal stiffness were reduced in comparison with the nulliparous group. Linearized collagen has been observed adjacent to mammary tumors with high expression of lysyl oxidase (LOX), a collagen crosslinking enzyme, and LOX activity is associated with stiffer mammary glands and higher tumor burden and grade (Levental et al., 2009). Additionally, tumor-associated collagen organization, defined by radially aligned collagen fibers, has been demonstrated to correlate with tumor invasion in a mouse model (Provenzano et al., 2008), and poor prognosis in breast cancer patients (Conklin et al., 2011). Thus, decreased collagen fiber linearization and stiffness adjacent to mammary ducts from parous rats might contribute to tumor suppression by reducing risk for local tumor cell invasion.
An important aspect of our work is the demonstration that fibrillar collagen I induces proliferation and invasive morphology through distinct pathways downstream of β1 integrin. Our proliferation data are consistent with other studies showing that fibrillar collagen I induces focal adhesion kinase (FAK)–ERK-mediated cell proliferation through β1 integrin activation (Barkan et al., 2010; Paszek et al., 2005; Provenzano et al., 2009). Here, we expand upon these studies by demonstrating that non-fibrillar collagen I also promotes tumor cell proliferation. However, mechanistic details are still elusive for how the β1-integrin–FAK signaling axis differentially interprets cues, depending on collagen organization, to govern downstream pathways leading to invasive cell behavior. Interestingly, others have recently demonstrated a relationship between β1 integrin distribution, cell morphology and metastatic growth using in vitro and in vivo models (Shibue et al., 2012). We show that collagen I organization alters the subcellular distribution of activated β1 integrin through a clathrin-mediated endocytosis mechanism. We find a ∼28% decrease in β1 integrin levels at the cell membrane in tumor cells cultured in fibrillar collagen I and a concomitant increase in the elongated-stellate morphology; phenotypes that are reversed when clathrin-mediated endocytosis is inhibited. These data are consistent with reports showing a ∼45% decrease in β1 integrin recycling and reduced non-small-cell lung cancer (NSCL) cell invasion upon knockdown of the Rab-coupling protein RCP/Rab11-FIP1, a key endocytotic mediator (Muller et al., 2009). Furthermore, tumor cell invasion in collagen-I-rich matrix is also dependent on matrix metalloproteinases (MMPs) such as MMP14 (Sabeh et al., 2004). Our results show that collagen I organization influences ECM-degrading enzymes, including MMP14, as expression was downregulated in non-fibrillar collagen I. A potential limitation of our 3D model, which combined fibrillar or non-fibrillar collagen I with laminin-rich Matrigel, is that laminin is not an ECM component of the intralobular stroma (Maller et al., 2010). Improved modeling of intralobular stroma will further facilitate our understanding of the role collagen I organization plays in the transition of breast cancer cells from an indolent to an invasive state.
In summary, our previous and current work demonstrates that changes in mammary fibrillar collagen density, organization and stiffness are part of the tissue remodeling induced by pregnancy in rodent mammary glands (Hattar et al., 2009). This current study suggests that parity-induced changes in fibrillar collagen organization reduce cellular programs associated with an invasive phenotype and provide a novel link between mammary collagen remodeling and ‘the protective effect’ of parity. This work may also enhance our understanding of collagen density in human breast cancer risk, as it suggests distinct roles based on collagen organization. Further exploration of the interplay between reproductive history, mammary collagen remodeling, and tumor cell behaviors may provide important information about a woman's lifetime risk for breast cancer and novel insights into breast cancer prevention.
Materials and Methods
Cell culture
Immortalized human mammary epithelial MCF12A cells, human breast cancer MCF10DCIS and MCF10DCIS.GFP cells were cultured in growth factor supplemented DMEM/F12 medium as previously described (Hattar et al., 2009; Lyons et al., 2011). MCF10DCIS and MCF10DCIS.GFP cells were generously provided by Kornelia Polyak (Dana-Farber Cancer Institute, MA). MCF10DCIS cells were selected because their transition from premalignant lesion to invasive breast carcinoma is dependent on the tissue microenvironment (Lyons et al., 2011). Murine mammary tumor D2.OR and D2.OR.GFP cells were cultured in complete DMEM high-glucose medium (Hyclone) with 10% fetal bovine serum as described previously (Morris et al., 1993). D2.OR and D2.OR.GFP cells were kindly provided to us by Ann Chambers (University of Western Ontario, London, Ontario, Canada). D2.OR cells were chosen because changes in ECM composition alter their proliferative activity (Barkan et al., 2010).
Fibrillar collagen characterization of human breast tissue
Human research was approved through the Colorado Multiple Institution IRB (COMIRB) and the source population was from the University of Colorado Hospital, Aurora, CO and The Shaw Cancer Center, Edwards, Colorado. All clinical investigations were conducted according to Declaration of Helsinki principles. Adjacent normal breast tissues were obtained from premenopausal women with primary breast cancers. Adjacent normal epithelial structures were defined as at least 5 mm away from the tumor and histologically normal in appearance, as independently determined by a clinical pathologist. For connective tissue content analysis, the age range was 29–44, with an average age of 35.8 for the nulliparous never-been pregnant group, and an average age of 37.8 for the parous group. For fibrillar collagen content analysis, the age range was the same, and the average age was 38.5 for the nulliparous group and 39.8 for the parous group. The parous group was defined as having 5 years or more since last childbirth. Intralobular connective tissue content was examined using H&E stain (n = 9 for nulliparous cases and n = 12 for parous cases) and fibrillar collagen content via Masson's trichrome stain (n = 6 for nulliparous cases and n = 5 for parous cases) in type 3 lobules as previously defined (Russo and Russo, 2004). Each case had 1–18 type 3 lobules analyzed, with an average of four lobules analyzed per case. A limitation of these analyses is that menstrual cycle data are lacking.
Harvesting rat mammary glands and isolation of mammary ECM
Animal procedures approved by the University of Colorado Institutional Animal Care and Use Committee. Sprague-Dawley female rats (Harlen) at 70±3 days of age were randomized into nulliparous and parous groups, with six rats per group. For the parous group, rats were bred and at 2 days post-parturition pup number was normalized to eight per dam. At 10 days lactation, pups were removed to initiate synchronized mammary gland involution. For the parous group, inguinal mammary glands were harvested one month post weaning and for the nulliparous group, from age-matched virgin rats. Rats between the nulliparous and parous were matched for estrus cycle status. Mammary glands were dissected, lymph nodes with adjacent mammary tissues removed and fixed in formalin or optimal cutting temperature compound (OCT)-embedded (Tissue-Tek), followed by immediate ethanol–dry-ice freezing. The remaining gland was snap-frozen for ECM isolation using published procedures (O'Brien et al., 2010a; Schedin et al., 2004).
Orthotopic tumor models
Orthotopic tumor cell injection into nulliparous and parous hosts
Six- to eight-week-old female mice from the ICR-strain of severe combined immunodeficiency (ICR-SCID) (Taconic, NY) were randomized into nulliparous and parous groups with 9 mice per group for D2.OR.GFP cell injections and 6–7 mice per group for MCF10DCIS.GFP cell injections. Mice in the parous groups were bred and allowed to proceed through pregnancy. Pup number was normalized to seven per dam within 2 days postpartum to allow synchronized weaning at day 10 of lactation. At six weeks post weaning, log phase 1×106 D2.OR.GFP cells or 2×105 MCF10DCIS.GFP cells were suspended in PBS (Hyclone) were injected into left and right number 4 inguinal mammary fat pads of age-matched nulliparous or parous mice (Lyons et al., 2011). Tumor growth was monitored using calipers and Illumatool (Lightools Research) bi-weekly.
Tumor cells co-injected with rat mammary matrix
Six-week-old ICR-SCID female mice (Taconic, CA) were randomized into four groups of 10: group 1, MCF10DCIS.GFP cells with nulliparous matrix; group 2, MCF10DCIS.GFP cells with parous matrix; group 3, D2.OR.GFP cells with nulliparous matrix; and group 4, D2.OR.GFP cells with parous matrix. Using the mammary-fat-pad model with the ICR-SCID mice, tumor cells were co-injected with 20 µl of 300 µg/ml of mammary ECM isolated from nulliparous or parous rats, as previously described (McDaniel et al., 2006). Tumor growth was monitor as described above. Tumor multiplicity was calculated on the basis of the number of tumors with a distance of at least 0.2 cm apart per mammary gland by using GFP-based chemiluminescence for live mice, with data supported by histological evaluation.
3D cell culture models
Model 1 was as follows. D2.OR cells were mixed with 200 µl of 200 µg/ml isolated rat mammary ECM and plated on a 3D Matrigel pad (BD Biosciences; Matrigel concentrations 9.7–10 mg/ml) and cultured for 4–6 days in a 96-well plate (Barkan et al., 2010). Model 2 is modified from Krause et al. (Krause et al., 2008). Briefly, 50 µl of 4 mg/ml Matrigel were spread onto a 24-well plate transwell insert (BD Biosciences) and incubated for 1 hour at 37°C prior to plating the cells. A total of 10,000 D2.OR cells or MCF10DCIS cells were suspended in 200 µl of 4 mg/ml Matrigel or in Matrigel mixed with 4 mg/ml or 8 mg/ml rat tail collagen I (BD Biosciences) at ratios of 1.5∶1 Matrigel to collagen I obtaining final concentrations of 1.6 mg/ml collagen I (1X) or 3.2 mg/ml (2X), respectively, and then plated on top of the thin 4 mg/ml Matrigel pad. The durations for these assays were 6 days for D2.OR cells and 8 days for MCF10DCIS cells unless noted otherwise.
All inhibitor treatments were performed using model 2. Chlorpromazine (5 µM) (Sigma) was added to medium only. U0126 (10 µM) (Promega) was added to medium and the 3D matrix pad. Vehicles were water and DMSO for chlorpromazine and U0126, respectively. Cells were replenished with new drug plus media every other day for a total of three cycles (day 0, 2 and 4). For β1 integrin blocking experiment, anti-β1 integrin antibody (10 µg/ml) (BD Biosciences) was added to 3D matrix pad in day 0 and the experiment duration was 4 days. Vehicle was a whole rat IgG (Jackson ImmunoResearch). For the β1 integrin blocking antibody experiment, morphological analysis was performed based on brightfield images. Each experiment were performed in triplicate and repeated at least twice. The data presented are from one representative experiment. Cells were imaged using an inverted microscope (Zeiss Axioscope 25). All samples were treated 0.1 mM bromodeoxyuridine (BrdU) (Sigma) at final concentration for 6 hours prior for fixing. Cells were fixed in methacarn (model 1) for 5 minutes or 10% Neutral Buffered Formalin (model 2) for 24 hours.
Cell lysates from 3D culture were generated according to the following protocol. Cells cultured in Matrigel or in a mixture of Matrigel and collagen I were washed with cold PBS, were triturated in 10 mM EGTA in PBS with protease and phosphatase inhibitors, and then transfer into eppendorf tubes. Samples were placed on ice on a rotary shaker for 1 hour and then centrifuged for 5 minutes at 2500 rpm. Pellets were resuspended in RIPA lysis buffer with protease and phosphatase inhibitors and placed on a rotary shaker for 15 minutes. Samples were centrifuged for 15 minutes at 14,000 rpm and supernatants collected.
Preparation of non-fibrillar collagen I
The NF collagen I was prepared by sonication of 4 mg/ml collagen I using six cycles of 60 seconds and 30 seconds breaks at 60% duty cycles and output 6 on a 450 W Branson Sonifier (Branson; Danbury).
Quantitative immunohistochemistry and immunofluorescence
Formalin or methacarn-fixed 3D cell culture pads were paraffin-embedded and sectioned to 4 µm sections. Slides were pre-treated in DIVA Decloaker antigen retrieval solution (Biocare) at 125°C under pressure for 5 minutes for all antibodies except activated β1 integrin, which was pre-treated with 0.5% Triton-X 100 (Sigma) for 5 minutes. Primary antibody incubations were for 1 hour at room temperature with 1∶90 for rat anti-activated β1 integrin antibody (BD Biosciences, 9EG7 clone); 1∶2000 for mouse anti-E-cadherin antibody (BD Biosciences) and 1∶100 for mouse anti-BrdU antibody (Dako). Signal was detected using Envision+ System anti-mouse or anti-rabbit secondary (Dako) or 1∶100 Alexa-Fluor-594-conjugated goat anti-rat IgG (Invitrogen) for non-conjugated primary antibody. For nuclear staining, Hoechst 33342 fluorescent stain (Thermo Scientific Pierce) was used. Fluorescence imaging was performed on a Zeiss Axiovert 200M and quantitative analysis was performed with Slide Book (version 4.0.1.3). For β1 integrin distribution analysis, 21–22 images (total of 37–45 structures) per group were taken from three wells per condition. For quantitative histological analyses, entire sections were imaged with Aperio ScanCope T3 scanner at 0.47 µm per pixel follow by down-sampling to a resolution of 1.5 µm per pixel to facilitate subsequent image manipulation (Lyons et al., 2011). E-cadherin immunohistochemistry stains were quantified by membrane staining intensity utilizing the Aperio color membrane algorithm (Aperio Technologies, Color Deconvolution Algorithm User's Guide, 2007) using 15 representative structures per well with a total of 3–4 wells for each condition. Statistical values were assessed based on the average junctional E-cadherin intensities from each well. In the BrdU analysis, the percentage positive cells to total number of cells were calculated based on n = 4 wells per condition. For the human breast tissue cohort, the amounts of connective tissue (H&E) and collagen (Masson's trichrome) were determined as percent of positive intralobular stain to total lobular area per case, with each reported data point representing a single case. n = 9–12 for human breast tissue for H&E and 5–6 for trichrome stain.
Immunoblot analyses
Immunoblotting (IB) for ECM and collagen I was performed as described previously (Schedin et al., 2004). Primary antibodies used for IB were: 1∶1000 rabbit anti-ERK1/2 (Millipore), 1∶1000 rabbit anti-phospho-ERK1/2 [Cell Signaling Technology (CST)], 1∶1000 rabbit anti-total β1 integrin (CST), 1∶1000 rabbit anti-cyclin D1 (CST), 1∶1000 rabbit anti-GAPDH (Sigma), 1∶1000 rabbit anti-decorin (Sigma) and 1∶1000 rabbit anti-collagen I (Abcam). Densitometry was performed using ImageJ software (version 1.45e). For IB for D2.OR cell lysates cultured in rat mammary ECM, three wells were pooled to one sample. For IB for D2.OR cell lysates cultured in Matrigel or in a mixture of Matrigel and collagen I, means were calculated from three separate wells per condition. Experiments were repeated twice.
Two-photon second-harmonic microscopy
Rat mammary gland tissues were imaged via two-photon excitation (TPE) and SHG in the Advanced Light Microscopy Core Facility at University of Colorado Anschutz Medical Campus. Images were captured using a confocal LSM 510 META (Carl Zeiss Inc.) equipped with a femtosecond-pulsed titanium–sapphire laser (Chameleon Ultra; Coherent) with tuning range 690 nm–1020 nm. Images sized at 89.9 µm×89.9 µm and were obtained via ZEN2009 software using an 100×, 1.4 NA Plan-Apochromat oil-immersion objective (Carl Zeiss Inc.). The excitation source was tuned for 800 nm to generate the SHG signal and the emission signal from the sample was collected by non-descanned detectors after being separated out by the dichroic mirror 425DCLP (Chroma Technologies) in the SHG signal and TPE signal. Fiber directionality was quantified by measuring the coherence factor with ImageJ (version 1.45e) and the plugin OrientationJ (Biomedical Image Group). Coherence factors were measured from six representative stromal areas (yellow boxes) that are evenly distributed around the terminal duct circumference in mammary glands. A total of 13 size-matched mammary ducts within right-side glands from seven rats for each group were used for this analysis.
Atomic force microscopy analysis
Tissue preparation
OCT-embedded frozen tissues were cut into 25-µm sections. Each section was fast-thawed by immersing in PBS at room temperature. Then each tissue section was covered with PBS that contained protease inhibitors and phosphatase inhibitors, as well as propidium iodide to visualize cellular content.
AFM measurements
This procedure is as described previously (Lopez et al., 2011). Briefly, AFM indentations were performed using MFP3D-BIO inverted optical AFM (Asylum research) mounted on a Nikon TE200-U inverted fluorescence microscope. Silicon nitride cantilevers were used for indentation with a spring constant of 0.06 N/m and a borosilicate glass spherical tip with a 5 µm diameter (Novascan Tech). The cantilever was calibrated using the thermal oscillation method for each session. Tissues were indented at a 20 µm/s loading rate, with a maximum force of 2 nN. AFM force maps were performed on 50 µm×50 µm fields. Adjacent stromal areas from one to three terminal ducts were measured per mammary gland. Mammary glands of 10 rats (1 gland per rat) were used for these analyses from two independent animal experiments. Data analyses were done using the Hertz model in Igor Pro (version 6.22A). The Poisson's ratio of 0.5 was used in the calculation of the Young's elastic modulus (Alcaraz et al., 2003).
LC-MS/MS-based proteomic analysis
LC-MS/MS analyses were performed in the proteomic mass spectrometry facility at University of Colorado Anschutz Medical Campus. A single batch of mammary ECM isolated from nulliparous and parous rats was used for mass spectrometry analyses. Each sample was analyzed five times on three different occasions. Prior to LC-MS/MS, 30–40 µg of each sample was separated by molecular mass via 1D gel electrophoresis using a Nupage™ gel with a 4–12% acrylamide gradient with a Bis-Tris buffer system (Invitrogen). The entire lane for each sample was cut into 17 bands, excised, washed, reduced, alkyated and then digested overnight at room temperature with sequencing-grade modified trypsin (Promega).
Nanoflow reverse-phase LC-MS/MS was performed using an Eksigent nanoLC-2D system (Eksigent) coupled to a LTQ Orbitrap-Velos mass spectrometer (Thermo Fisher). Data acquisition was performed using Xcalibur™ (Version 2.1) software. MS/MS spectra were extracted from raw data files and converted into Mascot generic files using an in-house script. These peak lists were searched against SwissProt and IPI databases using an in-house Mascot™ server (Version 2.2.06, Matrix Science). Mass tolerances were ±15 ppm for MS peaks, and ±0.6 Da for MS/MS fragment ions.
MS/MS spectra were then compiled into Scaffold™ (Version 3; Proteome Software) for qualitative and quantitative analyses. Additionally, Progenesis LC-MS (Nonlinear Dynamics) was utilized to measure peptide peak area intensities. Intra-normalization was performed for each individual analysis. Each normalized value for a specific protein represents the sum of unique peptide intensities at a specific band divided by the band with highest sum peptide intensities in one of the bands (number 1–17) for that protein. This normalization approach was chosen to control for inter-run variations for specific proteins owing to potential differences in the number of unique peptides identified among various analyses. Outliers were excluded in pairs using the Grubb's test with a significance level of P<0.05. Statistical analyses were performed using GraphPad Instat (version 3.05). For statistical analyses, P-values across groups were calculated based on one-way ANOVA tests and an unpaired Student's t-test was used for specific pairs in particular molecular mass or individual collagen chains.
3D cell culture transcriptome microarray analysis
D2.OR cells were cultured in Matrigel or Mat/1X Col I for 4 days. Four wells from each condition were pooled to make one sample for RNA isolation, and the microarray analyses were performed on three samples per group. At total of 750 µl of TRIzol®LS reagent (Invitrogen) was added to each 3D matrix pad (ratio 1∶4). The matrix pads were mechanically disrupted and cells were lysed by trituration. To generate a single sample, four wells were pooled in this point from each condition and transferred into 10-ml conical tubes, vortexed for 30 seconds, incubated for 3 minutes at room temperature, prior to being divided into 1.5 ml tubes and centrifuged at 12,000 g for 10 minutes at 4°C. TRIzol®LS-chloroform extraction was performed. One volume of 70% ethanol was added to the resulting upper (aqueous) layer. An RNeasy micro kit (QIAGEN) was utilized to isolate and purified RNA according to the manufacturer's protocol.
RNA integrity number scores were 10, and 260 nm:280 nm ratios ranged from 1.95 to 2.05 for all samples. cDNA was prepared using Ambion® WT expression kit (Invitrogen) for transcriptome analysis. Transcriptome profiling was performed with the Affymetrix GeneChip® Mouse Gene 1.0 ST Array. Raw microarray gene expression profiles were extracted and normalized by using the robust multiarray average (Irizarry et al., 2003) with the Affymetrix Power Tools program. Heatmaps were generated by matrix2png (Pavlidis and Noble, 2003). Genes with expression greater than 2-fold are considered as differentially expressed genes. Raw microarray data have been deposited in the NCBI Gene Expression Omnibus, and can be downloaded under the accession number GSE39539. Microarray validation was performed via RNA isolated from an independent experiment and was performed via quantitative RT-PCR on myiQ Single-Color Real Time PCR Detection system from Bio-Rad with custom primers from Integrated Device Technology (supplementary material Table S4).
Statistics
Statistical analyses were performed with GraphPad Instat (version 3.05) with unpaired Student's t-tests or one-way ANOVA with Bonferroni's post-hoc comparisons for selected pairs when more than two groups were present. Error bars in the figures represent standard deviations unless noted otherwise.
Supplementary Material
Acknowledgments
We thank Virginia F. Borges and the Young Women's Breast Cancer Translational Program for providing clinical specimens (University of Colorado Anschutz Medical Campus, UC AMC), Radu Moldovan (UC AMC Advanced Light Microscopy Core) for helping with the SHG imaging, Ryan Hill and Monika Dzieciatkowska (UC AMC) for assisting with mass spectrometry, Sonali Jindal (UC AMC) for pathological analyses, Pat Bell (UC AMC) for immunohistochemistry expertise, Clarissa Durand-Rougely (UC AMC) for immunohistochemistry analyses, An Doan (UC AMC Genomic Microarray Core) for assisting with microarray analysis, and James Lambert and S. Gail Eckhardt (UC AMC) for providing the U0126 inhibitor. We also thank Arthur Gutierrez-Hartmann and Paul Jedlicka (UC AMC) for critical input into the manuscript.
Footnotes
Author contributions
O.M. designed experiments, performed or partook in all experiments, interpreted data and was primary manuscript writer; K.C.H. performed the proteomic analysis and interpreted related data; T.R.L. performed mouse tumor studies and assisted with manuscript preparation; I.A. and V.M.W. performed AFM analysis and interpreted related data; R.P. assisted in evaluating integrin sub-cellular distribution; A.C.T. interpreted transcriptome microarray analysis; P.S. designed experiments, interpreted data and co-wrote the manuscript.
Funding
This work was mainly supported by an Idea Award from the US Department of Defense [grant number BC095850 to P.S.]. Additional support for this work was received from the National Institutes of Health (National Cancer Institute) [grant numbers R01 CA138818-01A1 to V.M.W., U01 ES019458 to Z.W. and V.M.W., R21 CA132741 to K.H.; UL1 RR025780 to Advanced Light Microscopy Core and Proteomic Mass Spectrometry Facility, P30 CA046934 to the Proteomic Mass Spectrometry Facility]. The work was also supported by an American Cancer Society New England Division Postdoctoral Fellowship Spin Odyssey [grant number PF-08-257-01-CSM to T.R.L.] and a Susan G Komen award [grant number PDF12230246 to I.A.]; and the Dobbs Charitable Fund, in memory of Connie Kazda Schedin (to P.S.). Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.121590/-/DC1
References
- Abrams T. J., Guzman R. C., Swanson S. M., Thordarson G., Talamantes F., Nandi S. (1998). Changes in the parous rat mammary gland environment are involved in parity-associated protection against mammary carcinogenesis. Anticancer Res. 18 6A, 4115–4121 [PubMed] [Google Scholar]
- Alcaraz J., Buscemi L., Grabulosa M., Trepat X., Fabry B., Farré R., Navajas D. (2003). Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 84, 2071–2079 10.1016/S0006-3495(03)75014-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge H. L., Meng X., Zhang G., Veit G., Sun M., Klement J. F., Beason D. P., Soslowsky L. J., Koch M., Birk D. E. (2009). Type XIV collagen regulates fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice. J. Biol. Chem. 284, 8427–8438 10.1074/jbc.M805582200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D., Kleinman H., Simmons J. L., Asmussen H., Kamaraju A. K., Hoenorhoff M. J., Liu Z. Y., Costes S. V., Cho E. H., Lockett S. et al. (2008). Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 10.1158/0008-5472.CAN-07-6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D., El Touny L. H., Michalowski A. M., Smith J. A., Chu I., Davis A. S., Webster J. D., Hoover S., Simpson R. M., Gauldie J. et al. (2010). Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 70, 5706–5716 10.1158/0008-5472.CAN-09-2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Barcellos-Hoff M. H. (1987). The influence of extracellular matrix on gene expression: is structure the message? J. Cell Sci. Suppl. 8, 327–343 10.1242/jcs.1987.Supplement_8.18 [DOI] [PubMed] [Google Scholar]
- Caswell P. T., Chan M., Lindsay A. J., McCaffrey M. W., Boettiger D., Norman J. C. (2008). Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 183, 143–155 10.1083/jcb.200804140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin M. W., Eickhoff J. C., Riching K. M., Pehlke C. A., Eliceiri K. W., Provenzano P. P., Friedl A., Keely P. J. (2011). Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 10.1016/j.ajpath.2010.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson K. G., Baribault H., Holmes D. F., Graham H., Kadler K. E., Iozzo R. V. (1997). Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 136, 729–743 10.1083/jcb.136.3.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearth R. K., Delgado D. A., Hiney J. K., Pathiraja T., Oesterreich S., Medina D., Dees W. L., Lee A. V. (2010). Parity-induced decrease in systemic growth hormone alters mammary gland signaling: a potential role in pregnancy protection from breast cancer. Cancer Prev. Res. (Phila.) 3, 312–321 10.1158/1940-6207.CAPR-09-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley S. I., Feng Y., Krishnamurthy R., Kim D. H., Celedon A., Longmore G. D., Wirtz D. (2010). A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. 12, 598–604 10.1038/ncb2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar R., Maller O., McDaniel S., Hansen K. C., Hedman K. J., Lyons T. R., Lucia S., Wilson R. S., Jr, Schedin P. (2009). Tamoxifen induces pleiotrophic changes in mammary stroma resulting in extracellular matrix that suppresses transformed phenotypes. BCR 11, R5 10.1186/bcr2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 10.1093/biostatistics/4.2.249 [DOI] [PubMed] [Google Scholar]
- Iyengar P., Espina V., Williams T. W., Lin Y., Berry D., Jelicks L. A., Lee H., Temple K., Graves R., Pollard J. et al. (2005). Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest. 115, 1163–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny P. A., Lee G. Y., Myers C. A., Neve R. M., Semeiks J. R., Spellman P. T., Lorenz K., Lee E. H., Barcellos-Hoff M. H., Petersen O. W. et al. (2007). The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 1, 84–96 10.1016/j.molonc.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S., Maffini M. V., Soto A. M., Sonnenschein C. (2008). A novel 3D in vitro culture model to study stromal-epithelial interactions in the mammary gland. Tissue Eng. Part C Methods 14, 261–271 10.1089/ten.tec.2008.0030 [DOI] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W. et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Sun L., Miller N., Nicklee T., Woo J., Hulse-Smith L., Tsao M. S., Khokha R., Martin L., Boyd N. (2005). The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol. Biomarkers Prev. 14, 343–349 10.1158/1055-9965.EPI-04-0490 [DOI] [PubMed] [Google Scholar]
- Lopez J. I., Kang I., You W. K., McDonald D. M., Weaver V. M. (2011). In situ force mapping of mammary gland transformation. Integr. Biol. 3, 910–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Mu E., Wei Y., Riethdorf S., Yang Q., Yuan M., Yan J., Hua Y., Tiede B. J., Lu X. et al. (2011). VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20, 701–714 10.1016/j.ccr.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Weaver V. M., Werb Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons T. R., O'Brien J., Borges V. F., Conklin M. W., Keely P. J., Eliceiri K. W., Marusyk A., Tan A. C., Schedin P. (2011). Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat. Med. 17, 1109–1115 10.1038/nm.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B., Cole P., Lin T. M., Lowe C. R., Mirra A. P., Ravnihar B., Salber E. J., Valaoras V. G., Yuasa S. (1970). Age at first birth and breast cancer risk. Bull. World Health Organ. 43, 209–221 [PMC free article] [PubMed] [Google Scholar]
- Maller O., Martinson H., Schedin P. (2010). Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. J. Mammary Gland Biol. Neoplasia 15, 301–318 10.1007/s10911-010-9189-6 [DOI] [PubMed] [Google Scholar]
- McDaniel S. M., Rumer K. K., Biroc S. L., Metz R. P., Singh M., Porter W., Schedin P. (2006). Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am. J. Pathol. 168, 608–620 10.2353/ajpath.2006.050677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D., Smith G. H. (1999). Chemical carcinogen-induced tumorigenesis in parous, involuted mouse mammary glands. J. Natl. Cancer Inst. 91, 967–969 10.1093/jnci/91.11.967 [DOI] [PubMed] [Google Scholar]
- Morris V. L., Tuck A. B., Wilson S. M., Percy D., Chambers A. F. (1993). Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clin. Exp. Metastasis 11, 103–112 10.1007/BF00880071 [DOI] [PubMed] [Google Scholar]
- Muller P. A., Caswell P. T., Doyle B., Iwanicki M. P., Tan E. H., Karim S., Lukashchuk N., Gillespie D. A., Ludwig R. L., Gosselin P. et al. (2009). Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327–1341 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]
- O'Brien J., Fornetti J., Schedin P. (2010a). Isolation of mammary-specific extracellular matrix to assess acute cell-ECM interactions in 3D culture. J. Mammary Gland Biol. Neoplasia 15, 353–364 10.1007/s10911-010-9185-x [DOI] [PubMed] [Google Scholar]
- O'Brien J., Lyons T., Monks J., Lucia M. S., Wilson R. S., Hines L., Man Y. G., Borges V., Schedin P. (2010b). Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am. J. Pathol. 176, 1241–1255 10.2353/ajpath.2010.090735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old W. M., Meyer-Arendt K., Aveline-Wolf L., Pierce K. G., Mendoza A., Sevinsky J. R., Resing K. A., Ahn N. G. (2005). Comparison of label-free methods for quantifying human proteins by shotgun proteomics. MCP 4, 1487–1502 10.1074/mcp.M500084-MCP200 [DOI] [PubMed] [Google Scholar]
- Onder T. T., Gupta P. B., Mani S. A., Yang J., Lander E. S., Weinberg R. A. (2008). Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 68, 3645–3654 10.1158/0008-5472.CAN-07-2938 [DOI] [PubMed] [Google Scholar]
- Park C. C., Zhang H., Pallavicini M., Gray J. W., Baehner F., Park C. J., Bissell M. J. (2006). Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 66, 1526–1535 10.1158/0008-5472.CAN-05-3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D. et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 10.1016/j.ccr.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Pavlidis P., Noble W. S. (2003). Matrix2png: a utility for visualizing matrix data. Bioinformatics 19, 295–296 10.1093/bioinformatics/19.2.295 [DOI] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Knittel J. G., Yan L., Rueden C. T., White J. G., Keely P. J. (2008). Collagen density promotes mammary tumor initiation and progression. BMC Med. 6, 11 10.1186/1741-7015-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Keely P. J. (2009). Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28, 4326–4343 10.1038/onc.2009.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinier K. S., Vacek P. M., Geller B. M. (2007). Risk factors for breast carcinoma in situ versus invasive breast cancer in a prospective study of pre- and post-menopausal women. Breast Cancer Res. Treat. 103, 343–348 10.1007/s10549-006-9375-9 [DOI] [PubMed] [Google Scholar]
- Russo J., Russo I. H. (1980). Susceptibility of the mammary gland to carcinogenesis. II. Pregnancy interruption as a risk factor in tumor incidence. Am. J. Pathol. 100, 497–512 [PMC free article] [PubMed] [Google Scholar]
- Russo J., Russo I. H. (2004). Development of the human breast. Maturitas 49, 2–15 10.1016/j.maturitas.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Russo J., Moral R., Balogh G. A., Mailo D., Russo I. H. (2005). The protective role of pregnancy in breast cancer. BCR 7, 131–142 10.1186/bcr1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J., Balogh G. A., Russo I. H. (2008). Full-term pregnancy induces a specific genomic signature in the human breast. Cancer Epidemiol. 17, 51–66 [DOI] [PubMed] [Google Scholar]
- Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S. et al. (2004). Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 167, 769–781 10.1083/jcb.200408028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P., Keely P. J. (2011). Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 3, a003228 10.1101/cshperspect.a003228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedin P., Mitrenga T., McDaniel S., Kaeck M. (2004). Mammary ECM composition and function are altered by reproductive state. Mol. Carcinog. 41, 207–220 10.1002/mc.20058 [DOI] [PubMed] [Google Scholar]
- Schonfeld S. J., Pfeiffer R. M., Lacey J. V., Jr, Berrington de González A., Doody M. M., Greenlee R. T., Park Y., Schairer C., Schatzkin A., Sigurdson A. J. et al. (2011). Hormone-related risk factors and postmenopausal breast cancer among nulliparous versus parous women: An aggregated study. Am. J. Epidemiol. 173, 509–517 10.1093/aje/kwq404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T., Weinberg R. A. (2009). Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. USA 106, 10290–10295 10.1073/pnas.0904227106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T., Brooks M. W., Inan M. F., Reinhardt F., Weinberg R. A. (2012). The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2, 706–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D. K., Pazik J. E., Dao T. L. (1988). Prevention of mammary carcinogenesis in rats by pregnancy: effect of full-term and interrupted pregnancy. Br. J. Cancer 57, 390–394 10.1038/bjc.1988.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specks U., Nerlich A., Colby T. V., Wiest I., Timpl R. (1995). Increased expression of type VI collagen in lung fibrosis. Am. J. Respir. Crit. Care Med. 151, 1956–1964 10.1164/ajrccm.151.6.7767545 [DOI] [PubMed] [Google Scholar]
- Takeda U., Utani A., Wu J., Adachi E., Koseki H., Taniguchi M., Matsumoto T., Ohashi T., Sato M., Shinkai H. (2002). Targeted disruption of dermatopontin causes abnormal collagen fibrillogenesis. J. Invest. Dermatol. 119, 678–683 10.1046/j.1523-1747.2002.01863.x [DOI] [PubMed] [Google Scholar]
- Wagner K. U., Boulanger C. A., Henry M. D., Sgagias M., Hennighausen L., Smith G. H. (2002). An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129, 1377–1386 [DOI] [PubMed] [Google Scholar]
- Wang L. H., Rothberg K. G., Anderson R. G. (1993). Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 10.1083/jcb.123.5.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.