Abstract
Cultured human lung cancer cell lines have been used extensively to dissect signaling pathways underlying cancer malignancy, including proliferation and resistance to chemotherapeutic agents. However, the ability of malignant cells to grow and metastasize in vivo is dependent upon specific cell-cell and cell-extracellular matrix (ECM) interactions, many of which are absent when cells are cultured on conventional tissue culture plastic. Previous studies have found that breast cancer cell lines show differential growth morphologies in three-dimensional (3D) gels of laminin-rich (lr) ECM, and that gene expression patterns associated with organized cell structure in 3D lrECM were associated with breast cancer patient prognosis. We show here that established lung cancer cell lines also can be classified by growth in lrECM into different morphological categories and that transcriptional alterations distinguishing growth on conventional tissue culture plastic from growth in 3D lrECM are reflective of tissue-specific differentiation. We further show that gene expression differences that distinguish lung cell lines that grow as smooth vs. branched structures in 3D lrECM can be used to stratify adenocarcinoma patients into prognostic groups with significantly different outcome, defining phenotypic response to 3D lrECM as a potential surrogate of lung cancer malignancy.
Insight, innovation, integration
The high incidence and poor prognosis of lung cancer has prompted substantial research towards identification of key effectors of lung cancer progression and metastasis which could be targeted therapeutically. A significant component of this research has involved the use of lung cancer cell lines, as these have been found to reflect many key genetic characteristics of the tumor of origin even after extensive culture. We demonstrate here that assay conditions previously shown to promote development of tissue-specific phenotypic characteristics in other cell types can be used with lung cancer cell lines to identify transcriptional alterations which are associated with response to 3D lrECM and which are predictive of lung adenocarcinoma patient prognosis. Our study describes an experimentally tractable model system in which potential effectors of lung cancer progression can be readily evaluated.
Introduction
Lung cancer is the leading cause of cancer death in the United States, and recent advances in early detection and improvements in treatment have had only a limited impact on overall prognosis.1 Non-small cell lung carcinoma (NSCLC) accounts for approximately 80% of lung cancer cases and has an overall 5-year survival rate of approximately 15;2 accordingly, there is significant need for new therapeutic approaches for treating NSCLC patients. Development of conditions for long-term propagation of isolated lung cancer cells in culture has led to more than 150 well characterized and widely distributed cell lines.3 As analyses of genomic alterations has shown that lung cancer cell lines often closely resemble the tumors from which they were derived 4, these cell lines represent potentially powerful tools both for identification of key processes involved in disease progression and as models for evaluating potential therapeutic strategies. However, the key features of malignancy, the ability to invade beyond the boundaries of the tumor and to metastasize to distant sites, are dependent upon cell-cell and cell-ECM interactions that are not well modeled in the conventional tissue culture conditions used to generate and propagate lung cancer cells.5 Growth in 3D lrECM assays has been found to model many of these interactions and has been used to identify tumor-associated processes which can be targeted to reduce cancer cell malignancy.6 Development of optimized methods for culturing mammary epithelial cell lines in 3D lrECM has revealed that nonmalignant breast cells develop into organized, growth-arrested acinar structures, whereas malignant cells continue to proliferate into disorganized cell masses, demonstrating that breast epithelial cell response to 3D lrECM reveals intrinsic characteristics.7 While the response of lung cancer cell lines in similar assays has not been as well characterized, an earlier comparison of normal bronchial tissue and three aggressive lung cancer cell lines in 3D lrECM revealed that the normal cells formed smooth, spheroid agglomerates, while cancer cells formed branching structures and invaded normal lung tissue explants.8 These results suggest that differential response of nonmalignant and malignant lung cancer cells to 3D lrECM could be used to define malignancy-associated pathways.
Transcriptional profiling of tumor biopsies from lung cancer patients has been used to identify biomarkers or sets of biomarkers that can be used to predict clinical outcome,9 to identify molecular markers of invasion10 and to classify lung cancer into distinct subtypes.11 Such studies can identify clinically useful prognostic features. However, even highly prognostic transcripts may not be directly associated with the processes leading to better or poorer outcome, and thus may not represent clinically useful therapeutic targets. By contrast, a model system which allows both identification of prognostic biomarkers as well as evaluation of the consequences of activating or suppressing specific pathways on the malignant cell characteristics might be a better approach to identify potential therapeutic targets. Such studies have been pursued for breast cancer: transcriptional profiling of a panel of breast cancer cell lines in 3D lrECM revealed characteristic gene alterations associated with colony morphology,12 and a transcriptional signature associated with cells that developed into a rounded, organized, and growth-arrested colony morphology was found to predict clinical outcome for breast cancer patients.13 As these studies employed in a highly tractable model system in which transcript expression can be easily modulated, evaluation of the impact of targeting specific pathways on the malignant growth phenotype could be easily assessed and used as an indicator of therapeutic potential. To test whether a similar relationship exists between lung cancer growth morphology in 3D lrECM and clinical outcome, we peformed transcriptional profiling of lung cancer cell lines grown on conventional tissue culture plastic and in 3D lrECM. We identified transcriptional patterns that distinguished growth on plastic from growth in 3D lrECM. We also identified a transcriptional signature that distinguished cells forming smooth, round colonies in 3D from those with a branching, invasive morphology, and determined that this signature could separate patients with lung adenocarcinoma into better, intermediate, and poorer outcome categories. These results demonstrate that growth of lung cancer cells in 3D lrECM can be used to investigate clinical characteristics of lung cancer progression and that these experimental models can be employed for discovery of novel prognostic biomarkers as well as evaluation of pathways potentially amenable to targeted therapies.
Results
3D lrECM growth characteristics of lung cancer cell lines
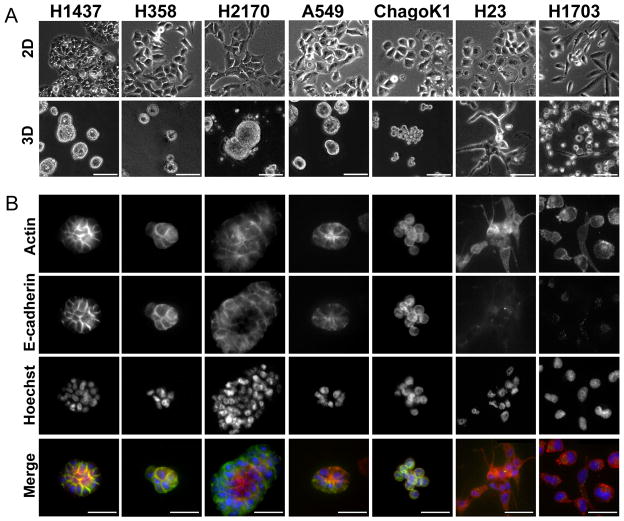
Nine commonly studied lung cancer cell lines (Table 1) were grown either on tissue culture plastic (2D) or in 3D culture on a thin layer of Matrigel covered with 2 % Matrigel dissolved in media (‘on top’ method).7 Cells were plated at equal density (5.0×104 per well)grown for five days. This time point was selected to provide distinct growth morphologies (Figure 1) while maintaining uniform proliferative status (assessed by Ki-67 staining, Supplemental Figure 1), so that subsequent transcriptional analyses would not be complicated by differential cell proliferation. Based on visual observation of growth patterns of lung cancer cells cultured in 3D lrECM, we classified the cells into two broadly distinct growth morphologies: smooth and branched (Fig. 1A). Smooth cell lines included H1437, H2170 and A549 (and H358, H460, not shown), which formed round or oval cell aggregates with strong cell-cell adhesion, and did not invade into the Matrigel. In contrast, H23 and H1703 (and H661, not shown) cell lines exhibited branched morphology, characterized by elongated cell bodies, and invasive protrusions. Chago-K1 cells developed into a distinct, grape-like morphology of poorly adhesive but noninvasive colonies. Immunofluorescent staining for filamentous actin and E-cadherin immunofluorescence showed distinct junctional staining in the smooth cells, while cells with the branched morphology generally showed cytoplasmic actin and little or no E-cadherin (Fig. 1B).
Table 1.
Characteristics of lung cancer cell lines used in study.
| Cell Line | 3D morphology | Tumor Type | Mutational status | Cigarette usage | E-cad | |
|---|---|---|---|---|---|---|
| TP53 | KRAS | |||||
| H1437 | Smooth | AC | Mt | Wt | S | + |
| H358 | Smooth | NSCLC | Mt | Mt | NS | + |
| H2170 | Smooth | SCC | Mt | Wt | NS | + |
| A549 | Smooth | AC | Wt | Mt | - | + |
| ChagoK1 | Grape-like | BC | Mt | Wt | - | + |
| H23 | Branching | AC | Mt | Mt | S | − |
| H1703 | Branching | NSCLC | Mt | Wt | S | − |
| H460 | Smooth | AC,LCC | Mt | Wt | NS | − |
| H661 | Branching | LCC | Mt | Wt | NS | − |
AC, Adenocarcinoma; NSCLC, Non Small Cell Lung Cancer; SCC, Squamous cell carcinoma; BC, bronchial carcinoma; LCC, Large cell carcinoma; Mt, mutant; Wt, wild type; S, smoker; NS, nonsmoker.
Figure 1. Morphologies of lung cancer cell lines grown in 3D.
A. Lung cancer cells adopt a flattened morphology when grown on tissue culture plastic (2D, top row), but can form smooth, round masses (H1437, H358, H2170, and A549), grape-like structures (ChagoK1), or invasive, branching structures (H23 and H1703) when grown in 3D lrECM cultures (3D, bottom row). Scale bar 50 mm. B. Expression of actin cytoskeleton (Actin, top row), E-cadherin (second row), cell nuclei (Hoechst, third row), and all three stains merged (Actin red, E-cadherin green, Hoecht blue; bottom row) of lung cancer cells grown in 3D. Scale bar 20 μm.
Analysis of gene expression of lung cancer cells in 2- and 3-dimensions
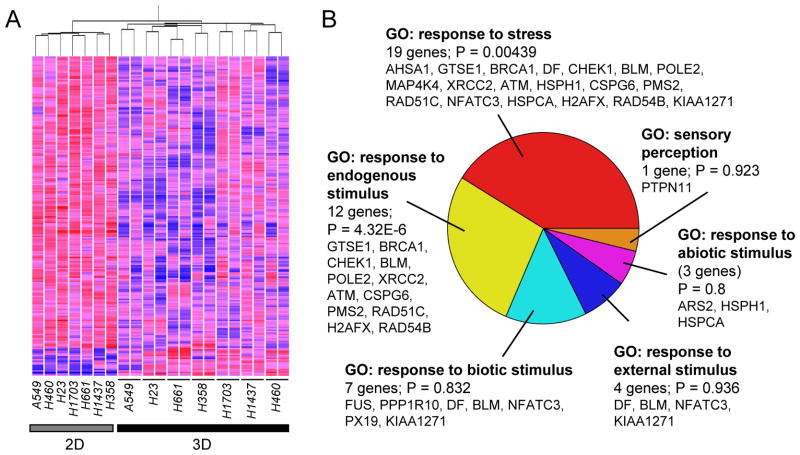
Gene expression profiles of seven of the lung cancer cell lines grown on conventional tissue culture plastic (2D) and in 3D lrECM (3D) were analyzed using Affymetrix GeneChip Human Genome U133 Plus 2.0 arrays. Two independent 3D culture samples were collected for each cell line for this experiment; replicates were not averaged but were treated as individual samples. We identified 228 transcripts that were significantly differentially expressed (p<0.05, ANOVA) between samples grown in 2D and 3D (Fig. 2A; Supplemental Table 1); of these, 208 were downregulated in 3D as compared to 2D, while 20 were upregulated in 3D. We used Gene Ontology annotations14 to determine whether specific molecular functions were overrepresented in the set of genes differentially expressed in 3D as compared to 2D (Supplemental Table 2). Among six biological process Gene Ontology classifications, genes associated with GO:50896 (response to stimulus) were apparently overrepresented (just missing statistical significance, p=0.0797); of the 27 differentially expressed genes annotated to GO:50896 (Figure 2B), 21 genes were associated with GO:6950 (response to stress; p=0.00439) and 14 genes with GO:9719 (response to endogenous stimulus; p=4.32e-6). The other gene ontology classifications did not reach statistical significance due to the small number of identified genes. That the majority of the differentially expressed genes were downregulated in 3D in combination with the significant association with response to stress and stimulus ontology categories suggests that culture on tissue culture plastic substrata leads to activation of common transcriptional programs in lung cancer cell lines with widely varying morphologies in 3D lrECM (Figure 1) and different tumor characteristics (Table 1).
Figure 2. Analysis of transcripts differentially expressed in lung cancer cell lines grown in 2D and 3D.
A. Hierarchical clustering of 228 transcripts that are significantly different (p<0.05, ANOVA) between lung cancer cell lines grown on tissue culture plastic (2D) or in 3D lrECM (3D). Red, elevated transcript expression; blue, decreased expression. B. Gene Ontology (GO) analysis of genes distinguishing 2D and 3D cells as subset of GO:8150: biological_process.
We subjected the list of differentially expressed genes to a NextBio (www.nextbio.com) meta-analysis, and identified data sets that were significantly associated with our list. Significant overlap was observed with data sets from differentiated vs. undifferentiated Caco-2 epithelial colorectal cancer cells (P = 3.4e-49, Supplemental Fig. 2A)15 and from polarizing differentiating MCF10A cells in a Transwell model (P = 4.5e-45; Supplemental Fig. 2B),16 consistent with the notion that even cells from long-term established lung cancer cell lines adopt a more differentiated phenotype when grown in 3D lrECM.
Analysis of gene expression of smooth vs. branched lung cancer cell lines in 3-dimensions
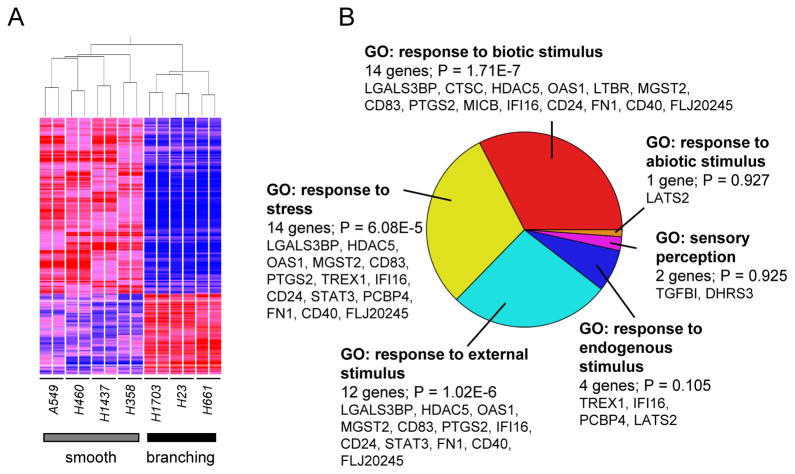
Culturing lung cancer epithelial cells in 3-dimensional conditions resulted in formation of distinct morphologies that grouped the majority of evaluated cell lines into smooth and branched morphologies. Gene expression profiles of seven lung cancer cell lines grown in duplicate experiments in 3D were analyzed. Comparison of transcriptional patterns between 4 cell lines categorized as smooth and 3 cell lines categorized as branched resulted in identification of 200 transcripts that were significantly differentially expressed (p<1e-5, ANOVA); of these, 63 transcripts were upregulated in branched morphology lines relative to smooth morphology cell lines, while 134 were downregulated in branched cell lines (Fig. 3A, Supplemental Table 3). We again used Gene Ontology annotations to classify differentially regulated genes (Supplemental Table 4), finding again that the most overrepresented biological process ontology category in our geneset was GO:50896 (response to stimulus; p=0.00199). Of the 34 genes annotated to GO:50896 (Fig. 3B), the most overrepresented subgroup was GO:9607 (response to biotic stimulus), consistent with the differential response of the smooth and branching morphology cell lines to the lrECM tumor-derived microenvironment. NextBio meta-analysis of the transcripts differentially expressed between smooth and branching morphologies showed significant overlap with data sets from lung cancer cells cultured with tobacco smoke condensate vs. controls (p=1.2e-10)17 (Supplemental Fig. 3A), as well as normal bronchial epithelial cells exposed cigarette smoke vs. non-exposed (P = 3.5e-10)18 (Supplemental Fig. 3B), suggesting that transcriptional programs induced by cigarette smoke, a known lung cancer carcinogen, might be associated with pathways controlling the invasive, branching morphology.
Figure 3. Analysis of transcripts differentially expressed in lung cancer cell lines that grow as smooth or invasive morphologies.
A. Hierarchical clustering of 200 transcripts that are significantly different (p<1e-5, ANOVA) between lung cancer cell lines that grow as smooth or invasive morphology in 3D culture. Red, elevated transcript expression; blue, decreased expression. B. Gene Ontology (GO) analysis of genes distinguishing smooth and invasive morphology as subset of GO:8150: biological_process.
A 20-gene 3-dimensional signature is associated with lung cancer patient outcome
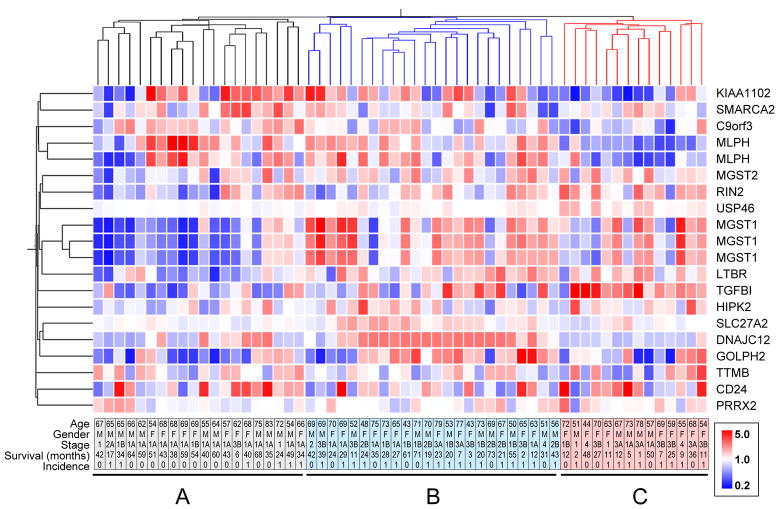
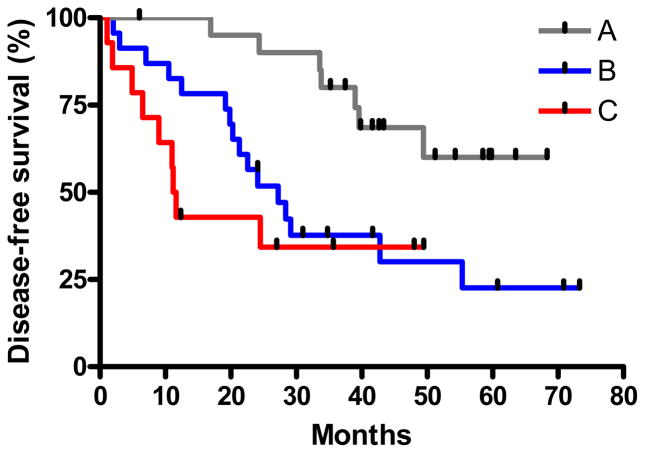
To assess the clinical relevance of the transcriptional patterns associated with the differential growth morphologies of the lung cancer cell lines, we defined a geneset containing the 20 most significantly differentially expressed (ANOVA, P = 5e-10) transcripts between the smooth and branching cell lines, and used this 20 transcript geneset to investigate a group of lung adenocarcinoma patients in the Duke cancer data set;19 this was facilitated by the fact that the Duke data set also had been analyzed using Affymetrix GeneChip Human Genome U133 Plus 2.0 arrays. Kaplan-Meier survival analyses performed for each of the individual transcripts in the dataset revealed that 7 transcripts were able to classify the patients into statistically significant better and poorer prognosis groups: MLPH-1 (p=0.0087), RIN2 (p=0.036), USP46 (p=0.037), MGST1-1 (p=0.0036), MGST1-2 (p=0.0011), MGST1-3 (p=0.004), LTBR (p=0.09), and SLC27 (p=0.032). (Supplemental Fig. 4). When we used expression levels of all 20 transcripts for hierarchical cluster analysis of the adenocarcinoma data set, we found that the patient data assembled into three groups, named here A, B and C, with 20, 24 and 14 patients per group, respectively (Fig. 4). While there was no statistical difference for average patient age or distribution of patient gender between the three subgroups (not shown), there was a substantial difference in the cancer stage, as stage 1 patients predominated group A, but progressively decreased in number in groups B and C, concomitant with an increasing number of more advanced cancers (Supplemental Fig. 5). Consistent with this distribution, analysis of the 3 groups by Kaplan-Meier analysis revealed a statistically significant difference (p=0.0053) in outcome between the three groups (Fig. 5). Disease-free survival at one year was 100% for patients in group A, but dropped to 80% and 40% for patients in groups B and C, respectively. Strikingly, although group C contained mostly patients with late stage disease diagnoses, five of them were diagnosed with Stage 1, 1A, or 1B disease; of these five, three showed relapse at 2, 5, and 11 months. These results indicate that assessment of prognosis using the 20 transcript geneset could potentially provide additional information beyond clinical-pathological parameters for patient diagnosis and assessment of treatment options.
Figure 4. Transcripts that best discriminate smooth vs. invasive morphology used to cluster lung cancer.
The 20 transcripts that most significantly different (p<5e-10) between lung cancer cells that grow as smooth or invasive were used to cluster 59 lung adenocarcinoma patients based on gene expression in Duke-Adeno dataset. Dendrograms on top and left represent degree of relatedness of individual samples (top) and gene probes (left). Rows, relative level of transcript expression (inset, color scale); columns, individual patients. Bottom plate contains age, gender, stage, survival (in months) and incidence information for individual patients. Top dendrogram branch colors represent different prognostic groups.
Figure 5. Transcripts that best discriminate smooth vs. invasive morphology classify lung cancer patients into differential outcome categories.
The 20 transcripts that most significantly different (p<5e-10) between lung cancer cells that grow as smooth or invasive morphologies cluster 59 lung adenocarcinoma patients into three groups with significantly different outcomes (p=0.0053). Group A, 20 patients; group B, 24 patients; group C, 14 patients.
Discussion
Currently, the preferred clinical method for assessing lung cancer prognosis and defining treatment decisions is the TNM staging system, which incorporates parameters of tumor size, spread to regional lymph nodes, and metastasis to distant sites.20 Patients classified as stage 1 (those with invasive cancer but no spread to regional lymph nodes or distant metastasis) are generally treated by surgical resection only, although disease relapse rates for these patients can be as high as 30%, and it may be that some of these patients could benefit from chemotherapy. Stage 2 patients (with spread to regional lymph nodes but no distant metastasis) are often treated with adjuvant chemotherapy, although parameters for which stage 2 patients would best benefit from this additional treatment have not been defined. Transcriptional profiling studies of clinical datasets have been pursued towards identifying prognostic gene signatures that can be used to appropriately individualize treatment for early stage NSCLC patients.9 Each of these studies have produced a different panel of diagnostic transcripts, with little overlap between the different signatures; this partially reflects differences in experimental and statistical methods,21 as well as the different characteristics of the analyzed patient datasets.22 Here, we have evaluated the potential of a geneset composed of the 20 transcripts which best differentiate cell lines that develop into smooth vs branched morphologies when grown in 3D lrECM. When applied to a clinical dataset of lung adenocarcinoma patients, the 20-transcript geneset was able to define three distinct prognostic groups with significant differences in patient outcome. While three of the five patients (60%) categorized as stage 1 in group C showed relapse with an average time to relapse of six months, only 5/13 (38%) of the stage 1 patients in group A showed relapse at an average time of 36 months; thus, the 20-transcript geneset provides additional prognostic ability beyond conventional staging methods. While the limited sample size precludes analysis of statistical significance, it is remarkable that a geneset that was derived from a completely different type of data is nevertheless associated with patient outcome. When considered in combination with previous studies showing that genesets derived from breast cancer cell lines grown in 3D lrECM can also be prognostic for breast cancer disease outcome,13 these results further reinforce the notion that cancer cell lines retain critical clinical information about the tumors from which they were derived that is revealed in a microenvironment that models the cellular interactions found in tumors.6, 23 It may be that additional refinements in the culture model could reveal additional phenotypic information, as with a recently published system which incorporated tissue fibroblasts and air-liquid interfaces in which immortalized nonmalignant bronchial epithelial cells were shown to develop along different differentiation lineages.24
Additional insight can also be found by examining individual components of the 20-transcript geneset used to classify the clinical dataset. SMARCA2 (also known as Brahma, or BRM), is a component of the SWI/SNF chromatin remodeling complex. In the clinical dataset, SMARCA2 showed increased expression in the better prognosis group A, but decreased expression in the poorer prognosis groups B and C. These results are consistent with studies showing that loss of SMARCA2 is correlated with poor prognosis in lung cancer patients25 and is a component of a breast cancer predictive signature.26 MGST1 (microsomal glutathione S-transferase 1) is downregulated in group A and upregulated in groups B and C; MGST1 was previously shown to be overexpressed in lung tumors developing in transgenic lung cancer models.27 That differences in MGST1 expression are by themselves prognostic of patient outcome (Supplemental Figure 4) as well as associated with altered morphological growth responses suggests that the 3D lrECM assay could be used to test therapeutic approaches targeting MGST1 as well as the pathways controlled by them, using morphological reversion as a potential endpoint. More generally, high throughput application7 of the lung cancer cell lines with the 3D lrECM assay could be used to screen compound libraries to identify novel agents capable of reversion of the malignant branched phenotype; tumor reversion is an emerging approach with considerable potential to augment existing anticancer therapies.28
It should be noted that not all of the members of the 20 transcript geneset were clearly associated with patient outcome. While some individual transcripts could be used to divide patients into high and low expressing groups with significantly different outcomes (e.g., MLPH1, MGST1, RIN2); in other cases, transcripts that were significantly differentially expressed between cell lines growing in 3D lrECM as smooth vs branching showed very low expression in the adenocarcinoma dataset (e.g., SLC27A2), or were not by themselves associated with differential outcome (e.g., HIPK2). However, even transcripts that did not by themselves predict outcome might nevertheless contribute to the prognostic characteristics of the 20 transcript geneset. Evaluation of manipulation of individual genes on growth morphology may provide a more definitive method for determining whether the differential expression in 3D is directly or indirectly related to the growth phenotype.
While there were many transcriptional alterations that distinguished cell lines showing smooth and branched morphology, there was also substantial overlap in transcriptional changes for all cell lines between growth on tissue culture plastic and growth in 3D lrECM. Gene expression analysis of lung cancer cells grown either in 2D or 3D culture revealed 228 differentially expressed transcripts that corresponded to 140 annotated genes. NextBio meta-analysis revealed that expression data of 2D vs. 3D grown cells negatively correlates with data from differentiated vs. undifferentiated Caco-2 cells,15 and with polarizing differentiating vs undifferentiated MCF10A cells.16 This indicates that the 3D lrECM-cultured cells are more similar to the differentiated phenotype of both cell lines, consistent with previous studies showing activation of tissue-relevant phenotypic characteristics for mammary epithelial cells grown in tissue-like context.23a Among individual transcripts in the 2D vs. 3D grown cells, SERPINB6 was the most consistently upregulated by growth in 3D lrECM (avg. 2D/3D expression=0.01, p=3.2e-11; Supplemental Table 1). SERPINB6 belongs to a family of serine proteinase inhibitors clade B, and while the its function has not been fully defined, a transcriptional study identified SERPINB6 as substantially and significantly upregulated during endothelial cell tubulogenesis in 3D lrECM,29 indicating the potential existence of overlapping pathways linking lung cancer cell lines and endothelial cells. Another transcript that was consistently upregulated in cells grown on 3D vs. 2D was RAB11A (avg. 2D/3D expression=0.44, p=0.015; Supplemental Table 1), a GTPase involved in apical recycling endosomes in polarized epithelial cells,30 implicating the transcriptional activation of molecules involved in directional protein sorting. Transcripts downregulated in 3D vs. 2D included PLK1 (Polo-like Kinase 1, avg 2D/3D expression=2.03, p=0.00032), a mitotic kinase which has been implicated as a regulator of growth into 3D lrECM,31 and MALAT 1 (Metastasis-associated lung adenocarcinoma transcript 1, avg 2D/3D expression=1.75, p=0.0097), a noncoding RNA associated with early-stage NSCLC32 and which as been found to control alternative splicing33 and lung cancer cell motility.34 While some transcripts showed increased expression in cells grown in 3D lrECM, the majority of significantly differentially expressed transcripts were downregulated in cells grown in 3D lrECM (Figure 2). How these transcriptional alterations are controlled in the lung cancer cell lines is unclear; however, previous studies have found activation of tissue-specific transcripts associated with general gene repression as a consequence of histone deacetylation in mammary epithelial cells grown in 3D lrECM,35 consistent with models indicating broad-scale chromatin alterations induced by growth in tissue structures.36 To what extent the specific molecular processes activated by 3D lrECM in mammary epithelial cells mirror those induced in lung cancer cell lines remains to be discovered.
Conclusions
Cultured lung cancer cell lines retain many of the genomic alterations found in the tumors from which they were derived and can be easily accessible for experimental replication; use of culture models simulating key aspects of the tumor microenvironment, such as the 3D lrECM model used here, facilitate development of multicellular structures reflective of the phenotypic alterations controlling cancer cell malignancy. Our findings further emphasize the importance of cell-cell and cell-ECM interactions as effectors of cellular function, and demonstrate how these principles can be applied to identify novel cancer-associated processes that are potential targets for therapeutic intervention.
Experimental section
Cell culture
A549 cells were cultured in Ham’s F12K medium with 10% FBS, 2 mM L-glutamine and 1.5 g/L sodium bicarbonate; H23, H358, H460, H661, H1703, H2170, and ChagoK1 cells were grown in RPMI supplemented with 10% FBS, 1 mM sodium pyruvate, 2mM L-glutamine, 10mM HEPES (Invitrogen), 4.5 g/L glucose and 1.5 g/L sodium bicarbonate. All media was also supplemented with 50 μg/ml gentamycin (all culture reagents from Invitrogen). Before plating for 3D lrECM cultures, cells were propagated for 7 days in 2D culture on plastic, and then seeded in growth media on top of a solidified layer of Matrigel (BD Biosciences), and then overlaid with a solution containing 2% matrigel, as was previously described.7 The cells were cultured for 5 days before fixation for immunofluorescence or harvesting RNA for transcriptional analysis.
Immunofluorescence
The 3D cultured cells were prepared for staining by subsequent washes with 15 % and 30 % sucrose solution in PBS, after which the cells were diluted in serum free culture media, and smeared on glass slides to air dry. The cells were fixed with 4 % formaldehyde, permeabilized with 0.1 % Triton X100 solution in PBS, and blocked for 1 hour in an immunofluorescence buffer (0.2 % Triton X100, 0.1 % BSA, 0.05 % Tween 20 in PBS) plus 5 % goat serum. Primary incubation with antibodies targeting Ki-67 or E-cadherin diluted 1:100 was performed for 2 hours. Secondary incubation used antibodies conjugated with Alexa Fluor 488, diluted 1:200 in blocking buffer and incubated for 1 hour. The cells were also stained with Hoechst 33342 1:10000 for 10 minutes, and Alexa fluor 594 phalloidin 1:500 for 30 minutes. All steps were performed at room temperature. All antibodies and cell staining reagents were from Invitrogen. Images were acquired using an Olympus IX71 fluorescent microscope. The percentage of Ki-67 positive cells with Hoechst stained nuclei was determined as an average staining of four fields of about 30–40 cells for each cell line.
Transcriptional analysis
After the cells were grown in either 2D or 3D culture for 5 days, RNA was isolated and analyzed using previously described methods.37 Briefly, RNA was labeled and hybridized to Affymetrix GeneChip Human Genome U133 Plus 2.0 arrays (Affymetrix) at the Mayo Clinic core facility. The GCRMA function of the GeneSpring software (Agilent) was used for processing, normalization and background correction. For identification of genesets that substantially and significantly distinguished 2D vs. 3D or smooth vs. ‘invasive’, transcripts were filtered for 2-fold average expression differences between categories and analyzed for ANOVA using parametric tests with variances not assumed equal (Welch t-test) and Benjamini and Hochberg False Discovery Rate multiple testing correction. Gene Ontology was analyzed using GeneSpring. The NextBio platform (www.nextbio.com) was utilized for meta-analysis of differentially expressed genes in order to identify significantly associated data sets. Kaplan-Meier survival curves were performed using GraphPad Prism.
Supplementary Material
Acknowledgments
We thank Dr. Evette Radisky for helpful discussions. We gratefully acknowledge the Mayo Clinic Advanced Genomic Technology Center Microarray Shared Resource. This work was supported by the National Institutes of Health grant R01 CA122086, by the James and Esther King Biomedical Research Program grant 07KN-09, and by the Office of Biological and Environmental Research of the Department of Energy.
Footnotes
Financial support: This work was supported by the National Institutes of Health grant R01 CA122086, by the James and Esther King Biomedical Research Program grant 07KN-09, and by the Office of Biological and Environmental Research of the Department of Energy.
References
- 1.Jemal A, Center MM, Ward E. The convergence of lung cancer rates between blacks and whites under the age of 40, United States. Cancer Epidemiol Biomarkers Prev. 2009;18:3349–52. doi: 10.1158/1055-9965.EPI-09-0740. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Gazdar AF, Girard L, Lockwood WW, Lam WL, Minna JD. Lung cancer cell lines as tools for biomedical discovery and research. J Natl Cancer Inst. 2010;102:1310–21. doi: 10.1093/jnci/djq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Wistuba, Bryant D, Behrens C, Milchgrub S, Virmani AK, Ashfaq R, Minna JD, Gazdar AF. Comparison of features of human lung cancer cell lines and their corresponding tumors. Clin Cancer Res. 1999;5:991–1000. [PubMed] [Google Scholar]; (b) Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–88. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Batran SE, Astner ST, Supthut M, Gamarra F, Brueckner K, Welsch U, Knuechel R, Huber RM. Three-dimensional in vitro cocultivation of lung carcinoma cells with human bronchial organ culture as a model for bronchial carcinoma. Am J Respir Cell Mol Biol. 1999;21:200–8. doi: 10.1165/ajrcmb.21.2.3205. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chen HY, Yu SL, Chen CH, Chang GC, Chen CY, Yuan A, Cheng CL, Wang CH, Terng HJ, Kao SF, Chan WK, Li HN, Liu CC, Singh S, Chen WJ, Chen JJ, Yang PC. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]; (b) Raponi M, Zhang Y, Yu J, Chen G, Lee G, Taylor JM, Macdonald J, Thomas D, Moskaluk C, Wang Y, Beer DG. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 2006;66:7466–72. doi: 10.1158/0008-5472.CAN-06-1191. [DOI] [PubMed] [Google Scholar]; (c) Shedden K, Taylor JM, Enkemann SA, Tsao MS, Yeatman TJ, Gerald WL, Eschrich S, Jurisica I, Giordano TJ, Misek DE, Chang AC, Zhu CQ, Strumpf D, Hanash S, Shepherd FA, Ding K, Seymour L, Naoki K, Pennell N, Weir B, Verhaak R, Ladd-Acosta C, Golub T, Gruidl M, Sharma A, Szoke J, Zakowski M, Rusch V, Kris M, Viale A, Motoi N, Travis W, Conley B, Seshan VE, Meyerson M, Kuick R, Dobbin KK, Lively T, Jacobson JW, Beer DG. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med. 2008;14:822–7. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE, Lin L, Chen G, Gharib TG, Thomas DG, Lizyness ML, Kuick R, Hayasaka S, Taylor JM, Iannettoni MD, Orringer MB, Hanash S. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–24. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]; (e) Kadara H, Behrens C, Yuan P, Solis L, Liu D, Gu X, Minna JD, Lee JJ, Kim E, Hong WK, Wistuba, Lotan R. A five-gene and corresponding protein signature for stage-I lung adenocarcinoma prognosis. Clin Cancer Res. 2011;17:1490–501. doi: 10.1158/1078-0432.CCR-10-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lau SK, Boutros PC, Pintilie M, Blackhall FH, Zhu CQ, Strumpf D, Johnston MR, Darling G, Keshavjee S, Waddell TK, Liu N, Lau D, Penn LZ, Shepherd FA, Jurisica I, Der SD, Tsao MS. Three-gene prognostic classifier for early-stage non small-cell lung cancer. J Clin Oncol. 2007;25:5562–9. doi: 10.1200/JCO.2007.12.0352. [DOI] [PubMed] [Google Scholar]; (g) Reed CE, Graham A, Hoda RS, Khoor A, Garrett-Mayer E, Wallace MB, Mitas M. A simple two-gene prognostic model for adenocarcinoma of the lung. J Thorac Cardiovasc Surg. 2008;135:627–34. doi: 10.1016/j.jtcvs.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Wan YW, Sabbagh E, Raese R, Qian Y, Luo D, Denvir J, Vallyathan V, Castranova V, Guo NL. Hybrid models identified a 12-gene signature for lung cancer prognosis and chemoresponse prediction. PLoS One. 2010;5:e12222. doi: 10.1371/journal.pone.0012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Peck K, Hong TM, Yang SC, Sher YP, Shih JY, Wu R, Cheng JL, Roffler SR, Wu CW, Yang PC. Global analysis of gene expression in invasion by a lung cancer model. Cancer Res. 2001;61:5223–30. [PubMed] [Google Scholar]
- 11.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen GD, Perou CM, Whyte RI, Altman RB, Brown PO, Botstein D, Petersen I. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 2001;98:13784–9. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH, Petersen OW, Gray JW, Bissell MJ. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, Bissell MJ. Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Res. 2006;66:7095–102. doi: 10.1158/0008-5472.CAN-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Martin KJ, Patrick DR, Bissell MJ, Fournier MV. Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS One. 2008;3:e2994. doi: 10.1371/journal.pone.0002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd M, Bressendorff S, Moller J, Olsen J, Troelsen JT. Mapping of HNF4alpha target genes in intestinal epithelial cells. BMC Gastroenterol. 2009;9:68. doi: 10.1186/1471-230X-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall AM, Pai VP, Sartor MA, Horseman ND. In vitro multipotent differentiation and barrier function of a human mammary epithelium. Cell Tissue Res. 2009;335:383–95. doi: 10.1007/s00441-008-0719-0. [DOI] [PubMed] [Google Scholar]
- 17.Hussain M, Rao M, Humphries AE, Hong JA, Liu F, Yang M, Caragacianu D, Schrump DS. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res. 2009;69:3570–8. doi: 10.1158/0008-5472.CAN-08-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maunders H, Patwardhan S, Phillips J, Clack A, Richter A. Human bronchial epithelial cell transcriptome: gene expression changes following acute exposure to whole cigarette smoke in vitro. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1248–56. doi: 10.1152/ajplung.00290.2006. [DOI] [PubMed] [Google Scholar]
- 19.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, Olson JA, Jr, Marks JR, Dressman HK, West M, Nevins JR. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–7. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 20.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–71. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 21.Boutros PC, Lau SK, Pintilie M, Liu N, Shepherd FA, Der SD, Tsao MS, Penn LZ, Jurisica I. Prognostic gene signatures for non-small-cell lung cancer. Proc Natl Acad Sci U S A. 2009;106:2824–8. doi: 10.1073/pnas.0809444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Boutros PC, Pintilie M, John T, Starmans MH, Der SD, Shepherd FA, Tsao MS, Jurisica I. Re: Gene expression-based prognostic signatures in lung cancer: ready for clinical use? J Natl Cancer Inst. 2010;102:1677–8. doi: 10.1093/jnci/djq385. author reply 1678–9. [DOI] [PubMed] [Google Scholar]; (b) Subramanian J, Simon R. Gene expression-based prognostic signatures in lung cancer: ready for clinical use? J Natl Cancer Inst. 2010;102:464–74. doi: 10.1093/jnci/djq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–35. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaughan MB, Ramirez RD, Wright WE, Minna JD, Shay JW. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74:141–8. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 25.(a) Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, Player A, Hong K, Settnek S, Gupta A, Buetow K, Hewitt S, Travis WD, Jen J. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res. 2004;10:4314–24. doi: 10.1158/1078-0432.CCR-03-0489. [DOI] [PubMed] [Google Scholar]; (b) Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res. 2003;63:560–6. [PubMed] [Google Scholar]
- 26.Bertucci F, Nasser V, Granjeaud S, Eisinger F, Adelaide J, Tagett R, Loriod B, Giaconia A, Benziane A, Devilard E, Jacquemier J, Viens P, Nguyen C, Birnbaum D, Houlgatte R. Gene expression profiles of poor-prognosis primary breast cancer correlate with survival. Hum Mol Genet. 2002;11:863–72. doi: 10.1093/hmg/11.8.863. [DOI] [PubMed] [Google Scholar]
- 27.Linnerth NM, Sirbovan K, Moorehead RA. Use of a transgenic mouse model to identify markers of human lung tumors. Int J Cancer. 2005;114:977–82. doi: 10.1002/ijc.20814. [DOI] [PubMed] [Google Scholar]
- 28.Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–95. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glesne DA, Zhang W, Mandava S, Ursos L, Buell ME, Makowski L, Rodi DJ. Subtractive transcriptomics: establishing polarity drives in vitro human endothelial morphogenesis. Cancer Res. 2006;66:4030–40. doi: 10.1158/0008-5472.CAN-05-3294. [DOI] [PubMed] [Google Scholar]
- 30.(a) Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem. 2007;282:23725–36. doi: 10.1074/jbc.M608531200. [DOI] [PubMed] [Google Scholar]
- 31.Radisky DC, Bissell MJ. Matrix metalloproteinase-induced genomic instability. Curr Opin Genet Dev. 2006;16:45–50. doi: 10.1016/j.gde.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 33.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, Ijiri K, Akimitsu N. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584:4575–80. doi: 10.1016/j.febslet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 35.(a) Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313:3066–75. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, Mossi R, Pujuguet P, Hager G, Bissell MJ. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol. 1998;18:2184–95. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pujuguet P, Radisky D, Levy D, Lacza C, Bissell MJ. Trichostatin A inhibits beta-casein expression in mammary epithelial cells. J Cell Biochem. 2001;83:660–70. doi: 10.1002/jcb.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Xu R, Spencer VA, Bissell MJ. Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors. J Biol Chem. 2007;282:14992–9. doi: 10.1074/jbc.M610316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.(a) Lelievre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci U S A. 1998;95:14711–6. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 37.Chen CS, Nelson CM, Khauv D, Bennett S, Radisky ES, Hirai Y, Bissell MJ, Radisky DC. Homology with vesicle fusion mediator syntaxin-1a predicts determinants of epimorphin/syntaxin-2 function in mammary epithelial morphogenesis. J Biol Chem. 2009;284:6877–84. doi: 10.1074/jbc.M805908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.