Abstract
The title compound, [Mn6(C7H4NO3)5(CH3CO2)2(C4H6N2)4.62(C3H7NO)1.38]·(C2H5)2O·C3H7NO·CH3OH·0.49H2O or MnII(OAc)2[15-MCMn(III)N(shi)-5](Me—Im)4.62(DMF)1.38·diethyl ether·DMF·MeOH·0.49H2O (where MC is metallacrown, −OAc is acetate, shi3− is salicylhydroximate, Me—Im is 1-methylimidazole, DMF is N,N-dimethylformamide, and MeOH is methanol), is comprised of five MnIII ions in the metallacrown ring and an MnII ion which is encapsulated in the central cavity. Four of the ring MnIII ions are six-coordinate with distorted octahedral geometries. Two of these MnIII ions have a planar configuration, while the other two MnIII have Λ absolute stereoconfiguration. The fifth MnIII is five-coordinated with distorted square-pyramidal geometry. Four of the ring MnIII ions each bind one 1-methylimidazole, while the final ring MnIII ion binds a DMF solvent molecule in an axial position and located in a trans position is either a Me—Im or a DMF molecule. The occupancy ratio of Me—Im to DMF is 0.62 (2) to 0.38 (2). The central MnII is seven-coordinate with a geometry best described as distorted face-capped trigonal–prismatic. DMF, diethyl ether, MeOH, and water molecules are located in the interstitial voids between the metallacrown molecules. The methanol molecule is positionally disordered [0.51 (1):0.49 (1)] and associated with a partially occupied water molecule [0.49 (1)]. This disorder is also associated with the positional disorder of the diethyl ether molecule [0.51 (1):0.49 (1)].
Related literature
For a general review of metallacrowns, see: Mezei et al. (2007 ▶). For related manganese and vanadium metallacrown structures, see: Lah & Pecoraro (1989 ▶) and Pecoraro (1989 ▶), respectively. For related Mn(II)[15-MCMn(III)N(shi)-5)] structures and synthetic procedures, see: Kessissoglou et al. (1994 ▶), Dendrinou-Samara et al. (2001 ▶, 2002 ▶, 2005 ▶); Emerich et al. (2010 ▶); Tigyer et al. (2011 ▶, 2012 ▶). For an explanation on how to calculate the s/h ratio, see: Stiefel & Brown (1972 ▶). For an explanation on how to calculate bond-valence-sum values, see: Liu & Thorp (1993 ▶). For an explanation on how to calculate the τ asymmetry parameter, see: Addison et al. (1984 ▶). For CELL_NOW software, see: Sheldrick (2008b
▶). 
Experimental
Crystal data
[Mn6(C7H4NO3)5(C2H3O2)2(C4H6N2)4.62(C3H7NO)1.38]·C4H10O·C3H7NO·CH4O·0.49H2O
M r = 1866.61
Triclinic,

a = 12.4181 (8) Å
b = 17.0108 (11) Å
c = 20.6627 (13) Å
α = 102.166 (4)°
β = 96.726 (4)°
γ = 107.496 (4)°
V = 3992.4 (5) Å3
Z = 2
Mo Kα radiation
μ = 1.01 mm−1
T = 100 K
0.30 × 0.23 × 0.15 mm
Data collection
Bruker SMART APEX CCD diffractometer
Absorption correction: multi-scan (TWINABS; Sheldrick, 2009 ▶) T min = 0.544, T max = 0.747
56608 measured reflections
18890 independent reflections
13018 reflections with I > 2σ(I)
R int = 0.134
Refinement
R[F 2 > 2σ(F 2)] = 0.087
wR(F 2) = 0.232
S = 1.04
18890 reflections
1146 parameters
93 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 1.07 e Å−3
Δρmin = −1.08 e Å−3
Data collection: APEX2 (Bruker, 2012 ▶); cell refinement: SAINT (Bruker, 2012 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008a ▶); program(s) used to refine structure: SHELXL2012 (Sheldrick, 2008a ▶) and SHELXLE Rev600 (Hübschle et al., 2011 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813015857/jj2164sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813015857/jj2164Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work was funded by the Shippensburg University Foundation (grant No. UGR2012/13–08) to BRT and CMZ. The diffractometer was funded by NSF grant No. 0087210, by Ohio Board of Regents grant No. CAP-491, and by YSU. The authors would like to thank Professor George M. Sheldrick for providing access to the beta version of SHELXL2012 prior to its official release.
supplementary crystallographic information
Comment
Metallacrowns were first recognized in 1989 by Pecoraro, and since then they have proven to be a versatile class of inorganic compounds (Pecoraro, 1989; Mezei et al., 2007). They have served as building blocks for 1-, 2-, and three-dimensional solids, displayed interesting relaxivity behavior, and served as selective-anion hosts (Mezei et al., 2007). In addition, the manganese-based 15-MC-5 compounds have shown enhanced antimicrobial properties compared to simple Mn-herbicide compounds (Kessissoglou et al., 1994; Dendrinou-Samara et al., 2001, 2002, 2005). These initial manganese-based 15-MC-5 compounds where made using pyridine to complete the coordination of the ring MnIII ions. However, recently it has been shown that imidazole and its derivatives can also be readily used to produce a manganese 15-MC-5 compound (Emerich et al., 2010; Tigyer et al. 2011, 2012).
Herein we report the synthesis, IR data, and single-crystal X-ray structure of the title compound [Mn6(C7H4NO3)5(C2H3O2)2(C4H6N2)4.62(C3H7NO)1.38].(C2H5)2O.C3H7NO.CH3OH.0.49H2O, 1, abbreviated as Mn(II)(OAc)2[15-MCMn(III)N(shi)-5](Me—Im)4.62(DMF)1.38.diethyl ether.DMF.MeOH.0.49H2O (where MC is metallacrown, -OAc is acetate, shi3- is salicylhydroximate, Me—Im is 1-methylimidazole, DMF is N,N-dimethylformamide, and MeOH is methanol). The molecule is nonplanar, which is typical of manganese-based 15-MC-5 complexes (Fig. 1). The MC framework of the molecule is comprised five shi3- ligands and five MnIII ions, which combine to form a -[MnIII—N—O]5– repeat unit. A MnII ion is captured in the central cavity of the MC and the MnII ion is tethered to the MC ring via two acetate ligands. Charge neutrality in the molecule is maintained by the five MnIII and one MnII cations and five shi3- and two acetate ligands.
Mn1 is located in the central cavity and is seven-coordinate with distorted face-capped trigonal prismatic geometry (Fig. 2). The geometry assignment is supported by both the calculated azimuthal angle (Φ) and the s/h ratio (Stiefel & Brown, 1972). These parameters can be used to distinguish an ideal trigonal prism and octahedron. In an ideal trigonal prism the angle between the atoms on opposite triangular faces is Φ = 0°, and the s/h ratio is 1.00. In an ideal octahedron the azimuthal angle equals 60°, and the s/h ratio is 1.22. To calculate these parameters the centroids of opposite triangular faces made by the donor oxygen atoms (O6, O9, and O18; O12, O15, and O16) were defined using the program Mercury (Fig. 3; Macrae et al., 2006). The azimuthal angles were measured between atoms on opposite faces through the centroids. To calculate the s/h ratio, the distance between the centroids was defined as h, and the distances between atoms on the same triangular face were defined as s. For Mn1 the Φ angles are 8.13°, 12.33°, and 15.98°, and the estimated average s/h ratio is 1.01±0.11. Thus, both the Φ angle and s/h parameters support a distorted faced-capped trigonal prismatic geometry. Mn1 is assigned a 2+ oxidation state which is supported by an average bond distance of 2.24 Å and a Bond Valence Sum (BVS) calculation of 1.92 (Liu & Thorp, 1993).
The ring Mn2 - Mn6 ions have various coordination modes and configurations (Fig. 4). Mn2 is five-coordinate (Fig. 4a) with distorted square pyramidal geometry. To evaluate the geometry about Mn2 the τ parameter was calculated (Addison et al., 1984). For an ideal square pyramidal geometry τ = 0, while for an ideal trigonal bipyramidal geometry τ = 1. For Mn2 τ is 0.21. Mn3 - Mn 6 are six-coordinate with distorted octahedral geometry. In addition, the coordination about these Mn can be described by their configurations. Mn3 (Fig. 4 b) and Mn6 (Fig. 4 e) have a propeller configuration of two chelate rings of different shi3- ligands with Λ absolute stereochemistry. Mn4 (Fig. 4c) and Mn5 (Fig. 4 d) adopt a planar (P) configuration, where two chelate rings of different shi3- ligands are located trans to each other. In addition, Mn2, Mn3, Mn5, and Mn6 each bind one 1-methylimidazole ligand, which is directed to the periphery of the metallacrown. Mn4 binds one DMF molecule in an axial position and located in a trans position is either a 1-methylimidazole or a DMF. The occupancy ratio of 1-methylimidazole to DMF is 0.62 (2) to 0.38 (2). Mn2 - Mn6 are assigned a 3+ oxidation state, which is supported by the average bond distances and BVS calculations. The average Mn-N/O bond distances for Mn2, Mn3, Mn4, Mn5, and Mn6 are 1.98 Å, 2.04 Å, 2.06 Å, 2.04 Å, and 2.05 Å, respectively, and the BVS calculations are 2.99, 3.09, 3.04, 3.11, and 3.09, respectively. In addition, Mn3 - Mn6 possess a Jahn-Teller axis, which is typical for high spin d4 MnIII ions.
Lastly DMF, diethyl ether, methanol, and water molecules are located in the interstitial voids between the metallacrown molecules. The methanol molecule is positional disordered [0.51 (1):0.49 (1)] and associated with a partially occupied water molecule [0.49 (1)]. This disorder is also associated with the positional disorder of the diethyl ether molecule [0.51 (1):0.49 (1)].
Experimental
Manganese(II) acetate tetrahydrate (99+%) was purchased from Acros Organics. Salicylhydroxamic acid (H3shi, 99%) and 1-methylimidazole (99%) were purchased from Alfa Aesar. Methanol (HPLC grade) was purchased from Pharmco-AAPer. N,N-dimethylformamide (Certified ACS grade) was purchased from BDH chemicals. Absolute diethyl ether was purchased from EMD Chemicals. All reagents were used as received and without further purification.
The compound {Mn(II)(OAc)2[12-MCMn(III)N(shi)-4](DMF)6.2DMF was prepared as previously reported (Lah & Pecoraro, 1989). Dark brown/black crystals were isolated and dried. Then the {Mn(II)(OAc)2[12-MCMn(III)N(shi)-4](DMF)6.2DMF compound (0.1 mmol) was dissolved in 20 ml of a 75:25 solution of DMF and methanol resulting in a dark brown solution. Following 25 µL of 1-methylimidazole was added and no change was observed. This solution was stirred for 5 minutes. Diffusion of diethyl ether into the solution at room temperature resulted in small black platelets suitable for X-ray analysis after 8 days. The percent yield was 6.8% based on {Mn(II)(OAc)2[12-MCMn(III)N(shi)-4](DMF)6.2DMF.
Elemental analysis for the dried material (accounting for the loss of the diethyl ether lattice solvent) C65.62H75.36Mn6N16.62O22.87 [FW = 1792.43 g/mol] found % (calculated); C 44.30 (43.97); H 4.10 (4.24); N 13.34 (12.99).
Refinement
The crystals under investigation were heavily intergrown and fragile and no single piece sufficiently large for XRD analysis could be obtained. Attempts to obtain single pieces from larger fragments through careful cutting were not successful due to the dark colour and fragility of the crystallites. Instead a sufficiently large fragment with three larger and a number of smaller moieties was chosen for analysis. The orientation matrices for the three largest moieties were identified using the program CELL_NOW (Sheldrick, 2008b) with the three components being not related by any obvious twin operations. The three components were integrated using SAINT (Bruker, 2012) resulting in the following statistics:
54454 data (16586 unique) involve domain 1 only, mean I/sigma 3.4
23789 data (11631 unique) involve domain 2 only, mean I/sigma 2.6
24251 data (11600 unique) involve domain 3 only, mean I/sigma 1.7
41797 data (20117 unique) involve 2 domains, mean I/sigma 3.0
25039 data (10789 unique) involve 3 domains, mean I/sigma 3.1
8 data (8 unique) involve 4 domains, mean I/sigma 1.2
The exact twin matrices identified by the integration program were found to be:
Matrix 1 → Matrix 2
0.96554 - 0.07897 0.03223
0.17039 1.03400 0.01296
-0.14548 - 0.01729 0.98326
Matrix 1 → Matrix 3
0.97038 - 0.06968 0.02764
0.16471 1.04753 0.07915
-0.18744 - 0.12396 0.95778
Matrix 2 → Matrix 3
1.00268 0.00911 - 0.00488
0.00169 1.01434 0.06707
-0.02835 - 0.10572 0.97641
The data were corrected for absorption using TWINABS (Sheldrick, 2009), and the structure was solved using direct methods with only the non-overlapping reflections of component 1. The structure was refined using the hklf 5 routine with all reflections of component 1 (including the overlapping ones) with a resolution better than 0.8 Å, resulting in BASF values of 0.301 (2) and 0.167 (2).
The total number of reflections given (_diffrn_reflns_number) is before the cutoff at 0.8 Å. The Rint value (_diffrn_reflns_av_R_equivalents) given is for these reflections and is based on agreement between observed single and composite intensities and those calculated from refined unique intensities and twin fractions before the cutoff at 0.8 Å (TWINABS).
One of the coordinated 1-methylimidazole ligands is partially replaced by a DMF molecule. Overlapping atoms were constrained to have identical ADPs and to be close to isotropic. The DMF molecule was restrained to have a geometry similar to that of another not disordered DMF molecule. The occupancy ratio refined to 0.61983 (2000) to 0.38017 (2000) in favor of the 1-methylimidazole molecule.
A methanol molecule is positional disordered with one of the molecules associated with a partially occupied water molecule. The disorder is associated with disorder of a diethyl ether molecule. Occupancy ratios of all three solvent molecules refined to essentially 1:1 (0.50926 (1100) to 0.49074 (1100)). The oxygen and carbon atoms of the methanol and water molecules were restrained to have similar ADPs (SIMU restraint in Shexltl).
Reflections 0 0 1 and 1 - 1 1 were obstructed by the beam stop and were omitted from the refinement.
Figures
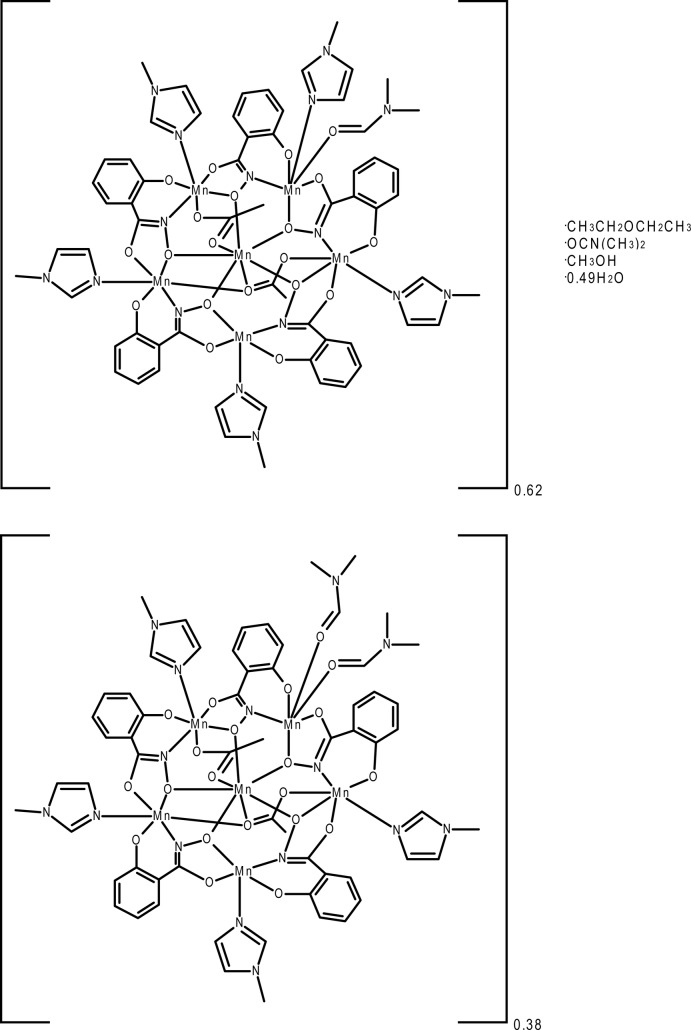
Fig. 1.
Single-crystal X-ray structure of Mn(II)(OAc)2[15-MCMn(III)N(shi)-5](Me—Im)4.62(DMF)1.38.diethyl ether.DMF.MeOH.0.49H2O (1). The thermal ellipsoid plot of 1 is at a 50% probability level. For Mn4 only the 1-methylimidazole is shown bound to the MnIII, since 1-methylimidazole possess a higher occupancy ratio compared to the coordinated DMF (0.62 (2):0.38 (2)). Hydrogen atoms and the lattice solvent molecules have been omitted for clarity. Color scheme for all figures: green - MnII and MnIII, red - oxygen, blue - nitrogen, and gray - carbon.
Fig. 2.

Side (a) and top (b) views of the first coordination sphere about Mn1 (2+ oxidation state) of 1. The thermal ellipsoid plots are at a 50% probability level.
Fig. 3.

Side (a) and top (b) views of the first coordination sphere about Mn1 of 1 demonstrating how the azimuthal anlge (Φ) was defined and calculated using the program Mercury (Macrae et al., 2006). The thermal ellipsoid plots are at a 50% probability level.
Fig. 4.
First coordination sphere about each MnIII ion of 1. a) Mn2 with distorted square pyramidal geometry b) Mn3 with Λ configuration c) Mn4 with planar configuration and 1-methylimidazole bound (0.62 (2) occupancy) d) Mn4 with planar configuration and DMF bound (0.38 (2) occupancy) e) Mn5 with planar configuration and f) Mn6 with Λ configuration. The thermal ellipsoid plots are at a 50% probability level. Hydrogen atoms have been omitted for clarity.
Crystal data
| [Mn6(C7H4NO3)5(C2H3O2)2(C4H6N2)4.62(C3H7NO)1.38]·C4H10O·C3H7NO·CH4O·0.49H2O | Z = 2 |
| Mr = 1866.61 | F(000) = 1920.8 |
| Triclinic, P1 | Dx = 1.552 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.4181 (8) Å | Cell parameters from 9924 reflections |
| b = 17.0108 (11) Å | θ = 2.2–27.6° |
| c = 20.6627 (13) Å | µ = 1.01 mm−1 |
| α = 102.166 (4)° | T = 100 K |
| β = 96.726 (4)° | Plate, black |
| γ = 107.496 (4)° | 0.30 × 0.23 × 0.15 mm |
| V = 3992.4 (5) Å3 |
Data collection
| Bruker SMART APEX CCD diffractometer | 13018 reflections with I > 2σ(I) |
| Radiation source: fine focus sealed tube | Rint = 0.134 |
| ω and phi scans | θmax = 26.4°, θmin = 1.3° |
| Absorption correction: multi-scan (TWINABS; Sheldrick, 2009) | h = −15→15 |
| Tmin = 0.544, Tmax = 0.747 | k = −21→20 |
| 56608 measured reflections | l = 0→25 |
| 18890 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.087 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.232 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0741P)2 + 15.4132P] where P = (Fo2 + 2Fc2)/3 |
| 18890 reflections | (Δ/σ)max < 0.001 |
| 1146 parameters | Δρmax = 1.07 e Å−3 |
| 93 restraints | Δρmin = −1.08 e Å−3 |
Special details
| Experimental. Di-µ-aceto-mono(dimethylformamide)pentakis(µ-N,2-dioxidobenzene-1-carboximidato)pentakis(1-methylimidazole)pentamanganese(III)manganese(II)–diethyl ether-dimethylformamide-methanol-water (1/1/1/0.49)FT–IR bands (KBr pellet, cm-1): 1669, 1653, 1598, 1570, 1500, 1437, 1421, 1389, 1320, 1258, 1243, 1146, 1102, 1033, 1025, 954, 926, 865, 753, 681, 669, 653, 616, 595, 577, 486, 469, 418, and 404. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles, and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refined as a 3-component twin. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Mn1 | 0.56026 (9) | 0.25912 (7) | 0.23833 (5) | 0.0193 (3) | |
| Mn2 | 0.58160 (10) | 0.32031 (7) | 0.40739 (6) | 0.0229 (3) | |
| Mn3 | 0.57536 (10) | 0.06313 (7) | 0.27180 (6) | 0.0220 (3) | |
| Mn4 | 0.79276 (10) | 0.23709 (8) | 0.15153 (6) | 0.0248 (3) | |
| Mn5 | 0.60155 (10) | 0.43514 (8) | 0.17163 (6) | 0.0225 (3) | |
| Mn6 | 0.28559 (9) | 0.25305 (8) | 0.22500 (6) | 0.0215 (3) | |
| O1 | 0.6762 (5) | 0.3379 (4) | 0.4890 (3) | 0.0313 (14) | |
| O2 | 0.6924 (5) | 0.1096 (3) | 0.3655 (3) | 0.0267 (13) | |
| O3 | 0.5555 (4) | 0.1697 (3) | 0.3059 (2) | 0.0241 (12) | |
| O4 | 0.6034 (5) | −0.0397 (3) | 0.2429 (3) | 0.0268 (13) | |
| O5 | 0.8200 (4) | 0.1295 (3) | 0.1493 (3) | 0.0231 (12) | |
| O6 | 0.6780 (4) | 0.1918 (3) | 0.2005 (2) | 0.0227 (12) | |
| O7 | 0.9041 (5) | 0.2796 (4) | 0.1026 (3) | 0.0319 (14) | |
| O8 | 0.7492 (5) | 0.4685 (3) | 0.1438 (3) | 0.0276 (13) | |
| O9 | 0.6274 (4) | 0.3267 (3) | 0.1616 (2) | 0.0223 (12) | |
| O10 | 0.5824 (5) | 0.5419 (4) | 0.1828 (3) | 0.0273 (13) | |
| O11 | 0.2929 (5) | 0.3835 (4) | 0.2420 (3) | 0.0279 (13) | |
| O12 | 0.4229 (4) | 0.3040 (3) | 0.1939 (2) | 0.0201 (11) | |
| O13 | 0.1525 (4) | 0.1935 (4) | 0.2509 (2) | 0.0266 (13) | |
| O14 | 0.4137 (4) | 0.2615 (3) | 0.4247 (2) | 0.0250 (12) | |
| O15 | 0.4878 (4) | 0.3109 (3) | 0.3253 (2) | 0.0232 (12) | |
| O16 | 0.3855 (4) | 0.1555 (3) | 0.1950 (2) | 0.0262 (12) | |
| O17 | 0.4274 (5) | 0.0358 (3) | 0.1872 (3) | 0.0278 (13) | |
| O18 | 0.6999 (4) | 0.3596 (3) | 0.3154 (3) | 0.0249 (12) | |
| O19 | 0.7094 (5) | 0.4754 (4) | 0.2770 (3) | 0.0287 (13) | |
| O21 | 1.0082 (8) | 0.7575 (6) | 0.1173 (4) | 0.077 (3) | |
| N1 | 0.6115 (5) | 0.2131 (4) | 0.3736 (3) | 0.0217 (14) | |
| N2 | 0.6810 (5) | 0.1129 (4) | 0.2135 (3) | 0.0236 (14) | |
| N3 | 0.7409 (5) | 0.3353 (4) | 0.1536 (3) | 0.0243 (15) | |
| N4 | 0.4572 (5) | 0.3925 (4) | 0.2015 (3) | 0.0193 (13) | |
| N5 | 0.3687 (5) | 0.2698 (4) | 0.3167 (3) | 0.0209 (14) | |
| N6 | 0.5978 (5) | 0.4465 (4) | 0.4367 (3) | 0.0266 (15) | |
| N7 | 0.5869 (6) | 0.5728 (4) | 0.4346 (3) | 0.0285 (16) | |
| N8 | 0.4444 (6) | 0.0020 (4) | 0.3156 (3) | 0.0278 (16) | |
| N9 | 0.2710 (6) | −0.0377 (5) | 0.3401 (4) | 0.0333 (18) | |
| N12 | 0.5058 (6) | 0.3922 (4) | 0.0623 (3) | 0.0261 (15) | |
| N13 | 0.3696 (6) | 0.3739 (5) | −0.0245 (4) | 0.0350 (18) | |
| N14 | 0.1925 (5) | 0.2156 (4) | 0.1289 (3) | 0.0222 (14) | |
| N15 | 0.0488 (5) | 0.1584 (4) | 0.0433 (3) | 0.0277 (16) | |
| N17 | 1.1778 (9) | 0.7400 (6) | 0.0974 (5) | 0.055 (2) | |
| C1 | 0.7412 (7) | 0.2946 (5) | 0.5097 (4) | 0.0273 (18) | |
| C2 | 0.8089 (7) | 0.3292 (6) | 0.5748 (4) | 0.033 (2) | |
| H2 | 0.8052 | 0.3805 | 0.6021 | 0.040* | |
| C3 | 0.8807 (8) | 0.2901 (6) | 0.6000 (5) | 0.039 (2) | |
| H3 | 0.9275 | 0.3149 | 0.6438 | 0.046* | |
| C4 | 0.8839 (7) | 0.2147 (6) | 0.5611 (4) | 0.036 (2) | |
| H4 | 0.9330 | 0.1874 | 0.5783 | 0.044* | |
| C5 | 0.8169 (7) | 0.1785 (6) | 0.4979 (4) | 0.030 (2) | |
| H5 | 0.8186 | 0.1257 | 0.4723 | 0.036* | |
| C6 | 0.7465 (7) | 0.2181 (5) | 0.4705 (4) | 0.0272 (18) | |
| C7 | 0.6808 (6) | 0.1773 (5) | 0.4001 (4) | 0.0244 (17) | |
| C8 | 0.6933 (7) | −0.0526 (5) | 0.2201 (4) | 0.0218 (17) | |
| C9 | 0.7137 (7) | −0.1284 (5) | 0.2253 (4) | 0.0277 (19) | |
| H9 | 0.6648 | −0.1667 | 0.2454 | 0.033* | |
| C10 | 0.8059 (8) | −0.1473 (5) | 0.2007 (4) | 0.031 (2) | |
| H10 | 0.8187 | −0.1988 | 0.2042 | 0.037* | |
| C11 | 0.8785 (7) | −0.0923 (5) | 0.1716 (4) | 0.031 (2) | |
| H11 | 0.9408 | −0.1058 | 0.1552 | 0.037* | |
| C12 | 0.8599 (7) | −0.0184 (5) | 0.1666 (4) | 0.0256 (18) | |
| H12 | 0.9101 | 0.0190 | 0.1464 | 0.031* | |
| C13 | 0.7674 (7) | 0.0042 (5) | 0.1907 (4) | 0.0256 (18) | |
| C14 | 0.7568 (6) | 0.0855 (5) | 0.1844 (4) | 0.0215 (16) | |
| C15 | 0.9623 (7) | 0.3627 (5) | 0.1109 (4) | 0.0240 (17) | |
| C16 | 1.0757 (7) | 0.3848 (6) | 0.0985 (5) | 0.036 (2) | |
| H16 | 1.1086 | 0.3418 | 0.0847 | 0.043* | |
| C17 | 1.1388 (8) | 0.4704 (6) | 0.1067 (5) | 0.040 (2) | |
| H17 | 1.2154 | 0.4856 | 0.0989 | 0.047* | |
| C18 | 1.0924 (8) | 0.5327 (6) | 0.1258 (6) | 0.046 (3) | |
| H18 | 1.1366 | 0.5908 | 0.1314 | 0.055* | |
| C19 | 0.9814 (8) | 0.5116 (6) | 0.1371 (5) | 0.037 (2) | |
| H19 | 0.9492 | 0.5553 | 0.1500 | 0.045* | |
| C20 | 0.9156 (7) | 0.4264 (5) | 0.1299 (4) | 0.0264 (18) | |
| C21 | 0.7958 (7) | 0.4102 (5) | 0.1423 (4) | 0.0265 (18) | |
| C22 | 0.5060 (7) | 0.5713 (5) | 0.2107 (4) | 0.0286 (19) | |
| C23 | 0.5196 (8) | 0.6574 (5) | 0.2185 (5) | 0.036 (2) | |
| H23 | 0.5829 | 0.6921 | 0.2042 | 0.044* | |
| C24 | 0.4455 (8) | 0.6936 (6) | 0.2458 (5) | 0.041 (2) | |
| H24 | 0.4584 | 0.7527 | 0.2507 | 0.050* | |
| C25 | 0.3511 (8) | 0.6445 (6) | 0.2665 (5) | 0.038 (2) | |
| H25 | 0.2998 | 0.6698 | 0.2858 | 0.046* | |
| C26 | 0.3327 (8) | 0.5587 (6) | 0.2587 (4) | 0.033 (2) | |
| H26 | 0.2671 | 0.5248 | 0.2717 | 0.040* | |
| C27 | 0.4097 (7) | 0.5201 (5) | 0.2316 (4) | 0.0271 (19) | |
| C28 | 0.3839 (7) | 0.4280 (5) | 0.2256 (4) | 0.0266 (18) | |
| C29 | 0.1312 (7) | 0.1794 (5) | 0.3100 (4) | 0.0247 (18) | |
| C30 | 0.0158 (7) | 0.1417 (6) | 0.3126 (4) | 0.032 (2) | |
| H30 | −0.0420 | 0.1291 | 0.2738 | 0.038* | |
| C31 | −0.0157 (7) | 0.1224 (6) | 0.3711 (4) | 0.032 (2) | |
| H31 | −0.0944 | 0.0962 | 0.3721 | 0.039* | |
| C32 | 0.0687 (8) | 0.1416 (6) | 0.4288 (4) | 0.039 (2) | |
| H32 | 0.0474 | 0.1284 | 0.4689 | 0.046* | |
| C33 | 0.1825 (7) | 0.1797 (6) | 0.4270 (4) | 0.031 (2) | |
| H33 | 0.2400 | 0.1918 | 0.4658 | 0.038* | |
| C34 | 0.2143 (7) | 0.2008 (5) | 0.3685 (4) | 0.0253 (18) | |
| C35 | 0.3384 (6) | 0.2455 (5) | 0.3703 (4) | 0.0224 (17) | |
| C36 | 0.3540 (8) | 0.0246 (6) | 0.3266 (4) | 0.033 (2) | |
| H36 | 0.3484 | 0.0787 | 0.3252 | 0.040* | |
| C37 | 0.4177 (9) | −0.0790 (6) | 0.3249 (5) | 0.039 (2) | |
| H37 | 0.4670 | −0.1123 | 0.3215 | 0.047* | |
| C38 | 0.3104 (9) | −0.1046 (6) | 0.3395 (5) | 0.043 (3) | |
| H38 | 0.2713 | −0.1578 | 0.3476 | 0.051* | |
| C39 | 0.1575 (8) | −0.0355 (7) | 0.3518 (6) | 0.046 (3) | |
| H39A | 0.1457 | 0.0152 | 0.3412 | 0.070* | |
| H39B | 0.1533 | −0.0333 | 0.3991 | 0.070* | |
| H39C | 0.0976 | −0.0868 | 0.3227 | 0.070* | |
| C44 | 0.4266 (7) | 0.4216 (6) | 0.0375 (4) | 0.032 (2) | |
| H44 | 0.4119 | 0.4705 | 0.0609 | 0.039* | |
| C45 | 0.4990 (8) | 0.3220 (6) | 0.0108 (4) | 0.038 (2) | |
| H45 | 0.5459 | 0.2873 | 0.0125 | 0.045* | |
| C46 | 0.4142 (9) | 0.3109 (7) | −0.0423 (5) | 0.043 (2) | |
| H46 | 0.3912 | 0.2676 | −0.0835 | 0.051* | |
| C47 | 0.2743 (9) | 0.3887 (7) | −0.0654 (5) | 0.049 (3) | |
| H47A | 0.2928 | 0.3932 | −0.1095 | 0.074* | |
| H47B | 0.2644 | 0.4418 | −0.0420 | 0.074* | |
| H47C | 0.2028 | 0.3410 | −0.0715 | 0.074* | |
| C48 | 0.0787 (6) | 0.1769 (5) | 0.1098 (4) | 0.0254 (18) | |
| H48 | 0.0268 | 0.1646 | 0.1396 | 0.030* | |
| C49 | 0.2341 (6) | 0.2238 (5) | 0.0704 (4) | 0.0253 (18) | |
| H49 | 0.3120 | 0.2503 | 0.0677 | 0.030* | |
| C50 | 0.1447 (8) | 0.1875 (6) | 0.0180 (4) | 0.037 (2) | |
| H50 | 0.1481 | 0.1832 | −0.0282 | 0.044* | |
| C51 | −0.0700 (7) | 0.1133 (6) | 0.0044 (4) | 0.035 (2) | |
| H51A | −0.1240 | 0.1078 | 0.0355 | 0.053* | |
| H51B | −0.0758 | 0.0565 | −0.0217 | 0.053* | |
| H51C | −0.0888 | 0.1460 | −0.0265 | 0.053* | |
| C52 | 0.5556 (7) | 0.4904 (5) | 0.4005 (4) | 0.0287 (18) | |
| H52 | 0.5096 | 0.4666 | 0.3565 | 0.034* | |
| C53 | 0.6594 (7) | 0.5046 (5) | 0.4962 (4) | 0.033 (2) | |
| H53 | 0.7000 | 0.4921 | 0.5324 | 0.040* | |
| C54 | 0.6531 (7) | 0.5821 (5) | 0.4953 (4) | 0.034 (2) | |
| H54 | 0.6879 | 0.6334 | 0.5301 | 0.041* | |
| C55 | 0.5553 (11) | 0.6383 (6) | 0.4112 (5) | 0.051 (3) | |
| H55A | 0.6230 | 0.6901 | 0.4213 | 0.077* | |
| H55B | 0.4952 | 0.6510 | 0.4339 | 0.077* | |
| H55C | 0.5264 | 0.6185 | 0.3623 | 0.077* | |
| C56 | 0.3561 (6) | 0.0741 (5) | 0.1823 (4) | 0.0237 (17) | |
| C57 | 0.2298 (7) | 0.0248 (5) | 0.1627 (5) | 0.035 (2) | |
| H57A | 0.1966 | 0.0393 | 0.1233 | 0.052* | |
| H57B | 0.1920 | 0.0394 | 0.2004 | 0.052* | |
| H57C | 0.2180 | −0.0365 | 0.1516 | 0.052* | |
| C58 | 0.7437 (7) | 0.4380 (5) | 0.3170 (4) | 0.0276 (19) | |
| C59 | 0.8493 (8) | 0.4901 (6) | 0.3721 (5) | 0.040 (2) | |
| H59A | 0.9188 | 0.4980 | 0.3526 | 0.060* | |
| H59B | 0.8535 | 0.4597 | 0.4072 | 0.060* | |
| H59C | 0.8434 | 0.5459 | 0.3919 | 0.060* | |
| C63 | 1.0639 (11) | 0.7248 (8) | 0.0824 (6) | 0.058 (3) | |
| H63 | 1.0231 | 0.6850 | 0.0408 | 0.070* | |
| C64 | 1.2447 (11) | 0.7980 (8) | 0.1637 (6) | 0.064 (3) | |
| H64A | 1.2936 | 0.7710 | 0.1848 | 0.096* | |
| H64B | 1.1916 | 0.8094 | 0.1930 | 0.096* | |
| H64C | 1.2929 | 0.8518 | 0.1569 | 0.096* | |
| C65 | 1.2376 (12) | 0.7004 (9) | 0.0527 (7) | 0.077 (4) | |
| H65A | 1.1816 | 0.6534 | 0.0171 | 0.115* | |
| H65B | 1.2881 | 0.6783 | 0.0781 | 0.115* | |
| H65C | 1.2838 | 0.7426 | 0.0322 | 0.115* | |
| O20 | 0.6594 (5) | 0.1644 (4) | 0.0531 (3) | 0.0323 (14) | |
| C60 | 0.5545 (7) | 0.1347 (5) | 0.0516 (4) | 0.032 (2) | |
| H60 | 0.5291 | 0.1407 | 0.0934 | 0.038* | |
| N16 | 0.4749 (6) | 0.0948 (4) | −0.0048 (3) | 0.0290 (16) | |
| C61 | 0.5092 (8) | 0.0841 (7) | −0.0693 (5) | 0.050 (3) | |
| H61A | 0.4738 | 0.0243 | −0.0953 | 0.076* | |
| H61B | 0.5932 | 0.1000 | −0.0626 | 0.076* | |
| H61C | 0.4840 | 0.1207 | −0.0941 | 0.076* | |
| C62 | 0.3540 (7) | 0.0622 (7) | −0.0048 (5) | 0.046 (3) | |
| H62A | 0.3124 | 0.0864 | −0.0340 | 0.069* | |
| H62B | 0.3409 | 0.0782 | 0.0413 | 0.069* | |
| H62C | 0.3262 | −0.0001 | −0.0215 | 0.069* | |
| N10 | 0.926 (2) | 0.2986 (16) | 0.2537 (10) | 0.018 (4) | 0.61983 (2000) |
| C40 | 0.9050 (18) | 0.298 (2) | 0.3148 (9) | 0.026 (4) | 0.61983 (2000) |
| H40 | 0.8322 | 0.2711 | 0.3245 | 0.031* | 0.61983 (2000) |
| C41 | 1.0418 (11) | 0.3449 (9) | 0.2625 (7) | 0.032 (3) | 0.61983 (2000) |
| H41 | 1.0806 | 0.3584 | 0.2272 | 0.038* | 0.61983 (2000) |
| C42 | 1.0926 (18) | 0.3686 (12) | 0.3292 (11) | 0.031 (4) | 0.61983 (2000) |
| H42 | 1.1719 | 0.3964 | 0.3488 | 0.037* | 0.61983 (2000) |
| N11 | 1.0021 (17) | 0.3431 (17) | 0.3619 (9) | 0.032 (3) | 0.61983 (2000) |
| C43 | 1.012 (3) | 0.352 (3) | 0.4357 (11) | 0.036 (4) | 0.61983 (2000) |
| H43A | 0.9349 | 0.3336 | 0.4463 | 0.055* | 0.61983 (2000) |
| H43B | 1.0529 | 0.4115 | 0.4602 | 0.055* | 0.61983 (2000) |
| H43C | 1.0547 | 0.3160 | 0.4491 | 0.055* | 0.61983 (2000) |
| O22 | 0.919 (3) | 0.304 (2) | 0.2376 (13) | 0.024 (7) | 0.38017 (2000) |
| C40B | 0.918 (3) | 0.303 (3) | 0.2977 (15) | 0.026 (4) | 0.38017 (2000) |
| H40B | 0.8482 | 0.2713 | 0.3083 | 0.031* | 0.38017 (2000) |
| N11B | 1.009 (3) | 0.343 (3) | 0.3485 (12) | 0.032 (3) | 0.38017 (2000) |
| C42B | 1.113 (3) | 0.398 (2) | 0.3362 (19) | 0.031 (4) | 0.38017 (2000) |
| H42A | 1.1787 | 0.3837 | 0.3544 | 0.037* | 0.38017 (2000) |
| H42B | 1.1227 | 0.4574 | 0.3583 | 0.037* | 0.38017 (2000) |
| H42C | 1.1076 | 0.3912 | 0.2875 | 0.037* | 0.38017 (2000) |
| C43B | 1.007 (6) | 0.343 (5) | 0.4180 (15) | 0.036 (4) | 0.38017 (2000) |
| H43D | 0.9357 | 0.3002 | 0.4208 | 0.055* | 0.38017 (2000) |
| H43E | 1.0116 | 0.3994 | 0.4436 | 0.055* | 0.38017 (2000) |
| H43F | 1.0732 | 0.3287 | 0.4367 | 0.055* | 0.38017 (2000) |
| C67 | 0.524 (2) | 0.885 (2) | 0.4934 (15) | 0.056 (9) | 0.50926 (1100) |
| H67A | 0.5447 | 0.8590 | 0.5288 | 0.084* | 0.50926 (1100) |
| H67B | 0.4699 | 0.8405 | 0.4553 | 0.084* | 0.50926 (1100) |
| H67C | 0.4871 | 0.9263 | 0.5114 | 0.084* | 0.50926 (1100) |
| C68 | 0.632 (4) | 0.931 (2) | 0.470 (4) | 0.070 (6) | 0.50926 (1100) |
| H68A | 0.6130 | 0.9652 | 0.4399 | 0.083* | 0.50926 (1100) |
| H68B | 0.6907 | 0.9695 | 0.5091 | 0.083* | 0.50926 (1100) |
| O23 | 0.6761 (11) | 0.8707 (9) | 0.4341 (7) | 0.042 (4) | 0.50926 (1100) |
| C69 | 0.779 (2) | 0.9130 (19) | 0.4118 (13) | 0.049 (5) | 0.50926 (1100) |
| H69A | 0.8372 | 0.9545 | 0.4504 | 0.059* | 0.50926 (1100) |
| H69B | 0.7610 | 0.9435 | 0.3784 | 0.059* | 0.50926 (1100) |
| C70 | 0.823 (2) | 0.8415 (19) | 0.3795 (12) | 0.072 (8) | 0.50926 (1100) |
| H70A | 0.7729 | 0.8086 | 0.3359 | 0.108* | 0.50926 (1100) |
| H70B | 0.8232 | 0.8037 | 0.4093 | 0.108* | 0.50926 (1100) |
| H70C | 0.9017 | 0.8668 | 0.3727 | 0.108* | 0.50926 (1100) |
| C67B | 0.527 (2) | 0.862 (2) | 0.4667 (16) | 0.059 (9) | 0.49074 (1100) |
| H67D | 0.5568 | 0.8294 | 0.4938 | 0.089* | 0.49074 (1100) |
| H67E | 0.5090 | 0.8312 | 0.4189 | 0.089* | 0.49074 (1100) |
| H67F | 0.4572 | 0.8692 | 0.4808 | 0.089* | 0.49074 (1100) |
| C68B | 0.618 (4) | 0.949 (2) | 0.477 (4) | 0.070 (6) | 0.49074 (1100) |
| H68C | 0.5947 | 0.9773 | 0.4431 | 0.083* | 0.49074 (1100) |
| H68D | 0.6219 | 0.9855 | 0.5224 | 0.083* | 0.49074 (1100) |
| O23B | 0.7272 (15) | 0.9433 (12) | 0.4718 (7) | 0.063 (5) | 0.49074 (1100) |
| C69B | 0.741 (2) | 0.920 (2) | 0.4032 (12) | 0.049 (5) | 0.49074 (1100) |
| H69C | 0.7267 | 0.9614 | 0.3789 | 0.059* | 0.49074 (1100) |
| H69D | 0.6857 | 0.8625 | 0.3796 | 0.059* | 0.49074 (1100) |
| C70B | 0.8652 (18) | 0.9207 (19) | 0.4061 (11) | 0.062 (8) | 0.49074 (1100) |
| H70D | 0.8836 | 0.8909 | 0.4394 | 0.092* | 0.49074 (1100) |
| H70E | 0.9178 | 0.9798 | 0.4192 | 0.092* | 0.49074 (1100) |
| H70F | 0.8735 | 0.8917 | 0.3616 | 0.092* | 0.49074 (1100) |
| O24 | 1.033 (2) | 0.7637 (18) | 0.2477 (12) | 0.069 (5) | 0.49074 (1100) |
| H24A | 0.98 (2) | 0.716 (6) | 0.246 (14) | 0.104* | 0.49074 (1100) |
| H24B | 1.066 (5) | 0.769 (19) | 0.215 (3) | 0.104* | 0.49074 (1100) |
| O25 | 0.8511 (16) | 0.6404 (11) | 0.2802 (10) | 0.057 (5) | 0.49074 (1100) |
| H25A | 0.8459 | 0.5927 | 0.2873 | 0.085* | 0.49074 (1100) |
| C72 | 0.8032 (19) | 0.6824 (14) | 0.3257 (10) | 0.050 (6) | 0.49074 (1100) |
| H72A | 0.8101 | 0.7384 | 0.3179 | 0.075* | 0.49074 (1100) |
| H72B | 0.8432 | 0.6899 | 0.3715 | 0.075* | 0.49074 (1100) |
| H72C | 0.7217 | 0.6492 | 0.3205 | 0.075* | 0.49074 (1100) |
| C71 | 0.880 (2) | 0.6747 (14) | 0.2613 (11) | 0.046 (6) | 0.50926 (1100) |
| H71A | 0.8977 | 0.6216 | 0.2546 | 0.069* | 0.50926 (1100) |
| H71B | 0.8191 | 0.6695 | 0.2241 | 0.069* | 0.50926 (1100) |
| H71C | 0.8529 | 0.6855 | 0.3041 | 0.069* | 0.50926 (1100) |
| O26 | 0.980 (2) | 0.7439 (17) | 0.2632 (12) | 0.069 (5) | 0.50926 (1100) |
| H26A | 0.9827 | 0.7503 | 0.2241 | 0.104* | 0.50926 (1100) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mn1 | 0.0181 (6) | 0.0201 (6) | 0.0178 (6) | 0.0066 (5) | 0.0045 (4) | 0.0000 (4) |
| Mn2 | 0.0220 (6) | 0.0225 (6) | 0.0200 (6) | 0.0084 (5) | 0.0012 (5) | −0.0033 (5) |
| Mn3 | 0.0233 (6) | 0.0202 (6) | 0.0210 (6) | 0.0079 (5) | 0.0066 (5) | −0.0002 (5) |
| Mn4 | 0.0236 (6) | 0.0234 (6) | 0.0313 (7) | 0.0111 (5) | 0.0132 (5) | 0.0063 (5) |
| Mn5 | 0.0222 (6) | 0.0208 (6) | 0.0266 (6) | 0.0102 (5) | 0.0097 (5) | 0.0039 (5) |
| Mn6 | 0.0177 (6) | 0.0257 (6) | 0.0178 (6) | 0.0059 (5) | 0.0040 (4) | 0.0004 (5) |
| O1 | 0.039 (3) | 0.030 (3) | 0.022 (3) | 0.019 (3) | −0.006 (2) | −0.006 (2) |
| O2 | 0.030 (3) | 0.025 (3) | 0.025 (3) | 0.014 (2) | 0.003 (2) | 0.001 (2) |
| O3 | 0.025 (3) | 0.022 (3) | 0.017 (3) | 0.006 (2) | 0.003 (2) | −0.007 (2) |
| O4 | 0.024 (3) | 0.020 (3) | 0.037 (3) | 0.009 (2) | 0.010 (3) | 0.003 (2) |
| O5 | 0.025 (3) | 0.017 (3) | 0.028 (3) | 0.006 (2) | 0.010 (2) | 0.006 (2) |
| O6 | 0.028 (3) | 0.017 (3) | 0.022 (3) | 0.005 (2) | 0.010 (2) | 0.004 (2) |
| O7 | 0.030 (3) | 0.028 (3) | 0.040 (3) | 0.010 (3) | 0.021 (3) | 0.005 (3) |
| O8 | 0.022 (3) | 0.027 (3) | 0.035 (3) | 0.010 (2) | 0.012 (2) | 0.004 (3) |
| O9 | 0.020 (3) | 0.022 (3) | 0.024 (3) | 0.008 (2) | 0.006 (2) | 0.002 (2) |
| O10 | 0.027 (3) | 0.030 (3) | 0.030 (3) | 0.016 (3) | 0.012 (3) | 0.006 (3) |
| O11 | 0.025 (3) | 0.029 (3) | 0.032 (3) | 0.014 (2) | 0.010 (2) | 0.003 (2) |
| O12 | 0.018 (3) | 0.020 (3) | 0.020 (3) | 0.005 (2) | 0.005 (2) | 0.003 (2) |
| O13 | 0.027 (3) | 0.033 (3) | 0.014 (3) | 0.003 (2) | 0.004 (2) | 0.004 (2) |
| O14 | 0.023 (3) | 0.028 (3) | 0.020 (3) | 0.012 (2) | 0.000 (2) | −0.002 (2) |
| O15 | 0.016 (2) | 0.024 (3) | 0.024 (3) | 0.004 (2) | 0.006 (2) | −0.002 (2) |
| O16 | 0.020 (3) | 0.028 (3) | 0.022 (3) | 0.007 (2) | −0.003 (2) | −0.005 (2) |
| O17 | 0.031 (3) | 0.025 (3) | 0.026 (3) | 0.012 (2) | 0.006 (2) | −0.001 (2) |
| O18 | 0.019 (3) | 0.023 (3) | 0.023 (3) | 0.002 (2) | 0.001 (2) | −0.005 (2) |
| O19 | 0.032 (3) | 0.029 (3) | 0.026 (3) | 0.012 (3) | 0.006 (2) | 0.005 (2) |
| O21 | 0.068 (6) | 0.097 (7) | 0.063 (5) | 0.054 (5) | −0.009 (5) | −0.011 (5) |
| N1 | 0.020 (3) | 0.024 (3) | 0.017 (3) | 0.005 (3) | 0.007 (3) | −0.003 (3) |
| N2 | 0.022 (3) | 0.014 (3) | 0.032 (4) | 0.005 (3) | 0.006 (3) | 0.000 (3) |
| N3 | 0.021 (3) | 0.024 (4) | 0.031 (4) | 0.011 (3) | 0.012 (3) | 0.006 (3) |
| N4 | 0.018 (3) | 0.017 (3) | 0.024 (3) | 0.008 (3) | 0.009 (3) | 0.004 (3) |
| N5 | 0.013 (3) | 0.019 (3) | 0.025 (3) | 0.000 (2) | 0.007 (2) | 0.000 (3) |
| N6 | 0.026 (3) | 0.024 (4) | 0.025 (3) | 0.008 (3) | 0.005 (3) | −0.003 (3) |
| N7 | 0.041 (4) | 0.021 (3) | 0.024 (3) | 0.013 (3) | 0.006 (3) | 0.002 (3) |
| N8 | 0.033 (4) | 0.023 (4) | 0.024 (4) | 0.005 (3) | 0.012 (3) | 0.001 (3) |
| N9 | 0.034 (4) | 0.035 (4) | 0.032 (4) | 0.010 (3) | 0.016 (3) | 0.008 (3) |
| N12 | 0.026 (4) | 0.032 (4) | 0.023 (3) | 0.013 (3) | 0.009 (3) | 0.005 (3) |
| N13 | 0.030 (4) | 0.049 (5) | 0.035 (4) | 0.022 (4) | 0.011 (3) | 0.017 (4) |
| N14 | 0.019 (3) | 0.027 (4) | 0.021 (3) | 0.010 (3) | 0.008 (3) | 0.003 (3) |
| N15 | 0.023 (3) | 0.031 (4) | 0.022 (3) | 0.010 (3) | −0.002 (3) | −0.007 (3) |
| N17 | 0.063 (6) | 0.058 (6) | 0.051 (6) | 0.025 (5) | 0.017 (5) | 0.021 (5) |
| C1 | 0.026 (4) | 0.022 (4) | 0.029 (4) | 0.005 (3) | 0.005 (3) | 0.002 (3) |
| C2 | 0.029 (5) | 0.039 (5) | 0.026 (4) | 0.011 (4) | −0.002 (4) | −0.001 (4) |
| C3 | 0.027 (5) | 0.054 (6) | 0.031 (5) | 0.013 (4) | −0.003 (4) | 0.007 (4) |
| C4 | 0.024 (4) | 0.051 (6) | 0.033 (5) | 0.013 (4) | 0.008 (4) | 0.007 (4) |
| C5 | 0.026 (4) | 0.036 (5) | 0.034 (5) | 0.015 (4) | 0.014 (4) | 0.008 (4) |
| C6 | 0.018 (4) | 0.029 (5) | 0.028 (4) | 0.001 (3) | 0.006 (3) | 0.003 (4) |
| C7 | 0.017 (4) | 0.023 (4) | 0.031 (4) | 0.003 (3) | 0.012 (3) | 0.004 (3) |
| C8 | 0.030 (4) | 0.016 (4) | 0.019 (4) | 0.011 (3) | 0.002 (3) | 0.000 (3) |
| C9 | 0.033 (5) | 0.021 (4) | 0.026 (4) | 0.008 (4) | 0.007 (4) | 0.002 (3) |
| C10 | 0.038 (5) | 0.019 (4) | 0.037 (5) | 0.011 (4) | 0.010 (4) | 0.007 (4) |
| C11 | 0.032 (5) | 0.026 (5) | 0.032 (5) | 0.015 (4) | 0.007 (4) | −0.003 (4) |
| C12 | 0.022 (4) | 0.023 (4) | 0.029 (4) | 0.008 (3) | 0.007 (3) | −0.001 (3) |
| C13 | 0.026 (4) | 0.024 (4) | 0.024 (4) | 0.011 (3) | 0.007 (3) | −0.004 (3) |
| C14 | 0.022 (4) | 0.017 (4) | 0.022 (4) | 0.002 (3) | 0.002 (3) | 0.004 (3) |
| C15 | 0.020 (4) | 0.031 (4) | 0.024 (4) | 0.008 (3) | 0.011 (3) | 0.010 (3) |
| C16 | 0.028 (5) | 0.046 (6) | 0.044 (6) | 0.023 (4) | 0.014 (4) | 0.012 (4) |
| C17 | 0.025 (5) | 0.043 (6) | 0.060 (7) | 0.015 (4) | 0.020 (4) | 0.020 (5) |
| C18 | 0.031 (5) | 0.033 (5) | 0.072 (8) | 0.003 (4) | 0.019 (5) | 0.016 (5) |
| C19 | 0.032 (5) | 0.024 (5) | 0.062 (7) | 0.013 (4) | 0.014 (5) | 0.016 (4) |
| C20 | 0.020 (4) | 0.030 (5) | 0.031 (5) | 0.011 (3) | 0.008 (3) | 0.008 (4) |
| C21 | 0.025 (4) | 0.030 (5) | 0.023 (4) | 0.012 (4) | 0.006 (3) | −0.002 (3) |
| C22 | 0.030 (4) | 0.030 (5) | 0.030 (4) | 0.019 (4) | 0.006 (4) | 0.003 (4) |
| C23 | 0.045 (6) | 0.017 (4) | 0.046 (6) | 0.009 (4) | 0.017 (5) | 0.005 (4) |
| C24 | 0.041 (6) | 0.032 (5) | 0.054 (6) | 0.019 (4) | 0.015 (5) | 0.005 (5) |
| C25 | 0.037 (5) | 0.036 (5) | 0.051 (6) | 0.024 (4) | 0.021 (5) | 0.006 (4) |
| C26 | 0.032 (5) | 0.031 (5) | 0.039 (5) | 0.017 (4) | 0.011 (4) | 0.001 (4) |
| C27 | 0.022 (4) | 0.028 (4) | 0.029 (4) | 0.014 (3) | 0.004 (3) | −0.004 (3) |
| C28 | 0.020 (4) | 0.032 (5) | 0.026 (4) | 0.010 (3) | 0.001 (3) | 0.002 (4) |
| C29 | 0.025 (4) | 0.023 (4) | 0.022 (4) | 0.006 (3) | 0.004 (3) | 0.001 (3) |
| C30 | 0.027 (4) | 0.036 (5) | 0.021 (4) | 0.003 (4) | 0.006 (3) | −0.003 (4) |
| C31 | 0.021 (4) | 0.041 (5) | 0.030 (5) | 0.005 (4) | 0.009 (4) | 0.006 (4) |
| C32 | 0.029 (5) | 0.056 (6) | 0.026 (5) | 0.009 (4) | 0.006 (4) | 0.008 (4) |
| C33 | 0.032 (5) | 0.036 (5) | 0.026 (4) | 0.011 (4) | 0.009 (4) | 0.006 (4) |
| C34 | 0.023 (4) | 0.028 (4) | 0.025 (4) | 0.012 (3) | 0.008 (3) | −0.002 (3) |
| C35 | 0.023 (4) | 0.021 (4) | 0.019 (4) | 0.010 (3) | 0.007 (3) | −0.006 (3) |
| C36 | 0.033 (5) | 0.031 (5) | 0.038 (5) | 0.012 (4) | 0.011 (4) | 0.009 (4) |
| C37 | 0.048 (6) | 0.031 (5) | 0.048 (6) | 0.019 (4) | 0.030 (5) | 0.010 (4) |
| C38 | 0.058 (7) | 0.028 (5) | 0.057 (6) | 0.022 (5) | 0.036 (5) | 0.014 (5) |
| C39 | 0.036 (5) | 0.044 (6) | 0.068 (7) | 0.017 (5) | 0.026 (5) | 0.020 (5) |
| C44 | 0.029 (5) | 0.042 (5) | 0.031 (5) | 0.019 (4) | 0.009 (4) | 0.009 (4) |
| C45 | 0.047 (6) | 0.043 (6) | 0.027 (5) | 0.021 (5) | 0.014 (4) | 0.006 (4) |
| C46 | 0.050 (6) | 0.049 (6) | 0.030 (5) | 0.021 (5) | 0.010 (5) | 0.003 (4) |
| C47 | 0.036 (6) | 0.067 (7) | 0.053 (7) | 0.021 (5) | 0.005 (5) | 0.031 (6) |
| C48 | 0.019 (4) | 0.034 (5) | 0.023 (4) | 0.015 (3) | 0.003 (3) | 0.000 (3) |
| C49 | 0.017 (4) | 0.030 (4) | 0.028 (4) | 0.010 (3) | 0.004 (3) | 0.001 (3) |
| C50 | 0.044 (5) | 0.050 (6) | 0.017 (4) | 0.018 (5) | 0.009 (4) | 0.003 (4) |
| C51 | 0.037 (5) | 0.033 (5) | 0.022 (4) | 0.001 (4) | 0.001 (4) | −0.004 (4) |
| C52 | 0.036 (5) | 0.024 (4) | 0.022 (4) | 0.010 (4) | 0.003 (3) | 0.000 (3) |
| C53 | 0.029 (4) | 0.035 (5) | 0.025 (4) | 0.010 (4) | −0.004 (3) | −0.008 (4) |
| C54 | 0.035 (5) | 0.029 (5) | 0.028 (4) | 0.007 (4) | 0.008 (4) | −0.009 (4) |
| C55 | 0.091 (9) | 0.028 (5) | 0.037 (5) | 0.029 (5) | 0.002 (5) | 0.004 (4) |
| C56 | 0.024 (4) | 0.025 (4) | 0.015 (4) | 0.004 (3) | 0.003 (3) | −0.003 (3) |
| C57 | 0.025 (4) | 0.021 (4) | 0.044 (5) | 0.001 (3) | −0.003 (4) | −0.006 (4) |
| C58 | 0.027 (4) | 0.028 (5) | 0.019 (4) | 0.004 (4) | 0.010 (3) | −0.007 (3) |
| C59 | 0.035 (5) | 0.032 (5) | 0.043 (5) | 0.011 (4) | −0.008 (4) | −0.001 (4) |
| C63 | 0.060 (8) | 0.056 (7) | 0.053 (7) | 0.018 (6) | −0.006 (6) | 0.012 (6) |
| C64 | 0.063 (8) | 0.056 (8) | 0.069 (8) | 0.014 (6) | 0.003 (7) | 0.018 (6) |
| C65 | 0.082 (10) | 0.095 (11) | 0.075 (9) | 0.036 (8) | 0.050 (8) | 0.039 (8) |
| O20 | 0.029 (3) | 0.036 (3) | 0.030 (3) | 0.010 (3) | 0.009 (3) | 0.004 (3) |
| C60 | 0.041 (5) | 0.028 (4) | 0.020 (4) | 0.017 (4) | −0.006 (4) | −0.007 (3) |
| N16 | 0.028 (4) | 0.033 (4) | 0.018 (3) | 0.009 (3) | 0.000 (3) | −0.005 (3) |
| C61 | 0.038 (5) | 0.055 (7) | 0.050 (6) | 0.013 (5) | 0.015 (5) | −0.003 (5) |
| C62 | 0.030 (5) | 0.059 (7) | 0.041 (5) | 0.015 (5) | 0.013 (4) | −0.006 (5) |
| N10 | 0.028 (7) | 0.025 (7) | 0.010 (8) | 0.017 (5) | 0.002 (6) | 0.010 (6) |
| C40 | 0.019 (6) | 0.030 (5) | 0.027 (8) | 0.009 (5) | 0.001 (6) | 0.007 (8) |
| C41 | 0.026 (4) | 0.032 (4) | 0.030 (6) | 0.011 (3) | 0.004 (4) | −0.005 (5) |
| C42 | 0.028 (7) | 0.031 (10) | 0.030 (6) | 0.009 (7) | 0.006 (5) | 0.000 (7) |
| N11 | 0.026 (4) | 0.032 (4) | 0.030 (6) | 0.011 (3) | 0.004 (4) | −0.005 (5) |
| C43 | 0.036 (6) | 0.048 (12) | 0.015 (11) | 0.010 (6) | 0.005 (11) | −0.007 (13) |
| O22 | 0.018 (9) | 0.028 (10) | 0.024 (11) | 0.004 (7) | −0.005 (8) | 0.009 (8) |
| C40B | 0.019 (6) | 0.030 (5) | 0.027 (8) | 0.009 (5) | 0.001 (6) | 0.007 (8) |
| N11B | 0.026 (4) | 0.032 (4) | 0.030 (6) | 0.011 (3) | 0.004 (4) | −0.005 (5) |
| C42B | 0.028 (7) | 0.031 (10) | 0.030 (6) | 0.009 (7) | 0.006 (5) | 0.000 (7) |
| C43B | 0.036 (6) | 0.048 (12) | 0.015 (11) | 0.010 (6) | 0.005 (11) | −0.007 (13) |
| C67 | 0.064 (15) | 0.063 (16) | 0.051 (15) | 0.019 (12) | 0.036 (12) | 0.025 (12) |
| C68 | 0.074 (14) | 0.084 (17) | 0.051 (16) | 0.024 (12) | 0.018 (9) | 0.019 (17) |
| O23 | 0.044 (8) | 0.041 (8) | 0.044 (8) | 0.017 (7) | 0.018 (7) | 0.008 (7) |
| C69 | 0.056 (11) | 0.059 (7) | 0.036 (7) | 0.019 (7) | 0.009 (8) | 0.020 (5) |
| C70 | 0.054 (15) | 0.12 (3) | 0.044 (14) | 0.032 (16) | 0.016 (12) | 0.016 (16) |
| C67B | 0.072 (19) | 0.07 (2) | 0.07 (2) | 0.049 (17) | 0.037 (18) | 0.037 (18) |
| C68B | 0.074 (14) | 0.084 (17) | 0.051 (16) | 0.024 (12) | 0.018 (9) | 0.019 (17) |
| O23B | 0.070 (12) | 0.085 (14) | 0.038 (9) | 0.031 (11) | 0.010 (9) | 0.015 (9) |
| C69B | 0.056 (11) | 0.059 (7) | 0.036 (7) | 0.019 (7) | 0.009 (8) | 0.020 (5) |
| C70B | 0.040 (13) | 0.09 (2) | 0.038 (13) | 0.011 (13) | −0.001 (11) | 0.012 (14) |
| O24 | 0.064 (14) | 0.079 (12) | 0.057 (11) | 0.020 (12) | 0.002 (9) | 0.015 (8) |
| O25 | 0.062 (11) | 0.037 (9) | 0.069 (12) | 0.010 (8) | 0.013 (9) | 0.022 (8) |
| C72 | 0.052 (13) | 0.053 (14) | 0.037 (12) | 0.004 (11) | 0.003 (10) | 0.016 (10) |
| C71 | 0.071 (17) | 0.030 (12) | 0.031 (12) | 0.017 (12) | −0.008 (11) | 0.007 (9) |
| O26 | 0.064 (14) | 0.079 (12) | 0.057 (11) | 0.020 (12) | 0.002 (9) | 0.015 (8) |
Geometric parameters (Å, º)
| Mn1—O18 | 2.211 (5) | C29—C30 | 1.396 (11) |
| Mn1—O6 | 2.226 (5) | C29—C34 | 1.399 (11) |
| Mn1—O15 | 2.237 (5) | C30—C31 | 1.387 (12) |
| Mn1—O9 | 2.244 (5) | C30—H30 | 0.9500 |
| Mn1—O12 | 2.246 (5) | C31—C32 | 1.400 (12) |
| Mn1—O3 | 2.265 (6) | C31—H31 | 0.9500 |
| Mn1—O16 | 2.280 (5) | C32—C33 | 1.375 (12) |
| Mn2—O1 | 1.851 (5) | C32—H32 | 0.9500 |
| Mn2—O15 | 1.889 (5) | C33—C34 | 1.397 (12) |
| Mn2—N1 | 1.970 (6) | C33—H33 | 0.9500 |
| Mn2—N6 | 2.045 (6) | C34—C35 | 1.492 (10) |
| Mn2—O14 | 2.132 (5) | C36—H36 | 0.9500 |
| Mn3—O4 | 1.874 (5) | C37—C38 | 1.362 (13) |
| Mn3—O3 | 1.901 (5) | C37—H37 | 0.9500 |
| Mn3—N2 | 2.005 (7) | C38—H38 | 0.9500 |
| Mn3—N8 | 2.067 (7) | C39—H39A | 0.9800 |
| Mn3—O2 | 2.119 (5) | C39—H39B | 0.9800 |
| Mn3—O17 | 2.245 (6) | C39—H39C | 0.9800 |
| Mn4—O7 | 1.869 (6) | C44—H44 | 0.9500 |
| Mn4—O6 | 1.912 (5) | C45—C46 | 1.368 (13) |
| Mn4—O5 | 1.951 (5) | C45—H45 | 0.9500 |
| Mn4—N3 | 1.957 (7) | C46—H46 | 0.9500 |
| Mn4—O22 | 2.08 (3) | C47—H47A | 0.9800 |
| Mn4—O20 | 2.308 (6) | C47—H47B | 0.9800 |
| Mn4—N10 | 2.35 (2) | C47—H47C | 0.9800 |
| Mn5—O10 | 1.871 (6) | C48—H48 | 0.9500 |
| Mn5—O9 | 1.938 (5) | C49—C50 | 1.345 (11) |
| Mn5—O8 | 1.942 (5) | C49—H49 | 0.9500 |
| Mn5—N4 | 1.945 (6) | C50—H50 | 0.9500 |
| Mn5—O19 | 2.267 (6) | C51—H51A | 0.9800 |
| Mn5—N12 | 2.282 (7) | C51—H51B | 0.9800 |
| Mn6—O13 | 1.864 (5) | C51—H51C | 0.9800 |
| Mn6—O12 | 1.916 (5) | C52—H52 | 0.9500 |
| Mn6—N5 | 1.967 (6) | C53—C54 | 1.349 (12) |
| Mn6—N14 | 2.043 (6) | C53—H53 | 0.9500 |
| Mn6—O11 | 2.144 (6) | C54—H54 | 0.9500 |
| Mn6—O16 | 2.379 (5) | C55—H55A | 0.9800 |
| O1—C1 | 1.337 (10) | C55—H55B | 0.9800 |
| O2—C7 | 1.279 (9) | C55—H55C | 0.9800 |
| O3—N1 | 1.419 (7) | C56—C57 | 1.498 (10) |
| O4—C8 | 1.321 (9) | C57—H57A | 0.9800 |
| O5—C14 | 1.310 (9) | C57—H57B | 0.9800 |
| O6—N2 | 1.434 (8) | C57—H57C | 0.9800 |
| O7—C15 | 1.343 (10) | C58—C59 | 1.526 (11) |
| O8—C21 | 1.288 (10) | C59—H59A | 0.9800 |
| O9—N3 | 1.406 (8) | C59—H59B | 0.9800 |
| O10—C22 | 1.332 (9) | C59—H59C | 0.9800 |
| O11—C28 | 1.284 (10) | C63—H63 | 0.9500 |
| O12—N4 | 1.404 (7) | C64—H64A | 0.9800 |
| O13—C29 | 1.333 (9) | C64—H64B | 0.9800 |
| O14—C35 | 1.296 (9) | C64—H64C | 0.9800 |
| O15—N5 | 1.404 (7) | C65—H65A | 0.9800 |
| O16—C56 | 1.281 (9) | C65—H65B | 0.9800 |
| O17—C56 | 1.255 (9) | C65—H65C | 0.9800 |
| O18—C58 | 1.272 (10) | O20—C60 | 1.241 (10) |
| O19—C58 | 1.254 (10) | C60—N16 | 1.338 (9) |
| O21—C63 | 1.219 (14) | C60—H60 | 0.9500 |
| N1—C7 | 1.325 (10) | N16—C62 | 1.436 (10) |
| N2—C14 | 1.320 (9) | N16—C61 | 1.438 (11) |
| N3—C21 | 1.335 (10) | C61—H61A | 0.9800 |
| N4—C28 | 1.325 (9) | C61—H61B | 0.9800 |
| N5—C35 | 1.319 (10) | C61—H61C | 0.9800 |
| N6—C52 | 1.329 (11) | C62—H62A | 0.9800 |
| N6—C53 | 1.373 (9) | C62—H62B | 0.9800 |
| N7—C52 | 1.343 (9) | C62—H62C | 0.9800 |
| N7—C54 | 1.367 (11) | N10—C40 | 1.32 (2) |
| N7—C55 | 1.441 (11) | N10—C41 | 1.38 (3) |
| N8—C36 | 1.320 (11) | C40—N11 | 1.36 (2) |
| N8—C37 | 1.377 (11) | C40—H40 | 0.9500 |
| N9—C36 | 1.341 (11) | C41—C42 | 1.37 (3) |
| N9—C38 | 1.366 (12) | C41—H41 | 0.9500 |
| N9—C39 | 1.467 (11) | C42—N11 | 1.38 (2) |
| N12—C44 | 1.328 (11) | C42—H42 | 0.9500 |
| N12—C45 | 1.395 (10) | N11—C43 | 1.49 (2) |
| N13—C44 | 1.344 (11) | C43—H43A | 0.9800 |
| N13—C46 | 1.351 (12) | C43—H43B | 0.9800 |
| N13—C47 | 1.489 (12) | C43—H43C | 0.9800 |
| N14—C48 | 1.337 (9) | O22—C40B | 1.25 (2) |
| N14—C49 | 1.386 (10) | C40B—N11B | 1.34 (2) |
| N15—C48 | 1.323 (10) | C40B—H40B | 0.9500 |
| N15—C50 | 1.355 (11) | N11B—C42B | 1.43 (2) |
| N15—C51 | 1.481 (10) | N11B—C43B | 1.44 (2) |
| N17—C63 | 1.344 (15) | C42B—H42A | 0.9800 |
| N17—C65 | 1.434 (14) | C42B—H42B | 0.9800 |
| N17—C64 | 1.487 (14) | C42B—H42C | 0.9800 |
| C1—C2 | 1.403 (11) | C43B—H43D | 0.9800 |
| C1—C6 | 1.405 (11) | C43B—H43E | 0.9800 |
| C2—C3 | 1.380 (13) | C43B—H43F | 0.9800 |
| C2—H2 | 0.9500 | C67—C68 | 1.53 (2) |
| C3—C4 | 1.377 (13) | C67—H67A | 0.9800 |
| C3—H3 | 0.9500 | C67—H67B | 0.9800 |
| C4—C5 | 1.370 (12) | C67—H67C | 0.9800 |
| C4—H4 | 0.9500 | C68—O23 | 1.41 (3) |
| C5—C6 | 1.391 (12) | C68—H68A | 0.9900 |
| C5—H5 | 0.9500 | C68—H68B | 0.9900 |
| C6—C7 | 1.489 (11) | O23—C69 | 1.44 (2) |
| C8—C9 | 1.409 (11) | C69—C70 | 1.54 (3) |
| C8—C13 | 1.412 (11) | C69—H69A | 0.9900 |
| C9—C10 | 1.400 (12) | C69—H69B | 0.9900 |
| C9—H9 | 0.9500 | C70—H70A | 0.9800 |
| C10—C11 | 1.381 (12) | C70—H70B | 0.9800 |
| C10—H10 | 0.9500 | C70—H70C | 0.9800 |
| C11—C12 | 1.367 (12) | C67B—C68B | 1.53 (3) |
| C11—H11 | 0.9500 | C67B—H67D | 0.9800 |
| C12—C13 | 1.426 (10) | C67B—H67E | 0.9800 |
| C12—H12 | 0.9500 | C67B—H67F | 0.9800 |
| C13—C14 | 1.458 (11) | C68B—O23B | 1.41 (3) |
| C15—C20 | 1.385 (11) | C68B—H68C | 0.9900 |
| C15—C16 | 1.413 (11) | C68B—H68D | 0.9900 |
| C16—C17 | 1.394 (13) | O23B—C69B | 1.43 (2) |
| C16—H16 | 0.9500 | C69B—C70B | 1.54 (3) |
| C17—C18 | 1.362 (13) | C69B—H69C | 0.9900 |
| C17—H17 | 0.9500 | C69B—H69D | 0.9900 |
| C18—C19 | 1.377 (12) | C70B—H70D | 0.9800 |
| C18—H18 | 0.9500 | C70B—H70E | 0.9800 |
| C19—C20 | 1.400 (12) | C70B—H70F | 0.9800 |
| C19—H19 | 0.9500 | O24—H24A | 0.85 (2) |
| C20—C21 | 1.491 (11) | O24—H24B | 0.85 (2) |
| C22—C23 | 1.394 (11) | O25—C72 | 1.36 (3) |
| C22—C27 | 1.425 (12) | O25—H25A | 0.8400 |
| C23—C24 | 1.365 (12) | C72—H72A | 0.9800 |
| C23—H23 | 0.9500 | C72—H72B | 0.9800 |
| C24—C25 | 1.391 (13) | C72—H72C | 0.9800 |
| C24—H24 | 0.9500 | C71—O26 | 1.43 (4) |
| C25—C26 | 1.378 (12) | C71—H71A | 0.9800 |
| C25—H25 | 0.9500 | C71—H71B | 0.9800 |
| C26—C27 | 1.416 (11) | C71—H71C | 0.9800 |
| C26—H26 | 0.9500 | O26—H26A | 0.8400 |
| C27—C28 | 1.477 (12) | ||
| O18—Mn1—O6 | 93.13 (19) | C27—C26—H26 | 119.4 |
| O18—Mn1—O15 | 70.27 (18) | C26—C27—C22 | 118.7 (8) |
| O6—Mn1—O15 | 148.3 (2) | C26—C27—C28 | 117.6 (8) |
| O18—Mn1—O9 | 87.6 (2) | C22—C27—C28 | 123.7 (7) |
| O6—Mn1—O9 | 77.54 (19) | O11—C28—N4 | 121.0 (7) |
| O15—Mn1—O9 | 126.44 (19) | O11—C28—C27 | 121.5 (7) |
| O18—Mn1—O12 | 114.48 (19) | N4—C28—C27 | 117.5 (7) |
| O6—Mn1—O12 | 137.17 (18) | O13—C29—C30 | 116.1 (7) |
| O15—Mn1—O12 | 74.31 (18) | O13—C29—C34 | 125.3 (7) |
| O9—Mn1—O12 | 71.99 (18) | C30—C29—C34 | 118.6 (8) |
| O18—Mn1—O3 | 89.1 (2) | C31—C30—C29 | 120.8 (8) |
| O6—Mn1—O3 | 77.94 (18) | C31—C30—H30 | 119.6 |
| O15—Mn1—O3 | 75.07 (19) | C29—C30—H30 | 119.6 |
| O9—Mn1—O3 | 155.02 (18) | C30—C31—C32 | 120.0 (8) |
| O12—Mn1—O3 | 131.25 (19) | C30—C31—H31 | 120.0 |
| O18—Mn1—O16 | 157.79 (19) | C32—C31—H31 | 120.0 |
| O6—Mn1—O16 | 101.13 (18) | C33—C32—C31 | 119.6 (9) |
| O15—Mn1—O16 | 89.02 (18) | C33—C32—H32 | 120.2 |
| O9—Mn1—O16 | 111.98 (19) | C31—C32—H32 | 120.2 |
| O12—Mn1—O16 | 65.01 (19) | C32—C33—C34 | 120.5 (8) |
| O3—Mn1—O16 | 77.50 (19) | C32—C33—H33 | 119.7 |
| O1—Mn2—O15 | 175.9 (2) | C34—C33—H33 | 119.7 |
| O1—Mn2—N1 | 89.4 (2) | C33—C34—C29 | 120.3 (7) |
| O15—Mn2—N1 | 94.2 (2) | C33—C34—C35 | 118.2 (7) |
| O1—Mn2—N6 | 87.0 (3) | C29—C34—C35 | 121.4 (7) |
| O15—Mn2—N6 | 89.0 (2) | O14—C35—N5 | 121.4 (7) |
| N1—Mn2—N6 | 163.1 (3) | O14—C35—C34 | 119.8 (7) |
| O1—Mn2—O14 | 103.4 (2) | N5—C35—C34 | 118.8 (7) |
| O15—Mn2—O14 | 78.2 (2) | N8—C36—N9 | 112.2 (8) |
| N1—Mn2—O14 | 95.8 (2) | N8—C36—H36 | 123.9 |
| N6—Mn2—O14 | 101.1 (2) | N9—C36—H36 | 123.9 |
| O4—Mn3—O3 | 176.2 (2) | C38—C37—N8 | 110.3 (8) |
| O4—Mn3—N2 | 88.5 (2) | C38—C37—H37 | 124.8 |
| O3—Mn3—N2 | 91.5 (2) | N8—C37—H37 | 124.8 |
| O4—Mn3—N8 | 89.7 (3) | C37—C38—N9 | 105.6 (8) |
| O3—Mn3—N8 | 91.0 (2) | C37—C38—H38 | 127.2 |
| N2—Mn3—N8 | 169.5 (3) | N9—C38—H38 | 127.2 |
| O4—Mn3—O2 | 97.5 (2) | N9—C39—H39A | 109.5 |
| O3—Mn3—O2 | 78.8 (2) | N9—C39—H39B | 109.5 |
| N2—Mn3—O2 | 99.1 (2) | H39A—C39—H39B | 109.5 |
| N8—Mn3—O2 | 91.4 (2) | N9—C39—H39C | 109.5 |
| O4—Mn3—O17 | 96.3 (2) | H39A—C39—H39C | 109.5 |
| O3—Mn3—O17 | 87.5 (2) | H39B—C39—H39C | 109.5 |
| N2—Mn3—O17 | 88.0 (2) | N12—C44—N13 | 112.1 (8) |
| N8—Mn3—O17 | 81.9 (2) | N12—C44—H44 | 124.0 |
| O2—Mn3—O17 | 164.7 (2) | N13—C44—H44 | 124.0 |
| O7—Mn4—O6 | 179.0 (2) | C46—C45—N12 | 109.5 (8) |
| O7—Mn4—O5 | 97.2 (2) | C46—C45—H45 | 125.2 |
| O6—Mn4—O5 | 82.2 (2) | N12—C45—H45 | 125.2 |
| O7—Mn4—N3 | 90.2 (3) | N13—C46—C45 | 106.4 (8) |
| O6—Mn4—N3 | 90.4 (2) | N13—C46—H46 | 126.8 |
| O5—Mn4—N3 | 171.3 (2) | C45—C46—H46 | 126.8 |
| O7—Mn4—O22 | 86.4 (10) | N13—C47—H47A | 109.5 |
| O6—Mn4—O22 | 94.4 (10) | N13—C47—H47B | 109.5 |
| O5—Mn4—O22 | 93.6 (11) | H47A—C47—H47B | 109.5 |
| N3—Mn4—O22 | 91.5 (11) | N13—C47—H47C | 109.5 |
| O7—Mn4—O20 | 90.9 (2) | H47A—C47—H47C | 109.5 |
| O6—Mn4—O20 | 88.3 (2) | H47B—C47—H47C | 109.5 |
| O5—Mn4—O20 | 86.3 (2) | N15—C48—N14 | 110.0 (7) |
| N3—Mn4—O20 | 88.9 (2) | N15—C48—H48 | 125.0 |
| O22—Mn4—O20 | 177.3 (10) | N14—C48—H48 | 125.0 |
| O7—Mn4—N10 | 91.7 (6) | C50—C49—N14 | 107.9 (7) |
| O6—Mn4—N10 | 89.0 (6) | C50—C49—H49 | 126.1 |
| O5—Mn4—N10 | 88.7 (7) | N14—C49—H49 | 126.1 |
| N3—Mn4—N10 | 95.7 (7) | C49—C50—N15 | 107.5 (7) |
| O20—Mn4—N10 | 174.6 (6) | C49—C50—H50 | 126.3 |
| O10—Mn5—O9 | 177.8 (2) | N15—C50—H50 | 126.3 |
| O10—Mn5—O8 | 95.9 (2) | N15—C51—H51A | 109.5 |
| O9—Mn5—O8 | 82.5 (2) | N15—C51—H51B | 109.5 |
| O10—Mn5—N4 | 89.4 (2) | H51A—C51—H51B | 109.5 |
| O9—Mn5—N4 | 92.2 (2) | N15—C51—H51C | 109.5 |
| O8—Mn5—N4 | 174.5 (3) | H51A—C51—H51C | 109.5 |
| O10—Mn5—O19 | 91.3 (2) | H51B—C51—H51C | 109.5 |
| O9—Mn5—O19 | 87.2 (2) | N6—C52—N7 | 110.9 (7) |
| O8—Mn5—O19 | 84.4 (2) | N6—C52—H52 | 124.6 |
| N4—Mn5—O19 | 93.7 (2) | N7—C52—H52 | 124.6 |
| O10—Mn5—N12 | 91.3 (2) | C54—C53—N6 | 109.4 (8) |
| O9—Mn5—N12 | 90.1 (2) | C54—C53—H53 | 125.3 |
| O8—Mn5—N12 | 91.3 (2) | N6—C53—H53 | 125.3 |
| N4—Mn5—N12 | 90.3 (2) | C53—C54—N7 | 106.9 (7) |
| O19—Mn5—N12 | 175.2 (2) | C53—C54—H54 | 126.6 |
| O13—Mn6—O12 | 174.6 (2) | N7—C54—H54 | 126.6 |
| O13—Mn6—N5 | 87.7 (2) | N7—C55—H55A | 109.5 |
| O12—Mn6—N5 | 93.5 (2) | N7—C55—H55B | 109.5 |
| O13—Mn6—N14 | 87.6 (2) | H55A—C55—H55B | 109.5 |
| O12—Mn6—N14 | 90.5 (2) | N7—C55—H55C | 109.5 |
| N5—Mn6—N14 | 171.0 (3) | H55A—C55—H55C | 109.5 |
| O13—Mn6—O11 | 107.3 (2) | H55B—C55—H55C | 109.5 |
| O12—Mn6—O11 | 77.9 (2) | O17—C56—O16 | 123.0 (7) |
| N5—Mn6—O11 | 93.7 (2) | O17—C56—C57 | 120.2 (7) |
| N14—Mn6—O11 | 95.0 (2) | O16—C56—C57 | 116.8 (7) |
| O13—Mn6—O16 | 107.0 (2) | C56—C57—H57A | 109.5 |
| O12—Mn6—O16 | 68.0 (2) | C56—C57—H57B | 109.5 |
| N5—Mn6—O16 | 83.3 (2) | H57A—C57—H57B | 109.5 |
| N14—Mn6—O16 | 90.7 (2) | C56—C57—H57C | 109.5 |
| O11—Mn6—O16 | 145.4 (2) | H57A—C57—H57C | 109.5 |
| C1—O1—Mn2 | 131.9 (5) | H57B—C57—H57C | 109.5 |
| C7—O2—Mn3 | 110.3 (5) | O19—C58—O18 | 126.0 (7) |
| N1—O3—Mn3 | 115.7 (4) | O19—C58—C59 | 117.7 (8) |
| N1—O3—Mn1 | 112.8 (4) | O18—C58—C59 | 116.2 (8) |
| Mn3—O3—Mn1 | 119.2 (2) | C58—C59—H59A | 109.5 |
| C8—O4—Mn3 | 129.1 (5) | C58—C59—H59B | 109.5 |
| C14—O5—Mn4 | 111.9 (5) | H59A—C59—H59B | 109.5 |
| N2—O6—Mn4 | 112.7 (4) | C58—C59—H59C | 109.5 |
| N2—O6—Mn1 | 124.0 (4) | H59A—C59—H59C | 109.5 |
| Mn4—O6—Mn1 | 123.2 (3) | H59B—C59—H59C | 109.5 |
| C15—O7—Mn4 | 124.9 (5) | O21—C63—N17 | 126.0 (11) |
| C21—O8—Mn5 | 111.0 (5) | O21—C63—H63 | 117.0 |
| N3—O9—Mn5 | 110.8 (4) | N17—C63—H63 | 117.0 |
| N3—O9—Mn1 | 117.3 (4) | N17—C64—H64A | 109.5 |
| Mn5—O9—Mn1 | 112.4 (2) | N17—C64—H64B | 109.5 |
| C22—O10—Mn5 | 130.5 (5) | H64A—C64—H64B | 109.5 |
| C28—O11—Mn6 | 110.2 (5) | N17—C64—H64C | 109.5 |
| N4—O12—Mn6 | 117.1 (4) | H64A—C64—H64C | 109.5 |
| N4—O12—Mn1 | 114.2 (4) | H64B—C64—H64C | 109.5 |
| Mn6—O12—Mn1 | 107.3 (2) | N17—C65—H65A | 109.5 |
| C29—O13—Mn6 | 132.4 (5) | N17—C65—H65B | 109.5 |
| C35—O14—Mn2 | 109.4 (5) | H65A—C65—H65B | 109.5 |
| N5—O15—Mn2 | 118.1 (4) | N17—C65—H65C | 109.5 |
| N5—O15—Mn1 | 112.2 (4) | H65A—C65—H65C | 109.5 |
| Mn2—O15—Mn1 | 109.8 (2) | H65B—C65—H65C | 109.5 |
| C56—O16—Mn1 | 130.8 (5) | C60—O20—Mn4 | 123.1 (5) |
| C56—O16—Mn6 | 133.1 (5) | O20—C60—N16 | 124.5 (8) |
| Mn1—O16—Mn6 | 92.22 (19) | O20—C60—H60 | 117.8 |
| C56—O17—Mn3 | 128.8 (4) | N16—C60—H60 | 117.8 |
| C58—O18—Mn1 | 128.1 (5) | C60—N16—C62 | 123.0 (7) |
| C58—O19—Mn5 | 135.8 (5) | C60—N16—C61 | 119.9 (7) |
| C7—N1—O3 | 113.8 (6) | C62—N16—C61 | 117.1 (7) |
| C7—N1—Mn2 | 132.3 (5) | N16—C61—H61A | 109.5 |
| O3—N1—Mn2 | 113.7 (4) | N16—C61—H61B | 109.5 |
| C14—N2—O6 | 112.2 (6) | H61A—C61—H61B | 109.5 |
| C14—N2—Mn3 | 130.6 (6) | N16—C61—H61C | 109.5 |
| O6—N2—Mn3 | 117.2 (4) | H61A—C61—H61C | 109.5 |
| C21—N3—O9 | 112.5 (6) | H61B—C61—H61C | 109.5 |
| C21—N3—Mn4 | 128.7 (5) | N16—C62—H62A | 109.5 |
| O9—N3—Mn4 | 118.5 (4) | N16—C62—H62B | 109.5 |
| C28—N4—O12 | 113.7 (6) | H62A—C62—H62B | 109.5 |
| C28—N4—Mn5 | 134.2 (6) | N16—C62—H62C | 109.5 |
| O12—N4—Mn5 | 112.1 (4) | H62A—C62—H62C | 109.5 |
| C35—N5—O15 | 112.7 (6) | H62B—C62—H62C | 109.5 |
| C35—N5—Mn6 | 134.0 (5) | C40—N10—C41 | 105.9 (19) |
| O15—N5—Mn6 | 113.1 (4) | C40—N10—Mn4 | 126.8 (19) |
| C52—N6—C53 | 105.7 (7) | C41—N10—Mn4 | 127.2 (14) |
| C52—N6—Mn2 | 126.9 (5) | N10—C40—N11 | 110.3 (18) |
| C53—N6—Mn2 | 127.3 (6) | N10—C40—H40 | 124.8 |
| C52—N7—C54 | 107.1 (7) | N11—C40—H40 | 124.8 |
| C52—N7—C55 | 126.0 (7) | C42—C41—N10 | 110.4 (17) |
| C54—N7—C55 | 126.9 (7) | C42—C41—H41 | 124.8 |
| C36—N8—C37 | 104.5 (7) | N10—C41—H41 | 124.8 |
| C36—N8—Mn3 | 126.1 (6) | C41—C42—N11 | 104.3 (17) |
| C37—N8—Mn3 | 127.8 (6) | C41—C42—H42 | 127.9 |
| C36—N9—C38 | 107.4 (8) | N11—C42—H42 | 127.9 |
| C36—N9—C39 | 126.4 (8) | C40—N11—C42 | 108.7 (15) |
| C38—N9—C39 | 126.2 (8) | C40—N11—C43 | 125.0 (18) |
| C44—N12—C45 | 104.2 (7) | C42—N11—C43 | 125.7 (19) |
| C44—N12—Mn5 | 125.3 (5) | N11—C43—H43A | 109.5 |
| C45—N12—Mn5 | 129.5 (6) | N11—C43—H43B | 109.5 |
| C44—N13—C46 | 107.8 (8) | H43A—C43—H43B | 109.5 |
| C44—N13—C47 | 125.3 (8) | N11—C43—H43C | 109.5 |
| C46—N13—C47 | 126.9 (8) | H43A—C43—H43C | 109.5 |
| C48—N14—C49 | 106.2 (6) | H43B—C43—H43C | 109.5 |
| C48—N14—Mn6 | 126.6 (5) | C40B—O22—Mn4 | 130 (3) |
| C49—N14—Mn6 | 127.3 (5) | O22—C40B—N11B | 125 (3) |
| C48—N15—C50 | 108.4 (7) | O22—C40B—H40B | 117.7 |
| C48—N15—C51 | 124.9 (7) | N11B—C40B—H40B | 117.7 |
| C50—N15—C51 | 126.7 (7) | C40B—N11B—C42B | 120 (2) |
| C63—N17—C65 | 122.6 (11) | C40B—N11B—C43B | 124 (3) |
| C63—N17—C64 | 119.2 (10) | C42B—N11B—C43B | 116 (3) |
| C65—N17—C64 | 118.2 (11) | N11B—C42B—H42A | 109.5 |
| O1—C1—C2 | 116.9 (7) | N11B—C42B—H42B | 109.5 |
| O1—C1—C6 | 124.4 (7) | H42A—C42B—H42B | 109.5 |
| C2—C1—C6 | 118.7 (8) | N11B—C42B—H42C | 109.5 |
| C3—C2—C1 | 121.1 (8) | H42A—C42B—H42C | 109.5 |
| C3—C2—H2 | 119.4 | H42B—C42B—H42C | 109.5 |
| C1—C2—H2 | 119.4 | N11B—C43B—H43D | 109.5 |
| C4—C3—C2 | 119.4 (8) | N11B—C43B—H43E | 109.5 |
| C4—C3—H3 | 120.3 | H43D—C43B—H43E | 109.5 |
| C2—C3—H3 | 120.3 | N11B—C43B—H43F | 109.5 |
| C5—C4—C3 | 120.7 (9) | H43D—C43B—H43F | 109.5 |
| C5—C4—H4 | 119.7 | H43E—C43B—H43F | 109.5 |
| C3—C4—H4 | 119.7 | C68—C67—H67A | 109.5 |
| C4—C5—C6 | 121.0 (8) | C68—C67—H67B | 109.5 |
| C4—C5—H5 | 119.5 | H67A—C67—H67B | 109.5 |
| C6—C5—H5 | 119.5 | C68—C67—H67C | 109.5 |
| C5—C6—C1 | 119.0 (8) | H67A—C67—H67C | 109.5 |
| C5—C6—C7 | 118.0 (7) | H67B—C67—H67C | 109.5 |
| C1—C6—C7 | 123.0 (8) | O23—C68—C67 | 111 (2) |
| O2—C7—N1 | 120.6 (7) | O23—C68—H68A | 109.5 |
| O2—C7—C6 | 120.6 (7) | C67—C68—H68A | 109.5 |
| N1—C7—C6 | 118.8 (7) | O23—C68—H68B | 109.5 |
| O4—C8—C9 | 116.9 (7) | C67—C68—H68B | 109.5 |
| O4—C8—C13 | 123.8 (7) | H68A—C68—H68B | 108.1 |
| C9—C8—C13 | 119.3 (7) | C68—O23—C69 | 111.1 (18) |
| C10—C9—C8 | 120.1 (8) | O23—C69—C70 | 106 (2) |
| C10—C9—H9 | 119.9 | O23—C69—H69A | 110.6 |
| C8—C9—H9 | 119.9 | C70—C69—H69A | 110.6 |
| C11—C10—C9 | 120.9 (8) | O23—C69—H69B | 110.6 |
| C11—C10—H10 | 119.5 | C70—C69—H69B | 110.6 |
| C9—C10—H10 | 119.5 | H69A—C69—H69B | 108.8 |
| C12—C11—C10 | 119.5 (8) | C69—C70—H70A | 109.5 |
| C12—C11—H11 | 120.3 | C69—C70—H70B | 109.5 |
| C10—C11—H11 | 120.3 | H70A—C70—H70B | 109.5 |
| C11—C12—C13 | 122.1 (8) | C69—C70—H70C | 109.5 |
| C11—C12—H12 | 119.0 | H70A—C70—H70C | 109.5 |
| C13—C12—H12 | 119.0 | H70B—C70—H70C | 109.5 |
| C8—C13—C12 | 118.1 (8) | C68B—C67B—H67D | 109.5 |
| C8—C13—C14 | 124.2 (7) | C68B—C67B—H67E | 109.5 |
| C12—C13—C14 | 117.7 (8) | H67D—C67B—H67E | 109.5 |
| O5—C14—N2 | 120.8 (7) | C68B—C67B—H67F | 109.5 |
| O5—C14—C13 | 120.2 (7) | H67D—C67B—H67F | 109.5 |
| N2—C14—C13 | 119.0 (7) | H67E—C67B—H67F | 109.5 |
| O7—C15—C20 | 123.0 (7) | O23B—C68B—C67B | 112 (2) |
| O7—C15—C16 | 117.7 (7) | O23B—C68B—H68C | 109.3 |
| C20—C15—C16 | 119.3 (8) | C67B—C68B—H68C | 109.3 |
| C17—C16—C15 | 119.3 (8) | O23B—C68B—H68D | 109.3 |
| C17—C16—H16 | 120.4 | C67B—C68B—H68D | 109.3 |
| C15—C16—H16 | 120.4 | H68C—C68B—H68D | 107.9 |
| C18—C17—C16 | 121.0 (8) | C68B—O23B—C69B | 113 (3) |
| C18—C17—H17 | 119.5 | O23B—C69B—C70B | 106 (2) |
| C16—C17—H17 | 119.5 | O23B—C69B—H69C | 110.5 |
| C17—C18—C19 | 120.1 (9) | C70B—C69B—H69C | 110.5 |
| C17—C18—H18 | 119.9 | O23B—C69B—H69D | 110.5 |
| C19—C18—H18 | 119.9 | C70B—C69B—H69D | 110.5 |
| C18—C19—C20 | 120.6 (8) | H69C—C69B—H69D | 108.7 |
| C18—C19—H19 | 119.7 | C69B—C70B—H70D | 109.5 |
| C20—C19—H19 | 119.7 | C69B—C70B—H70E | 109.5 |
| C15—C20—C19 | 119.7 (7) | H70D—C70B—H70E | 109.5 |
| C15—C20—C21 | 123.8 (7) | C69B—C70B—H70F | 109.5 |
| C19—C20—C21 | 116.4 (7) | H70D—C70B—H70F | 109.5 |
| O8—C21—N3 | 121.6 (7) | H70E—C70B—H70F | 109.5 |
| O8—C21—C20 | 120.0 (7) | H24A—O24—H24B | 120 (10) |
| N3—C21—C20 | 118.3 (7) | C72—O25—H25A | 109.5 |
| O10—C22—C23 | 118.0 (8) | O25—C72—H72A | 109.5 |
| O10—C22—C27 | 124.2 (7) | O25—C72—H72B | 109.5 |
| C23—C22—C27 | 117.8 (8) | H72A—C72—H72B | 109.5 |
| C24—C23—C22 | 122.5 (9) | O25—C72—H72C | 109.5 |
| C24—C23—H23 | 118.7 | H72A—C72—H72C | 109.5 |
| C22—C23—H23 | 118.7 | H72B—C72—H72C | 109.5 |
| C23—C24—C25 | 120.3 (9) | O26—C71—H71A | 109.5 |
| C23—C24—H24 | 119.8 | O26—C71—H71B | 109.5 |
| C25—C24—H24 | 119.8 | H71A—C71—H71B | 109.5 |
| C26—C25—C24 | 119.3 (8) | O26—C71—H71C | 109.5 |
| C26—C25—H25 | 120.3 | H71A—C71—H71C | 109.5 |
| C24—C25—H25 | 120.3 | H71B—C71—H71C | 109.5 |
| C25—C26—C27 | 121.3 (9) | C71—O26—H26A | 109.5 |
| C25—C26—H26 | 119.4 | ||
| N1—Mn2—O1—C1 | 4.1 (7) | C19—C20—C21—N3 | −162.5 (8) |
| N6—Mn2—O1—C1 | −159.4 (7) | Mn5—O10—C22—C23 | 173.4 (6) |
| O14—Mn2—O1—C1 | 99.9 (7) | Mn5—O10—C22—C27 | −8.2 (12) |
| N2—Mn3—O4—C8 | 25.0 (7) | O10—C22—C23—C24 | 179.3 (9) |
| N8—Mn3—O4—C8 | −165.4 (7) | C27—C22—C23—C24 | 0.8 (14) |
| O2—Mn3—O4—C8 | −74.0 (7) | C22—C23—C24—C25 | −0.8 (16) |
| O17—Mn3—O4—C8 | 112.8 (6) | C23—C24—C25—C26 | −0.5 (15) |
| O5—Mn4—O7—C15 | −149.1 (7) | C24—C25—C26—C27 | 1.7 (14) |
| N3—Mn4—O7—C15 | 35.6 (7) | C25—C26—C27—C22 | −1.6 (13) |
| O22—Mn4—O7—C15 | −55.9 (13) | C25—C26—C27—C28 | 179.5 (8) |
| O20—Mn4—O7—C15 | 124.5 (7) | O10—C22—C27—C26 | −178.1 (8) |
| N10—Mn4—O7—C15 | −60.2 (9) | C23—C22—C27—C26 | 0.4 (12) |
| O8—Mn5—O10—C22 | −169.4 (7) | O10—C22—C27—C28 | 0.8 (13) |
| N4—Mn5—O10—C22 | 8.8 (7) | C23—C22—C27—C28 | 179.2 (8) |
| O19—Mn5—O10—C22 | −84.9 (7) | Mn6—O11—C28—N4 | 0.5 (9) |
| N12—Mn5—O10—C22 | 99.1 (7) | Mn6—O11—C28—C27 | 179.4 (6) |
| N5—Mn6—O13—C29 | 5.2 (7) | O12—N4—C28—O11 | 2.3 (10) |
| N14—Mn6—O13—C29 | 177.5 (7) | Mn5—N4—C28—O11 | −179.7 (5) |
| O11—Mn6—O13—C29 | −88.0 (7) | O12—N4—C28—C27 | −176.6 (6) |
| O16—Mn6—O13—C29 | 87.5 (7) | Mn5—N4—C28—C27 | 1.4 (11) |
| N1—Mn2—O15—N5 | 91.7 (5) | C26—C27—C28—O11 | 2.4 (12) |
| N6—Mn2—O15—N5 | −104.9 (5) | C22—C27—C28—O11 | −176.5 (8) |
| O14—Mn2—O15—N5 | −3.4 (4) | C26—C27—C28—N4 | −178.7 (7) |
| N1—Mn2—O15—Mn1 | −38.7 (3) | C22—C27—C28—N4 | 2.4 (12) |
| N6—Mn2—O15—Mn1 | 124.7 (3) | Mn6—O13—C29—C30 | 171.1 (6) |
| O14—Mn2—O15—Mn1 | −133.8 (3) | Mn6—O13—C29—C34 | −7.5 (12) |
| Mn3—O3—N1—C7 | 7.7 (7) | O13—C29—C30—C31 | 178.5 (8) |
| Mn1—O3—N1—C7 | −134.4 (5) | C34—C29—C30—C31 | −2.8 (13) |
| Mn3—O3—N1—Mn2 | −177.2 (3) | C29—C30—C31—C32 | 0.7 (14) |
| Mn1—O3—N1—Mn2 | 40.6 (5) | C30—C31—C32—C33 | 0.2 (14) |
| Mn4—O6—N2—C14 | −4.7 (7) | C31—C32—C33—C34 | 1.2 (14) |
| Mn1—O6—N2—C14 | 178.8 (5) | C32—C33—C34—C29 | −3.3 (13) |
| Mn4—O6—N2—Mn3 | 174.5 (3) | C32—C33—C34—C35 | 177.4 (8) |
| Mn1—O6—N2—Mn3 | −1.9 (6) | O13—C29—C34—C33 | −177.4 (8) |
| Mn5—O9—N3—C21 | −11.7 (7) | C30—C29—C34—C33 | 4.1 (12) |
| Mn1—O9—N3—C21 | −142.7 (5) | O13—C29—C34—C35 | 1.9 (12) |
| Mn5—O9—N3—Mn4 | 174.0 (3) | C30—C29—C34—C35 | −176.6 (7) |
| Mn1—O9—N3—Mn4 | 43.0 (6) | Mn2—O14—C35—N5 | −4.2 (8) |
| Mn6—O12—N4—C28 | −4.3 (7) | Mn2—O14—C35—C34 | 177.4 (5) |
| Mn1—O12—N4—C28 | −130.9 (5) | O15—N5—C35—O14 | 1.7 (10) |
| Mn6—O12—N4—Mn5 | 177.2 (3) | Mn6—N5—C35—O14 | 175.8 (5) |
| Mn1—O12—N4—Mn5 | 50.6 (5) | O15—N5—C35—C34 | −179.9 (6) |
| Mn2—O15—N5—C35 | 2.3 (7) | Mn6—N5—C35—C34 | −5.8 (11) |
| Mn1—O15—N5—C35 | 131.6 (5) | C33—C34—C35—O14 | 2.0 (11) |
| Mn2—O15—N5—Mn6 | −173.1 (3) | C29—C34—C35—O14 | −177.3 (7) |
| Mn1—O15—N5—Mn6 | −43.8 (5) | C33—C34—C35—N5 | −176.4 (7) |
| Mn2—O1—C1—C2 | 176.2 (6) | C29—C34—C35—N5 | 4.3 (11) |
| Mn2—O1—C1—C6 | −3.2 (12) | C37—N8—C36—N9 | −1.9 (10) |
| O1—C1—C2—C3 | −178.6 (8) | Mn3—N8—C36—N9 | 164.7 (6) |
| C6—C1—C2—C3 | 0.9 (13) | C38—N9—C36—N8 | 1.5 (11) |
| C1—C2—C3—C4 | −1.6 (14) | C39—N9—C36—N8 | −177.1 (8) |
| C2—C3—C4—C5 | 0.2 (14) | C36—N8—C37—C38 | 1.6 (11) |
| C3—C4—C5—C6 | 1.8 (13) | Mn3—N8—C37—C38 | −164.7 (7) |
| C4—C5—C6—C1 | −2.4 (12) | N8—C37—C38—N9 | −0.7 (11) |
| C4—C5—C6—C7 | 176.9 (8) | C36—N9—C38—C37 | −0.4 (11) |
| O1—C1—C6—C5 | −179.5 (8) | C39—N9—C38—C37 | 178.2 (9) |
| C2—C1—C6—C5 | 1.0 (12) | C45—N12—C44—N13 | 1.4 (10) |
| O1—C1—C6—C7 | 1.3 (13) | Mn5—N12—C44—N13 | −168.2 (5) |
| C2—C1—C6—C7 | −178.2 (8) | C46—N13—C44—N12 | −1.1 (11) |
| Mn3—O2—C7—N1 | −6.3 (9) | C47—N13—C44—N12 | 178.7 (8) |
| Mn3—O2—C7—C6 | 174.7 (5) | C44—N12—C45—C46 | −1.2 (10) |
| O3—N1—C7—O2 | −0.2 (10) | Mn5—N12—C45—C46 | 167.7 (6) |
| Mn2—N1—C7—O2 | −174.1 (5) | C44—N13—C46—C45 | 0.3 (11) |
| O3—N1—C7—C6 | 178.8 (6) | C47—N13—C46—C45 | −179.6 (9) |
| Mn2—N1—C7—C6 | 4.9 (11) | N12—C45—C46—N13 | 0.6 (11) |
| C5—C6—C7—O2 | −2.3 (11) | C50—N15—C48—N14 | −1.8 (10) |
| C1—C6—C7—O2 | 176.9 (7) | C51—N15—C48—N14 | 178.4 (7) |
| C5—C6—C7—N1 | 178.7 (7) | C49—N14—C48—N15 | 2.2 (9) |
| C1—C6—C7—N1 | −2.1 (11) | Mn6—N14—C48—N15 | −176.9 (5) |
| Mn3—O4—C8—C9 | 157.2 (6) | C48—N14—C49—C50 | −1.9 (9) |
| Mn3—O4—C8—C13 | −23.7 (11) | Mn6—N14—C49—C50 | 177.3 (6) |
| O4—C8—C9—C10 | 178.5 (7) | N14—C49—C50—N15 | 0.8 (10) |
| C13—C8—C9—C10 | −0.7 (11) | C48—N15—C50—C49 | 0.5 (10) |
| C8—C9—C10—C11 | 0.4 (13) | C51—N15—C50—C49 | −179.6 (8) |
| C9—C10—C11—C12 | −0.1 (13) | C53—N6—C52—N7 | −0.5 (9) |
| C10—C11—C12—C13 | 0.1 (12) | Mn2—N6—C52—N7 | −177.4 (5) |
| O4—C8—C13—C12 | −178.5 (7) | C54—N7—C52—N6 | 0.5 (9) |
| C9—C8—C13—C12 | 0.7 (11) | C55—N7—C52—N6 | −179.1 (9) |
| O4—C8—C13—C14 | 2.7 (12) | C52—N6—C53—C54 | 0.3 (9) |
| C9—C8—C13—C14 | −178.2 (7) | Mn2—N6—C53—C54 | 177.2 (6) |
| C11—C12—C13—C8 | −0.4 (11) | N6—C53—C54—N7 | 0.0 (10) |
| C11—C12—C13—C14 | 178.5 (7) | C52—N7—C54—C53 | −0.3 (9) |
| Mn4—O5—C14—N2 | 3.4 (8) | C55—N7—C54—C53 | 179.3 (9) |
| Mn4—O5—C14—C13 | −178.1 (5) | Mn3—O17—C56—O16 | 47.4 (11) |
| O6—N2—C14—O5 | 0.8 (9) | Mn3—O17—C56—C57 | −130.8 (6) |
| Mn3—N2—C14—O5 | −178.2 (5) | Mn1—O16—C56—O17 | −6.5 (11) |
| O6—N2—C14—C13 | −177.7 (6) | Mn6—O16—C56—O17 | −157.8 (5) |
| Mn3—N2—C14—C13 | 3.2 (10) | Mn1—O16—C56—C57 | 171.8 (5) |
| C8—C13—C14—O5 | −171.6 (7) | Mn6—O16—C56—C57 | 20.5 (10) |
| C12—C13—C14—O5 | 9.5 (10) | Mn5—O19—C58—O18 | −20.6 (12) |
| C8—C13—C14—N2 | 6.9 (11) | Mn5—O19—C58—C59 | 158.0 (6) |
| C12—C13—C14—N2 | −171.9 (7) | Mn1—O18—C58—O19 | 5.9 (11) |
| Mn4—O7—C15—C20 | −32.3 (11) | Mn1—O18—C58—C59 | −172.7 (5) |
| Mn4—O7—C15—C16 | 148.8 (6) | C65—N17—C63—O21 | −178.3 (13) |
| O7—C15—C16—C17 | −179.9 (9) | C64—N17—C63—O21 | 3.5 (19) |
| C20—C15—C16—C17 | 1.1 (14) | Mn4—O20—C60—N16 | 177.6 (6) |
| C15—C16—C17—C18 | −0.6 (16) | O20—C60—N16—C62 | −179.2 (9) |
| C16—C17—C18—C19 | −0.2 (17) | O20—C60—N16—C61 | 0.0 (13) |
| C17—C18—C19—C20 | 0.7 (17) | C41—N10—C40—N11 | 0 (4) |
| O7—C15—C20—C19 | −179.6 (8) | Mn4—N10—C40—N11 | −178 (2) |
| C16—C15—C20—C19 | −0.6 (13) | C40—N10—C41—C42 | 4 (3) |
| O7—C15—C20—C21 | −0.7 (13) | Mn4—N10—C41—C42 | −178.4 (16) |
| C16—C15—C20—C21 | 178.2 (8) | N10—C41—C42—N11 | −6 (3) |
| C18—C19—C20—C15 | −0.2 (15) | N10—C40—N11—C42 | −4 (4) |
| C18—C19—C20—C21 | −179.2 (9) | N10—C40—N11—C43 | −176 (3) |
| Mn5—O8—C21—N3 | 5.0 (9) | C41—C42—N11—C40 | 6 (3) |
| Mn5—O8—C21—C20 | −173.0 (6) | C41—C42—N11—C43 | 178 (3) |
| O9—N3—C21—O8 | 4.6 (10) | Mn4—O22—C40B—N11B | 173 (4) |
| Mn4—N3—C21—O8 | 178.2 (5) | O22—C40B—N11B—C42B | 7 (9) |
| O9—N3—C21—C20 | −177.4 (6) | O22—C40B—N11B—C43B | 178 (7) |
| Mn4—N3—C21—C20 | −3.8 (11) | C67—C68—O23—C69 | −180 (4) |
| C15—C20—C21—O8 | −163.3 (8) | C68—O23—C69—C70 | 175 (4) |
| C19—C20—C21—O8 | 15.5 (12) | C67B—C68B—O23B—C69B | 80 (5) |
| C15—C20—C21—N3 | 18.6 (12) | C68B—O23B—C69B—C70B | 177 (2) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JJ2164).
References
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. G. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
- Bruker (2012). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Dendrinou-Samara, C., Alevizopoulou, L., Iordanidis, L., Samaras, E. & Kessissoglou, D. P. (2002). J. Inorg. Biochem. 89, 89–96. [DOI] [PubMed]
- Dendrinou-Samara, C., Papadopoulos, A. N., Malamatari, D. A., Tarushi, A., Raptopoulou, C. P., Terzis, A., Samaras, E. & Kessissoglou, D. P. (2005). J. Inorg. Biochem. 99, 864–875. [DOI] [PubMed]
- Dendrinou-Samara, C., Psomas, G., Iordanidis, L., Tangoulis, V. & Kessissoglou, D. P. (2001). Chem. Eur. J. 7, 5041–5051. [DOI] [PubMed]
- Emerich, B., Smith, M., Zeller, M. & Zaleski, C. M. (2010). J. Chem. Crystallogr. 40, 769–777.
- Hübschle, C. B., Sheldrick, G. M. & Dittrich, B. (2011). J. Appl. Cryst. 44, 1281–1284. [DOI] [PMC free article] [PubMed]
- Kessissoglou, D. P., Kampf, J. & Pecoraro, V. L. (1994). Polyhedron, 13, 1379–1391.
- Lah, M. S. & Pecoraro, V. L. (1989). J. Am. Chem. Soc. 111, 7258–7259.
- Liu, W. & Thorp, H. H. (1993). Inorg. Chem. 32, 4102–4105.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Mezei, G., Zaleski, C. M. & Pecoraro, V. L. (2007). Chem. Rev. 107, 4933–5003. [DOI] [PubMed]
- Pecoraro, V. L. (1989). Inorg. Chim. Acta 155, 171–173.
- Sheldrick, G. M. (2008a). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2008b). CELL_NOW University of Göttingen, Germany.
- Sheldrick, G. M. (2009). TWINABS University of Göttingen, Germany.
- Stiefel, E. I. & Brown, G. F. (1972). Inorg. Chem. 11, 434–436.
- Tigyer, B. R., Zeller, M. & Zaleski, C. M. (2011). Acta Cryst. E67, m1041–m1042. [DOI] [PMC free article] [PubMed]
- Tigyer, B. R., Zeller, M. & Zaleski, C. M. (2012). Acta Cryst. E68, m1521–m1522. [DOI] [PMC free article] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813015857/jj2164sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813015857/jj2164Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




