Abstract
Multisensory stimulation has been shown to alter the sense of body-ownership. Given that perceived similarity between one’s own body and those of others is crucial for social cognition, we investigated whether multisensory stimulation can lead participants to experience ownership over a hand of different skin colour. Results from two studies using introspective, behavioural and physiological methods show that, following synchronous visuotactile (VT) stimulation, participants can experience body-ownership over hands that seem to belong to a different racial group. Interestingly, a baseline measure of implicit racial bias did not predict whether participants would experience the RHI, but the overall strength of experienced body-ownership seemed to predict the participants’ post-illusion implicit racial bias with those who experienced a stronger RHI showing a lower bias. These findings suggest that multisensory experiences can override strict ingroup/outgroup distinctions based on skin colour and point to a key role for sensory processing in social cognition.
Keywords: Body-ownership, Rubber hand illusion, Multisensory, Social groups, Skin colour, Body image
1. Introduction
Recent studies on the role of multisensory integration for body-awareness have aptly demonstrated the malleability of body representations. In particular, bodily illusions such as the Rubber Hand Illusion (RHI, see Botvinick & Cohen, 1998) and the body swap illusion (Petkova & Ehrsson, 2008) suggest that synchronised visuo-tactile (VT) stimulation between one’s own body and a foreign body can change the sense of body-ownership. For example, in the RHI, seeing a fake hand being touched at the same time as the experimenter touches the participant’s own unseen hand causes the rubber hand to be experienced as part of one’s body. Several studies have shown that factors such as the corporeality of the stimulated object (Haans, IJsselsteijn, & de Kort, 2008; Tsakiris, Carpenter, James, & Fotopoulou, 2010), the anatomical congruency (Pavani, Spence, & Driver, 2000; Tsakiris & Haggard, 2005), the volumetric congruency (Pavani & Zampini, 2007), the postural congruency (Austen, Soto-Faraco, Enns, & Kingstone, 2004; Costantini & Haggard, 2007), and the spatial relation between viewed and felt body-part (Lloyd, 2007), modulate the induction of the RHI and the experience of body-ownership. For example, greater discrepancies between the visual form of the viewed object relative to the participant’s hand or between the posture of the rubber hand relative to the participant’s hand diminish or even abolish the RHI (Tsakiris et al., 2010).
Less is known however about how more surface level differences between the participants’ own hand and the rubber hand affect the RHI. One area of particular interest is whether the skin colour of the rubber hand compared to that of the participant has an effect on the inducement or strength of the RHI. Skin colour is a particularly salient difference between racial groups and, as such, appears to be a default method of separating others into ingroup and outgroup categories, participating in several social cognition processes (e.g., stereotyping) (Fiske & Neuberg, 1990; Hewstone, Hantzi, & Johnston, 1991; though see Kurzban, Tooby, & Cosmides, 2001). Therefore the extent to which skin colour affects the RHI provides a viable way of investigating whether social distinctions influence body-ownership (Farmer & Tsakiris, 2012).
The research issue of whether factors such as skin colour have an effect on body-ownership is further motivated by the findings of a number of recent studies. Using behavioural and neuroimaging methods these studies have shown that the activation of shared bodily representations for self and other (Gallese & Sinigaglia, 2010; Keysers & Gazzola, 2009; Rizzolatti & Fabbri-Destro, 2010; Thomas, Press, & Haggard, 2006) can be modulated by whether the other person being observed is considered to be a member of an ingroup or an outgroup (Désy & Théoret, 2007; Gutsell & Inzlicht, 2010; Molnar-Szakacs, Wu, Robles, & Iacoboni, 2007; Serino, Giovagnoli, & Làdavas, 2009). For example Serino and colleagues (Serino et al., 2009) investigated the effect of ingroup/outgroup distinctions on the visual remapping of touch (VRT), an effect in which the observation of touch on another’s body leads to greater sensitivity to tactile stimulation on one’s own body. Participants were more accurate in detecting touch when they observed touch delivered on the face of someone from the same ethnic or political group as themselves. The second of these findings is especially interesting as it involves a purely social distinction between self and other with no greater bodily dissimilarity (e.g. same skin colour) between a politician with the opposing views to one’s own than to one with the same views.
Other studies have used neuroimaging techniques to investigate the role of group membership in modulating shared bodily representations. For example, motor cortex excitability as measured by single-pulse transcranial magnetic stimulation (TMS) over the primary motor cortex, which presumably reflects activity within the human mirror neuron system involved in social cognition (Fadiga, Fogassi, Pavesi, & Rizzolatti, 1995), was greater when participants observed gestures performed by an actor of the same ethnic group compared to a member of an ethnic outgroup (Molnar-Szakacs et al., 2007). In contrast however Désy and Théoret (2007) found increased corticospinal activation when participants observed a hand of a different skin colour performing an action compared to a hand of their own colour. This discrepancy may be due to the different nature of the hand actions observed in the two studies; Molnar-Szakacs et al. (2007) used communicative gestures while Désy and Théoret (2007) used simple finger movements. Despite the differences, both studies point to differential sensorimotor responses to observed events occurring in an ingroup or outgroup body. A recent EEG study (Gutsell & Inzlicht, 2010) also supports the hypothesis that motor representations in the brain are sensitive to ingroup/outgroup distinctions. Using white participants the authors found activation over the motor cortex during the observation of racial ingroup members performing actions but not during observation of racial outgroup members performing the same actions. Moreover this effect was linked to participants’ level of racial prejudice and was greater for more strongly disliked racial outgroups compared to less disliked outgroups.
Finally, two recent studies have investigated the effect of racial group membership on the sensorimotor empathy for pain. Xu, Zuo, Wang, and Han (2009) found that the observation of members of a racial outgroup receiving painful stimuli led to less BOLD activation in brain areas involved in pain processing than did the observation of a racial ingroup. Avenanti, Sirigu, and Aglioti (2010) used single pulse TMS to observe corticospinal excitability in black and white participants observing a hand of either their own skin colour or a different skin colour being stabbed with a syringe and found that, while observation of a hand from a racial ingroup led to motor suppression, observation of a racial outgroup hand resulted in motor excitation. Additionally, motor excitation was positively correlated with the participants’ implicit racial bias as measured by an implicit association task (IAT) (Greenwald, McGhee, & Schwartz, 1998). This suggests that the modulation of sensorimotor empathy, rather than merely being due to the physical difference between the ingroup and outgroup hands, was related to the social attitudes of participants (Avenanti, Bueti, Galati, & Aglioti, 2005).
Given this evidence for a distinction between ingroup and outgroup in the activation of shared bodily representations the question that the current study seeks to address is whether changes in the sense of body-ownership induced by the RHI can also be elicited for a hand of a different skin colour. To date only two studies have commented on this question, but without testing this hypothesis directly. Longo, Schüür, Kammers, Tsakiris, and Haggard (2009) reported that the actual similarity of skin colour between the participants’ hands and the rubber hand, as measured by both third person ratings and skin luminosity, seemed to have no effect on the strength of the RHI. Similarly, Holmes, Snijders, and Spence (2006) examined the effect of visual exposure to a white rubber hand on participants reaching movements and found no difference between white and non-white participants in either the alteration of reaching movements or the sense of perceived body-ownership over a white rubber hand, suggesting that skin colour does not affect body-ownership.1 However, neither of these studies directly addressed the question of how skin colour impacts on feelings of body-ownership. Both Longo et al. (2009) and Holmes et al. (2006) used a white rubber hand, the skin colour of the culturally dominant group, and only included a small number of non-white participants (around 25% of all participants in both Longo et al. (2009) and Holmes et al. (2006; Holmes, 2011, personal communication) and neither study directly manipulated the skin colour of either the rubber hand used or the population tested.
The current study is the first to directly investigate in a systematic manner whether the inducement and strength of the RHI is modulated by the skin colour of the rubber hand. Rather than testing different racial groups, we opted for the direct control of the skin colour of the rubber hand. Therefore, across two experiments, we manipulated the skin colour of the rubber hand (i.e. white vs. black) to examine the extent to which white participants experienced a sense of body-ownership for a body-part from a same or different racial group. We quantified the change in body-ownership using introspective (i.e. RHI questionnaire), behavioural (i.e. proprioceptive drift) and physiological (i.e. skin conductance response, SCR) measures. Experiment 1 examined the effect of synchronous vs. asynchronous visuo-tactile (VT) stimulation on the experience of body-ownership for hands of both skin colours. Experiment 2 built on these findings by including a new condition in which participants observed synchronous stimulation of the hands from a third person perspective (3PP) in addition to the synchronous and asynchronous conditions in which the rubber hand appeared from a 1st person perspective (1PP) and also introduced a pre-VT stimulation measure of SCR in order to examine any differences in baseline SCR to threatening stimuli between the black and white rubber hands.
In order to examine whether the participants’ psychosocial attitudes had an effect on the strength of their feelings of body-ownership over the black hand, we measured their implicit bias in favour of people of white ethnicity compared to people of black ethnicity using the IAT for race (Greenwald et al., 1998). In Experiment 1 we also investigated whether there was a converse relationship between body-ownership and racial bias by asking participants to complete the same IAT again after the VT stimulation. In addition we also measured participants’ trait empathy using the Interpersonal Reactivity Index (IRI, Davis, 1983) to examine the relationship between empathy and the RHI and to determine whether those with greater empathic traits were more likely to feel ownership over a hand of a different skin colour.
2. Experiment 1
2.1. Methods
2.1.1. Participants
Twenty participants (mean age ± SD: 21 ± 2.49, 6 male) gave their informed consent to participate and were paid for their participation. All participants self identified as white. The study was approved by the Departmental Ethics Committee, Royal Holloway, University of London.
2.1.2. Design
The study used a repeated measures design with 3 factors, each with 2 levels. The first factor was the mode of VT stimulation between the participant’s hand and the rubber hand (synchronous vs. asynchronous); the second factor was the skin colour of the rubber hand (white hand vs. black hand); and the third factor was the type of stimulus appearing at the end of the VT stimulation (pain vs. touch). Therefore there were a total of 8 conditions. Each condition was presented once per participant and conditions were presented in a randomised order.
2.1.3. Procedure
Participants were asked to attend 2 experimental sessions within 7 days of each other. In the first session participants were asked to complete a demographic questionnaire and the IRI (Davis, 1983). Following this, participants carried out a computer administered version of the race-IAT. The associations between stimuli and response key and the order of associations (i.e. positive words and white faces or positive words and black faces) were counterbalanced across participants (Greenwald, Nosek, & Banaji, 2003; Greenwald et al., 1998). The data from the IATs were analysed using the improved IAT scoring algorithm recommended by Greenwald et al. (2003).
At the beginning of the second experimental session two electrodes were attached to the index and middle fingers of participant’s right hand in order to measure skin conductance response (SCR) to the stimulus (pain or touch) presented at the end of VT stimulation. SCR is a sensitive and valid indicator for arousal (Boucsein, 1992). Physiological signals were sampled at a rate of 250 Hz and amplified (AD Instruments).
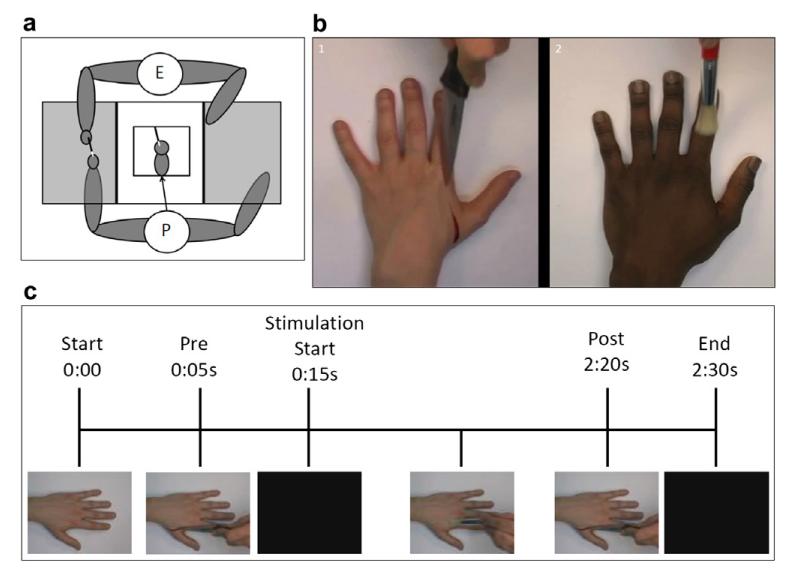
In each of the eight experimental conditions participants received VT stimulation while looking at a rubber hand. The technique used to deliver VT stimulation and collect participants proprioceptive judgements was identical to that used by Tsakiris and Haggard (2005, see Experiment 1). Participants sat in front of a table with a frame on it covered by a two way mirror. The two way mirror was used to make the rubber hand appear (during stimulation) and disappear (during proprioceptive judgements and questions). For each condition participants saw a ruler reflected on the mirror. The ruler was positioned 18 cm above the mirror, to appear at the same gaze depth as the rubber hand. Participants were asked, “Where is your index finger?” and in response, they verbally reported a number on the ruler. They were instructed to judge the position of their finger by projecting a parasagittal line from the centre of their fingertip to the ruler. During the judgments, there was no tactile stimulation, and the lights under the two-way mirror were switched off to make the rubber hand invisible, leaving only the ruler visible. Following the judgement, the lights under the two-way mirror were switched on so that participants could see the rubber hand, and the experimenter commenced VT stimulation for 120 s. During VT stimulation the participant’s left hand and the rubber hand were stimulated, on the index, middle and ring fingers alternately, from the knuckle to the tip with two identical paintbrushes with a frequency of approximately 1 Hz. In the synchronous condition the hands were brushed at the same time, while in the asynchronous condition they were brushed 180° out of phase.
After VT stimulation, the experimenter either, produced a hypodermic syringe and thrust it into the rubber hand’s index finger (pain stimulus), or produced a cotton-bud, and touched the rubber hand’s index finger with it (touch stimulus). To minimise the effect of surprise, participants were shown the syringe and cotton-bud before the experiment began and were assured that these would not touch their own hand. To control for the timing of events across conditions, the experimenter was listening to an audio file that prompted them when to cease VT stimulation and when to apply the pain or touch stimuli to the rubber hand.
Four seconds after the delivery of the pain or touch stimuli, the lights under the two-way mirror were switched off, so that participants could no longer see the rubber hand, and a second judgement of felt location was obtained. Participants were then asked to remove their left hand from the table, and to use a 7-point Likert Scale (ranging from −3, i.e. strongly disagree, to +3, i.e. strongly agree) to indicate their agreement on three statements related to their own subjective experience during each VT condition (see Fig. 1), which were adapted from Longo, Schüür, Kammers, Tsakiris, and Haggard (2008). Participants were also asked a fourth question on their perception of pain when the stimuli touched the rubber hand which was rated on a 7 point scale from 0 (no pain) to 6 (intense pain). Once this was completed the next block began. Blocks were presented in a random order across participants. Finally, once all blocks had been completed, participants again carried out the race IAT.
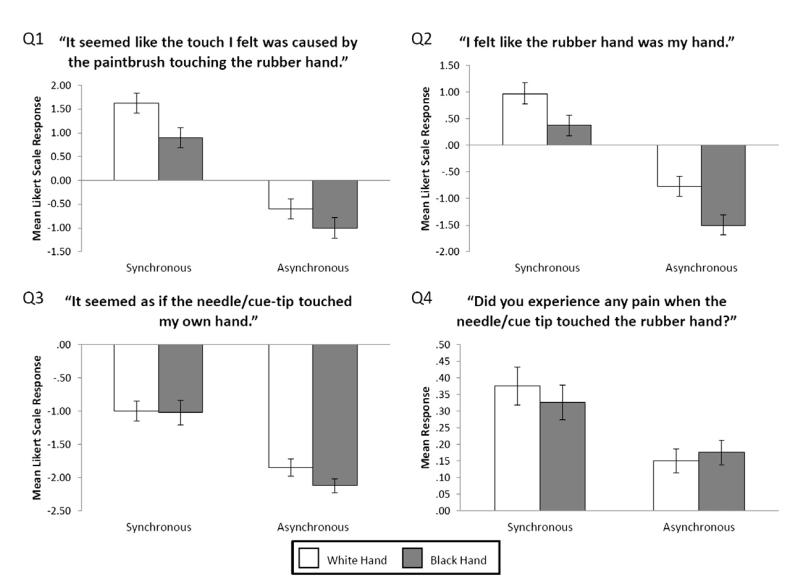
Fig. 1.
Mean questionnaire ratings (n = 20) averaged across pain and touch conditions. Error bars indicate standard error.
2.1.4. Analysis of behavioural and physiological data
Proprioceptive drift was calculated by subtracting participants’ pre-stimulation proprioceptive judgement from their post-stimulation proprioceptive judgement for each condition.
SCR values were calculated by taking the mean SCR from the 6 s starting 1 s after the appearance of the pain/touch stimuli and subtracting from this the mean SCR between 3 and 1 s before the appearance of the stimulus (baseline; Dimberg, 1990). This interval was chosen to be the region of interest, because changes in SCR normally occur between 1 and 2 s after stimulus onset, although the response can be delayed up to 5 s (Edelberg, 1967). Following standard guidelines for the analysis of electrodermal responses, SCR amplitudes below 0.05 μs were scored as zero responses (Boucsein, 1992; Dawson, Schell, & Filion, 2007). Participants who had zero responses in more than half of the trials in which the pain stimuli appeared were considered ‘null-responders’ and were excluded from the analysis (e.g. Phelps et al., 2001), which left 17 participants in the SCR analysis. All data were individually z-scored to control for individual differences in responsiveness (Boucsein, 1992; Venables & Christie, 1980).
2.2. Results
2.2.1. Introspective measures
In order to examine the effectiveness of the manipulation of body-ownership the number of people experiencing ownership over the white and black hand in the synchronous condition was calculated based on participants’ response to the statement “I felt like the rubber hand was my hand”. Participants with a mean response greater than 0 were considered to have felt body-ownership over the rubber hand while those with a response less than or equal to 0 were not. The manipulation was largely successful with 75% of participants experiencing body-ownership over the white rubber hand and 60% of participants experiencing body-ownership over the black rubber hand during synchronous VT stimulation across the pain and touch conditions.
In order to further analyse the relationship between skin colour and introspective judgements of body-ownership separate repeated measures ANOVAs were run on each of the four questions. Each ANOVA contained three within-subjects factors: the mode of VT stimulation (asynchronous/synchronous), the skin colour of the rubber hand (white/black), and the type of stimulus appearing at the end of the VT stimulation (pain/touch).
Both the first question “It seemed like the touch I felt was caused by the paintbrush touching the rubber hand.” and the second question “I felt like the rubber hand was my hand.” showed the same pattern of results (see Fig. 1). A significant main effect was found for the mode of VT stimulation (Q1: F(1,19) = 25.97, p < .001, MSE = 6.55, Q2: F(1,19) = 31.32, p < .001, MSE = 4.2), because overall synchronous VT stimulation resulted in significantly higher ratings compared to asynchronous stimulation across all levels of the other factors. A main effect was also found for the skin colour of the rubber hand (Q1: F(1,19) = 7.89, p < .01, MSE = 1.6, Q2: F(1,19) = 16.62, p < .01, MSE = 1.06), because overall the white rubber hand resulted in higher ratings across all levels of the other factors. For both questions, the main effect of type of stimulus and all interactions were not significant.
For both the third question “It seemed as if the needle/cue-tip touched my own hand.” and the fourth question “Did you experience any pain when the needle/cue tip touched the rubber hand?” a significant main effect of VT stimulation was found (Q3: F(1,19) = 31.32, p < .001, MSE = 2.99, Q4: F(1,19) = 4.89, p < .05, MSE = 0.29, see Fig. 1). This was because overall synchronous VT stimulation resulted in higher ratings across all levels of the other two factors. No other significant main effects or interactions were found for question 3 or 4.
Overall, the analysis of the psychometric data suggests that an ingroup rubber hand is more likely to be experienced as self-relevant independently of the pattern of VT stimulation, but at the same time, the critical manipulation of synchronous vs. asynchronous multisensory stimulation shows synchronous VT stimulation can elicit a sense of body-ownership independently of the skin colour of the rubber hand. In fact, we examined whether the magnitude of the change between synchronous and asynchronous conditions in the subjective experience captured by questions 1 and 2 was the same when looking a white rubber hand vs. a black rubber hand (averaged across pain and touch conditions). We therefore subtracted the ratings obtained in the asynchronous condition from those obtained in the synchronous condition, for each hand, and directly compared the resulting values which reflect the magnitude of change in the subjective experience between synchronous and asynchronous. The differences between black and white rubber hand conditions were not significant (for the first question, t(19) = 0.76, p > .05, and for the second question, t(19) = 0.58, p > .05), suggesting that the magnitude in the change of the experience between synchronous and asynchronous stimulation was comparable between the two different rubber hands.
2.2.2. Proprioceptive drift
In order to investigate the effect of skin colour on behavioural measures of body-ownership a repeated measures ANOVA with mode of VT stimulation (asynchronous/synchronous)× skin colour of rubber hand (white/black) × type of stimulus (pain/touch) was carried out on the proprioceptive drift values.
A significant main effect was found for the mode of VT stimulation (F(1,19) = 11.47, p < .05, MSE = 5.45; see Fig. 2a), because overall synchronous VT stimulation resulted in greater proprioceptive drift across all levels of the other two factors. No main effect of skin colour or type of stimulus was found and there were no significant interactions, suggesting that the increases in proprioceptive drift following synchronous stimulation were not affected by the skin colour of the rubber hand or the stimulus that appeared at the end of stimulation.
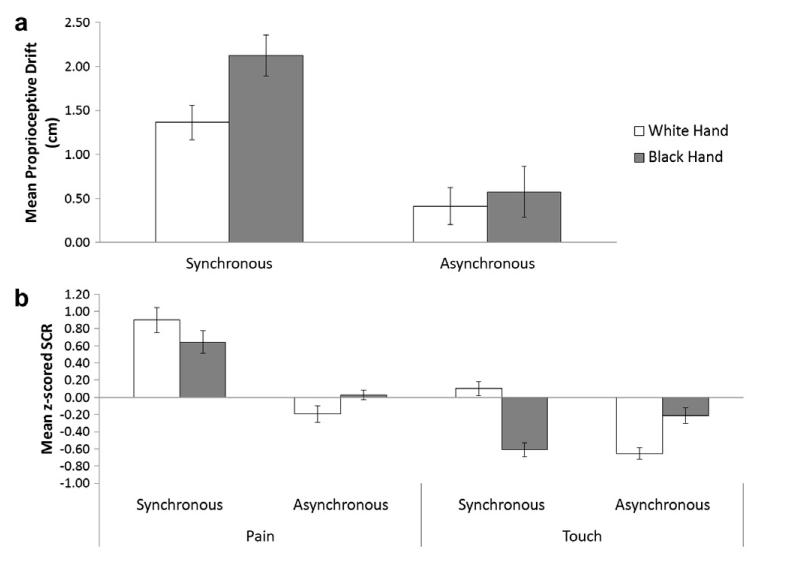
Fig. 2.
(a) Mean proprioceptive drift (n = 20) averaged across pain and touch conditions. Zero represents the felt position of the participant’s hand prior to VT stimulation. (b) Mean z-scored SCR in response to the threatening and non-threatening object approaching the rubber hand across conditions (n = 17). Error bars indicate standard errors.
2.2.3. Skin conductance responses
In order to assess whether physiological response to observing painful stimuli touching the rubber hand was modulated by the skin colour of the rubber hand, a mode of VT stimulation (asynchronous/synchronous)× skin colour of rubber hand (white/black) × type of stimulus (pain/touch) repeated measures ANOVA was run on the SCR values from each condition (see Fig. 2b). As indicated in the method section, participants who had responses of less than 0.05 μs in more than half of the trials in which the pain stimuli appeared were considered null-responders. Due to the exclusion of null-responders the sample for the SCR analysis was made up of 17 participants.
A main effect was found for type of stimulus (F(1,16) = 28.3, p < .001, MSE = 0.57), because the painful stimulus elicited higher SCRs than the observation of non-painful stimulus touching the rubber hand. A main effect was also found for the mode of VT stimulation (F(1,16) = 11.96, p = <.01, MSE = 0.76), because overall SCRs were higher following synchronous VT stimulation compared to asynchronous stimulation. An interaction between type of stimulus and stimulation was also found (F(1,16) = 5.38, p < .05, MSE = 0.72). Paired comparisons revealed that this interaction was due to significantly higher SCRs after synchronous stimulation than after asynchronous stimulation for the pain/white hand (t(16) = 2.52, p < .05), pain/black hand (t(16) = 2.27, p < .05) and touch/white hand (t(16) = 3.11, p < .01) conditions, but not for the touch/black hand conditions. Finally an interaction between colour and stimulation was found (F(1,16) = 8.76, p < .01). However, paired comparisons indicated that this interaction was due to significantly higher SCRs for the white hand compared to the black hand in the touch synchronous conditions (t(16) = 2.89, p < .05), and not in the pain conditions (t(16) = 0.59, p > .05), ruling out the possibility that synchronous stimulation elicited significantly higher SCRs for the painful stimulus when applied to the white hand than to the black hand. Given that it is unclear how to interpret SCR data to a non-threatening stimuli and the fact that the touch conditions were primarily included to control for participants becoming habituated to the appearance of painful stimuli we will not address this effect further in the paper. No main effect of skin colour was found and nor were there any other significant interactions.
2.2.4. Relationship between empathy and body-ownership for ingroup and outgroup hands
In order to analyse the factors that predicted the strength of the experience of body-ownership for the white and black rubber hands, the mean of each participant’s responses to question 2 (e.g. “I felt like the rubber hand was my hand”) of the questionnaire were averaged across the pain- and touch-synchronous stimulation conditions and the resulting values were used as dependent variables in two multiple regressions (entry method) using participants’ scores on the four sub-scales from the IRI as predictors. The regression carried out on ownership scores for the white rubber hand, produced an R2 of .78, (F(4,15) = 5.88, p < .01). This was due to a significant positive regression weight on the fantasy subscale of the IRI (B = 0.24, t(15) = 3.97, p < .001). The same regression carried out on ownership scores for the black rubber hand produced an R2 of .62, (F(4,15) = 6.17, p < .01). This was again due to a significant positive regression weight on the fantasy subscale of the IRI (B = 0.26, t(15) = 4.38, p < .001). In neither analysis were any of the other IRI subscales significant predictors.
2.2.5. Relationship between racial bias and Body-ownership for Ingroup and outgroup hands
In order to investigate whether racial bias, as measured with the IAT, had an effect on the experience of body-ownership for the white and black rubber hands, the mean of each participant’s responses to question 2 of the questionnaire were averaged across the pain- and touch-synchronous stimulation conditions and the resulting values were used as dependent variables in two linear regressions (entry method) using participants’ scores on the pre VT stimulation IAT as the predictor. Neither regression however, produced a significant model for the white rubber hand: R2 of .054 (F(1,18) = 2.08, p > .05), for the black rubber hand: R2 of −0.11, (F(1,18) = 0.79, p > .05)), suggesting that participants’ implicit racial bias did not predict whether they would experience the RHI for either the white or black hand.
To investigate whether feeling body-ownership over a hand of a different skin colour changed participant’s racial bias as measured with IAT, a multiple regression (entry method) was run with participants ratings for question 2 after synchronous VT stimulation (averaged across pain and touch conditions) for both the white and black rubber hands as predictors and their score on the post VT stimulation IAT as the dependent variable. This regression produced an R2 of .32, (F(2,17) = 3.96, p < .05) indicating that the stronger the sense of body-ownership participants experienced over the rubber hands the lower their post testing racial bias, as measured with the IAT. Interestingly, the experience of ownership over either the white (B = −0.11, t(17) = −1.5, p > .05) or the black (B = −0.01, t(17) = .09, p > .05) rubber hand were not individually significant predictors, suggesting that the post-RHI IAT score was predicted by a generalised experience of ownership over any rubber hand rather than exclusively by the experience of ownership of a white or black hand.
To investigate whether feeling body-ownership over a hand of a different skin colour led to a significant reduction in racial bias a measure of change in racial bias was calculated by subtracting the participants’ scores in the pre-stimulation IAT from their scores in the post stimulation IAT. The resulting values were entered as the dependent variable in a multiple regression (entry method) and participants ratings for question 2 after synchronous VT stimulation (averaged across pain and touch conditions) for both the white and black rubber hands were used as the predictors. The regression did not produce a significant model (R2 of −.07, (F(2,17) = 0.35, p > .05).
2.3. Discussion: Experiment 1
The results from Experiment 1 suggested that synchronous VT stimulation could lead to the feeling of body-ownership over a hand of a different skin colour as measured by introspective reports, behavioural and physiological measures. Analysis of the introspective reports showed that synchronous VT results in body-ownership independently of the skin colour. However, subjective reports also indicated that skin colour did have an effect on subjective feelings of body-ownership which was independent to that of multisensory stimulation. Unlike subjective reports, the behavioural and physiological data revealed no main effect of skin colour. For the SCR data, we observed significant differences between the synchronous and asynchronous stimulation for the pain stimulus for both the white and the black hand, consistent with the effect of stimulation observed on proprioceptive drift. In addition multiple regressions indicated a role of empathy in individual differences in the experience of the RHI for both white and black hands and that the overall strength of experienced body-ownership during the RHI seemed to predict the participants’ post-illusion implicit racial bias with those who experienced a stronger RHI showing a lower bias. Importantly, across all these measures, the effect of synchronous multisensory stimulation, over and above the mere presence of multisensory stimulation (i.e. asynchronous conditions), revealed comparable changes in the experience of body-ownership for hands of both skin colours, suggesting that synchronous stimulation can lead to ownership over a hand of a different skin colour.
It is possible however that the observed changes in introspective, behavioural and physiological measures of body-ownership were due to synchronous stimulation per se and not directly to the feeling of body-ownership. For example, it might be the case that increased SCRs to threatening stimuli reflect a purely attentional effect that result from the association of synchronous events occurring on one’s own body and on an external body-part, rather than a change in the experience of body-ownership per se. One way to dissociate the pure effect of synchrony from the effect of synchrony on body-ownership is to deliver synchronous VT stimulation under conditions that either induce body-ownership or not. To that end, in Experiment 2 we included an additional control condition at which participants received tactile stimulation on their own unseen hand, synchronous with that observed on the rubber hand, but the rubber hand was viewed from a third person perspective (3PP). It has been shown that this condition does not induce a sense of body-ownership (Ehrsson, Spence, & Passingham, 2004). Another potential limitation of Experiment 1, which we sought to address in Experiment 2, was the absence of a pre-stimulation baseline measure of SCR to observation of threatening objects approaching the hands. Obtaining pre-stimulation SCR measures is important for examining whether there are differences in baseline SCR to threatening stimuli between the black and white rubber hands, and for quantifying the change in SCR as a function of the colour of the rubber hand, the stimulation pattern and the experience of ownership. In order to address these weaknesses a second experiment was conducted.
3. Experiment 2
3.1. Methods
3.1.1. Participants
Forty-eight white participants (mean age ± SD: 26.5, ±8.34, 14 male) gave their informed consent to participate and were paid for their participation. All participants self identified as white. The study was approved by the Departmental Ethics Committee, Royal Holloway, University of London.
3.1.2. Design
The study used a repeated measures design with 2 factors. The first factor was the type of VT stimulation delivered to the participant’s hand and the rubber hand. The first two levels of this factor were the same as those in Experiment 1, that is synchronous and asynchronous stimulation delivered to the participant’s hand and a hand seen from a first person perspective (1PP). In addition another level was added in which synchronous VT stimulation was delivered to the participant’s hand and another hand which was rotated 180° from the position of the participants own hand, so that the hand was viewed from a third person perspective (3PP). Previous studies have demonstrated that the RHI does not occur when the rubber hand is viewed from this perspective (Ehrsson et al., 2004). The second factor was the skin colour of the hand (white hand vs. black hand). Therefore there were a total of six conditions. Each condition was presented once per participant and conditions were presented in a randomised order.
3.1.3. Materials
Rather than using a physical rubber hand as in Experiment 1, in this study videos of real hands were used in order to increase the accuracy of the timing of events; however, for the sake of simplicity, we will refer to the hands displayed in the video as “rubber” hands. Twelve videos were used in this study: male participants saw videos of male hands while female participants saw videos of female hands. For both male and female hands there was one video for each of the six conditions (see Fig. 3b).
Fig. 3.
(a) Experimental setup indicating positions of experimenter (E) and participant (P). (b) Examples of experimental stimuli: (1) White female hand being cut by knife. (2) Black male hand being stroked with paintbrush. (c) Time course of videos.
3.1.4. Procedure
At the beginning of the experiment, participants were asked to complete demographic and IRI questionnaires (Davis, 1983). Following this, participants carried out the same computer administered version of the IAT as used in Experiment 1. Following the completion of the IAT participants were asked to sit in front of the experimental setup (see Fig. 3a). In this setup the participant’s left hand was hidden beneath a sheet of card while a computer screen was visible with its centre at the midline of the participant’s body. Two electrodes were attached to the index and middle fingers of the participant’s right hand in order to measure SCR. In each of the six experimental conditions participants first viewed the hand in the video being cut by a knife in order to record a baseline measure of arousal to threatening stimuli prior to VT stimulation. Following this, participants received VT stimulation for 120 s while looking at the video of the hand. VT stimulation was delivered manually by the experimenter with the use of the same paintbrush as seen in the video. During VT stimulation the participant’s hand and the hand in the video were stimulated on the index finger in the same way, from the knuckle to the fingertip with a frequency of 1 Hz. In the synchronous condition, the participant’s hand was brushed at the same time as the hand in the video, while in the asynchronous condition it was brushed 180° out of phase with the video. Following VT stimulation, the video again showed the hand being cut by a knife in order to measure arousal to threatening stimuli subsequent to VT stimulation (see Fig. 3c). After each video, participants were asked to remove their left hand from the table and were asked the same 4 questions used in Experiment 1. Once this was completed the next block began. Blocks were presented in a random order across participants and each block was presented once per participant.
3.1.5. Analysis of physiological data
Mean SCR change in response to observation of the knife appearing was taken for both the appearance at the beginning (pre-stimulation) and the end (post-stimulation) of VT stimulation. In both cases SCR change was calculated, as in Experiment 1, by subtracting a baseline measure from the mean SCR over 6 s starting 1 s after the appearance of the knife. The baseline was the mean SCR between 3 and 1 s before the appearance of the knife. SCR amplitudes below 0.05 μs were scored as zero responses (Boucsein, 1992; Dawson et al., 2007). Participants who had zero responses in more than half of the post stimulation trials were considered ‘null-responders’ and were excluded from the analysis which left 42 participants in the SCR analysis. All data were individually z-scored to control for individual differences in responsiveness (Boucsein, 1992; Venables & Christie, 1980).
3.2. Results
3.2.1. Introspective measures
As in Experiment 1 the number of people experiencing ownership over the white and black hand in the synchronous 1PP condition was calculated based on participants’ response to the statement “I felt like the rubber hand was my hand”. Participants with a mean response greater than 0 were considered to have felt body-ownership over the rubber hand while those with a response less than or equal to 0 were not. The manipulation for the white hand was largely successful with 77% of participants experiencing body-ownership in the synchronous 1st person perspective (1PP) VT stimulation condition. For the black hand the manipulation was less successful than in Experiment 1, with only 50% participants experiencing body-ownership over the black hand during synchronous 1PP VT stimulation. Possible reasons for this discrepancy between the two experiments are addressed in the discussion.
In order to investigate the role of stimulation, perspective and skin colour on the strength of the RHI a 3 × 2 repeated measures ANOVA with type of VT stimulation (synchronous 1PP, asynchronous 1PP, synchronous 3PP) and skin colour of the viewed hand (white, black) as the factors was run for each of the four questions.
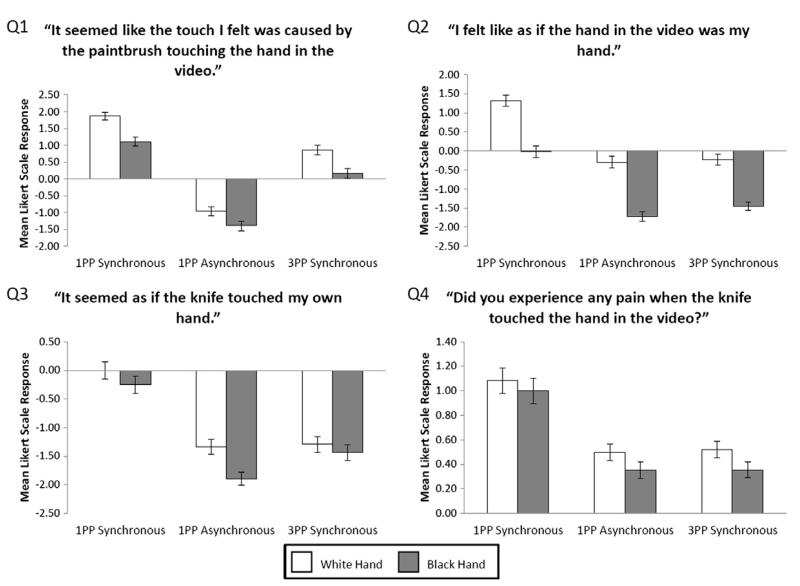
For the first question, “It seemed like the touch I felt was caused by the paintbrush touching the hand in the video”. Mauchly’s test indicated that the assumption of sphericity had been violated (χ2(2) = 10.06, p < .01), therefore degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = 0.84). A significant main effect was found for type of VT stimulation (F(1.67,78.57) = 75.68, p < .001, MSE = 2.76 s Fig. 4) because, overall, synchronous 1PP VT stimulation resulted in higher ratings than asynchronous 1PP stimulation (for the white hand t(47) = 9.55, p < .05, for the black hand t(47) = 8.62, p < .05) or synchronous 3PP stimulation (for the white hand t(47) = 4.92, p < .05, for the black hand t(47) = 3.49, p < .05, and synchronous 3PP stimulation resulted in higher ratings that asynchronous 1PP stimulation for the white hand t(47) = 5.71, p < .05, for the black hand t(47) = 4.9, p < .05. A main effect was also found for skin colour (F(1,47) = 18.84, p < .001, MSE = 1.53) because, after both synchronous and asynchronous VT stimulation ratings for the white hand were significantly higher than those for the black hand. There was no significant interaction between stimulation and skin colour, replicating the findings of Experiment 1.
Fig. 4.
Mean questionnaire answers (n = 48). Error bars indicate standard error.
For the second question, “I felt like as if the hand in the video was my hand.”, a significant main effect was found for type of VT stimulation (F(2,94) = 32.3, p < .001, MSE = 2.47, see Fig. 4) because, overall, synchronous 1PP VT stimulation resulted in higher ratings compared to both asynchronous 1PP stimulation and synchronous 3PP stimulation. A main effect was also found for skin colour (F(1,47) = 35.170, p < .001, MSE = 3.25) because, overall, the white hand resulted in higher ratings than the black hand. There was no significant interaction between stimulation and skin colour.
For both the third question, “It seemed as if the knife touched my own hand.” and the fourth question, “Did you experience any pain when the knife touched the hand in the video?”, Mauchly’s test indicated that the assumption of sphericity had been violated (Q3: χ2(2) = 12, p < .01, Q4: χ2(2) = 16.89, p < .001), therefore degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (Q3: ε = 0.81, Q4: ε = 0.77). In both questions a significant main effect of type of VT stimulation was found (Q3: F(1.63,76.45) = 27.19, p < .001, MSE = 2.76, Q4: F(1.53,71.9) = 28.53, p < .001, MSE = 0.83, see Fig. 4) because, overall, synchronous 1PP stimulation led to higher ratings than both asynchronous 1PP stimulation and synchronous 3PP stimulation. There was no effect of skin colour or any interaction between stimulation and skin colour.
As in Experiment 1 the analysis of the psychometric data suggests that an ingroup rubber hand is more likely to be experienced as self-relevant independently of the pattern of VT stimulation, but at the same time, the critical manipulation of synchronous vs. asynchronous multisensory stimulation shows synchronous VT stimulation leads to a greater sense of body-ownership independently of the skin colour of the rubber hand. As in Experiment 1 we examined whether the magnitude of the change between synchronous and asynchronous conditions in the subjective experience captured by questions 1 and 2 was the same when looking a white rubber hand vs. a black rubber hand. The differences between black and white rubber hand conditions were not significant (Q1: t(47) = 1.03, p > .05, Q2: t(47) = −0.29, p > .05), suggesting that the magnitude in the change of the experience between synchronous and asynchronous stimulation was comparable between the two different rubber hands. We also examined whether the magnitude of the change between synchronous 1PP and synchronous 3PP conditions in the subjective experience captured by questions 1 and 2 was the same when looking a white rubber hand vs. a black rubber hand. The differences between black and white rubber hand conditions were not significant (Q1: t(47) = 0.24, p > .05, Q2: t(47) = 0.38, p > .05), suggesting that the magnitude in the change of the subjective experience of ownership between 1st person and 3rd person perspectives was comparable between the two different rubber hands.
3.2.2. Skin conductance responses
In order to investigate the effect of stimulation, perspective and skin colour on physiological responses to the observation of painful stimuli touching the hand a 3 × 2 × 2 repeated measures ANOVA was run on the z-scored SCR data. The factors were: type of VT stimulation (1PP synchronous, 1PP asynchronous, 3PP synchronous), the skin colour of the hand (white, black) and the timing of the appearance of threatening stimuli (pre-VT stimulation, post-VT stimulation).
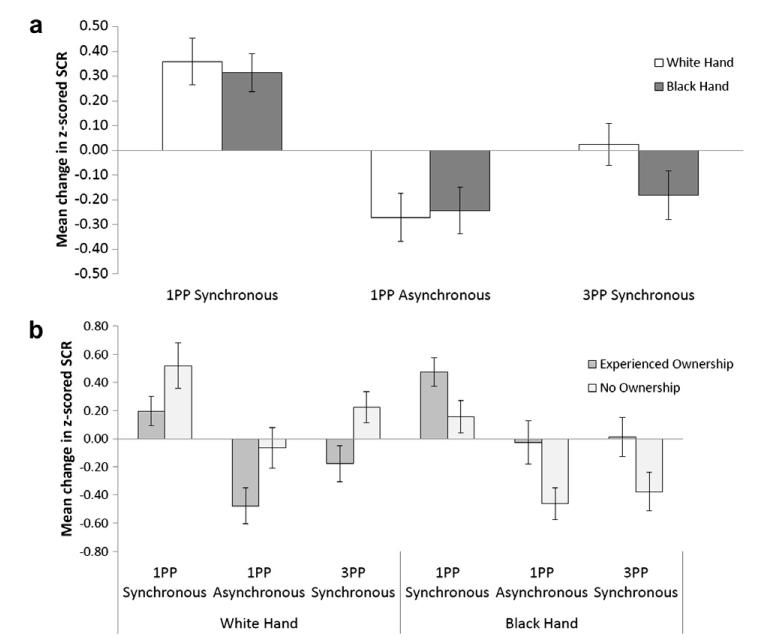
No significant main effects were found. However, a significant interaction was found between timing and type of VT stimulation (F(2,82) = 4.46, p < .05, MSE = 0.87) (see Fig. 5a). Pairwise comparisons revealed that this was driven by significant increases in the post- compared to pre-stimulation SCRs following synchronous 1PP VT stimulation, this effect was significant for the black hand (t(41) = −2.05, p < .05) at the two tail level and for the white hand at the 1 tailed level (t(41) = −1.88, p < .05). No other significant interactions were found. Importantly there was no difference between pre- and post-SCRs following either asynchronous 1PP (for white: t(41) = 1.39, p > .05. For black: t(41) = −1.28, p > .05) or synchronous 3PP (for white: t(41) = −0.14, p > .05. For black: t(41) = 0.93, p > .05) VT stimulation.
Fig. 5.
(a) Mean change in z-scored SCR in response to the threatening object approaching the rubber hand across conditions (n = 42). (b) Mean change in z-scored SCR in response to the threatening object following VT stimulation across conditions divided by the experienced ownership of the black hand (n = 21 who experienced body-ownership over the back hand, and n = 21 who did not experience body-ownership over the black hand). 0 represents prestimulation SCR. Error bars indicate standard error.
These results support our hypothesis that increased SCRs following synchronous 1PP VT stimulation are due to the experience of ownership rather than to a mere attentional effect due to synchronous VT stimulation.
3.2.3. Skin conductance responses by experience of body-ownership for the black hand
Due to the fact that half of the participants tested in this study did not experience feelings of body-ownership over the black hand, the relationship between feeling body-ownership and the black hand was further investigated. Participants were divided into two groups based on whether they reported ownership over the black hand or not as measured by their response to question 2 after synchronous 1PP VT stimulation with the black hand (a response greater than 0 was taken as indicating an experience of body-ownership). This resulted in two groups; 21 participants who experienced ownership of a black hand, and 21 who did not experience ownership of the black hand.
A 3 × 2 × 2 × 2 mixed factors ANOVA was then run on the z-scored SCR data. The three within subject factors were: the type of VT stimulation (1PP synchronous, 1PP asynchronous, 3PP synchronous), the skin colour of the hand (white, black) and the timing of the appearance of threatening stimuli (pre VT, post VT) and the between subjects factor was the experience of ownership over the black hand in the first person synchronous condition.
As in the previous analysis a significant interaction was found between timing and type of VT stimulation (F(2,80) = 4.35, p < .05, MSE = 0.89) (see Fig. 5b). A significant interaction was also found between timing, skin colour of the hand and whether or not the participant experienced body-ownership over the black hand (F(1,40) = 8.18, p < .01, MSE = 0.55). Pairwise comparisons suggested that this effect was driven by the fact that those who experienced ownership of the black hand showed higher SCRs to the black hand in the 1PP synchronous post-stimulation compared to 1PP pre-stimulation condition (t(20) = −2.33, p < .05). In contrast no significant differences were found among those who did not experience ownership of the black hand.
3.2.4. The role of empathy and racial bias in predicting body-ownership
As in Experiment 1 in order to investigate whether or not participants’ empathy predicted their experienced body-ownership for the white or the black rubber hand, participant’s ratings for question 2 in both the white and black hand synchronous 1PP condition were entered as the dependent variables in separate multiple regressions (entry method) using participant’s scores on the four sub scales from the IRI as predictors. One participant was excluded from these regressions as they failed to correctly complete the IRI. There were no significant results for the regression carried out on ownership scores for the white rubber hand, however the regression carried out on ownership scores for the black rubber hand produced an R2 of .23 (F(4,42) = 3.18, p < .05). This was due to a significant positive regression weight on both the fantasy subscale (B = 0.14, t(42) = 2.232, p < .05) and the personal distress subscale (B = 0.14, t(42) = 2.31, p < .05) of the IRI. None of the other IRI subscales were significant contributors to the model.
In order to investigate whether racial bias as measured with the IAT had an effect on the experience of body-ownership for the white and black rubber hands, participant’s ratings for question 2 in both the white and black hand synchronous 1PP condition were entered as the dependent variables in separate linear regressions (entry method) using participant’s scores on the pre VT stimulation IAT as the predictor. Neither regression however, produced a significant model (for the white hand: R2 of −.12 (F(1,46) = .46, p > .05, for the black hand R2 of −.02 (F(1,46) = .23, p > .05)).
4. General discussion
The two experiments reported in this paper are the first to directly investigate the extent to which the sense of body-ownership induced with the RHI depends on the skin colour of the observed hand. The findings of these studies demonstrate that multisensory stimulation can induce a sense of body-ownership over a rubber hand with the skin colour of a different racial group than that of the participant. In Experiment 1 it was found that synchronous, but not asynchronous, VT stimulation led to a feeling of body-ownership for both the white and black rubber hands as measured introspectively, behaviourally and physiologically. Almost two thirds of the participants in this experiment reported a sense of body-ownership over a black rubber hand, as well as showing the expected proprioceptive drift towards the rubber hand and increase in autonomic responses when viewing a painful stimulus being applied to either the white or the black rubber hand.
Experiment 2 expanded on these results by replicating the pattern of findings seen in Experiment 1 for both the introspective and physiological measures, but with improved controlled conditions. For example, the inclusion of a form of synchronous VT stimulation that does not induce body-ownership (i.e. 3PP, Ehrsson et al., 2004) was used to show that the observed increase in SCR is caused by the experience of body-ownership, rather than merely synchronous VT stimulation. This can be seen by the fact that, for hands of both skin colours, synchronous stimulation of a hand seen from a 3PP led to significantly lower introspective reports of body-ownership and SCRs to threatening stimuli that did stimulation of a hand seen from a 1PP. The link between experiencing ownership over the observed hand and increased SCR to threatening stimuli can also be seen in the finding that those participants who experienced body-ownership for the black hand showed a significantly higher increase in SCRs following 1PP synchronous stimulation of the black hand while participants who did not experience ownership showed no such increase.
One notable difference in the findings of Experiment 1 and 2 is that, while in Experiment 1 almost two thirds of participants reported feelings of ownership for the black hand, in Experiment 2 only half of all participants reported such feelings. One possible cause of this reduction in the number of participants experiencing body-ownership is the different nature of the stimuli used. While the first experiment involved the use of a physically present prosthetic hand the second used videos instead. A previous study by IJsselsteijn, de Kort, and Haans (2006) investigated differences in the strength of the RHI when induced using a physically present rubber hand or using a “virtual” projection of a rubber hand and found that the illusion was less vivid when using projection than when using the unmediated hand.
Interestingly, while we found no effect of skin colour on size of proprioceptive drift (Experiment 1) or SCR size (Experiments 1 and 2), introspective reports involving the sense of ownership and the location of tactile stimulation were affected by the skin colour of the observed hand, with participants reporting a more vivid experience of the RHI for the white than the black hand. Note, however, that the effect of skin colour was independent of stimulation as higher ratings on these two questionnaire items for the white hand were recorded after both synchronous and asynchronous VT stimulation and, in Experiment 2, for hands seen from both the 1PP and the 3PP. This general effect of skin colour implies that a hand of the same skin colour as that of the participant might result in a baseline effect of skin colour which “primes” higher cognitive processes of self-attribution involved in the sense of body-ownership, allowing an easier incorporation of the fake hand of same skin colour. Thus, although the skin colour of the rubber hand affected the perceived body-ownership, this effect was independent of that of VT stimulation or anatomical congruency. Rather it seems that these findings reflect a greater incorporation of a white hand into participants’ body in general rather than a stronger effect of VT stimulation in inducing body-ownership of the white hand. In fact the magnitude of the effect of stimulation, that is the difference between synchronous and asynchronous VT, was equal for both white and black hands, suggesting that multisensory stimulation can change the sense of body-ownership equally for hands of same or different skin colour.
The finding that skin colour does affect the subjective reports of induced body-ownership across both stimulation conditions is in line with other studies that have shown that the visual properties of the observed hand can affect the strength of the RHI (Haans et al., 2008; Pavani et al., 2000; Tsakiris et al., 2010; Tsakiris & Haggard, 2005, Austen et al., 2004; Costantini & Haggard, 2007; Pavani & Zampini, 2007) and with studies showing that mere visual exposure to an observed hand can influence both proprioception (Holmes et al., 2006) and tactile perception (Durgin, Evans, Dunphy, Klostermann, & Simmons, 2007). Tsakiris (2010) suggested that during the RHI the visual form of the external object is compared against a body model that contains a reference description of the visual, anatomical and structural properties of one’s own body. However, the findings from previous studies (Holmes et al., 2006; Longo et al., 2009) suggested that more surface level visual features like skin texture and colour do not enter into this comparison. The current study’s results provide partial support for this claim, in that observing VT stimulation of a hand with a different skin colour still led to the experience of ownership in the majority of participants. However, taken together with a study from Haans et al. (2008) which found stronger subjective judgements of ownership after multisensory stimulation of a rubber hand with a skin like texture compared to a rubber hand with a non-skin texture, the current results suggest that surface level features such skin colour and texture are used as a comparator at a late stage and might have a modulating effect on conscious perception of the ownership, although not on its behavioural or physiological correlates.
Several recent studies have suggested a link between the experience of body-ownership induced by RHI and empathy (Asai, Mao, Sugimori, & Tanno, 2011; Durgin et al., 2007; Schütz-Bosbach & Prinz, 2007). Asai et al. (2011) found a link between the empathic concern subscale and tendency to experience the RHI. The link between empathy and experiencing the RHI was also found in the present study, although in a slightly different way. In Experiment 1, the experience of the RHI for both the white and black hand was predicted by participants’ scores in the fantasy subscale (FS) of the IRI. The finding that participants’ FS score was a significant predictor for experiencing the RHI for the black hand was replicated in Experiment 2, which in addition indicated that the score on the personal distress subscale was also a significant predictor. Davis (1983) characterises the FS of the IRI as tapping into respondents’ tendencies to transpose themselves imaginatively into the feelings and actions of fictitious characters in books, movies, and plays. It is interesting to note that Rochat (2003), in characterising the different levels of self awareness that occur in adults, relates the ability to step outside one’s own embodied self and project one’s self into a novel or film to a state of confusion between self and world. The relationship between FS and experience of the RHI found in this study seems to corroborate this view suggesting that those who find it easier to step into the shoes of fictional characters may have a more malleable representation of their own body. Overall, these findings add to the evidence that, along with other factors e.g., schizotypy (Asai et al., 2011), body image dissatisfaction (Mussap & Salton, 2006), and interoceptive sensitivity (Tsakiris, Tajadura-Jiménez, & Costantini, 2011), empathy plays a significant role in determining individual differences in the vividness of the RHI. A relative strength of the present study is the that our results do not rely solely on participants’ introspective reports of the illusion (which may reflect individual differences in suggestibility or other unknown cognitive factors) but also on participants autonomic reaction to painful stimuli being administered to the rubber hand (Experiments 1 and 2) and on participants judgements of the proprioceptive location of their own hand (Experiment 1). In particular, the pattern of our physiological variable reflects a change in participants’ autonomic regulation that at least cannot be changed or controlled at will.
The role of empathy in the RHI ties into the question of whether social factors such as ingroup/outgroup identification can also play a role in the strength of the rubber hand illusion. The lack of any relationship between participants pre-illusion racial bias and the strength of the RHI suggests that, unlike sensorimotor empathy for pain (Avenanti et al., 2010), the processes involved in the generation of a sense of body-ownership seem immune to the effects of top-down socio-cognitive factors such as racial bias, at least as measured with the IAT.
More interesting however is the finding of an effect of experiencing the RHI on post-illusion racial bias. A number of recent studies have suggested that sensory processing might alter the perceived physical (Longo et al., 2008) and psychological similarity between self and other, and possibly between ingroup and outgroup members. For example, seeing a face being touched at the same time as your own face might change self-face recognition (Tsakiris, 2008) but also the felt closeness to the other (Paladino, Mazzurega, Pavani, & Schubert, 2010). Synchronous movement between people has also been shown to increase perceived rapport and affiliation (Hove & Risen, 2009; Lakin & Chartrand, 2003; Miles, Nind, & Macrae, 2009), to improve performance in joint action tasks (Valdesolo, Ouyang, & DeSteno, 2010) and to improve co-operation in economic games (Wiltermuth & Heath, 2009). Experiment 1 of the present study adds to this literature on the social effect of synchrony by showing, for the first time, that the experience of body-ownership as induced by synchronous VT stimulation can exert a bottom-up effect on participants perceptions of an outgroup. A multiple regression on the change in IAT score between pre- and post-stimulation IATs did not find that experiencing ownership of the rubber hand lead to a significant reduction in racial bias. However, it was found that when the ownership ratings for both the white and black rubber hand were entered as predictors into a multiple regression they significantly predicted participants’ post-testing racial bias. Neither predictor was significant independently of the other due to the fact that ownership scores for the white and black rubber hand were themselves highly correlated. There are two possible explanations for this finding; one is that the decrease in racial bias was caused by simply experiencing the RHI for a hand of any skin colour and the other is that the change in IAT scores was driven by ownership of the black rubber hand specifically but that due to the strong correlation between ownership of the black and white rubber hands both ownership scores predicted the decrease. Unfortunately the repeated-measures design used in this study means that it is not possible to determine which of these possibilities is correct. Nonetheless this finding is a promising first step towards demonstrating that synchronous multisensory stimulation can alter higher level socio-cognitive processes. Further investigation of this issue using a between subjects design to separate out the effect of multisensory stimulation of a hand with an ingroup as opposed to outgroup skin colour on implicit racial bias would help to resolve the ambiguity in the results found in the present study.
In conclusion, to the extent that race is a strong modulator of social cognition and its underlying neural processes, understanding if and how multisensory processing can alter self-representations across the boundaries of racial groups might be important for probing the sensorimotor basis of social cognition (Farmer & Tsakiris, 2012). The present study takes a first step towards that direction by showing that changes in body-awareness as a result of multisensory stimulation can go beyond one’s own skin colour.
Acknowledgments
European Platform for Life Sciences, Mind Sciences and Humanities of the Volkswagen Foundation, Experimental Psychology Society Small Grant (UK) and the European Research Council (ERC-2010-StG-262853) under the FP7.
Footnotes
Holmes et al.’s study did not induce the RHI using visuotactile stimulation but rather only through visual exposure to a rubber hand perceived as being in the spatial location of the participant’s own hand and overall participant’s in this study did not experience strong feelings of ownership over the rubber hand.
References
- Asai T, Mao Z, Sugimori E, Tanno Y. Rubber hand illusion, empathy, and schizotypal experiences in terms of self-other representations. Consciousness and Cognition. 2011;20(4):1744–1750. doi: 10.1016/j.concog.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Austen EL, Soto-Faraco S, Enns JT, Kingstone A. Mislocalizations of touch to a fake hand. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(2):170–181. doi: 10.3758/cabn.4.2.170. [DOI] [PubMed] [Google Scholar]
- Avenanti IA, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8:955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Current Biology. 2010;20(11):1018–1022. doi: 10.1016/j.cub.2010.03.071. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands “feel” touch that eyes see. Nature. 1998;391(6669):756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. Plenum; New York: 1992. [Google Scholar]
- Costantini M, Haggard P. The rubber hand illusion: sensitivity and reference frame for body-ownership. Consciousness and Cognition. 2007;16(2):229–240. doi: 10.1016/j.concog.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44(1):113–126. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3rd ed Cambridge University Press; New York: 2007. pp. 159–181. [Google Scholar]
- Désy M-C, Théoret H. Modulation of motor cortex excitability by physical similarity with an observed hand action. PloS One. 2007;2(10):e971. doi: 10.1371/journal.pone.0000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimberg U. Facial electromyography and emotional reactions. Psychophysiology. 1990;27:481–494. doi: 10.1111/j.1469-8986.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Durgin FH, Evans L, Dunphy N, Klostermann S, Simmons K. Rubber hands feel the touch of light. Psychological Science. 2007;18(2):152–157. doi: 10.1111/j.1467-9280.2007.01865.x. [DOI] [PubMed] [Google Scholar]
- Edelberg R. Electrical properties of the skin. In: Brown CC, editor. Methods in psychophysiology. Williams and Wilkins; Baltimore: 1967. pp. 1–53. [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305(5685):875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: A magnetic stimulation study. Journal of Neurophysiology. 1995;73(6):2608. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Farmer H, Tsakiris M. The bodily social self: A link between phenomenal and narrative selfhood. Review of Philosophy and Psychology. 2012;3:125–144. [Google Scholar]
- Fiske ST, Neuberg SL. A continuum of impression formation, from category-based to individuating processes: Influences of information and motivation on attention and interpretation. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 21. Academic Press; New York: 1990. pp. 1–74. [Google Scholar]
- Gallese V, Sinigaglia C. The bodily self as power for action. Neuropsychologia. 2010;48(3):746–755. doi: 10.1016/j.neuropsychologia.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality and Social Psychology. 1998;74(6):1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. Journal of Personality and Social Psychology. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Gutsell JN, Inzlicht M. Empathy constrained: Prejudice predicts reduced mental simulation of actions during observation of outgroups. Journal of Experimental Social Psychology. 2010;46(5):841–845. [Google Scholar]
- Haans A, IJsselsteijn WA, de Kort YAW. The effect of similarities in skin texture and hand shape on perceived ownership of a fake limb. Body Image: An International Journal of Research. 2008;5:389–394. doi: 10.1016/j.bodyim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Hewstone M, Hantzi A, Johnston L. Social categorization and person memory: The pervasiveness of race as an organizing principle. European Journal of Social Psychology. 1991;21:517–528. [Google Scholar]
- Holmes NP, Snijders HJ, Spence C. Reaching with alien limbs: Visual exposure to prosthetic hands in a mirror biases proprioception without accompanying illusions of ownership. Perception & Psychophysics. 2006;68(4):685–701. doi: 10.3758/bf03208768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove MJ, Risen JL. It’s all in the timing: Interpersonal synchrony increases affiliation. Social Cognition. 2009;27(6):949–960. [Google Scholar]
- IJsselsteijn WA, de Kort YAW, Haans A. Is this my hand I see before me? The rubber hand illusion in reality, virtual reality, and mixed reality. Presence, Teleoperators & Virtual Environments. 2006;15:455–464. [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: Vicarious activity for actions, emotions, and sensations. Current Opinion in Neurobiology. 2009;19(6):666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Kurzban R, Tooby J, Cosmides L. Can race be erased? Coalitional computation and social categorization. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15387–15392. doi: 10.1073/pnas.251541498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin JL, Chartrand TL. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychological Science. 2003;14(4):334–339. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- Lloyd DM. Spatial limits on referred touch to an alien limb may reflect boundaries of visuo-tactile peripersonal space surrounding the hand. Brain and Cognition. 2007;64(1):104–109. doi: 10.1016/j.bandc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Longo MR, Schüür F, Kammers MPM, Tsakiris M, Haggard P. What is embodiment? A psychometric approach. Cognition. 2008;107(3):978–998. doi: 10.1016/j.cognition.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Longo MR, Schüür F, Kammers MPM, Tsakiris M, Haggard P. Self awareness and the body image. Acta Psychologica. 2009;132(2):166–172. doi: 10.1016/j.actpsy.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Miles LK, Nind LK, Macrae CN. The rhythm of rapport: Interpersonal synchrony and social perception. Journal of Experimental Social Psychology. 2009;45(3):585–589. [Google Scholar]
- Molnar-Szakacs I, Wu AD, Robles FJ, Iacoboni M. Do you see what I mean? Corticospinal excitability during observation of culture-specific gestures. PloS One. 2007;2(7):e626. doi: 10.1371/journal.pone.0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussap AJ, Salton N. A “rubber-hand” illusion reveals a relationship between perceptual body image and unhealthy body change. Journal of Health Psychology. 2006;11(4):627–639. doi: 10.1177/1359105306065022. [DOI] [PubMed] [Google Scholar]
- Paladino M-P, Mazzurega M, Pavani Francesco, Schubert TW. Synchronous multisensory stimulation blurs self-other boundaries. Psychological Science. 2010;21(9):1202–1207. doi: 10.1177/0956797610379234. [DOI] [PubMed] [Google Scholar]
- Pavani F, Spence C, Driver J. Visual capture of touch: Out-of-the-body experiences with rubber gloves. Psychological Science. 2000;11(5):353–359. doi: 10.1111/1467-9280.00270. [DOI] [PubMed] [Google Scholar]
- Pavani F, Zampini M. The role of hand size in the fake-hand illusion paradigm. Perception. 2007;36(10):1547–1554. doi: 10.1068/p5853. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Ehrsson HH. If I were you: Perceptual illusion of body swapping. PloS One. 2008;3(12):e3832. doi: 10.1371/journal.pone.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Rizzolatti Giacomo, Fabbri-Destro M. Mirror neurons: From discovery to autism. Experimental Brain Research. 2010;200(3–4):223–237. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- Rochat P. Five levels of self-awareness as they unfold early in life. Consciousness and Cognition. 2003;12(4):717–731. doi: 10.1016/s1053-8100(03)00081-3. [DOI] [PubMed] [Google Scholar]
- Schütz-Bosbach S, Prinz W. Perceptual resonance: Action-induced modulation of perception. Trends in Cognitive Sciences. 2007;11(8):349–355. doi: 10.1016/j.tics.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Serino A, Giovagnoli G, Làdavas E. I feel what you feel if you are similar to me. PloS One. 2009;4(3):e4930. doi: 10.1371/journal.pone.0004930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Press C, Haggard P. Shared representations in body perception. Acta Psychologica. 2006;121(3):317–330. doi: 10.1016/j.actpsy.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. Looking for myself: Current multisensory input alters self-face recognition. PLoS One. 2008;3(12):e4040. doi: 10.1371/journal.pone.0004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia. 2010;48(3):703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Haggard P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. Journal of Experimental Psychology: Human Perception & Performance. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Carpenter L, James D, Fotopoulou A. Hands only illusion: Multisensory integration elicits sense of ownership for body parts but not for non-corporeal objects. Experimental Brain Research. 2010;204:343–352. doi: 10.1007/s00221-009-2039-3. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Tajadura-Jiménez A, Costantini M. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proceedings of the Royal Society B: Biological Sciences. 2011 doi: 10.1098/rspb.2010.2547. published, 5 January 2011. http://dx.doi.org/10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdesolo P, Ouyang J, DeSteno D. The rhythm of joint action: Synchrony promotes cooperative ability. Journal of Experimental Social Psychology. 2010;46(4):693–695. [Google Scholar]
- Venables PH, Christie MJ. Mechanisms, instrumentation, recording techniques, and quantification of responses. In: Prokasy WF, Raskin DC, editors. Electrodermal activity in psychological research. Academic Press; New York: 1973. pp. 1–124. [Google Scholar]
- Wiltermuth SS, Heath C. Synchrony and cooperation. Psychological Science. 2009;20(1):1–5. doi: 10.1111/j.1467-9280.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. The Journal of Neuroscience. 2009;29(26):8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]