Abstract
Efficient access to a large chemical space based on new scaffolds with defined 3D conformations and highly variable in the side chains is needed to find novel functional materials. Four heterocyclic scaffolds based on a four component Ugi reaction of α-amino acids, oxo components, isocyanides and primary or secondary amines suitably functionalized are described. A handful of examples are described for each scaffold.
Ivar Ugi, half a century ago, pioneered the use of multicomponent reaction technology (MCR) for the discovery of novel agents with preferred properties. 1 MCR is now widely recognized for its impact on drug discovery projects and is strongly endorsed by academia in addition to industry. 2 Recent reviews on chemistry and biology of MCRs give comprehensive proof for the increasing number of marketed products based on MCRs including, for boceprevir,3 Retosiban,4 or Mandipropamid, just to name a few. Based on the sheer number of theoretically accessible MCR products, recent trends in drug discovery are geared toward leveraging MCR chemistry for use in various virtual screening tools. 6 For example, the recently introduced ANCHOR.QUERY freeware offers >25 million virtual MCR compounds for structure and web-based drug design proposes. 6c While there are hundreds of different MCRs described in the chemical literature, only a few are highly variable in all starting materials, such as the Ugi, Passerini, and van Leusen reactions. 1, 7 The size of the chemical space of the different MCR scaffolds has major implications on the usefulness of a particular MCR serving as a suitable virtual search space. Amongst the different strategies for the design of molecular complexity using MCR chemistry8 the post-Ugi secondary cyclization has been a very fruitful strategy in the past to accomplish novel scaffolds.
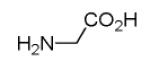
We have recently introduced a stereoselective Ugi-type reaction using four highly variable starting materials: primary or secondary amine 1, α-amino acid 2, oxo component 3, and isocyanide 4, thus comprising a novel truly 4-CR (Scheme 1). 9 Here, we would like to report on the design of Ugi post condensation reactions leading to four highly variable heterocyclic scaffolds.
Scheme 1.
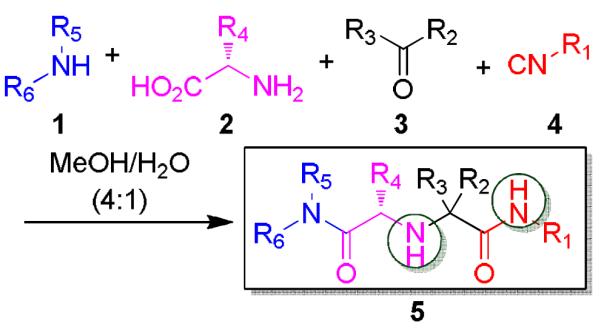
Previously reported Ugi 4-CR of α-amino acid, oxo-component, isocyanide and primary or secondary amine
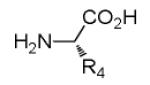
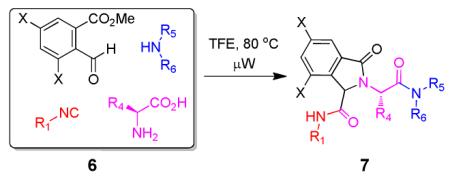
Due to the well-known functional group compatibility of the Ugi starting materials, we envisioned the construction of heterocyclic scaffolds involving the unique and reactive secondary amine formed during our Ugi reaction variation. Several intramolecular lactamizations based on different Ugi scaffolds have been reported in the past. 10 However, our synthesis differs from them by 1) yielding a different scaffold and One equivalent amount of amino acid, isocyanide, primary or secondary amine and methyl 2-formylbenzoate were added together in a microwave vial in one tenth molar concentration for one hour at 80°C. Crude reaction mixture was checked via supercritical fluid chromatography-mass spectrometry (SFC-MS) and both masses for the Ugi product and the newly cyclized product 7 could be seen from the mixture of 6. Work up was done to remove any unreacted amino acid and the reaction was heated again in ethanol. SFC-MS then revealed that only cyclized product 7 remained. Either one diastereomer precipitated out (7a and 7b) or crude mixture was purified via SFC (7c and 7d). When primary amines were used (7a and 7b), crude SFC traces revealed diastereomeric ratios of 7:3 and only the major diastereomer precipitated (see SI-21, SI-25). When secondary amines were used (7c and 7d), SFC traces using chiral cell OD revealed four stereoisomers in equal ratio, thus suggesting the racemization of the amino acid stereocenter by secondary amines as previously discovered. 9 (see SI-28, SI-29).
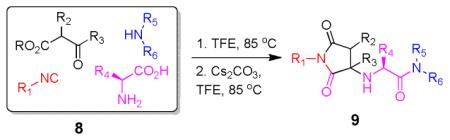
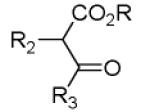
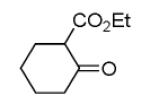
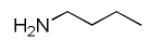
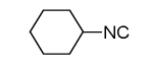
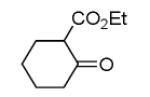
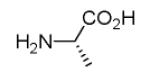
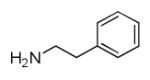
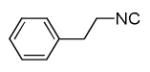
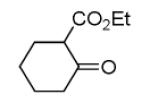
As reported previously, the Ugi reaction generally works well with aromatic and aliphatic aldehydes and ketones. Another available building block of suitable bifunctional orthogonal starting materials is the β-ketoester. In fact, when employing 2-oxocyclohexane carboxylic acid ethyl ester under heating conditions at 85°C, SFC-MS revealed the formation of the Ugi product as well as trace amounts of the corresponding pyrrolidinedione scaffold 9 (Table 2). Upon addition of 3 equivalents of cesium carbonate, complete cyclization of the Ugi product to the pyrrolidinedione 9 was observed. Benzyl 3-oxobutanoate also produced similar pyrrolidinedione 9d.
Table 2.
Cyclization of the Ugi 4-CR to yield the pyrrolidinedione scaffold
| Comp. | R1–NC |
|
|
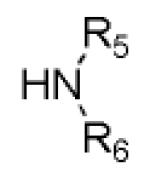
|
Yielda [%] |
|---|---|---|---|---|---|
| 9a |
|
|
|
|
40 |
| 9b |
|
|
|
|
49 |
| 9c |
|
|
|
|
45 |
| 9d |
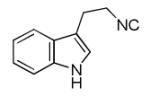
|
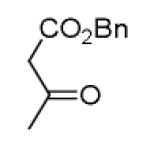
|
|
|
34 |
Overall yield in two steps in one pot (crude Ugi product was directly used for the cyclization).
Several analogous reactions were performed and all yielded the products in satisfactory yields (Table 2). The finding of this reaction is remarkable since several other orthogonal methods exist to access the pyrrolidinedione scaffold by isocyanide based MCRs. 11 Stereochemical analysis SFC-MS revealed that compound 9c gave a single diastereomer. However, 9b (9:1) and 9d (9.5:0.5) formed mixture of two distereomers (see SI-38,-41). The major distereomers were isolated using preparative TLC plate. Pyrrolidinedione 9a is exclusively formed as the cis relative stereoisomer. The x-ray structure of 9a shows the solid state conformation and also proves the overall structure of the scaffold (Fig 1).
Fig. 1.
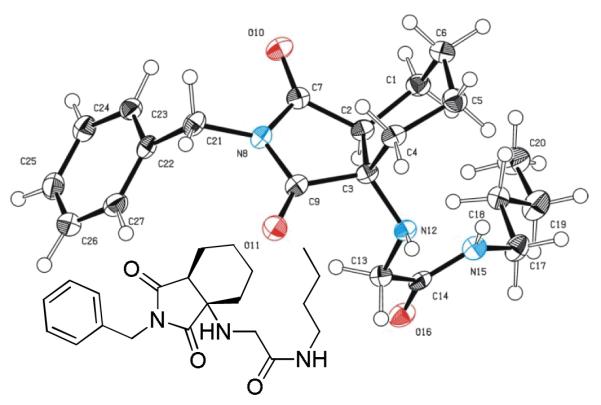
Structure of pyrrolidinedione product 9a in solid state.
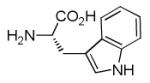
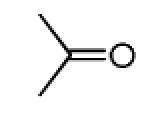
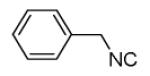
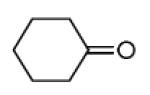
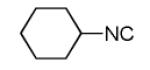
The Pictet-Spengler reaction recently became popular to perform Ugi post condensation reactions to yield complex polycyclic scaffolds. 12 For example the use of suitable starting materials allows for the convergent synthesis of the important schistosomiasis medication Praziquantel. 13 The required functional groups of the Pictet-Spengler reaction, an electron rich aromatic group such as tryptophan and an oxo group, can be conveniently introduced via the α-amino acid component and the primary amine component respectively (Table 3). By performing the Pictet-Spengler reaction under formic acid at room temperature conditions, we indeed could isolate the strained tricyclic 3,9-diazabicyclo[3.3.1]nonane system 11 from mixture 10. Several analogous reactions show the general character of this transformation (Table 3).
Table 3.
Pictet-Spengler cyclization of the Ugi 4-CR to yield the tricyclic 3,9-diazabicyclo[3.3.1] nonane scaffold
| Comp. | R1–NC |
|
Yielda [%] |
|---|---|---|---|
| 11a |
|
|
44 |
| 11b |
|
|
40 |
| 11c |
|
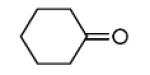
|
48 |
| 11d |
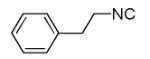
|
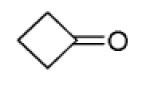
|
30 |
Overall yield in two steps in one pot (crude Ugi product was directly used for the Pictet-Spengler reaction).
Indolo annulated tricyclic 3,9-diazabicyclo[3.3.1]nonane systems or substructures thereof occur in several alkaloids, such as 11d. The herein described convergent synthesis could therefore be of use in the synthesis of the corresponding backbones. A crystal structure of 11d has been performed supporting the proposed scaffold structure (Fig 2).
Fig. 2.
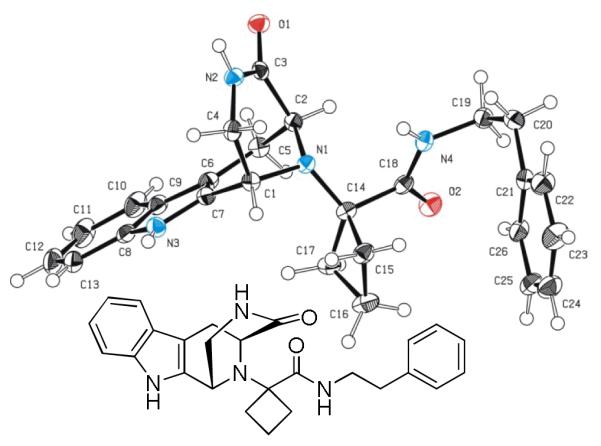
Structure of Ugi/Pictet-Spengler product 11d in solid state
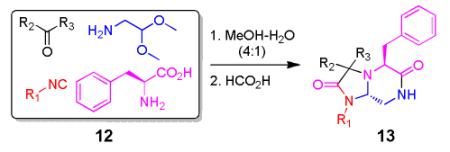
As a final example of how our new Ugi variation together with carefully chosen orthogonal functional groups in the other starting materials which can be used for the synthesis of novel and complex scaffolds involves the use of other electron rich aromatic α-amino acids such as phenylalanine (Table 4). Using similar conditions as seen in table 3 after replacing the amino acid as phenylalanine in the Ugi scaffold to yield the bicyclic tetrahydroimidazo[1,2-a]pyrazine-2,6(3H,5H)-dione scaffold 13 from mixture 12. Again, several performed syntheses show the generality of the reaction (Table 4). In all cases, the formation of only the trans diastereomer was observed. A crystal structure of 13c has been performed showing the formation of the trans stereoisomer and supporting the proposed overall scaffold structure (Fig 3).
Table 4.
Cyclization of the Ugi 4-CR to yield to yield bicyclic tetrahydroimidazo[1,2-a]pyrazine-2,6(3H,5H)-dione scaffold
| Comp. | R1–NC |
|
Yielda [%] |
|---|---|---|---|
| 13a |
|
|
63 |
| 13b |
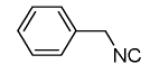
|
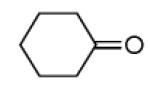
|
87 |
| 13c |
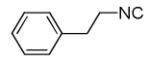
|
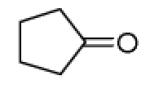
|
40 |
| 13d |
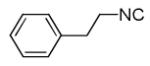
|
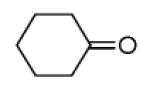
|
54 |
Overall yield in two steps in one pot (crude Ugi product was directly used for the cyclization).
Fig. 3.
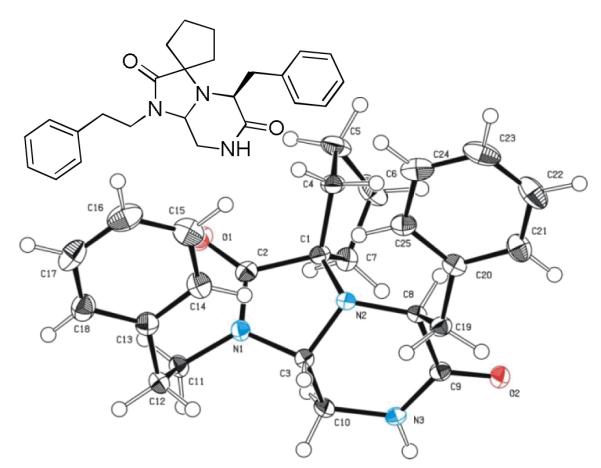
Bicyclic Ugi/condensation product 13c in solid state
Lastly, we were interested in the question of how some physiochemical properties of our scaffolds cluster and compare with other commonly used virtual screening libraries. Thus we calculated the descriptors cLogP, Rotatable Bonds, Molecular Weight, and other drug-like properties from a randomly generated library of 1000 compounds based on all four scaffolds (SI Fig 1-8). The majority of these compounds pass at least 3 out of 4 of Lipinski’s rule of 5; most of them however only fail the molecular weight rule. Interestingly the two later scaffolds (11 and 13) possess a low number of rotatable bonds and are highly stiff, increasing their chance of oral bio-availability. We were especially interested in the recently introduced shape descriptor principal moment-of-inertia (PMI) as a means to differentiate scaffolds based on shape. 14 These libraries were compared to 1000 randomly selected compounds from the Zinc database 15 and each library was found to possess more 3D characteristics than the Zinc compounds (Fig 4). As protein-protein interactions become an increasingly popular yet difficult target for pharmaceutical companies, novel chemical space needs to be explored.16 With novel scaffolds that possess more 3D features, the chances to successfully probe previously unmet medical needs greatly increases. Biological activities of compounds based on the new scaffolds will be reported in due course.
Fig. 4.
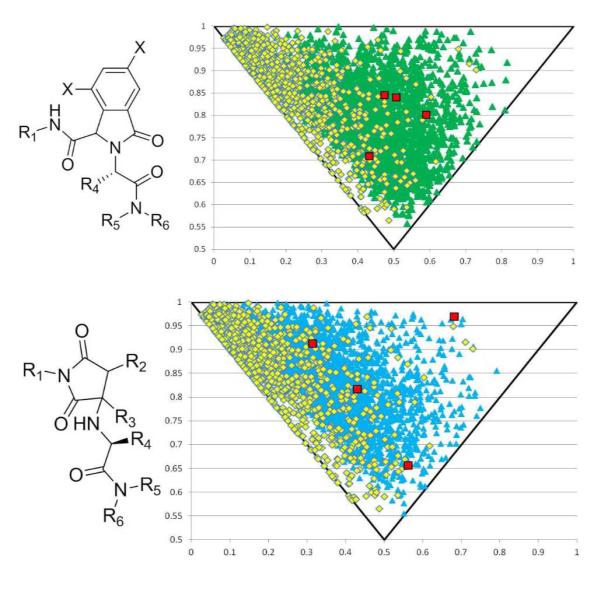
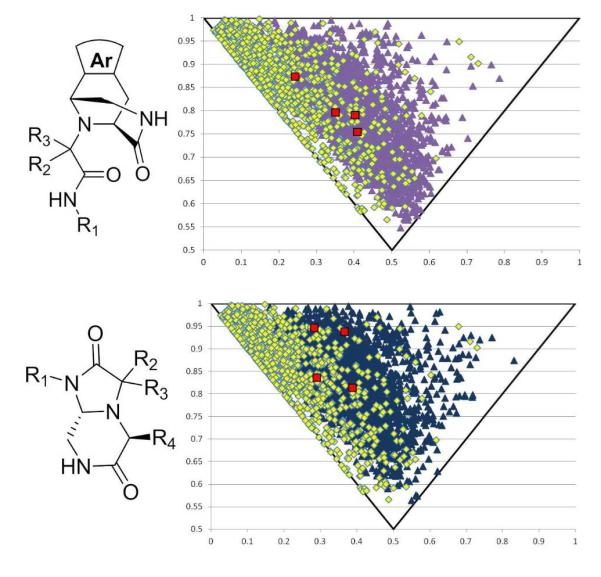
Principal Moment of Inertia (PMI) calculated for 1,000 randomly generated compounds for each of the four scaffolds 7 (green triangles), 9 (light blue triangles), 11 (purple triangles) and 13 (navy blue triangles) compared to 1,000 randomly chosen compounds from the Zinc Library15 (yellow squares). PMI of specific compounds from this paper can be seen in all four graphs as red squares.
Supplementary Material
Table 1.
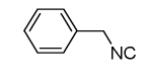
Cyclization of the Ugi 4-CR to yield the isoindolone scaffold
| Comp. | R1–NC |
|
|
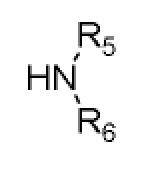
|
Yielda [%] |
|---|---|---|---|---|---|
| 7a |
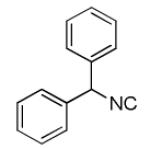
|
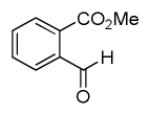
|
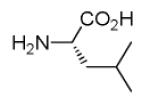
|
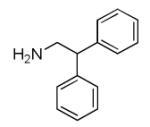
|
35 |
| 7b |
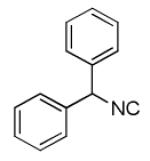
|
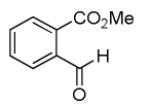
|
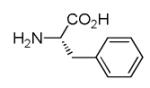
|
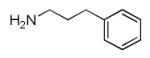
|
50 |
| 7c |
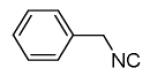
|
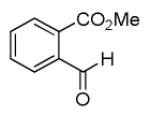
|
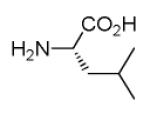
|
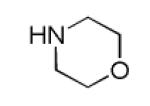
|
42 |
| 7d |
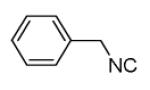
|
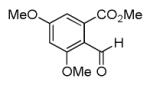
|
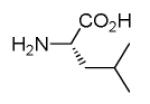
|
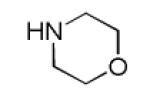
|
40 |
Overall yield in one step.
Acknowledgements
This work was supported partially by grants from NIH (1R21GM087617-01A1, 1P41GM094055-01, and 1R01GM09708201). KK acknowledge the ACS Medicinal Chemistry predoctoral fellowship (2011-2012).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures including the synthesis and characterizations of small molecules, crystal data, as well as the analysis of virtual libraries are provided. See DOI: 10.1039/b000000x/
Notes and references
- 1.Ugi I, Steinbrückner C. Angew. Chem. 1960;72:267–268. [Google Scholar]
- 2.Dömling A, Wang W, Wang K. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatraman S, Bogen SL, Arasappan A, Bennett F, Chen K, Jao E, Liu Y-T, Lovey R, Hendrata S, Huang Y, Pan W, Parekh T, Pinto P, Popov V, Pike R, Ruan S, Santhanam B, Vibulbhan B, Wu W, Yang W, Kong J, Liang X, Wong J, Liu R, Butkiewicz N, Chase R, Hart A, Agrawal S, Ingravallo P, Pichardo J, Kong R, Baroudy B, Malcolm B, Guo Z, Prongay A, Madison V, Broske L, Cui X, Cheng K-C, Hsieh Y, Brisson J-M, Prelusky D, Korfmacher W, White R, Bogdanowich-Knipp S, Pavlovsky A, Bradley P, Saksena AK, Ganguly A, Piwinski J, Girijavallabhan V, Njoroge FG. J. Med. Chem. 2006;49:6074–6086. doi: 10.1021/jm060325b. [DOI] [PubMed] [Google Scholar]
- 4.Liddle J, Allen MJ, Borthwick AD, Brooks DP, Davies DE, Edwards RM, Exall AM, Hamlett C, Irving WR, Mason AM, McCafferty GP, Nerozzi F, Peace S, Philp J, Pollard D, Pullen MA, Shabbir SS, Sollis SL, Westfall TD, Woollard PM, Wu C, Hickey DMB. Bioorg. Med. Chem. Lett. 2008;18:90–94. doi: 10.1016/j.bmcl.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Lamberth C, Jeanguenat A, Cederbaum F, De Mesmaeker A, Zeller M, Kempf H-J, Zeun R. Bioorg. Med. Chem. Lett. 2008;16:1531–1545. doi: 10.1016/j.bmc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 6 (a).Weber L, Wallbaum S, Broger C, Gubernator K. Angew. Chem. Int. Ed. 1995;34:2280–2282. [Google Scholar]; (b) Shoda M, Harada T, Kogami Y, Tsujita R, Akashi H, Kouji H, Stahura FL, Xue L, Bajorath J. J. Med. Chem. 2004;47:4286–4290. doi: 10.1021/jm040103i. [DOI] [PubMed] [Google Scholar]; (c) Koes D, Khoury K, Huang Y, Wang W, Bista M, Popowicz GM, Wolf S, Holak TA, Dömling A, Camacho CJ. PLoS ONE. 2012;7:e32839. doi: 10.1371/journal.pone.0032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 (a).Banfi L, Riva R. Org. React. John Wiley & Sons, Inc.; 2004. [Google Scholar]; (b) Van Leusen AM, Wildeman J, Oldenziel OH. J. Org. Chem. 1977;42:1153–1159. [Google Scholar]; (c) Passerini M, Simone L. Gazz. Chim. Ital. 1921;51:126–129. [Google Scholar]
- 8 (a).Weber L, Illgen K, Almstetter M. Synlett. 1999;1999:366–374. [Google Scholar]; (b) Ruijter E, Scheffelaar R, Orru RVA. Angew. Chem. Int. Ed. 2011;50:6234–6246. doi: 10.1002/anie.201006515. [DOI] [PubMed] [Google Scholar]; (c) Dömling A. Curr. Opin. Chem. Biol. 2000;4:318–323. doi: 10.1016/s1367-5931(00)00095-8. [DOI] [PubMed] [Google Scholar]
- 9.Khoury K, Sinha MK, Nagashima T, Herdtweck E, Dömling A. Angew. Chem. Int. Ed. 2012;51:10280–10283. doi: 10.1002/anie.201205366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10 (a).Marcos CF, Marcaccini S, Menchi G, Pepino R, Torroba T. Tetrahedron Lett. 2008;49:149–152. [Google Scholar]; (b) Zimmer R, Ziemer A, Gruner M, Brüdgam I, Hartl H, Reissig H-U. Synthesis. 2001;2001:1649–1658. [Google Scholar]; (c) Gunawan S, Ayaz M, De Moliner F, Frett B, Kaiser C, Patrick N, Xu Z, Hulme C. Tetrahedron. 2012;68:5606–5611. doi: 10.1016/j.tet.2012.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gunawan S, Keck K, Laetsch A, Hulme C. Mol. Diversity. 2012;16:601–606. doi: 10.1007/s11030-012-9373-2. [DOI] [PubMed] [Google Scholar]
- 11.Bossio R, Marcos CF, Marcaccini S, Pepino R. Synthesis. 1997;1997:1389–1390. [Google Scholar]
- 12 (a).Tyagi V, Khan S, Bajpai V, Gauniyal HM, Kumar B, Chauhan PMS. J. Org. Chem. 2012;77:1414–1421. doi: 10.1021/jo202255v. [DOI] [PubMed] [Google Scholar]; (b) Cano-Herrera MA, Miranda LD. Chem. Commun. 2011;47:10770–10772. doi: 10.1039/c1cc10759c. [DOI] [PubMed] [Google Scholar]; (c) Wang W, Ollio S, Herdtweck E, Dömling A. J. Org. Chem. 2010;76:637–644. doi: 10.1021/jo102058s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Znabet A, Zonneveld J, Janssen E, De Kanter FJJ, Helliwell M, Turner NJ, Ruijter E, Orru RVA. Chem. Commun. 2010;46:7706–7708. doi: 10.1039/c0cc02938f. [DOI] [PubMed] [Google Scholar]; (e) Wang W, Herdtweck E, Dömling A. Chem. Commun. 2010;46:770–772. doi: 10.1039/b917660h. [DOI] [PubMed] [Google Scholar]; (f) Liu H, Dömling A. J. Org. Chem. 2009;74:6895–6898. doi: 10.1021/jo900986z. [DOI] [PubMed] [Google Scholar]
- 13 (a).Cao H, Liu H, Dömling A. Chem. Eur. J. 2010;16:12296–12298. doi: 10.1002/chem.201002046. [DOI] [PubMed] [Google Scholar]; (b) Liu H, William S, Herdtweck E, Botros S, Dömling A. Chem. Biol. Drug Des. 2012;79:470–477. doi: 10.1111/j.1747-0285.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauer WH, Schwarz MK. J. Chem. Inf. Comput. Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- 15 (a).Irwin JJ, Shoichet BK. J. Chem. Inf. Model. 2005;45:177–182. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Coleman RG. J. Chem. Inf. Model. 2012;52:1757–2768. doi: 10.1021/ci3001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dömling A, Mannhold R, Kubinyi H, Folkers G. Protein-Protein Interactions in Drug Discovery (Methods and Principles in Medicinal Chemistry) Wiley-VCH; Weinheim, Germany: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


