Abstract
Although T Central Memory cells have been described as the most effective T-cell subtype against tumor growth, little is known about the requirements needed for their optimal ex vivo generation. Hence, our goal is to establish a protocol that will lead to consistent ex vivo generation of lymphocytes skewed toward a Central Memory phenotype. Antigen-specific T-cell lines were generated by ex vivo stimulation with Class-I and Class-II melanoma peptide pulsed Dendritic Cells in the presence of either IL-2 or IL-15 plus IL-21. Tumor specific lymphocytes of both Central Memory and effector characteristics were consistently generated from healthy donors and melanoma patients. IL15/IL21 cultures result in a cell population with a lower proportion of CD4+ CD25highFoxP3+ regulatory cells and higher number of CD8+ and CD56+ cells, and consequently render a higher yield of cells with a greater cytolytic activity and IFN-γ production against melanoma cell lines.
Keywords: T Central Memory, Melanoma, Adoptive immunotherapy, IL-2, IL-15, IL-21
1. Introduction
The potential antineoplastic efficacy of adoptive immunotherapy (AIT) with antigen-specific T-cells is supported by studies in mice and melanoma patients [1]. Moreover, there is increasing evidence that anti-tumor specific T-cells, obtained after multiple rounds of in vitro stimulation, are highly effective cytotoxic cells in vitro, but may be less effective in vivo than naïve T-cells or Memory T-cells [2-4]. Thus, the description of a reliable and relatively brief method for the ex vivo expansion of human T Central Memory cells (TCM), characterized by the presence of homing receptors in their membrane, as well as by their capacity to rapidly proliferate after an encounter with an antigen, is a subject of great interest [4,5].
Interleukin-2 (IL-2), a potent lymphocyte growth factor, has often been the cytokine of choice for the in vitro expansion of human T-cells, but recent reports have documented that in vivo administration of high doses of IL-2 increased the pool of FoxP3+ regulatory cells in patients with metastatic melanoma and other carcinomas [6-9]. In addition, the role of IL-2 in activation-induced cell death of T-cells and inhibition of memory CD8+ T-cell proliferation is well documented [10,11].
On the other hand, preclinical data in mice has shown that T-cells grown in the presence of IL-15 or IL-21 [12-14] developed a TCM phenotype (defined as CD45RO+ CD62L+) with higher in vivo anti-tumor activity than effector T-cells. Both human and mouse data indicates that IL-15 and IL-21 are able to support the proliferation and anti-tumor activity of CD8 cells in a synergistic way[15-17].
Researchers designing anti-tumor vaccines frequently focus on the CD8 compartment, neglecting the role ofCD4 helper cells and other lymphocyte subpopulations, despite the fact that CD4 T-cells are able to reject tumor cells in the absence of CD8 cells in some mouse models [18]. However, their main role is probably to support the activation and proliferation of CD8 T-cells [19], and, although the ultimate mechanism by which the CD4 cells provide their help is not yet totally understood, frequently it is mediated by CD40 cross-linking in the DC, and the subsequent “licensing” of the DC [20-22]. Similarly, the cross-talk between DC and NK and NKT cells, as well as their proven capacity to exert anti-tumor activity [23-26], indicates that the CD56+ population plays a potentially valuable role. We hypothesize that the combination of multiple lymphocyte subpopulations, specificities and states of differentiation may represent an ideal strategy directed against tumor growth and may circumvent tumor evasion mechanisms. Thus, we chose to expand the whole lymphocyte compartment, rather than purifying the CD8+ population.
In this study, we describe a protocol for the production of a lymphocyte population enriched for tumor specific T-cells. To achieve specific proliferation of anti-tumor lymphocytes, healthy donor or melanoma patient lymphocytes were stimulated with autologous matured DC (mDC) pulsed with a pool of Class-I and Class-II melanoma antigens in the presence of IL-2 or IL-15 and IL-21, followed by a non-specific anti CD3 plus IL-2 expansion. While the proportion of TCM cells in both groups were similar, lymphocytes cultured with IL15/IL21 expanded faster and the numbers of CD4+ CD25highFoxP3+ regulatory T-cells was three times lower. Lymphocytes induced in both protocols were antigen specific, as demonstrated by their ability to produce IFN-γ and exert cytolytic activity against a melanoma target, although the addition of IL15/IL21 to the cultures results in a significant increase in both lytic activity and IFN-γ production. Thus, we conclude that IL-15/IL-21 efficiently expands tumor-specific lymphocytes of both effector and memory phenotype, and that this population of lymphocytes exerts anti-tumor activity, offering the opportunity for use in melanoma clinical trials, obviating the negative factors associated with the use of IL-2.
2. Materials and methods
2.1. Human cells
PBMC were obtained from HLA-A2+ melanoma patients and healthy volunteers using an IRB approved protocol for apheresis. Lymphocyte and monocyte enriched fractions were isolated by elutriation, and cells were frozen in 10% human AB serum (Gemini Bio-Products, West Sacramento, CA) and 10% DMSO (Sigma, St. Louis, MO) and stored at −140°C. All subjects signed an informed consent form.
2.2. Peptides and cell lines
Peptides were prepared by New England Peptides (Gardner, MA), and the purity was >95% in all cases. Class-I peptides were MART1/Melan-A(26-352L) (ELAGIGILTV), NY-ESO-1(157-175) (SLLMWITQC), gp100(209-2172 M) (IMDQVPFSV), Tyrosinase(369-377) (YMDGTMSQV), and MAGE-A3(271-279) (FLWGPRALV) [27-31]. Class-II peptides were gp100(175-189) (GRAMLGTHTMEVTVY) and NY-ESO-1(157-170) (SLLMWITQCFLPVF) [32,33]. HLA-A2+ cell lines A375 (melanoma), SKOv3 (ovarian carcinoma), and the cell lines T2 and K562 were obtained from the American Type Culture Collection (ATCC) (Rockville, MD) and cultured following ATCC guidelines.
2.3. Generation of DC
Purified human monocytes were cultured in 75 ml gas-permeable VueLife culture bags (American Fluoroseal Corp., Gaithersburg, MD) with IL-4 (20 ng/mL) (PeproTech, Rocky Hill, NJ) and GM-CSF (500 IU/ml) (Berlex Laboratories, Montville, NJ) in serum free AIM-V media (GIBCO). Maturation of DC was induced by LPS (100 ng/ml) (List Biological Laboratories, Campbell, CA) and IFN-γ (20 ng/ml) (Schering, Kenilworth, NJ).
2.4. Generation of antigen-specific CTL
Lymphocytes were isolated from apheresis of HLA-A2+ healthy donors or melanoma patients. Matured DC were pulsed with a pool of peptides at 10 μg/mL in serum-free RPMI (Hyclone, South Logan, UT) for 2 h at room temperature. Autologous mDC were then added to lymphocytes at a ratio of 1:10 in 12 well tissue culture plates in RPMI supplemented with penicillin/streptomycin (50 lg/mL) and 10% human AB serum. Beginning 48 h later, CTL cultures were supplemented with either IL-2 (20 U/ml) (Novartis, Emoryville, CA) or IL-15 and IL-21 (10 μg/ml) (PeproTech). Fresh media and cytokines were added every 3-4 days. Ten days later cultures were re-stimulated with OKT3 (50 μg/ml) (Ortho Biotech Products, Bridgewater, NJ) and IL-2 (100 IU/ml) in the presence of irradiated autologous PBMC (1 × 106 cells/ml). The cell number was evaluated every 2-3 days, and the cells were diluted to a concentration of 0.5-1 × 106 cell/mL with fresh media and IL-2.
2.5. Flow cytometry
Flow cytometry was performed on a FACSCanto (BD Biosciences, San Jose, CA). Two phycoerythirin (PE)-labeled tetramers consisting of HLA-A2 folded with NY-ESO-1 and MAGE-A3 peptides were provided by the NIH Tetramer Core Facility. MART1/Melan-A and negative PE-labeled tetramers were purchased from Beckman Coulter. Fluorochrome conjugated anti-human antibodies were specific for CD3 (UCHT1), CD4 (OKT4), CD8 (RPA-T8), CD44 (IM7), CD45R0 (UCHL1), CD56 (MEM188), CD62L (Dreg56), CD80 (2D10) and IL-15Rα (JM7A4). All antibodies were from eBioscience (San Diego, CA) except CD56 (BD Biosciences). Apoptotic cells were analyzed by using Annexin V-apoptosis Detection Kit (eBioscience) according to the manufacturer’s instructions. Acquired data was analyzed using FlowJo software (Tree Star, Ashland, OR).
2.6. ELISPOT
Flat-bottomed, 96-well nitrocellulose-lined plates (Millipore, Bedford, MA) were coated with IFN-γ mAb (1-D1K) (Mabtech, Cincinnati, OH) and incubated o.n. at 4 °C. After washing with coating buffer, plates were blocked with 10% human AB serum for 2 h at 37 °C. Effector and target cells were incubated together for 20 h. After washing plates with 0.05% Tween 20 in PBS, a biotinylated secondary IFN-γ mAb (7-B6-1) (Mabtech) was added. After incubation for 2 h at 37 °C, the plates were washed and developed with streptavidin-alkaline phosphatase for 1 h at room temperature and then fresh substrate was added and the plates incubated for ≤5 min. Spots were counted with an Automated ELISPOT Reader System with KS 4.3 software (Carl Zeiss).
2.7. Cytotoxicity assays
Cytotoxic activity was analyzed in a standard Cr51 release assay as previously described [34]. Briefly, 1 106 target cells were incubated with 50 lCi of Cr51 for 2 ×h at37 °C. Labeled cells were washed three times then incubated with ranging numbers of effector cells. For the cold blocking experiments, 40 cold K562 per 1 Cr51 labeled target cell were added to every well. After a 4 h incubation, 100 ll of each supernatant was harvested and radioactivity counted.
2.8. Statistical analyses
Normality of distributions was assessed using the Saphiro-Wilk’s test. Normal distributions were compared by the Student’s t test; non-normal samplings were compared using the Mann-Whitney test. Values of p inferior to 0.05 were considered significant. All tests were done using Prism 5 software (GraphPad).
3. Results
3.1. Tumor-specific T-cells are readily expanded from healthy donors and melanoma patients
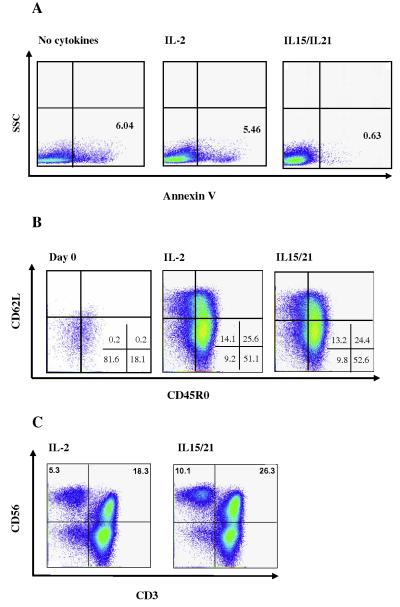
To evaluate the feasibility of ex vivo expansion of tumor specific Tcells, PBMC were obtained from HLA-A2+ healthy donors and melanoma patients. DC were matured with LPS and IFN-γ, so the high affinity sub-unit receptor IL-15α was up-regulated, enabling trans-presentation of IL-15 to the T-cells (Fig. 1) [35]. At the end of the enrichment phase (day 10) the total number of cells decreased in both the IL-2 and IL-15/IL-21 conditions compared to the starting number of lymphocytes (mean fold of expansion: IL-2 0.56; IL-15/IL-21 0.87). Cultures were then expanded with OKT3 and IL-2 in the presence of irradiated allogenic PBMC [36]. Five days after the re-stimulation (day 15), the total number of cells was evaluated. A significant cell expansion was achieved, especially in the IL-15/IL-21 group (IL-2 mean fold expansion 1.96; IL-15/IL-21 3.91). We found markedly lower number of apoptotic cells (Annexin V positive) in the IL15/IL21 cultures (Fig. 2a).
Fig. 1.
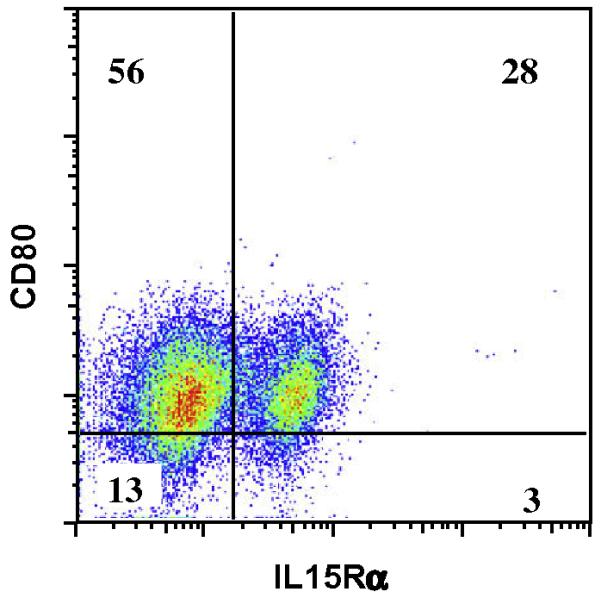
Matured DCs up-regulate CD80 and IL-15Rα. Monocyte derived DCs were grown in serum free media with IL-4 (20 μg/ml) and GM-CSF (500 IU/ml), and at day 6 they were matured or not with LPS (100 ng/ml) and IFN-γ (20 ng/ml). FACS analysis of matured DCs shows an up-regulation of CD80 and IL-15Rα after maturation. Shown are representative dot plots gated on CD11c+ cells and the numbers in the quadrants represent the percentages of the gated population.
Fig. 2.
Phenotypic analyses of cultures. (A) Lymphocytes from a melanoma patient were stimulated with autologous DC pulsed with a pool of Class-I and II peptides, and cultured in the presence of IL-2 (middle panel), IL-15/IL-21 (right panel) or no cytokines (left panel). The percentages of apoptotic cells were determined by Annexin V staining. (B) Lymphocytes from a melanoma patient were stimulated with autologous DC pulsed with a pool of Class-I and II peptides, and cultured in the presence of either IL-2 or IL-15/IL-21 and 10 days later were re-stimulated with αCD3, autologous PBMC and IL-2. Their phenotypes were analyzed at day 0 (left panel) and 5 days after the re-stimulation (day 15, middle and right panels). T-cells with a TCM phenotype were found in both culture conditions. Cells were pre-gated on CD3+ CD8+ cells. (c) Day 15 analysis of CD3 and CD56 on pre-gated CD8+ cells. Data shown is representative of two healthy donors and two melanoma patients.
We then examined the cell phenotype pre- and post-expansion. After 15 days of culture, we were able to identify CD8+ and CD4+ cells with a TCM phenotype, (CD45R0+ CD62L+) [37] in both culture conditions, with no significant difference between them (Table 1 and Fig. 2b). Finally, the percentage of CD56+ and CD8+ CD56+ cells was significantly higher in the IL-15/21 cultures (Table 1 and Fig. 2c).
Table 1.
Phenotypic analysis of expanded T-cells. Lymphocytes from two melanoma patients and two healthy donors were cultured in either IL-2 or IL-15 plus IL-21, in the presence or not of peptide pulsed autologous DC. Every condition was re-stimulated 10 days later with αCD3, autologous PBMC and IL-2. As a control we also include one condition without cytokines. The phenotypes of the cultured lymphocytes were analyzed 5 days after the re-stimulation. Numbers show the average percentage of positive cells. Bracketed numbers show the lowest and highest values.
| CD4+a | CD8+a | CD8+CD56+a | CD3–CD56+b | CD4+CD45RO+a | CD62L+c | CD8+CD45RO+a | CD62L+d | CD25HighFoxP3+e | |
|---|---|---|---|---|---|---|---|---|---|
| IL2 | 43.2 (17–62) | 47 (33–76) | 4.9 (0.2–17) | 3.9 (0.7–10.3) | 41(16–54) | 73.2 (65–81) | 30.7 (9–51) | 63.2 (44–72) | 6.0 (4.1–9.2) |
| IL15/21 | 34.3 (8–54) | 51.3 (42–66) | 10.1 (1–36) | 10.3 (7.4–13.3) | 29.5 (6.8–43) | 66.8 (60–78) | 33.3 (14–41) | 53.5 (42––59) | 1.8 (0.9–2.5) |
| IL2+DC | 55.5 (35–64) | 37.25 (29–59) | 5.3 (0.3–19) | 5.4 (1.5–7.8) | 53 (29–65) | 68.25 (64–71) | 24.3 (15–33) | 55.5 (45–71) | 7.0(5.8–8.1) |
| IL15/21+DC | 47.8 (22–66) | 44 (28–70) | 7.8 (0.4–28) | 8.2 (0.9–18) | 44.5 (18–64) | 72 (69–80) | 25 (15–38) | 54.8 (46–66) | 2.7 (1.5–3.3) |
| DC | 40.5 (21–59) | 52.8 (35–68) | 6.1 (0.2–21) | 9.9 (2.9–24.4) | 36.3 (20–54) | 65.3 (59–73) | 28.3 (17–42) | 54.8 (44–73) | 8 (6.2–9.7) |
Gated on CD3+ cells.
Gated on live cells.
Gated on CD4+CD45RO+ cells.
Gated on CD8+CD45RO+ cells.
Gated on CD3+CD4+ cells.
3.2. Addition of IL-15 and IL-21 effectively reduce the percentage of regulatory T-cell
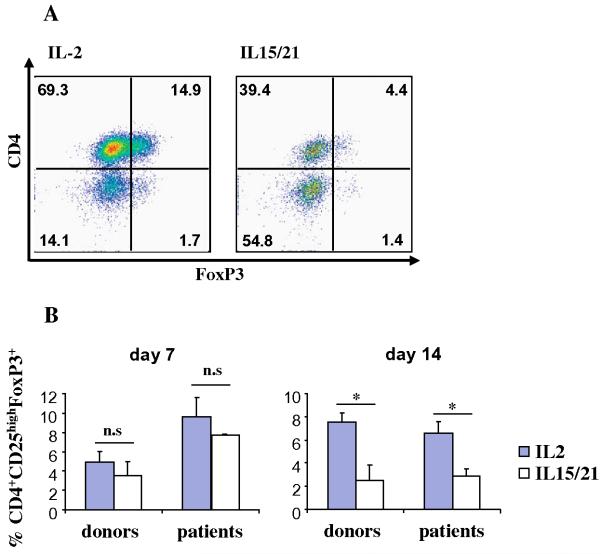
To determine whether the IL-2 or IL-15/IL-21 cytokines induce the proliferation of T regs, we evaluated the expression of CD25 and FoxP3 in the CD4 subpopulation at days 7 (during enrichment) and 15 (expansion phase) of the cultures. Interestingly, while at day 7 the proportion of CD4CD25highFoxP3+ cells were similar in both conditions, at day 14 to 15 we consistently detected a decrease in the proportion of T regs in the cultures with IL15/IL21 (Table 1 and Fig. 3).
Fig. 3.
Substitution of IL-2 by IL-15 plus IL-21 results in lower numbers of T regs. Lymphocytes from a melanoma patient were stimulated with autologous DC pulsed with a pool of Class-I and II peptides, and cultured in the presence of either IL-2 (left panel) or IL-15 plus IL-21 (right panel) and 10 days later were re-stimulated with αCD3, autologous PBMC and IL-2. (A) Phenotypic characterization of T regs. At day 15 of culture T-cells were stained for superficial markers CD3, CD4 and CD25 and then for intracellular FoxP3. Shown are representative dot plots gated on CD3+ CD25high cells. (B) The bars show percentages of T regs from two different patients and two healthy donors, analyzed at day 7 (left) and day 15 of the cultures (right).
3.3. The T-cells generated are specific against melanoma antigens
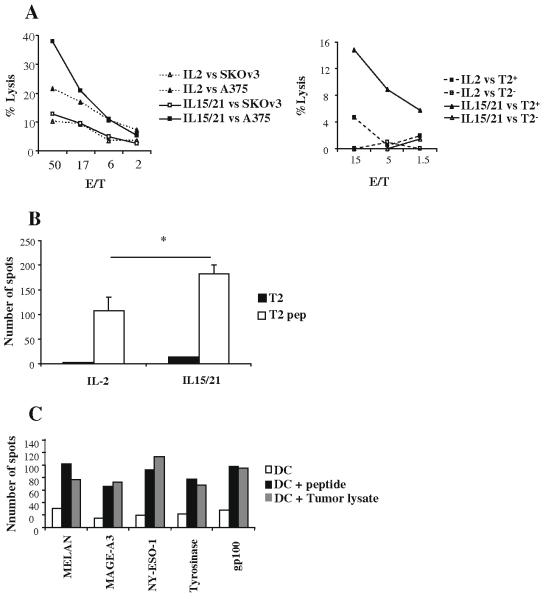
To examine whether the enrichment phase of the culture results in the expansion of a tumor specific T-cell population, we performed a Cr51 release assay against a melanoma cell line (A375) and an ovarian carcinoma cell line (SKOv3). As shown in Fig. 4a (left panel), cells expanded with the combination of IL-15/IL-21 had a higher lytic activity against the melanoma cells and had higher number of cells producing IFN-γ as measured by an ELISPOT assay (Fig. 4b). To see if the higher cytolytic activity in the IL-15/21 cultures was only due to the higher proportion of NK cells, we performed a cold blocking experiment, where an excess of cold NK target cell line K562 was added to every well. As shown in Fig. 4a (right panel), the IL-15/21 cultures still showed a higher lytic activity against peptide pulsed T2 cells when compared to the IL-2 cultures.
Fig. 4.
Expanded lymphocytes are specific against melanoma antigens. In (A left panel), lymphocytes from a melanoma patient were stimulated with peptide pulsed DC in the presence of IL-2 (dashed lines) or IL-15 plus IL-21 (solid lines) for 10 days, and expanded with αCD3 and IL-2 for another 5 days. Cytotoxic activity against A375 melanoma cell line (filled symbols) or an irrelevant SKOv3 ovarian carcinoma (open symbols) cell line was measured with a 4 h Cr51 release assay. Values represent triplicates at different E:T ratio. In (A, right panel) IL-2 (dashed lines) or IL15/21 (solid lines) expanded cultures were incubated with peptide pulsed or not T2 cells in the presence of cold K562, and cyototoxic activity was measured with a 4 h Cr51 release assay. Values represent triplicates at different E:T ratio. Data shown is representative of 2 independent experiments. In (B) we stimulated lymphocytes with IL-2 (left) or IL-15/21 (right) and then performed an IFN-γ ELISPOT against T2 cells (black columns) or peptide pulsed T2 (white columns). Columns represent the average of triplicate wells +/- SD. (C) Lymphocytes from a melanoma patient were cultured for 7 days with DC pulsed with the indicated individual peptides plus IL-2 and then we performed an IFN-γ ELISPOT against non pulsed DC (white columns), peptide pulsed DC (black columns) or melanoma A375 cell line tumor lysate (grey columns). Data representative of two different melanoma patients.
To test if any of the peptides used was able to induce superior cytotoxic activity, we stimulated lymphocytes from a healthy donor with DC pulsed with the individual Class-I peptides. Seven days later we performed an ELISPOT against unloaded DC, DC pulsed with the peptides or DC pulsed with a tumor lysate from the melanoma cell line. As shown in Fig. 4c, each of the individual peptides induced a specific immune response of similar intensity.
3.4. Emergence of tumor antigen specific CD8
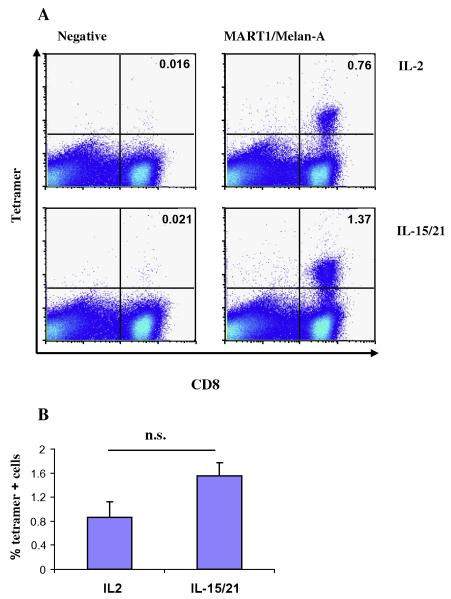
Using a MART1/Melan-A tetramer, we measured the percentage of tetramer specific cells for both the IL-2 and IL15/IL21 expanded cells. As shown in Fig. 5, although the differences were not statistically significant, we consistently found a higher percentage of tetramer positive CD8 cells when IL-15/IL-21 was used. This result was further confirmed with MAGE-A3 and NY-ESO-1 tetramers (data not shown).
Fig. 5.
MART1/Melan-A tetramer positive cells. (A) After 12 days of culture T-cells grown in IL-2 (upper panels) or IL15/IL21 (lower panels) were stained with a fluorochrome labeled MART1/Melan-A tetramer (right panels) or a negative control tetramer (left panels). Numbers denote the percentage of tetramer positive T-cells. Gated on CD3+ cells. (B) The bars show percentages of tetramer positive cells from four different samples.
4. Discussion
Despite the substantial progress that has been made in the last few decades in our understanding of the molecular and cellular events underlying T-cell mediated anti-tumor response [38], the clinical realization of effective therapeutic vaccines against vascularized tumors has not yet been widely achieved. Most of the protocols in which AIT is involved rely on the purification and further expansion of tumor-infiltrating lymphocytes (TIL), despite the inherent technical difficulty to obtain them in the most common malignancies. Even when TIL are obtained, in order to further expand their number, a rapid in vitro expansion protocol is required [2], and thus, the lymphocytes undergo a complete differentiation and acquire an effector phenotype, characterized by a high in vitro lytic activity [4]. However, some studies have documented that the adoptive transfer of TCM possessing enhanced lymphoid-homing properties provides superior immunity when compared with the transfer of other CD8 subpopulations [4,39]. Thus, the description of a reliable method for the production of large numbers of TCM from PBMC has recently become the subject of an extraordinary effort by the scientific community [23,40-44].
On the other hand, based probably on their ability to effectively kill tumor cells, but also by the concerns raised since the discovery of regulatory CD4 cells [45], CD8+ cells have become the main focus of several decades of research in anti-tumor immunotherapy, and consequently, most clinical trials rely on them. However, in the last few years a growing number of reports indicate that other lymphocyte subpopulations could have a positive impact when included in adoptive cellular therapy [18]. Consequently, we hypothesize that a mixture of T and effector CD8+ CM and CD4+ cells of different antigenic specificity, as well as NK and NKT cells will be better equipped to address the challenging environment associated with tumor development [46].
Here, we describe a new method for the expansion of anti-tumor lymphocytes based on the use of IL-15 plus IL-21 in the culture, in order to induce TCM cells [5,13], and the expansion of the whole lymphocyte compartment, instead of focusing merely on the CD8+ subpopulation. Due to the growing consensus that terminally differentiated T-cells are less effective in vivo than early effector or memory cells [4], we chose to give the cells only two cycles of in vitro stimulation. The first one was an enrichment phase, where T-cells were stimulated with autologous mDC pulsed with a pool of Class-I and Class-II melanoma antigens, followed by a non specific CD3 and IL-2 expansion. Our results demonstrate that the substitution of IL-2 with IL-15/IL-21 during the enrichment phase lead to a lower percentage of apoptotic cells, resulting in higher number of total cells after 14 days of culture. Contrary to our expectations, the percentage of TCM (CD45RO+ CD62L+) cells was not significantly different between groups. However, these results are in line with previous observations that low doses of IL-2 (1-5 ng/ml, in range with the 20 U/ml we were using) fail to develop fully differentiated effector cells [47-49]. According to other reports [50,51], the percentage of CD8 and CD56 cells, although variable between samples, was always higher when IL-15/IL-21 was used. Probably more relevant, the number of CD4+ CD25high- FoxP3+ cells after 2 weeks of culture was three times lower in the IL-15/IL-21 group.
Finally, the percentage of tetramer positive CD8 cells as well as the cytolytic activity and IFN-γ released against T2 targets loaded with melanoma peptides and tumor cell lines, was higher in the IL-15/IL-21 group. The very low cytolytic activity of the IL-2 cultures may be explained by the low dose of IL-2 in our cultures, in agreement with Manjunath’s and Mueller’s work [48,49]. Also, the greater proportion of NK cells could add to the higher cytolytic activity of the IL15/IL21 lymphocytes, as supported by cold blocking cytotoxic experiments.
In conclusion, we report a protocol for the efficient expansion of lymphocytes that, when cultured with the cytokines IL-15/IL-21, fulfill the in vitro desired characteristics of a heterogeneous mix of different lymphocyte subpopulations, specificities and states of differentiation, obviating the negative factors associated with the use of IL-2. The use of these expanded lymphocytes in an AIT treatment regimen may represent an effective strategy against tumor growth, and result in circumvention of tumor evasion mechanisms.
Acknowledgments
The authors wish to thank Dr. Randolph Noelle and Dr. Edward Usherwood for helpful discussions. We also thank the NIH Tetramer Core Facility for providing the NY-ESO-1 and MAGE tetramers. This work was supported by NIH Grants 5 RO1 CA95648 and 5 P20RR016437. Eduardo Huarte was partially supported by the Ramón Areces Foundation (Madrid, Spain).
Footnotes
5. Conflict of interest
The authors have no financial conflict of interest.
References
- [1].Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry RM, Marincola FM, Leitman SF, Seipp CA, Rogers-Freezer L, Morton KE, Nahvi A, Mavroukakis SA, White DE, Rosenberg SA. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J. Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Daudt L, Maccario R, Locatelli F, Turin I, Silla L, Montini E, Percivalle E, Giugliani R, Avanzini MA, Moretta A, Montagna D. Interleukin-15 favors the expansion of central memory CD8+ T cells in ex vivo generated, antileukemia human cytotoxic T lymphocyte lines. J. Immunother. 2008;31:385–393. doi: 10.1097/CJI.0b013e31816b1092. [DOI] [PubMed] [Google Scholar]
- [6].Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL. Characterization of CD4+ CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J. Clin. Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- [8].Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, Cost M, Cheng P, Chang A, Redman B, Herberman RB, Zou W. Interleukin-2 administration alters the CD4+ FOXP3+T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- [9].Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, Long LM, Bernstein D, Hill BJ, Douek DC, Berzofsky JA, Carter CS, Read EJ, Helman LJ, Mackall CL. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+ CD25+ regulatory T cells. Nat. Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- [10].Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ emory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- [11].Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- [12].Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;11:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- [15].Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J. Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- [16].Kishida T, Asada H, Itokawa Y, Cui FD, Shin-Ya M, Gojo S, Yasutomi K, Ueda Y, Yamagishi H, Imanishi J, Mazda O. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol. Ther. 2003;8:552–558. doi: 10.1016/s1525-0016(03)00222-3. [DOI] [PubMed] [Google Scholar]
- [17].Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005;201:139, 148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr. Opin. Immunol. 2009;2:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang LX, Shu S, Disis ML, Plautz GE. Adoptive transfer of tumor-primed, in vitro-activated, CD4+ T effector cells (TEs) combined with CD8+ TEs provides intratumoral TE proliferation and synergistic antitumor response. Blood. 2007;109:4865–4876. doi: 10.1182/blood-2006-09-045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- [21].Ribas A, Butterfield LH, Amarnani SN, Dissette VB, Kim D, Meng WS, Miranda GA, Wang HJ, McBride WH, Glaspy JA, Economou JS. CD40 cross-linking bypasses the absolute requirement for CD4 T cells during immunization with melanoma antigen gene-modified dendritic cells. Cancer Res. 2001;61:8787–8793. [PubMed] [Google Scholar]
- [22].Ahonen CL, Wasiuk A, Fuse S, Turk MJ, Ernstoff MS, Suriawinata AA, Gorham JD, Kedl RM, Usherwood EJ, Noelle RJ. Enhanced efficacy and reduced toxicity of multifactorial adjuvants ompared with unitary adjuvants as cancer vaccines. Blood. 2008;111:3116–3125. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meehan KR, Wu J, Webber SM, Barber A, Szczepiorkowski ZM, Sentman C. Development of a clinical model for ex vivo expansion of multiple populations of effector cells for adoptive cellular therapy. Cytotherapy. 2008;10:30–37. doi: 10.1080/14653240701762398. [DOI] [PubMed] [Google Scholar]
- [24].Vera M, Razquin N, Prieto J, Melero I, Fortes P, Gonzalez-Aseguinolaza G. Intratumoral injection of dendritic cells transduced by an SV40-based vector expressing interleukin-15 induces curative immunity mediated by CD8+ T lymphocytes and NK cells. Mol. Ther. 2005;12:950–959. doi: 10.1016/j.ymthe.2005.03.030. [DOI] [PubMed] [Google Scholar]
- [25].Stirnemann K, Romero JF, Baldi L, Robert B, Cesson V, Besra GS, Zauderer M, Wurm F, Corradin G, Mach JP, Macdonald HR, Donda A. Sustained activation and tumor targeting of NKT cells using a CD1d-anti-HER2-scFv fusion protein induce antitumor effects in mice. J. Clin. Invest. 2008;118:994–1005. doi: 10.1172/JCI33249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- [27].Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J. Exp. Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- [29].Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J. Exp. Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van der Bruggen P, Bastin J, Gajewski T, Coulie PG, Boel P, De Smet C, Traversari C, Townsend A, Boon T. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur. J. Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- [31].Wolfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde KH, Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur. J. Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- [32].Kobayashi H, Lu J, Celis E. Identification of helper T-cell epitopes that encompass or lie proximal to cytotoxic T-cell epitopes in the gp100 melanoma tumor antigen. Cancer Res. 2001;61:7577–7584. [PubMed] [Google Scholar]
- [33].Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc. Natl. Acad. Sci. USA. 2001;98:3964–3969. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huarte E, Sarobe P, Lu J, Casares N, Lasarte JJ, Dotor J, Ruiz M, Prieto J, Celis E, Borras-Cuesta F. Enhancing immunogenicity of a CTL epitope from carcinoembryonic antigen by selective amino acid replacements. Clin. Cancer Res. 2002;8:2336–2344. [PubMed] [Google Scholar]
- [35].Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc. Natl. Acad. Sci. USA. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- [38].Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- [39].Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- [40].Benlalam H, Vignard V, Khammari A, Bonnin A, Godet Y, Pandolfino MC, Jotereau F, Dreno B, Labarriere N. Infusion of Melan-A/Mart-1 specific tumor-infiltrating lymphocytes enhanced relapse-free survival of melanoma patients, Cancer Immunol. Immunother. 2007;56:515–526. doi: 10.1007/s00262-006-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dang Y, Knutson KL, Goodell V, dela Rosa C, Salazar LG, Higgins D, Childs J, Disis ML. Tumor antigen-specific T-cell expansion is greatly facilitated by in vivo priming. Clin. Cancer Res. 2007;13:1883–1891. doi: 10.1158/1078-0432.CCR-06-2083. [DOI] [PubMed] [Google Scholar]
- [42].Hirano N, Butler MO, Xia Z, Berezovskaya A, Murray AP, Ansen S, Nadler LM. Efficient presentation of naturally processed HLA class I peptides by artificial antigen-presenting cells for the generation of effective antitumor responses. Clin. Cancer Res. 2006;12:2967–2975. doi: 10.1158/1078-0432.CCR-05-2791. [DOI] [PubMed] [Google Scholar]
- [43].Oelke M, Moehrle U, Chen JL, Behringer D, Cerundolo V, Lindemann A, Mackensen A. Generation and purification of CD8+ Melan-A-specific cytotoxic T lymphocytes for adoptive transfer in tumor immunotherapy. Clin. Cancer Res. 2000;6:1997–2005. [PubMed] [Google Scholar]
- [44].Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- [46].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [47].Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- [48].Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mueller K, Schweier O, Pircher H. Efficacy of IL-2-versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur. J. Immunol. 2008;38:2874–2885. doi: 10.1002/eji.200838426. [DOI] [PubMed] [Google Scholar]
- [50].Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- [51].Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J. Immunol. 2000;164:1148–1152. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]