Abstract
In the title compound, C42H42N4O2, the complete molecule is generated by a crystallographic inversion centre. The porphyrin system exhibits a near planar macrocycle conformation with an average deviation from the least-squares plane of the 24 macrocycle atoms of 0.037 (5) Å. The phenyl ipso C atoms are positioned above and below the porphyrin plane by 0.35 (1) Å and the macrocycle shows evidence of in-plane rectangular elongation with N⋯N separations of 3.032 (5) and 2.803 (5) Å. Two intramolecular N—H⋯N hydrogen bonds occur.
Related literature
For the conformation of porphyrins, see: Scheidt & Lee (1987 ▶); Senge et al. (1997 ▶); Senge (2006 ▶). For the synthesis of such compounds, see: Wiehe et al. (2005 ▶).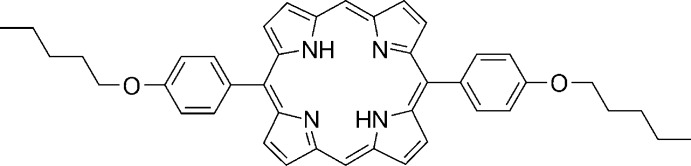
Experimental
Crystal data
C42H42N4O2
M r = 634.80
Triclinic,
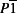
a = 9.5222 (6) Å
b = 9.5799 (6) Å
c = 10.2195 (6) Å
α = 67.777 (1)°
β = 88.063 (1)°
γ = 72.464 (1)°
V = 819.49 (9) Å3
Z = 1
Mo Kα radiation
μ = 0.08 mm−1
T = 90 K
0.30 × 0.10 × 0.08 mm
Data collection
Bruker SMART APEXII diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.97, T max = 0.99
9093 measured reflections
3606 independent reflections
2489 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.109
S = 1.04
3606 reflections
219 parameters
H-atom parameters constrained
Δρmax = 0.27 e Å−3
Δρmin = −0.23 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: XP in SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, 2R. DOI: 10.1107/S160053681301550X/zl2552sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681301550X/zl2552Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N21—H21⋯N24 | 0.88 | 2.50 | 3.033 (2) | 119 |
| N21—H21⋯N24i | 0.88 | 2.22 | 2.804 (2) | 123 |
Symmetry code: (i) 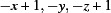 .
.
Acknowledgments
This work was supported by a grant from Science Foundation Ireland (SFI P.I. 09/IN.1/B2650).
supplementary crystallographic information
Comment
Many porphyrin structures with four meso substituents have been reported (Scheidt & Lee, 1987). The available number of structures for systems with only two meso residues is much smaller. In the context of an ongoing program on the conformational flexibilty of porphyrins (Senge, 2006) we are interested in a comparative analysis of 5,10-A2– and 5,15-A2-disubstituted porphyrins. The title compound is an example for the latter and exhibits a planar macrocycle with an average deviation from the least-squares-plane of the 24 macrocycle atoms of Δ24 = 0.037 (5) Å. The phenyl ipso carbon atoms are positioned above and below the porphyrin plane by 0.35 Å and the macrocycle shows evidence for in-plane distortion with N···N separations of 3.032 (5) and 2.803 (5) Å. This is similar to the situation found in 2,3,5,7,8,12,13,15, 17,18- decasubstituted porphyrins (Senge et al., 1997) where peri interaction between the meso and beta substituents occur. The molecules pack in parallel layers with the alkyl chains separating the macrocycles and only minimal π-aggregation.
Experimental
The compound was prepared as described by Wiehe et al. (2005) and crystallized from CH2Cl2/CH3OH.
Refinement
All nonhydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms were refined with a standard riding model (C—H distance 0.96 - 0.99 Å, Uiso = 1.2–1.5 times of parent atom). Pyrrole hydrogen atoms were located in difference maps and refined with isotropic thermal parameters.
Figures
Fig. 1.
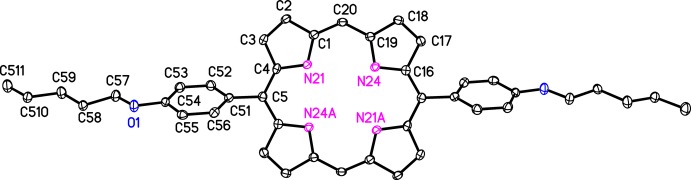
: Molecular structure of the title compound. Thermal ellipsoids are drawn at 50% probability level; hydrogen atoms have been omitted for clarity.
Crystal data
| C42H42N4O2 | Z = 1 |
| Mr = 634.80 | F(000) = 338 |
| Triclinic, P1 | Dx = 1.286 Mg m−3Dm = n/d Mg m−3Dm measured by not measured |
| Hall symbol: -P 1 | Melting point: n/d K |
| a = 9.5222 (6) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 9.5799 (6) Å | Cell parameters from 1771 reflections |
| c = 10.2195 (6) Å | θ = 4.5–60.7° |
| α = 67.777 (1)° | µ = 0.08 mm−1 |
| β = 88.063 (1)° | T = 90 K |
| γ = 72.464 (1)° | Parallelpiped, red |
| V = 819.49 (9) Å3 | 0.30 × 0.10 × 0.08 mm |
Data collection
| Bruker SMART APEXII diffractometer | 3606 independent reflections |
| Radiation source: fine-focus sealed tube | 2489 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.039 |
| Detector resolution: 8.3 pixels mm-1 | θmax = 27.1°, θmin = 2.2° |
| ω scans | h = −12→12 |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | k = −12→12 |
| Tmin = 0.97, Tmax = 0.99 | l = −13→13 |
| 9093 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.109 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0433P)2 + 0.1316P] where P = (Fo2 + 2Fc2)/3 |
| 3606 reflections | (Δ/σ)max < 0.001 |
| 219 parameters | Δρmax = 0.27 e Å−3 |
| 0 restraints | Δρmin = −0.23 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N21 | 0.64309 (14) | −0.19503 (16) | 0.46008 (14) | 0.0171 (3) | |
| H21 | 0.5815 | −0.1069 | 0.4627 | 0.047 (6)* | |
| N24 | 0.36841 (13) | −0.12143 (15) | 0.61217 (14) | 0.0160 (3) | |
| C1 | 0.62390 (16) | −0.34160 (19) | 0.52069 (17) | 0.0179 (4) | |
| C2 | 0.74472 (17) | −0.4492 (2) | 0.48647 (18) | 0.0190 (4) | |
| H2A | 0.7600 | −0.5592 | 0.5123 | 0.023* | |
| C3 | 0.83431 (17) | −0.36651 (19) | 0.41037 (17) | 0.0185 (4) | |
| H3A | 0.9229 | −0.4090 | 0.3740 | 0.022* | |
| C4 | 0.77200 (16) | −0.20511 (19) | 0.39479 (17) | 0.0169 (4) | |
| C5 | 0.83034 (16) | −0.07952 (19) | 0.33369 (17) | 0.0169 (4) | |
| C16 | 0.24129 (16) | −0.07563 (19) | 0.67302 (16) | 0.0164 (3) | |
| C17 | 0.18961 (17) | −0.2107 (2) | 0.74985 (18) | 0.0195 (4) | |
| H17A | 0.1041 | −0.2097 | 0.8006 | 0.023* | |
| C18 | 0.28721 (17) | −0.3376 (2) | 0.73457 (18) | 0.0198 (4) | |
| H18A | 0.2843 | −0.4437 | 0.7732 | 0.024* | |
| C19 | 0.39727 (16) | −0.28065 (19) | 0.64781 (17) | 0.0172 (4) | |
| C20 | 0.51314 (16) | −0.37959 (19) | 0.60556 (17) | 0.0185 (4) | |
| H20A | 0.5167 | −0.4877 | 0.6396 | 0.022* | |
| C51 | 0.98127 (17) | −0.11283 (19) | 0.28262 (17) | 0.0177 (4) | |
| C52 | 1.01657 (17) | −0.1671 (2) | 0.17315 (18) | 0.0206 (4) | |
| H52A | 0.9421 | −0.1859 | 0.1286 | 0.025* | |
| C53 | 1.15769 (17) | −0.1945 (2) | 0.12745 (18) | 0.0207 (4) | |
| H53A | 1.1792 | −0.2316 | 0.0527 | 0.025* | |
| C54 | 1.26742 (16) | −0.16720 (19) | 0.19238 (18) | 0.0188 (4) | |
| C55 | 1.23542 (17) | −0.11454 (19) | 0.30202 (17) | 0.0192 (4) | |
| H55A | 1.3103 | −0.0965 | 0.3467 | 0.023* | |
| C56 | 1.09475 (17) | −0.08822 (19) | 0.34664 (18) | 0.0192 (4) | |
| H56A | 1.0744 | −0.0526 | 0.4224 | 0.023* | |
| O1 | 1.41004 (11) | −0.18754 (14) | 0.15628 (12) | 0.0226 (3) | |
| C57 | 1.45008 (17) | −0.2298 (2) | 0.03660 (18) | 0.0227 (4) | |
| H57A | 1.3832 | −0.1524 | −0.0488 | 0.027* | |
| H57B | 1.4430 | −0.3366 | 0.0554 | 0.027* | |
| C58 | 1.60744 (17) | −0.2284 (2) | 0.01421 (18) | 0.0211 (4) | |
| H58A | 1.6702 | −0.2943 | 0.1048 | 0.025* | |
| H58B | 1.6106 | −0.1185 | −0.0147 | 0.025* | |
| C59 | 1.66924 (17) | −0.2919 (2) | −0.09922 (19) | 0.0249 (4) | |
| H59A | 1.6492 | −0.3934 | −0.0788 | 0.030* | |
| H59B | 1.6167 | −0.2157 | −0.1927 | 0.030* | |
| C510 | 1.83383 (17) | −0.3191 (2) | −0.10777 (18) | 0.0217 (4) | |
| H51A | 1.8868 | −0.3995 | −0.0159 | 0.026* | |
| H51B | 1.8546 | −0.2190 | −0.1236 | 0.026* | |
| C511 | 1.89275 (18) | −0.3744 (2) | −0.22522 (19) | 0.0277 (4) | |
| H51C | 2.0009 | −0.4029 | −0.2182 | 0.042* | |
| H51D | 1.8518 | −0.2889 | −0.3175 | 0.042* | |
| H51E | 1.8637 | −0.4673 | −0.2159 | 0.042* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N21 | 0.0139 (7) | 0.0134 (7) | 0.0229 (8) | −0.0023 (5) | 0.0014 (5) | −0.0075 (6) |
| N24 | 0.0136 (6) | 0.0145 (7) | 0.0195 (7) | −0.0030 (5) | −0.0003 (5) | −0.0071 (6) |
| C1 | 0.0159 (8) | 0.0165 (8) | 0.0220 (9) | −0.0040 (6) | −0.0007 (6) | −0.0088 (7) |
| C2 | 0.0184 (8) | 0.0141 (8) | 0.0241 (9) | −0.0023 (6) | 0.0006 (7) | −0.0090 (7) |
| C3 | 0.0148 (8) | 0.0197 (9) | 0.0206 (9) | −0.0017 (6) | 0.0021 (6) | −0.0103 (7) |
| C4 | 0.0119 (7) | 0.0192 (9) | 0.0184 (9) | −0.0012 (6) | 0.0004 (6) | −0.0087 (7) |
| C5 | 0.0151 (8) | 0.0190 (9) | 0.0171 (8) | −0.0047 (6) | 0.0008 (6) | −0.0078 (7) |
| C16 | 0.0156 (8) | 0.0186 (9) | 0.0146 (8) | −0.0046 (6) | 0.0001 (6) | −0.0065 (7) |
| C17 | 0.0179 (8) | 0.0206 (9) | 0.0199 (9) | −0.0071 (7) | 0.0040 (6) | −0.0071 (7) |
| C18 | 0.0193 (8) | 0.0156 (9) | 0.0237 (9) | −0.0066 (7) | 0.0022 (7) | −0.0059 (7) |
| C19 | 0.0153 (8) | 0.0159 (8) | 0.0201 (9) | −0.0046 (6) | −0.0004 (6) | −0.0066 (7) |
| C20 | 0.0180 (8) | 0.0136 (8) | 0.0236 (9) | −0.0054 (6) | −0.0001 (6) | −0.0063 (7) |
| C51 | 0.0171 (8) | 0.0144 (8) | 0.0191 (9) | −0.0031 (6) | 0.0028 (6) | −0.0052 (7) |
| C52 | 0.0183 (8) | 0.0214 (9) | 0.0221 (9) | −0.0066 (7) | 0.0012 (7) | −0.0080 (8) |
| C53 | 0.0232 (9) | 0.0212 (9) | 0.0189 (9) | −0.0060 (7) | 0.0045 (7) | −0.0100 (7) |
| C54 | 0.0146 (8) | 0.0146 (8) | 0.0222 (9) | −0.0026 (6) | 0.0047 (6) | −0.0036 (7) |
| C55 | 0.0193 (8) | 0.0165 (9) | 0.0204 (9) | −0.0057 (7) | −0.0015 (7) | −0.0053 (7) |
| C56 | 0.0197 (8) | 0.0182 (9) | 0.0184 (9) | −0.0034 (7) | 0.0021 (6) | −0.0078 (7) |
| O1 | 0.0168 (6) | 0.0286 (7) | 0.0251 (7) | −0.0071 (5) | 0.0065 (5) | −0.0135 (6) |
| C57 | 0.0202 (8) | 0.0268 (10) | 0.0229 (9) | −0.0062 (7) | 0.0069 (7) | −0.0127 (8) |
| C58 | 0.0180 (8) | 0.0183 (9) | 0.0233 (9) | −0.0047 (7) | 0.0060 (7) | −0.0050 (7) |
| C59 | 0.0204 (9) | 0.0312 (11) | 0.0260 (10) | −0.0096 (8) | 0.0071 (7) | −0.0135 (8) |
| C510 | 0.0185 (8) | 0.0216 (9) | 0.0237 (9) | −0.0046 (7) | 0.0049 (7) | −0.0088 (8) |
| C511 | 0.0209 (9) | 0.0360 (11) | 0.0324 (11) | −0.0105 (8) | 0.0071 (8) | −0.0190 (9) |
Geometric parameters (Å, º)
| N21—C1 | 1.370 (2) | C52—H52A | 0.9500 |
| N21—C4 | 1.3722 (19) | C53—C54 | 1.394 (2) |
| N21—H21 | 0.8800 | C53—H53A | 0.9500 |
| N24—C19 | 1.367 (2) | C54—O1 | 1.3710 (18) |
| N24—C16 | 1.3711 (19) | C54—C55 | 1.384 (2) |
| C1—C20 | 1.388 (2) | C55—C56 | 1.381 (2) |
| C1—C2 | 1.428 (2) | C55—H55A | 0.9500 |
| C2—C3 | 1.362 (2) | C56—H56A | 0.9500 |
| C2—H2A | 0.9500 | O1—C57 | 1.4335 (19) |
| C3—C4 | 1.427 (2) | C57—C58 | 1.511 (2) |
| C3—H3A | 0.9500 | C57—H57A | 0.9900 |
| C4—C5 | 1.399 (2) | C57—H57B | 0.9900 |
| C5—C16i | 1.412 (2) | C58—C59 | 1.527 (2) |
| C5—C51 | 1.496 (2) | C58—H58A | 0.9900 |
| C16—C5i | 1.412 (2) | C58—H58B | 0.9900 |
| C16—C17 | 1.457 (2) | C59—C510 | 1.515 (2) |
| C17—C18 | 1.348 (2) | C59—H59A | 0.9900 |
| C17—H17A | 0.9500 | C59—H59B | 0.9900 |
| C18—C19 | 1.448 (2) | C510—C511 | 1.514 (2) |
| C18—H18A | 0.9500 | C510—H51A | 0.9900 |
| C19—C20 | 1.396 (2) | C510—H51B | 0.9900 |
| C20—H20A | 0.9500 | C511—H51C | 0.9800 |
| C51—C52 | 1.394 (2) | C511—H51D | 0.9800 |
| C51—C56 | 1.403 (2) | C511—H51E | 0.9800 |
| C52—C53 | 1.389 (2) | ||
| C1—N21—C4 | 110.37 (13) | C54—C53—H53A | 120.3 |
| C1—N21—H21 | 124.8 | O1—C54—C55 | 114.91 (14) |
| C4—N21—H21 | 124.8 | O1—C54—C53 | 125.17 (15) |
| C19—N24—C16 | 105.16 (13) | C55—C54—C53 | 119.92 (14) |
| N21—C1—C20 | 126.59 (15) | C56—C55—C54 | 120.09 (15) |
| N21—C1—C2 | 106.65 (13) | C56—C55—H55A | 120.0 |
| C20—C1—C2 | 126.68 (15) | C54—C55—H55A | 120.0 |
| C3—C2—C1 | 108.13 (15) | C55—C56—C51 | 121.49 (16) |
| C3—C2—H2A | 125.9 | C55—C56—H56A | 119.3 |
| C1—C2—H2A | 125.9 | C51—C56—H56A | 119.3 |
| C2—C3—C4 | 108.17 (14) | C54—O1—C57 | 118.76 (12) |
| C2—C3—H3A | 125.9 | O1—C57—C58 | 106.88 (13) |
| C4—C3—H3A | 125.9 | O1—C57—H57A | 110.3 |
| N21—C4—C5 | 124.67 (14) | C58—C57—H57A | 110.3 |
| N21—C4—C3 | 106.62 (14) | O1—C57—H57B | 110.3 |
| C5—C4—C3 | 128.61 (14) | C58—C57—H57B | 110.3 |
| C4—C5—C16i | 123.29 (14) | H57A—C57—H57B | 108.6 |
| C4—C5—C51 | 118.72 (14) | C57—C58—C59 | 111.63 (14) |
| C16i—C5—C51 | 117.82 (14) | C57—C58—H58A | 109.3 |
| N24—C16—C5i | 125.69 (15) | C59—C58—H58A | 109.3 |
| N24—C16—C17 | 110.71 (14) | C57—C58—H58B | 109.3 |
| C5i—C16—C17 | 123.59 (14) | C59—C58—H58B | 109.3 |
| C18—C17—C16 | 106.36 (14) | H58A—C58—H58B | 108.0 |
| C18—C17—H17A | 126.8 | C510—C59—C58 | 113.42 (14) |
| C16—C17—H17A | 126.8 | C510—C59—H59A | 108.9 |
| C17—C18—C19 | 106.69 (15) | C58—C59—H59A | 108.9 |
| C17—C18—H18A | 126.7 | C510—C59—H59B | 108.9 |
| C19—C18—H18A | 126.7 | C58—C59—H59B | 108.9 |
| N24—C19—C20 | 126.46 (14) | H59A—C59—H59B | 107.7 |
| N24—C19—C18 | 111.07 (13) | C59—C510—C511 | 113.04 (14) |
| C20—C19—C18 | 122.44 (15) | C59—C510—H51A | 109.0 |
| C1—C20—C19 | 128.88 (15) | C511—C510—H51A | 109.0 |
| C1—C20—H20A | 115.6 | C59—C510—H51B | 109.0 |
| C19—C20—H20A | 115.6 | C511—C510—H51B | 109.0 |
| C52—C51—C56 | 117.34 (14) | H51A—C510—H51B | 107.8 |
| C52—C51—C5 | 123.54 (14) | C510—C511—H51C | 109.5 |
| C56—C51—C5 | 119.12 (15) | C510—C511—H51D | 109.5 |
| C53—C52—C51 | 121.78 (15) | H51C—C511—H51D | 109.5 |
| C53—C52—H52A | 119.1 | C510—C511—H51E | 109.5 |
| C51—C52—H52A | 119.1 | H51C—C511—H51E | 109.5 |
| C52—C53—C54 | 119.38 (16) | H51D—C511—H51E | 109.5 |
| C52—C53—H53A | 120.3 | ||
| C4—N21—C1—C20 | 174.18 (15) | C2—C1—C20—C19 | 176.51 (16) |
| C4—N21—C1—C2 | −2.51 (18) | N24—C19—C20—C1 | 0.4 (3) |
| N21—C1—C2—C3 | 1.54 (18) | C18—C19—C20—C1 | 178.43 (16) |
| C20—C1—C2—C3 | −175.14 (16) | C4—C5—C51—C52 | 63.4 (2) |
| C1—C2—C3—C4 | −0.05 (19) | C16i—C5—C51—C52 | −121.15 (18) |
| C1—N21—C4—C5 | −174.17 (15) | C4—C5—C51—C56 | −116.84 (18) |
| C1—N21—C4—C3 | 2.48 (18) | C16i—C5—C51—C56 | 58.6 (2) |
| C2—C3—C4—N21 | −1.45 (18) | C56—C51—C52—C53 | −0.7 (2) |
| C2—C3—C4—C5 | 175.01 (16) | C5—C51—C52—C53 | 179.07 (15) |
| N21—C4—C5—C16i | −2.8 (3) | C51—C52—C53—C54 | 0.0 (2) |
| C3—C4—C5—C16i | −178.73 (16) | C52—C53—C54—O1 | −179.13 (15) |
| N21—C4—C5—C51 | 172.32 (14) | C52—C53—C54—C55 | 0.5 (2) |
| C3—C4—C5—C51 | −3.6 (3) | O1—C54—C55—C56 | 179.34 (14) |
| C19—N24—C16—C5i | 179.15 (15) | C53—C54—C55—C56 | −0.4 (2) |
| C19—N24—C16—C17 | 0.37 (17) | C54—C55—C56—C51 | −0.4 (2) |
| N24—C16—C17—C18 | 0.14 (18) | C52—C51—C56—C55 | 0.9 (2) |
| C5i—C16—C17—C18 | −178.68 (15) | C5—C51—C56—C55 | −178.90 (15) |
| C16—C17—C18—C19 | −0.56 (18) | C55—C54—O1—C57 | −175.16 (14) |
| C16—N24—C19—C20 | 177.47 (16) | C53—C54—O1—C57 | 4.5 (2) |
| C16—N24—C19—C18 | −0.72 (17) | C54—O1—C57—C58 | 174.70 (13) |
| C17—C18—C19—N24 | 0.84 (19) | O1—C57—C58—C59 | 172.84 (13) |
| C17—C18—C19—C20 | −177.45 (15) | C57—C58—C59—C510 | −170.19 (15) |
| N21—C1—C20—C19 | 0.5 (3) | C58—C59—C510—C511 | −177.21 (15) |
Symmetry code: (i) −x+1, −y, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N21—H21···N24 | 0.88 | 2.50 | 3.033 (2) | 119 |
| N21—H21···N24i | 0.88 | 2.22 | 2.804 (2) | 123 |
Symmetry code: (i) −x+1, −y, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZL2552).
References
- Bruker (2005). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Scheidt, W. R. & Lee, Y. J. (1987). Struct. Bond. 64, 1–70.
- Senge, M. O. (2006). Chem. Commun. pp. 243–256. [DOI] [PubMed]
- Senge, M. O., Medforth, C. J., Forsyth, T. P., Lee, D. A., Olmstead, M. M., Jentzen, W., Pandey, R. K., Shelnutt, J. A. & Smith, K. M. (1997). Inorg. Chem. 36, 1149–1163. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wiehe, A., Shaker, Y. M., Brandt, J. C., Mebs, S. & Senge, M. O. (2005). Tetrahedron, 61, 5535–5564.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, 2R. DOI: 10.1107/S160053681301550X/zl2552sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681301550X/zl2552Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report


